Abstract

Alzheimer’s disease (AD) is a progressive degenerative disorder that results in a severe loss of brain cells and irreversible cognitive decline. Memory problems are the most recognized symptoms of AD. However, approximately 90% of patients diagnosed with AD suffer from behavioral symptoms, including mood changes and social impairment years before cognitive dysfunction. Recent evidence indicates that the dorsal raphe nucleus (DRN) is among the initial regions that show tau pathology, which is a hallmark feature of AD. The DRN harbors serotonin (5-HT) neurons, which are critically involved in mood, social, and cognitive regulation. Serotonergic impairment early in the disease process may contribute to behavioral symptoms in AD. However, the mechanisms underlying vulnerability and contribution of the 5-HT system to AD progression remain unknown. Here, we performed behavioral and electrophysiological characterizations in mice expressing a phosphorylation-prone form of human tau (hTauP301L) in 5-HT neurons. We found that pathological tau expression in 5-HT neurons induces anxiety-like behavior and alterations in stress-coping strategies in female and male mice. Female mice also exhibited social disinhibition and mild cognitive impairment in response to 5-HT neuron-specific hTauP301L expression. Behavioral alterations were accompanied by disrupted 5-HT neuron physiology in female and male hTauP301L expressing mice with exacerbated excitability disruption in females only. These data provide mechanistic insights into the brain systems and symptoms impaired early in AD progression, which is critical for disease intervention.
Keywords: Alzheimer’s disease, serotonin (5-HT), dorsal raphe nucleus, neuropsychiatric symptoms, tau, anxiety
Introduction
Alzheimer’s disease (AD), the most common form of dementia, is characterized by the accumulation of amyloid plaques and neurofibrillary tangles (NFTs), resulting in progressive neuronal degeneration.1 Cognitive impairment and memory loss are the most recognized symptoms of AD. However, neuropsychiatric disturbances, including social withdrawal, depression, anxiety, and aggression, are observed in 90% of patients2 and precede the onset of severe cognitive decline.3−6 These behavioral symptoms have been associated with an increased risk of conversion from mild cognitive impairment (MCI) to AD.7 Given the early occurrence of neuropsychiatric symptoms (NPS), understanding the mechanisms underlying NPS has the potential to significantly enhance our current understanding of AD symptomatology and pathology.
Serotonin (5-HT) plays a significant modulatory role in neuropsychological and cognitive function8,9 and has been increasingly recognized to play a role in early AD progression. Postmortem studies have reported early loss of 5-HT neurons along with NFTs in the raphe nucleus of AD patients.10−12 The dorsal raphe nucleus (DRN), where the majority of 5-HT neurons reside, is one of the first brain regions to show neurofibrillary pathology, even before the transentorhinal region in AD.13−17 Imaging studies have reported reduced volume18 and DRN functional connectivity in AD and MCI patients.19,20 A deficiency in 5-HT and early-stage serotonergic dysfunction may underlie the emergence of early NPS.21−23 5-HT neurons are also the main target of SSRIs, which have been associated with delayed progression from MCI to AD dementia24 and have been shown to be neuroprotective in AD animal models.25−27 Despite the association between 5-HT dysfunction and AD, little is known about how 5-HT neurons are affected in the early stages of the disease and how this impairment plays a role in pathology and behavioral symptoms.
Here, we sought to model the early neurofibrillary pathology in 5-HT neurons to understand its impact on 5-HT neuron function and behavior. To selectively induce tau pathology in 5-HT neurons, we infused a Cre-dependent adeno-associated virus (AAV) encoding the human tauP301L (hTauP301L) protein in the DRN of ePet1::Cre (ePet1hTauP301L) mice, enabling the targeted expression of a hyperphosphorylation-prone tau protein specifically in 5-HT neurons. We report the first evidence that 5-HT neuron-specific hTauP301L expression in young (2–4 months old) mice leads to deficits in the physiology of 5-HT neurons, resulting in increased anxiety-like behavior and altered stress-coping strategies. These data have important mechanistic implications for the emergence of preclinical symptoms prior to AD diagnosis.
Results and Discussion
Characterization of hTauP301L Expression in 5-HT Neurons
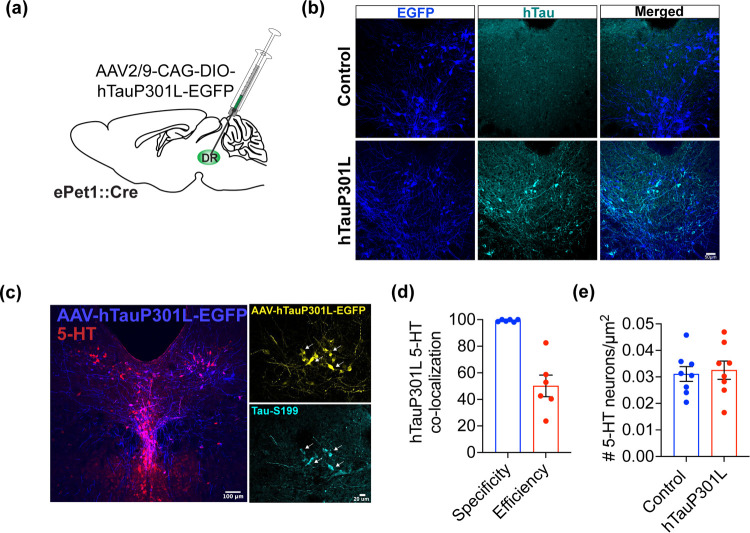
To induce pathological tau expression in 5-HT neurons, we infused a Cre-dependent AAV encoding the hyperphosphorylation-prone EGFP-fused hTauP301L protein into the DRN of 8-week old ePet1::Cre mice (ePet1hTauP301L) (Figure 1a). The controls received only the fluorescent reporter. We detected hTau expression in EGFP-positive cells of ePet1hTauP301L, whereas controls did not show hTau expression, as expected (Figure 1b). Three weeks after infusion, we verified the presence of EGFP expression in 5-HT neurons and its co-localization with phosphorylated tau expression (Figure 1c). We found that >90% of EGFP-positive cells also expressed 5-HT (specificity), while approximately 50% of 5-HT cells were co-labeled with EGFP (efficiency) (Figures 1d and S1a). To determine whether hTauP301L expression leads to the degeneration of 5-HT neurons, we perfused mice 8 weeks after viral infusion and analyzed the density of 5-HT neurons in the DRN. 5-HT neuron density was comparable between control and hTauP301L-infused mice, indicating that the expression of hTauP301L did not result in a detectable loss of 5-HT cells at this time point (unpaired t-test, p = 0.75) (Figure 1e). Moreover, when analyzed 8 weeks after viral infusion, hyperphosphorylated tau was mostly confined to neuronal processes (Figure S1b). We did not observe compensatory alterations in endogenous mouse Tau (mTau) expression in mice upon hTauP301L infusion (Figure S1c,d). Of note, it is possible that expression of hTauP301L has an impact on the number of 5-HT axons in target regions, with potential alterations in 5-HT release, which should be investigated in future studies.
Figure 1.
Expression of hTauP301L in DRN 5-HT neurons. (A) Schematic showing infusion of AAV2/9-CAG-DIO-hTauP301L-EGFP into the DRN of ePet1::Cre mice. (B) Representative images from a control and an ePet1hTauP301L mouse DRN immunostained for EGFP and hTau. Scale bar, 50 um. (C) Representative images showing co-localization of AAV2/9-CAG-DIO-hTauP301L-EGFP (blue) and serotonin (5-HT; red). Scale bar, 100 um. Right: Localization of S199 phosphorylation (cyan) in 5-HT neurons expressing hTauP301L (yellow). Scale bar, 20 um. (D) Quantification of specificity (% virus-labeled cells expressing 5-HT) and efficiency (% 5-HT cells coexpressing the virus) (n = 6 mice). (E) 5-HT neuron density between control (n = 8) and ePet1hTauP301L (n = 8) mice is comparable.
Our findings indicate that the initial somatic expression of hTauP301L in 5-HT neurons gradually becomes predominantly confined to axodendritic compartments over time, resembling the pathology observed in tauopathies (for review, see ref (28)). The absence of detectable neurodegeneration after a relatively early stage, specifically 8 weeks postviral infusion, in 4-month-old mice is consistent with the findings observed in mouse models displaying early tau pathology without neuronal or synaptic loss.29
hTauP301L Expression in 5-HT Neurons Leads to Anxiety-like Behavior
Tau-induced neurofibrillary changes have previously been detected in postmortem tissue from the DRN of AD patients; however, whether the pathological tau in 5-HT neurons during early adulthood contributes to the socioemotional dysfunction observed in the preclinical stages of AD remains to be elucidated. To determine the effect of phosphorylation-prone tau expression specifically in 5-HT neurons on anxiety-like behavior, we first infused Cre-dependent AAV encoding hTauP301L or EGFP in female and male ePet1::Cre mice and performed open-field and elevated maze tests 4 weeks after viral infusion. In the open-field test, female and male ePet1hTauP301L mice spent significantly less time in the center and greater time in the outer zones, suggesting increased anxiety-like behavior, compared with controls (Figure 2a–c). Overall, females spent less time in the center and more time in the outer zone than males (two-way ANOVA; Center zone effect of group: F(1, 34) = 4.85, p = 0.034, effect of sex: F(1, 34) = 10.32, p = 0.003; outer zone effect of group: F(1, 34) = 4.85, p = 0.034, effect of sex: F(1, 34) = 10.32, p = 0.003). hTauP301L females traveled a greater distance than did the control females, indicating higher levels of locomotor activity. There was no difference in locomotor activity in males in the open-field test (Figure S2a–c).
Figure 2.
hTauP301L expression in 5-HT neurons induces anxiety-like behavior. (A) Averaged heat maps showing the time spent in open field in control (n = 8 females, 12 males) and ePet1hTauP301L (n = 9 females, 9 males) mice. (B) Female and male ePet1hTauP301L mice spent less time in the center zone and, (C) greater time in the outer zone of the open field, indicating greater anxiety-like behavior. (D) Averaged heat maps showing the time spent in an elevated plus maze in control (n = 8 females, 12 males) and ePet1hTauP301L (n = 10 females, 9 males) mice. (E) Female and male ePet1hTauP301L mice spent less time in the open arms and (F) showed a tendency to remain more in the closed arms, indicating greater anxiety-like behavior. *p < 0.05, **p < 0.01, #p < 0. 1.
Two days after completion of the open field, we performed elevated plus maze (EPM). In this test, female and male ePet1hTauP301L mice spent significantly less time in the open arms and showed a tendency of increased time in the closed arms, compared with the controls, suggesting an enhanced anxiety-like behavior (two-way ANOVA; open arms, effect of group: F(1, 35) = 4.51, p = 0.041; effect of sex: F(1, 35) = 3.05, p = 0.089, closed arms, effect of group: F(1, 35) = 3.07, p = 0.089; effect of sex: F(1, 35) = 4.65, p = 0.038) (Figure 2d–f). There were no differences in the time spent within the central zone or the number of crossings through the center between groups (Figure S2d,e).
Altogether, these data show that selective expression of hTauP301L in 5-HT neurons during young adulthood results in greater anxiety-like behavior in female and male mice. Previous studies have demonstrated a significant association between anxiety and later cognitive impairment or dementia, indicating that anxiety may serve as a risk factor for AD development. Whether this association is a causal or a consequence of a neurodegenerative process in progression remains to be determined. Systematic reviews and meta-analyses have addressed this question by focusing on research that accounted for specific confounding variables, while excluding those that initially indicated cognitive impairment or depression as baseline factors.30,31 However, the mechanism underlying this association remains unclear. Early Braak stages, signifying initial neurofibrillary tangle pathology, have been linked to an increased likelihood of NPS development.17 The DRN has been identified as one of the key subcortical regions that shows tau-related cytoskeletal impairments and NFT pathology at these preclinical stages.13,15 Our data suggest that the presence of pathological tau in 5-HT neurons in the DRN is sufficient to induce anxiety, suggesting a potential mechanism for the onset of early-stage NPS. Importantly, DRN 5-HT neurons project to a variety of postsynaptic regions that modulate anxiety-like behavior and anxiety states, including but not limited to the basal32 and extended amygdala33 while amygdala is among the earlier regions to exhibit tau deposition in AD.34,35 Future studies focused on the effect of tau pathology on DRN-amygdala circuits will offer further insight into anxiety states exacerbated earlier in the disease progress.
hTauP301L Expression in 5-HT Neurons Alters Stress-Coping Strategies without Affecting Anhedonia
Preclinical and clinical studies have indicated that depression is a risk factor for cognitive decline and AD,36 while depressive symptoms manifest in 30% of patients diagnosed with AD.37,38 Because of the association between elevated tau burden and depression,39 we sought to determine whether pathological tau in 5-HT neurons results in depression-like symptoms, including behavioral despair or anhedonia. 5 to 6 weeks after surgery, ePet1hTauP301L mice and controls were subjected to tail suspension and forced swim tests. Originally designed for screening potential antidepressant compounds, these tests are used to assess struggle behaviors in the face of an inescapable stress-inducing condition.40 Analysis of immobility duration during the tail suspension test revealed that regardless of sex, ePet1hTauP301L mice spent significantly less time immobile, suggesting an increased active coping strategy (two-way ANOVA; effect of group: F(1, 35) = 14.85, p < 0.01, effect of sex: F(1, 35) = 2.35, p = 0.13) (Figure 3a). Over the duration of the test, immobility significantly increased in both groups; however, female and male ePet1hTauP301L mice remained more active than the controls (two-way ANOVA; females, effect of group: F(1, 96) = 9.9, p < 0.01; effect of time: F(5, 96) = 12.7, p < 0.01; males, effect of group: F(1, 114) = 18.78, p < 0.01, effect of time: F(5, 114) = 13.9, p < 0.01) (Figure 3b,c). In the forced swim test, female and male ePet1hTauP301L mice showed significantly reduced immobility, indicating increased active coping behavior (two-way ANOVA; effect of group: F(1, 35) = 10.12, p < 0.01, effect of sex: F(1, 35) = 0.07, p = 0.8) (Figure 3d). Immobility increased over time in both groups, with ePet1hTauP301L mice showing increased active coping behavior compared to the controls (two-way ANOVA; females, effect of group: F(1, 96) = 14.94, p < 0.01; effect of time: F(5, 96) = 23.02, p < 0.01; males, effect of group: F(1, 114) = 21.67, p < 0.01; effect of time: F(5, 114) = 67.83, p < 0.01) (Figure 3e,f). To assess whether the heightened active coping behavior in ePet1hTauP301L mice is potentially due to increased anxiety levels, control and ePet1hTauP301L mice received an acute dose of a prototypical anxiolytic and a 5-HT1A receptor agonist buspirone 30 min prior to FST.41 While there was no significant difference between vehicle- and buspirone-treated controls, buspirone significantly increased immobility in ePet1hTauP301L mice, suggesting a potential effect of anxiety levels on stress-coping behavior (Figure S3a,b).
Figure 3.
Altered stress-coping strategies without changes in anhedonia in response to 5-HT neuron-specific hTauP301L expression. (A) ePet1hTauP301L mice (n = 10 females, 9 males) spent less time immobile in TST, compared with controls (n = 8 females, 12 males). (B) Reduced immobility in TST in female ePet1hTauP301L mice over time. (C) Reduced immobility in TST in male ePet1hTauP301L mice over time, compared with controls. (D) ePet1hTauP301L mice (n = 10 females, 9 males) spent less time immobile in TST, compared with controls (n = 8 females, 12 males). (E) Reduced immobility in TST in female ePet1hTauP301L mice over time. (F) Reduced immobility in TST in male ePet1hTauP301L mice over time, compared with controls. (G) Sucrose preference did not differ between control and ePet1hTauP301L mice averaged over days or over consecutive days in (H) females and (I) males. **p < 0.01.
To determine whether hTauP301L expression in 5-HT neurons alters reward-related behavior, we next performed a sucrose preference test in single-housed control and ePet1hTauP301L mice. After acclimatization to two drinking from two different bottles, we calculated the amount of sucrose and water intake during the dark cycle over 16 h across two consecutive days. Both control and ePet1hTauP301L mice exhibited comparable levels of sucrose preference when averaged across days (Figure 3g) and over consecutive days (Figure 3h,i). We observed a tendency for increased average liquid (sucrose + water) intake in ePet1hTauP301L mice (Figure S4a) and a significant increase in liquid intake over subsequent days only in ePet1hTauP301L females (Figure S4b). However, this did not reach significance when controlling for body weight (Figure S4c). These data indicate that hTauP301L expression in 5-HT neurons does not lead to behavioral despair or a decreased preference for a palatable sucrose solution; however, female and male ePet1hTauP301L mice showed altered stress-coping strategies when compared to the controls. The observed changes in active coping strategies during tail suspension and forced swim tests in ePet1hTauP301L mice could potentially be attributed to heightened anxiety, which may serve as a confounding factor leading to increased locomotor activity in these tests.42 Whether the emergence of despair and anhedonia-like behaviors could result from prolonged expression of hTauP301L in 5-HT neurons or expression of hTauP301L in older mice remains to be determined.
The DRN sends dense 5-HT projections to the prefrontal cortex (PFC), which are integral to the modulation of prefrontal cortical activity and function through the interaction of multiple 5-HT receptors in pyramidal neurons and GABAergic interneurons.43 5-HT depletion in the mPFC disrupts GABA release within the corticolimbic circuitry leading to a reduction in passive coping behavior in FST.44 Therefore, a disruption of the 5-HT levels in the mPFC altering the top-down amygdala connectivity could contribute to the reduced immobility observed in ePet1hTauP301L mice, which remains to be investigated.
hTauP301L Expression in 5-HT Neurons Results in Sex-Dependent Alterations in Social Behavior and Cognition
The prodromal phase of AD is thought to involve NPS, in the absence of detectable cognitive symptoms.3,17 Among the preclinical symptoms, anxiety and depression are the most commonly observed and are considered robust indicators of subsequent AD development.30,31,45,46 While NPS is frequently observed throughout all stages, preclinical symptoms may include heightened agitation or impaired social functioning. These symptoms can appear during a relatively early pathological process, characterized by observable NFT pathology and a comparatively low amyloid-β burden.16,17,47 However, social impairments may become more pronounced with advanced pathology and the emergence of cognitive difficulties at a later stage during disease progression.
The DRN 5-HT neurons are activated by social interactions48 while 5-HT inputs from the DRN onto the cortical and subcortical postsynaptic regions are critical for the modulation of sociability and social stress susceptibility. Inhibition of 5-HT terminals in the ventral tegmental area results in a state of social vulnerability while activation of this pathway induces resilience in mice exposed to social stress.49 While 5-HT release increases in the anterior cingulate cortex (ACC) during social approach, inhibition of 5-HT terminals projecting from the DRN to the ACC disrupts social behavior as shown in monogamous mandarin voles.50 Because of the role of 5-HT in the modulation of social circuits, we hypothesized that tau pathology in 5-HT neurons would disrupt social behavior. To quantify social behavior, we performed a resident-intruder assay that involved a 5 min interaction in the home cage of an experimental mouse with a sex- and age-matched stranger conspecific51 (Figure 4a). Quantification of social sniffing of the stranger by the resident mouse revealed sex-dependent alterations in social behavior. Two-way ANOVA indicated a borderline increase in the number of social interaction bouts in ePet1hTauP301L mice compared to control mice (effect of group: F(1, 30) = 3.16, p = 0.086, effect of sex: F(1, 30) = 2.98, p < 0.09) (Figure 4b). However, analysis over time revealed that female ePet1hTauP301L mice spent significantly more time interacting with stranger mice than female controls (two-way ANOVA; females, effect of group: F(1, 75) = 6.67, p = 0.012; effect of time: F(4, 75) = 20.88, p < 0.01) (Figure 4c). In males, there was no significant difference in social interaction between the groups (two-way ANOVA; effect of group: F(1, 75) = 0.14, p = 0.71; effect of time: F(4, 75) = 7.47, p < 0.01) (Figure 4d). These data showed sex-dependent social disinhibition in ePet1hTauP301L mice compared with controls. Sex-dependent social abnormalities have been demonstrated in animal models of AD during the early or later stages of pathology.52,53 In the 3xTg-AD mouse model of AD, females developed social disinhibition at the age of 12 months and social withdrawal at an advanced age. In males, social disinhibition was not observed until the age of 18 months.54 The increased social interaction in female ePet1hTauP301L mice could potentially indicate early signs of aggression associated with AD.55,56 Mice deficient in 5-HT exhibit behavioral disinhibition and increased aggression toward conspecifics in a modified resident-intruder paradigm.57 While increased social interaction of female ePet1hTauP301L mice with intruders might indicate social disinhibition, the relatively short duration of the social interaction paradigm we utilized did not yield signs of aggressive behavior in ePet1hTauP301L mice. Whether social disinhibition in female ePet1hTauP301L mice progresses into social withdrawal, aggression, or social abnormalities emerge in males with prolonged hTauP301L expression remains to be determined.
Figure 4.
hTauP301L expression in 5-HT neurons leads to social disinhibition and cognitive impairment in females. (A) Schematic representation of resident-intruder social interaction test in home cage of control (n = 8 females, 7 males) and ePet1hTauP301L (n = 9 females, 10 males) mice. (B) Total number of interaction bouts in female and male ePet1hTauP301L mice compared with female controls. (C) Female ePet1hTauP301L mice spent greater time engaged in social sniffing of the novel conspecifics, compared to female controls. (D) Time spent in social sniffing was similar between male control and ePet1hTauP301L mice. (E) Schematic representation of Y-maze test (control; n = 7 females, 7 males, ePet1hTauP301L; n = 9 females, 10 males). (F) Female ePet1hTauP301L mice spent significantly less time in the novel arm in Y-maze forced alternation test, compared with female controls. (G) In Y-maze spontaneous alternation test, there was no difference between the groups. (H) Female and male ePet1hTauP301L mice traveled a greater distance compared with controls in Y-maze spontaneous alternation test. *p < 0.05, ns, nonsignificant.
To identify early cognitive alterations in ePet1hTauP301L mice, we performed Y-maze forced and spontaneous alternation tests to measure spatial working memory58 (Figure 4e). Control and ePet1hTauP301L mice were first tested in the forced alternation test in Y-maze with one of the arms obstructed during training. After 30 min, the mice were placed back in Y-maze with access to all three arms. The percentage of time spent in the novel arm was significantly reduced in female ePet1hTauP301L mice compared to female controls, while no differences were detected across all other groups (two-way ANOVA; group × sex interaction: F(1, 29) = 6.54, p = 0.016; Bonferroni’s multiple comparisons test: female control vs ePet1hTauP301L mice, p = 0.02) (Figure 4f). One week later, we performed a Y-maze spontaneous alternation test in a separate room, with access to different spatial cues. The quantification of spontaneous alternations revealed no significant differences between the groups (two-way ANOVA; effect of group: F(1, 29) = 0.03, p = 0.86; effect of sex: F(1, 29) = 0.16, p = 0.69) (Figure 4g). However, female and male ePet1hTauP301L mice traveled a greater distance in this test than control mice, suggesting that hyperactivity may be a confounding factor in the assessment of their performance (two-way ANOVA; effect of group: F(1, 29) = 4.84, p = 0.036; effect of sex: F(1, 29) = 0.99, p = 0.33) (Figure 4h). Overall, these data showed that female ePet1hTauP301L mice have deficits in cognitive function when compared with female controls, while males do not show differences in spatial working memory when assessed at this time point. Spatial working memory deficits have been demonstrated in mouse models of AD with amyloid or tau pathology.59−63 Moreover, in addition to the higher prevalence of AD in female sex, cognitive impairment associated with AD manifests earlier in women than in men.64,65 Additionally, women have higher levels of tau pathology across numerous brain regions, indicating greater vulnerability of the female sex to tau deposition and accelerated cognitive decline in the earlier stages of the disease.66 Taken together, we show that 5-HT neuron-specific hTauP301L expression in females results in impaired cognitive decline, while spatial working memory is spared in male mice with 5-HT neuron tau pathology. Future studies should determine whether the earlier cognitive deficit observed in female mice could be mediated in part by the disruption of 5-HT induced modulation of the medial PFC, which is essential for the representation of spatial working memory.67,68
Physiological Alterations in 5-HT Neurons in Response to hTauP301L
To determine how hTauP301L expression affects 5-HT neuron physiology, we performed whole-cell patch-clamp electrophysiology on DRN slices obtained from control and ePet1hTauP301L mice 4 weeks after viral infusion (Figure 5a). We examined the passive and active membrane characteristics of GFP-labeled 5-HT neurons to characterize the physiological differences between the DRN of the control and the DRN of ePet1hTauP301L mice. We found that the resting membrane potential (RMP) was significantly more depolarized in 5-HT neurons of female and male ePet1hTauP301L mice than in controls (two-way ANOVA; effect of group: F(1, 70) = 4.44, p = 0.039; effect of sex: F(1, 70) = 0.36, p = 0.55) (Figure 5b). Capacitance was significantly reduced in 5-HT neurons of female and male ePet1hTauP301L mice (two-way ANOVA; effect of group: F(1, 70) = 16.79, p < 0.01; effect of sex: F(1, 70) = 2.28, p = 0.13) (Figure 5c), while input resistance was comparable between the groups (two-way ANOVA; effect of group: F(1, 70) = 1.72, p = 0.19; effect of sex: F(1, 70) = 0.04, p = 0.84) (Figure 5d). Regarding active properties, the spike amplitude was significantly reduced in ePet1hTauP301L mice, with a significantly lower spike amplitude in females, compared with males (two-way ANOVA; effect of group: F(1, 70) = 27.77, p < 0.01, effect of sex: F(1, 70) = 5.65, p = 0.02) (Figure 5e). The spike threshold was slightly more depolarized in ePet1hTauP301L mice than in the controls, although this did not reach significance (two-way ANOVA; effect of group: F(1, 70) = 3.36, p = 0.071, effect of sex: F(1, 70) = 0.6, p = 0.44) (Figure 5f). We also detected sex-dependent differences in the intrinsic excitability of the ePet1hTauP301L 5-HT neurons. Compared to the controls, 5-HT neurons of female ePet1hTauP301L mice showed reduced firing in response to depolarizing current injections, indicating a disruption in their excitability (two-way repeated measures ANOVA, group × current interaction: F(20, 640) = 11.65, p < 0.01; Bonferroni’s multiple comparisons test: female control vs ePet1hTauP301L mice, ≥ 350 pA, all p < 0.05) (Figure 5g). The spike frequency of 5-HT neurons between male control and ePet1hTauP301L mice was comparable (two-way repeated measures ANOVA; effect of group: F(1, 38) = 0.02, p = 0.89; effect of current: F(1.446, 54.94) = 52.42, p < 0.01) (Figure 5h). Taken together, there were alterations in both the active and passive physiological properties of 5-HT neurons in female and male ePet1hTauP301L mice, with a significant reduction in the firing response of hTauP301L females only. These data indicated that hTauP301L elicits a disturbance in the physiological characteristics of both male and female mice. Notably, female mice exhibited a more pronounced disruption, characterized by a significant reduction in firing activity in response to excitatory inputs.
Figure 5.
hTauP301L expression disrupts the physiological characteristics of 5-HT neurons. (A) Schematic representation of a coronal brainstem section comprising the DRN (adapted from Paxinos and Franklin, 2001) where 5-HT neurons were recorded. Passive membrane properties including (B) resting membrane potential (RMP; mV), (C) membrane capacitance (pF), and (D) input resistance (Rinput; MΩ) are shown in control (females, n = 19 neurons; males, n = 16 neurons) and ePet1hTauP301L (females, n = 15 neurons; males, n = 24 neurons) mice. (E) Spike amplitude (mV) of 5-HT neurons from ePet1hTauP301L mice was significantly reduced compared to controls. (F) Spike threshold (mV) of 5-HT neurons from ePet1hTauP301L mice was slightly depolarized compared to controls. (G) IO curve (left) showing spike frequency in response to depolarizing current steps and representative current clamp traces (right) from 5-HT neurons of female mice. The firing frequency (Hz) of 5-HT neurons from female ePet1hTauP301L mice (n = 15 neurons) was reduced compared to that of female control mice (n = 19 neurons), indicating reduced excitability. (H) IO curve (left) showing spike frequency in response to depolarizing current steps and representative current clamp traces (right) from 5-HT neurons of male mice. The firing frequency (Hz) of 5-HT neurons was comparable between male control (n = 16 neurons) and ePet1hTauP301L (n = 24 neurons) mice. *p < 0.05, **p < 0.01, #p < 0. 1.
Conclusions
In summary, we demonstrated that 5-HT neuron-specific expression of a hyperphosphorylation-prone version of the human tau (hTauP301L) protein results in increased anxiety-like behavior and altered stress-coping strategies in female and male mice. Additionally, female mice expressing hTauP301L in 5-HT neurons exhibited social disinhibition and spatial working memory deficits, suggesting an impairment in social and cognitive function. We also detected alterations in the physiological characteristics of 5-HT neurons and a sex-dependent disruption in intrinsic excitability. Our data establish a connection between early-onset tau pathology in 5-HT neurons and the emergence of both behavioral and physiological impairments. These findings have important implications for early detection and diagnosis of Alzheimer’s Disease.
Methods
Animals
All experiments were performed in accordance with the guidelines established by the Canadian Council on Animal Care and approved by the Life and Environmental Sciences Animal Care Committee at the University of Calgary. Mice were housed under standard housing conditions (22–25 °C) on a 12:12 light/dark cycle with water and food ad libitum. Male and female heterozygous ePet1::Cre (JAX:012712) transgenic mice on a C57BL6/J background were used in all experiments to specifically target 5-HT neurons in the DRN. ePet1::Cre mice were paired with wild-type C57BL6/J mice for breeding. The mice were weaned on postnatal day 21 (PND 21) and housed with same-sex siblings in groups of 2–4.
Stereotaxic Surgery
Stereotaxic surgeries were performed after the PND 50. Mice were anesthetized in an induction chamber with 5% isoflurane and oxygen at a rate of 1 L/min. Once the absence of pedal withdrawal reflex was confirmed, they were transferred to a stereotaxic apparatus and supplemented with 2% isoflurane and oxygen (0.3 L/min). The skull was stabilized using ear bars, and the eyes were lubricated with eye gel. For analgesia, the mice were subcutaneously injected with 5 mg/kg meloxicam. The top of the skull was shaved and disinfected by using 70% ethanol and iodine. An anterior-posterior incision along the scalp was made to expose the skull, followed by 3% hydrogen peroxide application on the skull to visualize the bregma and lambda. Control mice received AAV2/9-CAG-Flex-eGFP (Addgene, cat # 59331-titer: 5 × 1013 vg/mL). For the expression of hTauP301L, the plasmid encoding EGFP-tagged human TauP301L was obtained from Addgene (#46908).29 Cre-dependent AAV2/9-CAG-DIO-hTauP301L-eGFP was prepared by the HBI Molecular Core Facility, University of Calgary (titer: 2.66 × 1013 vg/mL). For infusions, viruses were drawn up into the micropipette of a Drummond Nanoject automated nanoliter (nL) injector system attached to a stereotaxic arm. After ensuring that the bregma and lambda skull joints are at level, a drill was used to open a small hole on the skull at coordinates A/P: – 6.27 mm and ML: 0.0 mm from bregma. The micropipette was then inserted into the DRN at D/V: 4.2 mm at a 30° angle to avoid the aqueduct. A total of 800 nL of virus was injected at a rate of 20 nL/sec with a 5 s pause after injection of each 100 nL. 5 min after completion of injection, the micropipette was removed, and the skin was sutured. Each mouse was transferred to a holding cage over a heating pad for recovery. The mice were monitored after surgery, until they achieved complete consciousness and locomotion.
Histology and Immunohistochemistry
Mice were euthanized by intraperitoneal injection of pentobarbital (480 mg/kg). Once the absence of the pedal withdrawal and corneal blink reflex were confirmed, a transcardial perfusion was performed with 0.1 M phosphate buffer saline (PBS) followed by fixation with 4% paraformaldehyde (PFA). Brains were then carefully extracted and placed in PFA for 24 h. Brains were next transferred to a 30% sucrose solution until they were isotonic with the sucrose solution and sank. After freezing, brains were embedded onto the HM 430 sliding microtome stage using the Optimum Cutting Temperature Tissue Tek. 40 μm coronal sections were serially collected and stored in antifreeze (ethylene glycol) solution in a −20 °C freezer.
For immunostaining, every sixth section comprising the DRN was used. The staining procedure was performed over 3 days. On day 1, sections were washed 3 × 10 min in 0.1 M PBS on a shaker, followed by incubation in 5% Normal Donkey Serum (NDS) + 1% Triton-X-100 + 0.1 M PBS for 1 h. Next, sections were incubated in primary antibody solution for 24 h in the dark. The primary antibody solution contained 5% NDS + 0.5% Triton-X-100 + 0.1 M PBS + antibody (rabbit anti - pTau S199; 1:500; Thermo Fisher cat# 44779G, or rabbit anti - GFP; 1:500; Invitrogen cat# A11122) and/or 5-HT antibody (goat anti -5-HT; 1:500; Abcam cat# ab66047). On day 2, the sections were washed 3 × 10 min in 0.1 M PBS on a shaker. Next, sections were incubated in secondary antibody solution containing 5% NDS + 0.5% Triton-X-100 + 0.1 M PBS + donkey antirabbit A647 (1:500) (or donkey antirabbit A488 for GFP staining) and donkey antigoat A594 (1:500) for 24 h under dark. On day 3, sections were washed 3 × 10 min in 0.1 M PBS. Finally, sections were mounted on microscopy slides and coverslipped with PVA-DABCO mounting medium. Slides were stored in the dark, and imaging was performed after 24 h.
Sections were imaged by using an Olympus FV3000 confocal microscope. Images were acquired with 20× and 40× objectives, with the aperture set to 1 airy unit. Z-stack image sequences were maximum intensity projected as a 2D image using ImageJ software. 3–4 sections comprising the DRN were analyzed per mouse. Quantification of cells labeled with 5-HT or GFP was performed by using ImageJ software. Two mice were excluded from analysis due to possible missed injection, which was reflected in their histology. Only mice with accurate stereotaxic targeting and viral expression were included in the analyses.
Quantitative Real-Time PCR (qRT-PCR)
Four weeks after infusion with AAV2/9-CAG-Flex-eGFP or AAV2/9-CAG-DIO-hTauP301L-eGFP, the mice were sacrificed by cervical dislocation. Brains were extracted and rapidly frozen on dry ice. Tissue punches (1 mm diameter) from the frozen brainstem comprising the DR were collected. Total RNA was isolated from mouse brain tissue samples by first homogenizing in QIAzol reagent with subsequent chloroform extraction followed by further purification using the RNeasy micro kit according to the manufacturer’s instructions (Qiagen). Reverse transcription was performed using the Superscript VILO kit followed by real-time qPCR with 10 ng of cDNA per reaction using PowerUp SYBR green Mastermix on a Quantstudio 3 real-time qPCR instrument (Thermo Fisher). Intron-spanning primer sequences (Table S1) that could successfully differentiate between the human and mouse isoforms of MAPT gene were designed and validated by performing five, 10-fold serial dilutions using mouse brain cDNA with or without spiked in plasmid containing the human sequence, which yielded efficiencies of ∼110% and 5 orders of magnitude linear range for both primer sets. Both POLR2A and ACTB were obtained as predesigned qPCR primer assays from IDT, experimentally validated, and used as reference genes (Table S1). PCR specificity was confirmed by performing melt curve analysis. Data for individual experiments was analyzed using Quantstudio Design & Analysis version 2.6.0 software and the project was analyzed using the comparative Ct method69 with the relative quantification app on the Thermo Fisher connect platform (Table S2).
Behavior
Behavioral experiments started 4 weeks after surgeries. At least 2–3 days of rest were given between different tests. Tests were performed in three different cohorts. Cohorts 1 and 2 (control mice; n = 8 females, n = 12 males, ePet1hTauP301L mice; n = 8 females, n = 9 males) went through open field, elevated plus maze, tail suspension, forced swim, and sucrose preference tests. Cohorts 2 and 3 (control mice; n = 8 females, 7 males; ePet1hTauP301L mice; n = 9 females, 10 males) were included in social interaction and Y-maze tests.
Open-Field Test
The open-field test is used to assess locomotion and anxiety-like behavior in rodents.70 The apparatus consisted of a white opaque square box 40 cm × 40 cm × 30.5 cm (l × w × h) with a white corrugated plastic bottom. Each mouse was placed in a corner and allowed to freely explore for 15 min. Videos were recorded with an upright USB camera (ANY-maze) using ANY-Maze video tracking software (Stoelting Co.). The time spent in the outer and center zones as well as the total distance traveled were calculated using ANY-maze.
Elevated Plus Maze Test
The elevated plus maze is used to assess anxiety-like behavior in rodents and is based on the general avoidance of rodents from open and elevated spaces.71 The apparatus consisted of four identical arms, each measuring 35.5 cm, arranged in the shape of a plus sign, and raised 50 cm above the ground. Two opposite arms without walls were referred to as open arms, and the other two arms with walls around the outer perimeter formed the closed arms. At the start of the test, each mouse was placed in the center and allowed to explore for 15 min. The time spent in open and closed arms was calculated using ANY-maze video tracking software (Stoelting Co.).
Resident-Intruder Social Interaction Test
Home cage interaction is used to assess the sociability of freely moving rodents with novel conspecifics where interaction time indicates the sociability of the experimental mice. The test was performed in the home cage of the experimental mice. The cage mates were placed in a new cage (50% home cage and 50% new bedding), while the experimental animal in the home cage was taken to the test room. The test was conducted for 9 min. First 2 min served as the baseline during which the experimental mouse remained alone in his home cage. After 2 min, a novel age- and sex-matched conspecific was introduced. The mice were allowed to interact for 5 min. 2 min after removing the novel mouse, the test was completed. Video recordings were performed using ANY-maze video tracking software (Stoelting Co.). An experimenter blind to the conditions scored the interactions initiated by the experimental mouse. Social interaction time indicated the time the experimental mouse spent sniffing the novel mouse. The number of interaction bouts was calculated by counting the number of times the experimental mouse approached and sniffed the novel mouse.
Tail Suspension Test
The tail suspension test is used to assess stress-coping strategies in rodents. The apparatus consisted of a horizontal suspension bar 60 cm above ground with no objects around for mice to grasp. A 2 cm cylindrical tube was placed around the tail to prevent mice from grabbing onto their tail. Each experimental mouse was suspended from the bar using tape attached to the tail for a duration of 6 min. Video recordings were performed using the ANY-maze video tracking software (Stoelting Co.). Mobility was scored by an experiment blind to the conditions. Strong shaking of the body, movement of all four limbs similar to running, or arching to reach the tail or the suspension bar were defined as mobility. Immobility was calculated by subtracting the time spent immobile from the total duration of the test.
Forced Swim Test
The forced swim test is used to assess stress-coping strategies in rodents, and similar to the tail suspension test, decreased immobility indicates an active coping strategy. The apparatus consisted of a 2 L beaker filled 3/4 with tap water at 23–25 °C. The mice were slowly released into water and allowed to swim for 6 min. After the test was completed, the mice were gently dried using a paper towel and placed in temporary holding cages until all cage mates completed the test. Video recordings were performed using the ANY-maze video tracking software (Stoelting Co.). An experimenter blind to the conditions scored the mobility. Mobility was defined as vigorous lifting of the body or movement of at least two limbs. Movement of only one limb was not considered as movement. A separate group of mice received an intraperitoneal injection of vehicle (sterile water) (control; n = 2 females, n = 2 males, ePet1hTauP301L mice; n = 3 females, n = 2 males) or buspirone (5 mg/kg, Sigma) dissolved in sterile water41 (control; n = 3 females, n = 2 males, ePet1hTauP301L mice; n = 2 females, n = 3 males) 30 min prior to the forced swim test.
Sucrose Preference Test
The sucrose preference test is used to assess anhedonia-like behavior which relies on the inability to feel pleasure for rewards.72 The test was conducted over 4 days. On day 1 at 10:00 am, all experimental mice were single-housed and habituated to the presence of two 50 mL water bottles filled with tap water. On day 2 at 18:00 pm, one of the bottles was filled with 1.5% sucrose water, and the other bottle was filled with tap water. Both bottles were weighed and placed equidistant from the food pellets. On day 3, at 10:00 am, the bottles were weighed and filled with tap water. On day 3 at 18:00 pm, each bottle was filled with fresh 1.5% sucrose and tap water and bottle positions were switched. On day 4 at 10:00 am, the bottles were weighed and mice were placed back in their home cages. Sucrose preference was calculated by dividing the average amount of sucrose intake by the average amount of sucrose and water intakes across both days.
Y-Maze Forced Alternation Test
The Y-maze forced alternation test is used to assess spatial working memory by utilizing the innate exploratory tendency of rodents.58 A Y-shaped apparatus with three equal arms (30 cm each) was used for the test. The test was divided into two sections: S1 and S2. In S1, one of the arms was blocked off, and mice were allowed to freely explore the other two arms for 5 min. Afterward, the mice were placed in the home cage for 30 min. For S2, all three arms were accessible and mice were placed in the same starting arm as S1. The mice were allowed to explore the apparatus for 5 min. The time spent in each arm and the number of entries were recorded using the ANY-maze video tracking software (Stoelting Co.). Percent time in the novel arm was calculated as follows: [(time in novel arm)/(total test time – latency to exit the start arm)] × 100.
Y-Maze Spontaneous Alternation Test
The Y-maze spontaneous choice test is a variation of Y-maze forced alternation test which assesses spatial memory.58 In this variation, all of the arms of the apparatus were kept open. Each mouse was placed in one of the three arms to explore for 5 min. To avoid placement bias, the starting arm was kept different for each animal. To avoid recall of spatial cues from the Y-maze forced alteration test, this test was performed in a different room. The test was recorded via ANY-maze software, which was used to calculate the time spent in each arm along with the order of arm entry. Percent spontaneous alternation was calculated as follows: [(number of alternations)/(total arm entries – 2)] × 100.
Slice Electrophysiology
400 μm coronal slices comprising the dorsal raphe nucleus were obtained in ice-cold oxygenated sucrose-substituted artificial cerebrospinal fluid (aCSF) using a Leica VT1000 S vibratome. Slices were recovered in aCSF (128 mM NaCl, 10 mM d-glucose, 26 mM NaHCO3, 2 mM CaCl2, 2 mM MgSO4, 3 mM KCl, 1.25 mM NaH2PO4, pH 7.4) and kept saturated with 95% O2/5% CO2 at 30 °C for a minimum of 2 h. Recordings were performed at 30 °C in aCSF supplemented with 30 uM l-tryptophan, saturated with 95% O2/5% CO2, and perfused at a rate of 3–4 mL/min. The internal patch solution contained 120 mM potassium gluconate, 10 mM HEPES, 5 mM KCl, 2 mM MgCl2, 4 mM K2-ATP, 0.4 mM Na2-GTP, and 10 mM Na2-phosphocreatine, with pH adjusted to 7.3. Neurons were visualized using IR-DIC on an Olympus BX51WI microscope. 5-HT neurons were identified based on the expression of EGFP. Whole-cell recordings were obtained in current clamp mode using a MultiClamp 700B amplifier (Molecular Devices). Data were filtered at 4 kHz, digitized at 20 kHz using Digidata 1550B and Clampex software (Molecular Devices), and analyzed using the Clampfit software.
Statistical Analysis
The GraphPad Prism software was used to perform statistical analyses. Data were analyzed using two-way analysis of variance (ANOVA), repeated measures two-way ANOVA, or unpaired t tests. Two-way ANOVAs were performed with the group (control and hTauP301L) and sex (female and male) as factors. In the case of a significant interaction effect, Bonferroni corrections were made to control for Type 1 error. In open field, since one female ePet1hTauP301L mouse did not move, it was identified as an outlier using Grubb’s test and removed from the analysis. Statistical significances were set to p < 0.05. All data and figures were presented as mean ± standard error of the mean (SEM).
Acknowledgments
The authors thank Dr. Frank Visser for the development of the AAV2/9-CAG-DIO-hTauP301L-eGFP virus and qRT-PCR experiments. Funding for this study was provided by an Alzheimer’s Association Grant (AARG-22-917644) in partnership with Brain Canada to D.S. J.U.I. received funding from Alberta Innovates, and N.R. received Harley Hotchkiss-Samuel Weiss Postdoctoral Fellowship. Schematic images were created with BioRender.com.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschemneuro.3c00626.
Immunohistochemical characterization of hTauP301L in DRN; locomotor activity in open field and center time in elevated plus maze; effects of the serotonergic anxiolytic buspirone on the active coping strategy of control and ePet1hTauP301L mice in FST; amount of liquid consumed during the sucrose preference test; primers used for qRT-PCR; and data from the qRT-PCR analysis (PDF)
Author Contributions
# N.S.K. and J.U.I. contributed equally to this work. D.S., N.S.K., and J.U.I. designed the experiments and wrote the manuscript. N.S.K, J.U.I., and A.K.G performed behavioral and histological experiments. N.R. and S.J. performed the electrophysiological experiments and analysis. F.V. performed qRT-PCR experiments and analysis. N.F.J. and M.T. performed the surgeries.
The authors declare no competing financial interest.
Special Issue
Published as part of ACS Chemical Neurosciencevirtual special issue “Serotonin Research 2023”.
Supplementary Material
References
- Hyman B. T.; Phelps C. H.; Beach T. G.; Bigio E. H.; Cairns N. J.; Carrillo M. C.; Dickson D. W.; Duyckaerts C.; Frosch M. P.; Masliah E.; Mirra S. S.; Nelson P. T.; Schneider J. A.; Thal D. R.; Thies B.; Trojanowski J. Q.; Vinters H. V.; Montine T. J. National Institute on Aging-Alzheimer’s Association Guidelines for the Neuropathologic Assessment of Alzheimer’s Disease. Alzheimer’s Dementia 2012, 8 (1), 1–13. 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savva G. M.; Zaccai J.; Matthews F. E.; Davidson J. E.; McKeith I.; Brayne C.; Prevalence, Correlates and Course of Behavioural and Psychological Symptoms of Dementia in the Population. Br. J. Psychiatry 2009, 194 (3), 212–219. 10.1192/bjp.bp.108.049619. [DOI] [PubMed] [Google Scholar]
- Lyketsos C. G.; Carrillo M. C.; Ryan J. M.; Khachaturian A. S.; Trzepacz P.; Amatniek J.; Cedarbaum J.; Brashear R.; Miller D. S. Neuropsychiatric Symptoms in Alzheimer’s Disease. Alzheimer’s Dementia 2011, 7 (5), 532–539. 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringman J. M.; Liang L.-J.; Zhou Y.; Vangala S.; Teng E.; Kremen S.; Wharton D.; Goate A.; Marcus D. S.; Farlow M.; Ghetti B.; McDade E.; Masters C. L.; Mayeux R. P.; Rossor M.; Salloway S.; Schofield P. R.; Cummings J. L.; Buckles V.; Bateman R.; Morris J. C.; Early Behavioural Changes in Familial Alzheimer’s Disease in the Dominantly Inherited Alzheimer Network. Brain 2015, 138 (Pt 4), 1036–1045. 10.1093/brain/awv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier M. T. Treatment Strategies for the Behavioral Symptoms of Alzheimer’s Disease: Focus on Early Pharmacologic Intervention. Pharmacotherapy 2007, 27 (3), 399–411. 10.1592/phco.27.3.399. [DOI] [PubMed] [Google Scholar]
- Masters M. C.; Morris J. C.; Roe C. M. Noncognitive” Symptoms of Early Alzheimer Disease: A Longitudinal Analysis. Neurology 2015, 84 (6), 617–622. 10.1212/WNL.0000000000001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail Z.; Smith E. E.; Geda Y.; Sultzer D.; Brodaty H.; Smith G.; Agüera-Ortiz L.; Sweet R.; Miller D.; Lyketsos C. G.; Neuropsychiatric Symptoms as Early Manifestations of Emergent Dementia: Provisional Diagnostic Criteria for Mild Behavioral Impairment. Alzheimer’s Dementia 2016, 12 (2), 195–202. 10.1016/j.jalz.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Švob Štrac D.; Pivac N.; Mück-Šeler D. The Serotonergic System and Cognitive Function. Transl. Neurosci. 2016, 7 (1), 35–49. 10.1515/tnsci-2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargin D.; Jeoung H.-S.; Goodfellow N. M.; Lambe E. K. Serotonin Regulation of the Prefrontal Cortex: Cognitive Relevance and the Impact of Developmental Perturbation. ACS Chem. Neurosci. 2019, 10 (7), 3078–3093. 10.1021/acschemneuro.9b00073. [DOI] [PubMed] [Google Scholar]
- Hendricksen M.; Thomas A. J.; Ferrier I. N.; Ince P.; O’Brien J. T. Neuropathological Study of the Dorsal Raphe Nuclei in Late-Life Depression and Alzheimer’s Disease with and without Depression. Am. J. Psychiatry 2004, 161 (6), 1096–1102. 10.1176/appi.ajp.161.6.1096. [DOI] [PubMed] [Google Scholar]
- Yamamoto T.; Hirano A. Nucleus Raphe Dorsalis in Alzheimer’s Disease: Neurofibrillary Tangles and Loss of Large Neurons. Ann. Neurol. 1985, 17 (6), 573–577. 10.1002/ana.410170608. [DOI] [PubMed] [Google Scholar]
- Chen C. P.; Eastwood S. L.; Hope T.; McDonald B.; Francis P. T.; Esiri M. M. Immunocytochemical Study of the Dorsal and Median Raphe Nuclei in Patients with Alzheimer’s Disease Prospectively Assessed for Behavioural Changes. Neuropathol. Appl. Neurobiol. 2000, 26 (4), 347–355. 10.1046/j.1365-2990.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- Grinberg L. T.; Rüb U.; Ferretti R. E. L.; Nitrini R.; Farfel J. M.; Polichiso L.; Gierga K.; Jacob-Filho W.; Heinsen H.; The Dorsal Raphe Nucleus Shows Phospho-Tau Neurofibrillary Changes before the Transentorhinal Region in Alzheimer’s Disease. A Precocious Onset?. Neuropathol. Appl. Neurobiol. 2009, 35 (4), 406–416. 10.1111/j.1365-2990.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- Smith G. S.; Barrett F. S.; Joo J. H.; Nassery N.; Savonenko A.; Sodums D. J.; Marano C. M.; Munro C. A.; Brandt J.; Kraut M. A.; Zhou Y.; Wong D. F.; Workman C. I. Molecular Imaging of Serotonin Degeneration in Mild Cognitive Impairment. Neurobiol. Dis. 2017, 105, 33–41. 10.1016/j.nbd.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüb U.; Del Tredici K.; Schultz C.; Thal D. R.; Braak E.; Braak H. The Evolution of Alzheimer’s Disease-Related Cytoskeletal Pathology in the Human Raphe Nuclei. Neuropathol. Appl. Neurobiol. 2000, 26 (6), 553–567. 10.1046/j.0305-1846.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- Braak H.; Thal D. R.; Ghebremedhin E.; Del Tredici K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories from 1 to 100 Years. J. Neuropathol. Exp. Neurol. 2011, 70 (11), 960–969. 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- Ehrenberg A. J.; Nguy A. K.; Theofilas P.; Dunlop S.; Suemoto C. K.; Di Lorenzo Alho A. T.; Leite R. P.; Diehl Rodriguez R.; Mejia M. B.; Rüb U.; Farfel J. M.; de Lucena Ferretti-Rebustini R. E.; Nascimento C. F.; Nitrini R.; Pasquallucci C. A.; Jacob-Filho W.; Miller B.; Seeley W. W.; Heinsen H.; Grinberg L. T. Quantifying the Accretion of Hyperphosphorylated Tau in the Locus Coeruleus and Dorsal Raphe Nucleus: The Pathological Building Blocks of Early Alzheimer’s Disease. Neuropathol. Appl. Neurobiol. 2017, 43 (5), 393–408. 10.1111/nan.12387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-Y.; Tae W. S.; Yoon H.-K.; Lee B.-T.; Paik J.-W.; Son K.-R.; Oh Y.-W.; Lee M.-S.; Ham B.-J. Demonstration of Decreased Gray Matter Concentration in the Midbrain Encompassing the Dorsal Raphe Nucleus and the Limbic Subcortical Regions in Major Depressive Disorder: An Optimized Voxel-Based Morphometry Study. J. Affective Disord. 2011, 133 (1–2), 128–136. 10.1016/j.jad.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Greicius M. D.; Gennatas E. D.; Growdon M. E.; Jang J. Y.; Rabinovici G. D.; Kramer J. H.; Weiner M.; Miller B. L.; Seeley W. W. Divergent Network Connectivity Changes in Behavioural Variant Frontotemporal Dementia and Alzheimer’s Disease. Brain 2010, 133 (Pt 5), 1352–1367. 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett F. S.; Workman C. I.; Sair H. I.; Savonenko A. V.; Kraut M. A.; Sodums D. J.; Joo J. J.; Nassery N.; Marano C. M.; Munro C. A.; Brandt J.; Zhou Y.; Wong D. F.; Smith G. S. Association between Serotonin Denervation and Resting-State Functional Connectivity in Mild Cognitive Impairment. Hum. Brain Mapp. 2017, 38 (7), 3391–3401. 10.1002/hbm.23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M.; Gil-Bea F. J.; Diez-Ariza M.; Chen C. P. L.-H.; Francis P. T.; Lasheras B.; Ramirez M. J. Cholinergic-Serotonergic Imbalance Contributes to Cognitive and Behavioral Symptoms in Alzheimer’s Disease. Neuropsychologia 2005, 43 (3), 442–449. 10.1016/j.neuropsychologia.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lai M. K. P.; Tsang S. W.; Esiri M. M.; Francis P. T.; Wong P. T.-H.; Chen C. P. Differential Involvement of Hippocampal Serotonin1A Receptors and Re-Uptake Sites in Non-Cognitive Behaviors of Alzheimer’s Disease. Psychopharmacology 2011, 213 (2–3), 431–439. 10.1007/s00213-010-1936-2. [DOI] [PubMed] [Google Scholar]
- Khan K. M.; Balasubramanian N.; Gaudencio G.; Wang R.; Selvakumar G. P.; Kolling L.; Pierson S.; Tadinada S. M.; Abel T.; Hefti M.; Marcinkiewcz C. A. Human Tau-Overexpressing Mice Recapitulate Brainstem Involvement and Neuropsychiatric Features of Early Alzheimer’s Disease. Acta Neuropathol. Commun. 2023, 11 (1), 57. 10.1186/s40478-023-01546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C.; Wagner M.; Wolfsgruber S.; Ehrenreich H.; Schneider A.; Impact of SSRI Therapy on Risk of Conversion From Mild Cognitive Impairment to Alzheimer’s Dementia in Individuals With Previous Depression. Am. J. Psychiatry 2018, 175 (3), 232–241. 10.1176/appi.ajp.2017.17040404. [DOI] [PubMed] [Google Scholar]
- Cirrito J. R.; Disabato B. M.; Restivo J. L.; Verges D. K.; Goebel W. D.; Sathyan A.; Hayreh D.; D’Angelo G.; Benzinger T.; Yoon H.; Kim J.; Morris J. C.; Mintun M. A.; Sheline Y. I. Serotonin Signaling Is Associated with Lower Amyloid-β Levels and Plaques in Transgenic Mice and Humans. Proc. Natl. Acad. Sci. U. S. A. 2011, 108 (36), 14968–14973. 10.1073/pnas.1107411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y. I.; West T.; Yarasheski K.; Swarm R.; Jasielec M. S.; Fisher J. R.; Ficker W. D.; Yan P.; Xiong C.; Frederiksen C.; Grzelak M. V.; Chott R.; Bateman R. J.; Morris J. C.; Mintun M. A.; Lee J.-M.; Cirrito J. R. An Antidepressant Decreases CSF Aβ Production in Healthy Individuals and in Transgenic AD Mice. Sci. Transl. Med. 2014, 6 (236), 236re4 10.1126/scitranslmed.3008169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Gao Y.; Jiang L.; Chao F.-L.; Huang W.; Zhou C.-N.; Tang W.; Zhang L.; Huang C.-X.; Zhang Y.; Luo Y.-M.; Xiao Q.; Yu H.-R.; Jiang R.; Tang Y. Fluoxetine Attenuates the Impairment of Spatial Learning Ability and Prevents Neuron Loss in Middle-Aged APPswe/PSEN1dE9 Double Transgenic Alzheimer’s Disease Mice. Oncotarget 2017, 8 (17), 27676–27692. 10.18632/oncotarget.15398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J.; Lucas J. J.; Perez M.; Hernandez F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiol. Rev. 2004, 84 (2), 361–384. 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- Hoover B. R.; Reed M. N.; Su J.; Penrod R. D.; Kotilinek L. A.; Grant M. K.; Pitstick R.; Carlson G. A.; Lanier L. M.; Yuan L.-L.; Ashe K. H.; Liao D. Tau Mislocalization to Dendritic Spines Mediates Synaptic Dysfunction Independently of Neurodegeneration. Neuron 2010, 68 (6), 1067–1081. 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulpers B.; Ramakers I.; Hamel R.; Köhler S.; Oude Voshaar R.; Verhey F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am. J. Geriatr. Psychiatry 2016, 24 (10), 823–842. 10.1016/j.jagp.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Becker E.; Orellana Rios C. L.; Lahmann C.; Rücker G.; Bauer J.; Boeker M. Anxiety as a Risk Factor of Alzheimer’s Disease and Vascular Dementia. Br. J. Psychiatry 2018, 213 (5), 654–660. 10.1192/bjp.2018.173. [DOI] [PubMed] [Google Scholar]
- Sengupta A.; Holmes A. A Discrete Dorsal Raphe to Basal Amygdala 5-HT Circuit Calibrates Aversive Memory. Neuron 2019, 103 (3), 489–505.e7. 10.1016/j.neuron.2019.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz C. A.; Mazzone C. M.; D’Agostino G.; Halladay L. R.; Hardaway J. A.; DiBerto J. F.; Navarro M.; Burnham N.; Cristiano C.; Dorrier C. E.; Tipton G. J.; Ramakrishnan C.; Kozicz T.; Deisseroth K.; Thiele T. E.; McElligott Z. A.; Holmes A.; Heisler L. K.; Kash T. L. Serotonin Engages an Anxiety and Fear-Promoting Circuit in the Extended Amygdala. Nature 2016, 537 (7618), 97–101. 10.1038/nature19318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abiose O.; Deters K. D.; Young C.; Mormino E. C. Amygdala Tau in Preclinical Alzheimer’s Disease. Alzheimer’s Dementia 2020, 16 (S4), e046762 10.1002/alz.046762. [DOI] [Google Scholar]
- Stouffer K. M.; Grande X.; Duezel E.; Johansson M.; Creese B.; Witter M. P.; Miller M. I.; Wisse L. E. M.; Berron D. Amidst an Amygdala Renaissance in Alzheimer’s Disease. Brain 2023, awad411 10.1093/brain/awad411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyarko J. N. K.; Quartey M. O.; Baker G. B.; Mousseau D. D. Can Animal Models Inform on the Relationship between Depression and Alzheimer Disease?. Can. J. Psychiatry 2019, 64 (1), 18–29. 10.1177/0706743718772514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno N.; Homma A. What Is the Association between Depression and Alzheimer’s Disease?. Expert Rev. Neurother. 2009, 9 (11), 1667–1676. 10.1586/ern.09.106. [DOI] [PubMed] [Google Scholar]
- Byers A. L.; Yaffe K. Depression and Risk of Developing Dementia. Nat. Rev. Neurol. 2011, 7 (6), 323–331. 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babulal G. M.; Roe C. M.; Stout S. H.; Rajasekar G.; Wisch J. K.; Benzinger T. L. S.; Morris J. C.; Ances B. M. Depression Is Associated with Tau and Not Amyloid Positron Emission Tomography in Cognitively Normal Adults. J. Alzheimer’s Dis. 2020, 74 (4), 1045–1055. 10.3233/JAD-191078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk M. L.; de Kloet E. R. Coping with the Forced Swim Stressor: Current State-of-the-Art. Behav. Brain Res. 2019, 364, 1–10. 10.1016/j.bbr.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Lee K. M.; Coelho M. A.; Sern K. R.; Class M. A.; Bocz M. D.; Szumlinski K. K. Anxiolytic Effects of Buspirone and MTEP in the Porsolt Forced Swim Test. Chronic Stress 2017, 1, 2470547017712985 10.1177/2470547017712985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyan J.; Amir S. Too Depressed to Swim or Too Afraid to Stop? A Reinterpretation of the Forced Swim Test as a Measure of Anxiety-Like Behavior. Neuropsychopharmacology 2018, 43 (5), 931–933. 10.1038/npp.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R. Serotonergic Regulation of Neuronal Excitability in the Prefrontal Cortex. Neuropharmacology 2011, 61 (3), 382–386. 10.1016/j.neuropharm.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolina D.; Maran D.; Valzania A.; Conversi D.; Puglisi-Allegra S. Prefrontal/Amygdalar System Determines Stress Coping Behavior through 5-HT/GABA Connection. Neuropsychopharmacology 2013, 38 (10), 2057–2067. 10.1038/npp.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ownby R. L.; Crocco E.; Acevedo A.; John V.; Loewenstein D. Depression and Risk for Alzheimer Disease: Systematic Review, Meta-Analysis, and Metaregression Analysis. Arch. Gen. Psychiatry 2006, 63 (5), 530–538. 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri C. T.; Johnson L. G. Age-Related Cognitive Decline in Patients with Mood Disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32 (4), 962–967. 10.1016/j.pnpbp.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Braak H.; Del Tredici K. The Pathological Process Underlying Alzheimer’s Disease in Individuals under Thirty. Acta Neuropathol. 2011, 121 (2), 171–181. 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- Li Y.; Zhong W.; Wang D.; Feng Q.; Liu Z.; Zhou J.; Jia C.; Hu F.; Zeng J.; Guo Q.; Fu L.; Luo M. Serotonin Neurons in the Dorsal Raphe Nucleus Encode Reward Signals. Nat. Commun. 2016, 7, 10503 10.1038/ncomms10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou W.-J.; Song Y.-L.; Wu M.-Y.; Chen X.-T.; You Q.-L.; Yang Q.; Luo Z.-Y.; Huang L.; Kong Y.; Feng J.; Fang D.-X.; Li X.-W.; Yang J.-M.; Mei L.; Gao T.-M. A Discrete Serotonergic Circuit Regulates Vulnerability to Social Stress. Nat. Commun. 2020, 11 (1), 4218 10.1038/s41467-020-18010-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Zhang L.-Z.; He Z.-X.; Ma H.; Zhang Y.-T.; Xun Y.-F.; Yuan W.; Hou W.-J.; Li Y.-T.; Lv Z.-J.; Jia R.; Tai F.-D. Dorsal Raphe Nucleus to Anterior Cingulate Cortex 5-HTergic Neural Circuit Modulates Consolation and Sociability. eLife 2021, 10, e67638 10.7554/eLife.67638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M.; Terstege D. J.; Jamani N.; Tsutsui M.; Pavlov D.; Bugescu R.; Epp J. R.; Leinninger G. M.; Sargin D. Hypocretin/Orexin Neurons Encode Social Discrimination and Exhibit a Sex-Dependent Necessity for Social Interaction. Cell Rep. 2023, 42 (7), 112815 10.1016/j.celrep.2023.112815. [DOI] [PubMed] [Google Scholar]
- Pietropaolo S.; Delage P.; Lebreton F.; Crusio W. E.; Cho Y. H. Early Development of Social Deficits in APP and APP-PS1Mice. Neurobiol. Aging 2012, 33 (5), 1002.e17-27 10.1016/j.neurobiolaging.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Filali M.; Lalonde R.; Rivest S. Anomalies in Social Behaviors and Exploratory Activities in an APPswe/PS1Mouse Model of Alzheimer’s Disease. Physiol. Behav. 2011, 104 (5), 880–885. 10.1016/j.physbeh.2011.05.023. [DOI] [PubMed] [Google Scholar]
- Bories C.; Guitton M. J.; Julien C.; Tremblay C.; Vandal M.; Msaid M.; De Koninck Y.; Calon F. Sex-Dependent Alterations in Social Behaviour and Cortical Synaptic Activity Coincide at Different Ages in a Model of Alzheimer’s Disease. PLoS One 2012, 7 (9), e46111 10.1371/journal.pone.0046111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique H.; Hynan L. S.; Weiner M. F. Effect of a Serotonin Reuptake Inhibitor on Irritability, Apathy, and Psychotic Symptoms in Patients with Alzheimer’s Disease. J. Clin. Psychiatry 2009, 70 (6), 915–918. 10.4088/JCP.08m04828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki A.; Ueno H.; Sato N.; Shinjo H.; Morita Y. Serotonin Transporter Gene Polymorphism and BPSD in Mild Alzheimer’s Disease. J. Alzheimers. Dis. 2007, 12 (3), 245–253. 10.3233/JAD-2007-12306. [DOI] [PubMed] [Google Scholar]
- Angoa-Pérez M.; Kane M. J.; Briggs D. I.; Sykes C. E.; Shah M. M.; Francescutti D. M.; Rosenberg D. R.; Thomas D. M.; Kuhn D. M. Genetic Depletion of Brain 5HT Reveals a Common Molecular Pathway Mediating Compulsivity and Impulsivity. J. Neurochem. 2012, 121 (6), 974–984. 10.1111/j.1471-4159.2012.07739.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A.-K.; Guest P. C.; Sarnyai Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. 10.1007/978-1-4939-8994-2_10. [DOI] [PubMed] [Google Scholar]
- Hulshof L. A.; Frajmund L. A.; van Nuijs D.; van der Heijden D. C. N.; Middeldorp J.; Hol E. M. Both Male and Female APPswe/PSEN1dE9Mice Are Impaired in Spatial Memory and Cognitive Flexibility at 9 Months of Age. Neurobiol. Aging 2022, 113, 28–38. 10.1016/j.neurobiolaging.2021.12.009. [DOI] [PubMed] [Google Scholar]
- Wolf A.; Bauer B.; Abner E. L.; Ashkenazy-Frolinger T.; Hartz A. M. S. A Comprehensive Behavioral Test Battery to Assess Learning and Memory in 129S6/Tg2576 Mice. PLoS One 2016, 11 (1), e0147733 10.1371/journal.pone.0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedel C. J.; Patton J. M.; Miedel A. N.; Miedel E. S.; Levenson J. M. Assessment of Spontaneous Alternation, Novel Object Recognition and Limb Clasping in Transgenic Mouse Models of Amyloid-β and Tau Neuropathology. J. Visualized Exp. 2017, 123, 55523 10.3791/55523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J. D.; Kim Y. A.; Rafikian E. E.; Yang M.; Santa-Maria I. Marked Mild Cognitive Deficits in Humanized Mouse Model of Alzheimer’s-Type Tau Pathology. Front. Behav. Neurosci. 2021, 15, 634157 10.3389/fnbeh.2021.634157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.; Kim J.; Chang K.-A. Spatial Memory Deficiency Early in 6xTg Alzheimer’s Disease Mouse Model. Sci. Rep. 2021, 11 (1), 1334 10.1038/s41598-020-79344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws K. R.; Irvine K.; Gale T. M. Sex Differences in Cognitive Impairment in Alzheimer’s Disease. World J. Psychiatry 2016, 6 (1), 54–65. 10.5498/wjp.v6.i1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S.; Hendrie H. C.; Hall K. S.; Hui S. The Relationships between Age, Sex, and the Incidence of Dementia and Alzheimer Disease: A Meta-Analysis. Arch. Gen. Psychiatry 1998, 55 (9), 809–815. 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Buckley R. F.; Scott M. R.; Jacobs H. I. L.; Schultz A. P.; Properzi M. J.; Amariglio R. E.; Hohman T. J.; Mayblyum D. V.; Rubinstein Z. B.; Manning L.; Hanseeuw B. J.; Mormino E. C.; Rentz D. M.; Johnson K. A.; Sperling R. A. Sex Mediates Relationships Between Regional Tau Pathology and Cognitive Decline. Ann. Neurol. 2020, 88 (5), 921–932. 10.1002/ana.25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout J. J.; Griffin A. L. Representations of On-Going Behavior and Future Actions During a Spatial Working Memory Task by a High Firing-Rate Population of Medial Prefrontal Cortex Neurons. Front. Behav. Neurosci. 2020, 14, 151. 10.3389/fnbeh.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel P.; Hahn J.; Duvarci S.; Sigurdsson T. Prefrontal Pyramidal Neurons Are Critical for All Phases of Working Memory. Cell Rep. 2022, 39 (2), 110659 10.1016/j.celrep.2022.110659. [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D.; Livak K. J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3 (6), 1101–1108. 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Seibenhener M. L.; Wooten M. C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. J. Visualized Exp. 2015, (No. 96), e52434 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraeuter A.-K.; Guest P. C.; Sarnyai Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. Methods Mol. Biol. 2019, 1916, 69–74. 10.1007/978-1-4939-8994-2_4. [DOI] [PubMed] [Google Scholar]
- Liu M.-Y.; Yin C.-Y.; Zhu L.-J.; Zhu X.-H.; Xu C.; Luo C.-X.; Chen H.; Zhu D.-Y.; Zhou Q.-G. Sucrose Preference Test for Measurement of Stress-Induced Anhedonia in Mice. Nat. Protoc. 2018, 13 (7), 1686–1698. 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.