Abstract

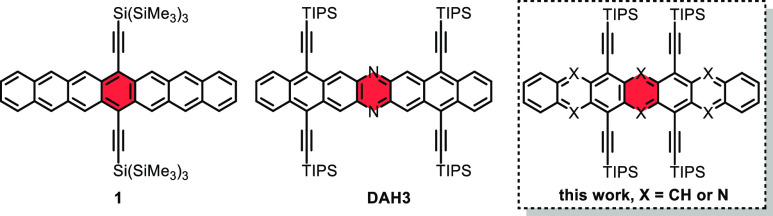
The symmetrical 7,16-diaza-6,8,15,17-tetrakis(triisopropylsilylethynyl)heptacene was obtained by a Pd-catalyzed reaction of a 2,3-diamino-1,4-diethynylanthracene with a 2,3-dibromo-1,4-diethynyl anthracene. Positioning the TIPS-ethynyl groups adjacent to the central ring suppresses dimerization via [4+4] cycloadditions and Diels–Alder reactions; the middle pyrazine ring renders this species stable to oxidation. A single crystal structure was obtained, and thin film transistors with μn = 0.042 cm2 V–1 s–1 were produced. Transposition of the alkynyl groups into the 5,8,15,18-positions with a quinoxaline unit in the center of the heptacene decreases the stability, as does the introduction of two more nitrogen atoms into the 5,18-positions. The hydrocarbon 6,8,15,17-tetrakis(triisopropylsilylethynyl)heptacene is reasonably stable with a half-life of 25 h in solution. Four correctly placed TIPS-ethynyl groups protect heptacene cores.
Pentacene, a benchmark p-channel semiconductor,1,2 is reasonably stable and processable by evaporation.3,4 Two TIPS-ethynyl groups at the central ring5 render it solution processable6−8 and add stability.9 Larger silyl groups (tBu3Si, (Me3Si)Si) give reasonably stable hexacenes10 and the marginally stable but isolable heptacene 1(11) (Figure 1). Isoelectronic azaacenes are obtained by fusing pyrazines onto the acene—double silylethynylation—generates stable azapentacenes12−16 and azahexacenes.17−19 In contrast to their hydrocarbon analogues,20−22 isolable azaheptacenes are unknown, despite Dutt’s claim in 1926.23,24DAH325 (Figure 1), the only reported azaheptacene, is reactive in solution. Upon crystallization, a mixture of butterfly dimers forms by [4+4] cycloaddition of the rings adjacent to the pyrazine.
Figure 1.
Known heptacene 1, diazaheptacene DAH3, and the (aza)heptacenes reported herein.
Azaacenes are, as a rule, more stable than acenes: Pyrazine rings increase the oxidation potential26,27 and retard oxidation/endo-peroxide formation, a major decomposition path of large acenes under ambient conditions.9,28 They prevent [4+4] or [4+2] cycloadditions (with ethynyl substituents)29,30 at the pyrazine ring,10,17 but how many pyrazines are needed and is there an optimum number? Can too many pyrazines decrease the azaacenes’ stability with spontaneous reduction to their dihydro species?17,31,32
Transposition of the silylethynyl groups toward the center results in DAH1, stable in solution and as a solid. The comparison of DAH1 to Hep highlights the nitrogens’ role in blocking degradation via oxidation. DAH1, DAH2, and DAH3(25) differ in the TIPS-ethynyls’ placement, and for Hep, DAH1, TAH, and HAH the nitrogen content in heptacenes with a fixed TIPS-ethynylation pattern (6, 8, 15, 17 positions) was investigated.
The syntheses of the five target (aza)acenes (Scheme 1) entails Pd-catalyzed Buchwald–Hartwig coupling33,34 of 2a(35) to 3a into DAH1-H2 (63%). Oxidation with MnO2 furnished DAH1 (72%) and byproduct S1 (10%, see SI, Figures S24, S36, and S37) after rearrangement of DAH1-H2. Combining 2a with 3b(25) leads to the non-centrosymmetric DAH2-H2. It is oxidized to DAH2 by MnO2 (85%). The synthesis of TAH-H2 and HAH-H2 exploits ortho-quinone 4, obtained from dibromodiiodoveratrole by demethylation and oxidation; 4 dimerizes spontaneously36,37 and was immediately combined with 2a or 2b(35) into 5a,b. 5a reacted with 5 equiv of TIPS-acetylene under Sonogashira38 conditions (50 °C) into multiply ethynylated N,N′-dihydro-intermediates, with 6a isolated in 39% yield. Surprisingly, 5b selectively transforms into 6b with 10 equiv of TIPS-acetylene at room temperature in 75% yield. 6a,b were Buchwald–Hartwig coupled33,34 to ortho-phenylenediamine to give TAH-H2 and HAH-H2. It is surprising that the Sonogashira/Buchwald–Hartwig coupling worked so well, considering halide selectivity and steric hindrance. MnO2 converted TAH-H2 into TAH in 5 min, while HAH-H2 was, even after 5 h, only partially oxidized into HAH. The azaheptacenes were isolated by column chromatography on silica. DAH1, DAH2, TAH, and HAH are microcrystalline solids, characterized by NMR, UV–vis, and IR spectroscopy, mass spectrometry, cyclic voltammetry, UPLC, and elemental as well as single-crystal structure analysis. Hep was synthesized according to the literature (see SI).39
Scheme 1. Synthesis of (Aza)heptacenes.
Figure S12 (SI) displays the UV–vis spectra of the N,N′-dihydro compounds. Upon oxidation (Figure 2a) we observe acene p-bands with absorption onsets for DAH1, DAH2, TAH, and HAH red-shifted to 1074, 1064, 1152, and 1045 nm, proof of azaheptacene formation (Table 1). TAH’s absorption is the most red-shifted, a consequence of its donor–acceptor character.40Hep is the most blue-shifted congener (absorption onset: 950 nm). The heptacene p-bands are broadened and/or display shoulders at the longest wavelengths, attributed to their arising diradical character,41 although their NMR spectra are well-resolved. The LUMOs of DAH1, HAH, and Hep are evenly distributed over the molecular skeleton, while the HOMO of TAH has small coefficients at the pyrazine rings,42 resulting in a decreased band gap (SI, Figure S1). Hep, DAH1, and DAH2 show two reduction and two oxidation waves (SI, Figure S5), while TAH and HAH only display two and three reduction events, respectively (SI, Figure S5). TAH’s and HAH’s electron affinities are <−4.10 eV; their radical anions might be stable in air and will be reported elsewhere.
Figure 2.
(a) Normalized UV–vis absorption spectra of (aza)heptacenes in DCM (10–5 mol L–1). (b) Time-dependent evolution of UV–vis spectra of DAH1 (10–5 mol L–1 in dry DCM) under ambient light and atmosphere. Inset: magnification of the p-bands. (c) Evolution of UV–vis absorption intensities at λabs,max for (aza)heptacenes under ambient conditions (UV–vis spectra: see SI, Figure S13).
Table 1. Photophysical and Calculated Properties of (Aza)heptacenes.
| compd | λabs ona [nm] | λabs maxb [nm] | Eg measc/Eg cald [eV] | E1/2red1e [V] | EAf/ELUMOg [eV] | IPh/EHOMOg [eV] |
|---|---|---|---|---|---|---|
| Hep | 950 | 865 | 1.30/1.22 | –1.15 | –3.65/-3.43 | –4.95/-4.65 |
| DAH1 | 1074 | 957 | 1.15/1.17 | –0.72 | –4.08/-3.71 | –5.23/-4.88 |
| DAH2 | 1064 | 917 | 1.17/1.19 | –0.77 | –4.03/–3.70 | –5.20/–4.89 |
| TAH | 1152 | 1011 | 1.08/1.12 | –0.69 | –4.11/–4.02 | –5.19/–5.14 |
| HAH | 1045 | 961 | 1.19/1.19 | –0.60 | –4.20/–4.19 | –5.39/–5.38 |
Onset of the lowest energy absorption maxima.
Most intense absorption of the p-band.
Optical gap calculated by λonset.
HOMO–LUMO gap calculated by DFT calculation.
First reduction potentials measured by cyclic voltammetry in DCM against Fc/Fc+ as the internal standard (−4.80 eV) using a Pt working electrode and Bu4NPF6 as electrolyte.
Electron affinities (EA) estimated from first reduction potentials; EA = −4.80 eV – Ered.43
FMO values calculated by DFT calculation44 (Gaussian16 B3LYP, def2TZVP; TMS groups were used instead of TIPS).
Ionization potential (IP) = EA – Eg meas.
DAH3, with the largest spacing between the TIPS-ethynyl substituents, is the computationally most stable isomer with respect to total energies neglecting decomposition (Table S1). Successively taken NMR (SI, Figures S6 to S11) and UV–vis spectra (Figures 2b,c and S13) illustrate the stability of DAH1, DAH2, and DAH3, highlighting the effect of the TIPS-ethynyl substituent pattern on diazaheptacenes. The NMR spectrum of DAH3 exhibited growing resonances attributed to butterfly dimers10,25 (≈85% consumption after 6 h). Under the same conditions, the spectra of DAH1 and DAH2 remained unchanged. According to UV–vis spectroscopy (Figures 2b,c and S13), the half-lives τ1/2 of DAH2 and DAH3 (DCM) are 4 days and 6 h, respectively. Solutions of DAH1 are unchanged after 7 d under ambient light and atmosphere (Figure 2b); that is, DAH1 is completely stable. Similar to heptacenes,11,20,22 the positions of the TIPS-ethynyl groups determine their stability; adjacent to the central ring they protect the center of the azaacene more effectively than those with more arene rings in between.
We studied the effect of N atom loading on stability. Adding another pyrazine moiety, TAH started to decompose after 7 d (I7d/I0 = 82% at λmax). HAH displayed a τ1/2 of 5 d; it was spontaneously reduced to its N,N′-dihydro species. In contrast to the decay channels of alkynylated acenes, this process is reversible: reoxidation proceeded quantitatively to HAH. Reduction of HAH was also observed under NMR conditions, and its spectrum was recorded under an inert atmosphere in the presence of PbO2. Removing the central pyrazine unit dramatically decreases the stability (τ1/2 Hep = 25 h, endoperoxide formed; see SI, Figures S67 and S68), although this is still reasonably stable for a heptacene, as it is only protected by four substituents compared to the most stable congener with six.22 In dilute solution, the rank order of stability is DAH1 > TAH > HAH ≈ DAH2 > Hep > DAH3 and depends upon the number of nitrogen atoms and placement of the TIPS-ethynyl substituents. In comparison to dilute solution, in the solid state, their stability increased with the rank order DAH1 > DAH2 > TAH > HAH > Hep > DAH3.
Specimens suitable for single-crystal structure analysis (SCRA) were grown by slow diffusion of methanol into chloroform (Hep polymorph I, DAH1, and TAH) or dichloromethane solutions (DAH2) or via cooling concentrated solutions in benzene (Hep polymorph II). Hep (polymorph II) and DAH2 crystallize as benzene and dichloromethane solvates, respectively (Table S2).
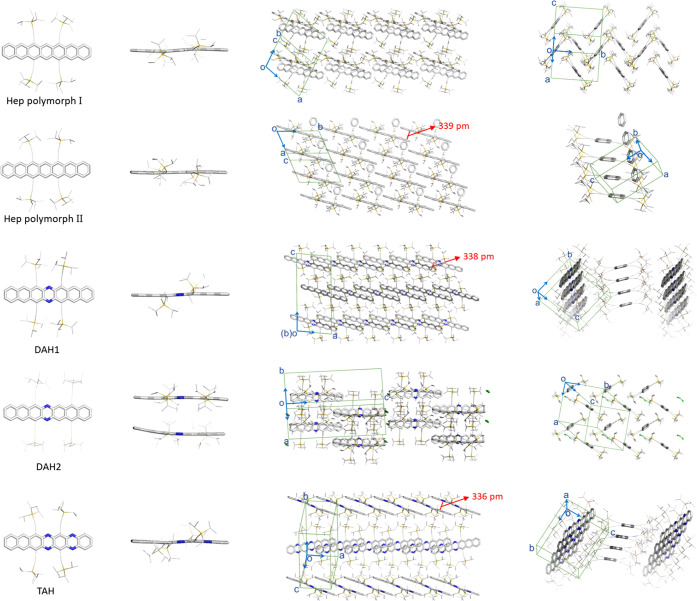
All of the acene backbones deviate from planarity (Figure 3); most are S-shaped. DAH2 contains two independent molecules per unit cell, one of which is strongly bent. The backbone of Hep (polymorph II) is almost planar. One-dimensional stacks extend along one direction with a mean π–π distance of 339 pm between the overlapping but slightly offset terminal rings. DAH1 and TAH exhibit an overlap of ∼3.5, three rings along the backbone, and columnar stacking with π–π distances of 0.34 Å. The noncentral pyrazine moiety in TAH is statistically disordered throughout the crystal lattice. Hep (polymorph I) and DAH2 lack π–π interactions due to their edge-to-face arrangement. DAH1 did not form a solvate when crystallized from benzene. In HAH hydrogen atoms at the central pyrazine rings occupy about 50% of the molecules (see SI, Figure S29) according to SCRA, providing evidence for its spontaneous reduction.
Figure 3.
Solid state structures (representative depictions) and packing motif obtained for (aza)heptacenes; hydrogen atoms are omitted for clarity. Note that for TAH, the position of the noncentral pyrazine is statistically disordered due to the centrosymmetry of the lattice. π-Stacking distances are estimated from the mean distances of the (aza)acene backbones. Corresponding atomic colors: carbon, gray; nitrogen, blue; silicon, yellow; chlorine, green.
The transfer integrals of all possible pairs of neighboring molecules (SCRA, ADF software package)45 and reorganization energies (four-point method, Gaussian1635) were used to compute the electron and hole transport mobilities μ (Table S3). Only the highest transfer integral of each compound is shown; all other transfer integrals, including images of the dimer pairs, are listed in the SI (Tables S4–S6, Figure S69). In comparison to hexaethynylheptacene,22Hep displays higher calculated mobilities: a smaller number of centrally placed TIPS-ethynyl substituents facilitates charge transport. Calculated hole mobilities of 2.6 cm2 V–1 s–1 (comparable to that of TIPS-Pen(8)) and electron mobilities of 3.5 cm2 V–1 s–1, suggest DAH1 is an attractive ambipolar semiconductor. TAH might be a promising n-type transporting material with theoretical electron mobilities up to 4.6 cm2 V–1 s–1, surpassing TIPS-TAP.46 These calculated mobilities are based on a diffusion model, which is valid only for crystal structures with a single molecule per unit cell of perfect translational symmetry and neglects the contribution of lattice phonons. These calculations provide the upper limit of charge carrier mobilities in the packing analyzed.47
DAH1 and TAH transport electrons in bottom gate/top contact field-effect transistors (Figure S70) with the best electron mobilities at 0.042 and 0.0031 cm2 V–1 s–1 (Table S7). Hep and DAH2 are ambipolar transport materials with μn-max = 0.023 cm2 V–1 s–1 and μp-max = 0.038 cm2 V–1 s–1 for Hep (DAH2: μn-max = 0.005 cm2 V–1 s–1 and μp-max = 0.0017 cm2 V–1 s–1). The discrepancies between the calculated and experimental values are due to the quality of the thin films and also the limitations of the calculations. Compared to hexaethynyl-heptacene,22 the mobility of Hep is 30 and 22 times higher for the n-channel and the p-channel transport, respectively. We expect the experimental mobilities of Hep, DAH1, and TAH to improve through device optimization. Thin film XRD analysis (see SI, Section 16) of Hep (polymorph I) and DAH2 suggests that the order in thin films and in the single crystal are identical. For DAH1 and TAH the diffraction patterns do not match the simulated diffractograms (single crystal), indicating that the molecules pack differently in thin films.
In conclusion, we have prepared stable and isolable azaheptacenes, claimed ≈100 years ago.23 The substitution pattern of DAH1 renders it immune to endoperoxide formation, butterfly dimerization, and Diels–Alder reactions. The correct placement of the silylethynyl substituents is critical for the azaheptacenes’ stability with respect to dimerization. In combination with a strategically placed central pyrazine, oxidation is suppressed. In solution, an increasing number of pyrazine units destabilizes azaheptacenes: We observe spontaneous reduction to the dihydro species as the main degradation pathway of these electron-poor systems. DAH1 and TAH, the most stable of any reported azaheptacene, are promising n-type semiconductors according to the calculated mobilities. We will further explore future applications of thin film transistors. The dibromides 6a,b are precursors to azaoctacenes and azanonacenes if they are coupled to diaminonaphthalenes and -anthracenes. We encourage the community to apply azaheptacenes as stability issues are solved.
Acknowledgments
We thank the DFG (SFB 1249) for generous support. We thank the state of Baden-Württemberg for support through bwHPC and the German Research Foundation (DFG) for support through grant no. INST40/575-1FUGG (JUSTUS 2 cluster). W. Zong thanks the Chinese Scholarship Council (CSC) for a scholarship. This paper is adapted from dissertations listed in refs (48−50).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c13629.
Additional synthetic procedures and characterization data of intermediates, additional spectra, X-ray crystallographic data, and details for theoretical calculations (PDF)
Experimental data are accessible via HeiData, an institutional repository from Heidelberg University, under DOI:10.11588/data/P9AVCU.
The authors declare no competing financial interest.
Supplementary Material
References
- Kitamura M.; Arakawa Y. Pentacene-based organic field-effect transistors. J. Phys.: Condens. Matter 2008, 20, 184011 10.1088/0953-8984/20/18/184011. [DOI] [Google Scholar]
- Tykwinski R. R. Synthesis of unsymmetrical derivatives of pentacene for materials applications. Acc. Chem. Res. 2019, 52, 2056–2069. 10.1021/acs.accounts.9b00216. [DOI] [PubMed] [Google Scholar]
- Ruiz R.; Choudhary D.; Nickel B.; Toccoli T.; Chang K.-C.; Mayer A. C.; Clancy P.; Blakely J. M.; Headrick R. L.; Iannotta S. Pentacene thin film growth. Chem. Mater. 2004, 16, 4497–4508. 10.1021/cm049563q. [DOI] [Google Scholar]
- Watanabe M.; Chang Y. J.; Liu S. W.; Chao T. H.; Goto K.; Islam M. M.; Yuan C. H.; Tao Y. T.; Shinmyozu T.; Chow T. J. The synthesis, crystal structure and charge-transport properties of hexacene. Nat. Chem. 2012, 4, 574–578. 10.1038/nchem.1381. [DOI] [PubMed] [Google Scholar]
- Anthony J. E.; Brooks J. S.; Eaton D. L.; Parkin S. R. Functionalized pentacene: improved electronic properties from control of solid-state order. J. Am. Chem. Soc. 2001, 123, 9482–9483. 10.1021/ja0162459. [DOI] [PubMed] [Google Scholar]
- Sakanoue T.; Sirringhaus H. Band-like temperature dependence of mobility in a solution-processed organic semiconductor. Nat. Mater. 2010, 9, 736–740. 10.1038/nmat2825. [DOI] [PubMed] [Google Scholar]
- Payne M. M.; Parkin S. R.; Anthony J. E.; Kuo C.-C.; Jackson T. N. Organic field-effect transistors from solution-deposited functionalized acenes with mobilities as high as 1 cm2/Vs. J. Am. Chem. Soc. 2005, 127, 4986–4987. 10.1021/ja042353u. [DOI] [PubMed] [Google Scholar]
- Park S. K.; Jackson T. N.; Anthony J. E.; Mourey D. A. High mobility solution processed 6, 13-bis (triisopropyl-silylethynyl) pentacene organic thin film transistors. Appl. Phys. Lett. 2007, 91, 063514 10.1063/1.2768934. [DOI] [Google Scholar]
- Fudickar W.; Linker T. Why triple bonds protect acenes from oxidation and decomposition. J. Am. Chem. Soc. 2012, 134, 15071–15082. 10.1021/ja306056x. [DOI] [PubMed] [Google Scholar]
- Purushothaman B.; Parkin S. R.; Anthony J. E. Synthesis and stability of soluble hexacenes. Org. Lett. 2010, 12, 2060–2063. 10.1021/ol100178s. [DOI] [PubMed] [Google Scholar]
- Payne M. M.; Parkin S. R.; Anthony J. E. Functionalized higher acenes: hexacene and heptacene. J. Am. Chem. Soc. 2005, 127, 8028–8029. 10.1021/ja051798v. [DOI] [PubMed] [Google Scholar]
- Li G.; Wu Y.; Gao J.; Wang C.; Li J.; Zhang H.; Zhao Y.; Zhao Y.; Zhang Q. Synthesis and physical properties of four hexazapentacene derivatives. J. Am. Chem. Soc. 2012, 134, 20298–20301. 10.1021/ja310131k. [DOI] [PubMed] [Google Scholar]
- Liang Z.; Tang Q.; Xu J.; Miao Q. Soluble and stable N-heteropentacenes with high field-effect mobility. Adv. Mater. 2011, 23, 1535–1539. 10.1002/adma.201004325. [DOI] [PubMed] [Google Scholar]
- Liu Y. Y.; Song C. L.; Zeng W. J.; Zhou K. G.; Shi Z. F.; Ma C. B.; Yang F.; Zhang H. L.; Gong X. High and balanced hole and electron mobilities from ambipolar thin-film transistors based on nitrogen-containing oligoacences. J. Am. Chem. Soc. 2010, 132, 16349–16351. 10.1021/ja107046s. [DOI] [PubMed] [Google Scholar]
- Miao S.; Appleton A. L.; Berger N.; Barlow S.; Marder S. R.; Hardcastle K. I.; Bunz U. H. F. 6,13-Diethynyl-5,7,12,14-tetraazapentacene. Chem.—Eur. J. 2009, 15, 4990–4993. 10.1002/chem.200900324. [DOI] [PubMed] [Google Scholar]
- Tverskoy O.; Rominger F.; Peters A.; Himmel H. J.; Bunz U. H. F. An efficient synthesis of tetraazapentacenes. Angew. Chem., Int. Ed. 2011, 50, 3557–3560. 10.1002/anie.201007654. [DOI] [PubMed] [Google Scholar]
- Lindner B. D.; Engelhart J. U.; Tverskoy O.; Appleton A. L.; Rominger F.; Peters A.; Himmel H. J.; Bunz U. H. F. Stable hexacenes through nitrogen substitution. Angew. Chem., Int. Ed. 2011, 50, 8588–8591. 10.1002/anie.201103676. [DOI] [PubMed] [Google Scholar]
- Engelhart J. U.; Lindner B. D.; Tverskoy O.; Rominger F.; Bunz U. H. F. Pd-catalyzed coupling of non-activated dibromoarenes to 2,3-diaminoarenes: formation of N,N′-dihydropyrazines. Chem.—Eur. J. 2013, 19, 15089–15092. 10.1002/chem.201303277. [DOI] [PubMed] [Google Scholar]
- Yue W.; Suraru S. L.; Bialas D.; Müller M.; Wurthner F. Synthesis and properties of a new class of fully conjugated azahexacene analogues. Angew. Chem., Int. Ed. 2014, 53, 6159–6162. 10.1002/anie.201403227. [DOI] [PubMed] [Google Scholar]
- Chun D.; Cheng Y.; Wudl F. The most stable and fully characterized functionalized heptacene. Angew. Chem., Int. Ed. 2008, 47, 8380–8385. 10.1002/anie.200803345. [DOI] [PubMed] [Google Scholar]
- Qu H.; Chi C. A stable heptacene derivative substituted with electron-deficient trifluoromethylphenyl and triisopropylsilylethynyl groups. Org. Lett. 2010, 12, 3360–3363. 10.1021/ol101158y. [DOI] [PubMed] [Google Scholar]
- Zeitter N.; Hippchen N.; Maier S.; Rominger F.; Dreuw A.; Freudenberg J.; Bunz U. H. F. Persistent ambipolar heptacenes and their redox species. Angew. Chem., Int. Ed. 2022, 61, e202200918 10.1002/anie.202200918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S. A theory of colour on the basis of molecular strain. The effect of chromophoric superposition. J. Chem. Soc. 1926, 129, 1171–1184. 10.1039/JR9262901171. [DOI] [Google Scholar]
- Badger G.; Pettit R. Polynuclear heterocyclic systems. Part IV. The linear pentacyclic compounds. J. Chem. Soc. 1951, 3211–3215. 10.1039/jr9510003211. [DOI] [Google Scholar]
- Engelhart J. U.; Tverskoy O.; Bunz U. H. F. A persistent diazaheptacene derivative. J. Am. Chem. Soc. 2014, 136, 15166–15169. 10.1021/ja509723q. [DOI] [PubMed] [Google Scholar]
- Liang Z.; Tang Q.; Xu J.; Miao Q. Soluble and stable N-heteropentacenes with high field-effect mobility. Adv. Mater. 2011, 23, 1535–1539. 10.1002/adma.201004325. [DOI] [PubMed] [Google Scholar]
- Ahrens L.; Tverskoy O.; Weigold S.; Ganschow M.; Rominger F.; Freudenberg J.; Bunz U. H. F. (Aza) Pentacenes clipped into a ring: stabilization of large (aza)acenes. Angew. Chem., Int. Ed. 2021, 60, 9270–9273. 10.1002/anie.202015348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A. R.; Bendikov M. Diels-Alder reaction of acenes with singlet and triplet oxygen-theoretical study of two-state reactivity. Chem. Commun. 2006, 1179–1181. 10.1039/b513597d. [DOI] [PubMed] [Google Scholar]
- Payne M. M.; Odom S. A.; Parkin S. R.; Anthony J. E. Stable, crystalline acenedithiophenes with up to seven linearly fused rings. Org. Lett. 2004, 6, 3325–3328. 10.1021/ol048686d. [DOI] [PubMed] [Google Scholar]
- Engelhart J. U.; Lindner B. D.; Tverskoy O.; Rominger F.; Bunz U. H. F. Partially fluorinated tetraazaacenes by nucleophilic aromatic substitution. J. Org. Chem. 2013, 78, 10832–10839. 10.1021/jo401824g. [DOI] [PubMed] [Google Scholar]
- Elter M.; Ahrens L.; Luo S. M.; Rominger F.; Freudenberg J.; Cao D. D.; Bunz U. H. F. Cata-annulated azaacene bisimides. Chem.—Eur. J. 2021, 27, 12284–12288. 10.1002/chem.202101573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M.; Beglaryan S. S.; Koser S.; Hahn S.; Tverskoy O.; Rominger F.; Bunz U. H. F. Dicyano-substituted diazaacenes. Chem.—Eur. J. 2017, 23, 7066–7073. 10.1002/chem.201700421. [DOI] [PubMed] [Google Scholar]
- Hahn S.; Koser S.; Hodecker M.; Seete P.; Rominger F.; Miljanic O. S.; Dreuw A.; Bunz U. H. F. Phenylene bridged cyclic azaacenes: dimers and trimers. Chem.—Eur. J. 2018, 24, 6968–6974. 10.1002/chem.201705704. [DOI] [PubMed] [Google Scholar]
- Netherton M. R.; Fu G. C. Air-stable trialkylphosphonium salts: simple, practical, and versatile replacements for air-sensitive trialkylphosphines. Applications in stoichiometric and catalytic processes. Org. Lett. 2001, 3, 4295–4298. 10.1021/ol016971g. [DOI] [PubMed] [Google Scholar]
- Li J. B.; Shen Y. Q.; Wan J. Q.; Yu X. L.; Zhang Q. C. Recent progress in the usage of phenazinediamine and its analogues as building blocks to construct large N-heteroacenes. Eur. J. Org. Chem. 2018, 2018, 3375–3390. 10.1002/ejoc.201800478. [DOI] [Google Scholar]
- Zong W.; Hippchen N.; Dittmar B.; Elter M.; Ludwig P.; Rominger F.; Freundenberg J.; Bunz U. H. F. Halogenated phenazinothiadiazoles: electron transporting materials. Asian J. Org. Chem. 2023, 12, e202300462 10.1002/ajoc.202300462. [DOI] [Google Scholar]
- Rolle C. J. III; Hardcastle K. I.; Soper J. D. Reactions of tetrabromocatecholatomanganese(III) complexes with dioxygen. Inorg. Chem. 2008, 47, 1892–1894. 10.1021/ic702390q. [DOI] [PubMed] [Google Scholar]
- Wang C.; Zhang J.; Long G.; Aratani N.; Yamada H.; Zhao Y.; Zhang Q. Synthesis, structure, and air-stable n-type field-effect transistor behaviors of functionalized octaazanonacene-8,19-dione. Angew. Chem., Int. Ed. 2015, 54, 6292–6296. 10.1002/anie.201500972. [DOI] [PubMed] [Google Scholar]
- Zeitter N.; Hippchen N.; Baur P.; Unterreiner T. V.; Rominger F.; Freudenberg J.; Bunz U. H. F. Pentacene to octacene: the limit of fourfold TIPS-ethynylation. Org. Mater. 2024, 10.1055/a-2241-0243. [DOI] [Google Scholar]
- Engelhart J. U.; Lindner B. D.; Schaffroth M.; Schrempp D.; Tverskoy O.; Bunz U. H. F. Substituted tetraaza- and hexaazahexacenes and their N,N′-dihydro derivatives: syntheses, properties, and structures. Chem.—Eur. J. 2015, 21, 8121–8129. 10.1002/chem.201500518. [DOI] [PubMed] [Google Scholar]
- Zeng Z.; Shi X.; Chi C.; Navarrete J. T. L.; Casado J.; Wu J. Pro-aromatic and anti-aromatic π-conjugated molecules: an irresistible wish to be diradicals. Chem. Soc. Rev. 2015, 44, 6578–6596. 10.1039/C5CS00051C. [DOI] [PubMed] [Google Scholar]
- Tang X.-D.; Liao Y.; Geng H.; Shuai Z.-G. Fascinating effect of dehydrogenation on the transport properties of N-heteropentacenes: transformation from p- to n-type semiconductor. J. Mater. Chem. 2012, 22, 18181–18191. 10.1039/c2jm33039c. [DOI] [Google Scholar]
- Cardona C. M.; Li W.; Kaifer A. E.; Stockdale D.; Bazan G. C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. 10.1002/adma.201004554. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams-Young D.; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, 2016.
- Velde G. T.; Bickelhaupt F. M.; Baerends E. J.; Guerra C. F.; Van Gisbergen S. J. A.; Snijders J. G.; Ziegler T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. 10.1002/jcc.1056. [DOI] [Google Scholar]
- Xu X.; Yao Y.; Shan B.; Gu X.; Liu D.; Liu J.; Xu J.; Zhao N.; Hu W.; Miao Q. Electron mobility exceeding 10 cm2V–1s–1 and band-like charge transport in solution-processed n-channel organic thin-film transistors. Adv. Mater. 2016, 28, 5276–5283. 10.1002/adma.201601171. [DOI] [PubMed] [Google Scholar]
- Stehr V.; Pfister J.; Fink R.; Engels B.; Deibel C. First-principles calculations of anisotropic charge-carrier mobilities in organic semiconductor crystals. Phys. Rev. B 2011, 83, 155208 10.1103/PhysRevB.83.155208. [DOI] [Google Scholar]
- Zong W.Synthesis and Characterization of Large Azaacenes and Stable Azaacene Radical Anions. Ph.D. Dissertation, Ruprecht-Karls-Universität Heidelberg, Germany, 2023 10.11588/heidok.00033641 (accessed 2024–01–25). [DOI] [Google Scholar]
- Hippchen N.Die Anwendung von Acenen und Azaacenes in organischen Feldeffekttranistoren. Dissertation, Universität Heidelberg, Heidelberg, Germany, 2023. [Google Scholar]
- Zeitter J. N.Pentacen bis Nonacen: Synthese, Charakterisierung und Anwendung. Ph.D. Thesis, Universität Heidelberg, Heidelberg, Germany, 2023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.