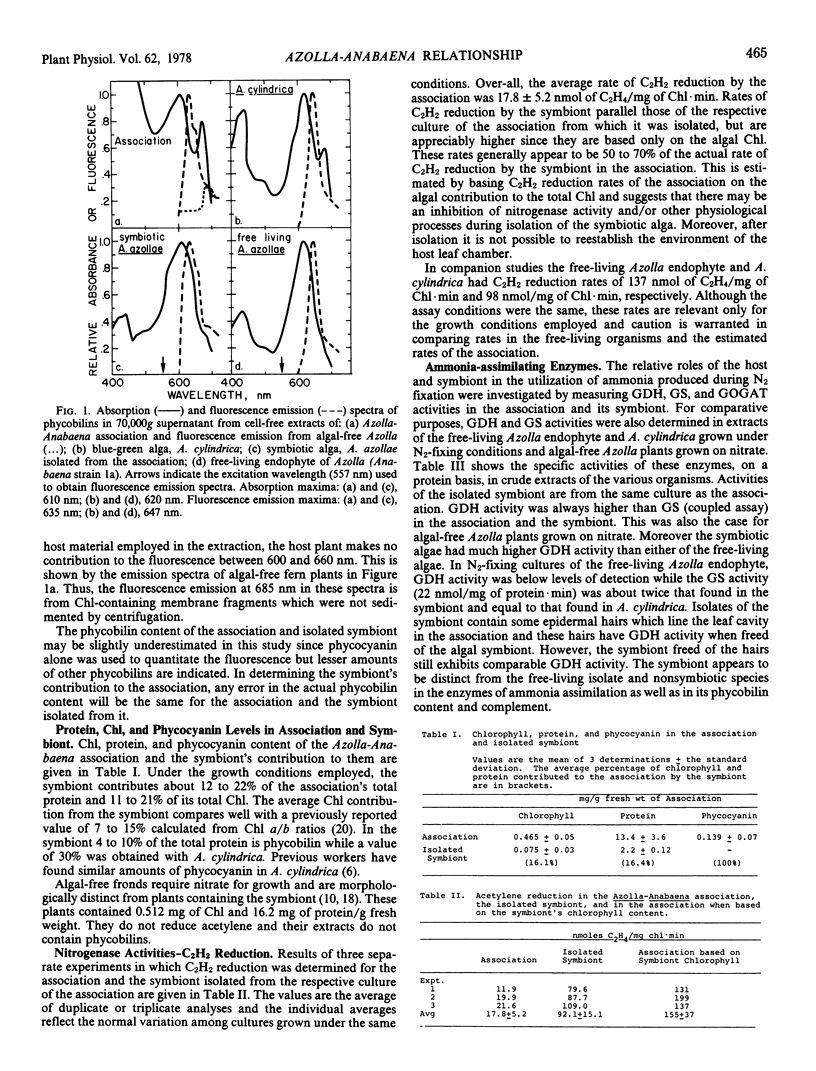
Abstract
The N2-fixing Azolla-Anabaena symbiotic association is characterized in regard to individual host and symbiont contributions to its total chlorophyll, protein, and levels of ammonia-assimilating enzymes. The phycocyanin content of the association and the isolated blue-green algal symbiont was used as a standard for this characterization. Phycocyanin was measured by absorption and fluorescence emission spectroscopy. The phycocyanin content and total phycobilin complement of the symbiotic algae were distinct from those of Anabaena cylindrica and a free-living isolate of the Azolla endophyte. The algal symbiont accounted for less than 20% of the association's chlorophyll and protein. Acetylene reduction rates in the association (based solely on the amount of algal chlorophyll) were 30 to 50% higher than those attained when the symbiont was isolated directly from the fern. More than 75% of the association's glutamate dehydrogenase and glutamine synthetase activities are contributed by the host plant. The specific activity of glutamate dehydrogenase is greater than that of glutamine synthetase in the association and individual partners. Both the host and symbiont have glutamate synthase activity. The net distribution of these enzymes is discussed in regard to the probable roles of the host and symbiont in the assimilation of ammonia resulting from N2 fixation by the symbiont.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fay P. Cell differentiation and pigment composition in Anabaena cylindrica. Arch Mikrobiol. 1969;67(1):62–70. doi: 10.1007/BF00413682. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A. Energy transfer in phycobilisomes from phycoerythrin to allophycocyanin. Biochim Biophys Acta. 1973 Apr 5;292(3):858–861. doi: 10.1016/0005-2728(73)90036-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MYERS J., KRATZ W. A. Relation between pigment content and photosynthetic characteristics in a blue-green algae. J Gen Physiol. 1955 Sep 20;39(1):11–22. doi: 10.1085/jgp.39.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson A., Rippka R., Kunisawa R. Heterocyst formation and nitrogenase synthesis in Anabaena sp. A kinetic study. Arch Mikrobiol. 1971;76(2):139–150. doi: 10.1007/BF00411788. [DOI] [PubMed] [Google Scholar]
- Newton J. W., Cavins J. F. Altered nitrogenous pools induced by the azolla-anabaena azolla symbiosis. Plant Physiol. 1976 Dec;58(6):798–799. doi: 10.1104/pp.58.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. A., Mayne B. C. The Azolla, Anabaena azollae Relationship: I. Initial Characterization of the Association. Plant Physiol. 1974 Jun;53(6):813–819. doi: 10.1104/pp.53.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. A., Mayne B. C. The Azolla, Anabaena azollae Relationship: II. Localization of Nitrogenase Activity as Assayed by Acetylene Reduction. Plant Physiol. 1974 Jun;53(6):820–824. doi: 10.1104/pp.53.6.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G. A., Toia R. E., Lough S. M. Azolla-Anabaena azollae Relationship: V. N(2) Fixation, Acetylene Reduction, and H(2) Production. Plant Physiol. 1977 Jun;59(6):1021–1025. doi: 10.1104/pp.59.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Miller R. E., Valentine R. C. Adenosine 3':5'-cyclic monophosphate control of the enzymes of glutamine metabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2922–2926. doi: 10.1073/pnas.69.10.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S., Milner L. A rapid radioactive assay for glutamine synthetase, glutaminase, asparagine synthetase, and asparaginase. Anal Biochem. 1970 Oct;37(2):429–438. doi: 10.1016/0003-2697(70)90069-2. [DOI] [PubMed] [Google Scholar]
- Tandeau de Marsac N. Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol. 1977 Apr;130(1):82–91. doi: 10.1128/jb.130.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- de Vasconcelos L., Fay P. Nitrogen metabolism and ultrastructure in Anabaena cylindrica. I. The effect of nitrogen starvation. Arch Mikrobiol. 1974 Mar 28;96(4):271–279. [PubMed] [Google Scholar]
- van Gorkom H. J., Donze M. Localization of nitrogen fixation in Anabaena. Nature. 1971 Nov 26;234(5326):231–232. doi: 10.1038/234231b0. [DOI] [PubMed] [Google Scholar]


