Abstract
This analysis describes the successes, challenges and opportunities to improve global vaccine safety surveillance as observed by the Vaccine Safety Working Group from its role as a platform of exchange for stakeholders responsible for monitoring the safety of vaccines distributed through the COVAX mechanism. Three key elements considered to be essential for ongoing and future pandemic preparedness for vaccine developers in their interaction with other members of the vaccine safety ecosystem are (1) the availability of infrastructure and capacity for active vaccine safety surveillance in low-income and middle-income countries (LMICs), including the advancement of concepts of safety surveillance and risk management to vaccine developers and manufacturers from LMICs; (2) more comprehensive mechanisms to ensure timely exchange of vaccine safety data and/or knowledge gaps between public health authorities and vaccine developers and manufacturers; and (3) further implementation of the concept of regulatory reliance in pharmacovigilance. These aims would both conserve valuable resources and allow for more equitable access to vaccine safety information and for benefit/risk decision-making.
Keywords: COVID-19, Vaccines
Summary box.
Infrastructures for the generation of evidence of vaccine safety have largely been developed to support regulatory and policy decision-making in national or regional immunisation programmes.
From the unique perspective of the Vaccine Safety Working Group of COVAX is an analysis of the challenges faced in the coordination of stakeholders in the safety surveillance of multiple new vaccines deployed in response to a global public health emergency.
Recommendations are intended to inform opportunities to improved collaboration of efforts to generate evidence of vaccine safety relevant to a greater diversity of global contexts.
Introduction
The SARS-CoV-2 pandemic resulted in unprecedented vaccine development using a diverse range of vaccine technologies, new and old. To date, 12 billion doses of COVID-19 vaccines have been rolled out worldwide and estimated to have saved 20 million lives.1 Given shortened clinical development timelines and emergency use approvals based on interim analyses, dependence on the global vaccine monitoring infrastructure for generating real-world evidence of safety had never been greater. Due to widespread and rapid deployment of multiple new vaccines, even rare adverse events following immunisation (AEFIs) could have the potential to affect large numbers of people daily, and their high visibility to impact public acceptance of vaccination. The global pharmacovigilance infrastructure therefore needed to ensure quick detection and effective management of any emergent safety signals.
The Vaccine Safety Working Group (VSWG) of COVAX was formed in November 2020 with representatives and consultants representing multiple stakeholders, including Brighton Collaboration, Developing Countries Vaccine Manufacturers Network (DCVMN), International Federation of Pharmaceutical Manufacturers and Associations, WHO and Coalition for Epidemic Preparedness Innovations (CEPI). Its primary objectives were to act as an open source of information for vaccine developers, to resolve common vaccine safety cross-project questions and challenges at speed, and to facilitate coordination within the ecosystem to maximise impact.
The aim of this analysis is to provide a description of the basic elements of vaccine safety surveillance systems and the reinforcements implemented to address the challenges of safety surveillance during the global immunisation campaign, to assess how well they have functioned, to describe specific challenges faced by vaccine developers, and to provide recommendations for an improved and robust response for vaccine safety surveillance for the next pandemic.
Global vaccine safety surveillance infrastructure
While both safety and effectiveness are of paramount importance during vaccine development, the limited study sample size and duration of follow-up of traditional phased clinical trials generally allow greater precision of efficacy over safety. The safety data collected in clinical development programmes characterise those adverse events which describe the ‘reactogenicity’ (‘tolerability’) of the vaccine. For example, current European Medicines Agency (EMA) guidance notes: ‘if a candidate vaccine contains components not previously included in licensed vaccines, it would be usual to aim for a safety database that is sufficient to estimate the frequency of uncommon adverse events (occurring in between 1/100 and 1/1000 vaccinated persons)’.2 Since vaccines are commonly used in millions (and billions for COVID-19) of recipients, the collection of additional safety data post-introduction is essential to further characterise the safety profile of a vaccine, to ensure an ongoing favourable benefit/risk balance, and to maintain public confidence.
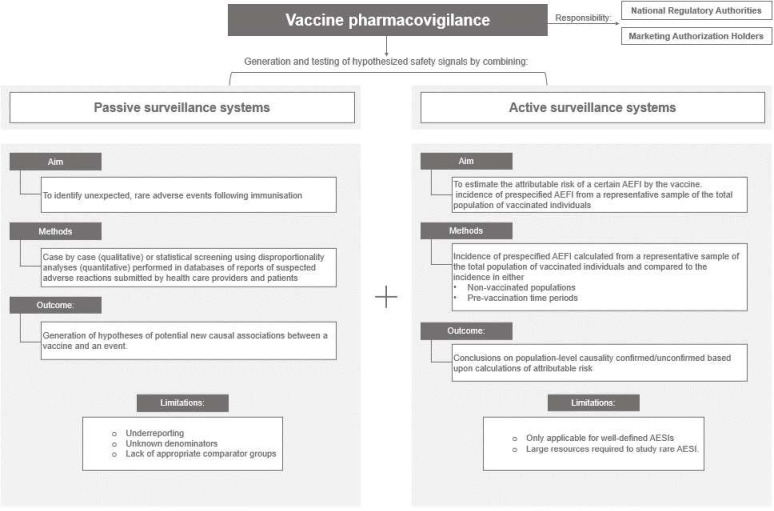
‘Vaccine pharmacovigilance’, as practised in high-income countries (HICs), is the shared responsibility of national regulatory authorities (NRAs), public health agencies and marketing authorisation holders. Comprehensive pharmacovigilance involves complementary passive and active surveillance systems for the generation and testing of hypothesised safety signals, respectively. Passive surveillance systems collect ‘spontaneous’ reports from healthcare providers and patients; statistical screening using disproportionality analyses in large databases of these reports allows for timely detection of signals of very rare AEFIs.3 Signal detection in passive surveillance databases generates hypotheses of potential causal associations between a vaccine and an event. Because passive surveillance is limited by issues such as under-reporting, unknown denominators and lack of appropriate comparator groups, active vaccine safety surveillance (AVSS) systems are needed to confirm or refute signals identified in passive systems and to estimate attributable risk and possible risk factors, if confirmed. Conclusions on causation to support regulatory and policy decision-making require adequately powered studies showing a higher incidence rate of AEFIs in vaccinees compared with control groups. The availability of standardised case definitions of adverse events of special interest (AESIs) and access to healthcare data (vaccination registries, medical records or insurance claims) are essential for AVSS. The limitations of AVSS include its utility only for known, well-defined AESIs and the large resources required to follow samples of large enough size and for long enough periods to study rare AESIs.4 The complementary nature of passive and active surveillance is displayed in figure 1.
Figure 1.
Vaccine pharmacovigilance is composed of two complementary systems, passive and active surveillance. AEFI, adverse event following immunisation; AESIs, adverse events of special interest.
Monitoring ‘vaccine safety’, as is more routinely performed in most low-income and middle-income countries (LMICs), generally aims to ensure high-quality production and administration of vaccines. The vision of the first WHO Global Vaccine Safety Blueprint (GVSB) was the provision of effective vaccine pharmacovigilance systems in all countries with at least minimal capacities at the national level. Implementation of this vision created surveillance infrastructure and processes executed though national immunisation programmes (NIPs). Collection and analysis of AEFI data in NIPs follow guidance provided by the WHO.5 Analyses of data are performed at different levels of the immunisation programme which have different objectives. Analysis of individual reports at the local level identifies cases requiring completion of AEFI investigation forms and subsequent individual-level causality assessment; while analysis of aggregate data at the national level is used for performance indicators to assess minimum country capacity for vaccine safety monitoring.6–8 In LMICs without common vaccine safety data platforms, NRAs tend to capture a relatively small number of AEFI reports, such as those reported directly by healthcare providers and/or those transferred by vaccine manufacturers. Furthermore, only reports within NRAs had been transferred into VigiBase, the global database established by the WHO Program for International Drug Monitoring. Analyses of safety data in many LMICs can thus be fragmented between expanded programmes on immunisation (EPI) and NRA and tend to be more descriptive in its approach.9 The limitations of this approach to monitoring ‘vaccine safety’ were acknowledged in the GVSB 2.0 (2021–2023) which was presented to key stakeholders at the Global Vaccine Safety Summit in December 2019.10 Central to this blueprint is a recognition that minimal capacity elements are not sufficient for the timely detection of vaccine safety signals and early post-marketing monitoring of novel products, and it provides objectives, strategies and an accountability framework to support the development of integrated pharmacovigilance systems to be implemented through the WHO regulatory strengthening network.
Infrastructure reinforcements to meet the challenges of the COVID-19 pandemic
To meet the demands of the pandemic, NRA in HICs, such as the US Food and Drug Administration (FDA), EMA and the national competent authorities in European Union (EU) member states, provided guidance to industry related to the development and licensure of COVID-19 vaccines. Given the circumstances of the public health crisis and shortened clinical development timelines, both US FDA and EMA provided specific guidance for COVID-19 vaccines, including considerations for pharmacovigilance and risk management plans (RMPs).11 12 ‘Consideration on core requirements for RMPs for COVID-19 vaccines’ from EMA detailed core safety elements required for inclusion into all RMPs and summary safety reports.13 Furthermore, initiatives such as BEST in the USA and ACCESS in the EU provided common protocols for the generation of background incidence of AESI and the performance of safety surveillance studies within existing large-linked databases of electronic healthcare data.14 15
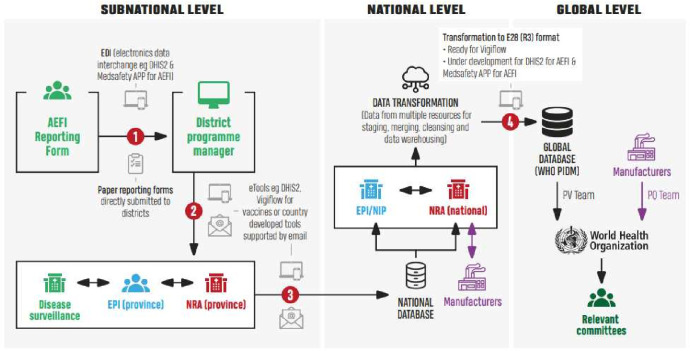
To support the less developed safety surveillance infrastructure in LMICs, the WHO rapidly responded with production of the COVID-19 vaccine safety surveillance manual.16 The intended audience of the manual was governments, global, regional and national staff from NIP, NRA, Ministries of Health, partners and pharmacovigilance centres, as well as vaccine manufacturers. The manual contains multiple modules providing guidance and tools to strengthen capacities and facilitate local, national, regional and global collaboration. Two key elements are the recommendations for data sharing between all relevant stakeholders and the implementation of the principles of regulatory reliance to pharmacovigilance. Sharing of data was prescribed between all relevant stakeholders in vaccine safety surveillance, and a generic strategy for data sharing at subnational, national and global levels was provided (figure 2). Reliance for pharmacovigilance is described as two activities: (1) reliance on processes, tools and methods by others (eg, signal detection, methods, templates for study protocols), and (2) reliance on product-specific regulatory activities or ‘mutual recognition’ (eg, assessment of marketing authorisation approval, assessment of post-authorisation safety protocols). Furthermore, a template for a cohort event monitoring safety study was provided.17
Figure 2.
Schematic representation of the structure for data sharing at the subnational, national and global levels as provided in the WHO COVID-19 vaccine safety surveillance manual. AEFI, adverse event following immunisation; EPI, expanded programmes on immunisation; NIP, national immunisation programme; NRA, national regulatory authority; PV, pharmacovigilance; PQ, prequalification; WHO PIDM, WHO Program for International Drug Monitoring.
The WHO Global Advisory Committee on Vaccine Safety (GACVS) created a COVID-19 Vaccine Safety Subcommittee that met regularly to assess new safety signals.18 The signal identification and initial review process was organised by the WHO Headquarters pharmacovigilance team, and the GACVS subcommittee provided a recommendation as well as advice on additional monitoring needs and public communication of safety information, if required. The creation of the COVAX VSWG was intended to complement the COVID-19 Vaccine Safety Subcommittee of GACVS, operating in direct communication with the WHO, with an aim to improve the implementation of pharmacovigilance activities in response to any arising safety concerns.
Analysis of the performance of the global vaccine safety surveillance during the COVID-19 pandemic
Developing a functional and well-integrated vaccine safety system is complex, challenging methodologically and requires considerable resources and time.19 20 The timely generation of real-world evidence on the safety profile of various COVID-19 vaccines described below was possible due to decades-long investments in creating functional pharmacovigilance systems including passive and active surveillance in several HICs, notably the USA,21 the UK,22 the EU,23 Canada24 and Australia.25 Serendipitiously, CEPI funded Brighton Collaboration’s Safety Platform for Emergency vACcines Project in May 2019 just prior to the pandemic.26 This allowed for the timely creation of a list of AESIs for the COVID-19 vaccines. Endorsement by GACVS ensured broad adoption/adaptation of a list of AESIs for pharmacovigilance of COVID-19 vaccines by many global stakeholders.
Reporting into passive surveillance systems has been unprecedented; in its final safety update on 8 December 2022, EMA reported that it had received over 1.6 million reports for all COVID-19 vaccines it had licensed for use.27 In its 2021 annual report to the European Commission, the EMA listed the identification of 11 unique signals for the COVID-19 vaccines.28 Furthermore, the EMA website reported updates to product information leaflets for the COVID-19 vaccines including additions of transverse myelitis, cutaneous small vessel vasculitis, hypoaesthesia/paraesthesia, extensive swelling of the vaccinated limb and tinnitus as possible adverse reactions.29
Two safety signals were of particular significance: (1) thrombotic thrombocytopenia syndrome/vaccine-induced thrombotic thrombocytopenia (TTS/VITT) for two adenoviral platform vaccines (AZD1222 and Ad26.COV2.S), and (2) myo/pericarditis for the mRNA platform vaccines.30–32 Regulators in HICs were able to rapidly define reporting rates and attributable risks of the COVID-19 vaccines used in their respective populations. NRAs and National Immunization Technical Advisory Groups provided benefit–risk policy frameworks, accommodating COVID-19 vaccine coverage, COVID-19 trends and reporting rates for the AESIs. This framework allowed stratified benefit–risk assessment and recommendation by age group and sex for the adenoviral and mRNA vaccines relative to the risks of TTS/VITT and myocarditis, respectively.33 Furthermore, active engagement of clinical scientists and their networks, especially haematologists with TTS/VITT, facilitated the production of guidance for the rapid identification and clinical management of patients with TTS.34 A potential impact of early identification and characterisation of the risk of TTS was the decrease in mortality observed in reports to EudraVigilance over time, from 47% in reports up to 28 March 2021 compared with 22% after this date.35
Above successes notwithstanding, several areas of vaccine safety systems in HICs need improvement. Given the known limitations of pre-approval clinical trials, unanticipated AESIs X should be expected when millions of doses of the new vaccine are administered in a global mass campaign. When AESIs X have emerged historically (eg, Guillain-Barre syndrome after swine influenza vaccine, narcolepsy after H1N1 influenza vaccine) and TTS/VITT and myocarditis after COVID-19 vaccines recently, the funding needed to understand their pathogenesis in a timely manner to optimally mitigate/prevent the risk has been lacking. Long delays of months to years between signal identification, hypothesis testing and elucidation of risk factors/mitigation strategies create a vacuum often filled with misinformation, contributing to vaccine hesitancy.36 The recently launched International Network of Special Immunisation Network seeks to address this gap in vaccine safety, through the application of a systems biology approach to uncover the pathogenesis of rare AESIs and inform vaccine development.37
The pandemic has served as a catalyst for surveillance system strengthening in LMICs. Training for both the public and healthcare professionals on the processes followed by the NRAs in ensuring the safety and effectiveness of the COVID-19 vaccines was implemented. Digital innovations, such as the MedSafety app, facilitated reporting of potential AEFIs in Ghana,38 and AEFI data triangulation and dashboard development supported public health decision-making in Nigeria.39 Regulators engaged in new regional collaborations for work sharing, most notably the African Union Smart Safety Surveillance (AU-3S) Project, involving Ghana, Nigeria, Ethiopia and South Africa.40 Signal detection on potential AEFIs from collaborating countries has been performed on a weekly basis, and summary review reports have been shared; such cooperation has allowed for the close monitoring of AEFIs that have been flagged elsewhere. Regulators have tried to accommodate the volume and complexity of RMPs and monthly safety reports which have been required by NRAs from HICs; however, it has not always been possible for these regulatory documents to be reviewed in a timely manner due to limited resources and relevant expertise in these regions.
Despite the above progress in systems strengthening in LMICs, the generation of evidence of safety from these regions remains limited, and the consequences of the lack of local safety data for the COVID-19 vaccines in the LMIC context have been described from Zimbabwe.41 Of the total of 4 500 000 reports contained within VigiBase as of October 2022, only 131 000 (3%) reports came from Africa and 434 000 (10%) from Asia.42 No signals of TTS/VITT with either the AZD1222 vaccine or Indian-manufactured Covishield (ChAdOx1_nCoV-19) have been identified in LMICs, where the majority of these vaccines have been used; only individual case reports have been published in the scientific literature.43 44 No safety signals for other WHO Emergency Use Listing Procedure-approved adenoviral-based vaccines (CanSino or Sputnik) or other inactivated COVID-19 vaccines (Sinopharm) not authorised in HICs have been communicated. Only two safety signals identified in VigiBase have been communicated through the WHO Pharmaceuticals Newsletter: myocarditis and hearing loss/tinnitus.45 46
A landscape survey and literature review to map AVSS activities globally and to identify vaccine safety evidence gaps was commissioned by the VSWG and executed by International Vaccine Access Center of Johns Hopkins University.47 Results revealed the implementation of AVSS activities in almost all WHO regions. However, the distribution of studies was heavily weighted to mRNA vaccines occurring in HICs. Furthermore, the most common AVSS activities in LMICs use a cohort event monitoring design in N<10 000 which can characterise the short-term adverse event profiles. In spite of the availability of a road map for international collaboration for safety monitoring of new vaccines in LMICs,48 a prior proof-of-concept study49 and operational lessons learnt,50 only two multicountry, hospital-based active surveillance studies for the estimation of risk of AESIs were ongoing in LMICs, and these were coordinated by international stakeholders such as the Pan American Health Organization51 and the ALIVE/Global Vaccine Data Network.52 Neither study involved vaccine developers or manufacturers, nor were they implemented as a pharmacovigilance activity as specified within an RMP.
Challenges for vaccine developers participating in the COVAX VSWG
One of the most significant challenges reported to the COVAX VSWG by vaccine developers in HICs (AstraZeneca, Moderna, Pfizer and J&J) related to a lack of coordination between global regulators which diluted global vaccine safety surveillance and risk management activities. Monthly safety reports were required by both the US FDA and EMA in addition to the routinely required periodical benefit–risk evaluation reports. Other requirements from non-US/non-EU countries introduced variability in consolidated vaccine safety information being provided to global health authorities. Individual health authority queries on safety topics became duplicative, often with only slight nuances between the requests, therefore not permitting the use of the same response for multiple regulators.
Regulatory requests to conduct multiple pharmacovigilance activities beyond global RMPs were resource-intensive and complicated by lack of infrastructure in LMICs to implement studies and by competition for the limited scientific resources, such as local clinical research organisations, to support protocol development and implementation. In some countries, particularly in LMICs, some pharmacovigilance activities were developed separately by NRAs and EPI with no or very limited data exchange, resulting in duplicate work by the pharmaceutical companies.
Vaccine developers from HICs have communicated the solutions they found during the pandemic and have made recommendations.53 For improving the generation of real-world evidence of safety, they suggest the development of geographically flexible, common protocols and/or joint company-sponsored platform observational studies for multiple vaccines, and they have issued a call for a collective strategy to build a network of sentinel sites in LMICs. To enable near real-time signal assessment, there is also the need to ensure availability of critical data, such as vaccine exposure data and background incidence rates of AESIs.
Vaccine developers and manufacturers in LMICs have struggled to meet specific requirements from both global and local NRA and NIPs. While a legal regulatory framework to reliably evaluate and monitor the quality and efficacy of vaccines may be present in many non-ICH (the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) regions/LMICs, legislation and regulations relating to pharmacovigilance are often weak or missing. A landscape analysis of members of the DCVMN highlights challenges which likely left many developers unprepared for the pandemic. A questionnaire was used to query the existing pharmacovigilance structures and practices of vaccine manufacturers in LMICs.54 Of the respondents (34 of 43), almost all (97%) respondents had procedures in place to define cases’ seriousness, a lower proportion had standard operating procedures on assessing causality, less than half had access to MedDRA (Medical Dictionary for Regulatory Activities) coding and only a quarter used Brighton Collaboration case definitions of vaccine-specific AEFIs.
In the absence of mature and robust pharmacovigilance and quality management systems, it is difficult for vaccine developers and manufacturers to perform vaccine safety surveillance and implement RMPs according to international standards. A lack of safety data exchange in LMICs from NIP and NRA has prevented local marketing authorisation holders from collecting enough reports to perform signal detection. Investment in local infrastructure is required for vaccine developers and manufacturers to implement pharmacovigilance activities as specified in RMPs with the purpose to allow periodical benefit–risk assessments.
Recommendations
A pandemic presents global challenges requiring integrated, rapid and global solutions. However, existing infrastructure is largely designed to support less acute national or regional priorities, or other focused interests. The COVID-19 pandemic provided an opportunity to begin an evolution to a more integrated global approach to pharmacovigilance and risk management. Vaccine developers from both HICs and LMICs would benefit from further efforts to build a collaborative global infrastructure to improve both the efficiency and the equity of safety evidence generation. There are lessons learnt from the COVAX VSWG that can inform preparations for responses to future pandemics.
Our conclusions and recommendations are in three mutually dependent areas:
Vaccine safety data generation following licensure for emergency use is critical for rapidly identifying and estimating the incidence of rare or very rare adverse events. Ensuring such vaccine safety data can rapidly be gathered from all relevant regions of the globe would allow ongoing and robust assessments of vaccine benefit–risk and confidence thereby underpinning successful vaccination campaigns. Progress in safety evidence generation in LMICs was facilitated by the guidance and templates provided by the WHO in the COVID-19 vaccine safety surveillance manual. However, vaccine developers and manufacturers in LMICs currently have limited capabilities in the practice of vaccine pharmacovigilance. Having complementary passive systems and targeted active approaches in all countries is likely neither feasible nor efficient globally. Therefore, it is imperative to appreciate the variance between local and regional pharmacovigilance systems and to strengthen both passive and active approaches where and when relevant. Sustaining the progress made towards active surveillance capacities during COVID-19 will be a key component of future pandemic preparedness.
Vaccine safety data sharing is essential between all stakeholders in the vaccine ecosystem to ensure equitable access to evidence for decision-making. For data to provide relevant insights for risk management, there must be comprehensive mechanisms in place to ensure vaccine safety data and/or knowledge of safety data gaps can be readily shared and used. Information exchange regarding post-licensure safety knowledge gaps could allow for collaborative efforts to generate the necessary data required for local regulatory benefit/risk decision-making. The resources required for efficient generation of high-quality evidence require involvement of the industry. Universal acceptance and use of harmonised tools, such as AESI case definitions and benefit/risk templates, would support both the capture of high-quality data as well as assure the shareability of vaccine safety data across stakeholders within countries and between global partners.
Further application of the principles of reliance across the ecosystem could minimise duplicate use of industry resources and compensate for disproportional global regulatory capabilities. Successful implementation of principles of reliance for registration and inspections exemplifies the potential of this concept55; further expansion of the concept of reliance in pharmacovigilance would be beneficial. Stakeholders within global vaccine safety surveillance should work together to develop consensus and develop guidance for further implementation of reliance to pharmacovigilance tasks related to signal detection, assessment of periodical benefit–risk evaluation reports, as well as safety updates to product labels.
Implementation of these recommendations will require collaboration and coordination between stakeholders within the global vaccine safety ecosystem. The WHO GVSB 2.0 (2019) has highlighted the need for early post-authorisation monitoring of novel products and an increased sensitivity in detecting vaccine safety signals in LMICs, and it has provided objectives, strategies and an accountability framework for the integration of vaccine surveillance into regulatory strengthening initiatives. Additional strategic considerations for preparedness would be harmonisation on critical elements in safety evidence generation, including endpoint definitions and protocols for active vaccine safety surveillance studies by the global vaccine safety community. Regional initiatives for regulatory reliance and work sharing, such as AU-3S, could be leveraged for provision of harmonised guidance for good pharmacovigilance practice in the event of public health emergencies and oversight of the implementation of RMPs. Building sustainable infrastructures and capacities for vaccine safety evidence generation requires large commitments and investment which are likely only possible through public health funding organisations or collective industry organisations and supported by political will.
Acknowledgments
The authors acknowledge the contributions of Ada Basterrica as the project manager for the VSWG, for her administrative contributions; and of Jodie Rogers, communication manager at CEPI, for her editorial comments and suggestions.
Footnotes
Handling editor: Seye Abimbola
Contributors: All authors contributed to the conceptualisation of the manuscript. REC, DB, PB, CJ-R, KH, AP and RTC made written contributions. MRB, EE, JM, LN, SP and PT provided critical review and revisions. REC prepared and submitted the publication.
Funding: The funding of the COVAX Vaccine Safety Working Group comes from CEPI.
Disclaimer: The author is a staff member of the World Health Organization. The author alone is responsible for the views expressed in this publication and they do not necessarily represent the views, decisions or policies of the World Health Organization.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
There are no data in this work.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical Modelling study. The Lancet Infectious Diseases 2022;22:1293–302. 10.1016/S1473-3099(22)00320-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Medicines Agency MA . Guideline on clinical evaluation of new vaccines, EMEA/CHMP/VWP/164653/2005, . 2005. Available: https://www.ema.europa.eu/en/clinical-evaluation-new-vaccines-scientific-guideline#current-effective-version---under-revision-section
- 3. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the vaccine adverse event reporting system (VAERS). Vaccine 2015;33:4398–405.:S0264-410X(15)00982-2. 10.1016/j.vaccine.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crawford NW, Clothier H, Hodgson K, et al. Active surveillance for adverse events following immunization. Expert Rev Vaccines 2014;13:265–76. 10.1586/14760584.2014.866895 [DOI] [PubMed] [Google Scholar]
- 5. Global Vaccine Safety Blueprint, Available: https://www.who.int/docs/default-source/documents/global-vaccine-safety-blueprint-1-2012.pdf [Accessed 25 Oct 2023].
- 6. WHO . AIDE-Mémoire on AEFI Investigation, Available: https://cdn.who.int/media/docs/default-source/pvg/global-vaccine-safety/new-aide-memoire-aefi.pdf?sfvrsn=66340a11_4 [Accessed 6 Sep 2022].
- 7. WHO . Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, . 2019. Available: https://www.who.int/publications/i/item/9789241516990 [Accessed 6 Sep 2022].
- 8. WHO . WHO/UNICEF joint reporting process, . 2016. Available: https://www.who.int/teams/immunization-vaccines-and-biologicals/immunization-analysis-and-insights/global-monitoring/who-unicef-joint-reporting-process [Accessed 6 Sep 2022].
- 9. Salman O, Topf K, Chandler R, et al. Progress in immunization safety monitoring — worldwide, 2010–2019. MMWR Morb Mortal Wkly Rep 2021;70:547–51. 10.15585/mmwr.mm7015a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Global Vaccine Safety Blueprint 2.0 (2021-2023), Available: https://www.who.int/publications/i/item/9789240036963 [Accessed 26 Oct 2023].
- 11. U.S . Department of health and human services food and Drug Administration center for Biologics evaluation and research. development and Licensure of vaccines to prevent COVID-19 guidance for industry. 2020. Available: https://www.fda.gov/media/139638/download [Accessed 10 Apr 2023].
- 12. EMA considerations on COVID-19 vaccine approval. EMA/592928/2020. Committee for human medicinal products (CHMP). Available: https://www.ema.europa.eu/en/documents/other/ema-considerations-covid-19-vaccine-approval_en.pdf [Accessed 10 Apr 2023].
- 13. Consideration on core requirements for Rmps of Covid19 vaccines. September 1, 2022. Available: https://www.ema.europa.eu/en/documents/other/consideration-core-requirements-rmps-covid-19-vaccines_en.pdf [Accessed 10 Apr 2023].
- 14. COVID-19 vaccine safety surveillance: active monitoring master protocol available at: COVID-19 vaccine safety active monitoring protocol (Bestinitiative.org) (February 10, 2021). n.d.
- 15. The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. Protocol: Background rates of Adverse Events of Special Interest for monitoring COVID-19 vaccines Version 1.1, . 2020. Available: https://www.encepp.eu/encepp/openAttachment/fullProtocol/37296 [Accessed 10 Apr 2023].
- 16. Licence: CC BY-NC-SA 3.0 IGO. In: COVID-19 vaccines: safety surveillance manual. second. Geneva: World Health Organization, 2021. [Google Scholar]
- 17. WHO . Protocol template to be used as template for observational study protocols: cohort event monitoring (CEM) for safety signal detection after vaccination with COVID-19 vaccines, . 2021. Available: https://apps.who.int/iris/handle/10665/342193 [Accessed 10 Apr 2023].
- 18. WHO . WHO weekly Epidemiological record; 2023. ;98:83–92. [Google Scholar]
- 19. Lasky T, Terracciano GJ, Magder L, et al. Association of the Guillain-Barre syndrome with the 1992-93 and 1993-94 influenza vaccines. N Engl J Med 1998;339:1797–802. 10.1056/NEJM199812173392501 [DOI] [PubMed] [Google Scholar]
- 20. Chen RT, Glanz J, Ch ST. 20: Pharmacoepidemiology studies of vaccine safety. In: Strom BL, Kimmel SE, Hennessy S, eds. Pharmacoepidemiology. 6th edition. Sussex: John Wiley & Sons, 2020: 437–95. 10.1002/9781119413431 [DOI] [Google Scholar]
- 21. Vaccine Safety . Vaccine safety Monitoring| CDC. Available: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/index.html [Accessed 20 Apr 2023].
- 22. publishing.service.Gov.UK . Green book: chapter 9 surveillance and monitoring. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/147870/Green-Book-Chapter-9.pdf [Accessed 20 Apr 2023].
- 23. EMA . Guideline on good Pharmacovigilance practices (GVP) - Product- or population-specific considerations I vaccines for prophylaxis against infectious diseases (Europa.EU). Available: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-product-population-specific-considerations-i-vaccines_en.pdf [Accessed 20 Apr 2023].
- 24. Canada.ca . Canadian adverse events following immunization surveillance system (CAEFISS). Available: https://www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html [Accessed 20 Apr 2023].
- 25. Australian government Department of health and aged care . Vaccine safety. Available: https://www.health.gov.au/topics/immunisation/about-immunisation/vaccine-safety [Accessed 20 Apr 2023].
- 26. CEPI . CEPI partners with Brighton collaboration to support safety assessment of vaccine candidates. Available: https://cepi.net/news_cepi/cepi-partners-with-brighton-collaboration-to-support-safety-assessment-of-vaccine-candidates-against-emerging-infectious-diseases/ [Accessed 20 Apr 2023].
- 27. European Medicines Agency . COVID-19 vaccines safety update December 2022. 2022. Available: https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccines-safety-update-8-december-2022_en.pdf [Accessed 10 Apr 2023].
- 28. European Medicines Agency . 2021 annual report on Eudravigilance for the European Parliament, the Council and the Commission. 2022. Available: https://www.ema.europa.eu/en/documents/report/2021-annual-report-eudravigilance-european-parliament-council-commission_en.pdf [Accessed 10 Apr 2023].
- 29. European Medicines Agency . Safety of COVID-19 vaccines. 2022. Available: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/safety-covid-19-vaccines [Accessed 10 Apr 2023].
- 30. Greinacher A, Thiele T, Warkentin TE, et al. Thrombotic thrombocytopenia after Chadox1 nCov‐19 vaccination. N Engl J Med 2021;384:2092–101.:NEJMoa2104840. 10.1056/NEJMoa2104840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after Chadox1 nCoV‐19 vaccination. N Engl J Med 2021;384:2124–30.:NEJMoa2104882. 10.1056/NEJMoa2104882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against COVID-19 in Israel. N Engl J Med 2021;385:2140–9.:NEJMoa2109730. 10.1056/NEJMoa2109730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Advisory Committee on Immunization Practices (ACIP) . ACIP Presentation Slides: Decmeber 16, 2021, . 2021. Available: https://www.cdc.gov/vaccines/acip/meetings/slides-2021-12-16.html
- 34. European Medicines Agency . EMA RAISES awareness of clinical care recommendations to manage suspected thrombosis with thrombocytopenia syndrome. 2021. Available: https://www.ema.europa.eu/en/news/ema-raises-awareness-clinical-care-recommendations-manage-suspected-thrombosis-thrombocytopenia [Accessed 6 Sep 2022].
- 35. van de Munckhof A, Krzywicka K, Aguiar de Sousa D, et al. Declining mortality of cerebral venous sinus thrombosis with thrombocytopenia after SARS-Cov-2 vaccination. Eur J Neurol 2022;29:339–44. 10.1111/ene.15113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen RT. Evaluation of vaccine safety after the events of 11 September 2001: role of cohort and case-control studies. Vaccine 2004;22:2047–53. 10.1016/j.vaccine.2004.01.023 [DOI] [PubMed] [Google Scholar]
- 37. Top KA, Chen RT, Levy O, et al. Advancing the science of vaccine safety during the Coronavirus disease 2019 (COVID-19) pandemic and beyond: launching an international network of special immunization services. Clin Infect Dis 2022;75(Suppl 1):S11–7. 10.1093/cid/ciac407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. The United Nations Development Programme . Med safety App for reporting side effects of medicines and other health products in Ghana. 2019. Available: https://www.undp.org/ghana/news/med-safety-app-reporting-side-effects-medicines-and-other-health-products-ghana [Accessed 6 Sep 2022].
- 39. Shragai T, Adegoke OJ, Ikwe H, et al. Implementation of data Triangulation and dashboard development for COVID-19 vaccine adverse event following Immunisation (AEFI) data in Nigeria. BMJ Glob Health 2023;8:e011006. 10.1136/bmjgh-2022-011006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. African Union Development Agency-NEPAD . African Union Smart Safety Surveillance (AU-3S): Spotlight Newsletter: Edition One, . 2021. Available: https://www.nepad.org/publication/african-union-smart-safety-surveillance-au-3s-spotlight-newsletter-edition-one [Accessed 6 Sep 2022].
- 41. Murewanhema G, Dzinamarira T, Madziva R, et al. SARS‐Cov ‐2 Vaccine‐Related adverse events in Zimbabwe: the need to strengthen Pharmacovigilance in Resource‐Limited settings. Pharmacoepidemiol Drug Saf 2022;31:379–80. 10.1002/pds.5393 [DOI] [PubMed] [Google Scholar]
- 42. Vigiaccess . Who. Available: https://www.vigiaccess.org/ [Accessed 31 Oct 2022].
- 43. Maramattom BV, Moidu FM, Varikkottil S, et al. Cerebral venous sinus thrombosis after Chadox1 vaccination: the first case of definite thrombosis with thrombocytopenia syndrome from India. BMJ Case Rep 2021;14:e246455. 10.1136/bcr-2021-246455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herrera-Comoglio R, Lane S. Vaccine-induced immune thrombocytopenia and thrombosis after the Sputnik V vaccine. N Engl J Med 2022;387:1431–2.:NEJMc2210813. 10.1056/NEJMc2210813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. WHO . WHO Pharmaceuticals Newsletter - N°4, 2021, . 2021. Available: https://www.who.int/publications/i/item/who-pharmaceuticals-newsletter---n-4-2021 [Accessed 16 May 2022].
- 46. WHO . WHO Pharmaceuticals Newsletter - N°1, 2022, . 2022. Available: https://apps.who.int/iris/handle/10665/351326 [Accessed 16 May 2022].
- 47. VIEW-Hub . The International Vaccine Access Center, Available: https://view-hub.org/vaccine/covid [Accessed 16 May 2022].
- 48. Izurieta HS, Zuber P, Bonhoeffer J, et al. Roadmap for the International collaborative epidemiologic monitoring of safety and effectiveness of new high priority vaccines. Vaccine 2013;31:3623–7. 10.1016/j.vaccine.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 49. Perez-Vilar S, Weibel D, Sturkenboom M, et al. WHO global vaccine safety-multi country collaboration. enhancing global vaccine Pharmacovigilance: proof-of-concept study on aseptic meningitis and immune Thrombocytopenic purpura following measles-Mumps containing vaccination. Vaccine 2018;36:347–54.:S0264-410X(17)30620-5. 10.1016/j.vaccine.2017.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Guillard-Maure C, Elango V, Black S, et al. WHO global vaccine safety-multi country collaboration. operational lessons learned in conducting a multi-country collaboration for vaccine safety signal verification and hypothesis testing: the global vaccine safety multi country collaboration initiative. Vaccine 2018;36:355–62.:S0264-410X(17)31011-3. 10.1016/j.vaccine.2017.07.085 [DOI] [PubMed] [Google Scholar]
- 51. Pan American Health Organization . Vaccine safety. Available: https://www.paho.org/en/topics/vaccine-safety [Accessed 15 Apr 2023].
- 52. Global Vaccine Data Network . African COVID-19 vaccine safety surveillance (Acvass). Available: https://www.globalvaccinedatanetwork.org/african-covid-19-vaccine-safety-surveillance-acvass [Accessed 15 Apr 2023].
- 53. Bauchau V, Davis K, Frise S, et al. Real-world monitoring of COVID-19 vaccines: an industry expert view on the successes, challenges, and future opportunities. Drug Saf 2023;46:327–33. 10.1007/s40264-023-01290-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hartmann K, Pagliusi S, Precioso A. Landscape analysis of Pharmacovigilance and related practices among 34 vaccine manufacturers from emerging countries. Vaccine 2020;38:5490–7.:S0264-410X(20)30788-X. 10.1016/j.vaccine.2020.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saint-Raymond A, Valentin M, Nakashima N, et al. Reliance is key to effective access and oversight of medical products in case of public health emergencies. Expert Rev Clin Pharmacol 2022;15:805–10. 10.1080/17512433.2022.2088503 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data in this work.