Abstract
Objectives
The role of the Controlling Nutritional Status (CONUT) scores in predicting the prognosis of lymphoma cases has been extensively explored, with no consistent results. The present meta-analysis focused on accurately evaluating whether CONUT could be used to predict the prognosis of lymphoma cases and its clinicopathological value.
Design
The present meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The prognostic significance of CONUT to overall survival (OS) and progression-free survival (PFS) in lymphoma was estimated by calculating pooled HRs with 95% CIs. The relationship between CONUT and clinicopathological characteristics was measured based on pooled ORs with 95% CIs.
Data sources
PubMed, Web of Science, Embase and Cochrane Library databases were comprehensively searched from inception through 24 March 2023.
Statistical methods
Either a random-effects model or a fixed-effects model was selected depending on the level of heterogeneity among the included studies.
Results
This meta-analysis enrolled seven articles, containing 2060 patients with lymphoma. According to the pooled analysis, a higher CONUT score significantly predicted poor OS (HR=1.94, 95% CI 1.46 to 2.57, p<0.001) as well as poorer PFS (HR=1.51, 95% CI 1.04 to 2.20, p=0.031). Furthermore, according to the combined analysis, a higher CONUT score was significantly associated with Ann Arbor stages III–IV (OR=3.75, 95% CI 2.96 to 4.75, p<0.001), an Eastern Cooperative Oncology Group performance status of 2–4 (OR=5.14, 95% CI 3.97 to 6.65, p<0.001), high-intermediate/high National Comprehensive Cancer Network International Prognostic Index (OR=8.05, 95% CI 5.11 to 12.66, p<0.001), B symptoms (OR=4.97, 95% CI 2.89 to 8.52, p<0.001), extranodal disease (OR=3.25, 95% CI 2.24 to 4.70, p<0.001), bone marrow involvement (OR=4.86, 95% CI 3.25 to 7.27, p<0.001) and elevated lactate dehydrogenase levels (OR=3.21, 95% CI 2.37 to 4.34, p<0.001).
Conclusions
According to our results, higher CONUT scores were significantly associated with poor OS and PFS in lymphoma.
Keywords: CONUT, lymphoma, meta-analysis, prognosis, evidence-based medicine
STRENGTHS AND LIMITATIONS OF THIS STUDY.
We performed a meta-analysis of seven studies, totalling 2060 patients, to investigate the association between the Controlling Nutritional Status (CONUT) score and prognosis of patients with lymphoma.
The relationships between CONUT scores and the clinicopathological characteristics of patients with lymphoma were determined in the stratified analysis.
The various cut-off values for CONUT scores in the included studies might be a source of heterogeneity in the results.
Introduction
Lymphoma accounted for 627 439 new cases of patients with cancer and 283 169 cancer-related deaths worldwide in 2020.1 Lymphomas are highly heterogeneous tumours that present with numerous clinical manifestations and are strikingly diverse genetically.2 They are classified as either non-Hodgkin’s lymphomas (NHL) or Hodgkin’s lymphomas, with various subtypes under these two umbrella terms.3 Moreover, in the Western hemisphere, NHL is the most common type of haematological malignancy, with an incidence of about 13 per 100 000 people.4 Although aggressive B cell lymphomas can be cured with chemotherapy and immunotherapy, most indolent lymphomas are incurable; however, they often achieve durable remissions requiring lifelong monitoring.5 Several advances have been made in the treatment of lymphoma, including stem cell transplantation, adoptive cell therapy and targeted therapy. However, refractory or recurrent tumours have limited the survival of patients with lymphoma. Therefore, there is an urgent need to identify novel diagnostic and prognostic markers that allow for accurate and early identification of high-risk patient groups with lymphomas.
Furthermore, systemic inflammation and malnutrition are related to dismal prognoses in many cancers.6–9 ‘Measures’ that represent the nutritional and inflammatory status of patients have been investigated as prognostic factors, including neutrophil to lymphocyte ratio,10 platelet to lymphocyte ratio,11 C reactive protein to albumin ratio,12 the Controlling Nutritional Status (CONUT) score13 and the Geriatric Nutritional Risk Index.14 CONUT scores are determined based on serum albumin content, peripheral lymphocyte count as well as total cholesterol content (online supplemental table S1). The CONUT system was first proposed in 2005 by Ignacio et al 15 and initially used as a screening approach to evaluating patients’ nutritional status. Additionally, CONUT is a significant prognostic factor in various types of cancer, such as breast cancer,16 hepatocellular carcinoma,17 urothelial carcinoma,18 colorectal cancer,19 hypopharyngeal cancer20 and prostate cancer.21 The legitimacy of using CONUT scores while predicting the prognosis of lymphoma cases has been widely investigated but no consistent results can be obtained.22–28 For instance, higher CONUT scores (usually ≥5) were reported to significantly predict the prognosis of lymphoma cases in some studies, whereas other scholars did not show a significant relationship between lymphoma prognosis and use of CONUT scores.23 Therefore, through a comprehensive literature review, the present meta-analysis focused on identifying the precise role of CONUT scores when estimating lymphoma prognoses. Moreover, we also investigated the association of CONUT with the clinicopathological characteristics of lymphoma.
bmjopen-2023-078320supp001.pdf (346.5KB, pdf)
Materials and methods
Patient and public involvement
No patients were involved.
Study guideline
The meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.29 The PRISMA checklist is shown in online supplemental table S2.
bmjopen-2023-078320supp004.pdf (66.8KB, pdf)
Literature search
We thoroughly searched PubMed, Web of Science, Embase and Cochrane Library databases from inception through 24 March 2023. To retrieve study literature related to the primary topic of our analysis, the following Medical Subject Headings (MeSH) terms were adopted and combined with text words, namely (controlling nutritional status or CONUT) and lymphoma. The detailed search strategies for each database are shown in online supplemental file 1. References in related articles were also reviewed, aiming to identify more work.
bmjopen-2023-078320supp002.pdf (28.3KB, pdf)
Study eligibility criteria
Studies conforming to the following criteria were included: (1) lymphoma confirmed by pathology; (2) reporting a relationship between CONUT scores and lymphoma survival outcomes; (3) providing enough information to predict HRs with 95% CIs; (4) a threshold was identified to stratify low and high CONUT scores; and (5) English studies. The following studies were excluded: (1) animal studies; (2) case reports, reviews, letters, conference abstracts and comments; and (3) studies with overlapped patients.
Data collection and quality evaluation
Two researchers (LL and LS) were responsible for collecting the data from the included articles, independently. Any discrepancy between them was resolved through mutual negotiation. The following data were collected from the qualified articles: first author, publication year, country, age, sample size, gender, study period, histology, study centre, cut-off value of CONUT, follow-up, survival outcomes and survival analysis, combined with HRs (95% CIs). In this meta-analysis, overall survival (OS) was the primary outcome, whereas progression-free survival (PFS) was the secondary outcome. Two researchers (LL and LS) were responsible for independently evaluating the quality of each enrolled study according to the Newcastle-Ottawa Scale (NOS).30 The NOS uses three factors: selection, comparability and outcome, with scores ranging from 1 to 9. Studies with NOS scores ≥6 were designated to be of high quality.
Statistical analysis
The role of CONUT scores in OS and PFS in lymphoma was analysed by calculating pooled HRs with 95% CIs. The Cochran Q test and I2 statistics were used to evaluate interstudy heterogeneity. An I2 of >50% and Q test with p<0.10 indicated studies with high heterogeneity. A random-effects model was adopted for studies with high heterogeneity, whereas a fixed-effects model was used for studies with low heterogeneity. Subgroup analyses were also carried out to identify the source of heterogeneity. Associations between CONUT scores and clinicopathological factors were measured based on pooled ORs with 95% CIs. Funnel plots and the Begg’s test were visually inspected to assess for potential publication bias. Statistical analyses were completed with Stata V.12.0 software. P<0.05 (two-tailed) stood for statistical significance.
Results
Literature screening
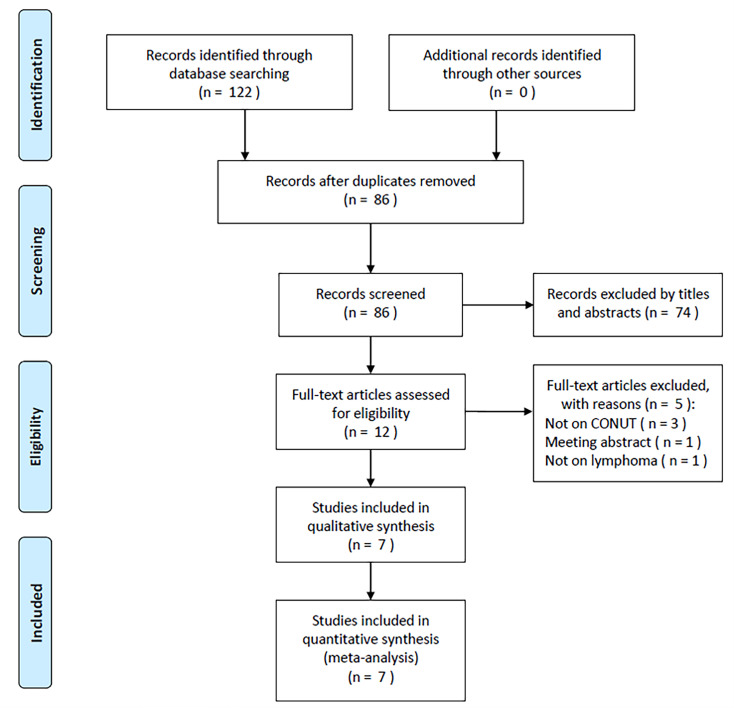
During the original literature review, 122 articles were initially included and 86 studies remained after removing duplicate records (figure 1). Another 74 articles were eliminated during title and abstract screening, and the remaining 12 were further examined with full-text reading. Later, five studies that did not focus on CONUT (n=3), were meeting abstracts (n=1) and did not study lymphoma (n=1) were eliminated. Ultimately, seven studies were enrolled in the present meta-analysis, involving 2060 patients with lymphoma22–28 (figure 1, online supplemental table S3).
Figure 1.
PRISMA flow diagram of this meta-analysis. CONUT, Controlling Nutritional Status; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
bmjopen-2023-078320supp005.pdf (37.4KB, pdf)
Eligible study features
Online supplemental table S3 presents the baseline features of the seven enrolled articles. The publication years of these articles ranged from 2020 to 2023. Three studies were carried out in Japan,22 23 27 two in China26 28 and two in Turkey.24 25 The enrolled articles all used the English language and were of retrospective design,22–28 with sample sizes between 81 and 615 patients (median: 266). Five were single-centre studies23–26 28 and two were multicentre trials.22 27 Four studies enrolled patients with diffuse large B cell lymphoma (DLBCL),22–25 whereas the remaining three included extranodal NK/T cell lymphoma,28 peripheral T cell lymphoma27 and lymphoma cases.26 Three studies adopted a CONUT cut-off value of ≥5,22 25 27 two used a cut-off of ≥224 28 and the two remaining each chose a cut-off of ≥423 and ≥7.26 All seven articles mentioned a relationship between CONUT scores and OS in lymphoma22–28 and three proved a relationship between CONUT and PFS.23 24 28 Five articles provided HRs with 95% CIs based on multivariable regressions23–27 and two offered the same based on univariable regressions.22 28 For all enrolled articles, the NOS scores ranged from 7 to 9, with a median of 8, suggesting high quality.
CONUT and OS in lymphoma
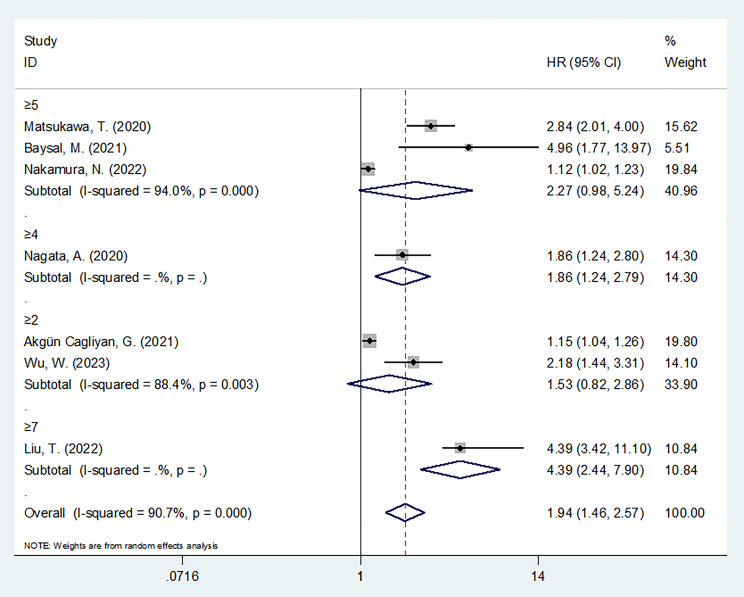
Seven articles, comprising 2060 cases,22–28 provided a relationship between CONUT and OS in lymphoma. We employed a random-effects model due to heterogeneity (I2=90.7%, p<0.001). As seen in figure 2 and online supplemental table S4, a pooled HR of 1.94 (95% CI 1.46 to 2.57, p<0.001) was obtained, which indicated a significant association between high CONUT scores and poor OS of lymphoma cases. Furthermore, subgroup analyses based on various factors were conducted (online supplemental table S4). An elevated CONUT score was significantly correlated with poor OS in the following subgroups: studies from China (p=0.002), sample size of ≥200 (p=0.013), single-centre studies (p=0.003), cut-off value of ≥4 (p=0.003), cut-off value of ≥7 (p<0.001) and those that included DLBCL cases (p=0.013) (online supplemental table S4).
Figure 2.
Forest plot depicting the association between CONUT and OS in lymphoma according to various cut-off values. The cut-off values in the studies are as follows: ≥2 in Akgün Çağlıyan et al 24 and Wu et al 28; ≥4 in Nagata et al 23; ≥5 in Matsukawa et al,22 Baysal et al 25 and Nakamura et al 27; and ≥7 in Liu et al.26 Overall estimate should be interpreted with caution give the varying cut-offs used in the different studies. CONUT, Controlling Nutritional Status; OS, overall survival.
bmjopen-2023-078320supp006.pdf (28.1KB, pdf)
CONUT and PFS in lymphoma
Altogether, three articles, involving 1116 cases,23 24 28 provided evidence on the prognostic role of CONUT in PFS. Due to heterogeneity (I2=80.3%, p=0.006), we used a random-effects model. The combined data from these studies indicated that elevated CONUT scores were significantly associated with shortened PFS in lymphoma (HR=1.51, 95% CI 1.04 to 2.20, p=0.031; online supplemental table S4, figure 3). Based on the subgroup analysis, high CONUT scores still significantly predicted PFS, regardless of subtype (p<0.05; online supplemental table S4). Moreover, elevated CONUT scores were also significantly correlated with poor PFS in studies from China (p<0.001) and Turkey (p<0.001) (online supplemental table S4).
Figure 3.
Forest plot depicting the association between CONUT and PFS in lymphoma according to various cut-off values. The cut-off values in the studies are as follows: ≥2 in Akgün Çağlıyan et al 24 and Wu et al 28; and ≥4 in Nagata et al. 23 Overall estimate should be interpreted with caution give the varying cut-offs used in the different studies. CONUT, Controlling Nutritional Status; PFS, progression-free survival.
CONUT and clinicopathological features
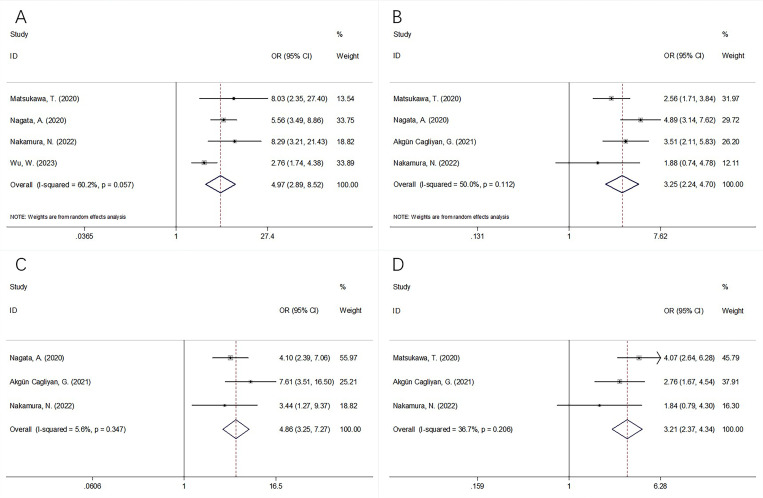
Five studies, comprising 1830 patients,22–24 27 28 reported a relationship between CONUT scores and the clinicopathological characteristics of lymphoma. According to the combined analyses in figures 4 and 5 and online supplemental table S5, higher CONUT scores were remarkably associated with Ann Arbor stages III–IV (OR=3.75, 95% CI 2.96 to 4.75, p<0.001), Eastern Cooperative Oncology Group performance status of 2–4 (OR=5.14, 95% CI 3.97 to 6.65, p<0.001), high to intermediate/high National Comprehensive Cancer Network International Prognostic Index (OR=8.05, 95% CI 5.11 to 12.66, p<0.001), B symptoms (OR=4.97, 95% CI 2.89 to 8.52, p<0.001), extranodal disease (OR=3.25, 95% CI 2.24 to 4.70, p<0.001), bone marrow involvement (OR=4.86, 95% CI 3.25 to 7.27, p<0.001) and elevated lactate dehydrogenase levels (OR=3.21, 95% CI 2.37 to 4.34, p<0.001). Nonetheless, CONUT scores were not significantly associated with gender (OR=1.04, 95% CI 0.71 to 1.53, p=0.823) in patients with lymphoma (figure 4, online supplemental table S5).
Figure 4.
Forest plots describing the correlation between CONUT and clinicopathological factors in lymphoma: (A) gender (male vs female); (B) Ann Arbor stage (III–IV vs I–II); (C) ECOG PS (2–4 vs 0–1); and (D) NCCN IPI (high-intermediate/high vs low/low-intermediate). CONUT, Controlling Nutritional Status; ECOG PS, Eastern Cooperative Oncology Group performance status; NCCN IPI, National Comprehensive Cancer Network International Prognostic Index.
Figure 5.
Forest plots describing the correlation between CONUT and clinicopathological factors in lymphoma: (A) presence of B symptoms (yes vs no); (B) extranodal disease (yes vs no); (C) bone marrow involvement (yes vs no); and (D) lactate dehydrogenase level (elevated vs normal). CONUT, Controlling Nutritional Status.
bmjopen-2023-078320supp007.pdf (19KB, pdf)
Publication bias
We created funnel plots with Begg’s test to examine possible publication bias for OS and PFS (online supplemental figure S1). Ultimately, the Begg’s test revealed an absence of significant publication bias for OS (p=0.230) and PFS (p=0.296).
Discussion
The effect of CONUT scores on the prognosis of lymphoma cases remains to be elucidated, according to prior investigations. This meta-analysis retrieved the most recent literature and collected data from seven eligible studies.22–28 This meta-analysis showed that elevated CONUT scores significantly predict dismal OS and inferior PFS in patients with lymphoma. Moreover, a high CONUT score can also be considered a risk factor for aggressive tumour biology in lymphoma. To our knowledge, this meta-analysis is the first to explore whether CONUT could be used when predicting the prognosis of lymphoma cases and to assess its clinicopathological value.
CONUT scores can be calculated according to serum albumin content, total lymphocyte count and total cholesterol content. A high CONUT score can be caused by decreased albumin levels, low lymphocyte counts and low cholesterol levels. Therefore, the mechanisms of the prognostic value of CONUT in lymphoma patient survival can be explained by the following aspects. First, albumin is at its highest abundance in plasma, which occupies around 50% of all proteins.31 Serum albumin level is frequently used to represent nutritional status and is closely associated with inflammation.32 Several human defence mechanisms are impaired by malnutrition, including anatomical barriers, humoral and cellular immunity, and phagocytic function. Serum albumin levels can be used to determine the prevalence of malnutrition.33 Hypoalbuminaemia can induce the activation of factors such as interleukin 1, interleukin 6 and tumour necrosis factor-α.34 35 A recent single-centre study showed that a low serum albumin level was a prognostic factor for an undesirable OS rate in patients with DLBCL.36 Another multicentre study also reported that consecutive hypoalbuminaemia was a simple and effective adverse prognostic factor in patients with DLBCL treated with the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) regimen.37 Second, lymphocytes are immune response cells which are essential for anticancer efficacy. Specifically, T lymphocytes recognise and kill tumour cells, thereby inhibiting proliferation and metastasis.38 Lymphocyte loss in the tumour microenvironment hinders the natural antitumour ability of the body, leading to immunity tolerance and escape by tumour cells.39 Furthermore, patients with lymphoma receiving chemotherapy could have low lymphocyte counts.35 Additionally, as an essential component of the cell membrane, cholesterol contributes plasma membrane dimension, organisation and fluidity, maintaining cellular function.40 Hypocholesterolaemia is significantly associated with lower levels of lymphocytes, total T cells and CD8+ cells, compared with hypercholesterolaemia.41 As serum cholesterol levels decrease, immune cells become unable to fight cancer cells due to the higher levels of cholesterol in cancer cell membranes.42 Hence, high CONUT scores are typically associated with tumour invasion and metastasis, along with dismal survival outcomes.
Recently, numerous meta-analyses have been conducted to explore whether CONUT scores can be used to predict the prognosis of various tumour types.43–45 Peng et al 43 showed that CONUT scores have a prognostic value for OS in patients with glioblastoma in their meta-analysis of 1406 cases. Xue et al 44 reported that a high CONUT score predicted dismal OS, cancer-specific survival (CSS) and recurrence-free survival (RFS) in upper tract urothelial carcinoma and renal cell carcinoma in their meta-analysis of 5410 cases. Another recent meta-analysis reported that higher CONUT scores had an unfavourable impact on OS, disease-free survival, CSS and PFS in lung cancer, relative to those with low CONUT scores.45 Ma et al 46 performed a meta-analysis enrolling 2294 patients and proved that higher CONUT scores remarkably predict the well-known dismal OS in pancreatic cancer. Additionally, as reported by Takagi et al, 47 higher CONUT scores predicted dismal OS, CSS and RFS in patients with colorectal cancer undergoing surgery. Therefore, the results of our meta-analysis were consistent with prior work on other cancers. We also analysed a meta-analysis that explored the prognostic role of CONUT scores in patients with haematological malignancies by Lu et al.48 Six studies were included in their meta-analysis and three investigated DLBCL. All three studies22–24 were included in the current meta-analysis, where we focused on lymphoma. Additionally, we analysed the association between CONUT scores and eight clinicopathological features in lymphoma which were not explored in the previous meta-analysis.48 Our meta-analysis included 2060 patients, which was more than that in the meta-analysis by Lu et al.48 Therefore, this study’s novelty, comprehensiveness and large sample size were strengths compared with the meta-analysis by Lu et al.48
Certain limitations should be noted in this meta-analysis. First, significant heterogeneity in the HRs for OS and PFS existed. For example, the HRs and 95% CIs from various countries were different from each other, which may be due to selection bias, sample size and study period. Therefore, we performed a subgroup analysis based on study country to explore its impact on the overall results. Although we used a random-effects model for this analysis, the source of heterogeneity could not be precisely traced. Second, our enrolled articles were of retrospective design, possibly introducing selection bias. Third, the threshold for CONUT scores were different between the included articles. Therefore, large-scale prospective studies should be conducted for further validation.
Conclusions
In summary, this meta-analysis provides concrete evidence that higher CONUT scores are significantly related to inferior OS and PFS in lymphoma.
bmjopen-2023-078320supp003.pdf (20.3KB, pdf)
Supplementary Material
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Contributors: LL and LS searched the database, judged the study eligibility and extracted the data. LL analysed the data and wrote the paper. LS designed the study and revised the paper. Both authors contributed to the article and approved the submitted version. LS is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The ethics committee and institutional review board waived a formal approval for the present work since it is based on published studies.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Roschewski M, Rossi D, Kurtz DM, et al. Circulating tumor DNA in lymphoma: principles and future directions. Blood Cancer Discov 2022;3:5–15. 10.1158/2643-3230.BCD-21-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao F, Hu J, Zhang J, et al. Prognostic value of peripheral blood lymphocyte/monocyte ratio in lymphoma. J Cancer 2021;12:3407–17. 10.7150/jca.50552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Etrych T, Braunova A, Zogala D, et al. Targeted drug delivery and theranostic strategies in malignant lymphomas. Cancers (Basel) 2022;14:626. 10.3390/cancers14030626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. He MY, Kridel R. Treatment resistance in diffuse large B-cell lymphoma. Leukemia 2021;35:2151–65. 10.1038/s41375-021-01285-3 [DOI] [PubMed] [Google Scholar]
- 6. Yang R, Chang Q, Meng X, et al. Prognostic value of systemic immune-inflammation index in cancer: a meta-analysis. J Cancer 2018;9:3295–302. 10.7150/jca.25691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liang R-F, Li J-H, Li M, et al. The Prognostic role of controlling nutritional status scores in patients with solid tumors. Clin Chim Acta 2017;474:155–8. 10.1016/j.cca.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 8. Li N, Tian G-W, Wang Y, et al. Prognostic role of the pretreatment C-reactive protein/albumin ratio in solid cancers: a meta-analysis. Sci Rep 2017;7:41298. 10.1038/srep41298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun KY, Chen SL, Xu JB, et al. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol 2014;140:1537–49. 10.1007/s00432-014-1714-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao Y, Zhang Z, Li Y, et al. Pretreatment neutrophil-to-lymphocyte ratio as a prognostic biomarker in unresectable or metastatic esophageal cancer patients with anti-PD-1 therapy. Front Oncol 2022;12:834564. 10.3389/fonc.2022.834564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu Y, Chen X, Song Y, et al. The platelet to lymphocyte ratio is a potential inflammatory marker predicting the effects of adjuvant chemotherapy in patients with stage II colorectal cancer. BMC Cancer 2021;21:792. 10.1186/s12885-021-08521-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bao Y, Yang J, Duan Y, et al. The C-reactive protein to albumin ratio is an excellent prognostic predictor for gallbladder cancer. Biosci Trends 2021;14:428–35. 10.5582/bst.2020.03326 [DOI] [PubMed] [Google Scholar]
- 13. Zhang Y, Kong FF, Zhu ZQ, et al. Controlling nutritional status (CONUT) score is a Prognostic marker in III-IV NSCLC patients receiving first-line chemotherapy. BMC Cancer 2023;23. 10.1186/s12885-023-10682-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakamoto T, Makinoya M, Sunaguchi T, et al. Geriatric nutritional risk index as a prognostic factor in patients with recurrent pancreatic cancer. PLoS ONE 2022;17:e0271073. 10.1371/journal.pone.0271073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ignacio de Ulíbarri J, González-Madroño A, de Villar NGP, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp 2005;20:38–45. [PubMed] [Google Scholar]
- 16. Zhu M, Chen L, Kong X, et al. Controlling nutritional status (CONUT) as a novel postoperative prognostic marker in breast cancer patients: a retrospective study. Biomed Res Int 2022;2022:3254581. 10.1155/2022/3254581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tamai Y, Iwasa M, Eguchi A, et al. The prognostic role of controlling nutritional status and skeletal muscle mass in patients with hepatocellular carcinoma after curative treatment. Eur J Gastroenterol Hepatol 2022;34:1269–76. 10.1097/MEG.0000000000002459 [DOI] [PubMed] [Google Scholar]
- 18. Une M, Ito M, Suzuki H, et al. Controlling nutritional status (CONUT) score and Sarcopenia as mutually independent Prognostic biomarkers in advanced urothelial carcinoma. Cancers (Basel) 2022;14:20.:5075. 10.3390/cancers14205075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H, Shin DM, Lee JH, et al. Combining Prognostic nutritional index (PNI) and controlling nutritional status (CONUT) score as a valuable Prognostic factor for overall survival in patients with stage I-III colorectal cancer. Front Oncol 2023;13:1026824. 10.3389/fonc.2023.1026824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin Q, Li C, Lin X, et al. Prognostic value of controlling nutritional status score in advanced Hypopharyngeal cancer. Laryngoscope 2023;133:2613–20. 10.1002/lary.30568 [DOI] [PubMed] [Google Scholar]
- 21. Arslan B, Ekinci S, Avci MA, et al. Controlling nutritional status score in predicting International society of Urological pathology score upgrading and biochemical recurrence after radical Prostatectomy. Asia Pac J Clin Oncol April 7, 2023. 10.1111/ajco.13951 [DOI] [PubMed] [Google Scholar]
- 22. Matsukawa T, Suto K, Kanaya M, et al. Validation and comparison of prognostic values of GNRI, PNI, and CONUT in newly diagnosed diffuse large B cell lymphoma. Ann Hematol 2020;99:2859–68. 10.1007/s00277-020-04262-5 [DOI] [PubMed] [Google Scholar]
- 23. Nagata A, Kanemasa Y, Sasaki Y, et al. Clinical impact of controlling nutritional status score on the prognosis of patients with diffuse large B-cell lymphoma. Hematol Oncol 2020;38:309–17. 10.1002/hon.2732 [DOI] [PubMed] [Google Scholar]
- 24. Akgün Çağlıyan G, Hacıoğlu S, Ünver Koluman B, et al. Is CONUT score a prognostic index in patients with diffuse large cell lymphoma Turk J Med Sci 2021;51:2112–9. 10.3906/sag-2101-406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baysal M, Bas V, Demirci U, et al. The utility of CONUT score in diffuse large B cell lymphoma patients. Niger J Clin Pract 2021;24:1194–9. 10.4103/njcp.njcp_429_20 [DOI] [PubMed] [Google Scholar]
- 26. Liu T, Hu R, Lv J, et al. Prognostic value of nutritional status in patients with human immunodeficiency virus infection-related lymphoma. Front Nutr 2022;9:1050139. 10.3389/fnut.2022.1050139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nakamura N, Kanemura N, Lee S, et al. Prognostic impact of the controlling nutritional status score in patients with peripheral T-cell lymphoma. Leuk Lymphoma 2022;63:1323–30. 10.1080/10428194.2021.2020777 [DOI] [PubMed] [Google Scholar]
- 28. Wu W, Ren K, Chen X, et al. A controlling nutritional status score is an independent predictor for patients with newly diagnosed nasal-type Extranodal NK/T-cell lymphoma based on Asparaginase-containing regimens. Cancer Med 2023;12:9439–48. 10.1002/cam4.5706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 30. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 31. Caraceni P, Tufoni M, Bonavita ME. Clinical use of albumin. Blood Transfus 2013;11:s18–25. 10.2450/2013.005s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Don BR, Kaysen G. Kaysen G: serum albumin: relationship to inflammation and nutrition. Semin Dial 2004;17:432–7. 10.1111/j.0894-0959.2004.17603.x [DOI] [PubMed] [Google Scholar]
- 33. Laky B, Janda M, Cleghorn G, et al. Obermair A: comparison of different nutritional assessments and body-composition measurements in detecting malnutrition among gynecologic cancer patients. Am J Clin Nutr 2008;87:1678–85. 10.1093/ajcn/87.6.1678 [DOI] [PubMed] [Google Scholar]
- 34. Chojkier M. Inhibition of albumin synthesis in chronic diseases: molecular mechanisms. J Clin Gastroenterol 2005;39(4 Suppl 2):S143–6. 10.1097/01.mcg.0000155514.17715.39 [DOI] [PubMed] [Google Scholar]
- 35. Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a Multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460–8. 10.1016/S1470-2045(11)70069-9 [DOI] [PubMed] [Google Scholar]
- 36. Hu X, Feng X, Wang H, et al. Association between serum albumin levels and survival in elderly patients with diffuse large B-cell lymphoma: a single-center retrospective study. Transl Cancer Res 2023;12:1577–87. 10.21037/tcr-23-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wei X, Zheng J, Zhang Z, et al. Consecutive hypoalbuminemia predicts inferior outcome in patients with diffuse large B-cell lymphoma. Front Oncol 2020;10:610681. 10.3389/fonc.2020.610681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bastid J, Bonnefoy N, Eliaou JF, et al. Lymphocyte-derived interleukin-17A adds another brick in the wall of inflammation-induced breast carcinogenesis. Oncoimmunology 2014;3:e28273. 10.4161/onci.28273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gooden MJM, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93–103. 10.1038/bjc.2011.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harikumar KG, Potter RM, Patil A, et al. Membrane cholesterol affects stimulus-activity coupling in type 1, but not type 2, CCK receptors: use of cell lines with elevated cholesterol. Lipids 2013;48:231–44. 10.1007/s11745-012-3744-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hughes DA, Townsend PJ, Haslam PL. Enhancement of the antigen-presenting function of monocytes by cholesterol: possible relevance to inflammatory mechanisms in extrinsic allergic alveolitis and atherosclerosis. Clin Exp Immunol 1992;87:279–86. 10.1111/j.1365-2249.1992.tb02988.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chimento A, Casaburi I, Avena P, et al. Cholesterol and its metabolites in tumor growth: therapeutic potential of Statins in cancer treatment. Front Endocrinol (Lausanne) 2018;9:807. 10.3389/fendo.2018.00807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peng J, Li X, Huang M, et al. Prognostic value of prognostic nutritional index score and controlling nutritional status score in patients with glioblastoma: a comprehensive meta-analysis. Front Oncol 2023;13:1117764. 10.3389/fonc.2023.1117764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xue W, Hu X, Zhang Y. The Association of controlling nutritional status (CONUT) score with survival in patients with surgically treated renal cell carcinoma and upper tract urothelial carcinoma: a systematic review and meta-analysis. Nutr Cancer 2022;74:1907–16. 10.1080/01635581.2021.1974894 [DOI] [PubMed] [Google Scholar]
- 45. Zhang C, Li XK, Cong ZZ, et al. Controlling nutritional status is a prognostic factor for patients with lung cancer: a systematic review and meta-analysis. Ann Palliat Med 2021;10:3896–905. 10.21037/apm-20-2328 [DOI] [PubMed] [Google Scholar]
- 46. Ma X, Zou W, Sun Y. Prognostic value of pretreatment controlling nutritional status score for patients with pancreatic cancer: a meta-analysis. Front Oncol 2021;11:770894. 10.3389/fonc.2021.770894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Takagi K, Buettner S, Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients with colorectal cancer: a systematic review and meta-analysis. Int J Surg 2020;78:91–6. 10.1016/j.ijsu.2020.04.046 [DOI] [PubMed] [Google Scholar]
- 48. Lu C, Chen Q, Fei L, et al. Prognostic impact of the controlling nutritional status score in patients with hematologic malignancies: a systematic review and meta-analysis. Front Immunol 2022;13:952802. 10.3389/fimmu.2022.952802 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-078320supp001.pdf (346.5KB, pdf)
bmjopen-2023-078320supp004.pdf (66.8KB, pdf)
bmjopen-2023-078320supp002.pdf (28.3KB, pdf)
bmjopen-2023-078320supp005.pdf (37.4KB, pdf)
bmjopen-2023-078320supp006.pdf (28.1KB, pdf)
bmjopen-2023-078320supp007.pdf (19KB, pdf)
bmjopen-2023-078320supp003.pdf (20.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data that support the findings of this study are available from the corresponding author upon reasonable request.