Abstract
Background
Generally, early-stage breast cancer has a good prognosis. However, if it spreads systemically, especially with pulmonary involvement, prospects worsen dramatically. Importantly, tumor-infiltrating T cells contribute to tumor control, particularly intratumoral T cells with a tissue-resident memory phenotype are associated with an improved clinical outcome.
Methods
Here, we use an adenoviral vector vaccine encoding endogenous tumor-associated antigens adjuvanted with interleukin-1β to induce tumor-specific tissue-resident memory T cells (TRM) in the lung for the prevention and treatment of pulmonary metastases in the murine 4T1 breast cancer model.
Results
The mucosal delivery of the vaccine was highly efficient in establishing tumor-specific TRM in the lung. Concomitantly, a single mucosal vaccination reduced the growth of pulmonary metastases and improved the survival in a prophylactic treatment. Vaccine-induced TRM contributed to these protective effects. In a therapeutic setting, the vaccination induced a pronounced T cell infiltration into metastases but resulted in only a minor restriction of the disease progression. However, in combination with stereotactic radiotherapy, the vaccine increased the survival time and rate of tumor-bearing mice.
Conclusion
In summary, our study demonstrates that mucosal vaccination is a promising strategy to harness the power of antitumor TRM and its potential combination with state-of-the-art treatments.
Keywords: breast cancer, vaccine, adjuvant, radiotherapy/radioimmunotherapy, memory
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Pulmonary metastases in breast cancer remain difficult to treat, resulting in poor survival rates. Intratumoral T cell infiltration, and in particular the presence of tissue-resident memory T cells (TRM), correlates with better clinical outcomes, but to date, no suitable vaccine approaches have been developed to induce such immune responses against endogenous tumor antigens in metastatic breast cancer.
WHAT THIS STUDY ADDS
The mucosal vaccination with an adjuvanted, adenoviral vector vaccine that delivers the endogenous tumor antigens AH1 and melanoma-associated antigen B results in robust antigen-specific TRM responses in the lung.
In a pulmonary breast cancer metastasis model, a single, prophylactic vaccination improves the survival time and rate, which is partially mediated by vaccine-induced TRM.
Therapeutic vaccination promotes strong infiltration of TRM into metastases and leads, in combination with stereotactic radiotherapy, to an improved survival of tumor-bearing mice.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our data demonstrate that mucosal tumor vaccines encoding appropriate tumor antigens are a promising strategy to generate tissue-resident, tumor-reactive T cell responses in pulmonary breast cancer metastases and may pave the way for a new generation of TRM-inducing vaccination modalities that harness these powerful T cell subtypes, especially in combination with established treatment options.
Background
Approximately 2.3 million new cases of breast cancer and 684,000-related deaths were reported worldwide for 2020, accounting for 11.7% of all cancer diagnoses.1 Recent improvements in screening and treatment approaches could increase the 5-year breast cancer survival rate to 85 %.2 However, a metastatic spread of tumor cells cannot always be prevented, with the lung as one of the most commonly affected sites. Despite new treatment options such as immunotherapy and personalized cancer therapies, the 5-year survival rates for metastatic breast cancer remain low and are particularly low for lung metastases at 12%.3 Several phase I and II clinical trials have evaluated breast cancer vaccines based on different vaccine platforms and delivering different tumor-associated antigens with limited success.4
Breast cancer exploits various mechanisms to suppress T cell responses, such as impairment of antigen presentation, induction of T cell exhaustion, or suppression of T cell infiltration.5 6 However, high levels of tumor-infiltrating lymphocytes (TIL) are associated with an improved prognosis in most solid cancers, including breast cancer.7 Today, it is well established, that a subset of those TIL show characteristics of tissue-resident memory T cells (TRM). A hallmark of TRM is a strict residency in healthy tissues and tumors peripheral tissues without a presence in circulation, contributing critically to peripheral immune surveillance. The tissue residency partly relies on the surface expression of retention molecules like CD69 or CD103, which are widely used as phenotypic markers for TRM.8 To establish tissue residency, local antigen is required in some tissues to promote upregulation of CD69, which in turn inhibits sphingosine-1-phosphate receptor 1-mediated tissue egress.9 CD103 expression is induced by transforming growth factor-β (TGF-β) signaling and is connected to an increased cytotoxic potential if a ligand is engaged.10 Despite the expression of programmed cell death protein 1 (PD1), a hallmark of T cell exhaustion, intratumoral TRM show a robust production of effector cytokines, degranulation, and cytotoxicity, enabling efficient tumor cell killing, suppression of tumor cell division, and the activation of innate immune cells.10 11 Consistent with this, intratumoral TRM are protective in the murine AT3 mammary carcinoma model12 and TIL with TRM gene signatures were found to be associated with an improved prognosis in patients with triple-negative breast cancer (TNBC) .7 12 Therefore, the specific induction of tumor antigen-specific TRM in the lung by a mucosal cancer vaccine has great potential for the prevention and treatment of lung metastases.
In previous studies, we have shown that mucosal vaccinations with adenoviral vectors adjuvanted with vector-encoded interleukin-1β (AdIL1β) efficiently establish antiviral TRM responses in the lung.13 14 The mucosal expression of IL-1β activates multiple checkpoints in the establishment of pulmonary TRM including activation of endothelial cells, upregulation of adhesion molecules and chemokines in the lung mucosa, recruitment of innate immune cells including migratory CD103+ dendritic cells (DC), priming of committed TRM progenitors, and finally, local differentiation of TRM supported by high levels of TGF-β.13 This broad inflammatory action of mucosal IL-1β may not only support the establishment of TRM but may also prove beneficial in overcoming cancer-related immunosuppression in the lung environment.
In this study, an adenoviral vector vaccine encoding two antigens of key tumor-associated antigen (TAA) families was evaluated as mucosal immunization in a murine TNBC lung metastasis model. We demonstrated that this mucosal vaccination strategy elicits tumor antigen-specific TRM in the lung and provides prophylactic protection against 4T1 lung metastases. Moreover, combined with stereotactic radiotherapy (RT), the therapeutic vaccination led to a partial control of existing lung metastases.
Methods
Vaccines
Replication-deficient Ad serotype 5 vector vaccines were produced according to the pAdEasy protocol.15 In brief, gene sequences for the TAA melanoma-associated antigen B (MAGE-b) (position 311–660 bp of complementary DNA), the murine leukemia virus gp70 AH1 peptide (single amino acid substitution at position, SPSYAYHQF; AH1A5) and thioredoxin were cloned into pShuttle and later inserted into pAdEasy-1 via homologous recombination. Antigen expression is initiated from a cytomegalovirus (CMV) immediate-early-1-promoter and transcription is terminated at a bovine growth hormone (BGH) polyadenylation signal. Correct insertion of the expression cassette into adenoviral vectors was confirmed by sequencing. Viral particles were purified after transfection and multiple infection cycles of HEK293A cells (Vivapure Adenopack Kit, Sartorius) and total virus concentration was assessed by optical density at 260 nm. Infectious particles were determined by Reed-and-Muench TCID50. The AdIL-β adjuvant vector and the Adempty control vector with no transgene expression were generated according to previous work.13
Immunizations
After 2 weeks of acclimatization, 7–8 weeks old female BALB/cJRj (Janvier) were immunized intranasally (i.n.) under general anesthesia (100 mg/kg ketamine and 13 mg/kg xylazine) by pipetting 50 µL of the vaccine in one nostril (1×107 IU vaccine vector, 2.5×106 IU adjuvant vector).
Tumor challenge
4T1-luc cells (kindly provided by Maria Hollmen, University of Helsinki) were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco) with 10% heat-inactivated fetal bovine serum (FBS; Anprotect) with 2 mM L-Glutamine (Gibco) and 1% streptomycin/penicillin (Gibco) in a humidified incubator at 5% CO2 and 37°C. Cells were detached with Trypsin and 5×104 cells in 150 µL phosphate-buffered saline (PBS) were injected intravenously into the median tail vein. Cage order was randomized during tumor injection to prevent time-dependent trends. Animals were euthanized with isoflurane at specific time points or as soon as they reached predefined humane endpoints assessed by a scoring system considering body condition score, general clinical symptoms and behavior as well as breathing activity.
If stated, FTY720 (Sigma-Aldrich) was added to the drinking water at a concentration of 2 µg/mL 3 days before the tumor challenge.
T cell analyses
To discriminate circulating and resident T cells, mice were injected intravenously with 2 µg anti-CD45-BV510 (clone 30-F11, BioLegend) 3 min before being euthanized with isoflurane followed by harvesting lungs and spleens. Lungs were cut and incubated in 2 mL R10 medium (Roswell Park Memorial Institute medium, RPMI, 1640, 10% fetal calf serum, FCS, 2 mM L-Glutamine, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, HEPES, 50 µM β-mercaptoethanol and 1% streptomycin/penicillin) supplemented with collagenase D (500 units) and DNase I (160 units) for 45 min at 37°C. Organs were mashed through a 70 µm cell strainer (Greiner Bio-One), washed with HBSS (Gibco) and erythrocytes were removed with an ammonium-chloride-potassium lysis (Gibco). For the following downstream methods, a fixed volume of the lung cell suspension (10–20% of total isolated lymphocytes; depending on the experiment) or 106 spleen cells were analyzed.
For the intracellular cytokine staining, cells were restimulated in 200 µL R10 medium supplemented with monensin (2 µM), anti-CD28 (1 µg/mL; eBioscience), anti-CD107a-FITC (1:200, clone 1D4B; BD) with or without AH1A5 peptide (SPSYAYHQF; GenScript) for 6 hours. Next, a surface staining with anti-CD8a-Pacific Blue (1:300, clone 53–6.7; BioLegend), anti-CD4-PerCP eFlour 710 (1:2000, clone RM4-5; eBioscience) and Fixable Viability Dye eFlour 780 (1:100; Thermo Fisher) in FACS-PBS (0.5% BSA, 2 mM EDTA, 1 mM NaN3, in PBS) for 20 min at 4°C was performed. Cells were fixed in 2% paraformaldehyde and permeabilized in FACS-PBS containing 1% saponin and anti-CD16/32 (1:200; Invitrogen). Cells were then stained intracellularly with anti-IL2-APC (clone JES6-5H4), anti-TNFα-PE-Cy7 (clone MPG-XT22) and anti-IFNγ-PE (clone XMG1.2; all 1:300, all BioLegend).
For phenotypic analyses, cells were stained with anti-CD8-PE (1:300, clone YTS169.4; Invitrogen), anti-CD127-FITC (1:500, clone A7R34; BioLegend), anti-CD69-PerCP-Cy5.5 (1:300, clone H1.2F3, BioLegend), anti-CD103-BV605 (1:100, clone 2E7; BioLegend), anti-KLRG1-PE-Cy7 (1:300, 2F1; Invitrogen), anti-CD44-BV711 (1:300, clone IM7; BioLegend), anti-CD45.2-PE-Dazzle (1:500, clone 104; BioLegend), and Fixable Viability Dye eFluor 780 (1:4000, Thermo Fisher) for 20 min at 4°C in FACS-PBS.
For dextramer analyses, cells were first stained with an APC-labeled H-2 Ld AH1 Dextramer (Immudex; 1:10 in FACS-PBS) for 20 min at 4°C followed by staining with anti-CD8-BV711 (1:300, clone 53–6.7; BioLegend), anti-CD4-BV605 (1:1000, clone RM4-5; BioLegend), anti-CD127-PE-Dazzle594 (1:300, clone A7R34; BioLegend), anti-CD69-BV421 (1:100, clone H1.2F3; BioLegend), anti-CD103-PE (1:100, clone 2E7; BioLegend), anti-KLRG1-PE-Cy7 (1:300, clone 2F1; Invitrogen), anti-CD44-PE-Cy5 (1:2000, clone IM7; BioLegend), and Fixable Viability Dye eFluor 780 (1:4000, Thermo Fisher) for 20 min at 4°C in FACS-PBS supplemented with brilliant stain buffer plus (BD).
Samples were acquired on an AttuneNXT (Thermo Fisher) or a Northern Lights flow cytometer (Cytek Biosciences) and analyzed using FlowJo (Tree Star).
Bioimaging
Ten min after receiving D-luciferin (150 µg/g intraperitoneally, Goldbio), isoflurane-anesthetized mice or resected lungs were imaged in an IVIS imaging system (PerkinElmer) and data was analyzed using the Living Image V.4.0 Software (PerkinElmer).
Analysis of tumor cells in lung cell suspensions
For the ex vivo luciferase assay, cells pelleted from 100 µL lung cell suspension were lysed in 80 µL Bright Glo Lysis Buffer (Promega) for 10 min at RT in a white 96-well plate (costar). Next, 20 µL Bright Glo substrate (Promega) was added 2 min before the chemiluminescence signal was captured on a microplate reader (VICTOR X5; PerkinElmer) and analyzed using 2030 Manager software (PerkinElmer).
In the clonogenic outgrow assay, lung cell suspensions were serially diluted in DMEM (Gibco) supplemented with 10% heat-inactivated FBS (Anprotect) with 2 mM L-Glutamine and 1% streptomycin/penicillin and 6-thioguanine (60 µM). One mL of each dilution was plated in a 6-well plate and incubated for 10 days at 37°C, 5% CO2, before colonies were assessed by crystal violet staining (0.1% crystal violet, 2.5% ethanol, 25% methanol in H2O). A mean colony number was determined by normalizing each countable dilution.
Immunohistochemistry
Mice were sacrificed by isoflurane inhalation and lungs were filled with 1 mL 20% sucrose in PBS and O.C.T. compound (1:2 mixture) before the trachea was closed with a thread (Sakura Finetek). Lungs and trachea were resected and incubated in 20% sucrose/PBS overnight at 4°C. Lungs were washed in sodium chloride and snap frozen in liquid nitrogen before 15 µm tissue sections were prepared using a cryostat. Sections were fixed in 1:1 acetone/methanol (5 min, 4°C), and tissues were surrounded with a PAP pen and rehydrated in PBS. Sections were incubated 2 hours at RT in PBS-T (0.05% Tween) supplemented with 5% FBS and anti-CD16/CD32 (10 µg/mL, clone 93, Invitrogen) before staining with anti-CD8α-AF488 (5 µg/mL, clone 53–6.7; BD) overnight at 4°C. Sections were mounted with ProLong Glass antifade including NucBlue (Hoechst 33342; Thermo Fisher Scientific). Images were captured with a 40× oil objective (PL APO, NA1.75) at an SP5X Leica laser scanning confocal microscope (Leica Camera AG) using LAS AF software V.2.7.3.9723 (Leica Camera AG) and analyzed using ImageJ software V.1.52p (Wayne Rasband). Additionally, tissue sections were stained with H&E for further analysis of tumor nodules. Stained tissue sections were scanned using a P1000 digital slide scanner (P1000, Gen.2. 3DHistech) and examined using QuPath16 V.0.4.2 and ImageJ V.1.52p (Wayne Rasband). Analysis of histopathological changes and tumor nodule examination were conducted by a pathologist.
Radiotherapy
Isoflurane-anesthetized mice were placed in a container manufactured for our study that provides an appropriate resting position for the irradiation process. The thorax was irradiated with a single dose of 5 Gray (Gy) in a Versa HD linear accelerator unit (6 MV, Elekta).
Study design and statistical analyses
Animals were randomly assigned to groups. Group sizes were decided with power analyses based on historical data or in pilot studies with a fixed group size of six animals. Each animal represents an experimental unit. Data was analyzed using Prism V.9.0 (GraphPad Software). Statistical analyses in graphs depict only statistically significant results.
Results
Mucosal but not systemic vaccination elicits localized T cell responses in the lung
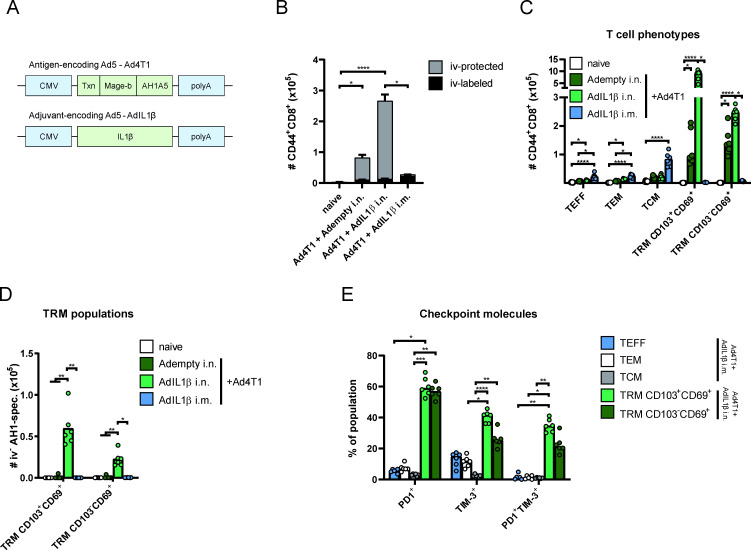
We generated a replication-deficient adenoviral vector vaccine (human adenovirus serotype 5) encoding a fragment of MAGE-b and the AH1 epitope of the endogenous murine leukemia virus gp70 protein, both highly expressed TAA in 4T1 tumors.17 18 In addition, the sequence of thioredoxin was fused to the N-terminus of the antigen sequence acting as an immunostimulatory scaffold protein (figure 1A).19 Mice were immunized either mucosally or intramuscularly (i.m.) with the vaccine vector (hereinafter referred to as Ad4T1) in combination with AdIL1β as a genetic adjuvant. To estimate the contributing effect of mucosal AdIL1β,13 a mucosal vaccination with Ad4T1 together with an empty adenoviral vector (Adempty) was applied. Here, the mice received an Adempty dose equivalent to the AdIL1β input. Five weeks after vaccination, CD8+ T cell responses were determined in lung and spleen cells by phenotypic analyses and multimer staining. Before harvesting the lung, we performed an intravascular staining with fluorophore-coupled anti-CD45 to discriminate TRM (protected from staining, iv-protected or iv−) from circulating T cells in following analyses (stained, iv-labeled or iv+; gating strategy shown in online supplemental figure 1A). Here it became evident that i.n. vaccination predominantly induced tissue-resident T cells, whereas i.m. vaccination did not yield any localized responses (figure 1B). Phenotypic analyses revealed a robust induction of CD103+CD69+ and CD103–CD69+ lung TRM in the Ad4T1+AdIL1β i.n. group (figure 1C), which was also confirmed for AH1-specific CD8+ T cells by dextramer staining (figure 1D, gating strategy shown in online supplemental figure 1B). AH1-specific TRM responses were low to undetectable without a co-delivery of AdIL1β (figure 1D). After i.m. vaccination, no TRM were observed but a low number of iv+ circulating memory phenotypes (effector, TEFF; effector memory, TEM; and central memory T cells, TCM) were sampled in the lung, probably passaging the lung vasculature at the sampling time point (figure 1C). Both CD103+CD69+ and CD103-CD69+ TRM populations displayed an expression of the checkpoint molecules PD1 and T cell immunoglobulin and mucin-domain containing-3 (TIM-3; figure 1E), which were described to be frequently expressed in TIL with a TRM profile,20 although this does not necessarily indicate functional exhaustion.10 In contrast, circulating T cell phenotypes after systemic immunization showed a substantially lower fraction of PD1-positive or TIM-3-positive cells.
Figure 1.
TAA-specific memory T cell subsets in the lung. BALB/c mice were vaccinated either intranasally (i.n.) or intramuscularly (i.m.) with Ad4T1 (107 IU) + AdIL1β or Adempty (2.5×106 IU) as adjuvant component (A) and sacrificed 35 days later for T cell analysis. CD44 was used as a marker for antigen-experienced T cells with the addition of an intravascular CD45 staining to discriminate circulating (iv+) and tissue-resident (iv–) memory cells. The bars represent the mean of each group±SEM (B). The phenotypic analysis of effector T cells (TEFF; CD127−KLRG1+), effector memory T cells (TEM; CD127+KLRG1+), central memory T cells (TCM; CD127+KLRG1−CD69−CD103−) and TRM cells (CD127+/−KLRG1−CD103+CD69+; C) with AH1-specific TRM within the iv− population (D). PD1 and TIM-3 expression within different T cell phenotypes. TEFF, TEM and TCM analyzed in Ad4T1+AdIL1β i.m. and TRM populations analyzed in Ad4T1+AdIL1β i.n. group (E). The gating strategies are shown in online supplemental figure 1. Bars display group means overlaid with individual data points (C–E); all groups n=6 (total 24 animals). Significances were determined using Kruskal-Wallis tests followed by Dunn‘s multiple comparisons test (B–E). P values indicate significant differences (*p<0.05; **p<0.005; ***p<0.0005; ****p<0.0001; only statistically significant comparisons shown). CMV, cytomegalovirus promotor; i.m., intramuscular; i.n., intranasal; iv, intravenous, PD1, programmed cell death protein 1; T cell immunoglobulin and mucin-domain containing-3, TIM-3; TRM, tissue-resident memory T cells.
jitc-2023-008652supp001.pdf (1.9MB, pdf)
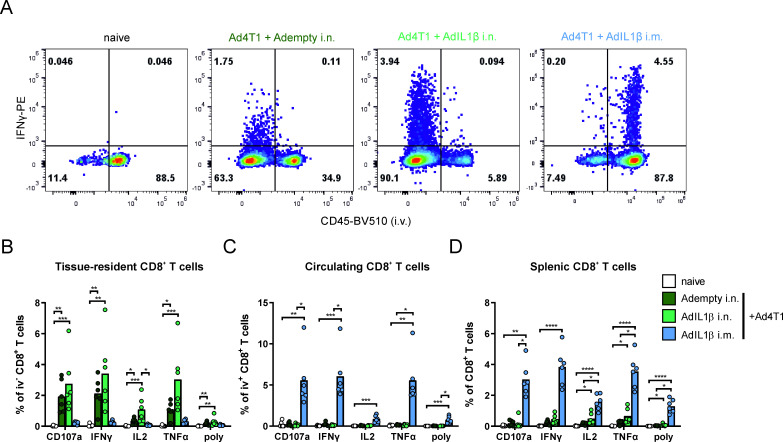
Next, we assessed the functionality of vaccine-induced T cells by ex vivo restimulation with the AH1A5 peptide followed by intracellular cytokine staining (gating strategy shown in online supplemental figure 2). Only the mucosal vaccine delivery induced significant levels of TAA-reactive iv− CD8+ T cells in the lung, as indicated by production of interferon (IFN)γ, IL2, and tumor necrosis factor α (TNFα) as well as degranulation indicated by CD107a staining (figure 2A,B). Opposed to this, systemic vaccination resulted in high levels of AH1-specific circulating T cells as shown in the iv+ T cell population sampled in the lung tissue (figure 2A,C) as well as in splenic CD8+ T cells (figure 2D), which was not seen to this extent after mucosal vaccination. After restimulation with a peptide pool covering a part of MAGE-b, we could not detect an antigen-specific T cell activation (data not shown). In conclusion, the route of vaccine delivery determines the localization of AH1-specific T cell responses, which are increased on co-administration of AdIL1β.
Figure 2.
Tumor antigen-specific CD8+ T cell responses. BALB/c mice were vaccinated either i.n. or i.m. as described in figure 1 and sacrificed 35 days later for T cell analysis. Representative flow cytometry plots for IFNγ production in iv+ and iv− CD8+ T cells of lung homogenates restimulated with an AH1 peptide (A). Responding CD8+ T cells were determined by an intracellular cytokine staining in spleen and lung homogenates (B–D). The gating strategy is shown in online supplemental figure 2. Bars show group means overlaid with individual data points; all groups n=6 (total 24 animals). Significances were determined using Kruskal-Wallis tests followed by Dunn‘s multiple comparisons test (B–D). P values indicate significant differences (*p<0.05; **p<0.005; ***p<0.0005; ****p<0.0001; only statistically significant comparisons shown). poly, polyfunctional T cell population positive for all assessed markers. IFN, interferon; i.m., intramuscular; i.n., intranasal; iv, intravenous; TNFα, tumor necrosis factor α.
Prophylactic immunization inhibits the growth of pulmonary breast cancer metastases and prolongs survival
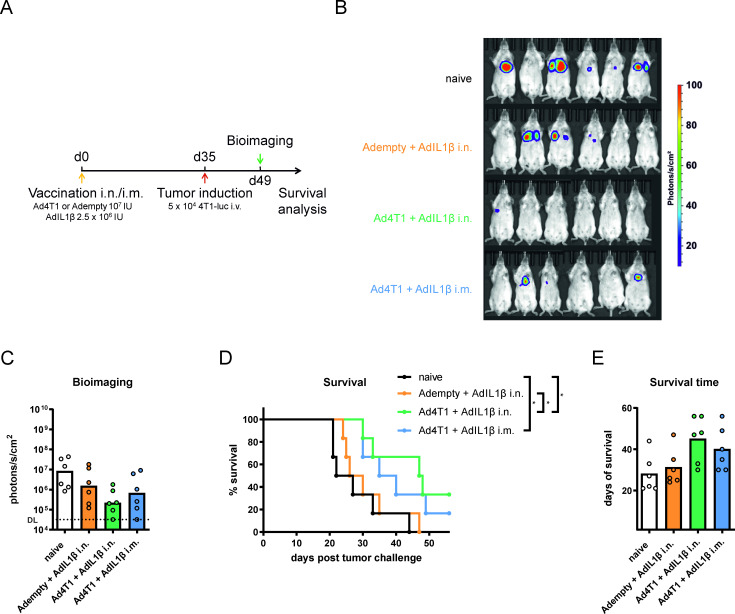
Next, we assessed the protective efficacy of the tumor vaccination. Based on the immunogenicity data, we immunized mice with Ad4T1+AdIL1β i.n. or i.m. In addition, we analyzed the delivery of AdIL1β without Ad4T1 to determine any effects of the adjuvant on protection that are not related to the delivery of the tumor vaccine. Five weeks later, mice were challenged with firefly luciferase-expressing 4T1 TNBC cells (4T1-luc) intravenously (figure 3A). 14 days after tumor induction, in vivo bioimaging showed by trend lower tumor burdens in the vaccinated groups compared with naïve mice (figure 3B,C). In line with these data, both i.n. and i.m. delivery of Ad4T1+AdIL1β resulted in a statistically significant improvement of the survival dynamics, while the mean survival time (47.5 and 37.5 days, respectively) was only by trend improved compared with the naïve group (24.5 days, figure 3D,E).
Figure 3.
Vaccine efficacy in a prophylactic setting. BALB/c mice were vaccinated with Ad4T1 (107 IU) + AdIL1β (2.5×106 IU) i.n. or i.m. or, alternatively, with Adempty (107 IU) + AdIL1β (2.5×106 IU) i.n. and challenged with 4T1-luc cells intravenously 35 days later. Survival was monitored for a period of 8 weeks (A). Bioimaging was performed on day 14 after tumor induction in an IVIS imaging system after the injection of mice with D-luciferin (B). Signal intensities are shown for individual animals in each group (C). The background signal is shown as a dashed line, indicating the detection limit (DL). Survival analysis over an 8-week period (D). The individual time until animals reach humane endpoints is shown together with group means. The experiment was terminated at day 56 and animals surviving to that time point were set to a survival of 56 days (E). Bars display group means overlaid with individual data points, all groups n=6 (total 24 animals). Significances were determined using Kruskal-Wallis tests followed by Dunn‘s multiple comparisons test (C, E). Significances in survival were determined using Gehan-Breslow-Wilcoxon-test (D). P values indicate significant differences (*p<0.05; only statistically significant comparisons shown). i.m., intramuscular; i.n., intranasal; IU, infectious units; iv, intravenous.
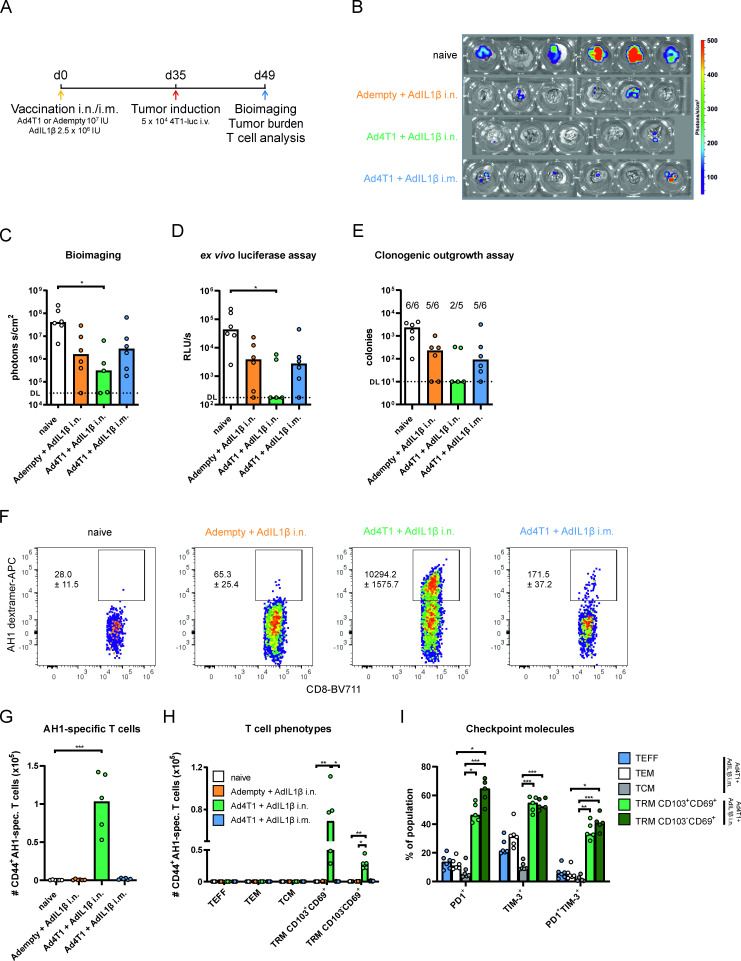
To confirm these results, pulmonary metastases were analyzed in-depth in a separate experiment. Whole lung bioimaging 14 days after metastasis induction showed that all treated groups, including the AdIL1β adjuvant control group, had lower median luciferase signals compared with unvaccinated mice (figure 4B,C). However, a statistically significant reduction was only detected in i.n. immunized mice that received Ad4T1 together with AdIL1β (figure 4C). Similar results were obtained by analyzing the luciferase activity in lung cell suspensions (figure 4D). To confirm these results in a second, luciferase-independent readout, the number of 4T1 cells in lung homogenates was quantified in a clonogenic outgrowth assay in presence of 6-thioguanine, a cytostatic drug the 4T1-luc cells are resistant to. All samples of unvaccinated animals displayed an outgrowth of 4T1 cells in this assay (figure 4E). In contrast, five out of six animals showed 4T1 cells in the lung in the i.m. vaccine and adjuvant-only groups, whereas the presence of 4T1 cells was only found in two out of five animals in the lung of Ad4T1+AdIL1β i.n. vaccinated animals.
Figure 4.
Analysis of the lung in the prophylactic vaccination setting. BALB/c mice were vaccinated i.n. or i.m. as described in figure 3 and challenged with 4T1-luc cells intravenously 35 days later. Lungs were harvested 14 days later for tumor burden and T cell analyses (A). Bioimaging of the resected lungs in the endpoint analysis of the experiment (n=5 for Ad4T1+AdIL1β, n=6 for other groups; total 23 animals; 1 animal lost during housing) in an IVIS imaging system after the injection of mice with D-luciferin (B). Intensities are shown for individual animals in each group (C). Ex vivo luciferase assay (D) and clonogenic assay (E) were performed with lung homogenates. The background signal is shown as a dashed line, indicating the detection limit (DL). AH1-specific cells are shown within the CD44+CD8+ population in exemplary dot plots with group mean values and SEM (F) and as individual counts per animal with group mean values (G). Effector (TEFF; CD127−KLRG1+), effector memory (TEM; CD127+KLRG1+) and central memory T cells (TCM; CD127+KLRG1−CD69−CD103−) as well as KLRG1−CD103+CD69+ TRM and KLRG1−CD103−CD69+ TRM within the CD44+CD8+AH1-spec. population (H). Gating strategies are shown in online supplemental figure 1 (no intravenous staining performed in this experiment). Programmed cell death protein 1 (PD1) and T cell immunoglobulin and mucin-domain containing-3 (TIM-3) expression within different T cell phenotypes. TEFF, TEM and TCM analyzed in Ad4T1+AdIL1β i.m. and TRM populations analyzed in Ad4T1+AdIL1β i.n. (I). Bars display group means overlaid with individual data points. Significances were determined using Kruskal-Wallis tests followed by Dunn‘s multiple comparisons test (C–E and G–I). P values indicate significant differences (*p<0.05; **p<0.005; ***p<0.0005; only statistically significant comparisons shown). i.m., intramuscular; i.n., intranasal; IU, infectious units; iv intravenous; spec., specific; TRM, tissue-resident memory T cells.
We further analyzed the quantity and the phenotype of T cells present in the lung at this time point. It became evident that the AH1-specific TRM response induced by i.n. vaccination with Ad4T1+AdIL1β was maintained throughout the tumor challenge without an obvious expansion or a major infiltration of circulating T cell phenotypes (figure 1D, figure 4F–H). Moreover, the presence of lung metastases did not influence the checkpoint expression status of TRM (figure 4I). The systemic immunization with the adjuvanted vaccine did not lead to a significant infiltration of T cells into the lung and neither did the tumor challenge itself lead to de novo induction of antigen-specific T cells (figure 4F–H). Therefore, a prophylactic mucosal vaccination supplemented with AdIL1β provides protection against pulmonary metastasis. This efficacy coincides with the presence of tumor-specific TRM responses in the lung, which are not seen after systemic immunization or by the presence of pulmonary lesions per se.
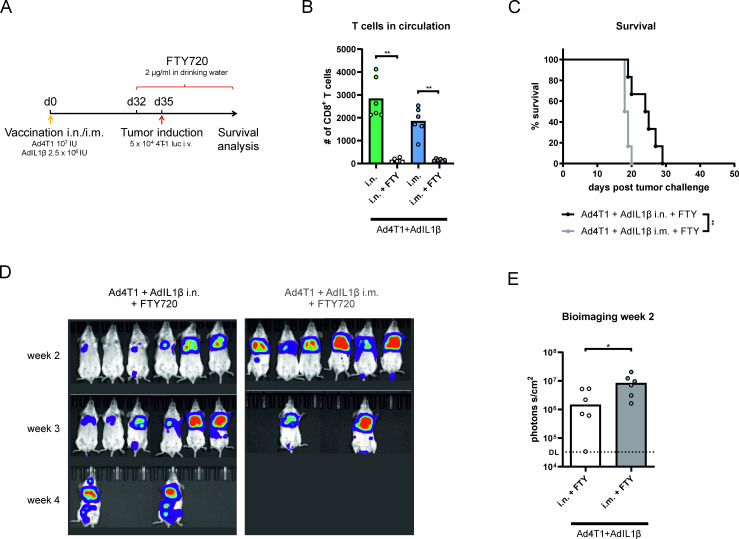
Vaccine-induced TRM contribute to vaccine efficacy
To assess the contribution of TRM to the protective capacity of the prophylactic vaccination, we used the compound FTY720 to sequester vaccine-induced T cells in lymphatic tissues and thereby inhibit a recruitment of circulating T cells into the lung on tumor challenge (figure 5A). A treatment with FTY720 beginning 3 days before tumor challenge resulted in an absence of CD8+ T cells in peripheral blood within 48 hours (figure 5B). In absence of T cell circulation, mucosally vaccinated animals still showed an improved survival (figure 5C) and a less pronounced metastatic growth in longitudinal bioimaging (figure 5D,E) compared with the systemically vaccinated group. Therefore, this experiment proves that vaccine-induced TRM contribute to the control of pulmonary 4T1 metastases. However, it should be noted that the survival rate of mucosally vaccinated animals was lower and the mean survival time shorter on FTY720 treatment compared with the previous experiment (figure 3D,E).
Figure 5.
Protective efficacy in the absence of circulating T cells. BALB/c mice were vaccinated i.n. or i.m. with Ad4T1 (107 IU) + AdIL1β (2.5×106 IU) and challenged with 4T1-luc cells intravenously 35 days later. The animals received FTY720-supplemented drinking water (2 µg/mL) 3 days before the tumor challenge to inhibit T cell circulation and the treatment was continued throughout the experiment (A). Number of CD8+ T cells in the blood of FTY720-treated animals compared with untreated animals was determined by flow cytometry analysis (B). Survival analysis over an 8-week period (C). Corresponding bioimaging (D) with quantification of luciferase signal at the 2-week time point (E). The background signal is shown as a dashed line. Statistical significances were determined using Mann-Whitney test (B, E) and Gehan-Breslow-Wilcoxon-test (C). All groups n=6 (total 12 animals). P values indicate significant differences (*p<0.05; **p<0.005; only statistically significant comparisons shown). i.m., intramuscular; i.n., intranasal; IU, infectious units; iv, intravenous.
Mucosal vaccination requires a combination with local radiotherapy to achieve therapeutic efficacy
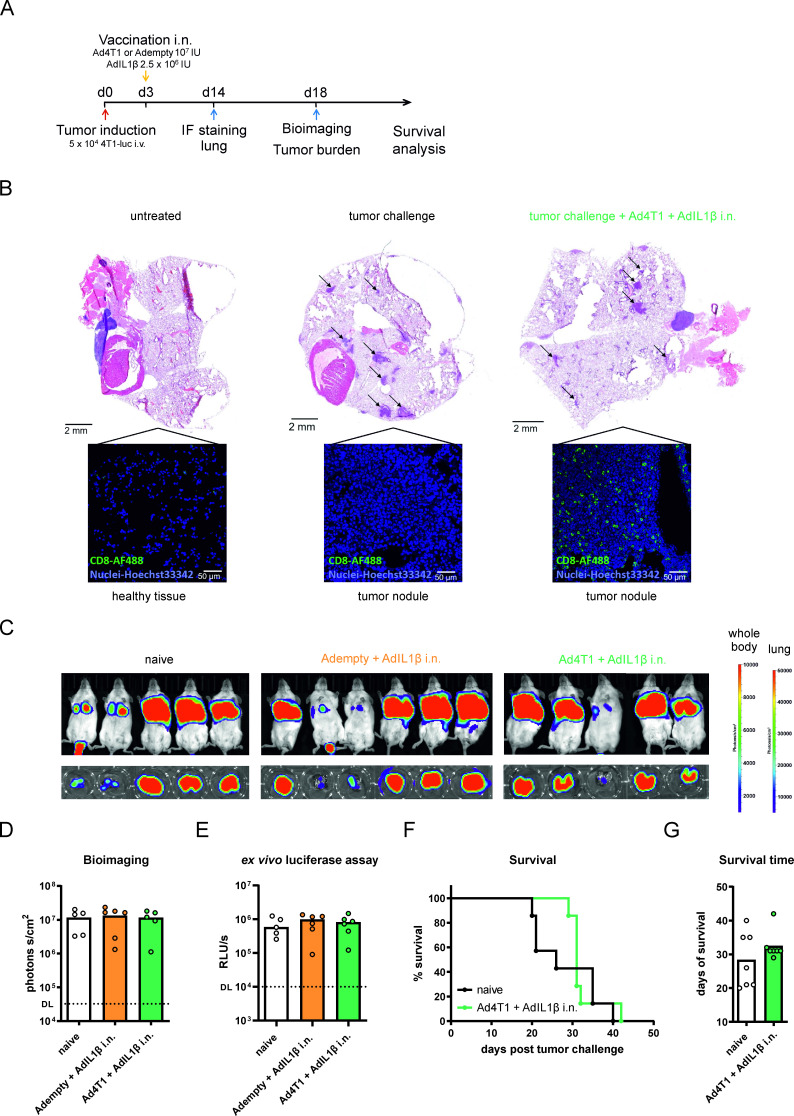
Next, we explored the vaccine efficacy of the mucosal vaccination strategy in a therapeutic setting. To this end, pulmonary 4T1-luc metastases were induced first, followed by the i.n. immunization with Ad4T1+AdIL1β 3 days later (figure 6A). In addition, the mucosal adjuvant AdIL1β was tested alone to analyze the direct effect of the cytokine on existing metastases. First of all, the ability of the mucosal vaccination to induce T cell infiltration into the metastases was analyzed 14 days after tumor challenge. Indeed, a pronounced presence of tumor-infiltrating CD8+ T cells was observed in tumor nodules in immunohistochemistry analyses of resected lungs from vaccinated but not naïve animals (figure 6B). Despite this pronounced localization of T cells within the tumor tissue, a therapeutic vaccination did not inhibit the growth of pulmonary metastases as seen by in vivo bioimaging 18 days after induction of metastases (figure 6C,D) and by measuring the luciferase signals in corresponding lung homogenates (figure 6E). Similarly, AdIL1β alone did not influence metastatic growth in the lung compared with naïve mice either. In a separate experiment, we analyzed the survival of vaccinated animals compared with naïve animals to see whether vaccination might result in a control of metastases at later time points. However, while a mucosal vaccination may have slightly increased the mean time to death, it did not protect animals from reaching humane endpoints (figure 6F,G). Thus, a therapeutic mucosal vaccination as a monotherapy was not successful in reducing tumor burden or in effectively improving survival, however, we did observe a pronounced T cell infiltration in the tumoral tissues.
Figure 6.
Vaccine efficacy after therapeutic vaccination. BALB/c mice were challenged with 4T1-luc cells intravenously and vaccinated i.n. with Ad4T1 (107 IU) + AdIL1β (2.5×106 IU) or Adempty (107 IU) + AdIL1β (2.5×106 IU) (A). Lungs of individual naive and i.n. immunized animals were resected 14 days later, embedded into O.C.T. and stained with anti-CD8 monoclonal antibody (green) for infiltrating T cells and Hoechst 33342 for nuclei (blue). Images were generated on a Leica SP5X confocal microscope (40×) with representative images shown. Tumor nodules are further displayed in H&E staining of respective lungs and are highlighted by indicating arrows (B). In vivo bioimaging on day 18 in an IVIS imaging system after the injection of mice with D-luciferin (C) with the corresponding quantification of the bioimaging (D) and an ex vivo luciferase assay was conducted to quantify the tumor burden (E). The background signal is shown as a dashed line. In a separate experiment, BALB/c mice were challenged with 4T1-luc cells intravenously and vaccinated i.n. with Ad4T1 (107 IU) + AdIL1β (2.5×106 IU) 3 days later. Survival analysis over an 8-week period (F). The individual time until animals reach humane endpoints is shown together with group means (G). Bars display group means overlaid with individual data points. Significances were determined using Kruskal-Wallis tests followed by Dunn‘s multiple comparisons (D, E) test or Mann-Whitney test (G). Significances in survival were determined using Gehan-Breslow-Wilcoxon test (F). No statistically significant differences were found. Groups n=6 (C, D and E, total of 18 animals, 1 naïve animal reached the humane endpoint on day 17; one Ad4T1+AdIL1β i.n. died shortly before bioimaging, data included for this animal in E but not D) and n=7 (F and G; total 14 animals). DL, detection limit; IL, interleukin; i.n., intranasal; IU, infectious units; i.v., intravenous; IF, immunofluorescence.
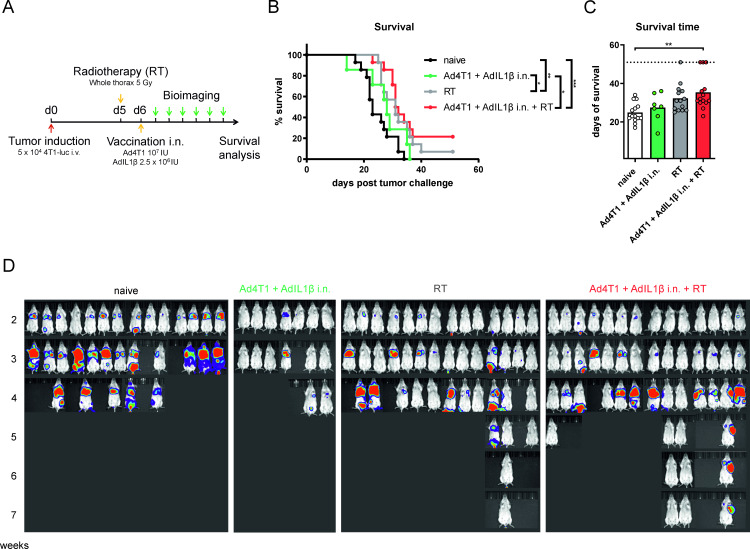
We speculated that the aggressive growth of the pulmonary metastases might outcompete the tumor-killing capacity of the vaccine-induced T cell response in a therapeutic setting. Therefore, stereotactic RT was employed to slow down tumor growth creating a longer therapeutic window for T cell-mediated tumor control. Tumor-bearing mice received a single, focused, stereotactic thorax irradiation of 5 Gy 5 days after metastasis induction, before the mucosal vaccination was administered to the respective groups the following day (figure 7A). Indeed, RT led to slower growth of metastases and a survival benefit in comparison to the untreated and vaccine-only groups (figure 7B–D). The combination of RT and mucosal immunization increased the survival rate (21% vs 7% in the RT-only group) up to 7 weeks after the tumor challenge, although the effect size was too small to reach statistical confidence. Only the combinatory treatment led to a statistically significant increase of the survival time (figure 7C). Altogether, these results show that combining RT with a mucosal vaccination might be a promising strategy against breast cancer lung metastases but further optimization of the treatment schedule is required.
Figure 7.
Vaccine efficacy after therapeutic vaccination in combination with RT. BALB/c mice were challenged with 4T1-luc cells intravenously and received whole thorax radiation with a single dose of 5 Gy on day 5. One day later, they were vaccinated i.n. with Ad4T1 (107 IU) + AdIL1β (2.5×106 IU) (A). Survival analysis over an 8-week period (B). The individual time until animals reach humane endpoints is shown together with group means. The experiment was terminated at day 56 and animals surviving to that time point were set to a survival of 56 days. Bars display group means overlaid with individual data points with significances determined using Kruskal-Wallis tests followed by Dunn‘s multiple comparisons test (C). Weekly in vivo bioimaging in an IVIS imaging system after the injection of mice with D-luciferin (D). Significances in survival were determined using Gehan-Breslow-Wilcoxon test (B). n=7 for Ad4T1+AdIL1β i.n., other groups n=14 (total 49 animals). P values indicate significant differences (*p<0.05; **p<0.005; ***p<0.0005). Gy, Gray; i.n., intranasal; IU, infectious units; i.v., intravenous; RT, radiotherapy.
Discussion
Fortunately, a diagnosis of breast cancer offers a comparatively good prognosis today. Unfortunately, this changes considerably when tumor cells spread to distant organs, particularly the lung.2 The intratumoral presence of TRM has been shown to mediate control of breast cancer in preclinical studies and to be associated with an improved prognosis in clinical studies.7 11 12 Therefore, vaccine approaches capable of specifically inducing localized immunity at cancer sites are promising interventions to prevent or treat pulmonary metastases. Here, we report on an efficient mucosal cancer vaccination and adjuvantation strategy that improves tumor control in different experimental settings.
In the first part of the present study, we show that a mucosal, but not a systemic, vaccine delivery leads to TAA-specific TRM in the lung. This is in line with the essential role of local antigen expression in the establishment of lung TRM.13 21 22 One possible reason for this may be the central role of migratory CD103+ DCs, which are involved in the priming of committed TRM precursors in draining lymph nodes23 and in promoting the final TRM differentiation in the lung through local antigen presentation.24 The inclusion of the genetic adjuvant AdIL1β significantly increased the induction of AH1-specific TRM compared with a non-adjuvanted mucosal vaccination. We have previously reported similar findings in the context of a mucosal influenza A vaccination strategy and described the activation of several essential checkpoints in the formation of lung TRM by the mucosal adjuvant including an enhanced recruitment of CD103+ DCs.13
Phenotypic analyses of the vaccine-induced response revealed a dominant CD103+CD69+ TRM phenotype that was enhanced by the adjuvant, whereas the CD103−CD69+ TRM response was generally lower. Mechanistically, it is tempting to speculate that the increased levels of TGF-β observed after the mucosal expression of IL-1β13 result from the increased presence of CD103+ DCs.24 Since TGF-β directly promotes an upregulation of CD103 on TRM, it may specifically favor a CD103+CD69+ TRM phenotype.24 25 Two recent fate mapping studies suggest a higher degree of plasticity or stemness in CD103− compared with CD103+ TRM, but were inconsistent regarding differential effector functionalities on bacterial or viral reinfection.26 27 However, several studies suggest a functional importance of CD103 expression in antitumor TRM. First, CD103 mediates localization and maintenance of TRM in tumors that are positive for its ligand E-cadherin.28 Notably, E-cadherin is expressed at high levels in 4T1 tumor cells and may even be important for the formation of lung metastases, despite the canonical view that E-cadherin is downregulated during epithelial-mesenchymal transition.29 Second, the interaction of CD103 with E-cadherin increases cytotoxicity and cytokine production by tightening the immunological synapse between TRM and tumor cells.30 Finally, the most relevant evidence arguing for a functional relevance of CD103 comes from clinical studies reporting a correlation between the intratumoral density of CD103+ TRM and an improved prognosis not observed with CD103− TIL.7 11 12
In the present study, a single, adjuvanted mucosal vaccination resulted in a significant reduction of pulmonary breast cancer metastases and improved survival when administered prophylactically. Moreover, mucosally vaccinated animals treated with FTY720 showed a reduced tumor burden and an improved survival compared to i.m. vaccinated, FTY720-treated littermates, indicating that vaccine-induced TRM contribute to the tumor control. Nevertheless, FTY720 treatment also decreased the protective efficacy of the mucosal vaccination suggesting that lung TRM inhibit metastatic growth, but are not sufficient to control the growth in the long-term. Considering a contribution of systemic T cells in the final clearance of lung metastases, a combination of mucosal and systemic vaccination may be a promising approach to exploit both T cell compartments and their characteristics in tumor vaccination. Such combinations should be evaluated in heterologous prime-boost schemes21 as well as in simultaneous, combined vaccination regimens.31 In general, the FTY720 treatment resulted in apparently more aggressive tumor growth, possibly due to immune impairment at mucosal surfaces32 or alteration of the pulmonary endothelial barrier function.33
Protective effects have also been reported with a mucosal nanoparticle vaccine against breast cancer metastasis34 and a mucosal protein vaccine against head and neck cancer in prophylactic settings.35 In these studies, tumor lysate and exogenous peptides were used as antigens. In the current study, we selected a MAGE family protein and an endogenous retroviral protein as clinically relevant endogenous TAA. MAGE proteins are of particular interest for tumor vaccination as they are expressed in human breast cancer biopsies but are otherwise silenced in most adult tissues.36 A MAGE protein has already been evaluated as an antigen in a phase I trial, resulting in antigen-specific T cells associated with a clinical response in some patients with melanoma.37 Endogenous retroviral proteins, such as those from human endogenous retroviruses (HERVs), are overexpressed in various cancers and patients with cancer exhibit HERV-directed immune responses.38 Therefore, they are considered promising targets for tumor vaccination. However, non-mutated TAA often lack immunogenicity when used for vaccination due to T cell tolerance against self-antigens.39 In the present study, 4T1 tumor-bearing mice did not mount endogenous AH1-specific T cell responses, underlining the T cell tolerance against AH140 and a potential immunosuppression by the TME. Vaccination and especially adjuvantation with AdIL1β could overcome these immunological barriers and efficiently induced AH1-specific responses. MAGE-b-specific responses were not observed on restimulation with MAGE-b peptides. However, earlier studies observed such responses in vaccinated BALB/c mice by ex vivo restimulation with MAGE-b-expressing cells as antigen source.18 Further studies are required to investigate the immunogenicity of this specific antigen in our context.
Mucosal vaccination did not significantly improve survival in the therapeutic setting, but we observed a strong T cell infiltration in histologic analyses. Such infiltration of “cold” 4T1 foci demonstrates the potential to shape the TME towards a “hot” tumor by selecting appropriate vaccines and adjuvants. CD8+ T cell infiltration is generally an important step towards tumor control as shown for Sipuleucel-T, the only approved cancer vaccine.41 In contrast to our study, Kandasamy et al were able to achieve partial control of pulmonary 4T1 metastases by therapeutic vaccination using a single-cycle influenza A virus as a vaccine vector.42 Importantly, human NY-ESO-1 (New York esophageal squamous cell carcinoma 1) was used as TAA artificially expressed by 4T1 cells and delivered by the vaccine. The use of such exogenous antigens circumvents the problem of pre-existing T cell tolerance probably resulting in stronger immune responses than observed after vaccination with endogenous TAA as used in our study. The majority of vaccine-induced TRM in our study expressed PD1 and TIM-3, both of which are associated with T cell exhaustion.43 However, these molecules are part of a specific TRM core profile20 that does not necessarily need to translate into inhibited effector functions, as has been demonstrated for TRM in infectious diseases22 and breast cancer.10 Nevertheless, the expression of checkpoint molecules in vaccine-induced TRM may render them susceptible to checkpoint ligand-mediated suppression in case of tumors positive for checkpoint ligands like PD1 ligand 1 (PD-L1). 4T1 tumor cells used in the current study only express low levels of PD-L1 and are resistant to checkpoint blockade treatment.44 It remains to be investigated whether immune checkpoint inhibitor treatment synergizes with our mucosal tumor vaccination strategy in other tumor models.
RT is an established therapeutic option for the treatment of many solid tumors due to its selective cytotoxic effects on rapidly dividing tumor cells. Besides this, RT has been proven to induce immune modulation.45 In addition to direct tumor killing, we believe that RT may have opened a longer therapeutic window for TRM development in our study. Typically, lung TRM require 7–14 days to fully differentiate13 and therefore may have developed too late after therapeutic vaccination in the highly aggressive 4T1 model without RT. However, RT has recently gained additional attention for its ability to modulate the immunosuppressive TME45 and has been shown to enhance the efficacy of therapeutic tumor vaccines by overcoming T cell tolerance.43 46 More specifically, E-cadherin expression has been shown to be upregulated by irradiation, potentially leading to a synergy of vaccine-induced CD103+ TRM with RT-induced E-cadherin in the TME.47 Moreover, RT-induced CXCL16 production by 4T1 cells might specifically attract CXCR6-expressing TRM cells.48 49 Future studies are needed to elaborate on the impact of these and other immunomodulatory effects of RT in the context of mucosal tumor vaccines and to optimize combinatory treatment schedules.
IL-1β is a pro-inflammatory cytokine with multifaceted effects. Some promote tumor growth by improving angiogenesis, endothelial activation, and recruitment of immunosuppressive immune cells,50 51 while others inhibit tumor growth by enhancing antitumor immune responses and preventing breast cancer metastasis by maintaining tumor cells in a mesenchymal state.52 These consequences of a chronic IL-1β-mediated inflammation may not be comparable to the use of this cytokine as a genetic adjuvant that induces short-term inflammation for only a few days.13 Neither prophylactic nor therapeutic administration of the adjuvant showed any tumorigenic activity in our study. Of note, although there are reports about adverse effects after mucosal administration of IL-1β in rodents,53 we did not observe long-term lung injury in a previous study using AdIL1β as a mucosal adjuvant.13 Several studies have evaluated the tolerability of systemically administered IL-1β in humans and reported dose-dependent toxicities including headache, fatigue, fever, chills and hypotension (reviewed in54). Subcutaneous administration is better tolerated than intravenous administration, suggesting that low and locally restricted levels of the cytokine are favorable to limiting the toxicity. Targeting the adjuvant to specific cell types has also been reported to improve tolerability in mice.55
We believe that mucosal vaccination strategies could be used in several clinical settings against breast cancer. First, mucosal vaccination could provide a novel intervention to combat detected lung metastases otherwise associated with a poor prognosis. Second, such vaccines could be used in semi-therapeutic, high-risk settings, where metastases have been detected in sentinel lymph nodes or other organs, but not in the lungs, to prevent the establishment and outgrowth of metastases. Third, mucosal immunity could also be induced preventively in low-risk patients with early-stage, non-invasive breast cancer. However, mucosal cancer vaccines that deliver appropriate TAA and adjuvants may also be used against pulmonary metastases from other primary tumor types or even against primary lung cancers.
In conclusion, we describe a mucosal vaccination approach that induces potent tumor antigen-specific TRM responses in the lung and that improves the control of pulmonary breast cancer metastasis in a prophylactic setting. A combinatory treatment with RT may be one option to increase vaccination efficacy in therapeutic situations. Future studies should optimize such vaccination approaches in terms of antigen selection, repeated vaccination schedules and optimized dose/fractionation of RT. Furthermore, the synergistic interplay of mucosal TRM with current first-line treatments is largely unexplored but has the potential to create a new generation of multimodal cancer therapies.
Acknowledgments
We thank the Preclinical Imaging Center Erlangen (PIPE) (University Hospital Erlangen), the Pathology and Comprehensive Cancer Center Erlangen-EMN (CCC) (University Hospital Erlangen, FAU Erlangen-Nuremberg) and the Preclinical Experimental Animal Centre (PETZ) (University Hospital Erlangen, FAU Erlangen-Nuremberg) for technical assistance. We further thank David Carnevale for graphical assistance and Maria Hollmen (Department of Medicine, Helsinki University Hospital, Finland) for generously providing the 4T1-cells.
Footnotes
Presented at: Data has been presented at the conferences ‘Recent insights into Immuno-Oncology’, Leuven, Belgium; ‘Joint Meeting DGFI and OGAI 2022’, Hannover, Germany and ‘Immunology2023’, Washington, DC, USA.56
Contributors: Conceptualization and design: FO, MT, UG and DL. Methodology: WB, CG, UG, BF, MR and CIG. Investigation: FO, AVA, PI, VV, LJ, AS, JTW, MR, A-SF, CIG, BF and DL. Visualization: FO and DL. Funding acquisition: MT and DL. Project administration: DL. Supervision: MT and DL. Writing: FO and DL. Guarantor: DL. Review and editing: all authors.
Funding: This project was supported by grants from the Interdisciplinary Center for Clinical Research, Medical Faculty, Friedrich-Alexander-University Erlangen-Nuremberg (ELAN P071 and Junior Project J100) and the Doktor Robert Pfleger-Stiftung. The work was further supported by the Deutsche Forschungsgemeinschaft (DFG) through the research training group RTG 2504 (project number: 401821119).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All mouse experiments were approved by the government of Lower Franconia and conducted according to the guidelines of the Federation of European Laboratory Animal Science Associations (FELASA) and the Society of Laboratory Animal Science (GV-SOLAS). Studies were carried out pursuant to the project licenses 55.2-2532-2-1262 and 55.2.2-2532-2-1538.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Weir HK, Carreira H, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2). The Lancet 2015;385:977–1010. 10.1016/S0140-6736(14)62038-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rabbani SA, Mazar AP. Evaluating distant metastases in breast cancer: from biology to outcomes. Cancer Metastasis Rev 2007;26:663–74. 10.1007/s10555-007-9085-8 [DOI] [PubMed] [Google Scholar]
- 4. Dafni U, Martín-Lluesma S, Balint K, et al. Efficacy of cancer vaccines in selected gynaecological breast and ovarian cancers: A 20-year systematic review and meta-analysis. Eur J Cancer 2021;142:63–82. 10.1016/j.ejca.2020.10.014 [DOI] [PubMed] [Google Scholar]
- 5. Luo N, Nixon MJ, Gonzalez-Ericsson PI, et al. DNA methyltransferase inhibition Upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat Commun 2018;9:1–11. 10.1038/s41467-017-02630-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie F, Zhou X, Su P, et al. Breast cancer cell-derived extracellular Vesicles promote Cd8+ T cell exhaustion via TGF-Β type II receptor signaling. Nat Commun 2022;13. 10.1038/s41467-022-31250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Savas P, Virassamy B, Ye C, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 2018;24:986–93. 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- 8. Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity 2014;41:886–97. 10.1016/j.immuni.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skon CN, Lee J-Y, Anderson KG, et al. Transcriptional downregulation of S1Pr1 is required for the establishment of resident memory Cd8+ T cells. Nat Immunol 2013;14:1285–93. 10.1038/ni.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Egelston CA, Avalos C, Tu TY, et al. Human breast tumor-infiltrating Cd8+ T cells retain Polyfunctionality despite PD-1 expression. Nat Commun 2018;9:4297. 10.1038/s41467-018-06653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganesan A-P, Clarke J, Wood O, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol 2017;18:940–50. 10.1038/ni.3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Virassamy B, Caramia F, Savas P, et al. Intratumoral Cd8+ T cells with a tissue-resident memory phenotype mediate local immunity and immune Checkpoint responses in breast cancer. Cancer Cell 2023;41:585–601. 10.1016/j.ccell.2023.01.004 [DOI] [PubMed] [Google Scholar]
- 13. Lapuente D, Storcksdieck Genannt Bonsmann M, Maaske A, et al. IL-1Β as Mucosal vaccine adjuvant: the specific induction of tissue-resident memory T cells improves the Heterosubtypic immunity against influenza A viruses. Mucosal Immunol 2018;11:1265–78. 10.1038/s41385-018-0017-4 [DOI] [PubMed] [Google Scholar]
- 14. Maier C, Fuchs J, Irrgang P, et al. Mucosal immunization with an adenoviral vector vaccine confers superior protection against RSV compared to natural immunity. Front Immunol 2022;13:920256. 10.3389/fimmu.2022.920256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He TC, Zhou S, da Costa LT, et al. A simplified system for generating recombinant Adenoviruses. Proc Natl Acad Sci U S A 1998;95:2509–14. 10.1073/pnas.95.5.2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bankhead P, Loughrey MB, Fernández JA, et al. Qupath: open source software for Digital pathology image analysis. Sci Rep 2017;7:16878. 10.1038/s41598-017-17204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deng W, Lira V, Hudson TE, et al. Recombinant Listeria promotes tumor rejection by Cd8+ T cell-dependent remodeling of the tumor Microenvironment. Proc Natl Acad Sci U S A 2018;115:8179–84. 10.1073/pnas.1801910115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim SH, Castro F, Gonzalez D, et al. Mage-B vaccine delivered by recombinant Listeria Monocytogenes is highly effective against breast cancer metastases. Br J Cancer 2008;99:741–9. 10.1038/sj.bjc.6604526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gödel P, Windmann S, Dietze KK, et al. Modification of one EPITOPE-flanking amino acid allows for the induction of friend Retrovirus-specific Cd8 + T cells by adenovirus-based immunization. J Virol 2012;86:12422–5. 10.1128/JVI.01607-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Djenidi F, Adam J, Goubar A, et al. Cd8+Cd103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a Prognostic factor for survival in lung cancer patients. J Immunol 2015;194:3475–86. 10.4049/jimmunol.1402711 [DOI] [PubMed] [Google Scholar]
- 21. Lapuente D, Fuchs J, Willar J, et al. Protective Mucosal immunity against SARS-Cov-2 after heterologous systemic prime-Mucosal boost immunization. Nat Commun 2021;12:6871. 10.1038/s41467-021-27063-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu T, Hu Y, Lee Y-T, et al. Lung-resident memory Cd8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 2014;95:215–24. 10.1189/jlb.0313180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iborra S, Martínez-López M, Khouili SC, et al. Optimal generation of tissue-resident but not circulating memory T cells during viral infection requires Crosspriming by DNGR-1+ Dendritic cells. Immunity 2016;45:847–60. 10.1016/j.immuni.2016.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wakim LM, Smith J, Caminschi I, et al. Antibody-targeted vaccination to lung Dendritic cells generates tissue-resident memory Cd8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 2015;8:1060–71. 10.1038/mi.2014.133 [DOI] [PubMed] [Google Scholar]
- 25. Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for Cd103+Cd8+ tissue-resident memory T cells of skin. Nat Immunol 2013;14:1294–301. 10.1038/ni.2744 [DOI] [PubMed] [Google Scholar]
- 26. Fung HY, Teryek M, Lemenze AD, et al. Cd103 fate mapping reveals that intestinal Cd103Tissue-resident memory T cells are the primary responders to secondary infection. Sci Immunol 2022;7:eabl9925. 10.1126/sciimmunol.abl9925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Hoesslin M, Kuhlmann M, de Almeida GP, et al. Secondary infections Rejuvenate the intestinal Cd103 + tissue-resident memory T cell pool . Sci Immunol 2022;7. 10.1126/sciimmunol.abp9553 [DOI] [PubMed] [Google Scholar]
- 28. Boutet M, Gauthier L, Leclerc M, et al. TGFβ signaling INTERSECTS with Cd103 integrin signaling to promote T-lymphocyte accumulation and antitumor activity in the lung tumor Microenvironment. Cancer Res 2016;76:1757–69. 10.1158/0008-5472.CAN-15-1545 [DOI] [PubMed] [Google Scholar]
- 29. Elisha Y, Kalchenko V, Kuznetsov Y, et al. Dual role of E-Cadherin in the regulation of invasive collective migration of Mammary carcinoma cells. Sci Rep 2018;8:4986. 10.1038/s41598-018-22940-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Franciszkiewicz K, Le Floc’h A, Boutet M, et al. Cd103 or LFA-1 engagement at the immune Synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell Effector functions. Cancer Res 2013;73:617–28. 10.1158/0008-5472.CAN-12-2569 [DOI] [PubMed] [Google Scholar]
- 31. Uddback IEM, Pedersen LMI, Pedersen SR, et al. Combined local and systemic immunization is essential for durable T-cell mediated Heterosubtypic immunity against influenza A virus. Sci Rep 2016;6:20137. 10.1038/srep20137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murphy CT, Hall LJ, Hurley G, et al. The Sphingosine-1-phosphate analogue Fty720 impairs Mucosal immunity and clearance of the Enteric pathogen Citrobacter Rodentium. Infect Immun 2012;80:2712–23. 10.1128/IAI.06319-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L, Chiang ET, Simmons JT, et al. Fty720-induced human pulmonary endothelial barrier Enhancement is mediated by C-Abl. Eur Respir J 2011;38:78–88. 10.1183/09031936.00047810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Donkor M, Choe J, Reid DM, et al. Nasal tumor vaccination protects against lung tumor development by induction of resident Effector and memory anti-tumor immune responses. Pharmaceutics 2023;15:445. 10.3390/pharmaceutics15020445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandoval F, Terme M, Nizard M, et al. Erratum: Mucosal imprinting of vaccine-induced Cd8+ T cells is crucial to inhibit the growth of Mucosal tumors (science Translational medicine. Sci Transl Med 2013;5:172ra20. 10.1126/scitranslmed.3004888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park JW, Kwon TK, Kim IH, et al. A new strategy for the diagnosis of MAGE-expressing cancers. J Immunol Methods 2002;266:79–86. 10.1016/s0022-1759(02)00105-9 [DOI] [PubMed] [Google Scholar]
- 37. Sahin U, Oehm P, Derhovanessian E, et al. An RNA vaccine drives immunity in Checkpoint-inhibitor-treated Melanoma. Nature 2020;585:107–12. 10.1038/s41586-020-2537-9 [DOI] [PubMed] [Google Scholar]
- 38. Müller MD, Holst PJ, Nielsen KN. A systematic review of expression and Immunogenicity of human endogenous retroviral proteins in cancer and discussion of therapeutic approaches. Int J Mol Sci 2022;23:1330. 10.3390/ijms23031330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Romero P, Banchereau J, Bhardwaj N, et al. The human vaccines project: A roadmap for cancer vaccine development. Sci Transl Med 2016;8:334ps9. 10.1126/scitranslmed.aaf0685 [DOI] [PubMed] [Google Scholar]
- 40. McWilliams JA, Sullivan RT, Jordan KR, et al. Age-dependent tolerance to an endogenous tumor-associated antigen. Vaccine 2008;26:1863–73. 10.1016/j.vaccine.2008.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor Microenvironment following preoperative Sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014;106:dju268. 10.1093/jnci/dju268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kandasamy M, Gileadi U, Rijal P, et al. Recombinant single-cycle influenza virus with exchangeable Pseudotypes allows repeated immunization to augment anti-tumour immunity with immune Checkpoint inhibitors. Elife 2023;12:1–21.:e76414. 10.7554/eLife.76414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jackson CM, Kochel CM, Nirschl CJ, et al. Systemic tolerance mediated by Melanoma brain tumors is reversible by radiotherapy and vaccination. Clin Cancer Res 2016;22:1161–72. 10.1158/1078-0432.CCR-15-1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sagiv-Barfi I, Kohrt HEK, Czerwinski DK, et al. Therapeutic antitumor immunity by Checkpoint blockade is enhanced by Ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A 2015;112:E966–72. 10.1073/pnas.1500712112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rückert M, Flohr A-S, Hecht M, et al. Radiotherapy and the immune system: more than just immune suppression. Stem Cells 2021;39:1155–65. 10.1002/stem.3391 [DOI] [PubMed] [Google Scholar]
- 46. Salomon N, Selmi A, Grunwitz C, et al. Local radiotherapy and E7 RNA-LPX vaccination show enhanced therapeutic efficacy in Preclinical models of Hpv16+ cancer. Cancer Immunol Immunother 2022;71:1975–88. 10.1007/s00262-021-03134-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Akimoto T, Mitsuhashi N, Saito Y, et al. Effect of radiation on the expression of E-Cadherin and Α-Catenin and invasive capacity in human lung cancer cell line in vitro. Int J Radiat Oncol Biol Phys 1998;41:1171–6. 10.1016/s0360-3016(98)00176-x [DOI] [PubMed] [Google Scholar]
- 48. Matsumura S, Wang B, Kawashima N, et al. Radiation-induced Cxcl16 release by breast cancer cells attracts Effector T cells. J Immunol 2008;181:3099–107. 10.4049/jimmunol.181.5.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karaki S, Blanc C, Tran T, et al. Cxcr6 deficiency impairs cancer vaccine efficacy and Cd8+ resident memory T-cell recruitment in head and neck and lung tumors. J Immunother Cancer 2021;9:e001948. 10.1136/jitc-2020-001948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Apte RN, Dotan S, Elkabets M, et al. The involvement of IL-1 in tumorigenesis, tumor Invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev 2006;25:387–408. 10.1007/s10555-006-9004-4 [DOI] [PubMed] [Google Scholar]
- 51. Kaplanov I, Carmi Y, Kornetsky R, et al. Blocking IL-1Β reverses the immunosuppression in Mouse breast cancer and Synergizes with anti–PD-1 for tumor Abrogation. Proc Natl Acad Sci U S A 2019;116:1361–9. 10.1073/pnas.1812266115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Castaño Z, San Juan BP, Spiegel A, et al. IL-1Β inflammatory response driven by primary breast cancer prevents metastasis-initiating cell Colonization. Nat Cell Biol 2018;20:1084–97. 10.1038/s41556-018-0173-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ganter MT, Roux J, Miyazawa B, et al. Interleukin-1Β causes acute lung injury via Αvβ5 and Αvβ6 integrin-dependent mechanisms. Circulation Research 2008;102:804–12. 10.1161/CIRCRESAHA.107.161067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dinarello CA. Biologic basis for Interleukin-1 in disease. Blood 1996;87:2095–147. 10.1182/blood.V87.6.2095.bloodjournal8762095 [DOI] [PubMed] [Google Scholar]
- 55. Van Den Eeckhout B, Van Hoecke L, Burg E, et al. Specific targeting of IL-1Β activity to Cd8+ T cells allows for safe use as a vaccine adjuvant. NPJ Vaccines 2020;5:64. 10.1038/s41541-020-00211-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Oltmanns FE, Antão AV, Irrgang P, et al. Mucosal vaccination with an adenoviral vector vaccine protects against pulmonary breast cancer metastasis by robust induction of tissue-resident memory T-cells in the lung. J Immunol 2023;210:145. 10.4049/jimmunol.210.Supp.145.22 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-008652supp001.pdf (1.9MB, pdf)
Data Availability Statement
Data are available upon reasonable request.