Abstract
Tau and α-synuclein aggregates are the main histopathological hallmarks present in Alzheimer’s disease (AD), Parkinson’s disease (PD), and other neurodegenerative disorders. Intraneuronal hyperphosphorylated tau accumulation is significantly connected to the degree of cognitive impairment in AD patients. In particular, the longest 2N4R tau isoform has a propensity to rapidly form oligomers and mature fibrils. On the other hand, misfolding of α-synuclein (α-syn) is the characteristic feature in PD and dementia with Lewy bodies (DLB). There is a strong crosstalk between the two prone-to-aggregation proteins as they coprecipitated in some brains of AD, PD, and DLB patients. Simultaneous targeting of both proteinaceous oligomers and aggregates is still challenging. Here, we rationally designed and synthesized benzothiazole- and indole-based compounds using the structural hybridization strategy between the benzothiazole N744 cyanine dye and the diphenyl pyrazole Anle138b that showed anti-aggregation activity towards 2N4R tau and α-syn, respectively. The anti-aggregation effect of the prepared compounds was monitored using the thioflavin-T (ThT) fluorescence assay, while transmission electron microscopy (TEM) was employed to detect fibrils upon the completion of a time-course study with the ThT assay. Moreover, the photo-induced crosslinking of unmodified protein (PICUP) assay was used to determine the formation of oligomers. Specifically, compounds 46 and 48 demonstrated the highest anti-aggregation activity by decreasing the ThT fluorescence to 4.0 and 14.8%, respectively, against α-syn. Although no noticeable effect on 2N4R tau oligomers, 46 showed promising anti-oligomer activity against α-syn. Both compounds induced a significantly high anti-aggregation effect against the two protein fibrils as visualized by TEM. Moreover, compound 48 remarkably inhibited α-syn inclusion and cell confluence using M17D cells. Collectively, compounds 46 and 48 could serve as a basic structure for further optimization to develop clinically active AD and PD disease-modifying agents.
Keywords: Alzheimer’s disease, Parkinson’s disease, Alpha-synuclein, Tau isoforms, Anti-oligomer agents
1. Introduction
The most prevalent neurodegenerative disorders impacting millions of patients and their caregivers annually are Alzheimer’s disease (AD) and Parkinson’s disease (PD).1,2 Classic AD symptoms include dementia, memory decline or loss, coherent speech difficulty, and general cognitive impairment.3,4 While in PD, the symptoms are mainly motorial including bradykinesia, tremor, and postural instability, however, nonmotor signs such as depression and sleep disturbance have also been experienced in some patients.5–7 The amyloid cascade hypothesis suggested that progressive deposition of amyloid-β (Aβ) protein is the pivotal hallmark in AD, and all the attendant pathological events such as neurofibrillary tangles (NFTs), formation of hyperphosphorylated tau protein, neuritic and glial cytopathology, and eventually dementia directly result from this deposition.8–10 The Food and Drug Administration (FDA) has recently approved lecanemab as an anti-Aβ monoclonal antibody for a breakthrough treatment of AD after its impressive clinical results in clearing Aβ and reducing cognitive impairment.11–13 However, infusion reactions and amyloid-related imaging abnormalities with edema or effusions (ARIA-E) are the most common adverse effects linked with lecanemab.14,15
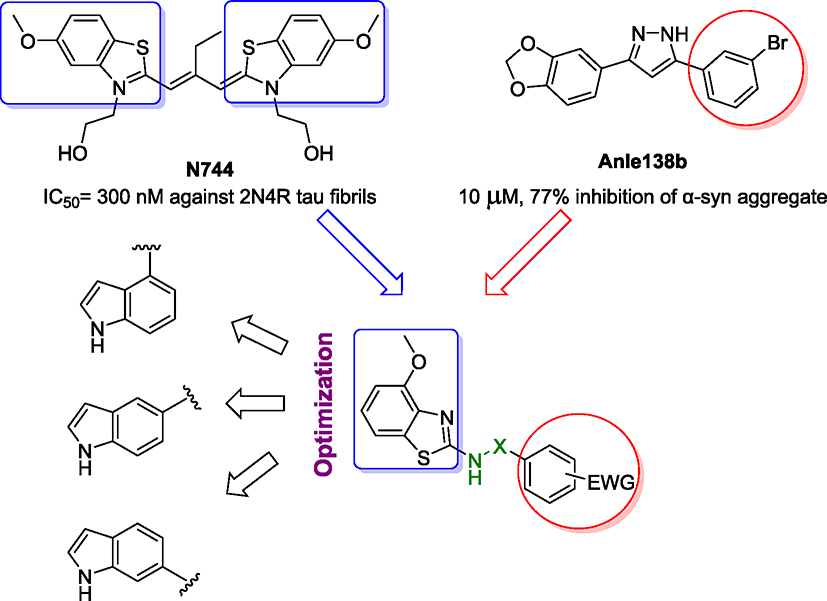
Recent findings emphasized that microtubule-associated protein tau (MAPT), hyperphosphorylated tau, and NFTs are more strongly linked with the degree of cognitive decline than with Aβ aggregates.16. In addition to AD, tau misfolding plays a role in the physiopathology of many neurodegenerative diseases such as frontotemporal dementia with Parkinsonism (FTDP-17), Pick’s disease, Down’s syndrome, and progressive supranuclear palsy (PSP), collectively termed tauopathies.17,18 Accordingly, tau has gained surprisingly high attention as a therapeutic target for AD and other tauopathies in the past few decades. Tau is present in six isoforms in the human brain which are categorized by the absence or the presence of 29-amino-acid N-terminal domain (0 N, 1 N, and 2 N) and the number of microtubule-binding C-terminal repeats (3R and 4R).19,20 Importantly, the 4R isoforms have faster aggregation rates and consequently higher propensity to produce neurodegeneration than the 3R variants.21 The benzothiazole cyanine dye N744 was identified as a strong tau aggregation inhibitor.22 N744 was found not only to inhibit the full-length (2N4R) tau aggregates (IC50 = 300 nM) but also remarkably induce the disaggregation of preformed filaments.23
In contrast, aberrant accumulation of intracellular proteinaceous aggregates of α-synuclein (α-syn) is the main histopathological characteristic of PD and dementia with Lewy bodies (DLB).24,25 α-Syn is a presynaptic acidic protein that plays potential roles in the management of synaptic vesicle release and neuronal survival.26 Under pathological conditions, misfolding of α-syn forms Lewy bodies (LBs) in the neurons.27 Braak and other colleagues have identified the correlation between LBs and disease progression.28,29 Recently, the diphenyl pyrazole Anle138b has been synthesized as a disease-modifying therapy for PD by inhibiting the formation of α-syn pathological aggregates (10 μM, 77 % inhibition of α-syn fibrils) in vitro as well as in vivo settings.30 Fluorescence measurements of Anle138b showed that significant fluorescence change occurred upon incubation with α-syn fibrils but not with monomers.31
As a corollary, there is a noticeable interplay between tau and LBs of α-syn as both aggregates coprecipitate in some brains of AD, PD, and DLB patients.32,33 Moreover, tau and α-syn synergistically facilitate the formation of each other’s aggregates in mouse models.34–36 The green tea catechin, (−)-epi-gallocatechin-3-gallate (EGCG), demonstrated significant anti-amyloidogenic characteristics against Aβ, tau, and α-syn aggregates.37 EGCG was able to reduce the fluorescence of Thioflavin-T (ThT) of approximately 90 %, limiting substantially the formation α-syn aggregates with a powerful inhibitory activity against oligomer formation.38,39 However, due to its polyphenol groups, EGCG exhibits a reduced membrane permeability and is subjected to high metabolic transformation which limit its clinical applications.40,41 Accordingly, there is an urgent need to design and prepare small molecules targeting both protein aggregates to reduce the progression of neurodegeneration.
Capitalization on the structures of N744 and Anle138b, benzothiazole and indole derivatives have been judiciously designed and synthesized by simple single-step reactions to reduce the 2N4R tau and α-syn aggregates. In particular, the indole derivatives with urea linker, 46 and 48, displayed the most promising results as a dual aggregation inhibitor for 2N4R tau and α-syn fibrils. Compounds 46 and 48 significantly decreased the ThT fluorescence to below 20 % against α-syn fibrils which are comparable to that of EGCG using similar assay conditions (see Supporting Information, Fig. S1). Moreover, 46 induced a noticeable inhibitory activity against α-syn oligomers, but not with the 2N4R tau oligomers. The use of transmission electron microscopy (TEM) revealed the powerful anti-aggregation properties of 46 and 48 on 2N4R tau and α-syn aggregates. In addition, 48 was highly effective in reducing α-syn inclusion using M17D cells.
2. Results and discussion
2.1. Design of the prepared compounds
Structural hybridization of two biologically active pharmacophores has been widely used as a common strategy to build up new hybrid scaffolds capable of binding to multitarget proteins.42–45 We selected the methoxy benzothiazole pharmacophore from N744 dye to guarantee the high affinity and inhibitory activity toward the β-sheet structures of 2N4R tau aggregates. On the other hand, structural analysis of Anle138b showed that the phenyl ring substituted with the electron-withdrawing (EWG) bromine atom has the highest activity against α-syn aggregates.30 Therefore, the elaborated compounds were designed by integrating the 4-methoxy benzothiazole moiety and various EWG-phenyl rings in one hybrid molecule. To further investigate the effect of the EWG groups, we also prepared the unsubstituted, electron-releasing, and 2-thiophenyl-containing counterparts (see Supporting Information, Table S1, Figures S2–S3). The two pharmacophores were separated by three different linkers: urea, amide, and sulfonamide. The most promising candidates were further optimized by replacing the benzothiazole ring with 4-,5-, and 6-substituted indole scaffolds (Fig. 1).
Fig. 1.
Design of methoxy benzothiazole and indole derivatives as dual 2N4R tau and α-syn aggregates inhibitors.
2.2. Chemistry
The designed compounds were synthesized using the commercially available 2-amino-4-methoxy benzothiazole (4-MBT). To prepare compounds with the urea linker, a quantitative amount of the appropriate phenyl isocyanate was used in dichloromethane (DCM). The amide or the sulfonamide counterparts were synthesized using the corresponding benzoyl chloride or benzene sulfonyl chloride, respectively, in the presence of anhydrous potassium carbonate and pyridine (Scheme 1). Then, the best candidates, with the 2-iodo and the 4-nitro sulfonamides and their urea analogs, were further modified by replacing the 4-MBT with 4-, 5-, or 6-amino indole (AI) using the same reaction conditions (Scheme 2). All products were obtained with moderate to quantitative yields (51–100 %).
Scheme 1.
Reagents and conditions: (a) DCM, r.t., 3–12 h, 71–100 %; (b) anhydrous K2CO3, pyridine, r.t., 8–10 h, 51–88 %.
Scheme 2.
Reagents and conditions: (a) DCM, r.t., 3–12 h, 79–100 %; (b) anhydrous K2CO3, pyridine, r.t., 3–12 h, 73–86 %.
2.3. Biophysical evaluation
2.3.1. Thioflavin-T (ThT) fluorescence assay
Thioflavin-T (ThT) is a fluorescent benzothiazole dye that upon binding to the cross β–sheet structures present in numerous prone-to-aggregate proteins. Upon binding, the dye results in a significant fluorescence quantum yield increase with a red shift in its excitation and emission spectra. The ThT assay was used not only to detect the presence of fibrils but also to measure the kinetics of the late-stage protein fibrillization process. Dense fibrils are formed at the plateau phase of the kinetic curve where equilibrium between disaggregation and aggregation occurs. Thus, the ThT assay was applied as a first-line screening step to monitor the antifibrillar activity of the newly synthesized 4-MBT compounds on α-syn protein. As expected, the unsubstituted, electron-releasing, and 2-thiophenyl-containing derivatives showed no activity against the α-syn aggregation via ThT fluorescence (Table S1). The 2-iodophenyl sulfonamide derivative 35 and the 4-nitrophenyl counterpart 42 demonstrated the highest anti-fibrillar activity as depicted by the decreasing ThT fluorescence to 5.0 and 9.5 %, respectively, compared to the 100 % control condition (Table 1). In order to identify the structural component that is crucial for biological activity, the benzothiazole moieties in 35 and 42 compounds were replaced by 4-, 5-, and 6-aminoindoles with sulfonamide or urea linkers (Table 2). Compounds 46 and 48, resulting from these structural modifications, induced comparable or slightly higher ThT fluorescence percentages compared to 35 and 42 (Fig. 2A). Importantly, compound 46 displayed superior activity against the earlier stage α-syn oligomer formation (discussed below). In addition, compound 46 showed a dose–response relationship with the α-syn anti-fibrillation assessment (Fig. 2B). Gradual increasing the compound concentration from 3.125 to 25 μM largely reduced the ThT fluorescence. Anti-fibrillary effects of compounds 46–48 is comparable to EGCG on α-syn and tau isoform 2N4R (Fig. S1).
Table 1.
The effect of the synthesized compounds containing the methoxy benzothiazole moiety (100 μM) on α-syn fibrils (2 μM) using thioflavin-T (ThT) fluorescence assay. The linker consisted of urea for compounds 1–14, amide for compounds 15–28, and sulfonamide for compounds 29–42.
| Compound ID | R | α-syn ThT % | Compound ID | R | α-syn ThT % |
|---|---|---|---|---|---|
|
| |||||
| 1 | 2-F | ≥ 200 | 8 | 4-I | 141.2 ± 14.5 |
| 2 | 4-F | 145.3 ± 8.0 | 9 | 3-CF3 | ≥ 200 |
| 3 | 2,4-F | 184.1 ± 12.3 | 10 | 4-CF3 | 94.8 ± 5.1 |
| 4 | 4-Cl | 172.8 ± 7.0 | 11 | 4-CN | ≥ 200 |
| 5 | 3,5-Cl | ≥ 200 | 12 | 2-NO2 | 64.4 ± 3.4 |
| 6 | 4-Br | 170.2 ± 7.1 | 13 | 3-NO2 | 78.1 ± 12.5 |
| 7 | 2-I | 124.8 ± 4.9 | 14 | 4-NO2 | 79.0 ± 12.8 |
| 15 | 2-F | 98.7 ± 16.9 | 22 | 4-I | ≥ 200 |
| 16 | 4-F | 103.0 ± 15.8 | 23 | 3-CF3 | 84.7 ± 8.2 |
| 17 | 2,4-F | 127.5 ± 18.0 | 24 | 4-CF3 | 115.9 ± 21.3 |
| 18 | 4-Cl | 116.4 ± 17.0 | 25 | 4-CN | 127.8 ± 21.8 |
| 19 | 3,5-Cl | 95.4 ± 9.4 | 26 | 2-NO2 | 102.0 ± 7.8 |
| 20 | 4-Br | ≥ 200 | 27 | 3-NO2 | 48.1 ± 8.8 |
| 21 | 2-I | 137.1 ± 20.9 | 28 | 4-NO2 | 45.4 ± 2.2 |
| 29 | 2-F | 64.9 ± 6.1 | 36 | 4-I | 42.8 ± 12.3 |
| 30 | 4-F | 70.4 ± 11.9 | 37 | 3-CF3 | 56.0 ± 3.5 |
| 31 | 2,4-F | 87.9 ± 8.9 | 38 | 4-CF3 | 53.8 ± 7.7 |
| 32 | 4-Cl | 40.3 ± 7.0 | 39 | 4-CN | 46.1 ± 1.6 |
| 33 | 3,5-Cl | 39.3 ± 6.7 | 40 | 2-NO2 | 41.0 ± 3.1 |
| 34 | 4-Br | 43.1 ± 6.5 | 41 | 3-NO2 | 40.1 ± 3.9 |
| 35 | 2-I | 5.8 ± 0.5 | 42 | 4-NO2 | 9.5 ± 1.8 |
Table 2.
The effect of the synthesized compounds containing different indole moieties (100 μM) on α-syn fibrils (2 μM) using thioflavin-T (ThT) fluorescence assay.

| ||||
|---|---|---|---|---|
|
| ||||
| Linker: 1) Urea for compounds 43–48; 2) Sulfonamide for compounds 49–54. | ||||
|
| ||||
| Compound ID | AR | R1 | R2 | α-syn ThT % |
|
| ||||
| 43 | 4-Aminoindole | 2-I | H | 38.3 ± 1.9 |
| 44 | 5-Aminoindole | 2-I | H | 40.3 ± 0.5 |
| 45 | 6-Aminoindole | 2-I | H | 83.4 ± 4.6 |
| 46 | 4-Aminoindole | H | 4-NO2 | 4.0 ± 0.2 |
| 47 | 5-Aminoindole | H | 4-NO2 | 2.9 ± 0.1 |
| 48 | 6-Aminoindole | H | 4-NO2 | 3.6 ± 1.3 |
| 49 | 4-Aminoindole | 2-I | H | 74.8 ± 8.0 |
| 50 | 5-Aminoindole | 2-I | H | 59.7 ± 6.8 |
| 51 | 6-Aminoindole | 2-I | H | 103.5 ± 13.1 |
| 52 | 4-Aminoindole | H | 4-NO2 | 83.9 ± 3.1 |
| 53 | 5-Aminoindole | H | 4-NO2 | 97.6 ± 20.7 |
| 54 | 6-Aminoindole | H | 4-NO2 | 63.5 ± 5.3 |
Fig. 2.
ThT fluorescence assay. The ThT kinetic curves of compounds 46, 47, and 48 (100 μM) when tested with: A. α-syn (2 μM); B. Dose-dependent inhibition of varying concentrations (3.125, 6.25, 12.5, 25, 50, 100 μM) of compound 46 on α-syn (2 μM) fibril formation using ThT fluorescence assay. For each concentration, triplicate data were gathered at the plateau phase from five consecutive time points. The error bars represent the individual standard error of the mean (SEM) for each condition.
2.3.2. Photo-Induced cross-linking of unmodified protein (PICUP) assay
Suppression of oligomer formation is a crucial end goal to validate the effectiveness of candidates targeting protein misfolding. The photo-induced cross-linking of unmodified proteins (PICUP) assay is a rapid and efficient photochemical technique that was used to study the early events of protein oligomerization and protofibril formation thanks to its potent ability to covalently cross-link unmodified protein assemblies. Compounds that showed the best anti-fibrillary activity, as monitored by ThT assay, were subjected to the α-syn PICUP assay (Fig. 3 and Fig. S2). The cross-linked α-syn in this assay consist of oligomers that approximately correspond to trimers (band size is between 35 and 40 kDa). Unlike 35 and 42, compound 46 showed promising anti-oligomer α-syn activity at a compound-to-protein ratio of 1:0.6. The compounds were further evaluated in a dose-dependent manner at varying concentrations (50, 100 and 200 μM) while the concentration of the tau protein remained at 10 μM. However, compounds 46, 47, and 48 showed no effect on inhibiting 2N4R or 0N4R tau oligomers induced by the PICUP assay (Fig. 4A and 4B, respectively). That could be explained by the large variation in the core structures between α-syn and tau isoforms. The ThT assay and PICUP assay results definitively confirmed the ability of compound 46 to inhibit the oligomers and fibrils of α-syn protein and mature fibrils of tau isoform 2N4R.
Fig. 3.
Inhibition of the α-syn oligomer formation induced by the PICUP assay. In the PICUP experiment, α-syn (60 μM) was cross-linked with varying concentrations of compounds 46, 47, and 48 (50, 100, and 200 μM). The optical density of the high molecular-weight bands represents α-syn oligomer while the lower bands represent the protein monomers. In particular, compound 46 reduced the development of α-syn oligomer-corresponding high molecular bands between 35 and 40 kDa. The control consisted of no light exposure and no Tris(2,2′-bipyridyl)ruthenium(II) chloride (Ru(bpy)3, a cross-linking agent).
Fig. 4.
Inhibition of the oligomer formation induced by the PICUP assay on two different tau isoforms (10 μM): A. 2N4R tau; B. 0N4R tau. In the experiment, we exposed the protein to different concentrations of compounds 46, 47, and 48 (50 μM, 100 μM, and 200 μM). None of the compounds demonstrated the ability to suppress the formation of tau oligomers using both tau isoforms. The control consisted of no light exposure and no Tris(2,2′-bipyridyl)ruthenium(II) chloride (Ru (bpy)3, a cross-linking agent).
2.3.3. In vitro transmission electron microscope (TEM) analysis
The top α-syn anti-fibrillar compounds were further tested against α-syn and tau (isoform 2N4R) fibrils using a transmission electron microscope (TEM) to further assess the anti-aggregation ability. Samples were pre-incubated for a period of 68 h (α-syn) and 72 h (tau) prior TEM analyses. Control with vehicle (DMSO 0.25 %) resulted in dense mature fibrils of α-syn and tau isoform 2N4R. TEM imaging of α-syn revealed that all three compounds (46, 47, and 48) significantly reduced the protein fibril formation (Fig. 5, upper row). Similar effect was observed with treated unfolded tau isoform 2N4R. Treatment with compounds 46–48 reduced formation of large dense accumulation of tau fibrils. A few short and fine needle-like fibrils (Fig. 5D and F) and rounded structures (Fig. 5H) were visualized after treatment of tau isoform 2N4R with compounds 46–48 in contrast to the control (Fig. 5, lower row).
Fig. 5.
TEM analysis of compounds 46, 47, and 48 (100 μM) on the inhibition of mature fibrils of α-syn (2 μM, upper panel) and tau isoform 2N4R (6 μM, lower panel). The unfolded protein was incubated with: A-B. DMSO (0.25 %); C-D. compound 46; E-F. compound 47; or G-H. compound 48. Incubation time was ~ 68 h and 72 h at 37° C for α-syn and tau (2N4R), respectively. Scale bars: 200 nm, magnification: 40 K.
2.4. α-Synuclein Inclusion-Forming neuroblastoma cell experiment
To examine the effects of the three compounds (46, 47, and 48) on cell viability and formation of α-syn inclusions, M17D neuroblastoma cells that express the inclusion-prone fusion protein α-Syn-S3K::YFP were used (Fig. 6). In addition, compounds 35, 42 were also examined using similar assay conditions (Fig. S3). Cells were incubated with different doses of compounds 24 h after plating. Then transgene expression was induced by doxycycline at 48 h and the effects of the compounds on inclusion formation were tested in a dose-dependent manner at the 96 h time-point. Compound 48 had the highest effect in the reduction of inclusions as the concentration increased. A similar trend was observed for its effect on cell confluence at a concentration range of 5 μM to 40 μM. While compounds 46 and 47 demonstrated a reasonable reducing effect on inclusions at varying concentrations, neither compound had an effect on cell confluence below 20 μM concentration, and only minor effects at 20 and 40 μM.
Fig. 6.
Inhibition of α-syn inclusion formation using M17D cells. The cells expressing the inclusion-prone α-Syn-3K::YFP fusion protein (dox-inducible) were treated with 0.1 % DMSO (vehicle; “0 μM”) as well as 1.25, 2.5, 5, 10, 20 and 40 μM of compounds 46 (A), 47 (B) and 48 (C) at t = 24 h after plating. Cells were induced with doxycycline at t = 48 h. Incucyte-based analysis of punctate YFP signals relative to 0.1 % DMSO was done at t = 96 h (N = 2 independent experiments, n = 6–12 individual wells total (0 μM, n = 12; 40, 20 and 10 μM, n = 6; all other concentrations, n = 12). Plot of confluence fold changes relative to DMSO vehicle (0 μM). D) Representative IncuCyte images of reporter cells treated with vehicle vs 5 μM compound 46, 47, and 48 (t = 96 h), green channel. Arrows indicate αS-rich YFP-positive inclusions. Scale bar, 50 μm. All data are presented as fold-changes relative to DMSO control + /− standard deviation. One-way ANOVA, Dunnett’s post-hoc test; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
3. Conclusion
Benzothiazole- and indole-based scaffold compounds were rationally designed and synthesized for dual inhibition of α-syn and tau (isoform 2N4R) aggregates. The elaborated compounds were prepared by hybridizing the active pharmacophore moieties of a strong tau (2N4R) aggregation inhibitor, N744 dye, and a powerful α-syn anti-aggregation Anle138b. ThT fluorescence assay revealed the benzothiazoles 35 and 42 significantly decreased the ThT fluorescence to 5.0 and 9.5 % towards α-syn fibrils. In addition, the ThT fluorescence was decreased to lower than 20 % by the indole-based urea compounds 46 and 48 towards α-syn fibrils. Compound 46 showed a noticeable inhibition of α-syn oligomer formation as assessed by the PICUP assay. The TEM confirmed the striking α-syn and tau (2N4R) anti-aggregation ability of compounds 46 and 48. Importantly, the α-syn inclusions were remarkably decreased after treatment with compound 48. Chemical refinement is now underway to further optimize the compounds to effectively abrogate the early-stage oligomer formation of both α-syn and tau isoform 2N4R.
4. Experimental procedures
4.1. General Information
All reagents and solvents were commercially available (Sigma Aldrich, St. Louis, MO; Thermo Scientific (formerly Alfa Aesar), Waltham, MA; Matrix Scientific, Columbia, SC; Ambeed, Arlington Hts, IL) and were used without further purification. Thin-layer chromatography (TLC) was used to monitor the reaction progress. Organic solutions were dried over anhydrous sodium sulfate. The solvents were evaporated on a Büchi rotavapor R-100 equipped with a Büchi V-100 vacuum controller. The nuclear magnetic resonance (NMR) spectra were recorded on a Bruker 500 MHz spectrometer. Proton chemical shifts are reported in parts per million (ppm) with the solvent reference relative to tetramethyl silane (TMS) employed as the internal standard (CDCl3, δ 7.26; DMSO‑d6, δ 2.54). The multiplicities of NMR signals are designated as s (singlet), d (doublet), dd (double doublet), t (triplet), q (quartet), br (broad), m (multiplet, for unresolved lines). High-resolution mass spectrometry (HRMS) of the compounds was carried out at the MSU Mass Spectrometry and Metabolomics Core Facility. Uncorrected melting points (mp) were scored using an electrothermal apparatus (Barnstead International, Dubuque, Iowa, USA).
4.2. Chemistry
4.2.1. General procedure for the synthesis of compounds with the urea linker (1–14, 43–48)
4-Methoxy-2-amino benzothiazole, 4-, 5-, or 6-amino indole (100 mg, 1.0 eq), and the appropriate phenyl isocyanate (1.0 eq) were dissolved in dichloromethane (DCM, 10 mL) and stirred for 4–12 h at room temperature. The precipitate was then filtered and washed with hexane (10 mL), DCM (2 × 10 mL), and diethyl ether (10 mL). The desired products were obtained in excellent to quantitative yields.
1-(2-Fluorophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl)urea (1).
Starting with 4-methoxy-2-amino benzothiazole and 2-fluoro phenyl isocyanate. Yield: 92%, white solid; 1H NMR (500 MHz, DMSO) δ 11.01 (s, 1H), 9.13 (s, 1H), 8.12 – 8.08 (m, 1H), 7.47 (dd, J = 7.9, 1.0 Hz, 1H), 7.30 – 7.25 (m, 1H), 7.23 – 7.15 (m, 2H), 7.14 – 7.05 (m, 1H), 6.95 (dd, J = 8.0, 1.0 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 157.9, 153.9, 151.9, 133.3, 126.8, 126.7, 125.2, 125.2, 124.5, 121.8, 115.8, 115.7, 113.9, 108.3, 56.2. Melting Point: 209.5 – 211.3 °C.
1-(4-Fluorophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl)urea (2).
Starting with 4-methoxy-2-amino benzothiazole and 4-fluoro phenyl isocyanate. Yield: 87%, white solid; 1H NMR (500 MHz, DMSO) δ 10.86 (s, 1H), 9.16 (s, 1H), 7.55 – 7.48 (m, 2H), 7.48 – 7.42 (m, 1H), 7.21 – 7.12 (m, 3H), 6.95 (dd, J = 8.1, 1.0 Hz, 1H), 3.89 (s, 3H). 13C NMR (126 MHz, DMSO) δ 159.4, 157.5, 151.6, 135.3, 133.1, 124.3, 121.4, 121.4, 116.0, 115.9, 113.9, 108.3, 56.2. Melting Point: 201.0 – 202.3 °C.
1-(2,4-Difluorophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl)urea (3).
Starting with 4-methoxy-2-amino benzothiazole and 2,4-difluoro phenyl isocyanate. Yield: 94%, white solid; 1H NMR (500 MHz, DMSO) δ 11.01 (s, 1H), 9.08 (s, 1H), 8.09 – 7.95 (m, 1H), 7.46 (dd, J = 8.0, 0.9 Hz, 1H), 7.38 – 7.32 (m, 1H), 7.18 (t, J = 8.0 Hz, 1H), 7.13 – 7.03 (m, 1H), 6.95 (d, J = 7.2 Hz, 1H), 3.89 (s, 3H). 13C NMR (126 MHz, DMSO) δ 157.3, 154.3, 151.8, 133.2, 124.5, 123.5, 123.2, 113.9, 111.8, 111.7, 108.3, 104.7, 104.5, 104.3, 56.2. Melting Point: 220.8 – 225.0 °C.
1-(4-Chlorophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl)urea (4).
Starting with 4-methoxy-2-amino benzothiazole and 4-chloro phenyl isocyanate. Yield: 82%, white solid; 1H NMR (500 MHz, DMSO) δ 10.94 (s, 1H), 9.27 (s, 1H), 7.53 (d, J = 8.8 Hz, 2H), 7.45 (dd, J = 7.9, 0.9 Hz, 1H), 7.36 (d, J = 8.8 Hz, 2H), 7.18 (t, J = 8.0 Hz, 1H), 6.95 (dd, J = 8.1, 1.0 Hz, 1H), 3.89 (s, 3H). 13C NMR (126 MHz, DMSO) δ 158.9, 158.5, 152.5, 151.5, 138.0, 133.0, 129.2, 127.0, 124.4, 121.8, 121.0, 113.9, 108.3, 56.2. Melting Point: 238.9 – 240.5 °C.
1-(3,5-Dichlorophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl) urea (5).
Starting with 4-methoxy-2-amino benzothiazole and 3,5-dichloro phenyl isocyanate. Yield: 91%, white solid; 1H NMR (500 MHz, DMSO) δ 11.38 (s, 1H), 9.47 (s, 1H), 7.64 – 7.57 (m, 2H), 7.44 (d, J = 8.0 Hz, 1H), 7.31 – 7.12 (m, 2H), 6.96 (d, J = 8.0 Hz, 1H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 152.5, 151.1, 142.3, 141.8, 134.6, 132.6, 124.5, 122.4, 117.6, 117.2, 114.0, 108.4, 56.2. Melting Point: 246.5 – 247.8 °C.
1-(4-Bromophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl)urea (6).
Starting with 4-methoxy-2-amino benzothiazole and 4-bromo phenyl isocyanate. Yield: 87%, white solid; 1H NMR (500 MHz, DMSO) δ 10.95 (s, 1H), 9.27 (s, 1H), 7.48 (s, 4H), 7.45 (dd, J = 8.0, 1.0 Hz, 1H), 7.18 (t, J = 8.0 Hz, 1H), 6.95 (dd, J = 8.1, 1.0 Hz, 1H), 3.89 (s, 3H). 13C NMR (126 MHz, DMSO) δ 158.4, 152.5, 151.5, 138.5, 133.0, 132.1, 124.4, 121.5, 121.4, 115.0, 113.9, 108.3, 56.2. Melting Point: 276.8 – 279.1 °C.
1-(2-Iodophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl)urea (7).
Starting with 4-methoxy-2-amino benzothiazole and 2-iodo phenyl isocyanate. Yield: 71%, white solid; 1H NMR (500 MHz, DMSO) δ 11.55 (s, 1H), 8.47 (s, 1H), 7.87 (dd, J = 7.9, 1.5 Hz, 1H), 7.82 (dd, J = 8.2, 1.5 Hz, 1H), 7.49 – 7.34 (m, 2H), 7.19 (t, J = 8.0 Hz, 1H), 6.99 – 6.87 (m, 2H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 158.1, 152.2, 151.9, 139.6, 139.3, 133.3, 129.3, 126.7, 124.4, 124.2, 113.9, 108.4, 92.8, 56.3. Melting Point: 224.0 – 225.2 °C.
1-(4-Iodophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl)urea (8).
Starting with 4-methoxy-2-amino benzothiazole and 4-iodo phenyl isocyanate. Yield: 85%, white solid; 1H NMR (500 MHz, DMSO) δ 10.93 (s, 1H), 9.24 (s, 1H), 7.64 (d, J = 8.7 Hz, 2H), 7.49 – 7.41 (m, 1H), 7.35 (d, J = 8.7 Hz, 2H), 7.18 (t, J = 8.0 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 3.89 (s, 3H). 13C NMR (126 MHz, DMSO) δ 158.4, 152.4, 151.5, 138.9, 138.0, 133.0, 127.2, 124.4, 121.6, 113.9, 108.3, 86.7, 56.2. Melting Point: 215.2 – 217.0 °C.
1-(4-Methoxybenzo[d]thiazol-2-yl)-3-(3-(trifluoromethyl) phenyl)urea (9).
Starting with 4-methoxy-2-amino benzothiazole and 3-trifluoromethyl phenyl isocyanate. Yield: 91%, white solid; 1H NMR (500 MHz, DMSO) δ 11.12 (s, 1H), 9.51 (s, 1H), 8.01 (s, 1H), 7.69 (d, J = 8.2 Hz, 1H), 7.55 (t, J = 8.0 Hz, 1H), 7.45 (d, J = 7.9 Hz, 1H), 7.38 (d, J = 7.7 Hz, 1H), 7.19 (t, J = 8.0 Hz, 1H), 6.96 (d, J = 7.9 Hz, 1H), 3.89 (s, 3H). 13C NMR (126 MHz, DMSO) δ 166.7, 151.7, 140.0, 130.6, 130.2, 129.9, 125.7, 124.4, 123.5, 123.1, 119.7, 115.4, 113.9, 108.3, 56.2.
1-(4-Methoxybenzo[d]thiazol-2-yl)-3-(4-(trifluoromethyl) phenyl)urea (10).
Starting with 4-methoxy-2-amino benzothiazole and 4-trifluoromethyl phenyl isocyanate. Yield: 97%, white solid; 1H NMR (500 MHz, DMSO) δ 11.03 (s, 1H), 9.53 (s, 1H), 7.72 (d, J = 8.4 Hz, 2H), 7.67 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 7.9 Hz, 1H), 7.19 (t, J = 8.0 Hz, 1H), 6.96 (d, J = 7.9 Hz, 1H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 142.8, 140.6, 140.0, 128.1, 126.7, 126.0, 124.5, 123.4, 121.7, 119.2, 113.9, 108.3, 56.2.
1-(4-Cyanophenyl)-3-(4-methoxybenzo[d]thiazol-2-yl)urea (11).
Starting with 4-methoxy-2-amino benzothiazole and 4-cyano phenyl isocyanate. Yield: 88%, white solid; 1H NMR (500 MHz, DMSO) δ 11.18 (s, 1H), 9.62 (s, 1H), 7.76 (d, J = 8.8 Hz, 2H), 7.70 (d, J = 8.8 Hz, 2H), 7.49 – 7.36 (m, 1H), 7.19 (t, J = 8.0 Hz, 1H), 6.96 (dd, J = 8.0, 0.9 Hz, 1H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 151.3, 143.6, 133.8, 132.7, 126.8, 124.5, 119.6, 119.4, 119.3, 114.0, 108.3, 104.9, 56.2. Melting Point: 252.4 – 252.9 °C.
1-(4-Methoxybenzo[d]thiazol-2-yl)-3-(2-nitrophenyl)urea (12).
Starting with 4-methoxy-2-amino benzothiazole and 2-nitro phenyl isocyanate. Yield: 99%, canary yellow solid; 1H NMR (500 MHz, DMSO) δ 12.17 (s, 1H), 9.92 (s, 1H), 8.28 (d, J = 8.4 Hz, 1H), 8.12 (d, J = 6.7 Hz, 1H), 7.79 – 7.69 (m, 1H), 7.47 (d, J = 7.9 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.20 (t, J = 8.0 Hz, 1H), 6.96 (d, J = 8.0 Hz, 1H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 13C NMR (126 MHz, DMSO) δ 138.9, 135.6, 134.0, 128.9, 126.0, 124.6, 124.0, 123.6, 118.9, 115.1, 113.9, 108.4, 56.3.
1-(4-Methoxybenzo[d]thiazol-2-yl)-3-(3-nitrophenyl)urea (13).
Starting with 4-methoxy-2-amino benzothiazole and 3-nitro phenyl isocyanate. Yield: 92%, white solid; 1H NMR (500 MHz, DMSO) δ 11.28 (s, 1H), 9.65 (s, 1H), 8.56 (s, 1H), 7.87–––7.81 (m, 2H), 7.58 (t, J = 8.2 Hz, 1H), 7.44 (d, J = 7.9 Hz, 1H), 7.18 (t, J = 8.0 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 159.0, 153.2, 151.3, 148.6, 140.5, 132.7, 130.6, 125.5, 124.5, 117.7, 113.9, 113.4, 110.3, 108.3, 56.2. Melting Point: 266.0 – 266.7 °C.
1-(4-Methoxybenzo[d]thiazol-2-yl)-3-(4-nitrophenyl)urea (14).
Starting with 4-methoxy-2-amino benzothiazole and 4-nitro phenyl isocyanate. Yield: 100%, off-white solid; 1H NMR (500 MHz, DMSO) δ 11.33 (s, 1H), 9.84 (s, 1H), 8.20 (d, J = 8.7 Hz, 2H), 7.76 (d, J = 8.7 Hz, 2H), 7.45 (d, J = 8.0 Hz, 1H), 7.20 (t, J = 8.0 Hz, 1H), 6.97 (d, J = 8.1 Hz, 1H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 151.2, 145.8, 142.2, 132.7, 125.5, 124.6, 121.9, 118.8, 114.0, 113.8, 108.6, 108.4, 56.2. Melting Point: 279.1 – 280.4 °C.
1-(1H-Indol-4-yl)-3-(2-iodophenyl)urea (43).
Starting with 4-amino indole and 2-iodo phenyl isocyanate. Yield: 95%, grey solid; 1H NMR (500 MHz, DMSO) δ 11.11 (s, 1H), 9.08 (s, 1H), 8.21 (s, 1H), 7.84 (d, J = 8.2 Hz, 1H), 7.80 (d, J = 7.4 Hz, 1H), 7.61 (d, J = 7.6 Hz, 1H), 7.37 – 7.32 (m, 1H), 7.30 (t, J = 2.8 Hz, 1H), 7.06 (d, J = 8.0 Hz, 1H), 6.99 (t, J = 7.9 Hz, 1H), 6.89 – 6.78 (m, 1H), 6.62 (s, 1H). 13C NMR (126 MHz, DMSO) δ 153.1, 140.5, 139.4, 137.0, 131.8, 129.0, 125.6, 124.5, 124.2, 122.0, 119.7, 108.6, 106.6, 98.6, 92.1. Melting Point: 207.0 – 208.4 °C.
1-(1H-Indol-5-yl)-3-(2-iodophenyl)urea (44).
Starting with 5-amino indole and 2-iodo phenyl isocyanate. Yield: 92%, grey solid; 1H NMR (500 MHz, DMSO) δ 10.93 (s, 1H), 9.18 (s, 1H), 7.87 (d, J = 8.2 Hz, 1H), 7.84 – 7.54 (m, 3H), 7.43 – 7.17 (m, 3H), 7.07 (d, J = 8.8 Hz, 1H), 6.87 – 6.69 (m, 1H), 6.34 (s, 1H). 13C NMR (126 MHz, DMSO) δ 153.2, 140.7, 139.4, 132.8, 131.7, 129.0, 128.2, 126.3, 125.0, 123.1, 115.1, 111.8, 110.5, 101.4, 91.2. Melting Point: 206.1 – 207.9 °C.
1-(1H-Indol-6-yl)-3-(2-iodophenyl)urea (45).
Starting with 6-amino indole and 2-iodo phenyl isocyanate. Yield: 100%, light grey solid; 1H NMR (500 MHz, DMSO) δ 10.93 (s, 1H), 9.31 (s, 1H), 7.88 (d, J = 8.2 Hz, 1H), 7.81 (t, J = 6.7 Hz, 3H), 7.42 (d, J = 8.4 Hz, 1H), 7.33 (s, 1H), 7.21 (t, J = 2.8 Hz, 1H), 6.87 (dd, J = 8.4, 1.9 Hz, 1H), 6.81 (d, J =7.5 Hz, 1H), 6.33 (t, J = 2.6 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 153.0, 140.6, 139.4, 136.7, 134.1, 129.0, 125.2, 125.0, 123.7, 123.2, 120.5, 112.3, 101.7, 101.4, 91.4. Melting Point: 213.1 – 215.3 °C.
1-(1H-Indol-4-yl)-3-(4-nitrophenyl)urea (46).
Starting with 4-amino indole and 4-nitro phenyl isocyanate. Yield: 89%, brown solid; 1H NMR (500 MHz, DMSO) δ 11.16 (s, 1H), 9.60 (s, 1H), 8.71 (s, 1H), 8.19 (d, J = 9.2 Hz, 2H), 7.72 (d, J = 9.2 Hz, 2H), 7.67 – 7.60 (m, 1H), 7.36 – 7.29 (m, 1H), 7.11 – 7.09 (m, 1H), 7.02 (t, J = 7.9 Hz, 1H), 6.55 (s, 1H). 13C NMR (126 MHz, DMSO) δ 152.4, 146.9, 141.4, 137.0, 131.2, 125.7, 124.8, 122.0, 119.7, 117.7, 108.5, 107.1, 98.2. HRMS m/z: calcd for C15H12N4O2, 296.0909; found, 296.0988, M + H+. Melting Point: 223.5 – 224.1 °C.
1-(1H-Indol-5-yl)-3-(4-nitrophenyl)urea (47).
Starting with 5-amino indole and 4-nitro phenyl isocyanate. Yield: 86%, beige-colored solid; 1H NMR (500 MHz, DMSO) δ 10.98 (s, 1H), 9.34 (s, 1H), 8.65 (s, 1H), 8.17 (d, J = 8.1 Hz, 2H), 7.86 – 7.54 (m, 3H), 7.45 – 7.18 (m, 2H), 7.11 (d, J = 8.6 Hz, 1H), 6.37 (s, 1H). 13C NMR (126 MHz, DMSO) δ 152.8, 147.4, 141.2, 133.1, 131.1, 128.2, 126.4, 125.6, 117.7, 115.5, 111.8, 111.1, 101.5. HRMS m/z: calcd for C15H12N4O2, 296.0909; found, 296.0992, M + H+. Melting Point: 248.5 – 250.1 °C.
1-(1H-Indol-6-yl)-3-(4-nitrophenyl)urea (48).
Starting with 6-amino indole and 4-nitro phenyl isocyanate. Yield: 79%, yellow solid; 1H NMR (500 MHz, DMSO) δ 10.96 (s, 1H), 9.36 (s, 1H), 8.80 (s, 1H), 8.18 (d, J = 9.2 Hz, 2H), 7.80 (s, 1H), 7.69 (d, J = 9.2 Hz, 2H), 7.43 (d, J = 8.4 Hz, 1H), 7.23 (t, J = 2.7 Hz, 1H), 6.90 (dd, J = 8.4, 2.0 Hz, 1H), 6.34 (s, 1H). 13C NMR (126 MHz, DMSO) δ 152.6, 147.2, 141.2, 136.6, 133.4, 125.6, 125.2, 124.1, 120.5, 117.7, 112.7, 102.1, 101.4. HRMS m/z: calcd for C15H12N4O2, 296.0909; found, 296.0993, M + H+. Melting Point: 247.3 – 248.9 °C.
4.2.2. General procedure for the synthesis of compounds with the amide or sulfonamide linker (15–42, 49–54)
The appropriate benzoyl chloride or its corresponding benzene sulfonyl chloride (1.0 eq) was added slowly to a stirred solution of 4-methoxy-2-amino benzothiazole, 4-, 5-, or 6-amino indole (100 mg, 1.0 eq) in pyridine (3 mL) at 0 °C. Potassium carbonate (1.5 eq) was added, and the reaction was allowed to stir overnight at room temperature. After completion, an aqueous solution of 1 N hydrochloric acid (7 mL) and dichloromethane (10 mL) was added to the reaction mixture and then extracted with DCM. The organic layers were then washed with saturated solutions of ammonium chloride (7 mL), sodium bicarbonate (7 mL), and brine (7 mL), respectively. Organic layers were collected, filtered over anhydrous magnesium sulfate, and concentrated in-vacuo. The crude was purified using column chromatography to obtain the desired products with moderate to good yields.
2-Fluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (15).
Starting with 4-methoxy-2-amino benzothiazole and 2-fluoro benzoyl chloride. Yield: 58%, white solid; 1H NMR (500 MHz, CDCl3) δ 8.32 – 8.11 (m, 1H), 7.63 – 7.56 (m, 1H), 7.44 (dd, J = 8.0, 0.9 Hz, 1H), 7.35 (d, J = 7.8, 1H), 7.30 (t, J = 8.0 Hz, 1H), 7.43 – 7.21 (m, 1H), 6.92 (dd, J = 8.0, 0.9 Hz, 1H), 4.04 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 161.3, 156.7, 152.1, 138.2, 135.2, 133.6, 132.3, 125.3, 125.3, 125.1, 116.7, 116.5, 113.5, 106.8, 55.9. Melting Point: 178.4 – 180.8 °C.
4-Fluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (16).
Starting with 4-methoxy-2-amino benzothiazole and 4-fluoro benzoyl chloride. Yield: 67%, white solid; 1H NMR (500 MHz, DMSO) δ 8.29 – 8.16 (m, 2H), 7.54 (dd, J = 8.0, 0.9 Hz, 1H), 7.39 (t, J = 8.8 Hz, 2H), 7.27 (t, J = 8.0 Hz, 1H), 7.00 (dd, J = 8.0, 0.9 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO) δ 172.5, 165.1, 157.7, 152.3, 133.3, 131.7, 131.6, 125.2, 116.3, 116.1, 113.9, 108.0, 56.1.
2,4-Difluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (17).
Starting with 4-methoxy-2-amino benzothiazole and 2,4-difluoro benzoyl chloride. Yield: 75%, white solid; 1H NMR (500 MHz, DMSO) δ 7.88 (td, J = 8.5, 6.5 Hz, 1H), 7.55 (dd, J = 8.0, 0.9 Hz, 1H), 7.50 – 7.39 (m, 1H), 7.32 – 7.21 (m, 2H), 7.01 (dd, J = 8.1, 1.0 Hz, 1H), 3.91 (s, 3H). 13C NMR (126 MHz, DMSO) δ 163.0, 159.8, 156.8, 152.4, 138.8, 133.3, 132.8, 125.4, 119.3, 119.2, 114.0, 112.4, 108.1, 105.5, 56.2. Melting Point: 147.6 – 149.1 °C.
4-Chloro-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (18).
Starting with 4-methoxy-2-amino benzothiazole and 4-chloro benzoyl chloride. Yield: 81%, white solid; 1H NMR (500 MHz, DMSO) δ 8.15 (d, J = 8.6 Hz, 2H), 7.62 (d, J = 8.6 Hz, 2H), 7.54 (dd, J = 8.0, 0.9 Hz, 1H), 7.27 (t, J = 8.0 Hz, 1H), 7.01 (dd, J = 8.1, 1.0 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO) δ 165.2, 152.4, 138.3, 133.4, 131.2, 131.1, 130.7, 129.3, 125.2, 120.8, 113.9, 108.0, 56.1.
3,5-Dichloro-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (19).
Starting with 4-methoxy-2-amino benzothiazole and 3,5-dichloro benzoyl chloride. Yield: 85%, white solid; 1H NMR (500 MHz, DMSO) δ 8.15 (s, 2H), 7.90 (s, 1H), 7.54 (dd, J = 8.0, 0.9 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.01 (d, J = 8.1 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO) δ 165.4, 157.7, 152.3, 135.7, 135.0, 133.2, 132.5, 128.3, 127.6, 125.4, 114.0, 108.1, 56.2.
4-Bromo-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (20).
Starting with 4-methoxy-2-amino benzothiazole and 4-bromo benzoyl chloride. Yield: 71%, white solid; 1H NMR (500 MHz, DMSO) δ 8.07 (d, J = 8.6 Hz, 2H), 7.75 (d, J = 8.6 Hz, 2H), 7.54 (dd, J = 7.9, 1.0 Hz, 1H), 7.27 (t, J = 8.0 Hz, 1H), 7.00 (dd, J = 8.1, 1.0 Hz, 1H), 3.91 (s, 3H). 13C NMR (126 MHz, DMSO) δ 165.4, 157.6, 152.4, 133.4, 132.2, 131.5, 130.8, 129.3, 127.3, 125.2, 113.9, 108.0, 56.1. Melting Point: 225.5 – 226.9 °C.
2-Iodo-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (21).
Starting with 4-methoxy-2-amino benzothiazole and 2-iodo benzoyl chloride. Yield: 75%, white solid; 1H NMR (500 MHz, DMSO) δ 7.95 (dd, J = 7.9, 1.1 Hz, 1H), 7.60 – 7.46 (m, 3H), 7.31 – 7.22 (m, 2H), 7.00 (dd, J = 8.1, 1.0 Hz, 1H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 168.4, 156.8, 152.5, 140.6, 139.8, 133.4, 132.4, 129.2, 128.6, 128.6, 125.3, 114.0, 108.1, 94.2, 56.2. Melting Point: 159.0 – 161.9 °C.
4-Iodo-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (22).
Starting with 4-methoxy-2-amino benzothiazole and 4-iodo benzoyl chloride. Yield: 88%, white solid; 1H NMR (500 MHz, DMSO) δ 7.94 – 7.91 (m, 2H), 7.91 – 7.88 (m, 2H), 7.67 (d, J = 8.4 Hz, 1H), 7.54 (dd, J = 7.9, 0.9 Hz, 1H), 7.27 (t, J = 8.0 Hz, 1H), 7.00 (dd, J = 8.0, 0.9 Hz, 1H), 3.91 (s, 3H). 13C NMR (126 MHz, DMSO) δ 167.4, 165.7, 157.6, 152.3, 138.1, 133.3, 131.5, 130.5, 125.2, 113.9, 108.0, 101.7, 56.1. Melting Point: 210.2 – 211.0 °C.
3-Trifluoromethyl-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (23).
Starting with 4-methoxy-2-amino benzothiazole and 3-trifluoromethyl benzoyl chloride. Yield: 67%, white solid; 1H NMR (500 MHz, CDCl3) δ 8.23 (s, 1H), 8.09 (d, J = 8.1 Hz, 1H), 7.74 (dd, J = 7.8, 1.7 Hz, 1H), 7.51 – 7.39 (m, 2H), 7.27 (d, J = 8.0 Hz, 1H), 6.69 (d, J = 8.0 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 164.8, 159.0, 151.4, 137.4, 133.1, 131.5, 131.1, 129.2, 125.4, 125.1, 124.4, 122.3, 113.5, 106.6, 55.0. Melting Point: 166.3 – 168.0 °C.
4-Trifluoromethyl-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (24).
Starting with 4-methoxy-2-amino benzothiazole and 4-trifluoromethyl benzoyl chloride. Yield: 73%, white solid; 1H NMR (500 MHz, DMSO) δ 8.31 (d, J = 8.0 Hz, 2H), 7.92 (d, J = 7.9 Hz, 2H), 7.56 (dd, J = 8.0, 0.9 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 7.02 (dd, J = 8.1, 1.0 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO) δ 172.5, 165.2, 152.4, 136.2, 133.3, 132.9, 129.7, 126.1, 125.4, 123.2, 114.0, 108.0, 56.2.
4-Cyano-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (25).
Starting with 4-methoxy-2-amino benzothiazole and 4-cyano benzoyl chloride. Yield: 72%, pale yellow solid; 1H NMR (500 MHz, DMSO) δ 8.26 (d, J = 8.0 Hz, 2H), 8.02 (d, J = 8.0 Hz, 2H), 7.55 (d, J = 7.9 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.01 (d, J = 8.0 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO) δ 165.0, 157.7, 152.3, 136.5, 133.3, 133.1, 130.4, 129.5, 125.3, 118.6, 115.4, 114.0, 108.0, 56.2. Melting Point: 223.3 – 224.1 °C.
2-Nitro-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (26).
Starting with 4-methoxy-2-amino benzothiazole and 2-nitro benzoyl chloride. Yield: 61%, off-white solid; 1H NMR (500 MHz, DMSO) δ 8.19 (dd, J = 8.2, 1.2 Hz, 1H), 7.90 – 7.78 (m, 3H), 7.57 (dd, J = 8.0, 1.0 Hz, 1H), 7.29 (t, J = 8.0 Hz, 1H), 7.01 (dd, J = 8.1, 1.0 Hz, 1H), 3.90 (s, 3H). 13C NMR (126 MHz, DMSO) δ 165.5, 156.7, 152.5, 146.9, 134.8, 133.4, 132.4, 130.6, 130.2, 125.4, 125.0, 125.0, 114.1, 108.3, 56.3. Melting Point: 233.7–235.3 °C.
3-Nitro-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (27).
Starting with 4-methoxy-2-amino benzothiazole and 3-nitro benzoyl chloride. Yield: 64%, white solid; 1H NMR (500 MHz, DMSO) δ 8.97 (s, 1H), 8.57 – 8.41 (m, 2H), 7.82 (t, J = 7.9 Hz, 1H), 7.53 (d, J = 7.9 Hz, 1H), 7.27 (t, J = 7.9 Hz, 1H), 7.00 (d, J = 8.0 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO) δ 164.5, 157.9, 152.2, 148.3, 135.0, 134.0, 133.3, 130.8, 127.6, 125.3, 123.7, 114.0, 110.1, 108.1, 56.2. Melting Point: 244.3 – 246.0 °C.
4-Nitro-N-(4-methoxybenzo[d]thiazol-2-yl)benzamide (28).
Starting with 4-methoxy-2-amino benzothiazole and 4-nitro benzoyl chloride. Yield: 78%, canary yellow solid; 1H NMR (500 MHz, DMSO) δ 8.33 (s, 4H), 7.55 (d, J = 8.0 Hz, 1H), 7.28 (t, J = 7.8 Hz, 1H), 7.01 (d, J = 7.8 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, DMSO) δ 164.8, 152.3, 150.2, 138.0, 133.3, 130.3, 129.3, 125.4, 124.1, 118.6, 114.0, 108.1, 56.2. Melting Point: 243.5 – 244.3 °C.
2-Fluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (29).
Starting with 4-methoxy-2-amino benzothiazole and 2-fluorophenyl sulfonyl chloride. Yield: 63%, brown solid; 1H NMR (500 MHz, CDCl3) δ 9.76 (s, 1H), 8.04 – 8.02 (m, 1H), 7.55 – 7.48 (m, 1H), 7.27 – 7.25 (m, 1H), 7.21 (t, J = 8.1 Hz, 1H), 7.17 – 7.09 (m, 2H), 6.87 (dd, J = 8.2, 0.9 Hz, 1H), 3.94 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.9, 160.5, 158.4, 146.0, 134.5, 129.5, 125.1, 124.1, 117.0, 116.8, 114.0, 108.3, 56.0. Melting Point: 195.3 – 197.1 °C.
4-Fluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (30).
Starting with 4-methoxy-2-amino benzothiazole and 4-fluoro phenylsulfonyl chloride. Yield: 74%, beige colored solid; 1H NMR (500 MHz, CDCl3) δ 8.03 – 7.94 (m, 2H), 7.18 (t, J = 8.1 Hz, 1H), 7.14 – 7.05 (m, 3H), 6.84 (d, J = 8.1 Hz, 1H), 3.91 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.1, 166.0, 163.9, 146.0, 137.9, 129.2, 129.2, 125.3, 125.1, 116.0, 115.8, 114.0, 108.4, 56.0. Melting Point: 166.1 – 167.4 °C.
2,4-Difluoro-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (31).
Starting with 4-methoxy-2-amino benzothiazole and 2,4-difluorophenyl sulfonyl chloride. Yield: 51%, beige-colored solid; 1H NMR (500 MHz, CDCl3) δ 9.77 (s, 1H), 8.09 – 7.99 (m, 1H), 7.21 (t, J = 8.1 Hz, 1H), 7.12 (dd, J = 8.1, 0.9 Hz, 1H), 6.96 (m, 1H), 6.91 – 6.83 (m, 2H), 3.94 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.9, 166.5, 164.5, 161.1, 159.2, 146.0, 131.2, 125.2, 125.1, 114.0, 108.3, 105.7, 105.5, 56.0. Melting Point: 188.2 – 190.2 °C.
4-Chloro-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (32).
Starting with 4-methoxy-2-amino benzothiazole and 4-chlorophenyl sulfonyl chloride. Yield: 64%, off-white solid; 1H NMR (500 MHz, DMSO) δ 7.83 (d, J = 8.7 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 7.34 (d, J = 8.0 Hz, 1H), 7.20 (t, J = 8.1 Hz, 1H), 7.02 (d, J = 8.1 Hz, 1H), 3.86 (s, 3H). 13C NMR (126 MHz, DMSO) δ 167.67, 146.91, 141.53, 137.51, 129.67, 128.23, 126.44, 125.07, 114.81, 109.60, 56.56. Melting Point: 153.9 – 155.3 °C.
3,5-Dichloro-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (33).
Starting with 4-methoxy-2-amino benzothiazole and 3.5-dichlorophenyl sulfonyl chloride. Yield: 75%, white solid; 1H NMR (500 MHz, CDCl3) δ 9.94 (s, 1H), 7.81 (d, J = 1.9 Hz, 2H), 7.43 (t, J = 1.9 Hz, 1H), 7.19 (d, J = 8.1 Hz, 1H), 7.10 (dd, J = 8.1, 0.9 Hz, 1H), 6.85 (d, J = 8.2 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.6, 146.1, 144.6, 135.6, 132.2, 125.4, 125.2, 125.2, 124.9, 114.0, 108.5, 56.1. Melting Point: 160.7 – 162.3 °C.
4-Bromo-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (34).
Starting with 4-methoxy-2-amino benzothiazole and 4-bromophenyl sulfonyl chloride. Yield: 79%. pale yellow solid; 1H NMR (500 MHz, CDCl3) δ 7.83 (d, J = 8.6 Hz, 2H), 7.58 (d, J = 8.6 Hz, 2H), 7.19 (t, J = 8.1 Hz, 1H), 7.10 (dd, J = 8.1, 0.9 Hz, 1H), 6.85 (dd, J = 8.2, 0.9 Hz, 1H), 3.92 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.2, 146.0, 140.8, 132.0, 128.1, 127.2, 125.3, 125.2, 125.1, 114.0, 108.4, 56.0. Melting Point: 175.6 – 177.1 °C.
2-Iodo-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (35).
Starting with 4-methoxy-2-amino benzothiazole and 2-iodo phenylsulfonyl chloride. Yield: 75%, white solid; 1H NMR (500 MHz, CDCl3) δ 9.68 (s, 1H), 8.31 (dd, J = 7.9, 1.7 Hz, 1H), 8.04 (dd, J = 7.8, 1.2 Hz, 1H), 7.48 (td, J = 7.6, 1.2 Hz, 1H), 7.22 – 7.11 (m, 3H), 6.87 (dd, J = 8.1, 0.9 Hz, 1H), 3.95 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 168.0, 145.9, 143.8, 142.3, 134.6, 133.0, 130.1, 128.1, 125.5, 125.1, 114.0, 108.3, 92.8, 56.0. HRMS m/z: calcd for C14H11IN2O3S2, 445.9256; found, 445.9351, M + H+. Melting Point: 224.9.0 – 226.5 °C.
4-Iodo-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (36).
Starting with 4-methoxy-2-amino benzothiazole and 4-iodophenyl sulfonyl chloride. Yield: 71%, beige colored solid; 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 8.6 Hz, 2H), 7.69 (d, J = 8.6 Hz, 2H), 7.19 (d, J = 8.1 Hz, 1H), 7.11 (dd, J = 8.1, 0.9 Hz, 1H), 6.86 (dd, J = 8.2, 0.9 Hz, 1H), 3.93 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.2, 145.9, 141.4, 138.0, 128.0, 125.3, 125.2, 125.1, 114.1, 108.4, 99.6, 56.0. Melting Point: 206.4 – 208.0 °C.
3-Trifluoromethyl-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (37).
Starting with 4-methoxy-2-amino benzothiazole and 3-trifluoromethylphenyl sulfonyl chloride. Yield: 68%, beige-colored solid; 1H NMR (500 MHz, CDCl3) δ 9.86 (s, 1H), 8.23 (d, J = 1.7 Hz, 1H), 8.17 (d, J = 7.9, 1H), 7.76 (dd, J = 7.7, 1.7 Hz, 1H), 7.59 (t, J = 7.9 Hz, 1H), 7.19 (t, J = 8.1 Hz, 1H), 7.10 (dd, J = 8.0, 0.9 Hz, 1H), 6.85 (d, J = 8.1 Hz, 1H), 3.91 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.5, 146.1, 142.9, 129.6, 129.0, 128.9, 125.3, 125.2, 125.2, 123.6, 123.6, 114.0, 108.5, 56.0. Melting Point: 156.3 – 158.0 °C.
4-Trifluoromethyl-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (38).
Starting with 4-methoxy-2-amino benzothiazole and 4-trifluoromethylphenyl sulfonyl chloride. Yield: 64%, beige-colored solid, 1H NMR (500 MHz, CDCl3) δ 9.75 (s, 1H), 8.10 (d, J = 8.1 Hz, 2H), 7.71 (d, J = 8.1, 2H), 7.21 (d, J = 8.1 Hz, 1H), 7.12 (dd, J = 8.1, 0.9 Hz, 1H), 6.86 (dd, J = 8.2, 0.9 Hz, 1H), 3.93 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.5, 146.1, 145.2, 134.1, 133.9, 127.0, 126.0, 125.9, 125.3, 114.1, 108.4, 56.0. Melting Point: 154.2 – 156.4 °C.
4-Cyano-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (39).
Starting with 4-methoxy-2-amino benzothiazole and 4-cyanophenyl sulfonyl chloride. Yield: 69%, brown solid. 1H NMR (500 MHz, DMSO) δ 8.12 – 7.92 (m, 4H), 7.35 (d, J = 7.9 Hz, 1H), 7.20 (t, J = 8.1 Hz, 1H), 7.02 (d, J = 8.2 Hz, 1H), 3.86 (s, 3H). 13C NMR (126 MHz, DMSO) δ 168.01, 147.11, 146.74, 133.79, 127.04, 125.15, 118.31, 115.05, 114.80, 110.96, 109.61, 56.57. Melting Point: 184.5 – 185.6 °C.
2-Nitro-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (40).
Starting with 4-methoxy-2-amino benzothiazole and 2-nitrophenyl sulfonyl chloride. Yield: 71%; brown solid; 1H NMR (500 MHz, CDCl3) δ 9.88 (brs, 1H), 8.32 – 8.23 (m, 1H), 7.73 – 7.63 (m, 3H), 7.22 (t, J = 8.1 Hz, 1H), 7.15 – 7.08 (m, 1H), 6.87 (dd, J = 8.1, 1.0 Hz, 1H), 3.94 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.9, 147.6, 146.1, 135.3, 133.2, 132.1, 130.3, 125.5, 125.4, 125.0, 124.3, 114.0, 108.4, 56.0. Melting Point: 201.6 – 203.1 °C.
3-Nitro-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (41).
Starting with 4-methoxy-2-amino benzothiazole and 3-nitrophenyl sulfonyl chloride. Yield: 59%, beige-colored solid; 1H NMR (500 MHz, CDCl3) δ 9.79 (s, 1H), 8.78 (t, J = 2.0 Hz, 1H), 8.38 – 8.33 (m, 1H), 8.32 – 8.29 (m, 1H), 7.68 (t, J = 8.0 Hz, 1H), 7.22 (t, J = 8.1 Hz, 1H), 7.13 (dd, J = 8.1, 0.9 Hz, 1H), 6.87 (dd, J = 8.1, 0.9 Hz, 1H), 3.94 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.7, 148.1, 146.1, 143.8, 132.2, 130.2, 126.8, 125.5, 125.2, 125.1, 121.8, 114.1, 108.5, 56.1. Melting Point: 166.3 – 167.8 °C.
4-Nitro-N-(4-methoxybenzo[d]thiazol-2-yl)benzenesulfonamide (42).
Starting with 4-methoxy-2-amino benzothiazole and 4-nitrophenyl sulfonyl chloride. Yield: 67%, pale yellow solid; 1H NMR (500 MHz, CDCl3) δ 8.28 (d, J = 8.9 Hz, 2H), 8.15 (d, J = 8.9 Hz, 2H), 7.22 (t, J = 8.1 Hz, 1H), 7.13 (dd, J = 8.0, 0.9 Hz, 1H), 6.87 (dd, J = 8.2, 0.9 Hz, 1H), 3.93 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 167.7, 149.8, 147.4, 146.2, 127.8, 125.5, 125.3, 125.2, 124.1, 114.1, 108.5, 56.1. HRMS m/z: calcd for C14H11N3O5S2, 365.0140; found, 365.0229, M + H+. Melting Point: 170.4 – 172.1 °C.
N-(1H-Indol-4-yl)-2-iodobenzene sulfonamide (49).
Starting with 4-amino indole and 2-iodophenyl sulfonyl chloride. Yield: 73%, grey solid; 1H NMR (500 MHz, DMSO) δ 11.09 (s, 1H), 10.25 (s, 1H), 8.06 (d, J = 7.7 Hz, 1H), 7.95 (d, J = 8.2 Hz, 1H), 7.45 (t, J = 7.5 Hz, 1H), 7.29 – 7.15 (m, 2H), 7.09 (d, J = 8.0 Hz, 1H), 6.88 (t, J = 7.8 Hz, 1H), 6.77 (d, J = 7.7 Hz, 1H), 6.74 (t, J = 2.6 Hz, 1H). 13C NMR (126 MHz, DMSO) δ 143.1, 142.7, 137.3, 134.1, 131.1, 129.1, 128.8, 125.0, 122.2, 121.5, 111.2, 108.9, 100.2, 93.8. Melting Point: 158.6 – 160.4 °C.
N-(1H-Indol-5-yl)-2-iodobenzene sulfonamide (50).
Starting with 5-amino indole and 2-iodophenyl sulfonyl chloride. Yield: 86%, brown solid; 1H NMR (500 MHz, CDCl3) δ 8.16 (s, 1H), 8.05 (dd, J = 7.9, 1.2 Hz, 1H), 7.93 (dd, J = 7.9, 1.6 Hz, 1H), 7.41 (d, J = 2.1 Hz, 1H), 7.32 – 7.27 (m, 1H), 7.26 – 7.19 (m, 2H), 7.18 (dd, J = 3.2, 2.5 Hz, 1H), 7.14 – 7.09 (m, 1H), 6.98 (dd, J = 8.6, 2.1 Hz, 1H), 6.48 – 6.43 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 142.0, 141.2, 134.4, 133.5, 132.0, 128.5, 128.1, 127.7, 125.5, 119.3, 116.7, 111.5, 102.9, 92.4. Melting Point: 163.2 – 164.4 °C.
N-(1H-Indol-6-yl)-2-iodobenzene sulfonamide (51).
Starting with 6-amino indole and 2-iodophenyl sulfonyl chloride. 78 %, reddish brown solid; 1H NMR (500 MHz, DMSO) δ 10.98 (s, 1H), 10.23 (s, 1H), 8.07 (dd, J = 7.8, 1.2 Hz, 1H), 7.97 (dd, J = 7.9, 1.6 Hz, 1H), 7.53 – 7.44 (m, 1H), 7.36 (d, J = 8.4 Hz, 1H), 7.24 – 7.17 (m, 3H), 6.82 (dd, J = 8.5, 2.0 Hz, 1H), 6.30 (s, 1H). 13C NMR (126 MHz, DMSO) δ 143.0, 142.4, 136.3, 134.2, 131.2, 131.2, 128.9, 125.9, 125.2, 120.8, 114.2, 104.3, 101.4, 93.7.
N-(1H-Indol-4-yl)-4-nitrobenzene sulfonamide (52).
Starting with 4-amino indole and 4-nitrophenyl sulfonyl chloride. Yield: 83%, yellow solid; 1H NMR (500 MHz, DMSO) δ 11.11 (s, 1H), 10.35 (s, 1H), 8.29 (d, J = 8.8 Hz, 2H), 7.95 (d, J = 8.8 Hz, 2H), 7.21 – 7.13 (m, 2H), 6.95 (t, J = 7.9 Hz, 1H), 6.82 (dd, J = 7.6, 0.9 Hz, 1H), 6.49 – 6.43 (m, 1H). 13C NMR (126 MHz, DMSO) δ 150.1, 146.4, 137.3, 128.8, 128.4, 125.5, 124.8, 123.1, 121.6, 113.4, 109.8, 99.6. Melting Point: 185.0 – 186.3 °C.
N-(1H-Indol-5-yl)-4-nitrobenzene sulfonamide (53).
Starting with 5-amino indole and 4-nitrophenyl sulfonyl chloride. Yield: 77%, yellow solid; 1H NMR (500 MHz, DMSO) δ 11.07 (s, 1H), 10.15 (s, 1H), 8.31 (d, J = 8.9 Hz, 2H), 7.90 (d, J = 8.9 Hz, 2H), 7.30 (t, J = 2.8 Hz, 1H), 7.25 (dd, J = 5.4, 3.2 Hz, 2H), 6.80 (dd, J = 8.7, 2.0 Hz, 1H), 6.37 – 6.30 (m, 1H). 13C NMR (126 MHz, DMSO) δ 150.1, 145.7, 134.4, 128.8, 128.5, 128.2, 126.9, 124.9, 117.9, 115.0, 112.3, 101.7. Melting Point: 142.1 – 144.5 °C.
N-(1H-Indol-6-yl)-4-nitrobenzene sulfonamide (54).
Starting with 6-amino indole and 4-nitrophenyl sulfonyl chloride. Yield: 76%, yellow solid; 1H NMR (500 MHz, DMSO) δ 11.01 (s, 1H), 10.27 (s, 1H), 8.32 (d, J = 8.9 Hz, 2H), 7.92 (d, J = 8.9 Hz, 2H), 7.36 (d, J = 8.4 Hz, 1H), 7.26 (t, J = 2.7 Hz, 1H), 7.19 – 7.13 (m, 1H), 6.72 (dd, J = 8.4, 1.9 Hz, 1H), 6.34 – 6.28 (m, 1H). 13C NMR (126 MHz, DMSO) δ 150.1, 145.6, 136.2, 130.7, 128.8, 126.3, 125.9, 124.9, 120.9, 115.1, 105.7, 101.4. Melting Point: 193.7 – 195.2 °C.
4.3. Biophysical experiments
4.3.1. Chemical and protein source
Thioflavin-T (ThT) were purchased from Alfa Aesar (Ward Hill, MA) for the α-syn ThT assays. Heparin sodium salt was purchased from Millipore-Sigma. Procuration of recombinant α-syn and tau 2N4R was from rPeptide (WatKinsville, GA). Concerning the preparation of tau 0N4R, a bacterial expression plasmid consisting of the vector pET30a carrying a cDNA encoding the human Tau 0N4R isoform was a kind gift of Dr. Benjamin Wolozin (Boston University). E. coli stock (Rosetta BL21 Ecoli (CamR) containing pET30a[0N4R tau wt] (KanR)) were grown in LB media supplemented with kanamycin (50 μg/mL) and chloramphenicol (50 μg/mL). Protein over-expression was induced by the addition of 1 mM IPTG for ~ 18 h at 37 °C, and cells were pelleted by centrifugation at 6,000 g for 15 min at 4 °C. The cells were resuspended in lysis buffer (10 mM Hepes pH 7.4, 50 mM NaCl, 1 mM MgCl2, 1 mM PMSF, 1X PIC, 0.5 mM DTT) and lysed by sonication at 30 sec On 1 min Off at ~ 30–45 % power for ~ 3–5 min. Lysate was centrifuged at 10000 rpm for 10 min at 4 °C and supernatant was transferred with 7.8 mL (total) of 3 M NaCl. The lysate was incubated for 10 min in 80 °C water bath, then cooled for 10 min in an ice bath. The lysate was centrifuge at 10000 rpm, 4 °C for 10 min and supernatant was transferred to new tubes. The supernatant was dialyzed overnight against cation exchange buffer (50 mM MES, 1 M NaCl, 1 mM DTT, pH 6.0). The dialysate was loaded onto a HiPrep SP HP column, and proteins were eluted with a linear gradient ranging from 50 mM to 1 M NaCl. Fractions containing tau isoform 0N4R were pooled, and the resulting protein solution was dialyzed against PBS (pH 7.4) and stored at − 80 °C. Similar procedure and plasmid vector construction were used to produce tau isoform 2N4R for the PICUP assays.
4.3.2. Thioflavin t (ThT) fluorescence assays
A technique commonly used to track the kinetics of α-syn fibril formation in response to various drug candidate treatments is the ThT fluorescence assay.46,47 The final concentration of the tested compounds was 100 μM while ThT was applied at a final concentration of 20 μM. The recombinant α-syn has been validated with proper quality control to confirm the monomeric state of the protein. α-Syn was dissolved in 20 mM Tris–HCl (pH 7.4) to a stock solution of 280 μM prior to resuspension in ThT buffer to obtain a final concentration of 2 μM (in each well). Compounds and ThT were first added to the wells. The kinetics of fibril formation begin when the α-syn is solubilized in the ThT buffer (10 mM PBS buffer (pH 7.4), supplemented with 0.5 mM SDS and 300 mM NaCl) and added to a non-treated black 96 well microplate with a transparent flat bottom. Each well was filled with a maximum volume of 150 μL buffer with one 3 mm borosilicate bead.48 The background fluorescence signal consisted of ThT in buffer and 0.25 % DMSO without α-syn. The excitation and emission wavelengths consisted of 440 and 485 nm, respectively. Measurements were acquired with Synergy HT multi-mode microplate reader (BioTek, Winooski, VT) and taken at 37 °C every 20 min with 10 sec shaking before reading the plate. Kinetics were of 40 to 70 h duration. Samples were measured in three replicates. For each time point, arbitrary units of fluorescence were calculated from the mean values normalized against the maximum value. The percentage of fluorescence intensity at the plateau phase in Tables 1–2 was expressed as mean ± SEM. Concerning the dose–response curve depicted in Figure 2, the data were plotted using GraphPad Prism.
4.3.3. Photo-induced cross-linking of unmodified proteins (PICUP) assay
α-Syn (resourced from rPeptide), 2N4R (produced in the lab), and 0N4R (produced in the lab) tau isoforms were diluted in 10 mM phosphate buffer to achieve final concentrations of 60 μM for α-syn and 10 μM for the tau isoforms.49 Compounds were tested at different concentration. Before light exposure, Ru(bpy) (300 μM final concentration) and ammonium persulfate (6 mM final concentration) were added to the protein solution in the presence or absence of compound to start the cross-linking process. In order to verify the progressive impact of our compounds on the suppression of protein oligomerization, controls unexposed to light or exempt of Ru(bpy) were used. The samples underwent quick light exposure using a 53 W (120 V) incandescent bulb in a homemade dark box. Light exposures consisted of 1 s and 60 s for the α-syn and tau isoforms, respectively. The ultimate capacity of each tube was 20 μL. 8.3 μL of Laemmli loading buffer containing 15% of 2-mercaptoethanol was added to the solution right away after light exposure, and the PICUP samples were then incubated at 95 °C for 10 min. On a 16% SDS-PAGE gel, the cross-linked samples were separated and Coomassie blue staining was used for protein band visualization.
4.3.4. Transmission electron microscopy
Tau isoform 2N4R (6 μM, resourced from rPeptide) mixed with DMSO (0.25 %) was incubated in a 10 mM PBS buffer (pH 7.4) for 72 h at 37 °C. α-Syn samples (at 2 μM) recovered from kinetic investigations of ThT fibril formation were also examined. A volume of 10 μL of each sample was put to a 400-mesh Formvar-carbon-coated copper grid (Electron Microscopy Sciences, Hatfield, PA) to prepare the grids for TEM analysis. The sample was incubated with the grids for 1 min before being rinsed three times with distilled water. A new solution of 1 % uranyl acetate was then applied for 1 min after being air-dried. The filter paper was used to absorb the solution, then grids were dried in the air. A transmission electron microscope (JEOL 1400 Flash, Japan) was used to analyze the grids, and micrographs were taken at a magnification of 40 k and an acceleration voltage of 100 kV.
4.4. α-Synuclein inclusion-forming neuroblastoma cell experiment
M17D-TR/αS-3 K::YFP neuroblastoma cells that are doxycycline (dox)-induced have been previously used for this assay.50 Compounds were introduced 24 h after the cells had been plated in 96-well plates at a density of 30,000 cells per well. αS-3 K::YFP transgene expression was stimulated 24 h later by adding culture medium dox at a final concentration of 1 μg per mL. The Incucyte Zoom 2000 platform (Essen Biosciences) was used to incubate the cells while taking continuous green bright field pictures. After 48 h of induction (96 h after plating), inclusion formation or growth endpoint analysis was carried out. The Incucyte processing definition “Inclusions” was developed as follows: parameters, fixed threshold, threshold (GCU) 50; edge split on; edge sensitivity 100; cleanup; hole fill (m2): 10; adjust size (pixels); filters; area (m2): max 50; mean intensity: min 60; integrated intensity: min 2000. The following processing definition of “Cells” was used to measure cell confluence: parameters, segmentation adjustment 0.7; Cleanup, all parameters are set to 0; filters, area (m2): min 345.00. A polyclonal antibody to GAPDH (Sigma-Aldrich, St. Louis, MO, G9545; 1:5000) and an S-specific monoclonal antibody 4B12 (Thermofisher, Waltham, MA; 1:1000) were used, as previously described, for the evaluation of protein expression by SDS-PAGE and Western blotting in the LiCor system.51.
5. Associated content
Additional data pertaining to the characterization of compounds (NMR, HRMS, IR) and additional biophysical (ThT, PICUP) and cell-based results are provided in Supporting Information.
Supplementary Material
Acknowledgement
The authors would like to acknowledge the professional services of Alicia Withrow at the Center for Advanced Microscopy at Michigan State University and the technical expertise of Eduardo Ramirez for PICUP experiments. We acknowledge the technical effort of Naseem Alfadhl for acquiring the IR spectra. We would like to thank Bejamin Wolozin (Boston University) for providing the constructs to produce tau isoforms 0N4R and 2N4R. J.S.F. support was provided by the National Institutes of Health (NIH) grants (AG070447, AG071985). U.D. was supported by NIH grants NS121826 and NS099328.
Abbreviations:
- Aβ
amyloid-β
- AD
Alzheimer’s disease
- AI
aminoindole
- DCM
dicloromethane
- dox
doxycycline
- EWG
electron-withdrawing group
- DLB
dementia with Lewy bodies
- FTDP-17
frontotemporal dementia with Parkinsonism
- MAPT
microtubule-associated protein tau
- 4-MBT
2-amino-4-methoxy benzothiazole
- NFTs
neurofibrillary tangles
- PD
Parkinson’s disease
- PICUP
photo-induced cross-linking of unmodified protein
- PSP
progressive supranuclear palsy
- RPD
relative pixel density
- SEM
standard error of the mean
- α-syn
α-synuclein
- ThT
thioflavin-T
- TEM
transmission electron microscopy
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Ahmed A. Elbatrawy: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Taiwo A. Ademoye: Writing – review & editing, Validation, Investigation, Formal analysis, Data curation. Heba Alnakhala: Writing – review & editing, Validation, Investigation, Formal analysis, Data curation. Arati Tripathi: Writing – review & editing, Validation, Supervision, Investigation, Formal analysis, Data curation. Ashique Zami: Writing – review & editing, Validation, Supervision, Data curation. Raluca Ostafe: Writing – review & editing, Validation, Supervision, Methodology, Formal analysis, Data curation. Ulf Dettmer: Writing – review & editing, Validation, Supervision, Methodology, Funding acquisition, Formal analysis, Data curation. Jessica S. Fortin: Writing – review & editing, Validation, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmc.2024.117613.
Data availability
No data was used for the research described in the article.
References
- 1.Association A, et al. 2010 Alzheimer’s disease facts and figures. Alzheimers Dement. 2010;6:158–194. 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Saez-Atienzar S, Masliah E. Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nat Rev Neurosci. 2020;21:433–444. 10.1038/s41583-020-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava S, Ahmad R, Khare SK. Alzheimer’s disease and its treatment by different approaches: A review. Eur J Med Chem. 2021:216. 10.1016/j.ejmech.2021.113320. [DOI] [PubMed] [Google Scholar]
- 4.Elkouzi A, Vedam-Mai V, Eisinger RS, Okun MS. Emerging therapies in Parkinson disease — repurposed drugs and new approaches. Nat Rev Neurol. 2019;15:204–223. 10.1038/s41582-019-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fil A, Cano-de-la-Cuerda R, Muñoz-Hellín E, Vela L, Ramiro-González M, Fernández-de-las-Peñas C. Pain in parkinson disease: A review of the literature. Parkinsonism Relat Disord. 2013;19:285–294. 10.1016/j.parkreldis.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Påhlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in Parkinson disease - Epidemiology, mechanisms and management. Nat Rev Neurol. 2012;8:35–47. 10.1038/nrneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 7.Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease—an evidence-based medicine review. Mov Disord. 2019;34:180–198. 10.1002/mds.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy J, Selkoe DJ. The amyloid hypothesis of alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297:353–356. [DOI] [PubMed] [Google Scholar]
- 9.Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: Time, space and “wingmen”. Nat Neurosci. 2015;18:800–806. 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8:595–608. 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancet T Lecanemab for Alzheimer’s disease: tempering hype and hope. Lancet. 2022;400:1899. 10.1016/S0140-6736(22)02480-1. [DOI] [PubMed] [Google Scholar]
- 12.van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early alzheimer’s disease. N Engl J Med. 2023;388:9–21. 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 13.Perneczky R, Jessen F, Grimmer T, et al. Anti-amyloid antibody therapies in Alzheimer’s disease. Brain. 2023;146:842–849. 10.1093/brain/awad005. [DOI] [PubMed] [Google Scholar]
- 14.Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: appropriate use recommendations. J Prev Alzheimers Dis. 2023;10:362–377. 10.14283/jpad.2023.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahase E Lecanemab trial finds slight slowing of cognitive decline, but clinical benefits are uncertain. BMJ. 2022;379, o2912. 10.1136/bmj.o2912. [DOI] [PubMed] [Google Scholar]
- 16.Giacobini E, Gold G. Alzheimer disease therapy - Moving from amyloid-β to tau. Nat Rev Neurol. 2013;9:677–686. 10.1038/nrneurol.2013.223. [DOI] [PubMed] [Google Scholar]
- 17.Medeiros R, Baglietto-Vargas D, Laferla FM. The role of tau in alzheimer’s disease and related disorders. CNS Neurosci Ther. 2011;17:514–524. 10.1111/j.1755-5949.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elbatrawy AA, Hyeon SJ, Yue N, et al. “turn-On” quinoline-based fluorescent probe for selective imaging of tau aggregates in alzheimer’s disease: rational design, synthesis, and molecular docking. ACS Sens. 2021;6:2281–2289. 10.1021/acssensors.1c00338. [DOI] [PubMed] [Google Scholar]
- 19.Gao Y, Tan L, Yu JT, Tan L. Tau in alzheimer’s disease: Mechanisms and therapeutic strategies. Curr Alzheimer Res. 2018;15:283–300. 10.2174/1567205014666170417111859. [DOI] [PubMed] [Google Scholar]
- 20.Mok SA, Condello C, Freilich R, et al. Mapping interactions with the chaperone network reveals factors that protect against tau aggregation. Nat Struct Mol Biol. 2018;25:384–393. 10.1038/s41594-018-0057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waheed Z, Choudhary J, Jatala FH, et al. The role of tau proteoforms in health and disease. Mol Neurobiol. 2023;60:5155–5166. 10.1007/s12035-023-03387-8. [DOI] [PubMed] [Google Scholar]
- 22.Chirita C, Necula M, Kuret J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry. 2004;43:2879–2887. 10.1021/bi036094h. [DOI] [PubMed] [Google Scholar]
- 23.Necula M, Chirita CN, Kuret J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: Implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry. 2005;44:10227–10237. 10.1021/bi050387o. [DOI] [PubMed] [Google Scholar]
- 24.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in lewy bodies. Nature. 1997;388:839–840. 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 25.Zeuner KE, Schäffer E, Hopfner F, Brüggemann N, Berg D. Progress of pharmacological approaches in parkinson’s disease. Clin Pharmacol Ther. 2019;105:1106–1120. 10.1002/cpt.1374. [DOI] [PubMed] [Google Scholar]
- 26.Breydo L, Wu JW, Uversky VN. α-Synuclein misfolding and Parkinson’s disease. Biochim Biophys Acta Mol basis Dis. 2012;1822:261–285. 10.1016/j.bbadis.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Henderson MX, Trojanowski JQ, Lee VMY. α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci Lett. 2019:709. 10.1016/j.neulet.2019.134316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rüb U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson’s disease (preclinical and clinical stages). J Neurol. 2002;249. 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Del TK, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 30.Wagner J, Ryazanov S, Leonov A, et al. Anle138b: A novel oligomer modulator for disease-modifying therapy of neurodegenerative diseases such as prion and Parkinson’s disease. Acta Neuropathol. 2013;125:795–813. 10.1007/s00401-013-1114-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deeg AA, Reiner AM, Schmidt F, et al. Anle138b and related compounds are aggregation specific fluorescence markers and reveal high affinity binding to α-synuclein aggregates. Biochim Biophys Acta Gen Subj. 2015;1850:1884–1890. 10.1016/j.bbagen.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 32.Dasari AKR, Kayed R, Wi S, Lim KH. Tau interacts with the C-terminal region of α-synuclein, promoting formation of toxic aggregates with distinct molecular conformations. Biochemistry. 2019;58:2814–2821. 10.1021/acs.biochem.9b00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colom-Cadena M, Gelpi E, Charif S, et al. Confluence of α-synuclein, tau, and β-amyloid pathologies in dementia with Lewy bodies. J Neuropathol Exp Neurol. 2013;72:1203–1212. 10.1097/NEN.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 34.Bhasne K, Sebastian S, Jain N, Mukhopadhyay S. Synergistic amyloid switch triggered by early heterotypic oligomerization of intrinsically disordered α-synuclein and tau. J Mol Biol. 2018;430:2508–2520. 10.1016/j.jmb.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Bassil F, Meymand ES, Brown HJ, et al. α-Synuclein modulates tau spreading in mouse brains. J Exp Med. 2021;218:e20192193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sengupta U, Guerrero-Muñoz MJ, Castillo-Carranza DL, et al. Pathological interface between oligomeric alpha-synuclein and tau in synucleinopathies. Biol Psychiatry. 2015;78:672–683. 10.1016/j.biopsych.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 37.Palhano FL, Lee J, Grimster NP, Kelly JW. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J Am Chem Soc. 2013;135: 7503–7510. 10.1021/ja3115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bieschke J, Russ J, Friedrich RP, et al. EGCG remodels mature α-synuclein and amyloid-β fibrils and reduces cellular toxicity. PNAS. 2010;107:7710–7715. 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wobst HJ, Sharma A, Diamond MI, Wanker EE, Bieschke J. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015;589:77–83. 10.1016/j.febslet.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mereles D, Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More Pitfalls than Promises? Int J Mol Sci. 2011;12:5592–5603. 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehmood S, Maqsood M, Mahtab N, et al. Epigallocatechin gallate: Phytochemistry, bioavailability, utilization challenges, and strategies. J Food Biochem. 2022;46. 10.1111/jfbc.14189. [DOI] [PubMed] [Google Scholar]
- 42.Sampath Kumar HM, Herrmann L, Tsogoeva SB. Structural hybridization as a facile approach to new drug candidates. Bioorg Med Chem Lett. 2020;30. 10.1016/j.bmcl.2020.127514. [DOI] [PubMed] [Google Scholar]
- 43.Gontijo VS, Viegas FPD, Ortiz CJC, et al. Molecular hybridization as a tool in the design of multi-target directed drug candidates for neurodegenerative diseases. Curr Neuropharmacol. 2020;18:348–407. 10.2174/1385272823666191021124443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivasiv V, Albertini C, Gonçalves AE, Rossi M, Bolognesi ML. Molecular hybridization as a tool for designing multitarget drug candidates for complex diseases. Curr Top Med Chem. 2019;19:1694–1711. 10.2174/1568026619666190619115735. [DOI] [PubMed] [Google Scholar]
- 45.Bérubé G An overview of molecular hybrids in drug discovery. Expert Opin Drug Discov. 2016;11:281–305. 10.1517/17460441.2016.1135125. [DOI] [PubMed] [Google Scholar]
- 46.Fortin JS, Shimanaka K, Saraswati AP, et al. Anti-fibrillization effects of sulfonamide derivatives on α-synuclein and hyperphosphorylated tau isoform 1N4R. J Mol Struct. 2022;1267. 10.1016/j.molstruc.2022.133574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maity S, Shimanaka K, Rivet LN, et al. In vitro characterization of urea derivatives to inhibit alpha-synuclein early-stage aggregation. J Mol Struct. 2022;1249. 10.1016/j.molstruc.2021.131569. [DOI] [Google Scholar]
- 48.Sipe JD, Cohen AS. Review: History of the amyloid fibril. J Struct Biol. 2000;130: 88–98. 10.1006/jsbi.2000.4221. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez E, Ganegamage SK, Elbatrawy AA, et al. 5-Nitro-1,2-benzothiazol-3-amine and N-Ethyl-1-[(ethylcarbamoyl)(5-nitro-1,2-benzothiazol-3-yl)amino]formamide Modulate α-Synuclein and Tau Aggregation. ACS Omega. 2023;8:20102–20115. 10.1021/acsomega.3c02668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Terry-Kantor E, Tripathi A, Imberdis T, et al. Rapid alpha-synuclein toxicity in a neural cell model and its rescue by a stearoyl-coa desaturase inhibitor. Int J Mol Sci. 2020;21:1–14. 10.3390/ijms21155193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imberdis T, Negri J, Ramalingam N, et al. Cell models of lipid-rich α-synuclein aggregation validate known modifiers of α-synuclein biology and identify stearoyl-CoA desaturase. PNAS. 2019;116:20760–20769. 10.1073/pnas.1903216116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.