Abstract
Grapevine (Vitis vinifera) is one of the most important perennial fruit plants. The variety Riesling stands out by developing a characteristic petrol-like odor note during aging, elicited by the aroma compound 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN). The UV-dependent TDN contents differ largely among Rieslings grown in the northern versus the southern hemisphere. Highest TDN concentrations were found in Australian Rieslings, where TDN is a scoring ingredient. In contrast, in Rieslings from Europe, for example, TDN may be a tending cause of rejection. A human receptor for TDN has been unknown. Here, we report on the identification of OR8H1 as a TDN-selective odorant receptor, out of a library of 766 odorant receptor variants. OR8H1 is selectively tuned to six carbon ring structures, identified by screening a collection of 180 key food odorants, using a HEK-293 cell-based cAMP luminescence assay equipped with the GloSensor technology.
Keywords: GPCRs, high throughput screening, off-flavor, wine
Introduction
The grapevine (Vitis vinifera) is one of the economically most important perennial fruit plants1 and appears in numerous different grape varieties. One of the world’s classic grape varieties is Riesling. The volatile compounds and their precursors, e.g, carotenoids, such as xanthophylls, particularly violaxanthin and neoxanthin,2 which are responsible for the characteristic aroma of Riesling wine, have been studied for many years.3−7 Different profiles of aroma-relevant volatiles and their precursors, such as carotenoid metabolites, mainly depend on the Riesling variety and viticultural area,2,3,8−11 shaping a Riesling-typical bouquet with floral, fruity, honey, but also petrol notes. A kerosene- or petroleum-like note in Riesling, which was first described by Simpson in 1978, is caused by 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN).12 TDN in moderate concentrations is a normal component of a matured Riesling wine’s bouquet.4,12−14 Moreover, it has been found that TDN occurs at higher concentrations in Riesling wines from the southern hemisphere, e.g., from South Africa or Australia.13−15 TDN median levels found for Hungarian Rieslings were in the area of 3.9–41.1 μg/L,16 wines from the “Mundus Vini 2015” sample originating mostly from Germany ranged from 0.4 to 14.8 μg/L free TDN and from 38.5 to 333.0 μg/L for total TDN.17 Sacks et al. (2012) showed for Riesling wines from New York state a range of 1.3–17.1 μg/L18 TDN among their samples. In contrast, Marais et al. (1992) described concentrations of TDN of 3–55 μg/L for Riesling wines from South Africa, and, thus, the southern hemisphere.15 Median TDN levels found for Australian Rieslings ranged between 14 and 88 μg/L TDN, with Black et al. (2012) reporting a min–max range of 2–255 μg/L TDN,13 which is higher as compared to the levels of TDN in, e.g., European Rieslings. The reason for that appears to be an increased sun/UV light exposure of Riesling grapes, which leads to altered relative amounts of carotenoids, an increase in xanthophylls as TDN precursors, and an increase in TDN and other C13-norisoprenoids, e.g., vitispirane.9,19−21 This, however, may be modulated by photoselective bunch zone shading during berry development,22 but also by, for example, the choice of Riesling clones and rootstocks.23 Yet, a climate change-induced petrol note in European Riesling wines due to higher TDN concentrations, however, may potentially be perceived as an off-flavor and may, therefore, result in the wine being rejected.15
Beyond wine, TDN has also been reported in a variety of other foods, such as arbutus distillates (arbutus spec. L.),24 passion fruit (Passiflora edulis Sims),25 peach (Prunus persica),26 raki,27 rum,28 strawberry (Fragaria × ananassa),29 and tomato paste (Solanum lycopersicum).30 TDN, however, has not been classified as KFO, according to Dunkel et al. (2014), yet.31 This is due to at least three strict criteria applied by Dunkel et al. (2014) to the aroma compounds to be included in their meta-analysis: (i) activity-based localization of the most potent odorants of serially diluted aroma extracts by GC-O, (ii) identification of key odorants by comparison of chromatographic retention times, mass spectrometric, and sensory information with those of independently synthesized reference compounds, and (iii) comprehensive quantitative determination of all key odorants using analytical methods such as stable isotope dilution analysis.31 Taking 2.3 μg/L as a basis for TDN odor threshold,32 the odor activity value of TDN is >1, throughout, based on quantitative data in Riesling.13,15−18 TDN, thus, may also be categorized as KFO in a forthcoming expanded meta-analysis.
The odor threshold of TDN originally was reported to be 20 μg/L,12 but Sacks et al. (2012) determined a detection threshold of 2 μg/L by using a spiked model wine.18 Recently, Ziegler et al. (2019) established detection thresholds in different matrices with a threshold for TDN of 2.3 μg/L in Riesling wine.32 Its detection and recognition start with the chemoreception of volatile TDN by its cognate odorant receptor(s) in the cilia of olfactory sensory neurons in the nose. A human receptor for TDN, however, has been unknown so far, neither could we find published evidence for a frequent specific anosmia for TDN, with phenotypes displaying 10–100-fold increased detection thresholds.33,34 A TDN-specific receptor, however, may potentially serve as a sensor for the identification (or authentication) of Riesling wines.
Here, we set out to identify and characterize the best responding human OR for TDN in a bidirectional screening approach, employing our KFO library of GC-pure compounds,35 and our IL-6-HaloTag-OR library,36,37 in a HEK-293 cell-based cAMP luminescence assay, equipped with GloSensor technology.38 This assay employs a genetically modified luciferase that becomes activated by an odorant-receptor-mediated cAMP signaling.38
Materials and Methods
Chemicals
The following chemicals were used: Dulbecco′s MEM medium (#F0435), FBS superior (#S0615), l-glutamine (#K0282), penicillin (10 000U/mL)/streptomycin (10 000U/mL) (#A2212), trypsin/EDTA solution (#L2143) (Biochrom, Berlin, Germany), calcium chloride dihydrate (#22322.295), d-glucose (#101174Y), DMSO (#83673.230), HEPES (#441476L), potassium chloride (#26764.230), and sodium hydroxide (#28244.295) (VWR Chemicals BDH Prolabo, Leuven, Belgium), sodium chloride (#1064041000, Merck, Darmstadt, Germany), D-luciferin (beetle) monosodium salt (#E464X, Promega, Madison, USA). The following odorants were used: 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN; CAS: 30364–38–6) (#ENAH97EDDC8E), 2-acetylnaphthalene (CAS: 93–08–3) (#134775), and methyl isoeugenol (CAS: 93–16–3) (#W247618, Sigma-Aldrich, Darmstadt, Germany). The KFOs used were as previously published39,40 (Table S1). Further details including the GC–O approach for the purity testing of the odorants prior to their use in the odorant receptor experiments are available in the literature.41
Molecular Cloning of Odorant Receptors
Mammalian orthologs of human OR8H1 have been identified via NCBI (https://www.ncbi.nlm.nih.gov/kis/info/how-are-orthologs-calculated/) and OrthoDB v11 (https://www.orthodb.org/). The protein-coding regions of the human (NCBI reference sequence: NM_001005199.1), chimpanzee (NCBI reference sequence: XM_009440400.4), and horse (NCBI reference sequence: NM_001391760.1) OR8H1 were amplified from genomic DNA by polymerase chain reaction (PCR) using gene-specific primers (Table S2), ligated with T4-DNA ligase (#M1804, Promega, Madison, USA) EcoRI/NotI (#R6017/ #R6435, Promega, Madison, USA) into the expression plasmid (#pFN210A SS-HaloTag CMV-neo Flexi-Vector, Promega, Madison, USA), and verified by Sanger sequencing (Eurofins Genomics, Ebersberg, Germany) using vector internal primers (Table S3). The chimp DNA was obtained from a saliva sample from an animal born to the Munich Zoological Garden before 2014. The horse DNA was obtained from a saliva sample.
In the same way, all human OR coding regions used in the present study (reference sequences and selected genetic variants, Tables S4 and S5) were cloned and ligated into the pFN210A expression plasmid and were purified using the PureYield Plasmid Midiprep System (#A2495, Promega, Madison, USA).
Cell Culture
We used HEK-293 cells42 (RRID:CVCL_0045), a human embryonic kidney cell-line, as a test cell system for the functional expression of recombinant ORs.38 HEK-293 cells were cultivated at 37 °C, 5% CO2, and 100% humidity in 4.5 g/L d-glucose containing DMEM with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/mL penicillin, and 100 U/mL streptomycin.
Luminescence Assay
Luminescence-based functional expression experiments in HEK-293 test cells were performed as described previously38 (for details, see Supporting Information). For receptor screening experiments, we used a cDNA expression plasmid library, comprising 766 cDNAs, coding for 386 human OR types (NCBI reference sequences), and 380 of their most frequent variants (Tables S4 and S5).
Data Analysis of the cAMP Luminescence Measurements of Odorant Concentration–Response Relations
The raw luminescence data obtained from the GloMax Discover detection system was analyzed using Excel (Microsoft Corp., Redmond, USA). These data have been published with Mendeley (doi: 10.17632/nzjwm89336.1). Data points of the basal level and data points after odorant application were each averaged. From each luminescence signal, the corresponding basal level was subtracted.
For concentration–response relations,
the baseline-corrected data set was normalized to the maximum amplitude
of the reference odorant-receptor pair. The data set for the mock
control was subtracted and EC50 values and curves were
derived from a weighted fit by fitting the function 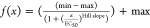 (43) to the data
by nonlinear regression (SigmaPlot 14.0, Systat Software), with the
constraint Hillslope ≤2.9 and with each data point weighted
by its reciprocal standard deviation (0.1/SD). All data are presented
as the mean ± SD.
(43) to the data
by nonlinear regression (SigmaPlot 14.0, Systat Software), with the
constraint Hillslope ≤2.9 and with each data point weighted
by its reciprocal standard deviation (0.1/SD). All data are presented
as the mean ± SD.
Flow Cytometry
The surface expression of recombinant ORs expressed in HEK-293 cells was evalutated by flow cytometry, as described earlier38 (for details, see the Supporting Information).
Bioinformatics
NCBI was used as a database for the retrieval of genetic information on Homo sapiens (human) odorant receptor genes (reference sequences) and their genetic variants (GRCh38).37 The phylogenetic reconstruction of ORs was performed with QIAGEN CLC Genomics Workbench 20.0 (https://digitalinsights.qiagen.com/), and MEGAX44 software. Therefore, in a first step, all human OR sequences were aligned using the ClustalW algorithm.45 The evolutionary history was inferred using the Maximum Likelihood method and JTT matrix-based model46 with 500 bootstrap replications.47 The tree is drawn to scale with branch lengths measured in the number of substitutions per site. Evolutionary analyses were conducted in MEGA X.44 For rooting the constructed tree, human rhodopsin (NCBI entry: NP_000530.1) was used as an out-group, since the origin of the opsins is more ancient than the origin of the OR gene family.48,49
Results/Discussion
OR8H1 Exclusively Responded to 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN)
An odorant receptor for TDN has been unknown so far. We, therefore, screened TDN at 100 μmol/L against an OR library comprising in total 766 cDNAs, coding for 386 human OR types and 230 frequent variants (Figure 1). In addition, another 150 frequent OR variants were screened with TDN at a concentration of 100 μmol/L (Figure S1). Notably, TDN exclusively activated a single OR above a 3σ-threshold: OR8H1. We then validated OR8H1 as a receptor for TDN by establishing its concentration–response relationship, yielding an EC50 value of 32.02 ± 6.09 μmol/L (Figure 2, Table 1).
Figure 1.
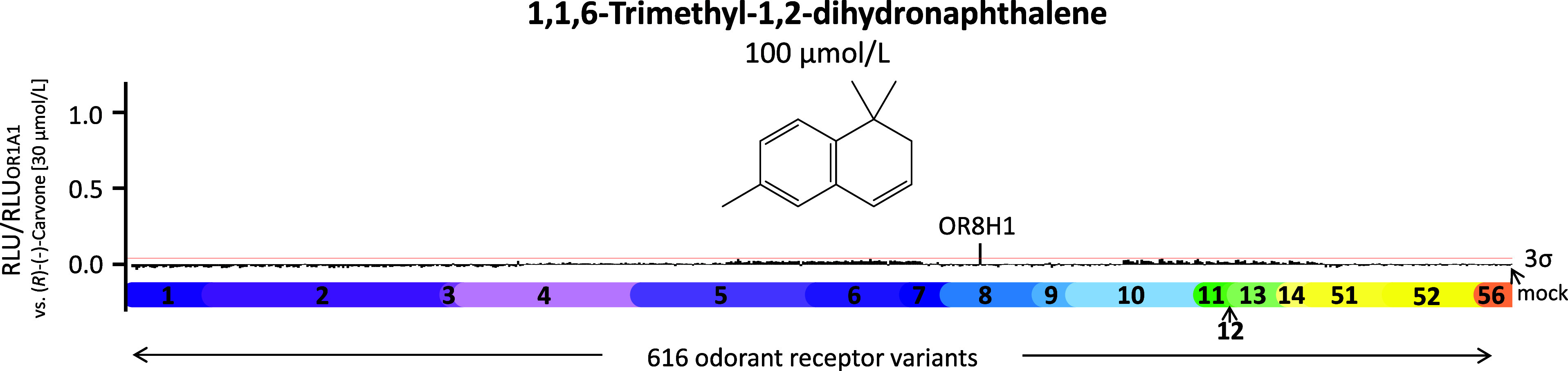
1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) exclusively activates OR8H1. Screening of 616 human OR variants with 100 μmol/L TDN. Shown is the mean (n = 1 in duplicate). Data were normalized to the response of OR1A1 to (R)-(−)-carvone (30 μmol/L). The different OR families are color-coded and sorted in ascending order. The red line indicates the 3σ-threshold. RLU = relative luminescence units. An expanded screening with further 150 variants is shown in Figure S1.
Figure 2.
OR8H1 concentration-dependently responded to TDN. Concentration–response relation of TDN on OR8H1. Data were mock control-subtracted, normalized to the highest signal, and displayed as mean ± SD (n = 3). RLU = relative luminescence units.
Table 1. EC50 Values, Odor Properties, and Comparison of OR8H1 Agonists with the Present Literature.
| no. | compound | EC50 in μmol/La | odor quality35 | odor threshold in water in μg/kg | functionally associated ORs |
|---|---|---|---|---|---|
| TDN (1,1,6-trimethyl-1,2-dihydronaphthalene) | 32.02 ± 6.09 | petrol-like | 1.132 | OR8H1this study | |
| 1 | 1-methyl-4-(prop-1-en-2-yl)benzene | 336.30 ± 74.86 | terpene-like | 8561 | OR8H1this study, OR2W140,50 |
| 2 | methyl eugenol (1,2-dimethoxy-4-prop-2-enylbenzene) | 113.95 ± 20.15 | grassy, harsh | 6862, 82063 | OR8H1this study, OR2J2,64 OR2J3,64 OR5K1,54,64,65 OR5K4,64 OR10G7,64 OR51E164 |
| 3 | 2-isopropyl-3-methoxypyrazine (2-methoxy-3-propan-2-ylpyrazine) | 147.49 ± 8.64 | earthy, pea-like | 0.003935 | OR8H1this study, OR5K154 |
| 4 | estragole (1-methoxy-4-prop-2-enylbenzene) | >600 | aniseed-like, licorice-like | 6.035 | OR8H1this study, OR1A1,40 OR2M4,66 OR2T34,66 OR2W1,40,50 OR52D167 |
| 5 | 2-methoxy-4-propylphenol | >600 | phenolic, clove-like | 1.9b35 | OR8H1this study |
| 6 | (R)-(−)-carvone ((5R)-2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one) | 192.7 ± 9.52 | mint-like | 5068 | OR8H1this study, OR1A1,40,69,70 OR2W1,40,50 OR5P364,69 |
| 7 | cuminaldehyde (4-Propan-2-ylbenzaldehyde) | 91.95 ± 11.69 | green71 | 40072 | OR8H1this study, OR2W150 |
| 8 | 1-p-menthene-8-thiol (2-(4-methylcyclohex-3-en-1-yl)propane-2-thiol) | 205.76 ± 80.75 | grapefruit-like, sulfuric | 0.000173 | OR8H1this study, OR2W150 |
Mean ± SD (n = 3).
OT in water/EtOH (60/40).
OR8H1 – A Receptor Selectively Tuned to a Distinct Group of Aromatic Compounds
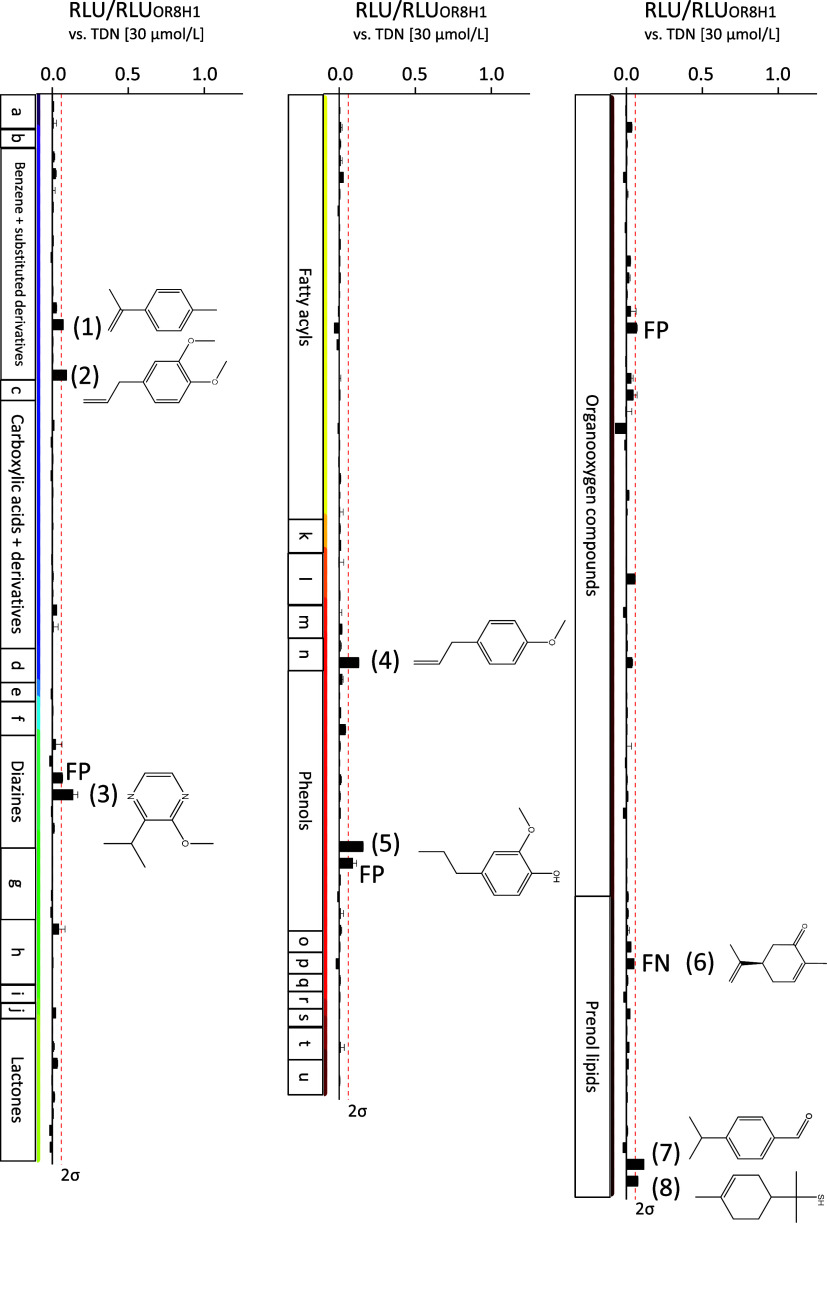
We then set out to elucidate the natural, food-related agonist profile of OR8H1, to characterize OR8H1 as either broadly tuned40,50 or narrowly tuned receptor.36,51 To characterize the KFO agonist spectrum of OR8H1, we used those food-aroma-relevant odorants initially published by Dunkel et al. (2014). We screened OR8H1 against 180 KFO compounds, which we sorted according to the ClassyFire classification,52 as described previously.50 Each KFO was tested at a concentration of 300 μmol/L in triplicate against OR8H1, and all receptor response amplitudes were normalized to the response of OR8H1 to 30 μmol/L TDN (Figure 3).
Figure 3.
OR8H1 is selectively tuned for molecules with six carbon rings. Screening of 180 KFOs against the human odorant receptor OR8H1. Shown are mean ± SD (n = 1 measured in triplicate). Mock control was subtracted. Data were normalized to the response of OR8H1 to TDN (30 μmol/L). The concentration of each KFO was 300 μmol/L. The red dashed line indicates the 2σ-threshold. RLU = relative luminescence units. FP = false positive (for concentration–response relation see Figure S2). FN = false negative (for concentration–response relation see Figure 4A). KFOs are grouped according to the ClassyFire classification. a = allyl sulfur compounds, b = azolines, c = benzodioxoles, d = cinnamic acids and derivatives, e = cinnamyl alcohols, f = coumarines and derivatives, g = dihydrofurans, h = heteroaromatic compounds, i = hydroxy acids and derivatives, j = indols and derivatives, k = organic disulfides, l = organic trisulfides, m = oxanes, n = phenol ethers, o = pyrans, p = pyridins and derivatives, q = pyrrolines, r = phenylpropanoic acids, s = thioacetals, t = thioethers, u = thiols.
Figure 3 shows the KFO activation pattern of OR8H1 that responded to seven KFOs out of five different chemical groups above a 2σ-threshold. We further identified one false-negative responder, (R)-(−)-carvone, which has been suggested previously as a potential agonist, being part of the OR8H1-activating “MCMP” odorant mixture.53 All of these compounds as well as TDN share a six ring basic structure, with most of them being aromatic compounds by a generic definition. These odorants are (1) 1-methyl-4-(prop-1-en-2-yl)benzene, (2) methyl eugenol, (3) 2-isopropyl-3-methoxypyrazine, (4) estragole, (5) 2-methoxy-4-propylphenol, (6) (R)-(−)-carvone, (7) cuminaldehyde, and (8) 1-p-menthene-8-thiol.
Indeed, all of them concentration-dependently activated OR8H1 (Figure 4A), with TDN being the most potent agonist, with an EC50 value of 32.02 ± 6.09 μmol/L, outperforming compounds (1)–(8) by at least half an order of magnitude (Figure 4A, Table 1).
Figure 4.
Aromatic compounds and cyclo-alkanes are agonists of OR8H1. (A) Concentration–response relations of cuminaldehyde (7), methyl eugenol (2), 2-isopropyl-3-methoxypyrazine (3), (R)-(−)-carvone (6), 1-p-menthene-8-thiol (8), 1-methyl-4-(prop-1-en-2-yl)benzene (1), 2-methoxy-4-propylphenol (5), and estragole (4) on OR8H1. Data were mock control-subtracted, normalized to the maximum response of OR8H1 to each compound, and displayed as mean ± SD (n = 3). (B) Concentration–response relations of 2-acetylnaphthalene and methyl isoeugenol on OR8H1. Data were mock control-subtracted, normalized to the response of OR8H1 to TDN (100 μmol/L), and displayed as mean ± SD (n = 3–5). RLU = relative luminescence units. To facilitate comparisons, the concentration–response relations of TDN on the human receptor were taken from Figure 2 (here in gray). (C) Radial, phylogenetic relationship of all human ORs with human rhodopsin (blue) as outgroup. Marked with colored dots are the deorphaned, phylogenetically related ORs OR9Q2,36 OR5M3,51 OR8D1,51,64 OR5K1,54 and OR8H1 (this study).
All of the compounds (1)–(8) and TDN display different odor qualities. For two compounds, 2-isopropyl-3-methoxypyrazine (3) and 1-p-menthene-8-thiol (8), odor thresholds (OTs) have been reported that are several orders of magnitude lower than the OT for TDN (Table 1). Moreover, ORs other than OR8H1 have been allocated previously to most of the compounds (1)–(8) (Table 1), one of which, for example, is broadly tuned receptor OR2W1 for five of the eight KFOs. Thus, it is likely that compounds (1)–(8) will each activate their own selective OR pattern, if screened against a comprehensive human OR library, which in all cases will include OR8H1.
It has been reported that 2-acetylnaphthalene, (R)-(−)-carvone, methyl isoeugenol, and 2-phenylethyl acetate activated OR8H1 as a mixture (“MCMP”), but the single compounds have not been tested on OR8H1.53 From the results of our present study, we may reject 2-phenylethyl acetate as an agonist of OR8H1, since it did not activate OR8H1 in our KFO screen (Figure 3), at least not up to 300 μmol/L. We further validated (R)-(−)-carvone as an agonist of OR8H1 (Figures 3 and 4A). Because of their structural similarity to the OR8H1 agonists TDN and methyl eugenol, both 2-acetylnaphthalene and methyl isoeugenol may also activate OR8H1. We have tested this and could validate 2-acetylnaphthalene as an agonist for OR8H1, but not methyl isoeugenol (Figure 4B).
Is OR8H1 a broadly or rather narrowly tuned receptor? Importantly, we had characterized previously the odorant receptor OR2W1 as the most broadly tuned human OR, so far, with a KFO-based promiscuity index (PI) – which is the number of bioassay-based agonists divided by the total number of tested compounds – of 0.38.50 In the present study, we tested OR8H1 against a largely identical set of 180 chemically diverse KFOs and one non-KFO (TDN) and identified nine agonists, which resulted in a PI of 0.05. This might suggest that OR8H1 is a rather narrowly tuned receptor. However, the chemical diversity index (CDI, number of chemical clusters represented by agonists divided by the total number of chemical clusters represented by the tested compounds) of OR8H1 is 0.12 (6 chemical agonist clusters out of 49 chemical clusters represented by our KFO library50), compared to a CDI of OR2W1 of 0.27.50 Given the chance of having missed potential agonist in our screening, we thus prefer to address OR8H1 as a selectively tuned receptor. In a tree displaying the phylogenetic relationships of human ORs, OR8H1 resides in the same clade as other previously identified, and highly selective ORs (Figure 4C), which, by their selectivities, are complementary to certain detection gaps in broadly tuned OR2W1.50 Examples are OR5M3 and OR8D1 detecting furanones,51 OR5K1 detecting alkylpyrazines,54 and OR9Q2 detecting 4-alkylphenols.36 Notably, the genes of OR5M3, OR8D1, OR8H1, and OR9Q2 all reside on chromosome 11q. Interestingly, OR8H1 is selective for TDN and detects both KFOs cuminaldehyde and methyl eugenol, compounds that did not activate broadly tuned OR2W1 (present study and50).
TDN Detection by OR8H1 Is an Evolutionary Conserved Receptor Function
OR8H1 has only a few orthologues in other species. We established concentration–response relations of TDN on OR8H1 orthologs from two species, Pan troglodytes [ptOR8H1] (chimpanzee) and Equus caballus [ecOR8H1] (horse), displaying a deduced protein sequence identity to human OR8H1 of 97% and 79%, respectively. However, only the chimp orthologue responded to TDN in a concentration-dependent manner and with the same EC50 (37.77 ± 29.09 μmol/L) as the human receptor, although with a ∼7-fold lower efficacy (Figure 5A). Response strength and EC50, however, did not correlate with the surface expression of all orthologs – both low- and nonresponding chimp and horse orthologs were significantly better expressed at the cell surface than the human receptor (Figure 5B).
Figure 5.
TDN function on OR8H1 is conserved among humans and chimpanzees. (A) Concentration–response relations of TDN on OR8H1 and the orthologs from horse (ecOR8H1) and chimp (ptOR8H1). Data were mock control-subtracted, normalized to the maximum response of human OR8H1 to TDN (100 μmol/L), and displayed as mean ± SD (n = 3). Note that for didactic reasons, the concentration–response relation of TDN on the human receptor was taken from Figure 2 (here in gray). (B) Bar chart showing the relative cell surface expression of OR8H1 and orthologs using the flow cytometry assay. Data is displayed as mean ± SD (n = 3). (C) Concentration–response relations of cuminaldehyde (7) and 1-p-menthene-8-thiol (8) on ptOR8H1. Data were mock control-subtracted, normalized to the maximum response of human OR8H1 to TDN (100 μmol/L), and displayed as mean ± SD (n = 3–4). To facilitate comparisons, the concentration–response relation of TDN on ptOR8H1 was taken from panel (A) (here in gray). RLU = relative luminescence units. ec, Equus caballus, pt, Pan troglodytes. Icons were created with BioRender.com.
Orthologous gene products across species supposedly display a conserved function (“heterospecic isofunctional homologs”55) and were suggested to be more functionally similar to each other than paralogs.56−59 This has been referred to as the “ortholog conjecture”.60 Our results on OR8H1 and at least one of the orthologues may support this notion, since (i) none of the human OR8H1 homologues (paralogues) responded to TDN in our screening assay and (ii) the chimp orthologue responded to TDN, cuminaldehyde (7), and 1-p-menthene-8-thiol (8) with a similar ranking of potencies (EC50) as observed with the human OR8H1 (Figure 5C).
A cautionary note: We might have missed other TDN-responsive ORs or responsive genetic variants because (i) our receptor screening experiments still did not include all known genetic variants of human ORs or (ii) some receptors in our OR library may not function with the assay used in our study. Further agonists of OR8H1 may be identified using QSAR, an expanded KFO library, or other comprehensive, biologically relevant odorant collections.
In summary, our dual screening strategy, in which a highly relevant food-related compound was tested against a comprehensive set of human ORs and their genetic variants, and vice versa, in which the solely identified OR was then tested against a comprehensive collection of KFOs, yielded a KFO-enriched and highly specific agonist profile of the newly characterized OR8H1, which is highly selective for TDN, the so-called petrol-note in Riesling.
Acknowledgments
We thank Antonella Di Pizio and Stephanie Frank for helpful discussions.
Glossary
Abbreviations used
- CDI
chemical diversity index
- GPCR
G-protein coupled receptor
- KFO
key food odorant
- OR
odorant receptor
- OT
odor threshold
- PI
promiscuity index
- TDN
1,1,6-trimethyl-1,2-dihydronaphthalene
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jafc.3c08230.
Additional experimental details on investigated odorants, oligonucleotides for molecular cloning, the cDNA expression plasmid OR library and the extended OR library screening as well as the validation of false-positive screening signals (PDF)
Author Present Address
∥ Tecan Deutschland GmbH, 74564 Crailsheim, Germany
Author Contributions
§ F.H. and T.F. contributed equally to this work.
The authors declare no competing financial interest.
Special Issue
Published as part of Journal of Agricultural and Food Chemistryvirtual special issue “13th Wartburg Symposium on Flavor Chemistry and Biology”.
Supplementary Material
References
- (OIV), I. O. o. V. a. W. Annual assessment of the world vine and wine sector in 2021. https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment_of_the_World_Vine_and_Wine_Sector_in_2021.pdf (25–08–2023),.
- Winterhalter P.; Gök R., TDN and β-damascenone: Two important carotenoid metabolites in wine. In Carotenoid cleavage products; ACS Publications: 2013; pp 125–137. [Google Scholar]
- Winterhalter P.; Sefton M. A.; Williams P. J. Volatile C-13-Norisoprenoid Compounds in Riesling Wine Are Generated from Multiple Precursors. Am. J. Enol. Vitic. 1990, 41, 277–283. 10.5344/ajev.1990.41.4.277. [DOI] [Google Scholar]
- Simpson R. F.; Miller G. C. Aroma Composition of Aged Riesling Wine. Vitis 1983, 22, 51–63. [Google Scholar]
- Winterhalter P.; Baderschneider B.; Bonnländer B., Analysis, Structure, and Reactivity of Labile Terpenoid Aroma Precursors in Riesling Wine. In Chemistry of Wine Flavor; American Chemical Society: 1998; Vol. 714, pp 1–12. [Google Scholar]
- Rapp A.; Volkmann C.; Niebergall H. Analysis of Volatile Aroma Compounds of Grapevine - Characterization of Riesling and Riesling Derived Cultivars. Vitis 1993, 32, 171–178. [Google Scholar]
- Tominaga T.; Baltenweck-Guyot R.; Gachons C. P. D.; Dubourdieu D. Contribution of Volatile Thiols to the Aromas of White Wines Made From SeveralVitis viniferaGrape Varieties. Am. J. Enol. Vitic. 2000, 51, 178–181. 10.5344/ajev.2000.51.2.178. [DOI] [Google Scholar]
- Rapp A. Possibilities of characterizing wine varieties by means of volatile flavor compounds. Dev. Food Sci. 1995, 37B, 1703. 10.1016/S0167-4501(06)80259-1. [DOI] [Google Scholar]
- Mendes-Pinto M. M. Carotenoid breakdown products the-norisoprenoids-in wine aroma. Arch. Biochem. Biophys. 2009, 483, 236–45. 10.1016/j.abb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Komes D.; Ulrich D.; Lovric T. Characterization of odor-active compounds in Croatian Rhine Riesling wine, subregion Zagorje. European Food Research and Technology 2006, 222, 1–7. 10.1007/s00217-005-0094-y. [DOI] [Google Scholar]
- Chisholm M. G.; Guiher L. A.; Vonah T. M.; Beaumont J. L. Comparison of Some French-American Hybrid Wines with White Riesling Using Gas-Chromatography Olfactometry. Am. J. Enol. Vitic. 1994, 45, 201–212. 10.5344/ajev.1994.45.2.201. [DOI] [Google Scholar]
- Simpson R. F.1,1,6-Trimethyl-1,2-dihydronaphthalene: an important contributor to the bottle aged bouquet of wine, 1978, 37.
- Black C.; Francis L.; Henschke P.; Capone D.; Anderson S.; Day M.; Holt H.; Pearson W.; Herderich M.; Johnson D. Aged Riesling and the development of TDN. Wine Vitic. J. 2012, 27, 20–26. [Google Scholar]
- Marais J. The significance of 1,1,6-trimethyl-1,2-dihydronaphthalene in the production of high quality Riesling wines. ACS Symp. Ser. 2001, 802, 273–284. 10.1021/bk-2002-0802.ch020. [DOI] [Google Scholar]
- Marais J.; Versini G.; van Wyk C. J.; Rapp A. Effect of Region on Free and Bound Monoterpene and C13-N orisoprenoid Concentrations in Weisser Riesling Wines. S. Afr. J. Enol. Vitic. 2017, 13, 71–77. 10.21548/13-2-2177. [DOI] [Google Scholar]
- Antal E.; Varga Z.; Kallay M.; Steckl S.; Bodor-Pesti P.; Fazekas I.; Solyom-Lesko A.; Kovacs B. Z.; Nagy B.; Szovenyi A. P.; Nyitrai-Sardy D. A. 1,1,6-Trimethyl-1,2-dihydronaphthalene Content of Riesling Wines in Hungary. ACS Omega 2023, 8, 36677–36685. 10.1021/acsomega.3c02445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gok R.; Bechtloff P.; Ziegler M.; Schmarr H. G.; Fischer U.; Winterhalter P. Synthesis of Deuterium-Labeled 1,1,6-Trimethyl-1,2-dihydronaphthalene (TDN) and Quantitative Determination of TDN and Isomeric Vitispiranes in Riesling Wines by a Stable-Isotope-Dilution Assay. J. Agric. Food Chem. 2019, 67, 6414–6422. 10.1021/acs.jafc.9b01428. [DOI] [PubMed] [Google Scholar]
- Sacks G. L.; Gates M. J.; Ferry F. X.; Lavin E. H.; Kurtz A. J.; Acree T. E. Sensory threshold of 1,1,6-trimethyl-1,2-dihydronaphthalene (TDN) and concentrations in young Riesling and non-Riesling wines. J. Agric. Food Chem. 2012, 60, 2998–3004. 10.1021/jf205203b. [DOI] [PubMed] [Google Scholar]
- Gerdes S. M.; Winterhalter P.; Ebeler S. E., Effect of Sunlight Exposure on Norisoprenoid Formation in White Riesling Grapes. In Carotenoid-Derived Aroma Compounds,; Winterhalter P.; Rouseff R. L., Eds. American Chemical Society: 2001; Vol. 802, pp 262–272. [Google Scholar]
- Kwasniewski M. T.; Vanden Heuvel J. E.; Pan B. S.; Sacks G. L. Timing of cluster light environment manipulation during grape development affects C13 norisoprenoid and carotenoid concentrations in Riesling. J. Agric. Food Chem. 2010, 58, 6841–9. 10.1021/jf904555p. [DOI] [PubMed] [Google Scholar]
- Nisar N.; Li L.; Lu S.; Khin N. C.; Pogson B. J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. 10.1016/j.molp.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Grebneva Y.; Bilogrevic E.; Rauhut D.; Herderich M. J.; Hixson J. L. Impacts of photoselective bunch zone shading on the volatile composition and sensory attributes for < i > Vitis vinifera</i> L. cv. Riesling. OENO One 2022, 56, 297–311. 10.20870/oeno-one.2022.56.3.5364. [DOI] [Google Scholar]
- Ziegler M.; Wegmann-Herr P.; Schmarr H. G.; Gok R.; Winterhalter P.; Fischer U. Impact of Rootstock, Clonal Selection, and Berry Size of Vitis vinifera sp. Riesling on the Formation of TDN, Vitispiranes, and Other Volatile Compounds. J. Agric. Food Chem. 2020, 68, 3834–3849. 10.1021/acs.jafc.0c00049. [DOI] [PubMed] [Google Scholar]
- Versini G.; Seeber R.; Dalla Serra A.; Sferlazzo G.; de Carvalho B.; Reniero F., Aroma compounds of arbutus distillates. In Food Flavors: Generation, Analysis and Process Influence, Proceedings of the 8th International Flavor Conference, Charalambous G., Ed. Elsevier: 1995; Vol. 37, pp 1779–1790. [Google Scholar]
- Murray K. E.; Shipton J.; Whitfield F. B. The chemistry of food flavour. I. Volatile constituents of passionfruit, < I > Passiflora edulis</I>. Aust. J. Chem. 1972, 25, 1921–1933. 10.1071/CH9721921. [DOI] [Google Scholar]
- Kemp T. R.; Stoltz L. P.; Packett L. V. Aromatic Hydrocarbons - Examination of Peach Fruit and Foliage Volatiles. Phytochemistry 1971, 10, 478. 10.1016/S0031-9422(00)94086-X. [DOI] [Google Scholar]
- Yavaş; Rapp A., Aroma components of raki. In Food Flavors: Generation, Analysis and Process Influence, Proceedings of the 8th International Flavor Conference, Charalambous G., Ed. Elsevier: 1995; Vol. 37, pp 1791–1811. [Google Scholar]
- Allan D. A. Less Volatile Alcohols, Esters and Hydrocarbons in a Raw Australian Rum. Ann. Technol. Agric. 1975, 24, 239–246. [Google Scholar]
- Stoltz L. P.; Kemp T. R.; Smith W. O.; Smith W. T.; Chaplin C. E. 1,2-Dihydro-1,1,6-Trimethylnaphthalene from Strawberry Oil. Phytochemistry 1970, 9, 1157. 10.1016/S0031-9422(00)85247-4. [DOI] [Google Scholar]
- Buttery R. G.; Teranishi R.; Flath R. A.; Ling L. C. Identification of Additional Tomato Paste Volatiles. J. Agric. Food Chem. 1990, 38, 792–795. 10.1021/jf00093a042. [DOI] [Google Scholar]
- Dunkel A.; Steinhaus M.; Kotthoff M.; Nowak B.; Krautwurst D.; Schieberle P.; Hofmann T. Nature’s chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angew. Chem., Int. Ed. Engl. 2014, 53, 7124–43. 10.1002/anie.201309508. [DOI] [PubMed] [Google Scholar]
- Ziegler M.; Gök R.; Bechtloff P.; Winterhalter P.; Schmarr H. G.; Fischer U. Impact of matrix variables and expertise of panelists on sensory thresholds of 1,1,6-trimethyl-1,2-dihydronaphthalene known as petrol off-flavor compound in Riesling wines. Food Qual Prefer 2019, 78, 103735 10.1016/j.foodqual.2019.103735. [DOI] [Google Scholar]
- Croy I.; Olgun S.; Mueller L.; Schmidt A.; Muench M.; Hummel C.; Gisselmann G.; Hatt H.; Hummel T. Peripheral adaptive filtering in human olfaction? Three studies on prevalence and effects of olfactory training in specific anosmia in more than 1600 participants. Cortex 2015, 73, 180–7. 10.1016/j.cortex.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Amoore J. E. Specific anosmia: a clue to the olfactory code. Nature 1967, 214, 1095–8. 10.1038/2141095a0. [DOI] [PubMed] [Google Scholar]
- Kreissl J.; Mall V.; Steinhaus P.; Steinhaus M.. Leibniz-LSB@TUM Odorant Database, Version 1.0. https://www.leibniz-lsb.de/datenbanken/leibniz-lsbtum-odorant-database/start/.
- Haag F.; Frey T.; Hoffmann S.; Kreissl J.; Stein J.; Kobal G.; Hauner H.; Krautwurst D. The multi-faceted food odorant 4-methylphenol selectively activates evolutionary conserved receptor OR9Q2. Food Chem. 2023, 426, 136492 10.1016/j.foodchem.2023.136492. [DOI] [PubMed] [Google Scholar]
- Wheeler D. L; Barrett T.; Benson D. A.; Bryant S. H.; Canese K.; Chetvernin V.; Church D. M.; DiCuccio M.; Edgar R.; Federhen S; Feolo M. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–d13. 10.1093/nar/gkm1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe F.; Frey T.; Fiedler J.; Geithe C.; Nowak B.; Krautwurst D. IL-6-HaloTag((R)) enables live-cell plasma membrane staining, flow cytometry, functional expression, and de-orphaning of recombinant odorant receptors. J. Biol. Methods 2017, 4, e81 10.14440/jbm.2017.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noe F.; Polster J.; Geithe C.; Kotthoff M.; Schieberle P.; Krautwurst D. OR2M3: A Highly Specific and Narrowly Tuned Human Odorant Receptor for the Sensitive Detection of Onion Key Food Odorant 3-Mercapto-2-methylpentan-1-ol. Chem. Senses 2017, 42, 195–210. 10.1093/chemse/bjw118. [DOI] [PubMed] [Google Scholar]
- Geithe C.; Noe F.; Kreissl J.; Krautwurst D. The Broadly Tuned Odorant Receptor OR1A1 is Highly Selective for 3-Methyl-2,4-nonanedione, a Key Food Odorant in Aged Wines, Tea, and Other Foods. Chem. Senses 2017, 42, 181–193. 10.1093/chemse/bjw117. [DOI] [PubMed] [Google Scholar]
- Czerny M.; Christlbauer M.; Christlbauer M.; Fischer A.; Granvogl M.; Hammer M.; Hartl C.; Hernandez N. M.; Schieberle P. Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odour qualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008, 228, 265–273. 10.1007/s00217-008-0931-x. [DOI] [Google Scholar]
- Graham F. L.; Smiley J.; Russell W. C.; Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen Virol 1977, 36, 59–74. 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- DeLean A.; Munson P. J.; Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am. J. Physiol. 1978, 235, E97–102. 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Stecher G.; Li M.; Knyaz C.; Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. D.; Higgins D. G.; Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–80. 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T.; Taylor W. R.; Thornton J. M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci 1992, 8, 275–82. 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Pattengale N. D.; Alipour M.; Bininda-Emonds O. R.; Moret B. M.; Stamatakis A. How many bootstrap replicates are necessary?. J. Comput. Biol. 2010, 17, 337–54. 10.1089/cmb.2009.0179. [DOI] [PubMed] [Google Scholar]
- Terakita A. The opsins. Genome Biol. 2005, 6, 213. 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura Y. Evolutionary dynamics of olfactory receptor genes in chordates: interaction between environments and genomic contents. Human genomics 2009, 4, 107–18. 10.1186/1479-7364-4-2-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag F.; Di Pizio A.; Krautwurst D. The key food odorant receptive range of broadly tuned receptor OR2W1. Food Chem. 2022, 375, 131680 10.1016/j.foodchem.2021.131680. [DOI] [PubMed] [Google Scholar]
- Haag F.; Hoffmann S.; Krautwurst D. Key Food Furanones Furaneol and Sotolone Specifically Activate Distinct Odorant Receptors. J. Agric. Food Chem. 2021, 69, 10999–11005. 10.1021/acs.jafc.1c03314. [DOI] [PubMed] [Google Scholar]
- Djoumbou Feunang Y.; Eisner R.; Knox C.; Chepelev L.; Hastings J.; Owen G.; Fahy E.; Steinbeck C.; Subramanian S.; Bolton E.; Greiner R.; Wishart D. S. ClassyFire: automated chemical classification with a comprehensive, computable taxonomy. J. Cheminform 2016, 8, 61. 10.1186/s13321-016-0174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N.; Yamano E.; Ishii A.; Tanaka M.; Nakamura J.; Watanabe Y. Involvement of the olfactory system in the induction of anti-fatigue effects by odorants. PLoS One 2018, 13, e0195263 10.1371/journal.pone.0195263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinek P.; Haag F.; Geithe C.; Krautwurst D. An evolutionary conserved olfactory receptor for foodborne and semiochemical alkylpyrazines. FASEB J. 2021, 35, e21638 10.1096/fj.202100224R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. Orthologs and paralogs - we need to get it right. Genome Biol. 2001, 2, INTERACTIONS1002. 10.1186/gb-2001-2-8-interactions1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinski K.; Botstein D. Orthology and functional conservation in eukaryotes. Annu. Rev. Genet 2007, 41, 465–507. 10.1146/annurev.genet.40.110405.090439. [DOI] [PubMed] [Google Scholar]
- Fang G.; Bhardwaj N.; Robilotto R.; Gerstein M. B. Getting started in gene orthology and functional analysis. PLoS Comput. Biol. 2010, 6, e1000703 10.1371/journal.pcbi.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaldon T.; Koonin E. V. Functional and evolutionary implications of gene orthology. Nat. Rev. Genet 2013, 14, 360–6. 10.1038/nrg3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov R. L.; Koonin E. V.; Lipman D. J. A genomic perspective on protein families. Science 1997, 278, 631–7. 10.1126/science.278.5338.631. [DOI] [PubMed] [Google Scholar]
- Nehrt N. L.; Clark W. T.; Radivojac P.; Hahn M. W. Testing the ortholog conjecture with comparative functional genomic data from mammals. PLoS Comput. Biol. 2011, 7, e1002073 10.1371/journal.pcbi.1002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masanetz C.; Grosch W. Key odorants of parsley leaves (Petroselinum crispum [Mill.] Nym. ssp. crispum) by odour-activity values. Flavour and Fragrance Journal 1998, 13, 115–124. . [DOI] [Google Scholar]
- Buttery R. G.; Black D. R.; Guadagni D. G.; Ling L. C.; Connolly G.; Teranishi R. California bay oil. I. Constituents, odor properties. J. Agric. Food Chem. 1974, 22, 773–777. 10.1021/jf60195a005. [DOI] [PubMed] [Google Scholar]
- Koff R. S.; Grady G. F.; Chalmers T. C.; Mosley J. W.; Swartz B. L. Viral hepatitis in a group of Boston hospitals. 3. Importance of exposure to shellfish in a nonepidemic period. N Engl J. Med. 1967, 276, 703–10. 10.1056/NEJM196703302761301. [DOI] [PubMed] [Google Scholar]
- Adipietro K. A.; Mainland J. D.; Matsunami H. Functional evolution of mammalian odorant receptors. PLoS Genet 2012, 8, e1002821 10.1371/journal.pgen.1002821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainland J. D.; Keller A.; Li Y. R.; Zhou T.; Trimmer C.; Snyder L. L.; Moberly A. H.; Adipietro K. A.; Liu W. L.; Zhuang H.; Zhan S.; Lee S. S.; Lin A.; Matsunami H. The missense of smell: functional variability in the human odorant receptor repertoire. Nat. Neurosci 2014, 17, 114–20. 10.1038/nn.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Kristeller D. C.; do Nascimento J. B. P.; Galante P. A. F.; Malnic B. Identification of agonists for a group of human odorant receptors. Front. Pharmacol. 2015, 6, 35. 10.3389/fphar.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz G.; Schlegel C.; Pernollet J. C.; Briand L. Comparison of odorant specificity of two human olfactory receptors from different phylogenetic classes and evidence for antagonism. Chem. Senses 2005, 30, 69–80. 10.1093/chemse/bji002. [DOI] [PubMed] [Google Scholar]
- Ohloff G.Importance of minor components in flavors and fragrances. 1978.
- Saito H.; Chi Q.; Zhuang H.; Matsunami H.; Mainland J. D. Odor coding by a Mammalian receptor repertoire. Sci. Signal 2009, 2, ra9. 10.1126/scisignal.2000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieki T.; Yamanaka Y.; Yoshikawa K. Functional analysis of human olfactory receptors with a high basal activity using LNCaP cell line. PLoS One 2022, 17, e0267356 10.1371/journal.pone.0267356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. S. Character impact odorants of Citrus Hallabong [(C. unshiu Marcov x C. sinensis Osbeck) x C. reticulata Blanco] cold-pressed peel oil. J. Agric. Food Chem. 2003, 51, 2687–92. 10.1021/jf021069o. [DOI] [PubMed] [Google Scholar]
- Pino J. A.; Mesa J. Contribution of volatile compounds to mango (L.) aroma. Flavour and Fragrance Journal 2006, 21, 207–213. 10.1002/ffj.1703. [DOI] [Google Scholar]
- Demole E.; Enggist P.; Ohloff G. 1-p-Menthene-8-thiol: A powerful flavor impact constituent of grapefruit juice (Citrus parodisi MACFAYDEN). Helv. Chim. Acta 1982, 65, 1785–1794. 10.1002/hlca.19820650614. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.