Summary
Background
Ensuring that individuals who are living with HIV rapidly initiate antiretroviral therapy (ART) is an essential step in meeting the 90–90-90 targets. We evaluated the feasibility and outcomes of rapid ART initiation in the Botswana Combination Prevention Project (BCPP). We aimed to establish whether simplified ART initiation with the offer of same-day treatment could increase uptake and reduce time from clinic linkage to treatment initiation, while maintaining rates of retention in care and viral suppression.
Methods
We did a quasi-experimental before and after study with use of data from the BCPP. The BCPP was a community-randomised HIV-prevention trial done in 30 communities across Botswana from Oct 1, 2013, to June 30, 2018. Participants in the 15 intervention clusters, who were HIV-positive and not already taking ART were offered universal HIV-treatment and same-day ART with a dolutegravir-based regimen at first clinic visit. This rapid ART intervention was implemented mid-way through the trial on June 1, 2016, enabling us to determine the effect of rapid ART guidelines on time to ART initiation and rates of retention in care and viral suppression at 1 year in the BCPP intervention group.
Findings
We assessed 1717 adults linked to study clinics before rapid ART introduction and 800 after rapid ART introduction. During the rapid ART period, 457 (57·1%, 95% CI 53·7–60·6) individuals initiated ART within 1 day of linkage, 589 (73·7%, 70·6–76·7) of 799 within 1 week, 678 (84·9%, 82·4–87·3) of 799 within 1 month, and 744 (93·5%, 91·6–95·1) of 796 within 1 year. Before the introduction of rapid ART, 163 (9·5%, 95% CI 8·2–11·0) individuals initiated ART within 1 day of linkage, 276 (16·1%, 14·4–17·9) within 1 week, 839 (48·9%, 46·5–51·3) within 1 month, and 1532 (89·2%, 87·7–90·6) within 1 year. 1 year after ART initiation, 1472 (90·5%, 87·4–92·8) of 1627 individuals who linked in the standard ART period were in care and had a viral load of less than 400 copies per mL, compared with 578 (91·6%, 88·1–94·1) of 631 in the rapid ART period (risk ratio 1·01, 95% CI 0·92–1·11).
Interpretation
Our findings provide support for the WHO recommendations for rapid ART initiation, and add to the accumulating evidence showing the feasibility, acceptability, and safety of rapid ART initiation in low-income and middle-income country settings.
Funding
US President’s Emergency Plan for AIDS Relief.
Introduction
Ensuring that individuals who are diagnosed with HIV infection rapidly initiate antiretroviral therapy (ART) is a crucial step in meeting the UNAIDs 90–90-90 targets;1,2 however, data from low-income and middle-income country settings have shown high rates of loss from the care cascade between HIV testing and ART initiation, or substantial delays to initiation of treatment.2–5 Many of these losses result from substantial barriers to ART initiation including the need for multiple clinic visits, repeated pre-ART counselling sessions, and delays in receiving baseline blood test results.6–10 A potential way to increase rates of ART initiation is to offer same-day ART to all individuals at their initial clinic visit—a strategy that became more feasible following the elimination of baseline CD4 cell count testing to determine treatment eligibility as well as the transition to a dolutegravir-containing first-line ART regimen.11
Several randomised controlled trials from low-income and middle-income countries have shown that rapid ART initiation is acceptable and feasible, and can increase rates of ART initiation, retention in care, and viral sup pression.12–15 In 2017, WHO updated treatment guidance to recommend rapid ART initiation (within ≤7 days) with the offer of same-day ART initiation in all individuals who were ready to start treatment.11 However, previous trials included enhancements to care beyond changes to ART timing, and also included CD4 cell count-based eligibility criteria, potentially limiting their generalis ability.2,16,17 Concerns also exist about ongoing retention in care after rapid ART initiation in patients with a high CD4 cell count.16,18
We evaluated the feasibility, uptake, and outcomes of rapid ART initiation with the offer of ART at first clinic visit in public sector ART clinics in Botswana in the intervention group of the cluster-randomised Botswana Combination Prevention Project (BCPP).19 We aimed to determine whether simplified ART initiation with the offer of same-day treatment could increase the uptake of ART and reduce time from clinic linkage to treatment initiation, while maintaining rates of retention in care and viral suppression.
Methods
Study design and participants
We did a quasi-experimental before and after study using data from the BCPP. The BCPP was a cluster-randomised HIV prevention trial done in 30 rural and peri-urban communities across Botswana with a total population of 180 000 people.19 The intervention strategy included community-wide, standardised, home-based, and mobile HIV testing campaigns, enhancement of routine testing in health facilities, and targeted outreach testing of all individuals aged 25 years and younger, as well as active linkage to care at local clinics for individuals with HIV who were not receiving ART. Expanded ART was provided at the time of clinic linkage.19 Study interventions were scaled up rapidly following trial commencement in 15 rural or peri-urban community clusters, which are the focus of this analysis, while 15 matched control community clusters received standard of care services (and are not included in this analysis). The mean community population was app roximately 6000 individuals.
Participants entered the BCPP cohort between Oct 1, 2013, and Oct 1, 2017, and interventions took place between Oct 30, 2013, and March 31, 2018. All 16–64-year-old community residents identified through community testing activities were assessed through a standard intake questionnaire and asked if they were HIV-positive. Individuals who did not know their status, did not have documentation of HIV-positive status, or did not have documentation of a negative HIV test within the preceding 3 months were offered rapid HIV-testing using KHB (Shanghai Kehua Bio-Engineering, Shanghai, China) and Unigold (Trinity Biotech, Bray, Ireland) parallel HIV tests. Discordant results were verified by laboratory testing with western blot. All participants provided verbal consent for HIV testing. The study was approved by the Centers for Disease Control and Prevention Institutional Review Board (Protocol #6475) and the Botswana Health Research and Develop ment Committee. The full protocol is available in the appendix (p 12).
Procedures
All newly identified and known individuals with HIV not on ART were counselled and referred to their local Ministry of Health community clinic for ART initiation and provided with linkage support services. Before the introduction of rapid ART initiation, individuals with HIV not on ART had a point-of-care CD4 cell count (PIMA CD4, Alere, Waltham, MA, USA) in the community at the initial community intake contact.
Before June 1, 2016, ART was initiated when an individual’s CD4 cell count was less than 350 cells per μL or they met criteria for WHO clinical stage III or IV for HIV Infection and Disease in Adults and Adolescents, based on Botswana’s Ministry of Health guidelines; or if they met the BCPP expanded ART eligibility criteria of HIV viral load of more than 10 000 copies per mL at any CD4 cell count, and from Aug 1, 2015, up until May 31, 2016, CD4 cell count less than 500 cells per μL (in line with updated WHO guidelines on the use of antiretroviral drugs for treating and preventing HIV infection). Standard procedures for ART initiation required three adherence counselling visits and baseline laboratory tests to be drawn and reviewed, thus requiring at least three clinic visits before ART initiation occurred. First-line therapy consisted of efavirenz, emtricitabine, and tenofovir as a single combination tablet.
Starting on June 1, 2016, all individuals with HIV were eligible and referred for ART, regardless of CD4 cell count or disease stage. Baseline viral load testing and point-of-care CD4 cell count testing were discontinued. The first-line regimen changed to emtricitabine and tenofovir as a combination tablet, plus dolutegravir. We concurrently introduced the rapid ART initiation intervention, with the offer of same-day ART initiation at first clinic visit after HIV testing (or following referral to the clinic for individuals who already knew their positive HIV status). A single nurse-led counselling session was done, and baseline blood tests including CD4 cell counts were taken at the initial clinic visit but providers were not required to await results before ART initiation. No further dedicated counselling sessions were given, with adherence counselling integrated into subsequent clinic visits. No additional changes to base line clinical assessment or opportunistic infection screening algorithms were made.
For the duration of the study, patients were seen 2 weeks after ART initiation for clinical review, then 3 months after ART initiation for viral load testing. Clinic appointments with viral load testing were then done every 6 months thereafter, unless the initial viral load was not suppressed, in which case patients would undergo repeat viral load testing 3 months later. CD4 cell count testing was done at baseline then annually unless the initial CD4 cell count was less than 200 cells per μL, in which case it was repeated after 6 months. ART and standard follow-up care were provided in public clinics by government staff. Patients who missed appoint ments or were lost to follow-up were contacted by clinic staff, either by telephone or, if possible, home visits, or tracked through a nationwide electronic medical records system to determine if they were obtaining care else where. At study initiation in 2013, each intervention clinic was supplemented by the Ministry of Health with an additional nurse to avoid congestion caused by additional community referrals, and a site coordinator whose role was to facilitate tracking and linkage of individuals who had tested positive for HIV in the community, and to ensure data were being collected for the study database.
Outcomes
The introduction of rapid ART provision mid-way through the study intervention period enabled us to examine the effect of rapid ART initiation guidelines on the uptake of ART and rates of retention in care and viral suppression. The analysis, planned at the time of rapid ART introduction, was restricted to individuals identified with HIV, not on ART, and linking for treatment in the 15 study intervention communities in which the rapid ART intervention was implemented. Data from control com munities were not included as the rapid ART intervention was not concurrently implemented at these sites, and detailed data for ART timing were not available. Primary outcome measures were time from first eligible ART clinic visit to ART initiation, and rates of retention in care and viral suppression (defined as a plasma viral load <400 copies per μL) at 1 year post-ART initiation. Given the potential for patients to have viral load testing at either 9 months or 12 months depending on viral suppression status at month 3, and to allow for delays in clinic attendance, a 90-day window was placed around the 1-year viral load timepoint. Patients were considered lost to follow-up if they had not attended any HIV-related clinic activity within 180 days of data censoring on June 28, 2018. Secondary outcomes included uptake of ART within 1 year of linkage, time from ART clinic linkage to HIV viral suppression, and mortality within 1 year of linkage.
Statistical analysis
The standard ART care cohort included any individual eligible for ART and linking to a clinic before June 1, 2016. The rapid ART start cohort included all individuals linked to a clinic from June 1, 2016, onwards following introduction of universal ART and rapid ART initiation guidelines. Data were summarised with use of frequencies and proportions with robust 95% CIs adjusted for clustering by community, and medians and IQRs. Rates of ART initiation were compared between groups using a hierarchical Cox proportional hazards model accounting for clustering by community through inclusion of a random-effects term. Individuals without the potential for a full year of follow-up were censored at the last potential follow-up time. Adjusted analyses included age, sex, and CD4 cell count as covariates. To account for the change in CD4-based ART initiation criteria, a stratified analysis was done restricted to individuals with CD4 cell counts of less than 350 cells per μL. An interrupted time-series analysis with a log-trans formed monthly aggregated ART timing data and proportion initiating ART within 7 days was done with use of the itsa function in Stata20 (appendix p 9).Segmented linear regression models estimated time or proportion each month and linear trends before and after rapid ART introduction, plus an estimate of the step-change at rapid ART introduction, with Newey-West SEs to handle autocorrelation and possible heteroskedasticity. Rates of retention in care and viral suppression at 1 year were summarised in the population with the potential for a full year of follow-up using proportions with robust 95% CIs adjusted for clustering by community, and compared between groups using a hierarchical mixed effects regression model incorporating a random effects term for community and adjustment for age, sex, and CD4 cell count. p values less than 0·05 were considered to be statistically significant. Data were analysed using Stata version 14 (StataCorp, College Station, TX, USA).
The study was monitored by an independent data and safety monitoring board, and prospectively registered at ClinicalTrials.gov, NCT01965470.
Role of the funding source
The funders of the study had no direct role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
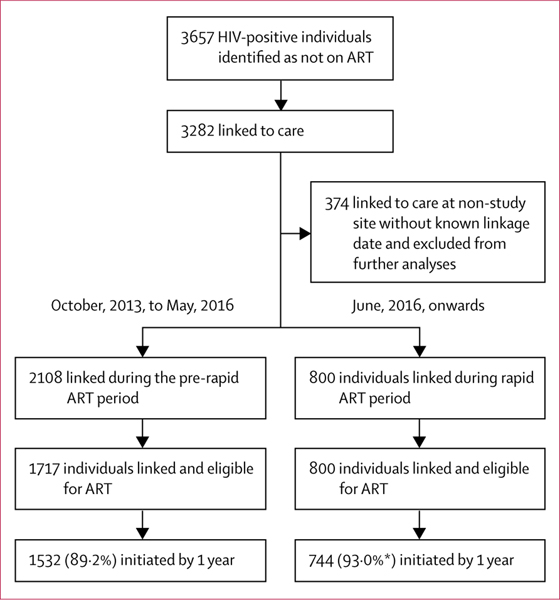
Between Oct 1, 2013, and March 31, 2018, BCPP research staff assessed HIV status in 61 655 community residents, of whom 13 328 (21·6%) were living with HIV. Of these, 3657 (27·4%) were not on ART; 3282 (89·7%) linked to care at an ART clinic a median of 6 days (IQR 3–35) after community assessment. 374 individuals linked to clinics outside the intervention communities who did not have reliably documented linkage or ART initiation dates and were excluded from further analysis. Of the remaining 2908 individuals included in the final analysis, 2108 linked during the standard ART period of whom 1717 (81·5%) were eligible for ART initiation, and 800 linked after the introduction of rapid ART initiation when all individuals with HIV were eligible for ART (figure 1). Baseline characteristics of study participants are shown in the table.
Figure 1: Trial profile.
ART=antiretroviral therapy. *The cumulative proportion on ART was 93·5% as not all individuals in the rapid ART group had completed 1 year of follow-up by the time of data censoring. In the pre-rapid ART period, 1465 (92·8%) of 1578 participants initiated efavirenz based regimens. In the rapid ART period 742 (99·1%) of 749 participants initiated dolutegravir based regimens.
Table:
Baseline characteristics
| Pre-rapid ART (n=1717) | Rapid ART (n=800) | p value | |
|---|---|---|---|
| Age, years | 37 (29–45) | 33 (26–41) | <0·0001 |
| Sex | |||
| Female | 1061 (61·8%) | 439 (54·9%) | 0·0010 |
| Male | 656 (38·2%) | 361 (45·1%) | ·· |
| Weight, kg* | 61 (54–71) | 61 (54–69) | 0·99 |
| New HIV diagnosis† | 812 (47·3%) | 484 (60·5%) | <0·0001 |
| Previous ART treatment | 108 (6·3%) | 54 (6·8%) | 0·020 |
| Baseline CD4 cell count, cells per μL‡ | 342 (232–472) | 344 (202–504) | 0·65 |
| Baseline creatinine, mmol/L | 67 (55–80) | 66 (55–77) | 0·52 |
| Baseline haemoglobin, g/dL L | 13·1 (11·8–14·3) | 13·4 (12·1–14·8) | 0·0010 |
| Baseline alanine aminotransferase, IU L | 17 (13–23) | 17 (12–24) | 0·78 |
Data are median (IQR) or n (%). Restricted to patients eligible for ART initiation. Data for age, sex, and previous HIV status were available for all participants. Baseline creatinine results were available in 1382 participants in the pre-rapid ART group and 572 participants in the rapid ART group. Baseline haemoglobin results were available in 1313 participants in the pre-rapid ART group and 635 participants in the rapid ART group. Baseline alanine aminotransferase results were available in 1288 participants in the pre-rapid ART group and 513 participants in the rapid ART group. p values for comparisons of proportions were derived from a hierarchical mixed effects regression models incorporating a random effects term for community. p values for comparisons of medians were derived from ranked testing (F-testing) of Somers’ D parameter estimates accounting for clustering by site. ART=antiretroviral therapy. IU=international units.
Weight was missing in 342 of 1717 participants in the pre-Rapid ART group and 232 of 800 participants in the rapid ART group.
Patients diagnosed as HIV-positive for the first time at study baseline testing.
Baseline CD4 cell counts were missing in 41 of 1717 participants in the pre-rapid ART group and 335 of 800 participants in the rapid ART group.
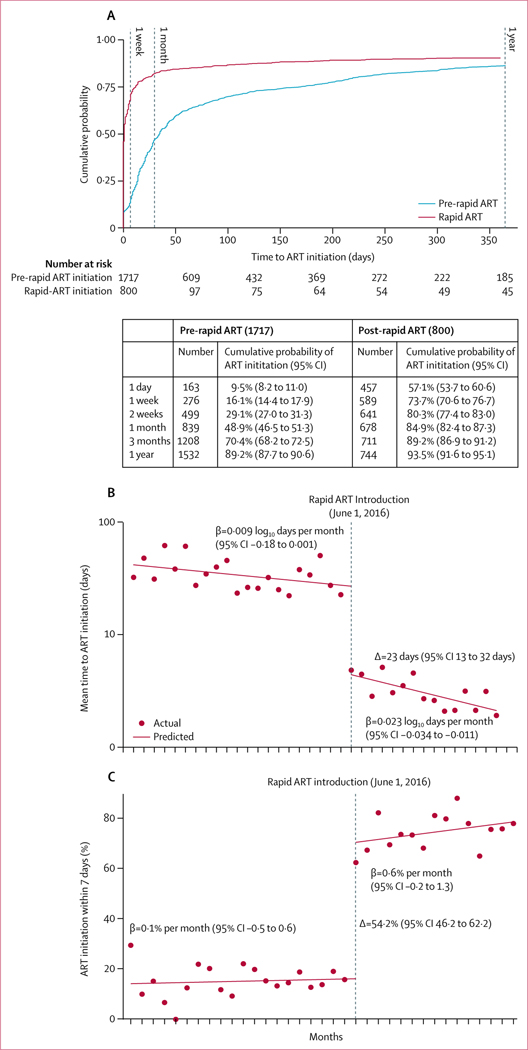
ART initiation occurred significantly more rapidly after the introduction of rapid ART guidelines on June 1, 2016 (figure 2A, adjusted hazard ratio 3·83, 95% CI 3·41–4·32 in hierarchical Cox proportional hazards model). During the rapid ART period, 457 (57·1%, 95% CI 53·7–60·6) linked individuals initiated ART within 1 day of linkage (ie, same-day, or the following morning if they presented to the clinic too late in the day to be seen by a prescriber), 589 (73·7%, 70·6–76·7) initiated within 1 week of linkage, and 678 (84·9%, 82·4–87·3) of 799 within 1 month; overall, 744 (93·5%, 91·6–95·1) of 796 individuals had initiated ART within the first year following linkage during the rapid ART period. Before the introduction of the rapid ART initiation guidelines, 276 (16·1%, 14·4–17·9) linked individuals initiated ART within 1 week of linkage, and 839 (48·9%, 46·5–51·3) within 1 month; overall, 1532 (89·2%, 87·7–90·6) individuals had initiated ART within the first year following linkage. Findings were very similar in analyses restricted to those with CD4 cell counts of 350 or less cells per μL, and CD4 cell counts of 500 or less cells per μL (appendix p 2). Analyses stratified by previous HIV diagnosis, sex, age, and CD4 cell count are shown in the appendix (p 7).
Figure 2: Time from linkage (first clinic visit) to ART initiation.
(A) Cumulative time to ART during the pre-rapid ART cohort (in blue) and following the introduction of rapid ART (in red), with cumulative probabilities in the table. (B) Results of an interrupted time-series analysis using log transformed monthly aggregate time-to-ART data. ART=antiretroviral therapy.
Interrupted time-series analyses with use of logtransformed monthly aggregate time-to-ART and monthly proportions initiating ART within 7 days of linkage showed significant step-changes following rapid ART introduction (figure 2B). There was no significant linear trend before introduction of rapid ART, but a significant decrease in time from linkage to ART initiation in the month following introduction of rapid ART initiation guidelines (Δ=–0·78 log10 days, 95% CI –0·93 to –0·63). Similarly, no trend was seen in the proportion of individuals initiating ART within 7 days during the standard ART initiation period (β coefficient 0·1% per month, 95% CI –0·5 to 0·7), but a significant increase in the month following introduction of rapid ART initiation guidelines (Δ=52·4%, 95% CI 46·2 to 62·2; p<0·0001; figure 2B, 2C). The Breusch-Godfrey test for auto correlation showed a p value of 0·14 at lag 1, and no evidence for autocorrelation at higher lag orders (6 tested, p>0·5 for all). Months with less than 15 ART initiations in total were excluded from both analyses to prevent any possible influence of statistical outliers due to small samples.
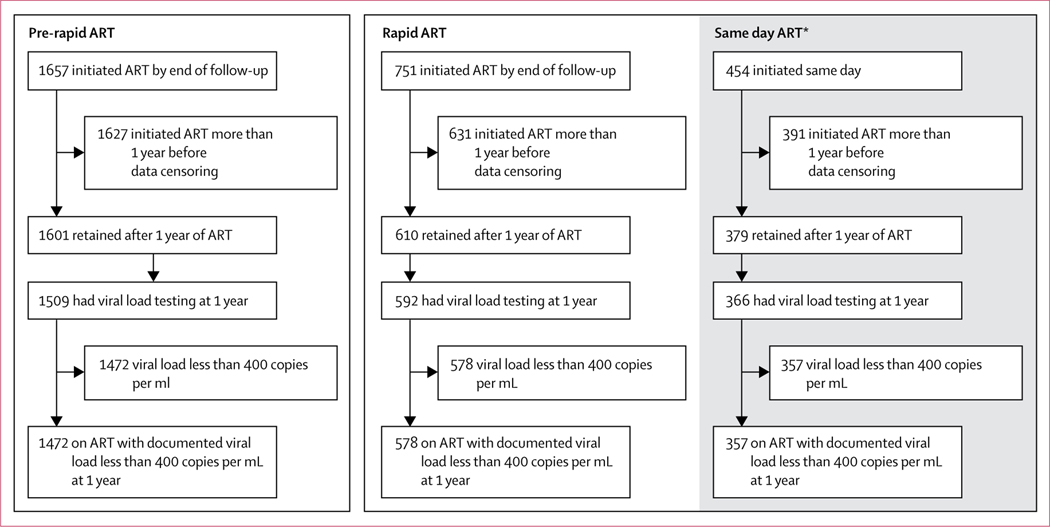
Retention in care at 1 year after ART initiation (limited to those individuals initiating ART at least 1 year before data censoring), and rates of viral suppression are shown in figure 3. Retention in care was 98·4% (1601 of 1627) at 1 year among individuals who linked before introduction of rapid ART initiation guidelines and initiated ART, and 96·7% (610 of 631) among individuals linking to care and initiating ART following introduction of rapid ART. 1-year viral load results were available for 1509 (92·7%) of 1627 individuals who linked and initiated ART in the standard ART period and 592 (93·8%) of 631 patients initiating during the rapid ART period, with viral suppression rates of 97·5% in the standard ART period and 97·6% during the rapid ART period. Overall, 90·5% (1472 of 1627, 95% CI 87·4–92·8) of individuals who linked in the standard ART period and initiated ART were in care and had a documented viral load of less than 400 copies per mL after 1 year of ART, compared with 91·6% (578 of 631, 88·1–94·1) of individuals who linked in the rapid ART period and initiated ART (risk ratio [RR] 1·01, 95% CI 0·92–1·11). Findings remained consistent in analysis adjusted for age, sex, and CD4 cell count (adjusted RR 1·04, 95% CI 0·93–1·17).
Figure 3: Retention in care and viral suppression at 1 year following standard or rapid ART initiation.
Analysis of retention and viral suppression at 1 year were restricted to individuals who had initiated ART at least 1 year before data censoring and had the potential for 1 year of follow-up. ART=antiretroviral therapy. *Those who initiated ART within 1 day of linkage.
Median time from linkage to documented viral suppression was significantly shorter following introduction of the rapid ART initiation guidelines, at 99 days (IQR 86–166) compared with 186 days (116–323) before rapid ART (p<0·0001). In the group linked to clinics before the introduction of rapid ART, 1276 (74·3%) of 1717 had reached viral suppression within a year of linkage, compared with 663 (82·9%) of 800 following the introduction of rapid ART (RR 1·12, 95% CI 1·02–1·23; adjusted RR 1·19, 1·06–1·33). During the standard (efavirenz-based) ART period, 1368 (82·7%) of 1654 participants who had initiated ART at least 6 months before data censoring had viral suppression within 6 months of ART initiation (median time from ART initiation to documented viral suppression was 112 days, IQR 84–194), compared with 643 (86·9%) of 740 during the rapid (dolutegravir based) ART period (median 93 days, IQR 82–139, RR 1·05, 95% CI 0·96–1·16; adjusted RR 1·09, 0·97–1·21).
12 (1·5%) of 800 patients who initiated ART following the introduction of rapid ART guidelines were found to have pre-ART creatinine clearance less than 60 mL per min (appendix p 8). Of these, nine initiated ART within the first 2 weeks, seven of whom started ART on the same day (ie, before availability of creatinine clearance results). Two of these nine patients had been given nonteno fovir-containing regimens (abacavir) due to a known history of renal impairment; three were switched to nontenofovir containing regimens (abacavir) on receipt of renal function results; and four remained on tenofovirbased regimens with stable renal function. Two of the nine patients died during the first year of follow-up. One patient had been given an abacavir-based regimen, and died of cervical carcinoma after 46 weeks of ART. The second patient was given a tenofovir-based regimen. Serum creatinine re mained stable at 91 mmol/L at week 2, and the attending clinicians opted to maintain the patient on tenofovir. The patient died after 20 weeks of ART of unknown causes. Overall mortality in the year following linkage was 1·2% (20 of 1717) in the standard ART period, and 1·0% (eight of 800) in the rapid ART period (RR 0·86, 95% CI 0·38–1·95; adjusted RR 0·88, 0·25–4·87).
Discussion
Simplified rapid ART initiation with the offer of same-day treatment at first ART clinic visit to all clinically stable patients was feasible in public ART clinics in Botswana, with more than half of patients initiating ART within 1 day of ART clinic linkage and 74% initiating within 1 week. Following the introduction of rapid ART, the proportion of patients established on ART increased from 49% to 85% at 1 month from clinic linkage, and from 89% to 94% at 1 year. The median time from clinic linkage to viral suppression was significantly reduced, with potential contributions from both more rapid initiation of ART and the switch to dolutegravir. Rates of retention in care and viral suppression were similarly high in individuals initiated on ART before the introduction of rapid ART and those initiated during the rapid ART period, with documented viral suppression after 1 year of ART in more than 90% of individuals in both groups.
Our findings provide support for the WHO recommendations of rapid ART initiation,11 adding to the accumulating evidence showing the feasibility, acceptability, and safety of rapid ART initiation in low-income and middle-income country settings, and provide some of the first evidence for high uptake rates among patients with high CD4 cell counts.3,21,22 Clinical trials from Haiti,14 Lesotho,15 South Africa,12,23 and Uganda13 have all shown the high uptake of same-day or rapid ART when offered to patients in clinic12–14,23 or community15 settings. In the RapIT trial in South Africa, 72% of individuals offered rapid ART initiation began on the same day, and 96% within 1 month.12 Similar figures were reported by Amanyire and colleagues from a cluster-randomised trial in Uganda,13 in which a clinic-level streamlined ART initiation intervention led to 71% of individuals initiating on the day of eligibility, compared with 18% in the control group. Even higher rates of 99% uptake of same-day ART have been reported from a clinic-based rapid ART initiation trial in Haiti,14 and 98% of participants in a community-based trial in Lesotho indicated readiness for same-day ART.15 These very high rates of same-day initiation (compared with 57% in our study) are generally in the context of selected populations; for example 225 (21%) of 1054 patients in the South African study were deemed too sick to participate; the Ugandan study only included patients once they had had CD4 cell count testing and clinical assessment and been deemed eligible for ART;13 and the Haitian study was also restricted to patients meeting clinical and CD4 cell count-based eligibility criteria.14 Initial data from analyses of uptake of rapid ART initiation in less selected populations in routine care settings in Africa show rates ranging from 25–70%, in keeping with our findings.21,22
Importantly, given potential concerns about higher attrition from care with rapid ART initiation and reductions in pre-ART counselling, particularly in individuals with high CD4 cell counts who might not perceive the need for treatment such as those in prevention of mother to child transmission of HIV Option B-plus programmes,16,18 our data also support the findings from these studies indicating that benefits of rapid ART initiation are sustained over time. Findings from the previous rapid ART studies in Haiti, Lesotho, South Africa, and Uganda have all indicated either equivalent or increased retention in care and viral suppression with rapid ART when compared with standard models of ART delivery.12–15 Our finding of 90% documented retention and viral suppression at 1 year following ART initiation closely matches data from the SEARCH trial in Uganda and Kenya, showing 89% retention in care among patients newly linked to care and rapidly initiated on ART.24
To the best of our knowledge, these are the first data reporting the outcomes of rapid ART initiation in a routine low-income and middle-income country care setting, without baseline screening blood tests, point-of-care CD4 cell counts, or additional assessment of asymptomatic patients. Although the safety of initiating ART while awaiting laboratory results has understandably been of concern to many clinicians,2 we did not document adverse patient outcomes arising from either initiation of tenofovir-based therapy in the absence of a serum creatinine result, or the development of immune reconstitution inflammatory syndromes in patients with low CD4 cell counts. Mortality within 1 year of linkage was 1% during both the standard ART and rapid ART periods.
The more rapid ART initiation and shorter time from linkage to viral suppression observed following implementation of rapid dolutegravir-based ART initiation in our study are likely to have both individual-level and population-level benefits. HIV transmission risk is highly dependent on viral load,25 with convincing evidence for marked reductions in transmission to sexual partners in individuals on effective ART.26 It is also probable that rapid ART can confer individual-level health benefits, particularly among individuals with low CD4 cell counts (<200 cells per μL) who are at a very high risk of death if ART is delayed for even a few weeks.27,28 We did not find a mortality reduction following rapid ART initiation in our setting, probably in part due to the relatively high median CD4 cell count at ART initiation and low numbers of individuals with CD4 cell counts of less than 200 cells per μL, meaning we did not have sufficient power to ascertain differences in morbidity and mortality. A further potential reason for the absence of an observed mortality benefit in our study is that during the standard ART period, individuals with low CD4 cell counts were identified with point-of-care counts, which would not be the case in routine care. Having been identified, these individuals were often quickly started on ART, lessening the effect of the introduction of rapid ART initiation guidelines in our study.
Our study had several limitations. Firstly, as a quasi-experimental before and after study, our pre-ART and post-rapid ART groups might not have been directly comparable. The group initiating rapid ART were more likely to be newly diagnosed with HIV, more likely to be men, and more likely to be younger; all factors previously associated with worse uptake of HIV services, suggesting we might have underestimated the effect of the rapid ART intervention.6,29–31 The before and after design also leads to the possibility of temporal confounding. Although there were no major changes in ART initiation procedures other than intro duction of rapid ART during the study, it is possible that clinic staff became more proficient at initiating patients as the study progressed; or that other study-specific or external factors influenced ART timing (eg, we did not have detailed information on rates of clinically suspected tuberculosis or pregnancy at baseline, both factors that might influence ART timing, although it is unlikely these differed by study period). The interrupted time-series analysis, done to examine this possibility, revealed no significant temporal trends in time to ART before rapid ART implementation suggesting that the assumptions necessary for causal inference regarding the effect of rapid ART guidelines on ART timing in the model were valid.20 Additionally, as rapid ART was implemented during the latter half of the trial, partici pants in the rapid ART group did not have the same length of time as those in the standard ART group to cycle back into care and be classified as on treatment after missing appointments, potentially leading to an underestimation of retention in care in the rapid ART period. Secondly, a change that was imple mented concurrently with rapid ART was the switch to dolutegravir-based ART. This change is unlikely to influence ART timing but is likely to have contributed to the more rapid viral suppression in the later cohort. Median time from ART initiation to viral suppression was slightly shorter in the rapid ART period when dolutegravir was the standard ART regimen but did not account for the major difference in time from linkage to viral suppression. Finally, results might not be directly gen eralisable to all African or low-income and middle-income country settings. Our study sites were rural or peri-urban, and the study population was 60% women with a median age of almost 35 years, and a relatively high median baseline CD4 cell count of 350 cells per μL. Although the study was done in public facilities, extra trained staff were placed by the Ministry of Health (with support from the study team) to deal with congestion at the clinics. Additional staff training was provided, and a higher level of monitoring and supervision was done than is standard in the government sector in Botswana. Most HIV testing was community based, with testing staff receiving specific training on delivering counselling messages facilitating rapid ART initiation.
In conclusion, we have shown that offering same-day ART to individuals presenting for HIV care in public sector clinics in Botswana leads to high rates of ART initiation, significantly reduced time from linkage to starting ART, and significantly reduced time to viral suppression. Rapid ART initiation was safe, even in the absence of baseline blood test results, and the benefits were sustained, with similar rates of retention in care and viral suppression after 1 year of ART to those seen with traditional models of care.
Supplementary Material
Research in context.
Evidence before this study
Strong evidence exists for both the patient-level health benefits of antiretroviral therapy (ART) and public health benefits of ART resulting from reduced HIV transmission. To realise these benefits, it is essential that individuals who are diagnosed with HIV rapidly initiate ART. However, data from low-income and middle-income settings have shown high rates of loss from the care cascade between HIV testing and ART initiation, or substantial delays to treatment initiation. A potential way to increase rates of ART initiation is to offer same-day ART to all individuals at their initial clinic visit. We searched PubMed, Embase, and PubMed Central for studies published in any language between Jan 1, 2000, and July 31, 2019, investigating the acceptability, feasibility, safety, and outcomes of rapid or same-day ART initiation in individuals with HIV presenting to treatment services (search terms included “HIV”; “antiretroviral therapy” or “ART”; and rapid, “same day” or “fast track”). We included all cohort studies or randomised controlled trials reporting outcomes of rapid or same-day ART interventions. Several randomised controlled trials from low-income and middle-income country settings have shown that rapid ART initiation, including treatment initiation at the first clinic visit, is acceptable and feasible, and can increase rates of ART initiation, retention in care, and viral suppression. However, previous trials included enhancements to care beyond changes to ART timing, and also included CD4 cell count eligibility criteria, potentially limiting their generalisability to more routine care settings in the test and treat era.
Added value of this study
We evaluated the feasibility and outcomes of rapid ART initiation with the offer of ART at first clinic visit in the context of the cluster-randomised Botswana Combination Prevention Project. Simplified rapid ART initiation with the offer of same-day treatment at first ART clinic visit was acceptable and feasible in public ART clinics in Botswana. To the best of our knowledge, these are the first data reporting the outcomes of rapid ART initiation in a routine low-income and middle-income country care setting, without baseline screening blood tests, point-of-care CD4 cell counts, or additional clinical assessments. Our findings add to the accumulating evidence showing the feasibility, acceptability, and safety of rapid ART initiation in low-income and middle-income country settings, and indicate that benefits of rapid ART initiation are sustained over time.
Implications of all the available evidence
The faster ART initiation and shorter time from clinic linkage to viral suppression observed following implementation of rapid ART initiation guidelines are likely to have both individual-level and population-level benefits. The findings support WHO guidance from 2017 recommending rapid ART initiation and could help HIV-treatment programmes in Africa and globally reach the ambitious UNAIDS 90–90-90 targets.
Acknowledgments
We thank all BCPP staff who contributed to the study, the Botswana Ministry of Health and Wellness for their input into the project, and the study participants. This project was supported by the United States President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of Cooperative Agreements U2G GH000073 and U2G GH000419. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the funding agencies. Preliminary results were presented at the Conference on Retroviruses and Opportunistic Infections, Seattle, USA, Feb 13–16, 2017, and at the International AIDS Society Conference in Amsterdam, Netherlands, July 23–27, 2018.
JNJ reports grants from the European and Developing Countries Clinical Trials Partnership and funding from the UK National Health Service (NHS) National Institute for Health Research (NIHR) using Official Development Assistance (ODA) funding through a Global Health Professorship (grant RP-2017–08-ST2–012) outside the submitted work. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, the Department of Health and Social Care, or other funding entities. SL reports grants from the United States President’s Emergency Plan for AIDS Relief/Centers for Disease Control and Prevention during the conduct of the study.
Footnotes
Declaration of interests
All other authors declare no competing interests.
Contributor Information
Refeletswe Lebelonyane, Botswana Ministry of Health and Wellness, Gaborone, Botswana.
Pamela Bachanas, Centers for Disease Control and Prevention, Division of Global HIV/AIDS and TB, Atlanta, GA, USA.
Lisa Block, Northrop Grumman, Atlanta, GA, USA.
Faith Ussery, Centers for Disease Control and Prevention, Division of Global HIV/AIDS and TB, Atlanta, GA, USA.
William Abrams, Centers for Disease Control and Prevention, Gaborone, Botswana.
Michelle Roland, Centers for Disease Control and Prevention, Gaborone, Botswana.
Joe Theu, Botswana Ministry of Health and Wellness, Gaborone, Botswana.
Max Kapanda, Botswana Ministry of Health and Wellness, Gaborone, Botswana.
Stembile Matambo, Botswana Ministry of Health and Wellness, Gaborone, Botswana.
Shahin Lockman, Harvard T H Chan School of Public Health, Boston, MA, US; Brigham and Women’s Hospital, Boston, MA, USA; Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Tendani Gaolathe, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; University of Botswana School of Medicine, Gaborone, Botswana.
Joseph Makhema, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana.
Janet Moore, Centers for Disease Control and Prevention, Division of Global HIV/AIDS and TB, Atlanta, GA, USA.
Prof Joseph N Jarvis, Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; Botswana– University of Pennsylvania Partnership, Gaborone, Botswana; Department of Clinical Research, Faculty of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, UK.
Data sharing
Anonymised individual participant data and a data dictionary defining each field in the dataset will be provided on request by the corresponding author. The dataset includes the de-identified participant data required to reproduce all analyses in the paper and associated analytical code. The statistical analysis plan can also be supplied on request. Data will be supplied in the form of a Stata data file and do file. These data will be made available from the time of publication and will be accessible to researchers who provide a methodologically sound proposal for any non-commercial use.
References
- 1.UNAIDS. 90–90–90—An ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS (UNAIDS). 2014. https://www.unaids.org/en/resources/documents/2017/90-90-90 (accessed May 23, 2019).
- 2.Rosen S, Fox MP, Larson BA, et al. Accelerating the uptake and timing of antiretroviral therapy initiation in sub-Saharan Africa: an operations research agenda. PLoS Med 2016; 13: e1002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bor J, Chiu C, Ahmed S, et al. Failure to initiate HIV treatment in patients with high CD4 counts: evidence from demographic surveillance in rural South Africa. Trop Med Int Health 2018; 23: 206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med 2011; 8: e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clouse K, Pettifor AE, Maskew M, et al. Patient retention from HIV diagnosis through one year on antiretroviral therapy at a primary health care clinic in Johannesburg, South Africa. J Acquir Immune Defic Syndr 2013; 62: e39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS 2012; 26: 2059–67. [DOI] [PubMed] [Google Scholar]
- 7.Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc 2012; 15: 17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed S, Autrey J, Katz IT, et al. Why do people living with HIV not initiate treatment? A systematic review of qualitative evidence from low- and middle-income countries. Soc Sci Med 2018; 213: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng EH, Bwana MB, Muyindike W, et al. Failure to initiate antiretroviral therapy, loss to follow-up and mortality among HIV-infected patients during the pre-ART period in Uganda. J Acquir Immune Defic Syndr 2013; 63: e64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maughan-Brown B, Kuo C, Galárraga O, et al. Stumbling blocks at the clinic: experiences of seeking HIV treatment and care in South Africa. AIDS Behav 2018; 22: 765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infectionRecommendations for a public health approach—Second edition. World Health Organization. Geneva; 2016. https://www.who.int/hiv/pub/arv/arv-2016/en/ (accessed May 23, 2019). [Google Scholar]
- 12.Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient’s first clinic visit: the RapIT randomized controlled trial. PLoS Med 2016; 13: e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV 2016; 3: e539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koenig SP, Dorvil N, Dévieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Med 2017; 14: e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: the CASCADE randomized clinical trial. JAMA 2018; 319: 1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng EH, Havlir DV. The science of rapid start–from the when to the how of antiretroviral initiation. PLoS Med 2017; 14: e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosen S, Maskew M, Brennan AT, et al. Improved simplified clinical algorithm for identifying patients eligible for immediate initiation of antiretroviral therapy for HIV (SLATE II): protocol for a randomized evaluation. Trials 2018; 19: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women (‘Option B+’) in Malawi. AIDS 2014; 28: 589–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makhema J, Wirth KE, Pretorius Holme M, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019; 381: 230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linden A Conducting interrupted time-series analysis for single- and multiple-group comparisons. Stata J 2015; 12: 480–500. [Google Scholar]
- 21.Tymejczyk O, Brazier E, Yiannoutsos CT, et al. Changes in rapid HIV treatment initiation after national “treat all” policy adoption in 6 sub-Saharan African countries: Regression discontinuity analysis. PLoS Med 2019; 16: e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross J, Sinayobye JD, Yotebieng M, et al. Early outcomes after implementation of treat all in Rwanda: an interrupted time series study. J Int AIDS Soc 2019; 22: e25279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens WS, Gous NM, MacLeod WB, et al. Multidisciplinary point-of-care testing in South African primary health care clinics accelerates HIV ART initiation but does not alter retention in care. J Acquir Immune Defic Syndr 2017; 76: 65–73. [DOI] [PubMed] [Google Scholar]
- 24.Brown LB, Havlir DV, Ayieko J, et al. High levels of retention in care with streamlined care and universal test and treat in East Africa. AIDS 2016; 30: 2855–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000; 342: 921–29. [DOI] [PubMed] [Google Scholar]
- 26.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One 2009; 4: e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS 2008; 22: 1897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornell M, Schomaker M, Garone DB, et al. Gender differences in survival among adult patients starting antiretroviral therapy in South Africa: a multicentre cohort study. PLoS Med 2012; 9: e1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai AC, Siedner MJ. The missing men: HIV Treatment scale-up and life expectancy in sub-Saharan Africa. PLoS Med 2015; 12: e1001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auld AF, Agolory SG, Shiraishi RW, et al. Antiretroviral therapy enrolment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults–seven African countries, 2004–2013. MMWR Morb Mortal Wkly Rep 2014; 63: 1097–103. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised individual participant data and a data dictionary defining each field in the dataset will be provided on request by the corresponding author. The dataset includes the de-identified participant data required to reproduce all analyses in the paper and associated analytical code. The statistical analysis plan can also be supplied on request. Data will be supplied in the form of a Stata data file and do file. These data will be made available from the time of publication and will be accessible to researchers who provide a methodologically sound proposal for any non-commercial use.