Abstract
Background
In the past few years, there has been a continuous rise in the occurrence of renal cell carcinoma (RCC), with RCC recurrence becoming the primary factor behind fatalities. Despite numerous clinical trials, the impact of different medications on the long-term survival of patients with RCC after surgery remains uncertain. This network meta-analysis aimed to evaluate the impact of various medications on the survival and safety of drugs in individuals with RCC following nephrectomy.
Methods
We conducted a thorough search in various databases, including CNKI, WAN FANG DATA, VIP, Web of Science, Cochrane Library (CENTRAL), PubMed, Scopus, and Embase, for articles published prior to June 2, 2023. This meta-analysis incorporated randomized controlled trials (RCTs).
Results
The analysis included 17 studies with 14,298 participants. The findings from the disease-free survival (DFS) analysis indicated that pembrolizumab demonstrated efficacy in enhancing DFS among patients with RCC following nephrectomy when compared to the placebo group (HR = 0.83, 95%CI 0.70 to 0.99). None of the drugs included in the study significantly improved overall survival (OS) and recurrence-free survival (RFS) after nephrectomy. For adverse events (AEs), sorafenib, pazopanib, sunitinib, and nivolumab plus ipilimumab interventions showed a higher incidence of adverse events compared with placebo.
Conclusion
The network meta-analysis yielded strong evidence indicating that pembrolizumab could potentially enhance DFS in patients with RCC following nephrectomy, surpassing the effectiveness of a placebo.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12894-024-01441-8.
Keywords: Renal cell carcinoma, Network meta-analysis, Adjuvant therapy, Efficacy
Introduction
The urinary system is commonly affected by renal cell carcinoma (RCC), a widespread malignant tumor. The locoregional disease is diagnosed in approximately 80% of patients [1]. Nevertheless, even after going through a surgical procedure, numerous individuals encounter a relapse, with recurrence rates over a span of 5 years varying from 10% in patients with low risk to as high as 68% in patients with high risk [2, 3]. Either partial or radical nephrectomy is the typical approach for treating locoregional RCC. Although numerous advancements have been in treating advanced illnesses in recent years, the effectiveness of post-nephrectomy adjuvant therapy remains unclear.
In patients with advanced disease, sunitinib and sorafenib, which are Vascular endothelial growth factor receptor (VEGFR) inhibitors, have demonstrated the ability to improve progression-free survival. Indeed, the prolonged utilization of these inhibitors in advanced stages has been noted to enhance the median overall survival period from 13 months to more than 29 months [4].
The probability of recurrence within the initial five years after the procedure was highest among patients who were considered free of disease following nephrectomy for RCC. Nearly all T4 patients experience relapse after nephrectomy, approximately 50% of T3 patients, and up to 26% of T2 patients, making the primary tumor stage a widely recognized prognostic indicator [5]. Increased tumor nuclear grade and the existence of sarcomatoid characteristics have also been recognized as separate variables linked to an elevated possibility of disease recurrence [6]. Patients with resectable soft tissue metastases at the time of diagnosis and primary kidney tumors (stage M1 disease) constitute another category of individuals who could potentially gain advantages from adjuvant therapy [7]. Even after successful removal of the kidney and complete removal of metastatic growths, individuals who are eligible for surgery still face a significant chance of experiencing a relapse and mortality within a span of 5 years post-operation, without any existing options for additional treatment.
In the United States of America (USA), the adjuvant therapy approval for patients at high risk of recurrence was granted to sunitinib, an anti-VEGF tyrosine kinase inhibitor(TKI), due to the S-TRAC trial’s results showing enhanced disease-free survival (DFS) in comparison to a placebo [8]. Nevertheless, this study did not demonstrate any significant benefit in terms of overall survival. Additional tests on anti-VEGF medications like pazopanib, axitinib, and sorafenib have failed to achieve their main effectiveness goals. At the same time, sunitinib has yielded inconsistent outcomes regarding DFS in randomized phase 3 trials [9–11]. Although sunitinib is not universally endorsed as a treatment in this context, there is insufficient strong evidence supporting its effectiveness for RCC.
The emergence of immune checkpoint inhibitors provides a new treatment option for tumor patients. Some researchers have found that nivolumab combined with ipilimumab can significantly improve the prognosis of RCC patients compared with the traditional targeted drug sunitinib [12]. Similarly, pembrolizumab combined with axitinib significantly improved patients’ objective response rate (ORR) [13]. However, the understanding of the application of adjuvant therapy after renal cancer surgery is still insufficient.
Despite some discrepancies in current research, more direct comparative studies are needed to evaluate the effectiveness of various agents. Researchers can utilize network meta-analysis to examine the efficacy of two different agents in studies with a placebo as the control group by incorporating direct and indirect comparisons. Hence, this research conducted an extensive assessment of the impacts of supplementary medications like sunitinib, sorafenib, and atezolizumab on survival measures such as DFS, overall survival (OS), and recurrence-free survival (RFS) in individuals following surgical removal of RCC via a meta-analysis involving multiple studies. The study also assessed the safety of these drugs to determine the best options for patients following RCC resection.
Methods
The research was carried out following the PRISMA guidelines, and the meta-analysis protocol can be found on the PROSPERO website, the registration ID is CRD42023440272. The meta-analysis process strictly follows the checklist of the Preferred Reporting Items for Systematic Review and Meta-Analyses guidelines (Supplement table I).
Search strategy
We searched multiple databases, such as CNKI (https://www.cnki.net/), WAN FANG DATA (https://www.wanfangdata.com.cn/), VIP (http://www.cqvip.com/), Web of Science (https://webofscience.clarivate.cn/), Cochrane Library (https://www.cochranelibrary.com/), PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.scopus.com/), and Embase (https://www.embase.com/), from inception to June 2, 2023, to find relevant studies. The search utilized the subsequent Mesh terms: (‘Kidney Tumors’ OR ‘Renal Tumors’ OR ‘Renal Malignancy’ OR ‘Kidney Malignancy’ OR ‘renal cell malignancy’ OR ‘renal cell tumor’ OR ‘kidney tumor’ OR ‘renal tumor’).
Selection criteria
In order to establish inclusion, the researchers applied the following criteria: (1) individuals diagnosed with RCC; (2) previous nephrectomy and/or metastasectomy leading to complete remission; (3) patients who received adjuvant therapy after nephrectomy and/or metastasectomy; (4) documentation of DFS, OS, RFS, AEs with a grade of ≥ 3; and (5) RCTs. The exclusion criteria included the following: (1) non-experimental studies, correspondences, evaluations, or summaries from conferences; (2) studies with only one group; (3) studies involving animals or laboratory investigations; and (4) repeated literature publications.
Extraction of data and evaluation of quality
Data from the included studies were independently extracted by two investigators (Guo LY and An T) using the Cochrane Risk of Bias 2.0 tool to evaluate the bias risk of each RCT. The senior reviewer (Huang ZX) resolved any inconsistencies. The collected data consisted of the primary author’s name, year of publication, patient count, medical condition, prescribed medications, the dosage of treatment, average duration of follow-up, severe AEs with a grade equal to or higher than 3, as well as the hazard ratios (HR) and corresponding 95% confidence intervals (CIs) for DFS, OS, and RFS.
Data analysis
In order to assess the existence of incongruity, tests for both incongruity and congruity were performed. I2 was primarily used to assess the degree of heterogeneity. When there was no difference between the results (I2 ≤ 50%), the fixed effects model was used for meta-analysis; otherwise, the random effects model was used. Following the elimination of notable clinical variability, a random-effects approach was employed for the meta-analysis. To assess whether the statistical significance was achieved for DFS, OS, and RFS between any pair, a net-league table (referred to as a matrix in algebra) was employed. STATA 14.0 MP was utilized to perform traditional meta-analyses on AEs graded ≥ 3. This process generated Napierian logarithm odds ratios (lnOR) and their corresponding standard error (selnOR) for each individual study. Subsequently, the lnHR and selnHR values for DFS, OS, and RFS, along with the lnOR and selnOR values for AEs, were entered into R 4.3.1. The Netmeta package performed data processing, network data plots, and forest plots sequentially.
Results
The studies that were included had certain characteristics
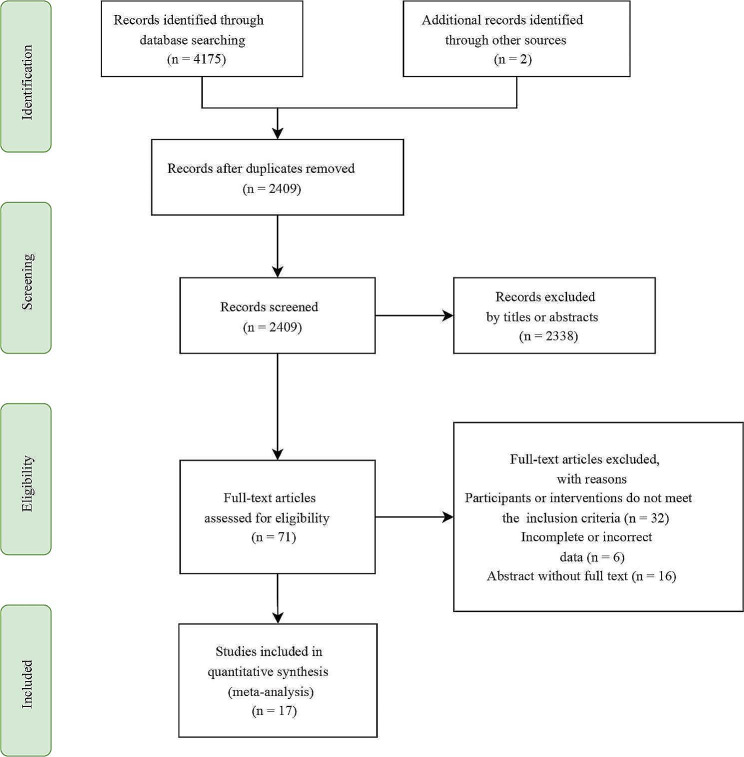
During the initial search period, we obtained a total of 4,177 publications published from 1977 to 2023. After removing duplicates and evaluating titles and abstracts, a total of 711 studies were considered suitable for a thorough examination, and eventually, 17 studies fulfilled our requirements (Fig. 1). Ultimately, all of the studies included 14,298 patients and compared 11 different treatments, specifically sunitinib, sorafenib, nivolumab in conjunction with ipilimumab, IL2 + IFN + 5FU, atezolizumab, pembrolizumab, brentuximab, tegafur in addition to uracil, thalidomide, and axitinib. We presented a comprehensive explanation of the included studies (Table 1). In these studies, all participants had fully recovered after undergoing a RCC nephrectomy, and the reported follow-up period varied from 24.1 to 112.9 months. Similarly, we tabulated the number of studies and patient samples included for different interventions (Table 2).
Fig. 1.
The flowchart shows the process of literature filtering
Table 1.
Characteristics of studies included in the network meta-analysis
| Study | Year of publication | Treatment | Sample size (T/C) | Median age | Gender (male/ female) | Follow up (month) | Therapeutic regimen | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Robert J Motzer [14] | 2023 | nivolumab+ipilimumab | 405 | 58 | 286/119 | 37 | nivolumab (240 mg) every 2 weeks for 12 doses plus ipilimumab (1 mg/kg) every 6 weeks for 4 doses | DFS/AE |
| placebo | 411 | 57 | 294/117 | 37 | ||||
| M. Aitchison [15] | 2014 | IL2 + IFN + 5FU | 154 | 57 | 107/47 | 72 | triple combination therapy (5-flurouracil, alpha-interferon (Roferon), interleukin-2(Proleukin)) no later than 12 weeks following surgery | DFS/OS |
| observation | 155 | 55 | 101/44 | 72 | ||||
| Sumanta Kumar Pal [7] | 2022 | atezolizumab | 390 | 61 | 287/103 | 60 | atezolizumab (1200 mg intravenous) once every 3 weeks for 16 cycles or 1 year | DFS/AE |
| placebo | 388 | 60 | 278/110 | 60 | ||||
| Robert J. Motzer [9] | 2021 | pazopanib | 769 | 58 | 537/232 | 76 | pazopanib or placebo for 1 year | OS |
| placebo | 769 | 59 | 554/215 | 76 | ||||
| Choueiri, Toni K [16] | 2021 | pembrolizumab | 496 | 60 | 347/149 | 24.1 | pembrolizumab(200 mg) once every 3 weeks for up to 17cycles (approximately 1 year) | DFS/AE |
| placebo | 498 | 60 | 359/139 | 24.1 | ||||
| Tim Eisen, [17] | 2020 | sorafenib | 639 | 58 | 458/181 | 78 | sorafenib 400 mg twice per day orally | DFS/OS/AE |
| sorafenib + placebo | 642 | 58 | 452/190 | 78 | ||||
| placebo | 430 | 58 | 306/124 | 78 | ||||
| Robert J. Motzer [18] | 2018 | sunitinib | 309 | 57 | 222/87 | 60 | sunitinib or placebo for nine cycles (_1 year) | DFS/OS/AE |
| placebo | 306 | 58 | 230/76 | 78 | ||||
| Naomi B Haas [5] | 2016 | sunitinib | 647 | 56 | 429/218 | 60 | sunitinib 50 mg per day orally, sorafenib 400 mg twice per day orally for 54 weeks | DFS/OS/AE |
| sorafenib | 649 | 55 | 437/212 | 60 | ||||
| placebo | 647 | 57 | 443/204 | 60 | ||||
| J Atzpodien [19] | 2005 | IL2 + IFN + 5FU | 135 | 59 | 97/38 | 51.6 | subcutaneous interleukin-2, interferon-alpha2a, and intravenous 5-fluorouracil for 8 weeks | RFS/OS |
| observation | 68 | 60 | 54/14 | 51.6 | ||||
| Karim Chamie [20] | 2017 | girentuximab | 433 | 58 | 276/157 | 54 | girentuximab, 50 mg (week 1), followed by weekly intravenous infusions of girentuximab, 20 mg (weeks 2–24) | DFS/OS/AE |
| placebo | 431 | 58 | 298/133 | 54 | ||||
| Giuseppe Procopio [21] | 2019 | sorafenib | 32 | 65 | 20/12 | 38 | sorafenib (standard dose 400 mg twice daily) for 52 wk | RFS/OS/AE |
| observation | 36 | 59 | 45/80 | 38 | ||||
| Thomas Powles [22] | 2021 | pembrolizumab | 496 | 60 | 347/149 | 30.1 | pembrolizumab 200 mg intravenously every 3 weeks for up to 17 cycles | DFS/OS/AE |
| Placebo | 498 | 60 | 359/139 | 30.1 | ||||
| Seiji Naito [23] | 1997 | tegafur + uracil | 33 | - | 22/11 | 112.9 | tegafur and uracil (300 to 600 mg as tegafur) every day for 2 years | RFS/AE |
| observation | 33 | - | 22/11 | 112.9 | ||||
| A. Mennitto [24] | 2021 | sorafenib | 32 | 65 | 20/12 | 42 | sorafenib (standard dose 400 mg twice daily) for 52 wk | RFS |
| observation | 36 | 59 | 45/80 | 42 | ||||
| Robert J. Motze [25] | 2017 | pazopanib600mg | 571 | 58 | 398/173 | 47.9 | pazopanib 800 mg once daily as the starting dose, then reduced to 600 mg once daily | DFS/OS/AE |
| placebo | 564 | 58 | 400/164 | |||||
| pazopanib800mg | 198 | 56 | 139/59 | 47.9 | ||||
| placebo | 205 | 60 | 154/51 | 47.9 | ||||
| Naomi B. Haas [26] | 2017 | sunitinib | 358 | 58 | 243/115 | 60 | sunitinib (50 mg), sorafenib (800 mg) for 1 year | DFS/OS |
| sorafenib | 355 | 57 | 248/107 | 60 | ||||
| placebo | 356 | 58 | 254/102 | 60 | ||||
| M. Gross-Goupil [11] | 2018 | axitinib | 363 | 58 | 280/83 | 31 | axitinib 5 mg twice-daily oral | DFS/AE |
| placebo | 361 | 58 | 250/11 | 31 |
DFS, disease-free survival; OS, overall survival; RFS, recurrence-free survival; AE, adverse event
Table 2.
The number of studies and patient samples included for different interventions
| Treatment | Study | Sample size |
|---|---|---|
| nivolumab + ipilimumab | 1 | 405 |
| IL2 + IFN + 5FU | 2 | 289 |
| atezolizumab | 1 | 390 |
| pazopanib | 2 | 1538 |
| pembrolizumab | 2 | 992 |
| sorafenib | 5 | 2349 |
| sunitinib | 3 | 1314 |
| girentuximab | 1 | 433 |
| tegafur + uracil | 1 | 33 |
| axitinib | 1 | 363 |
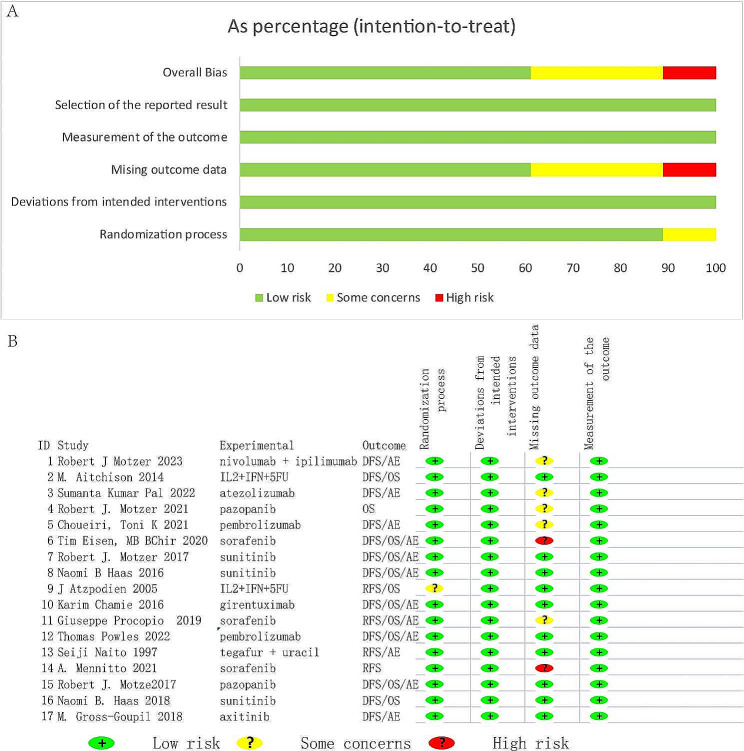
Potential for bias in research
Out of all the research conducted, 6 were categorized as open-label. The method of randomization was not provided in 1 article, and 7 articles mentioned withdrawals of over 20% during the follow-up period. The details of the risk of bias assessment for each study are presented in Fig. 2 (Fig. 2A and B).
Fig. 2.
Risk of bias for all randomized controlled trials included in this study. (A) Bar chart of bias; (B) Risk of bias summary. DFS, Disease-free survival; OS, Overall survival; RFS, Recurrence-free survival; AEs, adverse events; IL2 + IFN + 5FU, interleukin-2 + interferon-alpha2a + 5-fluorouracil
Survival analysis of DFS, OS, and RFS
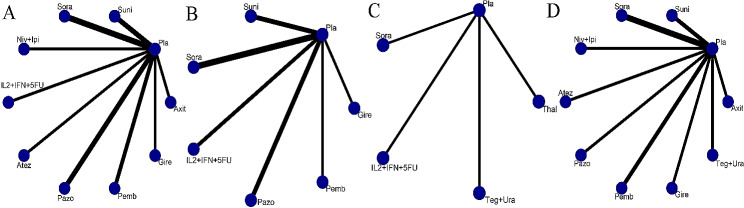
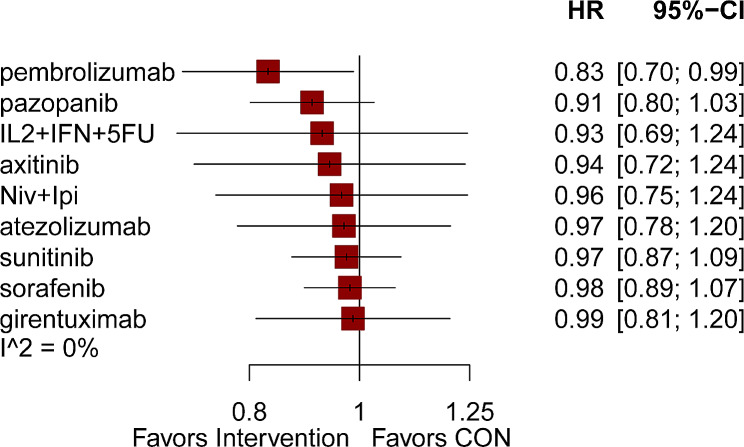
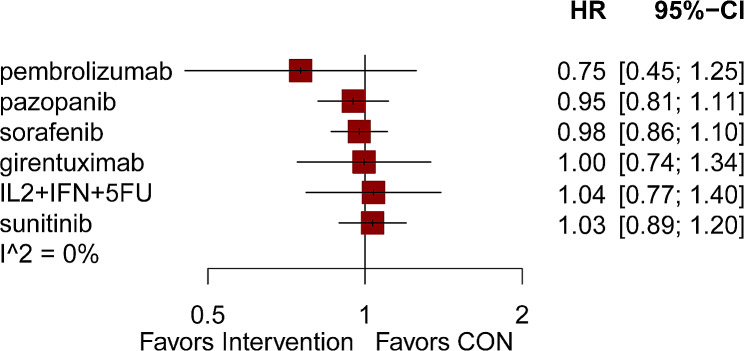
The comparative relationship between different interventions was shown in a network diagram (Fig. 3A-D). Among the 17 articles, 12 provided information on the HRs concerning DFS [5, 7, 11, 14–18, 20, 22, 25, 26]. We compared the 10 interventions included in the network diagram, both directly and indirectly. The most used agent was sorafenib, the most common comparison was between sorafenib and placebo. The intervention measures that exhibited notable distinctions compared to the placebo were pembrolizumab (HR = 0.83, 95%CI 0.70 to 0.99). Adjuvant treatments such as pazopanib, interleukin-2 + interferon-alpha2a + 5-fluorouracil (IL2 + IFN + 5FU), axitinib, nivolumab plus ipilimumab, atezolizumab, sunitinib, sorafenib, and girentuximab had no effect on DFS(HRs from 0.91 to 0.99)(Fig. 4). After conducting a network comparison, a grand total of 45 pairwise comparisons were obtained. The findings indicated that there was no notable disparity observed in DFS among these treatments. Table displays the precise outcomes (Table 3).
Fig. 3.
Network diagrams of outcome indicators. (A) Disease-free survival (DFS); (B) Overall survival (OS); (C) Recurrence-free survival (RFS); (D) adverse events (AEs). Pla, placebo; Suni, sunitinib; Sora, sorafenib; Niv + Ipi, nivolumab + ipilimumab; IL2 + IFN + 5FU, interleukin-2 + interferon-alpha2a + 5-fluorouracil; Atez, atezolizumab; Pazo, pazopanib; Pemb, pembrolizumab; Gire, girentuximab; Teg + Ura, tegafur + uracil; Thal, thalidomide; Axit, axitinib
Fig. 4.
Effects of different interventions on disease-free survival
Table 3.
The NMA presents the impact of each intervention for disease-free survival
| Placebo | 1.03 (0.92,1.15) | 1.02 (0.93,1.12) | 1.04 (0.80,1.34) | 1.08 (0.80,1.45) | 1.03 (0.83,1.28) | 1.10 (0.97,1.25) | 1.20 (1.01,1.43) | 1.01 (0.83,1.23) | 1.06 (0.81,1.40) |
| 1.03 (0.92,1.15) | sunitinib | NA | NA | NA | NA | NA | NA | NA | NA |
| 1.02 (0.93,1.12) | 0.99 (0.86,1.15) | sorafenib | NA | NA | NA | NA | NA | NA | NA |
| 1.04 (0.80,1.34) | 1.01 (0.76,1.33) | 1.02 (0.78,1.33) | nivolumab + ipilimumab | NA | NA | NA | NA | NA | NA |
| 1.08 (0.80,1.45) | 1.05 (0.77,1.44) | 1.06 (0.78,1.44) | 1.04 (0.71,1.54) | IL2 + IFN + 5FU | NA | NA | NA | NA | NA |
| 1.03 (0.83,1.28) | 1.00 (0.79,1.28) | 1.01 (0.80,1.28) | 1.00 (0.71,1.39) | 0.96 (0.66,1.38) | atezolizumab | NA | NA | NA | NA |
| 1.10 (0.97,1.25) | 1.07 (0.91,1.27) | 1.08 (0.92,1.26) | 1.06 (0.80,1.41) | 1.02 (0.74,1.40) | 1.07 (0.83,1.37) | pazopanib | NA | NA | NA |
| 1.20 (1.01,1.43) | 1.17 (0.95,1.44) | 1.18 (0.97,1.44) | 1.16 (0.85,1.58) | 1.12 (0.79,1.57) | 1.17 (0.88,1.54) | 1.09 (0.88,1.35) | pembrolizumab | NA | NA |
| 1.01 (0.83,1.23) | 0.99 (0.79,1.24) | 0.99 (0.80,1.23) | 0.98 (0.71,1.35) | 0.94 (0.66,1.34) | 0.98 (0.73,1.31) | 0.92 (0.73,1.16) | 0.84 (0.65,1.09) | girentuximab | NA |
| 1.06 (0.81,1.40) | 1.03 (0.77,1.39) | 1.04 (0.78,1.39) | 1.02 (0.70,1.49) | 0.98 (0.66,1.47) | 1.03 (0.73,1.46) | 0.97 (0.71,1.30) | 0.88 (0.64,1.22) | 1.05 (0.75,1.47) | axitinib |
11 of the 17 articles provided OS findings [5, 9, 15, 17–22, 25, 26]. Figure 3 presented a network graph that included 7 interventions, which were compared directly and indirectly (Fig. 3B). The thickness of the lines in the network graph represents the number of studies included, from which we can see that the most commonly used drug was sorafenib, and the most common comparison was between sorafenib and placebo. Adjuvant therapies including pembrolizumab, pazopanib,sorafenib, girentuximab, IL2 + IFN + 5FU and sunitinib could not influence the OS(HRs from 0.75 to 1.04) (Fig. 5). After conducting a network analysis, a grand total of 21 pairwise comparisons were obtained. The findings indicated no notable distinction was observed in OS among these treatments (Table 4).
Fig. 5.
The impact of various interventions on overall survival
Table 4.
The NMA presents the impact of each intervention for overall survival
| Placebo | 0.97 (0.83,1.12) | 1.03 (0.91,1.16) | 0.96 (0.72,1.30) | 1.05 (0.90,1.23) | 1.33 (0.80,2.21) | 1.00 (0.75,1.35) |
| 0.97 (0.83,1.12) | sunitinib | NA | NA | NA | NA | NA |
| 1.03 (0.91,1.16) | 1.06 (0.87,1.29) | sorafenib | NA | NA | NA | NA |
| 0.96 (0.72,1.30) | 1.00 (0.72,1.39) | 0.94 (0.68,1.30) | IL2 + IFN + 5FU | NA | NA | NA |
| 1.05 (0.90,1.23) | 1.09 (0.88,1.35) | 1.03 (0.84,1.25) | 1.09 (0.78,1.53) | pazopanib | NA | NA |
| 1.33 (0.80,2.21) | 1.37 (0.81,2.33) | 1.30 (0.77,2.19) | 1.38 (0.76,2.49) | 1.26 (0.74,2.15) | pembrolizumab | NA |
| 1.00 (0.75,1.35) | 1.04 (0.75,1.44) | 0.98 (0.71,1.35) | 1.04 (0.69,1.58) | 0.95 (0.68,1.33) | 0.76 (0.42,1.36) | girentuximab |
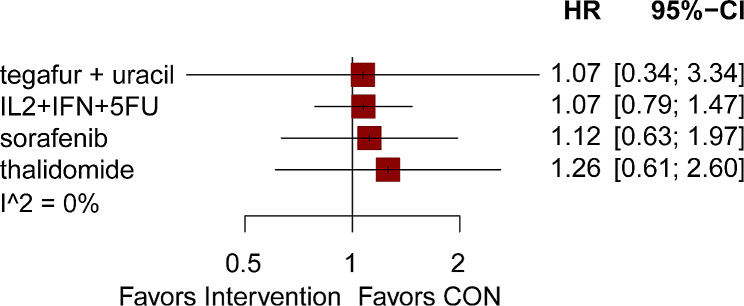
Of the 17 articles, 4 provided information on RFS outcomes [19, 21, 23, 24]. Figure 3 displays a network graph comparing the 4 interventions, both directly and indirectly (Fig. 3C). In terms of RFS, the frequency of the four drugs was the same. Adjuvant therapies including tegafur plus uracil, IL2 + IFN + 5FU, sorafenib, and thalidomide could not influence the RFS(HRs from 1.07 to 1.26) (Fig. 6). After conducting a network analysis, a grand total of 10 pairwise comparisons were obtained. The findings indicated that there was no notable distinction observed in RFS among these interventions (Table 5).
Fig. 6.
Effects of different interventions on recurrence-free survival
Table 5.
The NMA presents the impact of each intervention for recurrence-free survival
| placebo | 0.90 (0.51,1.58) | 0.93 (0.68,1.27) | 0.93 (0.30,2.91) | 0.79 (0.38,1.64) |
|---|---|---|---|---|
| 0.89 (0.51,1.58) | sorafenib | NA | NA | NA |
| 0.93 (0.68,1.27) | 1.04 (0.54,1.99) | IL2 + IFN + 5FU | NA | NA |
| 0.93 (0.30,2.91) | 1.04 (0.29,3.72) | 1.00 (0.31,3.26) | tegafur + uracil | NA |
| 0.79 (0.38,1.64) | 0.89 (0.35,2.23) | 0.85 (0.39,1.88) | 0.85 (0.22,3.27) | thalidomide |
Analysis of AEs (grade ≥ 3)
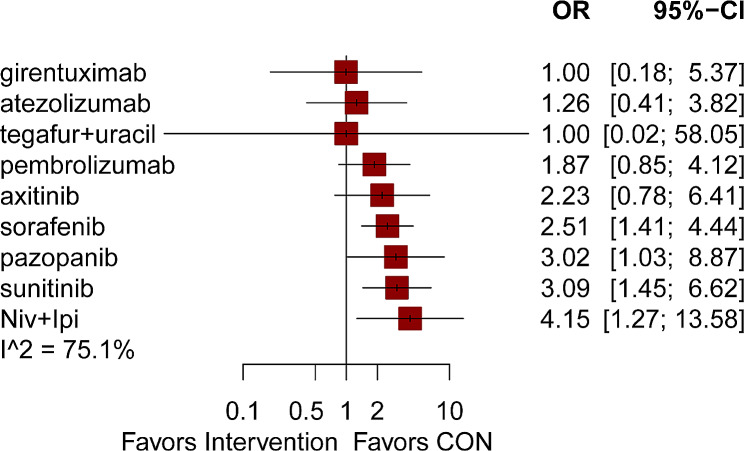
Out of the 17 articles, AEs of grade ≥ 3 were reported in 12 of them [5, 7, 11, 14, 16–18, 20–23, 25]. A network graph in Fig. 3 also displayed comparisons between 10 interventions (Fig. 3D). The most used drug in this part of the study was sorafenib, with the most compared between sorafenib and placebo. Most drugs were found to be more toxic than placebos. The intervention measures that exhibited significant variations in comparison to placebo. Adjuvant treatments including sorafenib, pazopanib, sunitinib, and nivolumab plus ipilimumab suggested a higher likelihood of AEs (ORs 2.51 to 4.15). The remaining adjuvant treatments, including girentuximab, atezolizumab, tegafur plus uracil, pembrolizumab, and axitinib, did not affect AEs (ORs 1.0 to 2.23) (Fig. 7). After conducting a network comparison, a grand total of 45 pairwise comparisons were obtained. The findings indicated no notable distinction was observed in the AEs among these treatments (Table 6).
Fig. 7.
Effects of different interventions on adverse events
Table 6.
The NMA presents the impact of each intervention for adverse events
| Placebo | 0.32 (0.15,0.69) | 0.40 (0.22,0.71) | 0.24 (0.07,0.79) | 0.80 (0.26,2.42) | 0.33 (0.11,0.97) | 0.54 (0.24,1.18) | 1.00 (0.19,5.42) | 1.00 (0.02,58.05) | 0.45 (0.16,1.29) |
|---|---|---|---|---|---|---|---|---|---|
| 0.32 (0.15,0.69) | sunitinib | NA | NA | NA | NA | NA | NA | NA | NA |
| 0.40 (0.22,0.71) | 1.23 (0.48,3.20) | sorafenib | NA | NA | NA | NA | NA | NA | NA |
| 0.24 (0.07,0.79) | 0.75 (0.18,3.05) | 0.60 (0.16,2.25) | nivolumab + ipilimumab | NA | NA | NA | NA | NA | NA |
| 0.80 (0.26,2.42) | 2.46 (0.64,9.47) | 1.99 (0.57,6.96) | 3.30 (0.65,16.77) | atezolizumab | NA | NA | NA | NA | NA |
| 0.33 (0.11,0.97) | 1.02 (0.27,3.83) | 0.83 (0.24,2.81) | 1.37 (0.28,6.82) | 0.42 (0.09,1.96) | pazopanib | NA | NA | NA | NA |
| 0.54 (0.24,1.18) | 1.66 (0.55,4.96) | 1.34 (0.51,3.56) | 2.22 (0.53,9.24) | 0.67 (0.17,2.63) | 1.62 (0.42,6.15) | pembrolizumab | NA | NA | NA |
| 1.00 (0.19,5.42) | 3.11 (0.49,19.74) | 2.52 (0.42,14.92) | 4.17 (0.53,32.72) | 1.26 (0.17,9.50) | 3.03 (0.41,22.42) | 1.88 (0.29,12.06) | girentuximab | NA | NA |
| 1.00 (0.02,58.05) | 3.09 (0.05,192.80) | 2.51 (0.04,151.46) | 4.15 (0.06,285.53) | 1.26 (0.02,84.73) | 3.02 (0.05,201.79) | 1.87 (0.03,117.00) | 1.00 (0.01,80.82) | tegafur + uracil | NA |
| 0.45 (0.16,1.29) | 1.39 (0.38,5.10) | 1.12 (0.34,3.74) | 1.86 (0.38,9.11) | 0.56 (0.12,2.61) | 1.36 (0.30,6.13) | 0.84 (0.22,3.13) | 0.45 (0.06,3.26) | 0.45 (0.01,29.81) | axitinib |
Discussion
As more and more clinical studies explore additional treatments for removing RCC, there is a simultaneous increase in clinical reports discussing various therapies [27]. However, we need to further clarify which adjuvant therapy is best for RCC. Conventional meta-analyses, limited to pairwise comparisons, might need more methodological assistance in determining the most efficacious intervention. Network meta-analysis allows for the comparison of various interventions [28]. Hence, this research utilized network meta-analysis for the initial occasion to evaluate the effectiveness and security of different supplementary therapies following surgical removal of RCC.
Regarding DFS, pembrolizumab was the only adjuvant drug that exhibited a noteworthy enhancement in comparison to a placebo (HR = 0.83, 95% CI 0.70 to 0.99); the remaining adjuvant drugs did not display any significant impact. Furthermore, given the inconclusive outcomes from previous sunitinib trials, our investigation determined sunitinib’s HR to be 0.97, with a 95% CI ranging from 0.87 to 1.09. Considering these results, we exercise prudence when considering the utilization of sunitinib to improve DFS in individuals who have undergone surgery for RCC. Previous research has not witnessed any enhancement in OS and RFS when employing supplementary medications. In our study, we reached a consistent finding that additional medications did not significantly improve postoperative OS and RFS results for individuals diagnosed with RCC when compared to a placebo. Due to the extended survival period following surgery in individuals with RCC, certain clinical studies might not have documented significant occurrences of OS. Hence, it might be essential to create novel medications and conduct more clinical experiments to validate the possible enhancement in OS and RFS among individuals who have undergone surgery for RCC.
In terms of AEs with a grade of ≥ 3, the included interventions of sorafenib, pazopanib, sunitinib, and nivolumab plus ipilimumab showed a higher incidence of adverse reactions compared to a placebo. The safety comparison between different interventions did not demonstrate a significant difference. First-line treatment should not include sorafenib, pazopanib, sunitinib, or nivolumab plus ipilimumab as they were determined to have no effect on improving DFS, OS, and RFS in patients who underwent RCC resection.
These findings indicate that among the existing drugs, pembrolizumab improves DFS in patients following RCC resection, while other drugs do not significantly enhance survival. Checkpoint inhibitors and TKIs are two different anticancer drugs with different mechanisms of action and effects in the treatment of tumors. Checkpoint inhibitors activate the patient’s own immune system to attack tumor cells by disarming immune checkpoints such as PD-1 and CTLA-4 [29]. This mechanism of immune activation can lead to a durable immune response and show significant therapeutic effects in multiple tumor types. Checkpoint inhibitors can trigger a long-lasting immune response, meaning that the immune system is able to recognize and attack tumor cells even after drug treatment is stopped. In contrast, the efficacy of TKIs is often associated with the presence and continued use of the drug. Checkpoint inhibitors can activate multiple types of immune cells, including T cells, B cells, and natural killer cells, leading to a more comprehensive anti-tumor immune response. TKIs inhibit the growth and spread of tumor cells mainly by interfering with signal transduction pathways. Tumor cells often evade the effects of TKIs through a variety of mechanisms, such as the development of drug-resistant mutations and the activation of alternative signaling pathways. However, checkpoint inhibitors, by boosting the activity of the immune system, can respond to situations in which tumor cells escape, thereby reducing the development of drug resistance.
RCC patients have significantly elevated levels of VEGF-A compared to patients with other types of cancer, indicating that RCC is a tumor that is rich in blood vessels [30]. Moreover, TKIs can improve vascularization, directly or indirectly increasing immune infiltration. Significant results were shown in the treatment of metastatic RCC with immune checkpoint inhibitors in the year 2016 [27]. Hence, the collective utilization of various medications exhibits a more potent ability to combat tumors, and the integration of additional supportive treatments alongside pembrolizumab presents a novel method to prolong the survival of patients who have undergone surgery for RCC. However, treatment-related toxicity must also be considered when using multiple adjunctive therapies in combination, as immune checkpoint inhibitors are susceptible to immune-related adverse events, while TKIs have chronic toxicity. While combination drug therapy may have more significant toxicity than single therapy, treatment plans that control toxicity within an acceptable range still have significant potential for application.
Conclusion
The network meta-analysis results showed that pembrolizumab was successful in enhancing DFS in patients who underwent surgery for RCC compared to a placebo. Additionally, the treatment did not lead to any significant toxicity. The assessment is a valuable guide for postoperative adjuvant therapy in individuals diagnosed with RCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
GLY designed the experiment, processed the data, and wrote the article. AT processed the data and carried out statistical analysis. HZX evaluated the literature. CT reviewed and revised the article. All authors reviewed the manuscript.
Funding
No project funding was received for this study.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Larroquette M, Peyraud F, Domblides C, Lefort F, Bernhard JC, Ravaud A, Gross-Goupil M. Adjuvant therapy in renal cell carcinoma: current knowledges and future perspectives. Cancer Treat Rev. 2021;97:102207. doi: 10.1016/j.ctrv.2021.102207. [DOI] [PubMed] [Google Scholar]
- 2.Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797. doi: 10.1136/bmj.g4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Role of molecular markers in the diagnosis and therapy of renal cell carcinoma. Urology. 2005;66(5 Suppl):1–9. doi: 10.1016/j.urology.2005.06.112. [DOI] [PubMed] [Google Scholar]
- 4.Albiges L, Choueiri T, Escudier B, Galsky M, George D, Hofmann F, Lam T, Motzer R, Mulders P, Porta C, et al. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol. 2015;67(1):100–10. doi: 10.1016/j.eururo.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Haas NB, Manola J, Uzzo RG, Flaherty KT, Wood CG, Kane C, Jewett M, Dutcher JP, Atkins MB, Pins M, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387(10032):2008–16. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delahunt B, Eble JN, Egevad L, Samaratunga H. Grading of renal cell carcinoma. Histopathology. 2019;74(1):4–17. doi: 10.1111/his.13735. [DOI] [PubMed] [Google Scholar]
- 7.Pal SK, Uzzo R, Karam JA, Master VA, Donskov F, Suarez C, Albiges L, Rini B, Tomita Y, Kann AG, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2022;400(10358):1103–16. doi: 10.1016/S0140-6736(22)01658-0. [DOI] [PubMed] [Google Scholar]
- 8.Staehler M, Motzer RJ, George DJ, Pandha HS, Donskov F, Escudier B, Pantuck AJ, Patel A, DeAnnuntis L, Bhattacharyya H, et al. Adjuvant sunitinib in patients with high-risk renal cell carcinoma: safety, therapy management, and patient-reported outcomes in the S-TRAC trial. Ann Oncol. 2018;29(10):2098–104. doi: 10.1093/annonc/mdy329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Russo P, Haas N, Doehn C, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, Lee JL, Lim HY, et al. Adjuvant Pazopanib Versus Placebo after Nephrectomy in patients with localized or locally Advanced Renal Cell Carcinoma: final overall survival analysis of the phase 3 PROTECT trial. Eur Urol. 2021;79(3):334–8. doi: 10.1016/j.eururo.2020.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 2011;108(10):1556–63. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 11.Gross-Goupil M, Kwon TG, Eto M, Ye D, Miyake H, Seo SI, Byun SS, Lee JL, Master V, Jin J, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Annals Oncology: Official J Eur Soc Med Oncol. 2018;29(12):2371–8. doi: 10.1093/annonc/mdy454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Tannir NM, McDermott DF, Aren Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthelemy P, Porta C, George S, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med. 2018;378(14):1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulieres D, Melichar B, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med. 2019;380(12):1116–27. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Russo P, Grunwald V, Tomita Y, Zurawski B, Parikh O, Buti S, Barthelemy P, Goh JC, Ye D, et al. Adjuvant nivolumab plus ipilimumab versus placebo for localised renal cell carcinoma after nephrectomy (CheckMate 914): a double-blind, randomised, phase 3 trial. Lancet. 2023;401(10379):821–32. doi: 10.1016/S0140-6736(22)02574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aitchison M, Bray CA, Van Poppel H, Sylvester R, Graham J, Innes C, McMahon L, Vasey PA. Adjuvant 5-flurouracil, alpha-interferon and interleukin-2 versus observation in patients at high risk of recurrence after nephrectomy for renal cell carcinoma: results of a phase III randomised European Organisation for Research and Treatment of Cancer (Genito-Urinary Cancers Group)/National Cancer Research Institute trial. Eur J Cancer. 2014;50(1):70–7. doi: 10.1016/j.ejca.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Choueiri TK, Tomczak P, Park SH, Venugopal B, Ferguson T, Chang YH, Hajek J, Symeonides SN, Lee JL, Sarwar N, et al. Adjuvant pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021;385(8):683–94. doi: 10.1056/NEJMoa2106391. [DOI] [PubMed] [Google Scholar]
- 17.Eisen T, Frangou E, Oza B, Ritchie AWS, Smith B, Kaplan R, Davis ID, Stockler MR, Albiges L, Escudier B, et al. Adjuvant Sorafenib for Renal Cell Carcinoma at Intermediate or High Risk of Relapse: results from the SORCE Randomized Phase III Intergroup Trial. J Clin Oncol. 2020;38(34):4064–75. doi: 10.1200/JCO.20.01800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Ravaud A, Patard JJ, Pandha HS, George DJ, Patel A, Chang YH, Escudier B, Donskov F, Magheli A, et al. Adjuvant sunitinib for high-risk renal cell Carcinoma after Nephrectomy: subgroup analyses and updated overall survival results. Eur Urol. 2018;73(1):62–8. doi: 10.1016/j.eururo.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atzpodien J, Schmitt E, Gertenbach U, Fornara P, Heynemann H, Maskow A, Ecke M, Wöltjen HH, Jentsch H, Wieland W, et al. Adjuvant treatment with interleukin-2- and interferon-alpha2a-based chemoimmunotherapy in renal cell carcinoma post tumour nephrectomy: results of a prospectively randomised trial of the German Cooperative Renal Carcinoma Chemoimmunotherapy Group (DGCIN) Br J Cancer. 2005;92(5):843–6. doi: 10.1038/sj.bjc.6602443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamie K, Donin NM, Kloepfer P, Bevan P, Fall B, Wilhelm O, Stoerkel S, Said J, Gambla M, Hawkins RE, et al. Adjuvant Weekly Girentuximab following nephrectomy for high-risk renal cell Carcinoma the ARISER Randomized Clinical Trial. Jama Oncol. 2017;3(7):913–20. doi: 10.1001/jamaoncol.2016.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Procopio G, Apollonio G, Cognetti F, Miceli R, Milella M, Mosca A, Chiuri VE, Bearz A, Morelli F, Ortega C, et al. Sorafenib Versus Observation following Radical Metastasectomy for Clear-cell renal cell carcinoma: results from the phase 2 Randomized Open-label RESORT Study. Eur Urol Oncol. 2019;2(6):699–707. doi: 10.1016/j.euo.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Powles T, Tomczak P, Park SH, Venugopal B, Ferguson T, Symeonides SN, Hajek J, Gurney H, Chang YH, Lee JL, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(9):1133–44. doi: 10.1016/S1470-2045(22)00487-9. [DOI] [PubMed] [Google Scholar]
- 23.Naito S, Kumazawa J, Omoto T, Iguchi A, Sagiyama K, Osada Y, Hiratsuka Y. Postoperative UFT adjuvant and the risk factors for recurrence in renal cell carcinoma: a long-term follow-up study. Kyushu University Urological Oncology Group. Int J Urol. 1997;4(1):8–12. doi: 10.1111/j.1442-2042.1997.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 24.Mennitto A, Verzoni E, Cognetti F, Miceli R, Milella M, Mosca A, Chiuri VE, Bearz A, Morelli F, Ortega C, et al. Radical metastasectomy followed by sorafenib versus observation in patients withclear cell renal cell carcinoma: extended follow -up of efficacy results from the randomized phase II RESORT trial. Expert Rev Clin Pharmacol. 2021;14(2):261–8. doi: 10.1080/17512433.2021.1879639. [DOI] [PubMed] [Google Scholar]
- 25.Motzer RJ, Haas NB, Donskov F, Gross-Goupil M, Varlamov S, Kopyltsov E, Lee JL, Melichar B, Rini BI, Choueiri TK, et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo after Nephrectomy in patients with localized or locally Advanced Renal Cell Carcinoma. J Clin Oncol. 2017;35(35):3916–23. doi: 10.1200/JCO.2017.73.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haas NB, Manola J, Dutcher JP, Flaherty KT, Uzzo RG, Atkins MB, DiPaola RS, Choueiri TK. Adjuvant treatment for high-risk clear cell renal Cancer: updated results of a high-risk subset of the ASSURE Randomized Trial. JAMA Oncol. 2017;3(9):1249–52. doi: 10.1001/jamaoncol.2017.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martini A, Fallara G, Pellegrino F, Cirulli GO, Larcher A, Necchi A, Montorsi F, Capitanio U. Neoadjuvant and adjuvant immunotherapy in renal cell carcinoma. World J Urol. 2021;39(5):1369–76. doi: 10.1007/s00345-020-03550-z. [DOI] [PubMed] [Google Scholar]
- 28.Mori K, Mostafaei H, Sari Motlagh R, Pradere B, Quhal F, Laukhtina E, Schuettfort VM, Kramer G, Abufaraj M, Karakiewicz PI, et al. Systemic therapies for metastatic hormone-sensitive prostate cancer: network meta-analysis. BJU Int. 2022;129(4):423–33. doi: 10.1111/bju.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B, Chan HL, Chen P. Immune Checkpoint inhibitors: basics and challenges. Curr Med Chem. 2019;26(17):3009–25. doi: 10.2174/0929867324666170804143706. [DOI] [PubMed] [Google Scholar]
- 30.Peyraud F, Larroquette M, Ravaud A, Gross-Goupil M. New insights into Adjuvant Therapy in Renal Cell Carcinoma: is the chapter of VEGF inhibitors definitely closed? Eur Urol. 2021;80(3):269–74. doi: 10.1016/j.eururo.2021.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.