Abstract
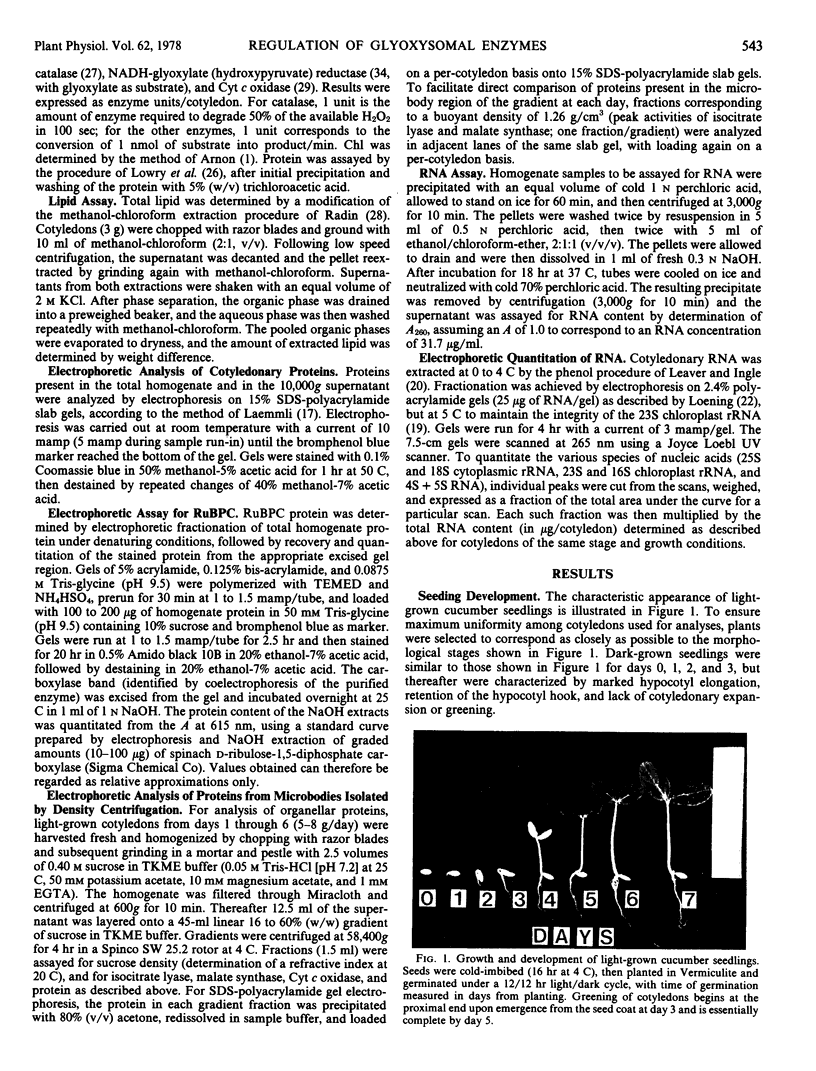
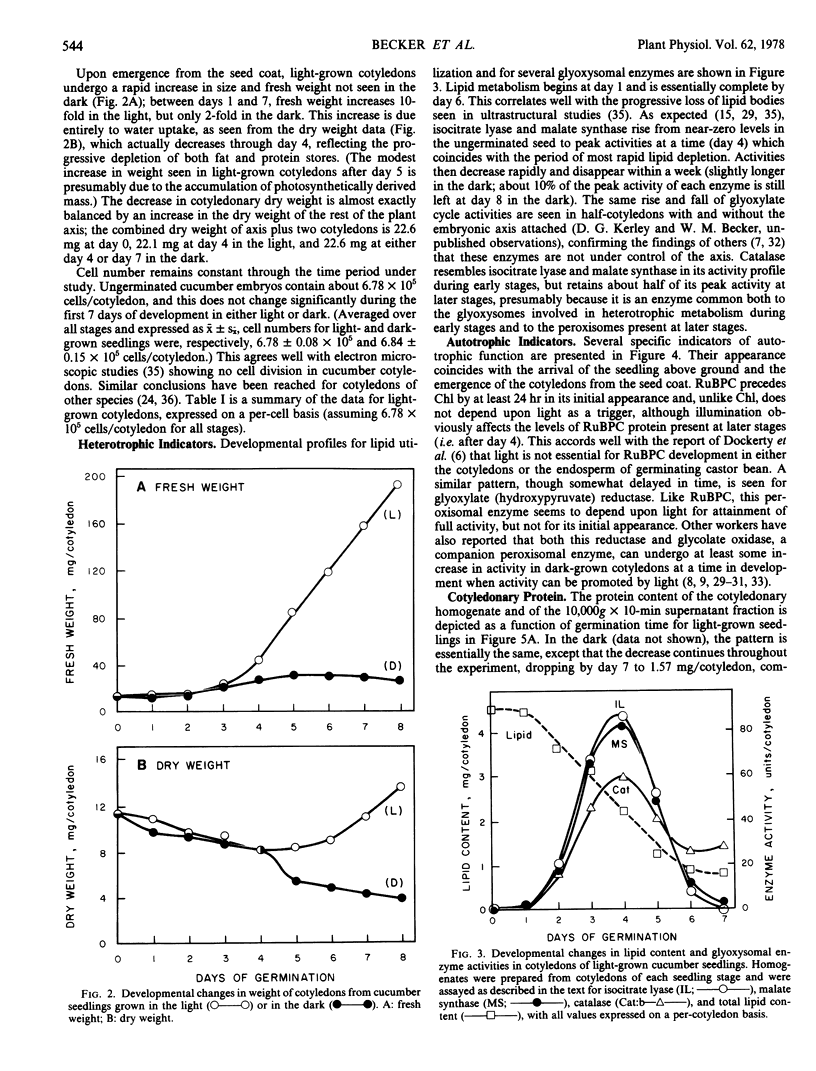
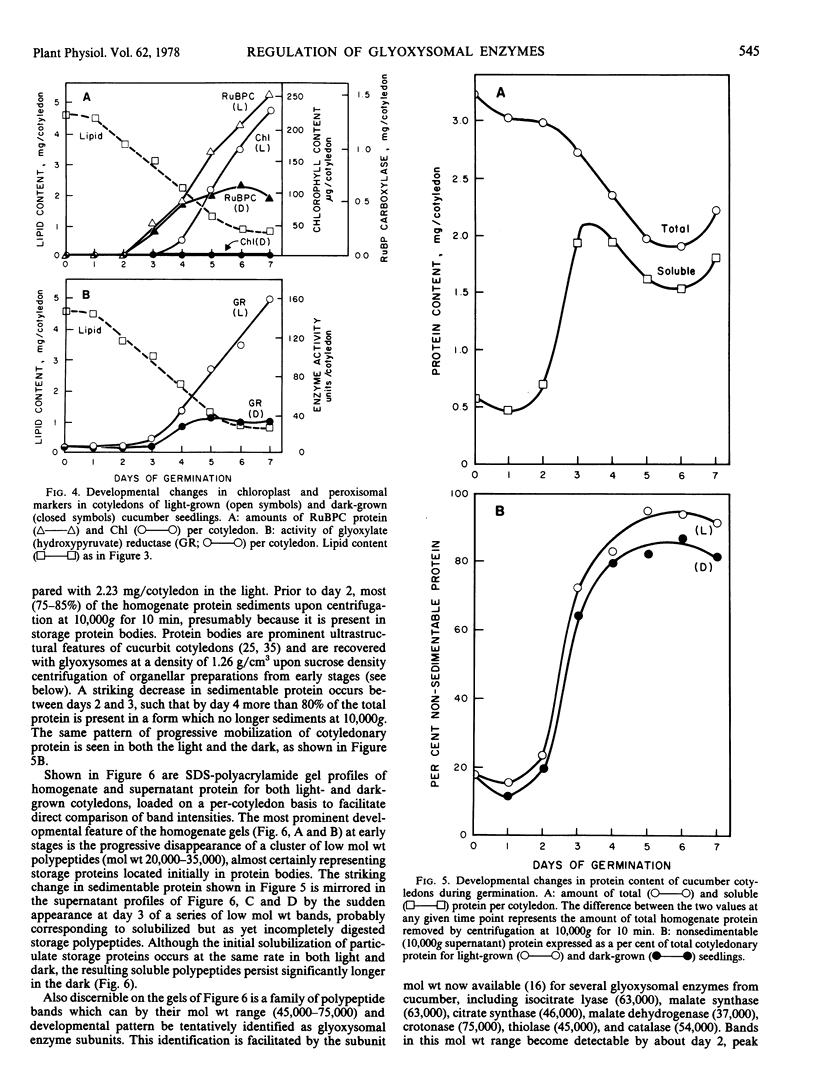
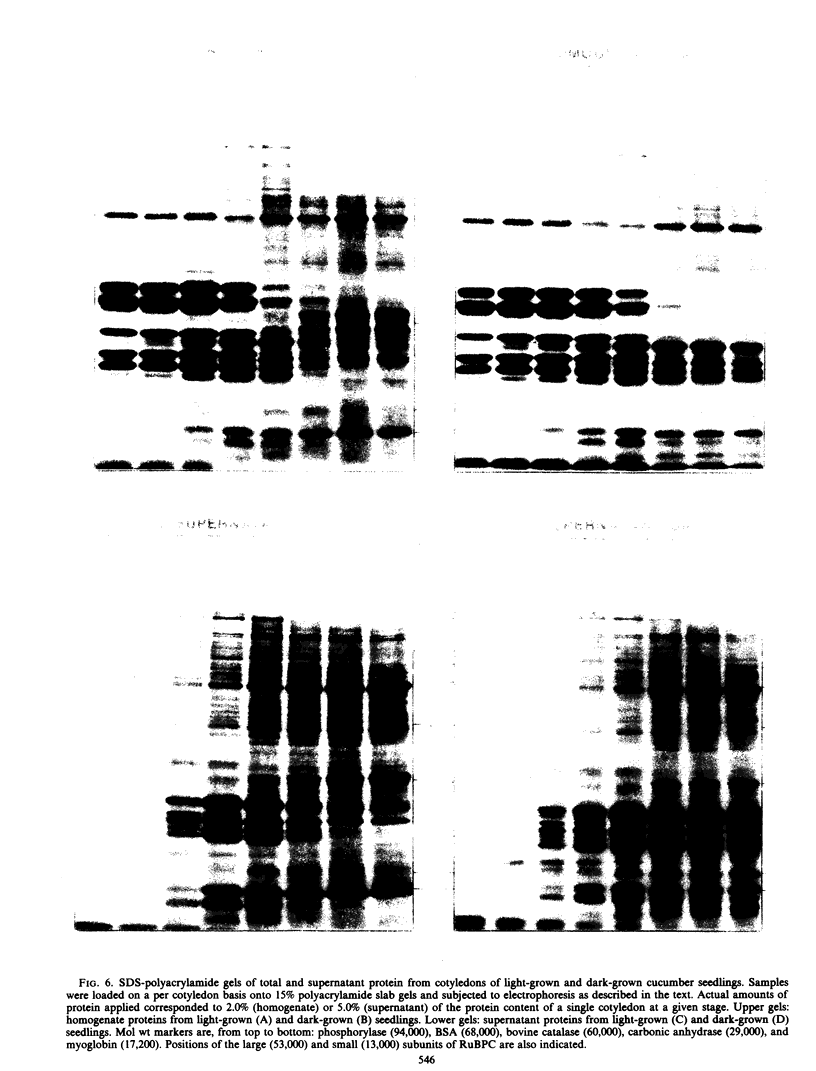
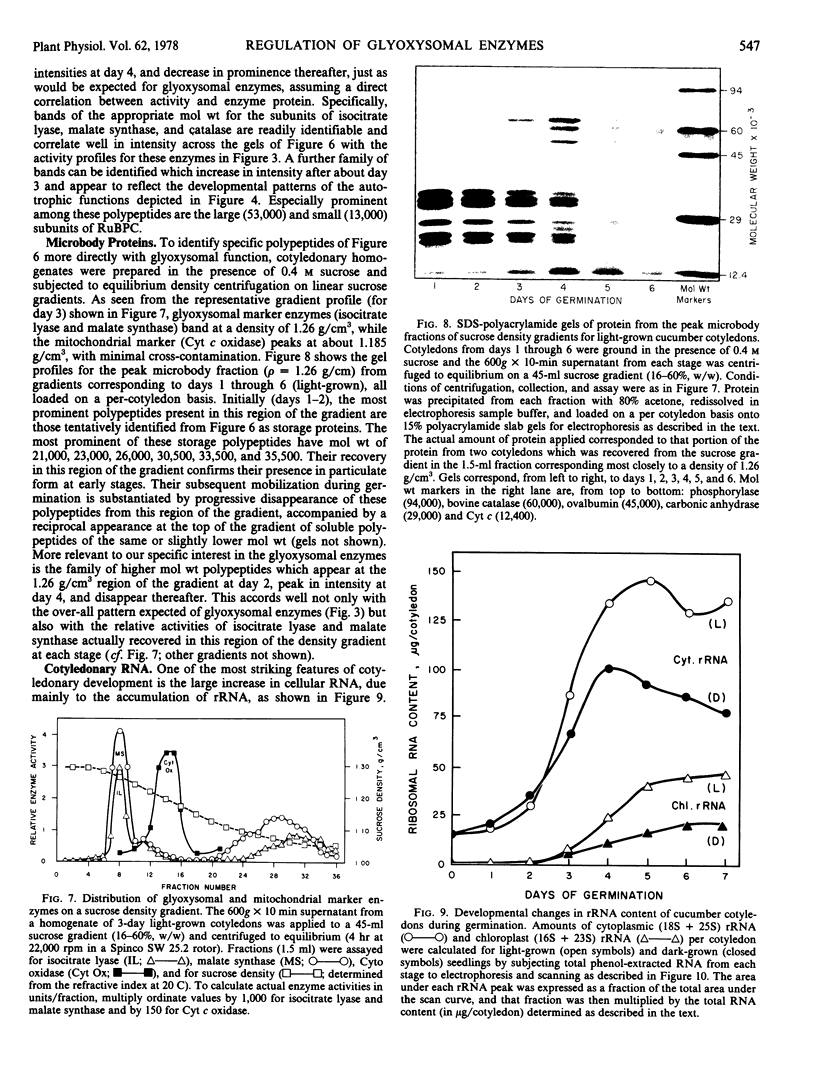
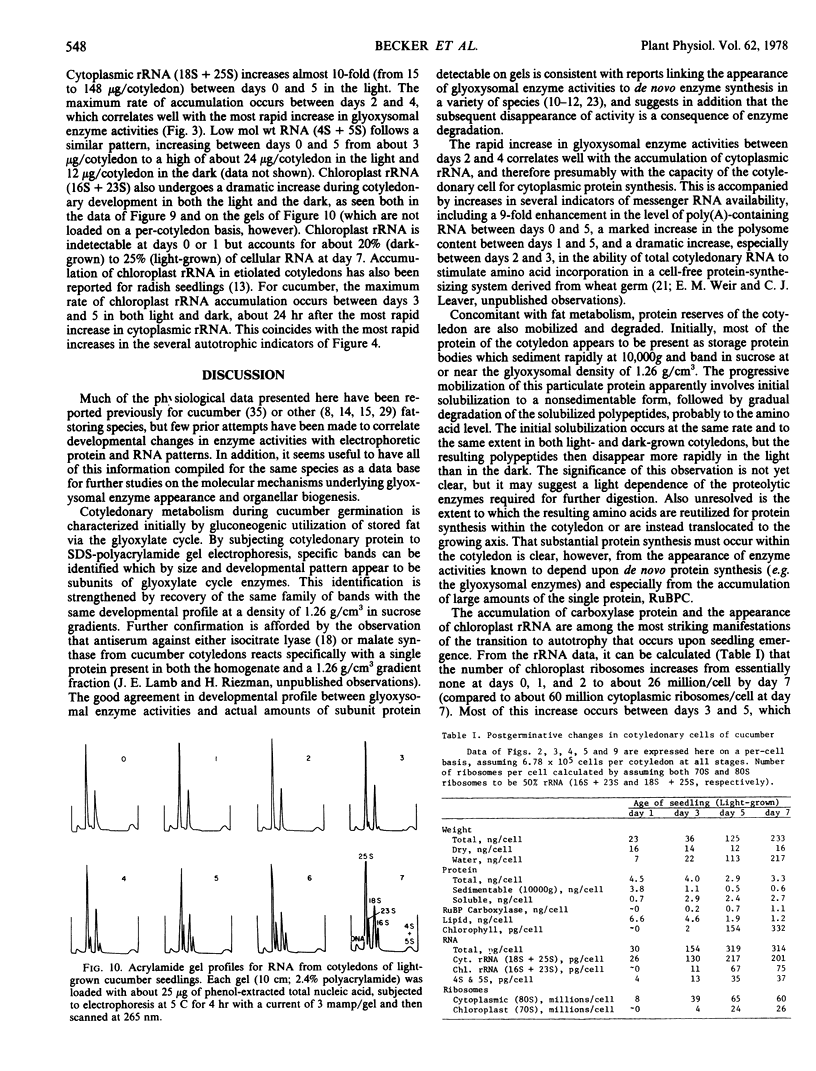
Developmental patterns of glyoxylate cycle and photosynthetic activities have been correlated with electrophoretic profiles of cotyledonary RNA and protein in both light- and dark-grown cucumber seedlings (Cucumis sativus L.) Cytoplasmic rRNA increases 10-fold between days 0 and 5, and the steepest increase coincides with the most rapid rise in activities of the glyoxysomal enzymes, isocitrate lyase and malate synthase. Chloroplast rRNA and ribulose bisphosphate (RuBP) carboxylase begin rising at day 3, followed about a day later by increases in glyoxylate reductase activity and chlorophyll content. Of these phototrophic indicators, only chlorophyll requires light for its initial appearance. Sodium dodecyl sulfate gel electrophoresis of total and soluble cotyledonary protein showed several developmental patterns, including: (a) progressive disappearance of storage protein present initially in particulate form; (b appearance and subsequent disappearance of a family of polypeptides identified by molecular weight, developmental profile, and density gradient centrifugation as subunits of glyoxysomal enzymes; and (c) appearance and progressive increase (in both light- and dark-grown cotyledons) of the large and small subunits of RuBP carboxylase, as well as other polypeptides presumably of chloroplast and peroxisomal origin.
Full text
PDF

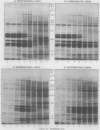
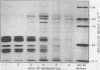
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers H. Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann N Y Acad Sci. 1969 Dec 19;168(2):313–324. doi: 10.1111/j.1749-6632.1969.tb43118.x. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Beevers H. Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constitutents and catalytic capacity. J Biol Chem. 1969 Jul 10;244(13):3507–3513. [PubMed] [Google Scholar]
- Dockerty A., Lord J. M., Merrett M. J. Development of ribulose-1,5-diphosphate carboxylase in castor bean cotyledons. Plant Physiol. 1977 Jun;59(6):1125–1127. doi: 10.1104/pp.59.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gientka-Rychter A., Cherry J. H. De Novo Synthesis of Isocitritase in Peanut (Arachis hypogaea L.) Cotyledons. Plant Physiol. 1968 Apr;43(4):653–659. doi: 10.1104/pp.43.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J. The effect of light and inhibitors on chloroplast and cytoplasmic RNA synthesis. Plant Physiol. 1968 Nov;43(11):1850–1854. doi: 10.1104/pp.43.11.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., Beevers H. The development of microbodies (glyoxysomes and leaf peroxisomes) in cotyledons of germinating watermelon seedlings. Plant Physiol. 1975 Feb;55(2):258–264. doi: 10.1104/pp.55.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T., McGregor D. I., Beevers H. Development of enzymes in the cotyledons of watermelon seedlings. Plant Physiol. 1973 Jan;51(1):66–71. doi: 10.1104/pp.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köller W., Kindl H. Glyoxylate cycle enzymes of the glyoxysomal membrane from cucumber cotyledons. Arch Biochem Biophys. 1977 May;181(1):236–248. doi: 10.1016/0003-9861(77)90502-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Ingle J. The molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1971 Jun;123(2):235–243. doi: 10.1042/bj1230235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver C. J. Molecular integrity of chloroplast ribosomal ribonucleic acid. Biochem J. 1973 Sep;135(1):237–240. doi: 10.1042/bj1350237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo C. P. Evidence for de novo synthesis of isocitratase and malate synthesis in germinating peanut cotyledons. Plant Physiol. 1968 Apr;43(4):660–664. doi: 10.1104/pp.43.4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trelease R. N., Becker W. M., Gruber P. J., Newcomb E. H. Microbodies (Glyoxysomes and Peroxisomes) in Cucumber Cotyledons: Correlative Biochemical and Ultrastructural Study in Light- and Dark-grown Seedlings. Plant Physiol. 1971 Oct;48(4):461–475. doi: 10.1104/pp.48.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]