Abstract
Endocannabinoid-mediated suppression of inhibitory synapses has been hypothesized but not yet demonstrated to occur in vivo, due to the difficulty of tracking endocannabinoid dynamics and synaptic plasticity during behavior. In mice navigating a linear track, we observed location-specific endocannabinoid signaling in hippocampal CA1 place cells, detected both in the postsynaptic membrane and the presynaptic inhibitory axons. All-optical in vivo interrogation of inhibitory synaptic potentials revealed that postsynaptic depolarization is followed by a suppression of inhibitory synaptic potentials. Furthermore, interneuron-specific cannabinoid receptor deletion altered place cell tuning. Therefore, rapid, postsynaptic, activity-dependent endocannabinoid signaling modulates inhibitory synapses on the time scale of seconds during behavior.
One-Sentence Summary:
Place cell activation in behaving mice triggers endocannabinoid signaling, suppressing inhibitory inputs.
Strong depolarization of neurons can induce a transient suppression of their inhibitory synaptic inputs in acute brain slices (1, 2). Such retrograde, activity-dependent suppression of GABAergic synapses, called depolarization-induced suppression of inhibition (DSI), is mediated by endocannabinoid (eCB) signaling (3–5). In vitro studies have shown that robust postsynaptic calcium increase during DSI triggers eCB synthesis and the retrograde activation of cannabinoid type-1 receptors (CB1s), which in turn suppresses GABA release. In CA1 region of the hippocampus, the highest CB1 expression is found on axons of perisomatically projecting GABAergic basket cells that also express cholecystokinin (CCKBCs) (6–8). On the contrary, the other major basket cell type, parvalbumin expressing basket cells (PVBCs), do not express CB1s. Correspondingly, DSI is maximally potent at CCKBC inputs to pyramidal cells and is capable of completely muting these synapses (9, 10).
DSI has been hypothesized to also occur in vivo, however, the specific neuronal activity patterns that give rise to DSI remain unknown (11). When mammals navigate their environment, individual hippocampal pyramidal cells discharge at specific place fields (11, 12), and several observations are consistent with the possibility that place cell firing in behaving animals may engage a DSI-like phenomenon. In vitro, externally imposed place cell-like activity can drive DSI (13), and disinhibition of the postsynaptic cells by DSI can facilitate excitatory synapse plasticity (14, 15). In vivo, place cell formation is supported by reduced inhibition (16, 17). A potential role of DSI in hippocampal place field properties has been proposed (18), however, the steps that would underlie a retrograde, eCB-mediated, DSI-like plasticity in vivo have remained speculative, and the hypothesis that DSI contributes to place cell disinhibition has remained untested. Here, we used optical methods in mice navigating a linear track to test: 1- Whether place cell activity in behaving animals is sufficient to trigger eCB synthesis in the postsynaptic cell; 2- whether eCB signals affect presynaptic CB1s on GABAergic terminals in vivo; and 3- if DSI-like plasticity can modulate place cell activity patterns.
Location-specific endocannabinoid signaling by place cells
The genetically encoded G-protein coupled Receptor Activation Based eCB reporter (GRABeCB2.0) enables the recording eCB dynamics with high spatial resolution in vivo (19, 20). Endocannabinoid mobilization during DSI depends on postsynaptic calcium influx (21). In order to characterize eCB signaling related to calcium transients, we expressed GRABeCB2.0 and the red-shifted calcium sensor, jRGECO1a (22) in CA1 neurons. We carried out 2-photon dual calcium and eCB imaging in the pyramidal layer while mice ran several laps on a linear treadmill track with tactile cues (Fig. 1A) (23). We segmented regions of interests (ROIs) corresponding to neuronal somata (most of which in the pyramidal layer are expected to belong to pyramidal cells) (24), and measured calcium and eCB signals in the same ROIs. We analyzed calcium transients by finding peaks on traces of fluorescence change over baseline (ΔF/F) (Fig. 1B). Transient eCB signals were detected concomitant with calcium peaks (Fig. 1B), with a peak delayed by 1.04 ± 0.16 s relative to calcium, and an average decay time constant of 3.53 ± 0.75 s. To investigate which eCB ligand contribute to the transients, we performed the latter analysis on datasets we previously recorded in the presence of ligand-specific inhibitors of eCB synthesis or metabolism (20). Calcium peak-coupled eCB transients were suppressed by inhibiting the synthesis of 2-arachidonoylglycerol (2-AG), the eCB species involved in CA1 DSI in vitro (25). Furthermore, eCB transient durations were extended after we treated mice with JZL 184 to inhibit monoacylglycerol lipase (MGL) and thus 2-AG degradation (25, 26) (Fig. S1A–C). Conversely, manipulations altering the synthesis or degradation of the other major eCB species, anandamide (AEA), had no effect on the in vivo eCB transients (Fig. S1D–F).
Fig. 1. Rapid endocannabinoid signaling in the hippocampus in vivo.

(A) GRABeCB2.0 and jRGECO1a were expressed in CA1 neurons. Head-fixed mice ran on a linear treadmill during multiphoton imaging. (B) Event-aligned average single-cell calcium and eCB responses during calcium transients. Plot shows mean responses (line) ± SEM (shaded area), n = 4 sessions from 4 vehicle-treated mice, 607 ± 241 ROIs per session, 5.2 ± 1.1 peaks per ROI. Labels show decay time constants of exponential fits. (C) Analysis of place cells. Average tuning curves (solid black line) were calculated for each session by aligning location-averaged place cell traces on their preferred location. (D) Average spatial tuning curves (±SEM) are shown centered on the preferred location of place cells (red: calcium) together with the tuning curves of eCB signals from the same cells (blue) or after shuffling cells within sessions (grey). One-sided, one-sample t-test with alternative hypothesis μ > 0: p = 5.67e-5, n = 4 male mice; shuffle: p = 0.88. Plots show average tuning curves (line) ± SEM (shaded area), n = 4 sessions from 4 drug-naïve mice, 161 ± 35 place cell ROIs per session.
Next, in order to investigate eCB dynamics specifically in place cells, we identified place cells by calculating location-specific average calcium signals (Fig. 1C). Average eCB signals were elevated around the same track locations where calcium was high in the same individual place cells (Fig. 1D). These results indicate that eCB mobilization in place cells during exploration is specific to the cell’s preferred location. In contrast, non-place cells had lower calcium and accompanying eCB transient amplitudes compared to place cells in the same field of view (Fig. S1G).
Although the molecular mechanisms of retrograde eCB transport are not precisely understood, there is general agreement that DSI requires the postsynaptically generated eCBs to engage presynaptic CB1s on interneuronal terminals impinging on the activated neuron (4, 27). Thus, we specifically allowed the expression of GRABeCB2.0 only in interneurons using Dlx5/6-Cre transgenic mice (28) to enable presynaptic eCB measurements (Fig. S2A). The distribution of GRABeCB2.0, a chimera of CB1 and a green fluorescent protein (GFP) variant, resembled membrane-enriched CB1 targeting (29) in interneuron axon terminals, with no detectable postsynaptic expression in principal cells, and relatively low expression in interneuron somata (Fig. S2B–F).
For simultaneously imaging somatic calcium and axonal eCB transients, we combined interneuronal GRABeCB2.0 and pan-neuronal, red-shifted calcium sensor expression (Fig. 2A, S2E). We generated somatic, putatively postsynaptic calcium (postCa) ROI sets as above and measured nearby axonal, putatively presynaptic eCB (preeCB) signals after enlarging the somatic ROIs (Fig. 2A). Similar to eCB signals measured in place cell somata (Fig. 1D), preeCB signals in interneuronal axons surrounding place cells were elevated at the same track locations where postCa was high (Fig. 2B–C). These results indicate that place cell activations during behavior are accompanied by eCB signaling at perisomatic inhibitory axons.
Fig. 2. Spatially tuned presynaptic endocannabinoid signals in the hippocampus in vivo.

(A) Labeling strategy for in vivo imaging. Interneuronal GRABeCB2.0 and pan-neuronal jRGECO1a expression were combined. Bottom panels: segmentation approach. Neuron cell bodies were segmented in the jRGECO1a channel (postCa). The ROIs were enlarged by binary dilation for measuring signals in the neighboring axons in the GRABeCB2.0 channel (preeCB). (B) Average spatial tuning curves (±SEM) are shown centered on the preferred location of place cells (red: calcium) together with the tuning curves of eCB signals from the corresponding preECB ROIs (blue) or after shuffling ROIs within sessions (grey), n = 18 sessions from 5 mice, 193 ± 130 ROIs per session. (C) Quantification of signal intensity at the preferred location. Boxes: median ± interquartile range; whiskers: non-outlier range; markers: recording sessions. preeCB: p = 0.003, n = 5 mice, 3 males and 2 females; shuffle: p = 0.84. (D) Spatial tuning curves are shown after injecting mice with JZL-184 to inhibit the enzymatic breakdown of the eCB 2-AG by monoacylglycerol-lipase (MGL), or after vehicle injection. (E) Quantification of location-specific preECB signals, p = 0.022, Mann-Whitney test, n = 11 Vehicle sessions from 5 mice and 3 JZL sessions from 3 mice.
Similarly to DSI in vitro (30) and calcium transient-related postECB signals in vivo (Fig S1B), location-specific preECB signals around place cells were magnified by pharmacological inhibition of 2-AG degradation (Fig. 2D–E), consistent with a prominent role of 2-AG in inhibitory axon eCB signaling, while not ruling out the partial involvement of other eCBs such as AEA.
Postsynaptic activity-dependent modulation of inhibitory postsynaptic potentials
Retrograde eCB signaling through CB1 inhibits CCKBC to pyramidal cell synapses in vitro (9, 31). Based on our results showing eCB transients time-locked to calcium transients, we expected to observe an activity-dependent modulation of CCKBC synapses. We utilized a CCKBC-specific (Sncg-FlpO) mouse line to test this hypothesis (32) and developed an all-optical method to probe synaptic transmission between CCKBCs and postsynaptic neurons. These animals express the FlpO recombinase enzyme specifically in gamma-synuclein (Sncg) expressing cells. Sncg is expressed selectively in CCKBC; therefore, FlpO will be expressed specifically in this cell population in Sncg-FlpO mice. We expressed FlpO-dependent excitatory opsin (sombC1C2TG) (33) in CCKBCs and a soma-localized genetically encoded voltage indicator (GEVI, somQuasAr6a) (34) in sparsely labeled CA1 neurons in Sncg-FlpO mice (Fig. 3A, S3A). We imaged GEVI in awake mice head-fixed on a spherical treadmill while activating CCKBCs with photostimulation (Fig. 3B). Brief CCKBC activation elicited time-locked CA1 neuronal hyperpolarization, consistent with optogenetically evoked inhibitory postsynaptic potentials (oeIPSP, Fig. 3C).
Fig. 3. Inhibitory synaptic plasticity in behaving mice.
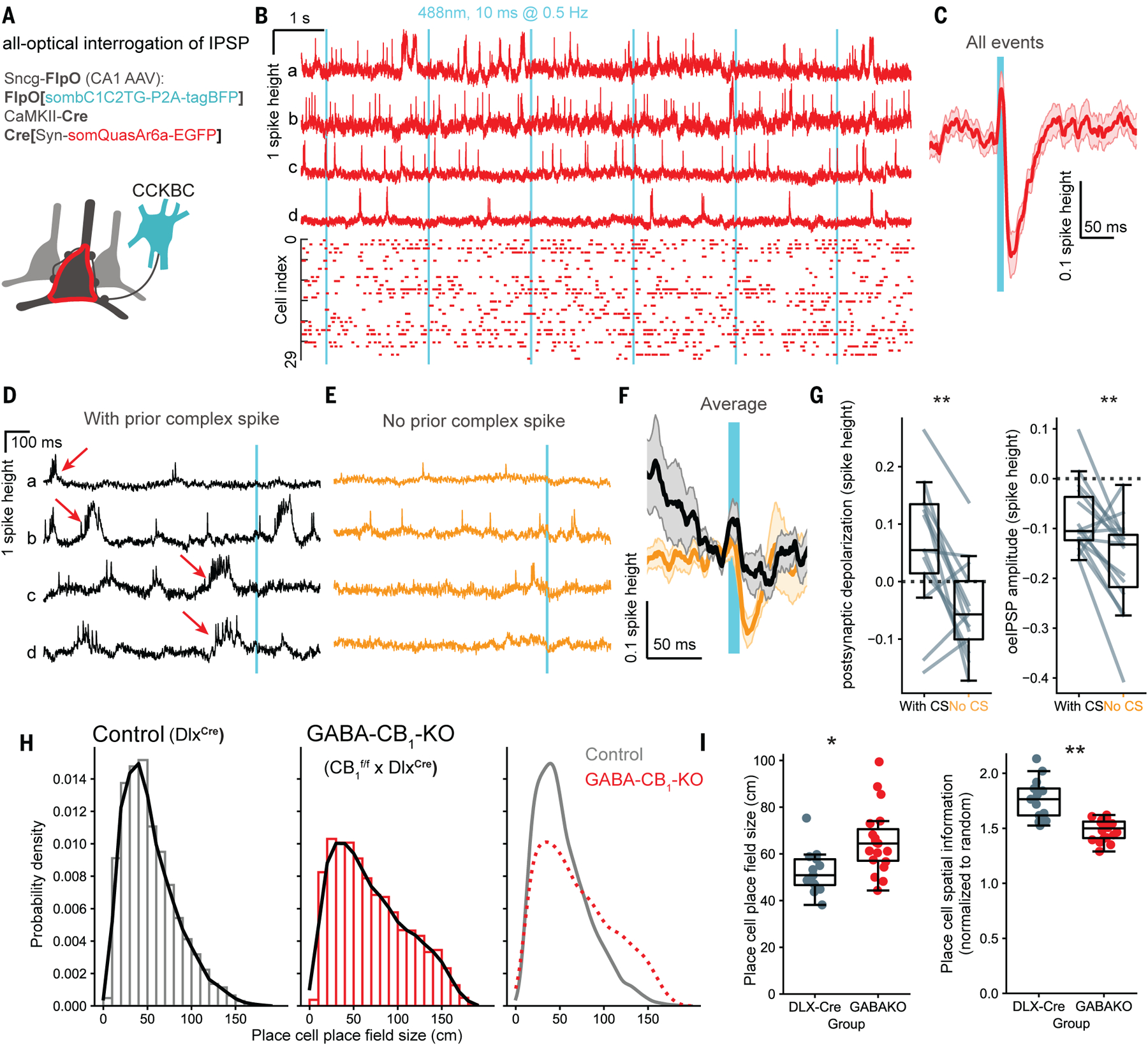
(A) Labeling strategy for all-optical assay of CCKBC synaptic function in vivo. (B) Top: example unfiltered fluorescence traces from four CA1 neurons (a–d). Bottom: spike raster (n = 30 neurons, 5 mice). Cyan bars: CCKBC photostimulation onset (488 nm, 10 ms duration, 9.5–20 mW/mm2, 0.5 Hz). (C) Mean subthreshold postsynaptic waveforms following presynaptic CCKBC photostimulation (n = 30 neurons, 5 mice). (D) Unfiltered example traces of plateau-driven complex spikes (red arrows) preceding photostimulation events (E) Additional example traces from the same cells as in (D), without complex spikes occurring within 1 sec before the stimulation. (F) Stimulus-triggered average (mean ± SEM) oeIPSP (black: with CS; orange: without CS). (G) Quantification of neuronal depolarization before stimulation and oeIPSP amplitudes (negative values), during trials with or without preceding complex spikes (depolarization: p = 0.0076, paired t-test, n = 15 cells, from 4 mice; oeIPSP amplitude: p = 0.0045). (H) Histograms of place field sizes of individual place cells in control mice and after cell type-specific CB1 KO in GABAergic neurons (GABA-CB1-KO). n = 420 ± 254 place cells, 5 control and 3 GABA-CB1-KO animals. (I) Quantification of place cell place field size and spatial information. n =13 sessions from 2 male and 2 female control mice; 19 sessions from 3 male GABA-CB1-KO mice. Markers and box plots show individual sessions (boxes: median ± interquartile range, whiskers: non-outlier range). Place field size: p = 0.032, χ2(1) = 4.59; spatial information: p = 0.004, χ2(1) = 8.5, linear mixed effects models and likelihood ratio test.
Plateau-driven complex spikes in CA1 pyramidal cells are particularly important for synaptic plasticity (12, 33, 35). We identified plateau-driven complex spikes with voltage imaging, and then grouped the photostimulation-induced responses based on the presence or absence of complex spikes during the 1 second before the stimulus (Fig. 3D–E). Whereas oeIPSPs were detectable in the absence of a preceding complex spike (Fig 3E), the same postsynaptic cells showed reduced oeIPSPs after complex spikes (Fig 3D, F, G). As expected, the average postsynaptic depolarization before the CCKBCs stimulus was higher in the presence of complex spikes (Fig. 3G). Together, these results demonstrate a transient suppression of CCKBC inhibition after complex spikes, consistent with a DSI-like mechanism.
Interneuron cannabinoid receptors modulate place cell activity patterns
Taken together, the above results provide evidence for postsynaptic neuronal activity-dependent modulation of CCKBC synapses in vivo. A suppression of inhibition could disinhibit place cells during place field traversal, contributing to location-specific place cell activity (16, 36). In order to determine whether preventing inhibitory synaptic eCB signaling may lead to altered place fields, we knocked out CB1 selectively in forebrain GABAergic neurons (GABA-CB1-KO, lacking CB1 from perisomatic and dendritic interneurons) (28) (Fig. S3B), and recorded place cell calcium signals during a spatial navigation task as mice foraged for a water reward. Both control (Dlx-Cre) and GABA-CB1-KO mice exhibited spatially tuned calcium signals, suggesting that CB1 expression by GABAergic neurons is not required for place field formation per se (Fig. S3C–D). However, we observed a widening of place fields in GABA-CB1-KO mice relative to mice with intact CB1 expression (Fig. 3H–I). Analyzing properties of individual place cells revealed that in the absence of interneuron CB1 expression, place cells were active over a larger fraction of the belt, and altogether encoded less spatial information (Fig. 3I, S3C–J). In GABA-CB1-KO, place cells fired less reliably lap-to-lap, and had fewer calcium transients near the preferred location (Fig. S3H–I). As a population, place cells in GABA-CB1-KO encoded mouse location less accurately compared to control, despite the similar ratio of place cells (Fig. S3E,J). The observed changes in place cell activity patterns are consistent with the reported impaired spatial learning performance of GABA-CB1-KO mice (37) and mice with perturbed CCKBC development (38).
In this study, we report (1) rapid eCB signals time-locked to calcium transients in hippocampal neurons including place cells, both in the postsynaptic membrane and the presynaptic inhibitory axons; (2) modulation of CCKBC synapses correlated to past postsynaptic activity; and (3) diminished place cell place field properties in the absence of eCB signaling at inhibitory synapses. Together, our results demonstrate that an eCB-mediated, DSI-like plasticity is capable of rapid modulation of inhibition in vivo on the behaviorally relevant time scale of seconds. Due to the selective expression of CB1 at synapses of CCK but not PV expressing interneurons, DSI may enable recently activated place cells to maintain elevated excitability without suppressing the ability of PVBC synapses to synchronize the PC population activity dynamics to theta- and gamma oscillations (39, 40). Such a selective, lasting suppression of inhibition involving CB1 signaling may also contribute to maintaining an eligibility trace for non-Hebbian activity-dependent plasticity (41).
Supplementary Material
Acknowledgments:
We thank Anna Ortiz, Charlotte Porter, Sandra Linder and Kishandra Patron for technical and administrative support. Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding:
National Institutes of Health grant R01NS99457 to IS
National Institutes of Health grant R01NS131728 to IS
National Institutes of Health grant R01NS133381 to IS
National Institutes of Health grant R00NS117795 to BD
National Institutes of Health grant K99MH132871 to LZF
National Institutes of Health grant K99NS126725 to JSF
Knight Initiative for Brain Resilience grant KCG-116 to IS
McNair Scholarship from the McNair Medical Institute at The Robert and Janice McNair Foundation to BD
Helen Hay Whitney Fellowship to LZF
Burroughs Wellcome Fund Career Award at the Scientific Interface to LZF.
Stanford University Bio-X Undergraduate Summer Research Program to CW
Grants from the NIMH, NIDA, NSF, and the Gatsby, Fresenius, AE, Tarlton, and NOMIS Foundations to KD.
Footnotes
Competing interests: IS declares unrelated consultant activity for Actio Biosciences, CODA Biotherapeutics, MapLight Therapeutics, Praxis Precision Medicines, and Ray Therapeutics. KD declares unrelated consultant activity for MapLight Therapeutics and Stellaromics.
Data and materials availability:
All data, code, and materials are available from the authors upon reasonable request.
References and Notes
- 1.Llano I, Leresche N, Marty A, Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 6, 565–574 (1991). [DOI] [PubMed] [Google Scholar]
- 2.Pitler T, Alger B, Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci 12, 4122–4132 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreitzer AC, Regehr WG, Cerebellar Depolarization-Induced Suppression of Inhibition Is Mediated by Endogenous Cannabinoids. J Neurosci 21, RC174–RC174 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson RI, Nicoll RA, Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M, Presynaptic Inhibition Caused by Retrograde Signal from Metabotropic Glutamate to Cannabinoid Receptors. Neuron 31, 463–475 (2001). [DOI] [PubMed] [Google Scholar]
- 6.Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF, Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci Official J Soc Neurosci 19, 4544–58 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM, Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Marsicano G, Lutz B, Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci 11, 4213–4225 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Földy C, Neu A, Jones MV, Soltesz I, Presynaptic, Activity-Dependent Modulation of Cannabinoid Type 1 Receptor-Mediated Inhibition of GABA Release. J Neurosci 26, 1465–1469 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S-H, Ledri M, Tóth B, Marchionni I, Henstridge CM, Dudok B, Kenesei K, Barna L, Szabó SI, Renkecz T, Oberoi M, Watanabe M, Limoli CL, Horvai G, Soltesz I, Katona I, Multiple Forms of Endocannabinoid and Endovanilloid Signaling Regulate the Tonic Control of GABA Release. J. Neurosci 35, 10039–10057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Keefe J, Dostrovsky J, The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 34, 171–175 (1971). [DOI] [PubMed] [Google Scholar]
- 12.Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC, Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat. Neurosci 18, 1133–1142 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubruc F, Dupret D, Caillard O, Self-tuning of inhibition by endocannabinoids shapes spike-time precision in CA1 pyramidal neurons. J Neurophysiol 110, 1930–1944 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carlson G, Wang Y, Alger BE, Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci 5, 723–724 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Chevaleyre V, Castillo PE, Endocannabinoid-Mediated Metaplasticity in the Hippocampus. Neuron 43, 871–881 (2004). [DOI] [PubMed] [Google Scholar]
- 16.Valero M, Navas-Olive A, de la Prida LM, Buzsáki G, Inhibitory conductance controls place field dynamics in the hippocampus. Cell Rep. 40, 111232 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rolotti SV, Ahmed MS, Szoboszlay M, Geiller T, Negrean A, Blockus H, Gonzalez KC, Sparks FT, Canales ASS, Tuttman AL, Peterka DS, Zemelman BV, Polleux F, Losonczy A, Local feedback inhibition tightly controls rapid formation of hippocampal place fields. Neuron 110, 783–794.e6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund TF, Katona I, Piomelli D, Role of Endogenous Cannabinoids in Synaptic Signaling. Physiol Rev 83, 1017–1066 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Dong A, He K, Dudok B, Farrell JS, Guan W, Liput DJ, Puhl HL, Cai R, Wang H, Duan J, Albarran E, Ding J, Lovinger DM, Li B, Soltesz I, Li Y, A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo. Nat. Biotechnol 40, 787–798 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farrell JS, Colangeli R, Dong A, George AG, Addo-Osafo K, Kingsley PJ, Morena M, Wolff MD, Dudok B, He K, Patrick TA, Sharkey KA, Patel S, Marnett LJ, Hill MN, Li Y, Teskey GC, Soltesz I, In vivo endocannabinoid dynamics at the timescale of physiological and pathological neural activity. Neuron 109, 2398–2403.e4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimotodani Y, Ohno-Shosaku T, Kano M, Ca2+-assisted receptor-driven endocannabinoid release: mechanisms that associate presynaptic and postsynaptic activities. Curr Opin Neurobiol 17, 360–365 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, Patel R, Macklin JJ, Bargmann CI, Ahrens MB, Schreiter ER, Jayaraman V, Looger LL, Svoboda K, Kim DS, Sensitive red protein calcium indicators for imaging neural activity. Elife 5, e12727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danielson NB, Zaremba JD, Kaifosh P, Bowler J, Ladow M, Losonczy A, SublayerSpecific Coding Dynamics during Spatial Navigation and Learning in Hippocampal Area CA1. Neuron 91, 652–665 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaifosh P, Zaremba JD, Danielson NB, Losonczy A, SIMA: Python software for analysis of dynamic fluorescence imaging data. Front Neuroinform 8, 80 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hashimotodani Y, Ohno-shosaku T, Maejima T, Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocanabinoid release. 54, 58–67 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Long JZ, Nomura DK, Cravatt BF, Characterization of Monoacylglycerol Lipase Inhibition Reveals Differences in Central and Peripheral Endocannabinoid Metabolism. Chem Biol 16, 744–753 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albarran E, Sun Y, Liu Y, Raju K, Dong A, Li Y, Wang S, Südhof TC, Ding JB, Postsynaptic synucleins mediate endocannabinoid signaling. Nat. Neurosci 26, 997–1007 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monory K, Massa F, Egertová M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave K-A, During M, Klugmann M, Wölfel B, Dodt H-U, Zieglgänsberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B, The Endocannabinoid System Controls Key Epileptogenic Circuits in the Hippocampus. Neuron 51, 455–466 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dudok B, Barna L, Ledri M, Szabó SI, Szabadits E, Pintér B, Woodhams SG, Henstridge CM, Balla GY, Nyilas R, Varga C, Lee S-H, Matolcsi M, Cervenak J, Kacskovics I, Watanabe M, Sagheddu C, Melis M, Pistis M, Soltesz I, Katona I, Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat. Neurosci 18, 75–86 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan B, Wang W, Long JZ, Sun D, Hillard CJ, Cravatt BF, Liu Q, Blockade of 2Arachidonoylglycerol Hydrolysis by Selective Monoacylglycerol Lipase Inhibitor 4-Nitrophenyl 4-(Dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) Enhances Retrograde Endocannabinoid Signaling. J. Pharmacol. Exp. Ther 331, 591–597 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glickfeld LL, Scanziani M, Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci 9, 807–815 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudok B, Klein PM, Hwaun E, Lee BR, Yao Z, Fong O, Bowler JC, Terada S, Sparks FT, Szabo GG, Farrell JS, Berg J, Daigle TL, Tasic B, Dimidschstein J, Fishell G, Losonczy A, Zeng H, Soltesz I, Alternating sources of perisomatic inhibition during behavior. Neuron 109, 997–1012.e9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan LZ, Kim DK, Jennings JH, Tian H, Wang PY, Ramakrishnan C, Randles S, Sun Y, Thadhani E, Kim YS, Quirin S, Giocomo L, Cohen AE, Deisseroth K, All-optical physiology resolves a synaptic basis for behavioral timescale plasticity. Cell 186, 543–559.e19 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian H, Davis HC, Wong-Campos JD, Park P, Fan LZ, Gmeiner B, Begum S, Werley CA, Borja GB, Upadhyay H, Shah H, Jacques J, Qi Y, Parot V, Deisseroth K, Cohen AE, Video-based pooled screening yields improved far-red genetically encoded voltage indicators. Nat. Methods 20, 1082–1094 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epsztein J, Brecht M, Lee AK, Intracellular Determinants of Hippocampal CA1 Place and Silent Cell Activity in a Novel Environment. Neuron 70, 109–120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Royer S, Zemelman BV, Losonczy A, Kim J, Chance F, Magee JC, Buzsáki G, Control of timing, rate and bursts of hippocampal place cells by dendritic and somatic inhibition. Nat. Neurosci 15, 769–775 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albayram Ö, Passlick S, Bilkei-Gorzo A, Zimmer A, Steinhäuser C, Physiological impact of CB1 receptor expression by hippocampal GABAergic interneurons. Pflügers Archiv - European J Physiology 468, 727–737 (2016). [DOI] [PubMed] [Google Scholar]
- 38.del Pino I, Brotons-Mas JR, Marques-Smith A, Marighetto A, Frick A, Marín O, Rico B, Abnormal wiring of CCK+ basket cells disrupts spatial information coding. Nat Neurosci 20, 784–792 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varga C, Golshani P, Soltesz I, Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc. Natl. Acad. Sci 109, E2726–E2734 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JRP, Jonas P, Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc. Natl. Acad. Sci 99, 13222–13227 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milstein AD, Li Y, Bittner KC, Grienberger C, Soltesz I, Magee JC, Romani S, Bidirectional synaptic plasticity rapidly modifies hippocampal representations. Elife 10, e73046 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holtmaat A, Bonhoeffer T, Chow DK, Chuckowree J, Paola VD, Hofer SB, Hübener M, Keck T, Knott G, Lee W-CA, Mostany R, Mrsic-Flogel TD, Nedivi E, Portera-Cailliau C, Svoboda K, Trachtenberg JT, Wilbrecht L, Long-term, high-resolution imaging in the mouse neocortex through a chronic cranial window. Nature Protocols 4, 1128–1144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW, Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat. Neurosci 13, 1433–1440 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pnevmatikakis EA, Giovannucci A, NoRMCorre: An online algorithm for piecewise rigid motion correction of calcium imaging data. J. Neurosci. Methods 291, 83–94 (2017). [DOI] [PubMed] [Google Scholar]
- 45.Mukamel EA, Nimmerjahn A, Schnitzer MJ, Automated Analysis of Cellular Signals from Large-Scale Calcium Imaging Data. Neuron 63, 747–760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, code, and materials are available from the authors upon reasonable request.


