Abstract
p73 is a recently identified member of the p53 family. Previously it was shown that p73 can, when overproduced in p53-defective tumor cells, activate p53-responsive promoters and induce apoptosis. In this report we describe the generation of anti-p73 monoclonal antibodies and confirm that two previously described p73 isoforms are produced in mammalian cells. Furthermore, we show that these two isoforms can bind to canonical p53 DNA-binding sites in electrophoretic mobility shift assays. Despite the high degree of similarity between p53 and p73, we found that adenovirus E1B 55K, simian virus 40 T, and human papillomavirus E6 do not physically interact with p73. The observation that viral oncoproteins discriminate between p53 and p73 suggests that the functions of these two proteins may differ under physiological conditions. Furthermore, they suggest that inactivation of p73 may not be required for transformation.
p53 is the most frequently mutated human tumor suppressor gene identified to date. In model systems, restoration of p53 function in tumor cells leads either to a cell cycle block or to apoptosis. These activities have been linked to the ability of p53 to bind to specific DNA sequences and activate transcription. Among the genes that are activated by p53 are the cyclin-dependent kinase inhibitor p21 and a family of genes involved in oxidative stress (10, 36).
Recently, Kaghad et al. serendipitously identified a cDNA encoding a novel p53-like protein (22). The protein, called p73, is approximately 60% identical to p53 in the region corresponding to the p53 DNA-binding domain. Furthermore, all of the residues that contact DNA in the p53-DNA crystal structure are conserved in p73 (7). In addition, the N terminus of p73 is similar (∼25% identity) to the N-terminal transactivation domain of p53, and the C terminus of p73 contains a region which is comparably similar to a C-terminal oligomerization domain within p53 (34, 44, 48). Finally, the genomic organizations of p53 and p73 are highly similar, suggesting that they have a common ancestry (22). In keeping with this high degree of similarity, p73 can, at least when overproduced, activate transcription from p53-responsive promoters and induce tumor cell apoptosis (20, 22).
The p73 gene maps to chromosome 1p36, a region that is frequently deleted in a variety of human cancers (22). Nonetheless, to date no mutations have been identified in the remaining p73 allele in such tumors. Thus, p73, unlike many tumor suppressor gene products such as the retinoblastoma protein (pRB) and p53, does not appear to conform to a two-hit model of tumorigenesis. It has been suggested that this may be due to epigenetic silencing of the p73 allele retained in tumors (22). Alternatively, it is possible that p73 is not a tumor suppressor gene product and is not the relevant target of 1p36 deletions.
A number of unrelated DNA tumor viruses can inactivate pRB. Inactivation of pRB leads to derepression of E2F-responsive promoters, which, in turn, allows quiescent cells to enter S phase. In model systems, untimely derepression of E2F-responsive promoters can also lead to both p53-dependent and p53-independent apoptosis (17, 25, 35, 37, 43, 50). These viruses, however, encode a variety of antiapoptotic proteins. For example, polyomaviruses, papillomaviruses, and adenoviruses encode T, E6, and E1B 55K proteins, respectively, which bind to and inactivate p53 (1, 3, 8, 11, 16, 19, 26, 30, 32, 40, 42, 49, 51). Indeed, p53 was first identified as a cellular T-binding protein (26, 30). In this manner, the host DNA replication machinery is usurped for viral replication.
T, E6, and E1B each target different regions of p53. T binds to p53’s core DNA-binding domain (27, 38), whereas E1B 55K binds to, and silences, p53’s N-terminal transactivation domain (4, 23, 29, 51). E6 interacts with p53’s core DNA-binding domain and C terminus and targets p53 for ubiquitin-dependent proteolysis (28, 31, 32, 39, 40). In short, viral oncoproteins have been valuable reagents for studying the structure and functions of p53. Given the apparent similarity of p53 and p73, we asked whether viral oncoproteins that inactivate p53 were likewise capable of inactivating p73. Surprisingly, none of these viral oncoproteins interact with p73. Thus, small DNA tumor viruses can discriminate between p53 and p73.
MATERIALS AND METHODS
Cell lines and transfections.
SK-N-SH, SK-N-AS, SK-N-DZ, and IMR-32 neuroblastoma cells, SK-N-MC neuroepithelioma cells, and IM-9 myeloma cells were obtained from the American Type Culture Collection (Rockville, Md.) and were grown in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum at 37°C in the presence of 10% CO2. COS monkey kidney cells, 293 human embryonic kidney cells, and SAOS2 osteosarcoma cells were from the laboratory stocks of David Livingston and were maintained in Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum at 37°C in the presence of 10% CO2. Transfections were performed by the N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES)-buffered saline (BBS) method as described earlier (21). Briefly, cells growing in monolayers were transfected when approximately 70 to 80% confluent with 20 to 30 μg of plasmid DNA. The DNA precipitates were removed 18 h later and the cells were fed fresh medium. Protein and reporter assays were performed 24 h later.
Plasmids.
The mammalian expression plasmids encoding hemagglutinin (HA)-tagged versions of simian p73α and p73β were described earlier (20). The human p53 cDNAs in pGEX2T-p53 (wild type) and pGEX2T-p53 (135Tyr) (18) (gifts of John Huibregtse and Peter Howley) were excised by digestion with BamHI and EcoRI and ligated into pcDNA3-HA that had been cut with these two enzymes to make pcDNA3-HAp53 and pcDNA3-HAp53(135Tyr), respectively.
The mammalian expression plasmid pSG5-T was described previously (52). pSG5-T(dl357-370), a gift of Herta Chao, was made by replacing the PflMI-PstI cDNA fragment in pSG5-T, containing the wild-type p53-binding site, with the corresponding fragment from a cDNA encoding T dl357-370 (24), which contains a mutant p53-binding site. pGEX2T-E6 was a gift of Peter Howley. pGEX2T-E4 (ORF 6/7) was a gift of Joseph Nevins. 2XRGC-CAT was a gift of Erik Flemington and was described previously (20). pRcCMV-HAp51A was a gift of Shuntaro Ikawa (33). The corresponding p51 isoform resembles p73β.
Antibodies.
Monoclonal antibodies were generated by standard techniques (14). RBF/DNJ (BnR) mice were immunized with either glutathione S-transferase (GST)–p73α (amino acids [aa] 380 to 637) for the ER antibodies or GST-p73β (aa 380 to 499) for the GC antibodies. The antibodies were screened based on their ability to immunoprecipitate 35S-radiolabelled p73 (α and β) translation products and their ability to detect the original GST immunogens by Western blot analysis. ER13, ER15, and GC15 were selected for further characterization as described in Results.
Murine monoclonal anti-HA (12CA5) was obtained from Boehringer Mannheim. Murine monoclonal anti-T (PAb419) (13) and monoclonal anti-p53 (PAb421) hybridoma cells were a gift from Ed Harlow. Hybridoma supernatants were prepared by standard techniques (14).
Purified anti-p53 PAb1801 (Ab-2) murine monoclonal antibody was obtained from Oncogene Science. Purified anti-adenovirus type 5 E1B 55K (Ab-1) rat monoclonal antibody was obtained from Calbiotech. Purified rabbit anti-HA (Y11) antiserum was obtained from Santa Cruz.
Immunoprecipitation and immunoblot analysis.
To detect endogenous p73, cells growing in monolayers were removed from plastic dishes by scraping and collected, along with floating cells, by centrifugation. The cells were then washed once with ice-cold phosphate-buffered saline and lysed, except where indicated, in EBC buffer (50 mM Tris [pH 8.0], 120 mM NaCl, 0.5% Nonidet P-40) supplemented with aprotonin (11.5 μg/ml), phenylmethylsulfonyl fluoride (50 μg/ml), NaF (100 mM), and Na orthovanadate (0.2 mM). In some experiments the cells were lysed in Triton 1% buffer (25 mM Tris [pH 7.4], 25 mM NaCl, 1% Triton X-100) or radioimmunoprecipitation assay buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1.0% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS]) supplemented with the same inhibitors. For some transfection experiments, cells were lysed directly in EBC buffer without scraping. Lysates were clarified by centrifugation at 14,000 × g for 15 min at 4°C. Protein concentrations were determined by the Bradford method.
For immunoblot analysis, proteins were resolved by SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose (Bio-Rad). The filters were blocked in TBST (10 mM Tris [pH 8.0], 150 mM NaCl, 0.05% Tween 20 [Sigma]) supplemented with 5% (wt/vol) powdered milk for 1 h at room temperature and then incubated with primary antibody. Anti-p73 hybridoma supernatants were used at a dilutions of 1:50 to 1:100 (vol/vol) overnight at 4°C. Anti-HA (12CA5) hybridoma supernatant was used at a dilution of 1:50 (vol/vol) for 1 h at room temperature. Anti-p53 (PAb421) hybridoma supernatant was used at a dilution of 1:100 (vol/vol) overnight at 4°C. Purified anti-p53 (Ab-2) was used at a dilution of 1:1,000 (vol/vol) for 1 h at room temperature. The filters were then washed three times with TBST at room temperature and incubated with a horseradish peroxidase-conjugated goat antimouse antibody (Pierce) diluted 1:5,000 (vol/vol) in TBST supplemented with 2% (wt/vol) powdered milk and 1% goat serum for 1 h at room temperature. The filters were then washed three times with TBST, and bound secondary antibody was detected by enhanced chemiluminescence with Supersignal (Pierce) according to the manufacturer’s protocol.
For the experiments depicted in Fig. 3A and 6A, monoclonal antibodies were directly coupled to protein A-Sepharose prior to immunoprecipitation as described elsewhere (14). Briefly, hybridoma supernatants were incubated overnight at 4°C with protein A-Sepharose that had been preequilibrated in 50 mM Tris (pH 8.0)–150 mM NaCl–5 mM EDTA, 0.1% Nonidet P-40. The Sepharose was then resuspended in 0.1 M sodium borate (pH 9.0) containing 40 mM dimethylpimelimidate. Following a 1-h incubation at room temperature, the reaction was stopped by resuspending the Sepharose in 40 mM ethanolamine in 0.1 M sodium borate (pH 8.0). One hour later, the Sepharose was washed with NETN (20 mM Tris [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40) and stored in NETN with 0.01% merthiolate. Aliquots of Sepharose containing approximately 1 μg of coupled antibody were used per immunoprecipitation reaction.
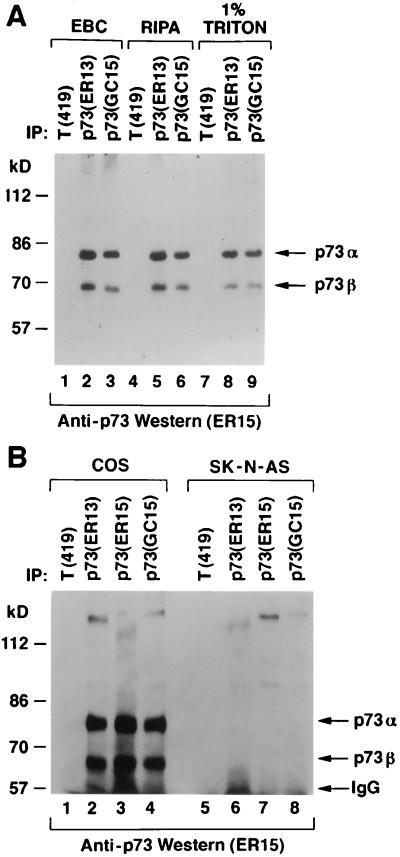
FIG. 3.
Identification of p73α and p73β in cell extracts. (A) COS cells were lysed in the presence of 0.5% Nonidet P-40 (EBC buffer) (lanes 1 to 3), in radioimmunoprecipitation assay buffer (lanes 4 to 6), or in the presence of 1% Triton (lanes 7 to 9) and immunoprecipitated (IP) with a control antibody (anti-T [PAb419]), anti-p73 (ER13), or anti-p73 (GC15) as indicated. Antibodies were cross-linked to protein A-Sepharose prior to immunoprecipitation. (B) COS cells (lanes 1 to 4) and SK-N-AS cells (lanes 5 to 8) were lysed in EBC buffer and immunoprecipitated with a control antibody (anti-T [PAb419]), anti-p73 (ER13), anti-p73 (ER15), or anti-p73 (GC15) as indicated. For both panels, Bound proteins were resolved by electrophoresis in an SDS–8% polyacrylamide gel and immunoblotted with anti-p73 (ER15) by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence.
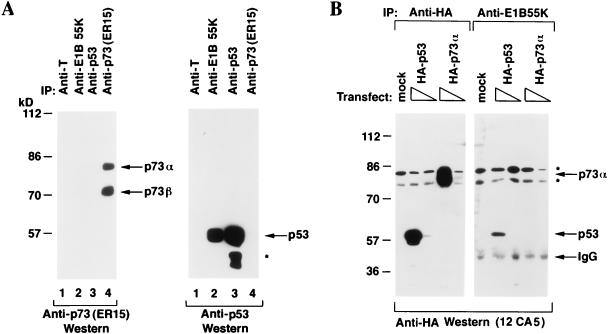
FIG. 6.
Differential binding of p53 and p73 to E1B 55K. (A) 293 human embryonic kidney cells were lysed and immunoprecipitated (IP) with the indicated antibodies. Specifically bound proteins were resolved by electrophoresis in an SDS–10% polyacrylamide gel and detected by immunoblotting with anti-p73 antibody (left panel) or anti-p53 antibody (right panel). Bound antibodies were detected by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence. The asterisk indicates faster-migrating species, previously noted by others, that interact with anti-p53 antibodies. (B) 293 human embryonic kidney cells were transfected with 1 or 10 μg, as indicated by the triangles, of expression plasmids encoding HA-tagged p53 (lanes 2, 7, and 8) or HA-tagged p73α (lanes 4, 5, 9, and 10) or with 10 μg of the backbone expression plasmid (lanes 1 and 6). Cell extracts were prepared and immunoprecipitated with anti-HA (lanes 1 to 5) or anti-E1B 55K antibodies (lanes 6 to 10). Specifically bound proteins were resolved by electrophoresis in an SDS–10% polyacrylamide gel and detected by immunoblotting with anti-HA antibody (12CA5) by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence. The asterisks indicate nonspecific bands.
To immunoprecipitate endogenous p73, approximately 1 mg of EBC cell extract was incubated with 50 μl of anti-p73 or anti-T hybridoma supernatant, 1 μg of rat monoclonal anti-E1B, or 1 μg of anti-p53 (Ab-2). To immunoprecipitate ectopically produced proteins, 250 μl of EBC extract, corresponding to approximately one-half of the cells grown in a nearly confluent 100-mm-diameter dish, was incubated with 50 μl of anti-HA or anti-T hybridoma supernatant, 1 μg of anti-HA rabbit polyclonal antibody (Santa Cruz), or 1 μg of anti-E1B monoclonal antibody. Immunoprecipitation of in vitro translation products was performed with 5 μl of in vitro translation product and 50 μl of hybridoma supernatant in 500 μl of NETN. In some experiments the in vitro translation products were first denatured by boiling in 50 μl of 2% SDS–50 mM Tris (pH 8.0). Immunoprecipitates were collected on protein A-Sepharose, washed five times with NETN, and eluted by boiling in SDS-containing sample buffer.
In vitro translation.
In vitro translation was performed with the TNT translation system (Promega) according to the manufacturer’s protocol.
Electrophoretic mobility shift assay.
A 23-mer oligonucleotide corresponding to a wild-type (5′-AGCTTAGGCATGTCTAGGCATGTCTA-3′) p53 DNA-binding site (10) was synthesized, annealed to a complementary synthetic oligonucleotide in vitro, and radiolabelled with [γ-32P]ATP by using polynucleotide kinase (New England Biolabs). DNA binding reactions were performed in 20 μl containing 2 μl of the indicated protein in vitro translation product (or unprogrammed reticulocyte lysate), 10 μl of 2× DNA binding buffer (50 mM Tris [pH 7.5], 40% glycerol, 100 mM KCl, 100 mM dithiothreitol, 2 mg of bovine serum albumin per ml, and 0.2% [vol/vol] Triton X-100), 1 ng of radiolabelled p53 DNA-binding site, and 300 ng of salmon sperm DNA. Where indicated, 40 ng of nonlabelled wild-type or sequence-scrambled (sense strand, 5′-CCAGCTAGCAGGCAGCATCAGTACTT) duplex oligonucleotides was added as specific and nonspecific competitors, respectively. Antibody supershift experiments were performed by adding 2 μl of a 1:10 (vol/vol) dilution of the indicated hybridoma supernatant. Binding reaction mixtures were incubated for 15 min at room temperature and then resolved by electrophoresis at 4°C in a 4% nondenaturing polyacrylamide gel containing 0.5× Tris-borate-EDTA buffer. Electrophoresis was conducted for 2 to 3 h at 200 V. The gels were then dried and exposed to film at −70°C with an intensifying screen.
p53 degradation assay.
The E6 destruction and GST-E6 binding assays were performed as described elsewhere (1, 31, 40).
CAT assay.
SAOS2 cells were transiently transfected with 5 μg of reporter plasmid, 2 μg of pCMV-βgal, 0.5 μg of p73 or p53 expression plasmid, and 10 μg of wild-type or mutant large-T-antigen plasmid. The total amount of DNA was brought up to 24 μg with pRcCMV carrier plasmid (Invitrogen). At 48 h after transfection, chloramphenicol acetyltransferase (CAT) assays were performed as described previously (21).
RESULTS
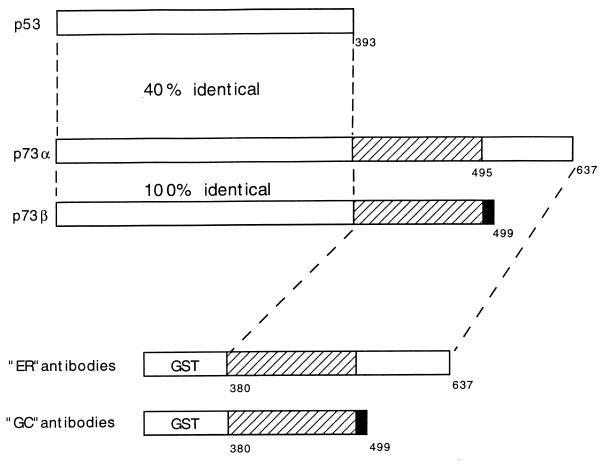
In order to study the p73 protein, we generated monoclonal anti-p73 antibodies. Two alternatively spliced p73 mRNA isoforms, designated p73α and p73β, were identified by Kaghad et al. (22). The p73β isoform lacks exon 13. Consequently, p73β contains a frameshift and terminates prematurely after the addition of a 5-aa sequence not present in the alpha isoform. Bacterial expression plasmids that encoded GST fused to the C terminus of either simian p73α or p73β were made (Fig. 1). These fusion proteins, which were designed so as to minimize the likelihood of generating antibodies that would cross-react with p53, were then used to immunize mice. Hybridomas were generated by standard techniques, and monoclonal antibodies were screened based on their ability to immunoprecipitate p73, but not p53, in vitro translation products. At least three such monoclonal anti-p73 antibodies, named ER13, ER15, and GC15, were identified in this fashion.
FIG. 1.
GST-p73 fusion proteins used to generate monoclonal antibodies. Shown schematically are p53, p73α, and p73β. Mice were immunized with GST fused to either the C terminus of p73α (ER mice) or the C terminus of p73β (GC mice). The p73β cDNA lacks exon 13, resulting in a frameshift at residue 495. p73β therefore contains five residues (black box) not present in p73α.
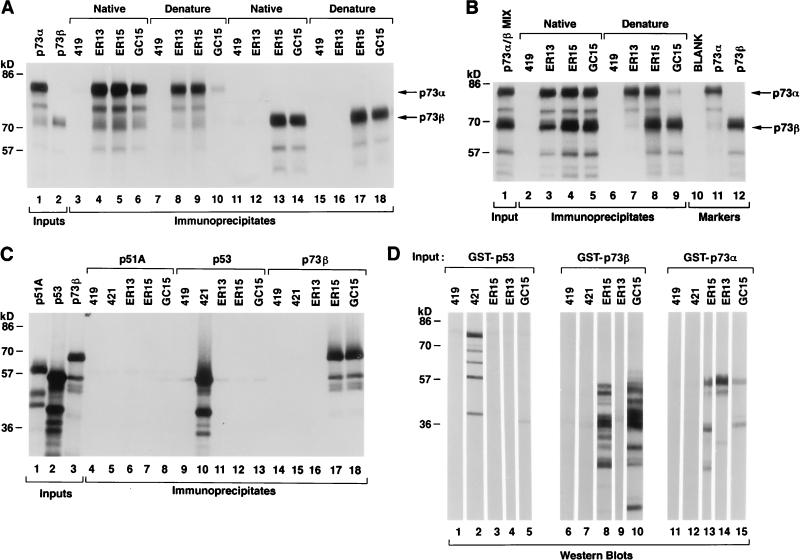
ER13 bound to p73α, but not p73β, in immunoprecipitation and immunoblot assays (Fig. 2A and D), suggesting that the ER13 epitope is located within the C-terminal region that is unique to p73α (Fig. 1). Of note, however, ER13 did immunoprecipitate p73β in the presence of p73α under native, but not denaturing, conditions (Fig. 2B [compare lanes 3 and 7] and 3 and data not shown). This suggests that p73α and p73β can heterodimerize in solution. ER15 and GC15 bound to both p73α and p73β in immunoprecipitation and immunoblot assays (Fig. 2A and D). The ability of GC15 to immunoprecipitate p73α, however, was diminished under denaturing conditions (Fig. 2A, compare lanes 6 and 10). This, coupled with the different band patterns observed in the immunoblot assays shown in Fig. 2D, implies that ER15 and GC15 recognize different epitopes common to p73α and p73β (note that multiple bands are seen in Fig. 2D due to limited proteolysis of GST fusion proteins during their production and purification). None of the anti-p73 antibodies recognized p53 or the recently identified p53-related protein p51/Ket (33, 41, 46) (Fig. 2C and D).
FIG. 2.
Characterization of anti-p73 antibodies. (A) p73α and p73β 35S-labelled in vitro translation products were immunoprecipitated with either control (anti-T; PAb419) or anti-p73 (ER13, ER15, and GC15) antibodies, as indicated, under native (lanes 3 to 6 and 11 to 14) or denaturing (lanes 7 to 10 and 15 to 18) conditions. Two microliters of the indicated in vitro translation product was loaded directly in lanes 1 and 2. (B) p73α and p73β were cotranslated in vitro in the presence of [35S]methionine and immunoprecipitated with either control (anti-T; PAb419) or anti-p73 (ER13, ER15, and GC15) antibodies, as indicated, under native (lanes 2 to 5) or denaturing (lanes 6 to 10) conditions. Two microliters of the indicated in vitro translation product was loaded directly in lanes 1, 11, and 12. (C) p51A, p53, and p73β 35S-labelled in vitro translation products were immunoprecipitated with either control (anti-T; PAb419) or anti-p73 (ER13, ER15, and GC15) antibodies, as indicated. Two microliters of the indicated in vitro translation product was loaded directly in lanes 1 to 3. For panels A to C, proteins were resolved by SDS-polyacrylamide gel electrophoresis and detected by fluorography. (D) GST-p53 (lanes 1 to 5), GST-p73β (aa 380 to 499) (lanes 6 to 10), and GST-p73α (aa 380 to 637) (lanes 11 to 15) were produced in bacteria, purified by using glutathione-Sepharose, resolved by SDS-polyacrylamide gel electrophoresis in preparative wells, and transferred to a membrane. The membrane was cut into strips and immunoblotted with either control (anti-T; PAb419) or anti-p73 (ER13, ER15, and GC15) antibodies, as indicated. Bound antibody was detected colorimetrically with an alkaline phosphatase-conjugated antimouse antibody.
It has not been formally proven that mammalian cells produce both p73α and p73β proteins. To address this, COS monkey kidney cells were lysed with a variety of detergents and immunoprecipitated with anti-p73 antibodies, or a control antibody, under conditions of antibody excess (Fig. 3A, lanes 1 to 9; Fig. 3B, lanes 1 to 4). Specifically bound proteins were detected by immunoblot analysis with an anti-p73 antibody (ER15). Two proteins, with apparent molecular masses of ∼80 and 70 kDa, were detected in the anti-p73, but not control, immunoprecipitates. These two proteins comigrated with the in vitro translation products of simian p73α and p73β cDNAs, respectively, under a variety of SDS-polyacrylamide gel electrophoresis conditions (data not shown). As an additional specificity control, SK-N-AS human neuroblastoma cells, which do not produce detectable levels of p73 mRNA (22), were analyzed in parallel (Fig. 3B, lanes 5 to 8). As expected, these cells lacked the 80- and 70-kDa species (Fig. 3B, compare lanes 6 to 8 with lanes 2 to 4). We have thus far been unable to purify enough of the 80- and 70-kDa species to perform partial proteolytic peptide mapping or to obtain peptide sequence data. Nonetheless, the facts that they (i) are recognized by multiple, independent, anti-p73 monoclonal antibodies, (ii) comigrate with p73α and p73β in vitro translation products, and (iii) are absent in a cell line that lacks p73 mRNA strongly suggest that they are p73α and p73β, respectively.
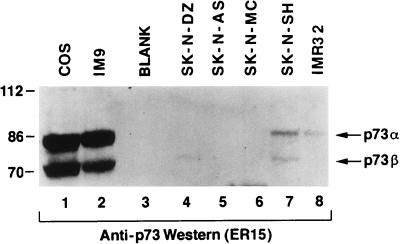
p73 maps to chromosome 1p36, a region that is frequently deleted in a variety of human tumors, including neuroblastomas (5, 45, 47). Although no intragenic p73 mutations have been identified to date, it has been suggested that the p73 gene locus might be subject to epigenetic regulation. In the next set of experiments, whole-cell extracts were prepared from COS cells, IM9 human myeloma cells, and a panel of neuroblastomas and neuroepitheliomas (Fig. 4). These extracts were resolved by SDS-polyacrylamide gel electrophoresis and immunoblotted with an anti-p73 antibody (ER15). p73α and p73β were readily detected in both COS cell extracts and IM9 cell extracts (Fig. 4, lanes 1 and 2). Thus, the failure to detect p73α and p73β in SK-N-AS cells (Fig. 3B, lanes 6 to 8, and 4, lane 5) cannot be attributed to species differences. p73α and p73β were either absent or barely detectable in all of the neuroblastoma and neuroepithelioma cell lines tested (Fig. 4, compare lanes 4 to 8 with lanes 1 and 2). Some of these cell extracts, including those prepared from COS, SK-N-SH, and SK-N-DZ cells, contained faster-migrating species that reacted with the ER15 antibody (data not shown). Whether these proteins represent additional p73 isoforms, additional p53/p73 family members, or unrelated proteins that share the ER15 epitope is currently under investigation. We find that most human tumor cell lines, like the neuroblastoma cell lines studied here, produce low or undetectable levels of p73α and p73β (unpublished data).
FIG. 4.
p73α and p73β protein levels in neuroblastoma cell lines. Cell extracts prepared from the indicated cell lines were resolved by electrophoresis in an SDS–8% polyacrylamide gel and immunoblotted with an anti-p73 antibody (ER15). Each lane contained ∼300 μg of total cell extract. Bound antibody was detected by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence.
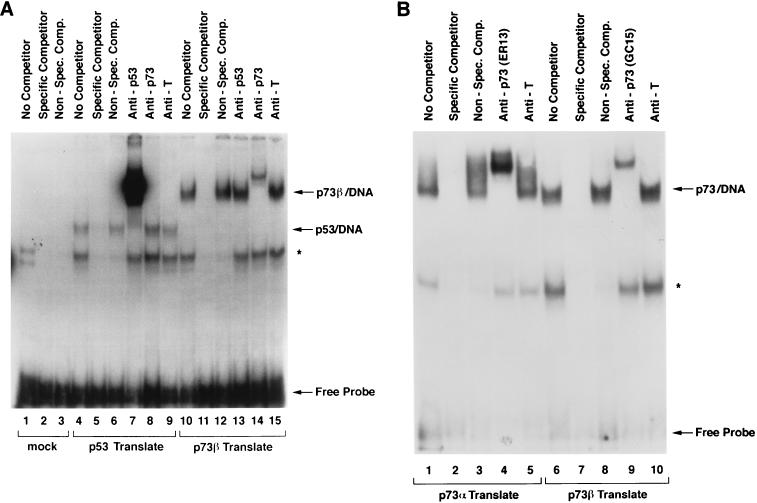
The availability of anti-p73 antibodies allowed us to next test directly whether p73 could, as predicted, bind to canonical p53 DNA-binding sites. A radiolabelled p53 DNA-binding site was incubated with either p53 (Fig. 5A, lanes 4 to 9) or p73β (lanes 10 to 15) synthesized in vitro by using a rabbit reticulocyte lysate. Unprogrammed rabbit reticulocyte lysate served as a negative control (lanes 1 to 3). As expected, p53 bound to this site (Fig. 5A, lane 4, arrow). This complex was specific, as it was abolished in the presence of excess, unlabelled competitor DNA that contained p53-binding sites and was unaffected by a competitor DNA that lacked such sites (Fig. 5A, compare lanes 6 and 5 to lane 4). In contrast, the complex indicated by the asterisk, detectable even with unprogrammed reticulocyte lysate, was abolished by both specific and nonspecific competitor DNA. The complex labelled p53/DNA contained p53, as it was supershifted by an anti-p53 antibody (Fig. 5A, lane 7) but not by anti-p73 or control (anti-T) antibodies (lanes 8 and 9). Similarly, p73β formed a specific complex with DNA that was supershifted by an anti-p73 antibody but not by the anti-p53 and anti-T antibodies (Fig. 5A, lanes 10 to 15). Comparable results were obtained with p73α (Fig. 5B, compare lanes 1 to 5 with lanes 6 to 10). Thus, as predicted, p73α and p73β can bind to p53 DNA-binding sites in a specific manner.
FIG. 5.
p73 binds to canonical p53 DNA-binding sites. A 32P-radiolabelled p53 DNA-binding site was incubated with p53 in vitro translation product (A, lanes 4 to 9), p73α in vitro translation product (A, lanes 10 to 15, and B, lanes 1 to 5), p73β in vitro translation product (B, lanes 6 to 10), or unprogrammed reticulocyte lysate (A, lanes 1 to 3) and subjected to electrophoretic mobility shift analysis. Binding reactions were carried out in the presence of a vast molar excess of unlabelled specific or nonspecific competitor DNA where indicated. Anti-p53, anti-p73, or a control antibody (anti-T) was added prior to the electrophoretic mobility shift assay as indicated. The asterisk indicates a nonspecific complex.
These results, together with earlier reports, suggest that p73 can, at least when overproduced, mimic the ability of p53 to bind to DNA, to activate transcription, and to inhibit tumor cell growth. DNA tumor viral oncoproteins have been invaluable reagents for elucidating the normal functions of tumor suppressor proteins, such as pRB and p53. Simian virus 40 (SV40) T antigen binds to the central region of p53 responsible for DNA binding (27, 38). The adenovirus E1B 55K protein interacts with residues within the N-terminal p53 transactivation domain that are highly conserved between p73 and p53 (4, 23, 29, 51). The human papillomavirus E6 protein interacts with the C terminus and the core structure of p53 (18, 31, 32, 39, 40). Given the high degree of similarity between p53 and p73, we next asked whether these viral oncoproteins could likewise physically interact with p73.
293 human embryonic kidney cells are stably transformed with a fragment of the adenovirus genome and produce E1B 55K (12). In the first set of experiments, these cells were immunoprecipitated with an anti-E1B 55K or control monoclonal antibody (Fig. 6A, lanes 2 and 1, respectively). The immunoprecipitates were resolved by SDS-polyacrylamide gel electrophoresis and immunoblotted with an anti-p73 antibody (left panel) or anti-p53 antibody (right panel). Anti-p73 and anti-p53 immunoprecipitates prepared in parallel served as additional controls (lanes 4 and 3, respectively). As expected, p53 coimmunoprecipitated with E1B 55K (Fig. 6A, lane 2, right panel). In contrast, p73 was not detectable in anti-E1B 55K immunoprecipitates (Fig. 6A, lane 2, left panel) despite the presence of p73 in these cells (Fig. 6A, lane 4, left panel).
These results left open the possibility that the failure to detect an association between p73 and E1B 55K reflected differences in the abundances of p73 and p53 in these cells. To address this concern, 293 cells were transiently transfected with an empty expression plasmid (Fig. 6B, lanes 1 and 6) or plasmids encoding HA-tagged p53 (lanes 2, 3, 7, and 8) or p73α (lanes 4, 5, 9, and 10). Cell extracts were prepared and immunoprecipitated with anti-HA antibody (lanes 1 to 5) or anti-E1B 55K (lanes 6 to 10) under conditions of antibody excess. Bound proteins were detected by anti-HA immunoblot analysis. Both p73α and p53 were produced at comparable levels (compare lanes 2 and 3 to lanes 4 and 5, respectively). Despite this, p53 was readily detected in anti-E1B 55K immunoprecipitates, whereas p73 was not (compare lane 7 to lane 9). Thus, we have been unable to detect stable complex formation between E1B 55K and p73α. Similar results were obtained for p73β (data not shown).
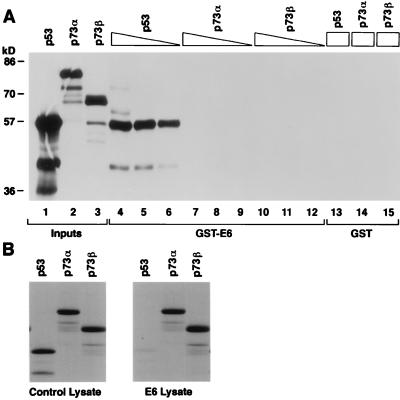
To determine whether E6 could interact with p73, we first performed GST pull-down assays with GST-E6 (Fig. 7A). As expected, radiolabelled p53 bound to GST-E6, whereas p73α and p73β did not (compare lanes 4 to 6 with lanes 7 to 12). None of these proteins bound to GST alone (lanes 13 to 15). Next we used an in vitro degradation assay developed by Scheffner et al. (40). This assay exploits the fact that rabbit reticulocyte lysate contains all of the proteins required for ubiquitin-dependent proteolysis of p53. Coincubation of bacterially produced E6 with radiolabelled p53 in vitro translation product led to virtual disappearance of the latter within 30 min (Fig. 7B, compare right and left panels). In contrast, under identical assay conditions, recombinant E6 had no discernible effect on the stability of p73α or p73β (Fig. 7B, compare right and left panels). Thus, E6 can not physically interact with p73 and is unable to target it for ubiquitin-dependent proteolysis.
FIG. 7.
E6 targets p53, but not p73, for degradation in vitro. (A) p53, p73α, and p73β 35S-labelled in vitro translation products were incubated with immobilized GST-E6 (lanes 4 to 12) or GST (lanes 13 to 15) as indicated. Each binding reaction mixture contained 40 μl (lanes 4, 7, 10, and 13 to 15), 20 μl (lanes 5, 8, and 11), or 10 μl (lanes 6, 9, and 12) of in vitro translation product. Specifically bound proteins were resolved by SDS-polyacrylamide gel electrophoresis and detected by fluorography. Five microliters of the indicated in vitro translation product was loaded directly in lanes 1 to 3. (B) p53, p73α, and p73β 35S-labelled in vitro translation products were incubated at 30°C with a lysate prepared from bacteria producing GST-E6 (right panel) or GST-E4 (left panel). The proteins were then resolved by electrophoresis in an SDS–10% polyacrylamide gel and detected by fluorography.
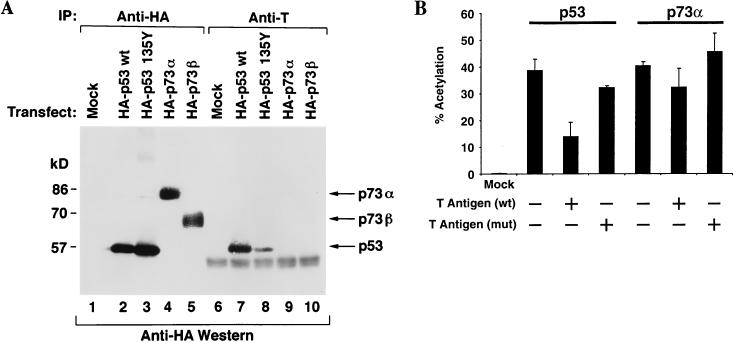
T antigen binds to the region that is most highly conserved between p53 and p73 (27, 38). This region has been implicated in p53’s ability to bind to specific DNA sequences and is also frequently altered in human tumors (2, 15, 34, 48). p73 was first identified in COS cells, a cell line that stably produces T antigen. We noted, however, that p73 was not present in anti-T immunoprecipitates prepared from these cells (Fig. 3A, lanes 1, 4, and 7, and Fig. 3B, lane 1), raising the possibility that T antigen does not bind to p73. To address this further, COS cells were transiently transfected with plasmids encoding HA-tagged versions of wild-type p53, a tumor-derived p53 mutant (p53 135Tyr) (6), p73α, and p73β or with the empty vector (Fig. 8A). Cell extracts were prepared and immunoprecipitated with anti-HA antibody (lanes 1 to 5) or anti-T antibody (lanes 6 to 10). Bound proteins were resolved by SDS-polyacrylamide gel electrophoresis and immunoblotted with an anti-HA antibody. All of the ectopically expressed proteins were produced at comparable levels (compare lanes 2 through 5). As expected, T antigen bound to wild-type p53 and, to a lesser extent, to the tumor-derived p53 mutant (lanes 7 and 8). In contrast, p73 was not detectable in the anti-T immunoprecipitates (lanes 9 and 10).
FIG. 8.
Differential binding of p53 and p73 to SV40 T. (A) COS cells, which stably produce SV40 T, were transfected with expression plasmids encoding the indicated HA-tagged proteins or with the backbone expression plasmid (Mock). Cell extracts were prepared and immunoprecipitated (IP) with anti-HA (lanes 1 to 5) or anti-T (lanes 6 to 10) antibodies. Specifically bound proteins were resolved by electrophoresis in an SDS–10% polyacrylamide gel and detected by immunoblotting with anti-HA antibody (12CA5) by using a horseradish peroxidase-conjugated antimouse antibody and enhanced chemiluminescence. (B) SAOS2 cells were transfected a CAT reporter plasmid containing a minimal promoter consisting of two p53-binding sites upstream of a TATA box together with expression plasmids for wild-type p53 or p73α. In each case, the amount of CAT activity, in the absence of T antigen, was set to 100%. Where indicated, the cells were also transfected with a plasmid encoding wild-type (wt) or mutant (mut) T antigen. Error bars represent one standard deviation.
In the final set of experiments, SAOS2 cells were transfected with a reporter plasmid in which the CAT gene was placed under the control of a p53-responsive promoter (2XRGC-CAT) along with expression plasmids encoding either p53 or p73α (Fig. 8B). These cells have both copies of the p53 gene deleted and consequently do not contain p53 protein (9). In addition, they contain either no or barely detectable levels of p73 (reference 22 and data not shown). In keeping with earlier studies, both p53 and p73α activated this reporter (20). Coexpression of wild-type T, but not a T-antigen mutant (dl357-370) that is incapable of binding to p53 (24), reproducibly inhibited the ability of p53 to activate this reporter. In contrast, neither wild-type T nor the T mutant significantly inhibited p73 function in these assays. Note that these experiments were performed with a 20-fold excess of T-antigen expression plasmid relative to the p53 or p73 expression plasmid. Similar results were obtained when a reporter containing the p53-responsive p21 promoter was used instead of 2XRGC-CAT (data not shown). In summary, both the biochemical data and transcriptional activation data suggest that T antigen, like E1B and E6, discriminates between p53 and p73.
DISCUSSION
Viral oncoproteins, as well as high-affinity monoclonal antibodies, have proven to be invaluble reagents for studying the functions of tumor suppressor proteins. In this report, we have described the generation and validation of multiple anti-p73 monoclonal antibodies. Using these reagents, we have confirmed that the two previously recognized p73 isoforms are produced in mammalian cells and can, as predicted, bind to canonical p53 DNA-binding sites.
To date no intragenic p73 mutations have been identified in human cancer cells. It has been suggested, however, that p73 is monoallelically expressed (22). If this was true, then loss of the transcriptionally active allele, as a result of a chromosomal deletion, might contribute to human carcinogenesis. On the other hand, others have found evidence of biallelic p73 expression in human T cells. It remains to be determined whether monoallelic expression of p73 might be restricted to certain tissues or whether the ability to imprint the p73 locus might itself be a polymorphic trait. p73 transcript analysis is further complicated by the existence of alternative promoters as well as of alternatively spliced mRNAs (4a). In summary, while a role for epigenetic control of p73 expression cannot be excluded, there are currently no genetic data that firmly establish that p73 is a tumor suppressor gene product.
At the protein level, we found that many tumor cell lines, including neuroblastoma cell lines, produce low levels of p73α and p73β. In the absence of p73 gene mutations, however, it is impossible to know whether this reflects pathology or physiology. Thus, one possibility is that p73α and p73β are, indeed, suppressed epigenetically. Alternatively, it is possible that the precursor cells that give rise to these tumors normally produce low levels of p73α and p73β. In addition, it is possible that p73 levels fall as a result of, rather than as a cause of, the malignant transformation of these cells.
One would have predicted that viral oncoproteins that inactivate p53 would also inactivate p73 if p53 and p73 played similar roles in cells. A potential precedent would be the inactivation of multiple retinoblastoma protein family members by adenovirus E1A, SV40 T, and human papillomavirus E7. Instead, we were unable to detect stable complexes between these proteins and p73 by using both biochemical and functional assays. One possible explanation is that p73 is not produced in those cells that are susceptible to transformation by these viruses. We found, however, that p73 is produced in COS cells and 293 cells. These cell lines are transformed by T and the left half of the adenovirus genome, respectively. Thus, stable binding of T or E1B 55K to p73 is apparently not necessary for transformation.
The high degree of similarity to p53 might have led to viral oncoprotein binding even if, as suggested above, inactivation of p73 is not required for transformation. In other words, if not by natural selection, then p73 might have bound to adenovirus E1B 55K, SV40 T, or human papillomavirus E6 by chance. The observation that viral oncoproteins discriminate between p53 and p73 raises the possibility that preservation of one or more p73 functions facilitates viral replication. In this light, the high levels of p73 seen in COS cells and 293 cells raise two interesting possibilities. The first is that high levels of p73 render a cell permissive for viral replication or transformation. The second is that p73 is induced following transformation with these agents.
In summary, despite the similarity between p53 and p73, only the former is a recurrent target of mutations in human cancer and a direct target of viral oncoproteins. This suggests that p53 and p73 are not functionally equivalent. In this regard, experiments performed to date suggest that p73, unlike p53, is not induced by DNA damage. Thus, p53 may have uniquely evolved to respond to genotoxic stress. Clearly, additional experiments are needed to decipher the normal functions of p73 and to determine whether it plays a role in human carcinogenesis.
ACKNOWLEDGMENTS
M.C.M. and C.A.J. contributed equally to this work.
We thank Arnold Berk, Herta Chao, Erik Flemington, Ed Harlow, Peter Howley, John Huibregste, Shuntaro Ikawa, and Joseph Nevins for reagents, Sorab Dalal for help with the GST-E6 binding assays, Ratna K. Vadlamudi for help with the p53 degradation assays, and members of the Kaelin laboratory for useful discussions.
M.C.M. was supported by an NIH training grant to the Cancer Biology Program at the Dana-Farber Cancer Institute. W.G.K. received support from the NIH and Novartis Pharmaceuticals and is a Howard Hughes Medical Institute assistant investigator.
REFERENCES
- 1.Band V, Dalal S, Delmolino L, Androphy E J. Enhanced degradation of p53 protein in HPV-E6 and BPV-1 E6 immortalized human mammary epithelial cells. EMBO J. 1993;12:1847–1852. doi: 10.1002/j.1460-2075.1993.tb05833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bargonetti J, Manfredi J J, Chen X, Marshak D R, Prives C. A proteolytic fragment from the central region of p53 has marked sequence-specific DNA-binding activity when generated from wild-type but not oncogenic mutant p53 protein. Genes Dev. 1993;7:2565–2574. doi: 10.1101/gad.7.12b.2565. [DOI] [PubMed] [Google Scholar]
- 3.Bargonetti J, Reynisdottir I, Friedman P N, Prives C. Site-specific binding of wild-type p53 to cellular DNA is inhibited by SV40 T antigen and mutant p53. Genes Dev. 1992;6:1886–1898. doi: 10.1101/gad.6.10.1886. [DOI] [PubMed] [Google Scholar]
- 4.Braithwaite A W, Blair G E, Nelson C C, McGovern J, Bellett A J. Adenovirus E1b-58 kD antigen binds to p53 during infection of rodent cells: evidence for an N-terminal binding site on p53. Oncogene. 1991;6:781–787. [PubMed] [Google Scholar]
- 4a.Caput, D. Unpublished data.
- 5.Cheng N C, Van Roy N, Chan A, Beitsma M, Westerveld A, Speleman F, Versteeg R. Deletion mapping in neuroblastoma cell lines suggests two distinct tumor suppressor genes in the 1p36 region, only one of which is associated with N-myc amplification. Oncogene. 1995;10:291–297. [PubMed] [Google Scholar]
- 6.Chiba I, Takahashi T, Nau M M, D’Amico D D, Curiel D T, Mitsudomi T, Buchhagen D L, Carbone D, Piantadosi S, Koga H, Reissman P T, Slamon D J, Holmes E C, Minna J D. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Oncogene. 1990;5:1603–1610. [PubMed] [Google Scholar]
- 7.Cho Y, Gorina S, Jeffrey P D, Pavletich N P. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–55. doi: 10.1126/science.8023157. [DOI] [PubMed] [Google Scholar]
- 8.Debbas M, White E. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 1993;7:546–554. doi: 10.1101/gad.7.4.546. [DOI] [PubMed] [Google Scholar]
- 9.Diller L, Kassel J, Nelson C, Gryka M, Litwak G, Gebhardt M, Bressac B, Ozturk M, Baker S, Vogelstein B, Friend S. p53 functions as a cell cycle control protein in osteosarcoma. Mol Cell Biol. 1990;10:5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 11.Farmer G, Bargonetti J, Zhu H, Fridman P, Prywes R, Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992;358:83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- 12.Graham F L, van der Eb A. A new technique for the assay of infectivity of human adenovirus 5 DNA. J Virol. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 13.Harlow E, Crawford L V, Pim D C, Williamson N M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 15.Hollstein M K, Rice K, Greenblatt M S, Soussi T, Fucks R, Sorlie T, Hovig E, Smith-Sorenson B, Montesano R, Harris C C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994;22:3551–3555. [PMC free article] [PubMed] [Google Scholar]
- 16.Hoppe-Seyler F, Butz K. Repression of endogenous p53 transactivation function in HeLa cervical carcinoma cells by human papillomavirus type 16 E6, human mdm-2, and mutant p53. J Virol. 1993;67:3111–3117. doi: 10.1128/jvi.67.6.3111-3117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh J, Fredersdorf S, Kouzarides T, Martin K, Lu X. E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 1997;11:1840–1852. doi: 10.1101/gad.11.14.1840. [DOI] [PubMed] [Google Scholar]
- 18.Huibregtse J M, Scheffner M, Howley P M. A cellular protein mediates association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO. 1991;10:4129–4135. doi: 10.1002/j.1460-2075.1991.tb04990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang D, Srinivasan A, Lozano G, Robbins P D. SV40 T antigen abrogates p53-mediated transcriptional activity. Oncogene. 1993;8:2805–2812. [PubMed] [Google Scholar]
- 20.Jost C, Marin M, Kaelin W G. p73 is a human p53-related protein that can induce apoptosis. Nature. 1997;389:191–194. doi: 10.1038/38298. [DOI] [PubMed] [Google Scholar]
- 21.Jost C A, Ginsberg D, Kaelin W G. A conserved region of unknown function participates in the recognition of E2F family members by the adenovirus E4 ORF 6/7 protein. Virology. 1996;220:78–90. doi: 10.1006/viro.1996.0288. [DOI] [PubMed] [Google Scholar]
- 22.Kaghad M, Bonnet H, Yang A, Creancier L, Biscan J-C, Valent A, Minty A, Chalon P, Lelias J-M, Dumont X, Ferrara P, McKeon F, Caput D. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 1997;90:809–819. doi: 10.1016/s0092-8674(00)80540-1. [DOI] [PubMed] [Google Scholar]
- 23.Kao C C, Yew P R, Berk A J. Domains required for in vitro association between the cellular p53 and the adenovirus 2 E1B 55K proteins. Virology. 1990;179:806–814. doi: 10.1016/0042-6822(90)90148-k. [DOI] [PubMed] [Google Scholar]
- 24.Kierstead T D, Tevethia M J. Association of p53 binding and immortalization of primary C57BL/6 mouse embryo fibroblasts by using simian virus 40 T-antigen mutants bearing internal overlapping deletion mutations. J Virol. 1993;67:1817–1829. doi: 10.1128/jvi.67.4.1817-1829.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalik T, DeGregori J, Schwarz J, Nevins J. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J Virol. 1995;69:2491–2500. doi: 10.1128/jvi.69.4.2491-2500.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane D P, Crawford L V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278:261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Fields S. Identification of mutations in p53 that affect its binding to SV40 large T antigen by using the yeast two-hybrid system. FASEB J. 1993;7:957–963. doi: 10.1096/fasebj.7.10.8344494. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Coffino P. High-risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol. 1996;70:4509–4516. doi: 10.1128/jvi.70.7.4509-4516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin J, Chen J, Elenbaas B, Levine A J. Several hydrophobic amino-acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994;8:1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- 30.Linzer D I H, Levine A J. Characterization of a 54K Dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979;17:43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- 31.Mansur C P, Marcus B, Dalal S, Androphy E J. The domain of p53 required for binding HPV 16 E6 is separable from the degradation domain. Oncogene. 1995;10:457–465. [PubMed] [Google Scholar]
- 32.Mietz J A, Unger T, Huibregtse J M, Howley P M. The transcriptional transactivation function of wild-type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO. 1992;11:5013–5020. doi: 10.1002/j.1460-2075.1992.tb05608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osada M, Ohba M, Kawahara C, Ishioka C, Kanamaru R, Katoh I, Ikawa Y, Nimura Y, Nakagawara A, Obinata M, Ikawa S. Cloning and functional analysis of human p51, which structurally and functionally resembles p53. Nat Med. 1998;4:839–843. doi: 10.1038/nm0798-839. [DOI] [PubMed] [Google Scholar]
- 34.Pavletich N P, Chambers K A, Pabo C O. The DNA-binding domain of p53 contains the four conserved regions and the major mutation hot spots. Genes Dev. 1993;7:2556–2564. doi: 10.1101/gad.7.12b.2556. [DOI] [PubMed] [Google Scholar]
- 35.Phillips A, Bates S, Ryan K, Helin K, Vousden K. Induction of DNA synthesis and apoptosis are separable functions of E2F-1. Genes Dev. 1997;11:1853–1863. doi: 10.1101/gad.11.14.1853. [DOI] [PubMed] [Google Scholar]
- 36.Polyak K, Xia Y, Zweier J, Kinzler K, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997;389:300–305. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 37.Qin X Q, Livingston D M, Kaelin W G, Adams P. Deregulated E2F1 expression leads to S-phase entry and p53-mediated apoptosis. Proc Natl Acad Sci USA. 1994;91:10918–10922. doi: 10.1073/pnas.91.23.10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruppert J, Stillman B. Analysis of a protein-binding domain of p53. Mol Cell Biol. 1993;13:3811–3820. doi: 10.1128/mcb.13.6.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheffner M, Takahashi T, Huibregtse J, Minna J, Howley P. Interaction of the human papillomavirus type 16 E6 oncoprotein with wild-type and mutant human p53 proteins. J Virol. 1992;66:5100–5105. doi: 10.1128/jvi.66.8.5100-5105.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. The E6 oncoprotein encoded by human papillomaviruses types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 41.Schmale H, Bamberger C. A novel protein with strong homology to the tumor suppressor p53. Oncogene. 1997;15:1363–1367. doi: 10.1038/sj.onc.1201500. [DOI] [PubMed] [Google Scholar]
- 42.Segawa K, Minowa A, Sugaswa K, Takano T, Hanaoka F. Abrogation of p53-mediated transactivation by SV40 large T antigen. Oncogene. 1993;8:543–548. [PubMed] [Google Scholar]
- 43.Shan B, Lee W-H. Deregulated expression of E2F-1 induces S-phase entry and leads to apoptosis. Mol Cell Biol. 1994;14:8166–8173. doi: 10.1128/mcb.14.12.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sturzbecher H W, Maimets T, Chumakov P, Brain R, Addison C, Simanis V, Rudge K, Philp R, Grimaldi M, Court W, Jenkins J R. p53 interacts with p34 cdc2 in mammalian cells: implication for cell cycle control and oncogenesis. Oncogene. 1990;5:795–801. [PubMed] [Google Scholar]
- 45.Takeda O, Homma C, Maseki N, Sakurai M, Kanda N, Schwab M, Nakamura Y, Kaneko Y. There may be two tumor suppressor genes on chromosome arm 1p closely associated with biologically distinct subtypes of neuroblastoma. Genes Chrom Cancer. 1994;10:30–39. doi: 10.1002/gcc.2870100106. [DOI] [PubMed] [Google Scholar]
- 46.Trink B, Okami K, Wu L, Sriuranpong V, Jen J, Sidransky D. A new human p53 homologue. Nat Med. 1998;4:747. doi: 10.1038/nm0798-747. [DOI] [PubMed] [Google Scholar]
- 47.Versteeg R, Caron H, Cheng N C, van der Drift P, Slater R, Westerveld A, Voute P A, Delattre O, Laureys G, Van Roy N, Speleman F. 1p36: every subband a suppressor. Eur J Cancer. 1995;31A:538–541. doi: 10.1016/0959-8049(95)00037-j. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Reed M, Wang P, Stenger J E, Mayr G, Anderson M E, Schwedes J F, Tegtmeyer P. p53 domains: identification and characterization of two autonomous DNA-binding regions. Genes Dev. 1993;7:2575–2586. doi: 10.1101/gad.7.12b.2575. [DOI] [PubMed] [Google Scholar]
- 49.Werness B A, Levine A J, Howley P M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 50.Wu X, Levine A J. p53 and E2F1 cooperate to mediate apoptosis. Proc Natl Acad Sci USA. 1994;91:3602–3606. doi: 10.1073/pnas.91.9.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yew P R, Berk A J. Inhibition of p53 transactivation required for transformation by adenovirus early E1B protein. Nature. 1992;357:82–85. doi: 10.1038/357082a0. [DOI] [PubMed] [Google Scholar]
- 52.Zalvide J, DeCaprio J A. Role of pRB-related proteins in simian virus 40 large T-antigen-mediated transformation. Mol Cell Biol. 1995;15:5800–5810. doi: 10.1128/mcb.15.10.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]