Abstract
Background and Aims
Marine macroalgae (‘seaweeds’) are critical to coastal ecosystem structure and function, but also vulnerable to the many environmental changes associated with anthropogenic climate change (ACC). The local habitat conditions underpinning observed and predicted ACC-driven changes in intertidal macroalgal communities are complex and probably site-specific and operate in addition to more commonly reported regional factors such as sea surface temperatures.
Methods
We examined how the composition and functional trait expression of macroalgal communities in SW England varied with aspect (i.e. north–south orientation) at four sites with opposing Equator- (EF) and Pole-facing (PF) surfaces. Previous work at these sites had established that average annual (low tide) temperatures vary by 1.6 °C and that EF-surfaces experience six-fold more frequent extremes (i.e. >30 °C).
Key Results
PF macroalgal communities were consistently more taxon rich; 11 taxa were unique to PF habitats, with only one restricted to EF. Likewise, functional richness and dispersion were greater on PF-surfaces (dominated by algae with traits linked to rapid resource capture and utilization, but low desiccation tolerance), although differences in both taxon and functional richness were probably driven by the fact that less diverse EF-surfaces were dominated by desiccation-tolerant fucoids.
Conclusions
Although we cannot disentangle the influence of temperature variation on algal ecophysiology from the indirect effects of aspect on species interactions (niche pre-emption, competition, grazing, etc.), our study system provides an excellent model for understanding how environmental variation at local scales affects community composition and functioning. By virtue of enhanced taxonomic diversity, PF-aspects supported higher functional diversity and, consequently, greater effective functional redundancy. These differences may imbue PF-aspects with resilience against environmental perturbation, but if predicted increases in global temperatures are realized, some PF-sites may shift to a depauperate, desiccation-tolerant seaweed community with a concomitant loss of functional diversity and redundancy.
Keywords: Anthropogenic climate change, aspect, biodiversity–ecosystem functioning, extreme temperatures, functional redundancy, functional traits, rocky shore, seaweed
INTRODUCTION
Globally, ecosystems face unprecedented pressures from a combination of habitat loss, over-exploitation, invasive species and a rapidly changing climate (MEA, 2005; Venter et al., 2016; Parmesan et al., 2022). The potential impact of these changes on biodiversity and ecosystem service provision is well established (Isbell et al., 2017; Paul et al., 2020). Central to the biodiversity–ecosystem functioning (BEF) relationship is the notion of ‘functional complementarity/redundancy’; i.e. how the impact of species losses on ecosystem function and resilience may be compensated for if functionally similar organisms are present but increase drastically once all species within a functional group are lost (Yachi & Loreau, 1999; Naeem, 2002; Seddon et al., 2016). Although several studies have demonstrated the robustness of the functional redundancy concept, it is apparent that assessment and prediction of the ecological consequences of environmental change for the contribution of functional diversity to the BEF relationship differs between ecosystems and environments (Cardinale, 2011; Fetzer et al., 2015; Dolbeth et al., 2019).
Capturing the effects of environmental perturbation upon functional redundancy and the BEF in field conditions is challenging. Manipulation of single and multiple key environmental factors such as temperature and precipitation is possible (Firth and Williams, 2009), but inevitably limited to imposing a short-duration ‘pulse’ or a limited range of relatively fixed experimental treatments (compared to ambient), in a single habitat type (Pfisterer and Schmid, 2002; Allison, 2004; Grime et al., 2008; Vetter et al., 2020). Although microbial microcosms offer a tractable way to manipulate, replicate and control species (functional) composition and multiple external environmental conditions (Fetzer et al., 2015; Banitz et al., 2020), they are a poor surrogate for the very different biological responses of plants and animals to fluctuating environments in natural field conditions (Fetzer et al., 2015). Not least of these differences is the fact that the regeneration biology of many eukaryotes is cued to variations and interactions of climate extremes, rather than changes in average temperature or precipitation (Parmesan and Hanley, 2015; Parmesan et al., 2022). In addition, shifts in species geographical distributions of the kind associated with anthropogenic climate change (ACC) and particularly with extreme weather events (Crisp, 1964; Firth et al., 2015, 2021; Filbee-Dexter et al., 2020) impose rapid changes in local community structure and ecosystem functioning (Usinowicz and Levine, 2018; Aguilera et al., 2020; Vetter et al., 2020).
Taken together, these issues highlight the importance of comparative, field‐based studies to better understand and predict the consequences of environmental variation for ecosystem functioning. The challenge is finding ecological communities in different habitats where natural fluctuations and extremes in critical environmental factors, for example temperature, occur. Aspect (i.e. north–south orientation) may be one such opportunity.
In terrestrial systems, variation in species distributions has long been associated with habitat orientation towards the sun (Cantlon, 1953; Nevo, 2012). Recent studies in marine intertidal systems also highlight considerable local differences in thermal conditions and species assemblages on Pole-facing (PF) versus Equator-eacing (EF) surfaces (Seabra et al., 2011; Firth et al., 2016; Lima et al., 2016). Moreover, when compared at relatively local scales (i.e. PF- and EF-slopes are metres apart), other confounding factors (resource and propagule availability, precipitation, disturbance, etc.) probably remain relatively constant, while natural average and extreme temperatures vary significantly. For example, Amstutz et al (2021) reported average annual low tide (i.e. ‘air’) temperatures 1.6 °C higher on EF than PF rock surfaces, with high extremes (i.e. >30 °C) six times more frequent on EF-surfaces. Unsurprisingly, community composition also varied with aspect, but the effect was apparent both within (e.g. more patellid and trochid grazing molluscs on PF-surfaces), as well as between (e.g. more carnivorous dogwhelks on PF-surfaces, but fewer filter-feeding barnacles) trophic levels. Moreover, Amstutz et al. (2021) also observed aspect-related variation in reproductive phenology and thermal stress responses in two patellid limpet species.
In demonstrating substantial variation in average and extreme temperature conditions over just a few meters, Amstutz et al. (2021) highlighted the excellent potential intertidal gullies have for elucidating the effect of ACC-linked warming on community pattern and process. However, their study focused on intertidal invertebrate abundance and did not consider how functional traits within communities varied with aspect. Marine macroalgae (‘seaweeds’) play a critical role in ecological structure and functioning in the intertidal and coastal shelf environments (Ling et al., 2009; Johnson et al., 2011; Pessarrodona et al., 2022), but are especially vulnerable to the physico-chemical changes associated with ACC (Harley et al., 2012; Smale et al., 2013). As a result, dramatic changes in macroalgal assemblages have been observed (Wernberg et al., 2011; Vale et al., 2021; Smale et al., 2022), although the majority of range shifts and species turnover responses reported in the literature are attributed to regional increases in sea surface temperatures (SSTs) and their effects upon subtidal algae. Given the importance of local habitat (e.g. surface topography and aspect) for organism behaviour, abundance and distribution, and provision of refugia from the environmental stresses that characterize the intertidal zone (Helmuth et al., 2006; Johnson et al., 2008; Bracewell et al., 2018; Jurgens et al., 2022), our ability to predict intertidal community and species response to ACC must consider these factors (Barton et al., 2019; Amstutz et al., 2021; Vale et al., 2021). Consequently, the aim of this study was to elucidate how ACC-linked warming is likely to affect community assembly and processes in intertidal macroalgal assemblages and subsequent shifts in the trait characteristics possessed by seaweeds on cooler PF- and warmer EF-aspects. To do this, we investigated how intertidal aspect (and the temperature variation associated with PF- and EF-slope orientation) influenced intertidal macroalgae communities, specifically testing the predictions that (1) community composition and taxon richness vary between EF- and PF-surfaces and that, consequently, (2) functional diversity, and thence redundancy, also vary with aspect.
METHODS
Study sites and sampling
Intertidal surveys were carried out on four natural rocky shores on the north and south coasts of the southwest peninsula of England: Bude (50.836 667, −4.556 944) and Croyde (51.133 889, −4.243 889) on the north coast and Bantham (50.276 944, −3.884 722) and South Milton Sands (50.253 889, −3.861 944) on the south coast. All sites share similar geology (Upper Palaeozoic, inter-bedded shales and sandstones) with strata tilted at 80–90° (i.e. vertical dip) running perpendicular (i.e. east–west strike) to the shoreline and where erosion of softer sediments had created a series of almost-vertical 1-5-m-high gullies with Pole- (north-) or Equator- (south-) facing rock surfaces (Amstutz et al., 2021).
At each location, four gullies were haphazardly selected, but with the proviso that they provided access to long sections of opposing PF and EF vertical rock surfaces at mid-shore level. This spatial configuration reduced the possibility of variation in wave exposure between EF and PF rock surfaces. In summer 2016 (June and July) and winter (January/February) 2017, 12 0.5 × 0.5-m (0.25-m2) quadrats were haphazardly positioned along each of four gullies at each site, such that PF- and EF-quadrats opposed each other at the same relative shore height in each gully (a total 192 quadrats per season). Quadrats were placed on vertical, flat surfaces, avoiding crevices, pools and other microhabitats, and the overall percentage cover of all component macroalgae was estimated.
Analysis of community structure
We identified seaweeds in the field as accurately as possible, lumping problem taxa to genera where necessary [hereafter referred to as operational taxonomic units (OTUs) rather than ‘species’]. We estimated the number of OTUs in each gulley by averaging across quadrats, and tested for an effect of ‘Aspect’, ‘Season’ and ‘Site’ (fixed effects) using a Poisson error generalized linear model in R v.4.0 (R Core Team, 2021). The model was simplified using single term deletions based upon change in Akaike’s information criterion (AIC) > 2; estimated marginal means tests (R package emmeans, Lenth, 2020) were used to derive pair-wise comparisons between aspects within sites where appropriate.
To test the hypothesis that macroalgal assemblages differed across aspects in the different sites we used pairwise contrasts within a mixed-model PERMANOVA implemented in PRIMER v.6, with ‘Aspect’ and ‘Season’ as fixed factors and ‘Site’ as a random factor. To minimize effects of abundant taxa, data were 4th-root transformed prior to calculation of a similarity matrix based upon Bray–Curtis distances. The pairwise contrast compared aspects within sites across different seasons; data imbalances in terms of numbers of quadrats (e.g. zero counts) led us to average macroalgal data to the gully level, and thus each ‘Aspect’ × ‘Site’ × ‘Season’ had n = 4 and N = 64. Patterns of similarity and difference were visualized using the ‘ordiplot’ function in the R package vegan (Oksanen et al., 2020).
Analysis of functional traits
Functional diversity was estimated in two complementary ways to account for the functional contributions of the considerable number of gullies supporting fewer than three algal taxa (in which cases some functional diversity metrics cannot be calculated), and to allow us to incorporate encrusting alga for which we lacked functional trait data in the database we employed.
First we used the extensive database of functional trait scores provided by Mauffrey et al. (2020a, b) to calculate values of Functional Richness (FRic) (Villéger et al., 2008) and Functional Dispersion (FDis) (Laliberté and Legendre, 2010), as measures of functional alpha and beta diversity respectively, using dbFD in the R package FD (Laliberté and Legendre, 2010; Laliberté et al., 2014). FRic is a measure of the total extent of niche space occupied by an assemblage and is distinct from the number of functional groups present. FDis is a measure of the mean distance of all species to the weighted centroid of the community in trait space. Mauffrey et al.’s (2020b) database includes traits linked to photosynthetic capacity, structure and space use that together provide information about resource acquisition, productivity and competitive dominance, desiccation and herbivory tolerance, and resistance to water movement. We selected 11 of the 12 traits employed by Mauffrey et al. (2020b) (we excluded the presence of pneumatocysts, as when sampled, none of our taxa possessed them). Functional diversity measures were calculated based upon Euclidean distances using standardized, abundance-weighted trait scores (where relevant). Where OTUs at the species level in our dataset had traits not present in the Mauffrey et al. (2020b) database, we attributed trait scores either by averaging across congeners in the database or by using multivariate imputation by chained equations (MICE package: van Buuren and Groothuis-Oudshoorn, 2011) provided that >70% of species scores were present. We tested these measures of functional diversity using the same linear modelling approach applied to taxonomic species richness, transforming data to achieve homogeneity of variances where necessary.
Second, we allocated the 24 macroalgal OTUs recorded to the ‘Emergent Functional Groups’ described by Mauffrey et al. (2020a). We included additional functional groups for encrusting red and brown algae respectively, in order to compare at the gully level how the rate of accumulation of observed functional group richness responds to increasing taxonomic species richness in the two different aspects (see Supplementary Data Table S1). We also performed this comparison for FRic and FDis, testing the heterogeneity of response in diversity accumulation using type III sum of squares (SS) linear models.
We hypothesized that rates of accumulation of functional groups/diversity per OTU would not differ if the degree of relative functional richness per OTU is consistent across aspects (see Micheli and Halpern, 2005). The lower the rate of accumulation of functional groups/diversity, the more likely the OTUs are to be functionally redundant.
RESULTS
Aspect and community structure
Although total algal cover varied little on PF-slopes with season (~3 %), on EF-slopes cover more than doubled between summer (~8 %) and winter (~17 %). These values are fairly typical of the moderately exposed, barnacle- and limpet-dominated shores of SW England. Twenty-four OTUs were recorded, and of these, 11 were present only on PF-surfaces (Supplementary Data Table S1). Two taxa (Lithophyllum incrustans and Lomentaria articulata) were also noticeably more common (i.e. more than an average 5 % difference) on PF-slopes in summer and/or winter surveys. Only one species (Blidingia minima) was restricted to EF-aspects, and this was a single record, but Fucus sp. (hereafter ‘fucoids’ – combined because individuals were often too small to be confidently identified to species) were especially noteworthy in being consistently more abundant on EF- than PF-slopes (average 1.6 % EF vs. 0.5 % PF in summer and 1.7 % EF vs. 0.8 % PF in winter). Three entire gulley sites were devoid of macroalgae, and these were excluded from further analyses due to their disproportionate leverage values.
PF-surfaces were more species-rich than EF-surfaces in all sites (Fig. 1), supporting an average of 3.7 more OTUs (‘Aspect’ Wald χ2 = 22.866; P < 0.001), while summer samples supported on average 1.1 more OTUs than winter samples (‘Season’ χ2 = 1.972; P = 0.160). Sites did not differ significantly (χ2 = 3.422; P = 0.181), and no significant interactions involving ‘Aspect’ were found (‘Aspect’ × ‘Season’ χ2 = 0.325; P = 0.569; ‘Aspect’ × ‘Site’ χ2 = 2.942; P = 0.230).
Fig. 1.
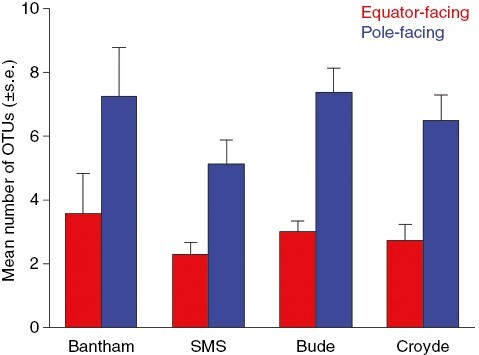
The influence of rock surface aspect on mean (±s.e., averaged across two seasons) species richness (determined using ‘operational taxonomic units’ – OTUs) of intertidal macroalgal communities sampled at four sites around the SW peninsula of England (SMS = South Milton Sands).
Unsurprisingly, algal assemblages varied across all four sites (PERMANOVA PsuF3,48 = 4.0475; P < 0.001), but while there was a seasonal effect (PsuF1,48 = 8.9749; P = 0.0385), we also found a consistent influence of aspect on community composition (PsuF1,48 = 16.211; P = 0.0289). Contrasts of aspects within sites reinforced this picture (Fig. 2; Supplementary Data Fig. 1a and b), indicating highly significant differences in algal assemblages between aspects in both seasons at Bude (t = 3.8256; P < 0.001), Croyde (t = 2.8244; P < 0.001) and South Milton (t = 3.0468; P < 0.001). The pattern of difference was less marked at Bantham (t = 1.931; P = 0.0207), largely as a result of variability attributable to EF gullies lacking macroalgal colonization. We detected no significant interactions between ‘Site’, ‘Season’ and ‘Aspect’. Collectively, there was strong support for our hypothesis that macroalgal community composition and species (OTU) richness vary between EF- and PF-surfaces.
Fig. 2.
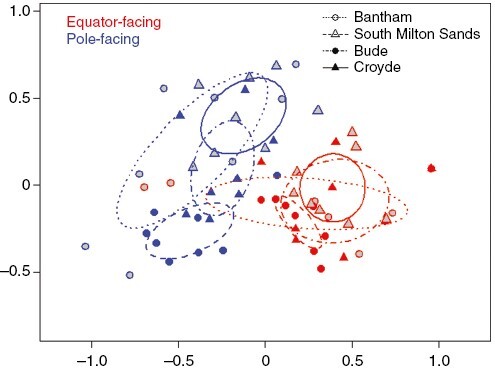
The influence of surface aspect on intertidal macroalgal communities sampled at four sites (symbols and line types) across the SW peninsula of England in 2016. nMDS was based on Bray–Curtis dissimilarity matrix; ellipses indicate standard deviations around group centroids, stress = 0.168.
Functional trait variation and redundancy
Following a square-root transformation to homogenize variance prior to analysis by linear modelling, we established that ‘Aspect’ was a significant predictor of number of functional groups (F1,40 = 20.113, P < 0.001). There was, however, no influence of ‘Season’ (F1,40 = 0.0418, P = 0.8391) or ‘Site’ (F2,40 = 0.5479, P = 0.5824) and no significant interaction between ‘Aspect’ and ‘Season’ (F1,40 = 0.2832, P = 0.5976). Consequently, the emergent pattern of more functional units on PF-slopes was consistent across seasons and sites (Fig. 3A).
Fig. 3.
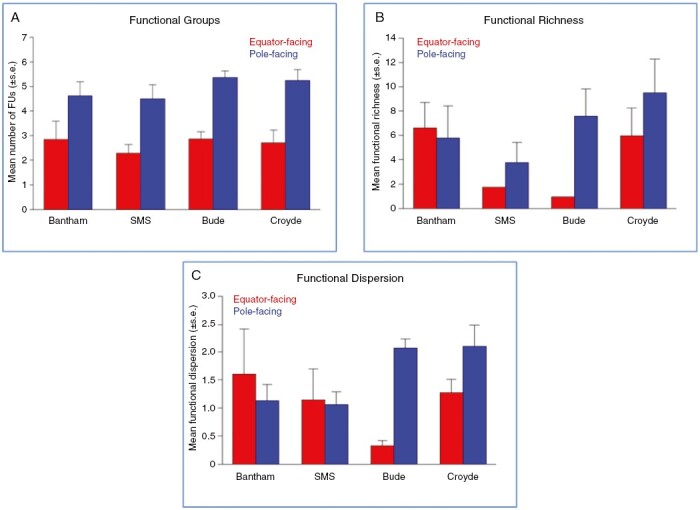
The influence of rock surface aspect on (A) mean (±s.e.) functional group number, (B) functional richness (FRic; Villéger et al., 2008) and (C) functional dispersion (FDis; Laliberté and Legendre, 2010), of intertidal macroalgae communities sampled at four sites around the SW peninsula of England (SMS = South Milton Sands); data from both seasons are aggregated.
By contrast, although there was a tendency towards higher functional richness (FRic) on PF-slopes at three sites (Fig. 3B), we established that FRic did not vary with ‘Aspect’ (F1,26 = 0.6286, P = 0.435) or ‘Site’ (F3,26 = 2.0277, P = 0.1346). There was a seasonal effect (F1,26 = 0.6.4361, P = 0.0175), probably attributed to the influence of homogeneous data for two of the sites, South Milton Sands and Bude on EF-slopes. There was no ‘Site’ × ‘Aspect’ interaction (F3,26 = 2.2047, P = 0.1115). For functional dispersion (FDis), there were no significant effects of ‘Aspect’ (F1,40 = 2.4159, P = 0.1280), ‘Season’ (removed during model simplification) or ‘Site’ (F2,40 = 0.7764, P = 0.4669) and no ‘Site’ × ‘Aspect’ interaction (F2,40 = 2.0204, P = 0.1454).
A Poisson error GLM comparing how functional group richness responded to increasing taxonomic (OTU) richness suggested that PF-slopes accumulated functional groups less quickly than EF-aspects (Fig. 4A), but this effect was (marginally) non-significant (χ2 = 3.3893; P = 0.0656). Following square-root transformation, analysis of functional richness (FRic) data with a type III SS linear model also failed to show any increase concomitant with the accumulation of OTUs (F1,31 = 0.0519; P = 0.0821), despite a positive trend on PF-slopes (Fig. 4B). Similar analysis of functional dispersion (FDis) data, however, revealed a significant difference (F1,57 = 8.761; P = 0.004) in the steeper rate of increase on EF- compared to PF-slopes (Fig. 4C).
Fig. 4.
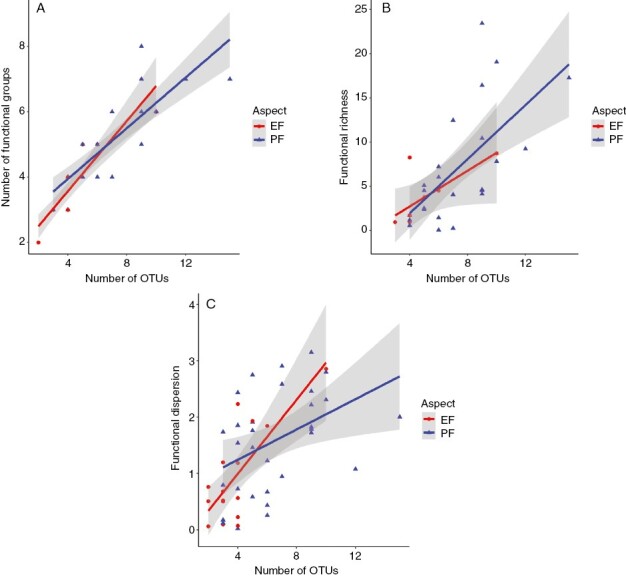
The influence of rock surface aspect on the accumulation of additional (A) macroalgal functional groups, (B) functional richness (FRic; Villéger et al., 2008) and (C) functional dispersion (FDis; Laliberté and Legendre, 2010), associated with a concomitant increase in the number of ‘operational taxonomic units’ (OTUs) for intertidal macroalgae communities at four sites in SW England; data from both seasons are aggregated.
Consequently, we conclude that while functional group richness achieved higher maxima on PF-aspects, the rate of per OTU accumulation of functional groups was lower; i.e. OTUs (‘species’) were packed more tightly into trait space, on cooler, PF-aspects and are, accordingly, displaying greater functional redundancy than EF-aspects. This in turn supports our second hypothesis that macroalgal functional diversity, and thus functional redundancy, varies with intertidal aspect.
DISCUSSION
Understanding variation in the functional diversity of primary producers across environmental gradients can provide essential information about how community structure and ecosystem functioning respond to anthropogenic stressors and environmental change (Cappellatti et al., 2020; Kühn et al., 2021; Westerband et al., 2021a, b). Our study revealed major differences, not only in overall macroalgal cover and OTU (‘species’) composition, but also considerable variation in functional diversity and redundancy between PF- and EF-slopes separated by just a few metres. Intuitively, it seems likely that the difference in temperature regimes (i.e. 1.6 °C annual average, seasonal average and six-fold variation in extremes) between EF- and PF-surfaces (Amstutz et al., 2021) is pivotal in explaining the observed differences in the macroalgal community. What we cannot disentangle is the relative importance of temperature on algal ecophysiology weighed against the role played by the various other intertidal organisms that influence algal settlement, establishment and persistence. For example, Amstutz et al., (2021) found a higher barnacle (Chthamalus sp.) abundance on EF-aspects, corroborating a reported tolerance of heat and desiccation stress in the Cirripedia (Southward, 1958; Wethey, 1983). It remains unclear, however, whether Chthamalus reduced the area available for macroalgal settlement because of an ecophysiological tolerance of warmer slopes or was simply taking advantage of reduced algal cover. Similarly, higher limpet and trochid abundance on PF-slopes (Firth et al., 2016; Amstutz et al., 2021) may signpost how ecophysiological responses in the grazer community influence macroalgal species distributions and trait expression, or instead reflect macroalgae abundance and food and shelter provision for the grazers.
While more research is needed to determine the ecological mechanisms explaining how and why algal abundance and distribution vary between PF- and EF-surfaces, our results underline how macroalgal functional diversity and redundancy reflect differences in environmental conditions. Not only did functional group richness achieve higher diversity on PF-aspects, but the rate of functional group accumulation per OTU was slower, indicating that OTUs packed more tightly into PF-aspect trait space. Moreover, the comparative accumulation rates of alpha (FRic) and beta (FDis) functional diversity signal that EF-aspects show greater change in beta diversity per unit change of alpha diversity. Taken together these findings point to the conclusion that PF-aspects harbour greater functional redundancy; that is, ecosystem function in the more diverse PF-aspects is more robust to species loss since functionally similar macroalgae are present to compensate (Yachi and Loreau, 1999; Naeem, 2002; Safi et al., 2011; Seddon et al., 2016).
Given the relative paucity of OTUs on EF-aspects (few plots supported more than one or two species of thermo-tolerant macroalgae), it is unsurprising that we found greater effective functional redundancy on the richer PF assemblages. Our understanding of why these differences emerged can be informed by an examination of the OTU trait expression on different aspects. Fucoids [assigned by Mauffrey et al. (2020a) into ‘Emergent Functional Group (EFG) 2’] dominated EF-plots. This group is characterized by species with a longer and more branched thallus with high surface area to perimeter (SA:P) and C:N ratios, traits indicative of high light capture potential, resistance to desiccation/herbivory, capacity for resource retention and allocation to structural rather than photosynthetic tissues (Mauffrey et al., 2020a). Of these emergent trait characteristics, resistance to desiccation and resource retention would seem to be the most valuable for any alga experiencing comparatively high mean and extreme temperatures on EF-aspects.
Although representation varied with season (see Supplementary Data Table S1), PF-aspects were generally dominated by OTUs assigned by Mauffrey et al. (2020a) to ‘EFGs’ 7, 8 or 9. Group 7 (comprising here Blidingia minima, Porphyra sp. and Ulva sp.) is characterized by high specific thallus area (STA), a trait linked to relatively high investment in photosynthetic over structural tissue. ‘EFG 8’ (Membranoptera alata and Osmundea sp.) similarly possesses traits associated with relatively rapid resource utilization [i.e. comparatively high STA, thallus surface area to volume (SA:V) ratio and N content]. ‘EFG 9’ (Cladophora sp., Lomentaria articulata and Rhodothamniella floridula) is also characterized by relatively high SA:V and N content. All three EFGs therefore express traits linked to relatively high investment in rapid resource capture and utilization, rather than structural resistance to environmental stressors such as desiccation or herbivory.
Given the comparative abundance of limpets and trochids on PF-aspects (Amstutz et al., 2021), apparently low grazing resistance in these EFGs seems counterintuitive. Although limpets do consume macroalgae directly (Davies et al., 2007; Lorenzen, 2007; Notman et al., 2016), like trochids, their main mode of feeding is a surface scraper of microscopic biofilms (Crothers, 2001; Jenkins et al., 2001). Consequently, the comparatively higher grazer abundance on PF-aspects may not directly impact established macroalgae (indeed, the most abundant species in our surveys, especially PF-aspects, Lithophyllum incrustans, is a ‘crustose’ rhodophyte highly resistant to surface grazing). This assertion does, however, raise an important point. If grazer activity is principally focused on rock-surface biofilms, then grazer selective pressure could well be more pronounced at the algal establishment phase. Consequently, we may need to focus on trait expression in gametophytes and newly settled sporophytes to fully understand algal–grazer–environment interactions (Martins et al., 2023).
Although further research, perhaps including the potential interactive effects of tidal height, exposure and surface heterogeneity (refugia), is needed to elucidate whether and how observed temperature differences influence algal distributions and trait expression, our study highlights the value that local variation in aspect can play in our understanding of ACC-stressors on community assembly and function (see also Amstutz et al., 2021). We also highlight the importance of ‘free air’ temperatures, rather than the more commonly studied SSTs, on intertidal species biology (see also Firth et al., 2011; Seabra et al., 2016). Only by considering the more extreme temperature variation and stress experienced by intertidal organisms when they are emersed are we likely to understand how ACC affects shifts in species distributions. Diaz-Acosta et al. (2021), for example, noted that although warm-water species had increased along the shores of the northwest Iberian Peninsula, physiological responses (e.g. photosynthesis) did not always match observed distributional shifts in response to increasing SSTs. By revealing major variation in algae trait expression and functional redundancy between warmer FF- and cooler PF-aspects, we signpost how even an average air temperature difference of 1.6 °C could affect intertidal community assembly and functional resilience. Our results suggest that for cooler microsites (e.g. PF-aspects and higher latitude shores), a shift to a less species-rich, desiccation-tolerant (fucoid-dominated), seaweed community is likely even if the lower range of air temperature increases predicted by contemporary models (e.g. the IPCC SSP 2.6 scenario) are realized this century. With this, a reduction in functional diversity and loss of functional redundancy may make these simplified systems more susceptible to additional environmental perturbation in a globally changing world.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following.
Table S1: Summary of variation in relative abundance (percentage cover) of macroalgal species encountered in four paired gullies sited at four locations in SW England according to aspect (PF, Pole-facing; and EF, Equator-facing), for summer 2016 and winter 2017. Assignment of the ‘Putative Functional Group’ follows Mauffrey et al. (2020a). Fig. S1. The influence of surface aspect on intertidal macroalgal communities sampled at four sites (symbols and line types) across the SW peninsula of England in (a) summer 2016 (June and July) and (b) winter 2017 (January/February). nMDS based upon Bray–Curtis dissimilarity matrix; ellipses indicate standard deviations around group centroids.
ACKNOWLEDGMENTS
The authors thank Professor Camille Parmesan for guidance with project development.
Contributor Information
Axelle Amstutz, School of Biological and Marine Sciences, University of Plymouth, Drakes Circus, Plymouth, PL4 8AA, UK.
Louise B Firth, School of Biological and Marine Sciences, University of Plymouth, Drakes Circus, Plymouth, PL4 8AA, UK.
Andy Foggo, School of Biological and Marine Sciences, University of Plymouth, Drakes Circus, Plymouth, PL4 8AA, UK.
John I Spicer, School of Biological and Marine Sciences, University of Plymouth, Drakes Circus, Plymouth, PL4 8AA, UK.
Mick E Hanley, School of Biological and Marine Sciences, University of Plymouth, Drakes Circus, Plymouth, PL4 8AA, UK.
FUNDING INFORMATION
The project was funded by a School of Biological & Marine Science (University of Plymouth) post-graduate studentship award to A.A.
LITERATURE CITED
- Aguilera MA, Valdivia N, Broitman BR, Jenkins SR, Navarrete SA.. 2020. Novel co‐occurrence of functionally redundant consumers induced by range expansion alters community structure. Ecology 101: e03150. [DOI] [PubMed] [Google Scholar]
- Allison G. 2004. The influence of species diversity and stress intensity on community resistance and resilience. Ecological Monographs 74: 117–134. [Google Scholar]
- Amstutz A, Firth LB, Spicer JI, Hanley ME.. 2021. Facing up to climate change: community composition varies with aspect and surface temperature in the rocky intertidal. Marine Environmental Research 172: 105482. [DOI] [PubMed] [Google Scholar]
- Banitz T, Chatzinotas A, Worrich A.. 2020. Prospects for integrating disturbances, biodiversity and ecosystem functioning using microbial systems. Frontiers in Ecology and Evolution 8: 21. [Google Scholar]
- Barton MG, Clusella-Trullas S, Terblanche JS.. 2019. Spatial scale, topography and thermoregulatory behaviour interact when modelling species’ thermal niches. Ecography 42: 376–389. [Google Scholar]
- Bracewell SA, Clark G, Johnston EL.. 2018. Habitat complexity effects on diversity and abundance differ with latitude: an experimental study over 20 degrees. Ecology 99: 1964–1974. [DOI] [PubMed] [Google Scholar]
- Cantlon JE. 1953. Vegetation and microclimates on north and south slopes of Cushetunk Mountain, New Jersey. Ecological Monographs 23: 241–270. [Google Scholar]
- Cappelatti L, Mauffrey ARL, Griffin JN.. 2020. Functional diversity of habitat formers declines scale-dependently across an environmental stress gradient. Oecologia 194: 135–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale BJ. 2011. Biodiversity improves water quality through niche partitioning. Nature 472: 86–89. [DOI] [PubMed] [Google Scholar]
- Crisp DJ. 1964. The effects of the severe winter of 1962-63 on marine life in Britain. Journal of Animal Ecology 33: 165–210. [Google Scholar]
- Crothers JH. 2001. Common topshells: an introduction to the biology of Osilinus lineatus with notes on other species in the genus. Field Studies 10: 115–160. [Google Scholar]
- Davies AJ, Johnson MP, Maggs CA.. 2007. Limpet grazing and loss of Ascophyllum nodosum canopies on decadal time scales. Marine Ecology Progress Series 339: 131–141. [Google Scholar]
- Diaz-Acosta L, Barreiro R, Provera I, Piñeiro-Corbeira C.. 2021. Physiological response to warming in intertidal macroalgae with different thermal affinity. Marine Environmental Research 169: 105350. [DOI] [PubMed] [Google Scholar]
- Dolbeth M, Crespo D, Leston S, Solane M.. 2019. Realistic scenarios of environmental disturbance lead to functionally important changes in benthic species-environment interactions. Marine Environmental Research 150: 104770. [DOI] [PubMed] [Google Scholar]
- Fetzer I, Johst K, Schäwe R, Banitz T, Harms H, Chatzinotas A.. 2015. The extent of functional redundancy changes as species’ roles shift in different environments. Proceedings of the National Academy of Sciences of the United States of America 112: 14888–14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbee-Dexter K, Wernberg T, Grace SP, et al. 2020. Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Scientific Reports 10: 13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth LB, Williams GA.. 2009. The influence of multiple environmental stressors on the limpet Cellana toreuma during the summer monsoon season in Hong Kong. Journal of Experimental Marine Biology and Ecology 375: 70–75. [Google Scholar]
- Firth LB, Knights AM, Bell SS.. 2011. Air temperature and winter mortality: implications for the persistence of the invasive mussel, Perna viridis in the intertidal zone of the south-eastern United States. Journal of Experimental Marine Biology and Ecology 400: 250–256. [Google Scholar]
- Firth LB, Mieszkowska N, Grant LM, et al. 2015. Historical comparisons reveal multiple drivers of decadal change of an ecosystem engineer at the range edge. Ecology and Evolution 5: 3210–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth LB, White FJ, Schofield M, et al. 2016. Facing the future: the importance of substratum features for ecological engineering of artificial habitats in the rocky intertidal. Marine and Freshwater Research 67: 131–143. [Google Scholar]
- Firth LB, Harris D, Blaze JA, et al. 2021. Specific niche requirements underpin multidecadal range edge stability, but may introduce barriers for climate change adaptation. Diversity and Distributions 27: 668–683. [Google Scholar]
- Grime JP, Fridley JD, Askew AP, Thompson K, Hodgson JG, Bennett CR.. 2008. Long‐term resistance to simulated climate change in an infertile grassland. Proceedings of the National Academy of Sciences USA 105: 10028–10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley CD, Anderson KM, Demes KW, et al. 2012. Effects of climate change on global seaweed communities. Journal of Phycology 48: 1064–1078. [DOI] [PubMed] [Google Scholar]
- Helmuth B, Mieszkowska N, Moore P, Hawkins SJ.. 2006. Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annual Review of Ecology, Evolution, and Systematics 37: 373–404. [Google Scholar]
- Isbell F, Gonzalez A, Loreau M, et al. 2017. Linking the influence and dependence of people on biodiversity across scales. Nature 546: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SR, Arenas F, Arrontes J, et al. 2001. European-scale analysis of seasonal variability in limpet grazing activity and microalgal abundance. Marine Ecology Progress Series 211: 193–203. [Google Scholar]
- Johnson MP, Hanley ME, Frost NJ, Moseley MWJ, Hawkins SJ.. 2008. The persistent spatial patchiness of limpet grazing. Journal of Experimental Marine Biology & Ecology 365: 136–141. [Google Scholar]
- Johnson CR, Banks SC, Barrett NS, et al. 2011. Climate change cascades: Shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology 400: 17–32. [Google Scholar]
- Jurgens LJ, Ashlock LW, Gaylord B.. 2022. Facilitation alters climate change risk on rocky shores. Ecology 103: e03596. [DOI] [PubMed] [Google Scholar]
- Kühn P, Ratier Backes A, Römermann C, Bruelheide H, Haider S.. 2021. Contrasting patterns of intraspecific trait variability in native and non-native plant species along an elevational gradient on Tenerife, Canary Islands. Annals of Botany 127: 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laliberté E, Legendre P.. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91: 299–305. [DOI] [PubMed] [Google Scholar]
- Laliberté E, Legendre P, Shipley B.. 2014. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-12. https://cran.r-project.org/web/packages/FD/index.html (22 March 2023, date last accessed).
- Lenth RV. 2020. emmeans: estimated marginal means, aka least-squares means. R package version 1.5.3. https://CRAN.R-project.org/package=emmeans (22 March 2023, date last accessed).
- Lima FP, Gomes F, Seabra R, et al. 2016. Loss of thermal refugia near equatorial range limits. Global Change Biology 22: 254–263. [DOI] [PubMed] [Google Scholar]
- Ling SD, Johnson CR, Frusher SD, Ridgway K.. 2009. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proceedings of the National Academy of Sciences 106: 22341–22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen S. 2007. The limpet Patella vulgata L at night in air: effective feeding on Ascophyllum nodosum monocultures and stranded seaweeds. Journal of Molluscan Studies 73: 267–274. [Google Scholar]
- Martins N, Coleman MA, Wernberg T, Roleda MY.. 2023. Opening the black box of kelps: Response of early life stages to anthropogenic stressors. Frontiers in Marine Science 9: 1133857. 10.3389/fmars.2022.1133857 [DOI] [Google Scholar]
- Mauffrey ARL, Cappelatti L, Griffin JN.. 2020a. Seaweed functional diversity revisited: confronting traditional groups with quantitative traits. Journal of Ecology 108: 2390–2405. [Google Scholar]
- Mauffrey ARL, Cappelatti L, Griffin JN.. 2020b. Functional diversity of seaweeds revisited: confronting traditional groups with quantitative traits. Dryad Digital Repository. 10.5061/dryad.nvx0k6dpn [DOI]
- Micheli F, Halpern BS.. 2005. Low functional redundancy in coastal marine assemblages. Ecology Letters 8: 391–400. [Google Scholar]
- Millennium Ecosystem Assessment. 2005. Ecosystems and human well-being: biodiversity synthesis. Washington, DC: World Resources Institute. [Google Scholar]
- Naeem S. 2002. Biodiversity equals instability? Nature 416: 23–24. [DOI] [PubMed] [Google Scholar]
- Nevo E. 2012. ‘Evolution Canyon’, a potential microscale monitor of global warming across life. Proceedings of the National Academy of Sciences USA 109: 2960–2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notman GM, McGill RA, Hawkins SJ, Burrows MT.. 2016. Macroalgae contribute to the diet of Patella vulgata from contrasting conditions of latitude and wave exposure in the UK. Marine Ecology Progress Series 549: 113–123. [Google Scholar]
- Oksanen J, Blanchet FG, Friendly M, et al. 2020. Vegan: community ecology package. R package version 2:5–5. https://cran.r-project.org/web/packages/vegan/index.html (21 March 2023, date last accessed).
- Parmesan C, Hanley ME.. 2015. Plants and climate change: complexities and surprises. Annals of Botany 116: 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmesan C, Morecroft MD, Trisurat Y, et al. 2022. Terrestrial and freshwater ecosystems and their services. In: Pörtner HO, Roberts DC, Tignor M, et al. eds. Climate change 2022: impacts, adaptation, and vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Paul C, Hanley N, Meyer ST, Fürst C, Weisser WW, Knoke T.. 2020. On the functional relationship between biodiversity and economic value. Science Advances 6: eaax7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessarrodona A, Asis J, Filbee-Dexter K, et al. 2022. Global seaweed productivity. Scientific Advances 8: eabn2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfisterer AB, Schmid B.. 2002. Diversity-dependent production can decrease the stability of ecosystem functioning. Nature 416: 84–86. [DOI] [PubMed] [Google Scholar]
- R Core Team 2021. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Safi K, Cianciaruso MV, Loyola RD, Brito D, Armour-Marshall K, Diniz-Filho JAF.. 2011. Understanding global patterns of mammalian functional and phylogenetic diversity. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences 366: 2536–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra R, Wethey DS, Santos AM, Lima FP.. 2011. Side matters: microhabitat influence on intertidal heat stress over a large geographical scale. Journal of Experimental Marine Biology and Ecology 400: 200–208. [Google Scholar]
- Seabra R, Wethey DS, Santos AM, Gomes F, Lima FP.. 2016. Equatorial range limits of an intertidal ectotherm are more linked to water than air temperature. Global Change Biology 22: 3320–3331. [DOI] [PubMed] [Google Scholar]
- Seddon N, Mace GM, Naeem S, et al. 2016. Biodiversity in the Anthropocene: prospects and policy. Proceedings of the Royal Society B: Biological Sciences 283: 20162094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale DA, Burrows MT, Moore P, et al. 2013. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecology & Evolution 3: 4016–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale DA, Teagle H, Hawkins SJ, et al. 2022. Climate-driven substitution of foundation species causes breakdown of a facilitation cascade with potential implications for higher trophic levels. Journal of Ecology 110: 2132–2144. [Google Scholar]
- Southward AJ. 1958. Note on the temperature tolerance of some intertidal animals in relation to environmental temperature and geographic distribution. Journal of the Marine Biological Association of the United Kingdom 37: 49–66. [Google Scholar]
- Usinowicz J, Levine JM.. 2018. Species persistence under climate change: a geographical scale coexistence problem. Ecology Letters 21: 1589–1603. [DOI] [PubMed] [Google Scholar]
- Vale CG, Arenas F, Barreiro R, Piñeiro-Corbeira C.. 2021. Understanding the local drivers of beta-diversity patterns under climate change: The case of seaweed communities in Galicia, North West of the Iberian Peninsula. Diversity & Distributions 27: 1696–1705. [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K.. 2011. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software 45: 1–67. [Google Scholar]
- Venter O, Sanderson EW, Magrach A, et al. 2016. Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nature Communications 7: 12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter VMS, Kreyling J, Dengler J, et al. 2020. Invader presence disrupts the stabilizing effect of species richness in plant community recovery after drought. Global Change Biology 26: 3539–3551. [DOI] [PubMed] [Google Scholar]
- Villéger S, Mason NWH, Mouillot D.. 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89: 2290–2301. [DOI] [PubMed] [Google Scholar]
- Wernberg T, Thomsen MS, Tuya F, Kendrick GA.. 2011. Biogenic habitat structure of seaweeds change along a latitudinal gradient in ocean temperature. Journal of Experimental Marine Biology and Ecology 400: 264–271. [Google Scholar]
- Westerband AC, Funk JL, Barton KE.. 2021a. Intraspecific trait variation in plants: a renewed focus on its role in ecological processes. Annals of Botany 127: 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerband A, Knight TM, Barton KE.. 2021b. Intraspecific trait variation and reversals of trait strategies across key climate gradients in native Hawaiian plants and non-native invaders. Annals of Botany 127: 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wethey DS. 1983. Geographic limits and local zonation: the barnacles Semibalanus (Balanus) and Chthamalus in New England. Biological Bulletin 165: 330–341. [Google Scholar]
- Yachi S, Loreau M.. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proceedings of the National Academy of Sciences of the United States of America 96: 1463–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


