Abstract
Background and Aims
Worldwide, invasive species are spreading through marine systems at an unprecedented rate with both positive and negative consequences for ecosystems and the biological functioning of organisms. Human activities from shipping to habitat damage and modification are known vectors of spread, although biological interactions including epibiosis are increasingly recognized as potentially important to introduction into susceptible habitats.
Methods
We assessed a novel mechanism of spread – limpets as transporters of an invasive alga, Sargassum muticum, into beds of the seagrass Zostera marina – and the physiological impact of its invasion. The association of S. muticum with three limpet species and other habitats was assessed using intertidal surveys on rocky shores and snorkelling at two seagrass sites in the UK. A 4-year field study tested the effect of S. muticum on Z. marina shoot density, dry weight and phenolic compounds (caffeic and tannic acid) content, and a laboratory experiment tested the impact of S. muticum on nutrient partitioning (C/H/N/P/Si), photosynthetic efficiency (Fv/Fm) and growth of Z. marina.
Results
On rocky shores 15 % of S. muticum occurrences were attached to the shells of live limpets. In seagrass beds 5 % of S. muticum occurrences were attached to the shells of dead limpets. The remainder were attached to rock, to cobblestones, to the seagrass matrix or embedded within the sand. Z. marina density and phenolics content was lower when S. muticum co-occurred with it. Over 3 years, photosynthetic responses of Z. marina to S. muticum were idiosyncratic, and S. muticum had no effect on nutrient partitioning in Z. marina.
Conclusions
Our results show limpets support S. muticum as an epibiont and may act as a previously unreported transport mechanism introducing invaders into sensitive habitats. S. muticum reduced production of phenolics in Z. marina, which may weaken its defensive capabilities and facilitate proliferation of S. muticum. The effect of S. muticum on Z. marina photosynthesis requires further work but having no effect on the capacity of Z. marina to sequester nutrients suggests a degree of resilience to this invader.
Keywords: Biochemistry, ecosystem engineer, invasion, limpet, Sargassum muticum, seagrass, vector, Zostera marina, allelopathy
INTRODUCTION
Ecosystem engineers have profound effects on ecosystem structure and functioning in terrestrial and aquatic ecosystems globally (Jones et al., 1994; Crooks, 2002; Emery-Butcher et al., 2020). When an invasive species is classed as both a habitat-forming and habitat-modifying ecosystem engineer, not only does it compete with native species (Mooney and Cleland, 2001; Morriën et al., 2010), but it may also create novel habitat (Rodriguez 2006; Byers et al., 2012; Firth et al., 2021), and modify abiotic conditions such as light, temperature or sediment deposition (Levin et al., 2006; McKinney and Goodell, 2010) that can have cascading environmental, economic and social impacts (Thomsen, 2010; Gribben et al., 2019; Wood et al., 2022; for a review see Guy-Haim et al., 2018). The presence or activity of an ecosystem engineer can also modify access to resources or biochemical conditions present within an environment (Jones et al., 1994; Lim et al., 2020).
Whilst human activities such as shipping, habitat loss, fragmentation and proliferation of artificial structures are known to enhance invasive spread in aquatic environments (With, 2002; Bishop et al., 2017; O’Shaughnessy et al., 2020; Adomako et al., 2021), some invasive species employ novel methods of spread between habitats across landscape scales. For instance, some species ‘hitch-hike’ as epibionts on the bills and feet of migratory birds (Green, 2016) and the carapaces of sea turtles (Harding et al., 2011). Similarly, the increasing amounts of plastic flotsam in marine environments are providing durable novel substrata facilitating the spread of invasives (Kiessling et al., 2015; Treneman et al., 2018). Following the 2011 Japanese earthquake and tsunami, Carlton et al. (2017) documented the transport of 289 living Japanese coastal marine species from 16 phyla on floating objects that travelled thousands of kilometres across the Pacific Ocean to the shores of North America. With ever-increasing landscape connectivity and corresponding proliferation of invasive species (e.g. Clubley et al., 2023), we are witnessing the homogenization of biota, changes in ecosystem functioning and the increased emergence of novel ecosystems (Hobbs et al., 2006, 2009; Bulleri et al., 2020).
Seagrass beds globally are particularly susceptible to invasion by macroalgae (Williams, 2007; Gallucci et al., 2012; Thomsen et al., 2012), and much work has been done on the impacts of invasive Caulerpa species. Whilst many authors have asserted that Caulerpa taxifolia can cause the regression of seagrasses (e.g. Boudouresque et al., 1995; Stafford and Bell, 2006; Glardon et al., 2008; Peirano et al., 2011), and negative impacts on seagrass-associated biota (Wright et al., 2007; Gribben et al., 2009; Byers et al., 2010), others have found no effect (for a review see Glasby et al., 2013). Where competition does occur, Caulerpa overgrows seagrass rhizomes; interacting with both the below- and above-sediment surface tissues, affecting nutrient acquisition and light availability (Ceccherelli et al., 2000). Previous research in the Mediterranean found that the native seagrass Posidonia oceanica increases its production of secondary metabolites (phenolic compounds such as caffeic acid) in response to the invasion of C. taxifolia into the seagrass beds (Dumay et al., 2004), ultimately allocating more resources to production of defensive mechanisms than to growth (Pergent et al., 2008). This was the first documented example of marine allelopathy between a seagrass and a macroalga.
In the UK, the invasive Japanese wireweed Sargassum muticum has successfully invaded seagrass (Zostera marina) beds (Fig. 1A; Druehl, 1973; Tweedley et al., 2008). Whilst it was previously thought that S. muticum required a hard substrate for attachment, thus limiting its ability to invade seagrass beds (North, 1973), it is now known that it can spread from rocky habitats into seagrass beds through the drifting of detached fertile branches with air-filled gas bladders (Engelen et al., 2015), or through ‘stone-walking’, whereby individuals attached to small stones may become buoyant and dispersed by local currents (Critchley, 1983). Firth et al. (2023) suggested that limpets may also be an important vector of spread of S. muticum from rocky shores. They reported that 24 % of 143 S. muticum individuals were attached to limpet shells (Fig. 1B; 83 % attached to the China limpet Patella ulyssiponensis, 17 % attached to the common limpet P. vulgata) on rocky shores. Like stone-walking, if a limpet that supports S. muticum becomes detached from the rock (Fig. 1C), the canopy provides buoyancy, thus enabling the shell to be transported to seagrass beds where it may become anchored though entanglement in the seagrass rhizome matrix or burial in sand or mud. To our knowledge, no research has focused on the importance of limpets as vectors of spread for S. muticum into Z. marina beds. Despite repeated concerns about the impacts of S. muticum on Z. marina beds since 1973 (Druehl, 1973; den Hartog, 1997), to our knowledge only a single paper has experimentally examined any impacts. DeAmicis and Foggo (2015) found that the epibiotic assemblages on the blades of Z. marina plants in plots that were invaded by S. muticum were significantly different from control uninvaded plots. Quantification of the impacts of S. muticum on the structure and functioning of seagrass beds remains a major knowledge gap.
Fig. 1.

Images of Sargassum muticum (A) colonizing a Zostera marina bed (photo credit Georgie Bull); (B) attached to the limpet Patella ulyssiponensis on a rocky shore (photo credit Louise Firth); (C) attached to a detached limpet shell washed up on the beach (photo credit Tony Legg).
The overarching aim of this study was to assess the role of limpets as vectors of spread of S. muticum into seagrass (Z. marina) beds and to quantify the impacts on a range of seagrass traits. First, we conducted field surveys to examine the association between S. muticum and attachment substrata (limpet shells, rock, cobbles, other substrata) both on rocky shores and in seagrass beds. Whilst both surveys were largely exploratory, we hypothesized that at least some S. muticum would be attached to limpet shells in both habitats and that shells of P. ulyssiponensis would be more important as an attachment substrate than P. vulgata. Second, a 4-year manipulative field experiment examined the impact of S. muticum on Z. marina phenolic compound production and density. We hypothesized that over time the relative phenolic content of Z. marina would be higher, and that density would be lower in the presence of S. muticum than without it. Finally, multiple laboratory experiments were conducted in a controlled environment to determine how S. muticum affects Z. marina photosynthesis [chlorophyll fluorescence output (Fv/Fm)], growth rates, and nutrient (C, H, N, P and Si) partitioning and allocation within its various tissue types (root-rhizome, leaf sheath and blade). We hypothesized that over time the presence of S. muticum would reduce Z. marina photosynthesis and growth rates and that it would alter nutrient partitioning and allocation within tissue types.
MATERIALS AND METHODS
Study system
All surveys and experiments in this study were conducted in Devon, SW England. The region is home to several seagrass beds located in shallow water (Green et al., 2018). Cellars Cove (50.31018, −4.06676) is known to support ~0.14 ha of Z. marina with densities reaching 6.7 ± 7.01 plants per 50 cm2 (Green et al., 2018). These beds have also supported S. muticum since 1976 (Boalch and Potts, 1977). The total seagrass extent within the Salcombe–Kingsbridge ria (50.23129, −3.77330) meanwhile was estimated at 6.3 ha (in 2008) with known shoot density averages of 240 shoots m–2 (Tweedley et al., 2008). The seagrass beds at Cawsand (50.33184, −4.19860) are the largest in the area covering 28.67 ha (Jenkin et al., 2021). The surveys of limpets as vectors for spread of S. muticum were conducted at Cellar Beach and Cawsand in summer 2022. Due to difficulties obtaining permission for sampling, Salcombe–Kingsbridge was not sampled. All experimental work on the impacts of S. muticum on Z. marina was conducted in the Salcombe–Kingsbridge ria between 2007 and 2011.
Are limpets potential vectors of spread of S. muticum in Z. marina beds?
To assess the potential for limpets to act as vectors of spread of S. muticum into Z. marina beds, surveys were conducted in Cawsand and Cellars Cove which had both rocky shores and seagrass beds adjacent to one another. To test the potential association between S. muticum and patellid limpets on rocky shores, three 45-min searches were conducted across 25-m transect lines (n = 3, ~1 m on either side of the transect line was observed as a sample area) in each of the lower, middle and upper regions of both rocky shore study sites during low-water spring tides. For every S. muticum thallus that was located, the ‘attachment’ substratum was noted [i.e. limpet (P. vulgata, P. ulyssiponensis, P. depressa), bedrock platform or loose cobblestones].
To test the association between S. muticum and limpet shells in seagrass beds, subtidal snorkelling surveys were conducted across shore-perpendicular weighted 25-m transect lines (n = 32) during low water slack of spring tides. Water depths at Cellars Cove and Cawsand survey sites ranged from ~<1–3.0 and ~3.3–3.6 m, respectively. Transect lines were systematically snorkelled along and ~1 m on either side of the transect line was observed as a sample area. For every S. muticum thallus that was located, the ‘attachment’ substratum was noted (as above, but also including ‘seagrass matrix’ which classifies a habitat created by intertwined blades and rhizomes of Z. marina forming a matt-like area that can be colonized by various settling species; Tanner, 2006; Tweedley et al., 2008).
Field study: what are the impacts of S. muticum upon Z. marina density and phenolic compound production?
Long-term field experimental set up.
To investigate how S. muticum potentially affects Z. marina, a 4-year field study (March 2007 to March 2011) was conducted in the Salcombe–Kingsbridge ria. Twenty permanent 1 × 1-m quadrats were established at a depth of 0.5 m below chart datum: ten each for two experimental treatments: with and without S. muticum (hereafter ‘Z+S’ and ‘Z’, respectively). Two similarly sized S. muticum individuals (~75–90 cm long) were harvested intact from nearby locations and attached to 25 × 25-cm plastic grids using cable ties; and two grids were secured within ten randomly selected permanent quadrats using reinforcing bar ‘hooks’, driven deep into the sediment. Control (Z) treatments were established by affixing two ‘empty’ grids within the remaining ten quadrats. Seagrass blades were carefully pulled through all grids to remain upright within the water column and not trapped underneath. Any additional S. muticum individuals that colonized the control Z treatments were removed during each sampling session.
Density measurements.
Four permanent 70-m-long shore-parallel transects were established between mean low water low (MLWL) and 1.2 m below MLWL. Z. marina densities were determined by sampling 12 randomly located 1-m2 quadrats along each transect. Within each quadrat, four 0.25 × 0.25-m sub-samples were taken by counting the number of individual shoots per area. Data were averaged to produce the mean Z. marina density per 1-m2 quadrat. After sampling Z. marina densities, the number of S. muticum plants along each transect was counted based on individual holdfasts present in a 1-m-wide strip centred on the transect. The mean number of S. muticum plants within the field site was calculated and used as a proxy for overall S. muticum densities within the estuary. To compare quadrat and transect Z. marina densities (i.e. manipulated vs. unmanipulated), quadrat densities from the same months that transect sampling occurred were averaged to produce the mean quadrat density for that sampled date.
Phenolic compound measurement.
Seagrass samples were collected within the established permanent quadrats every 6–8 weeks from three seasons (spring: March–May, summer: June–August and autumn: September–October, the active growing period for S. muticum) over a 4-year period (2007–2010). Three randomly selected shoot samples from each quadrat were harvested by cutting the blades just above the basal meristem; these were bagged and brought to the laboratory, where they were processed immediately. All blades were measured (length and width) and the blades used for phenolics assay were gently scraped clean of epibiota and frozen at −20 °C.
To quantify the percentage dry weight (% DW) content of caffeic (CA) and tannic acid (TA) equivalents within blade tissues, samples were dried at 65 °C for 24 h, ground and ~150 mg of weighed sample was extracted in 50 % MeOH for 24 h in a dark refrigerator at 4 °C. Phenols in blade tissue were assayed using an adapted Folin-Ciocalteu colorimetric assay (Harrison and Durance, 1989; Hargrave et al., 2017), processed in triplicate and read against CA and TA standard dilution series at 725 and 765 nm respectively using a Unicam Helios Epsilon spectrophotometer (Unicam Ltd, Cambridge, UK).
Statistical analyses.
To test the effect of S. muticum on Z. marina density, data from the permanent quadrats were analysed using a mixed model univariate generalized linear model (GLM) in SPSS 19 with the mean seagrass density per quadrat as the dependent variable. The GLM had three factors, ‘treatment’ and ‘year’ were designated as fixed with two (Z+S and Z) and four (2007, 2008, 2009 and 2010) levels respectively, but we set ‘season’ as a random factor with two levels (spring and autumn), nested within ‘year’, because we wanted to capture the overall growth season mean density of shoots, and thus ‘season’ here is akin to a temporal ‘block’. We were also unable to access the site in both seasons in all years due to tidal variations, meaning the final samples were not orthogonally distributed. We experimented with including a first-order autocorrelation term in the model to account for the repeated disturbance of the permanent quadrats, but this addition did nothing to the model fit or its interpretation. Type III sums of squares were used and Student-Newman-Keuls (SNK) post hoc tests were performed for ‘years’. Conformity to assumptions of normality and homogeneity of variances were confirmed by plots of fits and residuals. Pairwise comparisons with Tukey’s honest significant difference (HSD) tests using estimated marginal means were used to identify significant differences between the Z+S and Z treatments within the interaction term ‘year × treatment’ (P < 0.05).
To test the effect of S. muticum on overall seagrass phenolics, we used a three-factor PERMANOVA in PRIMER ver. 6.1. based on standardized CA and TA equivalents, using Euclidian distances and factors: Treatment (two levels: Z+S, Z; fixed), Year (four levels: 2007–2010, fixed) and Season (three levels: spring, summer, autumn, random due to lack of orthogonality as described above). Unrestricted permutations of raw data, type III sums of squares and 9999 permutations were set as design parameters (Anderson et al., 2008).
Laboratory study: what are the impacts of S. muticum on Z. marina photosynthetic performance, growth and nutrient partitioning?
Laboratory experimental conditions.
To investigate the impacts of S. muticum on Z. marina nutrient partitioning and physiological responses, four, 3- to 4-week laboratory experiments using wild-harvested Z. marina were undertaken annually from 2008 to 2011 in a constant temperature (CT) room. Seagrass shoots were hand-harvested locally in early spring and acclimated to laboratory conditions for 2 weeks in aerated tanks at in situ densities (~160 plants m−2). Ten glass tanks (30 × 23 × 39 cm; 27-L capacity) of seawater were partitioned into two, unequally sized compartments (60:40) by 1-cm grid plastic fencing to allow for water exchange while keeping algae or control seagrass shoots from physically interacting with the focal Z. marina plants. Experimental samples were all collected from the seagrass in the large tank compartment. Three treatments were established in 2008 and 2009: Z. marina + S. muticum (Z+S), Z. marina only (Z) and a biomass control, Z. marina + Z. marina (Z+Z) (Fig. 2). After 2009, only the Z+S and Z treatments were tested, following preliminary analysis indicating a lack of biomass (Z+Z vs. Z) effect. After epiphytes were gently removed by lightly scraping with a razor blade, five Z. marina shoots [each ~16–18 g wet weight (WW)] were anchored into the larger compartment of each tank to maintain similar seagrass biomass and spring in situ densities. One S. muticum individual (~60 g WW) attached to a small stone was added to the smaller compartment of each tank for the Z+S treatment. For the Z+Z biomass control treatment, five to seven additional Z. marina shoots (each ~12 g WW total biomass) were added to the smaller compartment. The Z treatment consisted of five Z. marina shoots anchored within the larger tank compartment only.
Fig. 2.

Laboratory experimental design using three treatments (left to right): Zostera marina + Sargassum muticum (Z+S), Z. marina only (Z) and Z. marina + Z. marina (Z+Z) (photo credits: Stacey DeAmicis).
Chlorophyll fluorescence measurements.
Chlorophyll fluorescence was used to examine effects of S. muticum proximity upon photosynthetic efficiency of the seagrass. In 2008, shoots were held at 15 ± 2 °C in a CT room with ~55–60 μmol m−2 s−1 photosynthetic photon flux density (PPFD) on a 16-h/8-h light/dark (16L:8D) cycle (equivalent to 3.17–3.46 quanta mol m−2 d−1). In 2009, we were able to increase the photosynthetic photon flux density (PPFD) to ~95–110 μmol m−2 s−1 (equivalent to 4.1–4.32 quanta mol m−2 d−1); the duration of irradiance was shorted to 12L:12D and the experimental temperature was lowered to 10 ± 2 °C to match the ambient conditions as the experiments occurred earlier in the spring than in 2008. To determine the maximum photochemical efficiency of the photosystem II (PSII) apparatus in dark-adapted seagrass blades, (Fv/Fm) measurements were recorded over a 5-s period at 100 % light intensity on four dates (T = 0, and weekly thereafter) throughout the experiment using an MK2 Plant Efficiency Analyser (PEA meter) (Hansatech Instruments Ltd, King’s Lynn, UK). Once tanks had been drained for a water change, five randomly selected green blades per tank were dark adapted using leaf clips for at least 15 min before readings were taken. The mean Fv/Fm for each tank at each date was used for statistical analysis to avoid pseudo-replication.
Growth measurements.
All blades in five individual shoots were punctured with a fine needle at the blade–sheath interface at the start of the experiment and again after ~14 d. Growth was measured as the distance the hole had grown away from the interface (Westera and Lavery, 2006). Length measurements between the top of the sheath and the puncture holes were taken on two dates, once at ~2 weeks after the start of the experiment and again at the end of the experiment. Growth data from each shoot were summed to produce the total production per shoot; data from the five shoots measured in each tank were then averaged to give the mean total production per shoot per tank. The mean total production per shoot per tank d−1 was calculated by dividing the total production per shoot per tank by the number of days from the initial hole punch.
Tissue nutrient measurements.
Three Z. marina tissue types (root-rhizome, sheath-meristem region and blades) were harvested at the end of the 2008 laboratory experiment and analysed separately for carbon and nitrogen content. These analyses were carried out to determine nutrient partitioning and allocation within each tissue type. Details of analytical chemistry are given in Supplementary Data S1.
Statistical analyses.
The effect of S. muticum on Z. marina chlorophyll fluorescence (Fv/Fm) was analysed in SPSS 19 using repeated-measures type III sums of squares linear models. The combined 2008 and 2009 analysis utilized a two-way design where both ‘year’ with two (2008 and 2009) and ‘treatment’ with three (Z+S, Z and Z+Z) levels respectively were fixed factors; ‘date’ was designated the within-subject factor with four levels (T = 0, 1, 2 and 3). Box’s test of equality of covariance matrices was used to ensure that the observed covariance matrices of the dependent variables were consistent across groups prior to analyses using type III sums of squares. Mauchly’s test of sphericity was also used to ensure that analytical assumptions were met, and where indicated, corrected degrees of freedom were employed using the Greenhouse–Geisser correction (Field, 2009). Conformity of data within each time class to assumptions of homogeneity of variances were confirmed using visual inspection of fits and residuals. Pairwise comparisons were used to identify significant differences between the Z+S, Z and Z+Z treatments (P < 0.05) using Tukey’s HSD based upon estimated marginal means.
The effect of S. muticum on Z. marina growth [total production per shoot per tank d−1 (in mm)] was analysed in SPSS 19 using a repeated-measures lm with type III sums of squares and tests for conformity to analytical assumptions (as described above). To avoid pseudo-replication, multiple data per tank were amalgamated and a single datum per tank was used as the replicate. The combined 2008 and 2009 analysis used a two-way design where both ‘year’ with two (2008 and 2009) and ‘treatment’ with three (Z+S, Z and Z+Z) levels respectively were fixed factors. ‘Date’ was set as the within-subject factor with two levels (mid- and end experiment measurements). SNK post hoc tests were performed for ‘year’ and pairwise comparisons were used to identify significant differences between ‘treatments’ (P < 0.05) using Tukey’s HSD based upon estimated marginal means.
The effect of S. muticum on Z. marina carbon and nitrogen partitioning was analysed using type III sums of squares univariate lms in SPSS 19; data were tested using a two-factor design, with ‘treatment’ with three (Z+S, Z and Z+Z) and ‘tissue’ types with three (root-rhizome, sheath and blade) levels respectively as fixed factors. As reproductive tissue was not equally produced amongst treatments and perhaps was a stress response, reproductive tissue was not included in the analysis.
RESULTS
Are limpets potential vectors of spread of S. muticum into Z. marina beds?
S. muticum was attached to limpet shells at both rocky shores sampled. These were primarily restricted to rockpools in the mid- to low shore. Of the 654 S. muticum individuals that were observed across both locations, 95 (15 %) were attached to limpet shells, whilst 517 (79 %) and 42 (6 %) were attached to the rock platform and loose cobblestones, respectively. A total of 384 were found at Cellars Cove (299 on rock, 43 on limpet shells, 42 on cobblestones) and 270 at Cawsand (218 on rock, 52 on limpet shells, none on cobblestones). P. ulyssiponensis was the only limpet species found to support S. muticum epibionts.
S. muticum was only observed in the seagrass bed at Cellars Cove. No individuals were found at Cawsand. Of the 168 individuals observed at Cellars Cove, eight (5 %) were attached to limpet shells (five on P. ulyssiponensis, three on P. vulgata), with the remaining 160 attached to a range of substrata (78 on cobbles, 69 in sand, nine in seagrass matrix, four on submerged rock). One limpet shell was observed to still have the soft body of the limpet still in the shell. This suggests that S. muticum settled on the limpet while it was alive and attached to a rocky substrate. This contrasts with the alternative scenario with the shell being deposited in the seagrass matrix first and colonized by S. muticum subsequently.
What are the impacts of S. muticum on Z. marina density and phenolic compounds?
Z. marina shoot densities in the permanent quadrats ranged from 162.9 ± 9 to 273.8 ± 16 shoots m−2 for the Z+S treatment and 136.1 ± 11 to 306.1 ± 22 shoots m−2 for the Z treatment (summarized in Fig. 3A). Results indicated significantly lower Z. marina density within the Z+S treatment permanent quadrats (P < 0.001) than in the control Z treatment quadrats, with significant treatment effects evident particularly in the final 2 years of the study. Densities within the quadrats broadly increased over time and a similar pattern emerged for S. muticum in the transects (linear model F1,30 = 13.840, P < 0.001; Fig. 3B) whilst Z. marina densities across the transects remained relatively stable (Fig. 3A).
Fig. 3.
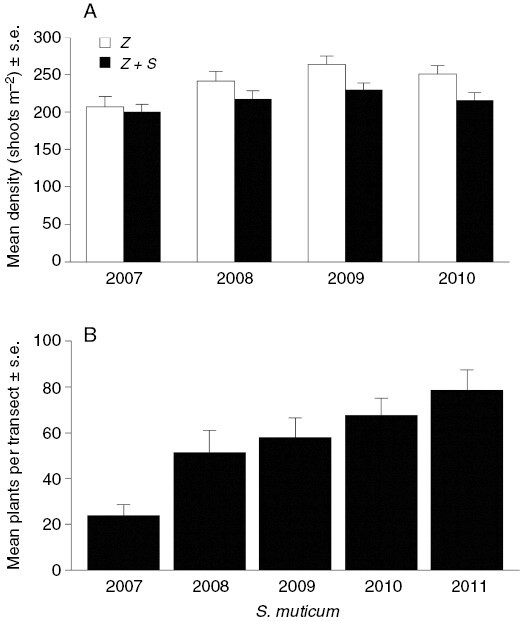
Mean (±s.e.) Zostera marina shoot densities in (A) two permanent quadrat treatments from 2007 to 2010: Z. marina + S. muticum (Z+S) and Z. marina only (Z). Results represent the annual mean density for each treatment (calculated across all seasons). Annual mean transect densities for (A) S. muticum and (B) Z. marina from 2007–2011 field data. Z. marina results are reported as the mean number of shoots m−2 averaged across all transects for each year and S. muticum results are reported as the mean number of plants in a 1-m-wide strip centred on the transect and averaged across all transects for each year. Sampling occurred only in autumn and spring in 2007 and 2011, respectively; all n = 18 per quadrat per year.
Phenolic contents in Z. marina varied throughout the study with average CA equivalents ranging from 1.39 to 1.48 % dry weight (DW) and TA equivalent contents ranging from 1.76 to 1.89 % DW. There was a significant main effect of treatment for both analyses (Fig. 4A and B), with Z. marina shoots in the Z+S treatment exhibiting significantly lower % DW phenolic content, for both CA (P < 0.05) and TA equivalents, (P < 0.01) than shoots in the Z treatment across all years.
Fig. 4.
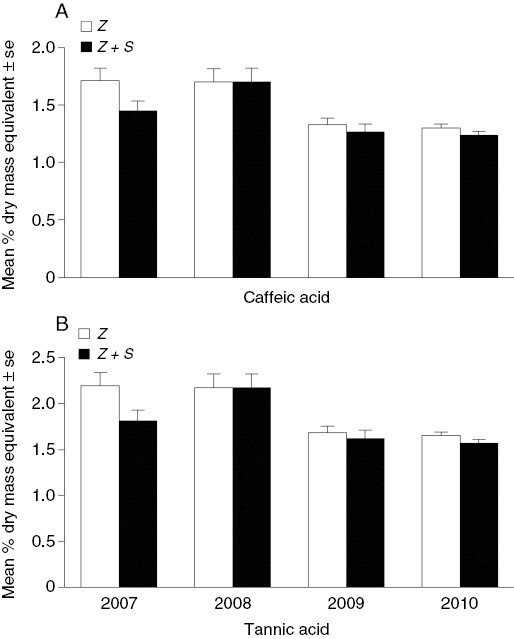
Mean (±s.e.) percentage dry weight for (A) caffeic and (B) tannic acid content of Zostera marina from long-term field study for two treatments: Z. marina + S. muticum (Z+S: n = 39, 40, 50, 60) and Z. marina only (Z: n = 40, 40, 50, 60) from 2007 to 2010.
What are the impacts of S. muticum on Z. marina photosynthetic efficiency, growth and nutrient partitioning?
Chlorophyll fluorescence analyses revealed significant differences between treatments with and without S. muticum (Fig. 5; P < 0.01), but pairwise comparisons indicated these differences only occurred in 2008 (P < 0.001) and were less prominent in the comparison of the S. muticum treatment with the biomass control treatment.
Fig. 5.
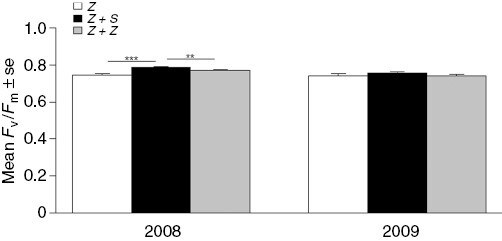
Mean Fv/Fm (±s.e.) from combined 2008–2009 data for three treatments (Z, Z+S and Z+Z), all n = 40. *Significant difference (P < 0.05).
Repeated-measures GLM indicated that there were no differences between responses of growth in the different treatments or years to the passage of time in the laboratory (Supplementary Data Fig. S1). Neither was there a main effect of treatment. Across the time periods, mean growth was 35.46 (Z+S treatment), 35.18 (Z treatment) and 32.10 (Z+Z treatment) mm per shoot per tank d−1. There was a significant interaction between Treatment and Year, but this was attributable to the biomass control treatment differing from the control; there was no indication of any effect involving the Z+S treatment.
Significant differences were also found in nutrient (carbon and nitrogen, Supplementary Data Fig. S2) partitioning amongst functional regions of the shoots (P < 0.05); again, pairwise comparisons indicated that these differences lay between the biomass control treatment (Z+Z) and the Sargassum treatment (Z+S) and between Z+Z and the unmanipulated seagrass (Z) treatments (P < 0.05) but not between Z+S and Z treatments. Plots of tissue N against C:N ratio indicated a lack of nutrient limitation.
DISCUSSION
S. muticum was found living attached to limpets both on rocky shores and in seagrass (Z. marina) beds suggesting that limpets may represent a vector of spread for S. muticum across landscapes from rocky shores into seagrass beds. Of all the Z. marina traits that were assessed, S. muticum was only found to have a negative effect on Z. marina density and phenolic compounds (both CA and TA equivalents), but there was little evidence of any effect on Z. marina photosynthesis [chlorophyll fluorescence output (Fv/Fm)], growth rates, and nutrient (C, H, N, P and Si) partitioning and allocation within its various tissue types (root-rhizome, leaf sheath and blade).
Despite limpets only accounting for attachment substrata for S. muticum in 5 % of cases in seagrass beds, they appear to be more important as attachment substrate on natural rocky shores (15 % of individuals were attached to limpets; see Firth et al., 2023 who reported 24 %). We found that S. muticum was attached to both P. ulyssiponensis and P. vulgata in seagrass beds, but it was only observed on P. ulyssiponensis on rocky shores. An emerging body of evidence is revealing that P. ulyssiponensis represents important habitat for algal epibionts (Pereira et al., 2022; Firth et al., 2023; see also Martins et al., 2014 for closely related P. aspera), particularly in relatively exposed conditions where densities of P. ulyssiponensis are high, and consequently grazing of the primary rock substrata is high. Firth et al. (2023) suggested that this is due to P. ulyssiponensis exhibiting aggressive behaviour towards limpet competitors, preventing mutual grazing on their shells, thereby indirectly providing an associational refuge for algae on their shells. More research is required to ascertain the exact mechanism underpinning this emergent pattern.
No S. muticum was found in the Z. marina beds at Cawsand. This could possibly be due to recent introduction of advanced mooring systems (Solandt, 2022), which aim to prevent the disturbance of seagrass by lifting mooring chains off the seabed. The introduction of these moorings has seen a reduction in anchor disturbance and scarring in the Cawsand Bay seagrass beds and an increase in seagrass density around mooring points (Solandt, 2022). In contrast, Cellars Cove experiences high levels of recreational boating traffic particularly during the summer months (peak growing seasons for both Z. marina and S. muticum) and is not home to any fixed moorings, so boats must anchor disturbing and uprooting nearby seagrass and leaving the area exposed and susceptible to invasion. Survey observations from Cellars Cove saw patchy distributions of seagrass with scar-like marks from anchoring with many of these cleared patches colonized by small sprouts of carpet-like S. muticum (T. Watts, pers. observ.). Whilst we did not quantify percentage cover of Z. marina in our transects in the 2022 survey, it is likely that this may have been due to high Z. marina densities at Cawsand preventing S. muticum from successfully invading the beds there (T. Watts, pers. observ.). Large numbers of individuals were observed on the adjacent rocky shore (n = 270), suggesting that lack of supply was not a limiting factor in this instance. In the Mediterranean, resistance to the invasion of Caulerpa cylindracea has been attributed to native seagrass Posidonia oceanica shoot density, suggesting that some factors correlated with the canopy structure must be involved in the reduced capacity of C. cylindracea to penetrate the meadows, such as space limitation, water motion, nutrient supply or canopy shading (Ceccherelli et al., 2000). Future work should quantify densities of both invader and recipient habitat.
Results from the long-term transect analysis indicated that S. muticum had little influence on naturally occurring Z. marina densities. Densities of Z. marina within the permanent experimental quadrats, however, showed a significant decrease, perhaps indicating shoot density declines in proximity to the invader, potentially driven by reduced irradiance levels. With decreasing Z. marina densities, infaunal communities may shift to greater numbers of hard-bodied taxa, as hard-bodied taxa are prevented from burrowing within the seagrass root-rhizome matrix more than soft-bodied taxa (Orth et al., 1984). Such an increase in hard-bodied taxa into native seagrass beds may exacerbate further invasion of S. muticum (Strong et al., 2006) and other non-native taxa (e.g. Codium fragile; Thomsen and McGlathery, 2006; Drouin et al., 2016).
Our results revealed that Z. marina phenolic content is suppressed in the presence of S. muticum. Nutrient limitation may potentially have influenced macrophyte biology and biochemistry, but no effects of nutrient limitation were found, suggesting that phenolic production within the Z. marina shoots, or lack thereof, was not a direct result of a Redfield ratio imbalance. These results contrast with findings for P. oceanica, which showed an increase in phenolic production with increasing invasive macroalgal interactions (Dumay et al., 2004; Pergent et al., 2008). Collectively, our findings indicate that macroalgal invasions into seagrass beds may have subtle, yet synergistic influences upon the physiology of seagrass, potentially leading indirectly to insidious consequences such as changing Z. marina’s defensive barrier to wasting disease (Harrison, 1982; Vergeer et al., 1995). Seagrass die-off due to disease may then potentially aid the facilitation and spread of invasive species as new ‘patches’ become available for additional colonization (den Hartog, 1997).
Signalling through the production of inceptive chemicals such as phenolics may be just one mode in which plants communicate. Release of water-soluble phenolic compounds into the water column from seagrass tissue may not deter or limit an invading alga (Zapata and McMillan, 1979; McMillan et al., 1980), as phenolics can quickly dissipate within the water column. A more effective delivery method would be to release phenolic compounds into the sediment (Zapata and McMillan, 1979) via roots and rhizomes, but as S. muticum is a non-rhizomatous alga, any allelopathic defences produced by Z. marina may have little influence in directly deterring the continued spread of S. muticum. Given the apparent conservation of pathways producing phenols in phaeophytes and land plants, and evidence for common transduction pathways associated with the octadecanoid signalling pathway common to both (e.g. Coleman et al., 2007), it is perhaps unsurprising that evidence exists for allelopathic consequences of close juxtaposition of the alga and the angiosperm. For this reason, further research into the exact pathways or signal transduction mechanisms underpinning this ‘communication’ in the marine environment are needed.
The data accumulated in this study are akin to circumstantial evidence in a murder trial, not quite a ‘smoking gun’ but the villain of the piece has certainly been placed squarely in the frame. There are weak forces in ecology that when coupled with unnatural forces, such as anthropogenic disturbances, can combine to have profound effects within ecosystems. The individual results have been mixed; each on its own may not unequivocally communicate the negative effects of S. muticum’s invasion on Z. marina, but when considered collectively, they do. Although more than 4000 plant species have been introduced to the USA and Canada over the past 400 years, there is no evidence that even one ‘native’ species has been driven to extinction (Davis et al., 2003). This, however, should not negate concern over the continued proliferation and spread of S. muticum. It is clear from the present study that there is still much to learn regarding the effects of invasion of S. muticum into Z. marina meadows. As with most scientific investigations, the present study has raised as many questions as it has answered. More research is required to examine the multitude of possible impacts of S. muticum on vulnerable seagrass beds.
SUPPLEMENTARY DATA
Supplementary data are available at Annals of Botany online and consist of the following.
Figure S1: Mean growth (mm per tank d−1, ±s.e.) from three laboratory study with three treatments: Zostera marina only (Z), Zostera marina + Sargassum muticum (Z+S) and Z. marina + Z. marina (Z+Z) repeated across two years (2008, 2009); n = 10 tanks per treatment per year.
Figure S2. Mean tissue contents (a = carbon, b = nitrogen) in three regions of the seagrass (blades, roots, sheaths) in each of three laboratory treatments: Zostera marina only (Z), Z. marina + Sargassum muticum (Z+S), and Z. marina + Z. marina (Z+Z); n = 10 per treatment other than for blades, where reproductive activity resulted in seven, seven and four replicates respectively in the three treatments.
ACKNOWLEDGEMENTS
We thank the Salcombe Harbour Authority who kindly granted permission to work in the shallow subtidal region of the Salcombe–Kingsbridge system, Natural England who gave permission for collection of seagrass for laboratory experiments, and the proprietors of Sunny Cliff Hotel Apartments and John Morris who provided parking, staging and access to the foreshore.
Contributor Information
Louise B Firth, School of Biological and Marine Sciences, University of Plymouth, Plymouth, UK.
Andy Foggo, School of Biological and Marine Sciences, University of Plymouth, Plymouth, UK.
Thomas Watts, School of Biological and Marine Sciences, University of Plymouth, Plymouth, UK.
Antony M Knights, School of Biological and Marine Sciences, University of Plymouth, Plymouth, UK.
Stacey deAmicis, School of Biological and Marine Sciences, University of Plymouth, Plymouth, UK.
FUNDING
The long-term study was funded by the Jack Kent Cooke Graduate Scholarship awarded by the Jack Kent Cooke Foundation: http://www.jkcf.org/.
LITERATURE CITED
- Adomako MO, Alpert P, Du DL, Yu FH.. 2021. Effects of fragmentation of clones compound over vegetative generations in the floating plant Pistia stratiotes. Annals of Botany 127: 123–133. doi: 10.1093/aob/mcaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ, Gorley RN, Clarke KR.. 2008. Permanova+ for Primer: Guide to software and statistical methods. Plymouth: PRIMER-E Ltd. [Google Scholar]
- Bishop MJ, Mayer-Pinto M, Airoldi L, et al. 2017. Effects of ocean sprawl on ecological connectivity: impacts and solutions. Journal of Experimental Marine Biology and Ecology 492: 7–30. doi: 10.1016/j.jembe.2017.01.021. [DOI] [Google Scholar]
- Boalch GT, Potts GW.. 1977. The first occurrence of Sargassum muticum (Yendo) Fensholt in the Plymouth area. Journal of the Marine Biological Association of the United Kingdom 57: 29–31. doi: 10.1017/s0025315400021202. [DOI] [Google Scholar]
- Boudouresque CF, Meinesz A, Riberta MA, Ballesteros E.. 1995. Spread of the green alga Caulerpa taxifolia (Caulerpales, Chlorophyta) in the Mediterranean: possible consequences of a major ecological event. Scientia Marina 59: 21–29. [Google Scholar]
- Bulleri F, Batten S, Connell SD, et al. 2020. Human pressures and the emergence of novel marine ecosystems. Oceanography and Marine Biology: an Annual Review 58: 456–535. [Google Scholar]
- Byers JE, Wright JT, Gribben PE.. 2010. Variable direct and indirect effects of a habitat‐modifying invasive species on mortality of native fauna. Ecology 91: 1787–1798. doi: 10.1890/09-0712.1. [DOI] [PubMed] [Google Scholar]
- Byers JE, Gribben PE, Yeager C, Sotka EE.. 2012. Impacts of an abundant introduced ecosystem engineer within mudflats of the southeastern US coast. Biological Invasions 14: 2587–2600. doi: 10.1007/s10530-012-0254-5. [DOI] [Google Scholar]
- Carlton JT, Chapman JW, Geller JB, et al. 2017. Tsunami-driven rafting: Transoceanic species dispersal and implications for marine biogeography. Science 357: 1402–1406. doi: 10.1126/science.aao1498. [DOI] [PubMed] [Google Scholar]
- Ceccherelli G, Piazzi L, Cinelli F.. 2000. Response of the non-indigenous Caulerpa racemosa (Forsskål) J. Agardh to the native seagrass Posidonia oceanica (L.) Delile: effect of density of shoots and orientation of edges of meadows. Journal of Experimental Marine Biology and Ecology 243: 227–240. doi: 10.1016/s0022-0981(99)00122-7. [DOI] [Google Scholar]
- Coleman RA, Ramchunder SJ, Moody AJ, Foggo A.. 2007. An enzyme in snail saliva induces herbivore-resistance in a marine alga. Functional Ecology 21: 101–106. [Google Scholar]
- Clubley CH, Firth LB, Wood LE, Bilton DT, Silva TA, Knights AM.. 2023. Science paper or big data? Assessing invasion dynamics using observational data. Science of the Total Environment 877: 162754. doi: 10.1016/j.scitotenv.2023.162754. [DOI] [PubMed] [Google Scholar]
- Critchley AT. 1983. The establishment and increase of Sargassum muticum (Yendo) Fensholt populations within the Solent area of southern Britian. II. An investigation of the increase in canopy cover of the alga at low water. Botanica Marina 26: 547–552. [Google Scholar]
- Crooks JA. 2002. Characterizing ecosystem‐level consequences of biological invasions: The role of ecosystem engineers. Oikos 97: 153–166. doi: 10.1034/j.1600-0706.2002.970201.x. [DOI] [Google Scholar]
- Davis T, Llanes F, Volesky B, Mucci A. 2003. Metal selectivity of Sargassum spp. and their alginates in relation to their alpha-L-guluronic acid content and conformation. Environmental Science & Technology 37: 261–267. [DOI] [PubMed] [Google Scholar]
- DeAmicis S, Foggo A.. 2015. Long-term field study reveals subtle effects of the invasive alga Sargassum muticum upon the epibiota of Zostera marina. PLoS One 10: e0137861. doi: 10.1371/journal.pone.0137861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog C. 1997. Is Sargassum muticum a threat to eelgrass beds? Aquatic Botany 58: 37–41. doi: 10.1016/s0304-3770(97)00007-7. [DOI] [Google Scholar]
- Drouin A, McKindsey CW, Johnson LE.. 2016. Dynamics of recruitment and establishment of the invasive seaweed Codium fragile within an eelgrass habitat. Marine Biology 163: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druehl LD. 1973. Marine transplantations. Science 179: 12. doi: 10.1126/science.179.4068.12. [DOI] [PubMed] [Google Scholar]
- Dumay O, Costa J, Desjobert JM, Pergent G.. 2004. Variations in the concentration of phenolic compounds in the seagrass Posidonia oceanica under conditions of competition. Phytochemistry 65: 3211–3220. doi: 10.1016/j.phytochem.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Emery‐Butcher HE, Beatty SJ, Robson BJ.. 2020. The impacts of invasive ecosystem engineers in freshwaters: A review. Freshwater Biology 65: 999–1015. doi: 10.1111/fwb.13479. [DOI] [Google Scholar]
- Engelen AH, Serebryakova A, Ang P, et al. 2015. Circumglobal invasion by the brown seaweed Sargassum muticum. Oceanography and Marine Biology: An Annual Review 53: 81–126. [Google Scholar]
- Field A. 2009. Discovering Statistics Using SPSS, 3 edn. London: SAGE Publications Ltd. [Google Scholar]
- Firth LB, Duff L, Gribben PE, Knights AM.. 2021. Do positive interactions between marine invaders increase likelihood of invasion into natural and artificial habitats? Oikos 130: 453–463. doi: 10.1111/oik.07862. [DOI] [Google Scholar]
- Firth LB, Clubley C, McGrath A, et al. 2023. If you can’t beat them, join them: limpet shells as refugia from grazing & competition pressure. Oceanography and Marine Biology: An Annual Review 61: 329–362. [Google Scholar]
- Gallucci F, Hutchings P, Gribben P, Fonseca G.. 2012. Habitat alteration and community-level effects of an invasive ecosystem engineer: a case study along the coast of NSW, Australia. Marine Ecology Progress Series 449: 95–108. doi: 10.3354/meps09547. [DOI] [Google Scholar]
- Glardon C, Walters LJ, Quintana-Ascencio P, McCauley L, Stam WT, Olsen JL.. 2008. Predicting risks of invasion of macroalgae in the genus Caulerpa in Florida. Biological Invasions 10: 1147–1157. [Google Scholar]
- Glasby TM. 2013. Caulerpa taxifolia in seagrass meadows: killer or opportunistic weed? Biological Invasions 15: 1017–1035. doi: 10.1007/s10530-012-0347-1. [DOI] [Google Scholar]
- Green AJ. 2016. The importance of waterbirds as an overlooked pathway of invasion for alien species. Diversity and Distributions 22: 239–247. doi: 10.1111/ddi.12392. [DOI] [Google Scholar]
- Green A, Chadwick MA, Jones PJS.. 2018. Variability of UK seagrass sediment carbon: Implications for blue carbon estimates and marine conservation management. PLoS One 13: e0204431. doi: 10.1371/journal.pone.0204431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribben PE, Wright JF, O’Connor WA, Doblin MA, Eyre B, Steinberg PD.. 2009. Reduced performance of native infauna following recruitment to a habitat-forming invasive marine alga. Oecologia 158: 733–745. [DOI] [PubMed] [Google Scholar]
- Gribben PE, Angelini C, Altieri AH, Bishop MJ, Thomsen MS, Bulleri F.. 2019. Facilitation cascades in marine ecosystems: a synthesis and future directions. Oceanography and Marine Biology: An Annual Review 57: 127–168. [Google Scholar]
- Guy‐Haim T, Lyons DA, Kotta J, et al. 2018. Diverse effects of invasive ecosystem engineers on marine biodiversity and ecosystem functions: A global review and meta‐analysis. Global Change Biology 24: 906–924. doi: 10.1111/gcb.14007. [DOI] [PubMed] [Google Scholar]
- Harding JM, Walton WJ, Trapani CM, Frick MG, Mann R.. 2011. Sea turtles as potential dispersal vectors for non-indigenous species: the veined rapa whelk as an epibiont of loggerhead sea turtles. Southeastern Naturalist 10: 233–244. doi: 10.1656/058.010.0204. [DOI] [Google Scholar]
- Hargrave MS, Foggo A, Pessarrodona A, Smale DA.. 2017. The effects of warming on the ecophysiology of two co-existing kelp species with contrasting distributions. Oecologia 183: 531–543. doi: 10.1007/s00442-016-3776-1. [DOI] [PubMed] [Google Scholar]
- Harrison PG. 1982. Control of microbial growth and of amphipod grazing by water- soluble compounds from leaves of Zostera marina. Marine Biology 67: 225–230. doi: 10.1007/bf00401288. [DOI] [Google Scholar]
- Harrison PG, Durance C.. 1989. Seasonal variation in phenolic content of eelgrass shoots. Aquatic Botany 35: 409–413. doi: 10.1016/0304-3770(89)90012-0. [DOI] [Google Scholar]
- Hobbs RJ, Arico S, Aronson J, et al. 2006. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography 15: 1–7. doi: 10.1111/j.1466-822x.2006.00212.x. [DOI] [Google Scholar]
- Hobbs RJ, Higgs E, Harris JA.. 2009. Novel ecosystems: implications for conservation and restoration. Trends in Ecology & Evolution 24: 599–605. doi: 10.1016/j.tree.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Jenkin A, sturgeon S, Trundle C. 2021. Acoustic survey of the seagrass bed at Cawsand within the Plymouth sound and Estuaries Special Area of Conservation Field Report. Hayle, UK: Cornwall Inshore Fisheries and Conservation Authority (Cornwall IFCA). [Google Scholar]
- Jones CG, Lawton JH, Shachak M.. 1994. Organisms as ecosystem engineers. Oikos 69: 373–386. doi: 10.2307/3545850. [DOI] [Google Scholar]
- Kiessling T, Gutow L, Thiel M.. 2015. Marine litter as habitat and dispersal vector. In Bergmann M, Gutow L, Klage M, eds. Marine Anthropogenic Litter. Cham: Springer, 141–181. [Google Scholar]
- Levin LA, Neira C, Grosholz ED.. 2006. Invasive cordgrass modifies wetland trophic function. Ecology 87: 419–432. doi: 10.1890/04-1752. [DOI] [PubMed] [Google Scholar]
- Lim HS, Fraser A, Knights AM.. 2020. Spatial arrangement of biogenic reefs alters boundary layer characteristics to increase risk of microplastic bioaccumulation. Environmental Research Letters 15: 064024. doi: 10.1088/1748-9326/ab83ae. [DOI] [Google Scholar]
- Martins GM, Faria J, Furtado M, Neto AI.. 2014. Shells of Patella aspera as ‘islands’ for epibionts. Journal of the Marine Biological Association of the United Kingdom 94: 1027–1032. doi: 10.1017/s0025315414000447. [DOI] [Google Scholar]
- McKinney AM, Goodell K.. 2010. Shading by invasive shrub reduces seed production and pollinator services in a native herb. Biological Invasions 12: 2751–2763. doi: 10.1007/s10530-009-9680-4. [DOI] [Google Scholar]
- McMillan C, Zapata O, Escobar L.. 1980. Sulphated phenolic-compounds in seagrasses. Aquatic Botany 8: 267–278. [Google Scholar]
- Mooney HA, Cleland EE.. 2001. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences of the United States of America 98: 5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriën E, Engelkes T, Macel M, Meisner A, Van der Putten WH.. 2010. Climate change and invasion by intracontinental range-expanding exotic plants: the role of biotic interactions. Annals of Botany 105: 843–848. doi: 10.1093/aob/mcq064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North WJ. 1973. Regulating marine transplantation. Science 179: 1181. doi: 10.1126/science.179.4079.1181-a. [DOI] [PubMed] [Google Scholar]
- Orth RJ, Heck KL Jr, van Montfrans J.. 1984. Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7: 339–350. doi: 10.2307/1351618. [DOI] [Google Scholar]
- O’Shaughnessy KA, Hawkins SJ, Yunnie AL, et al. 2020. Occurrence and assemblage composition of intertidal non-native species may be influenced by shipping patterns and artificial structures. Marine Pollution Bulletin 154: 111082. doi: 10.1016/j.marpolbul.2020.111082. [DOI] [PubMed] [Google Scholar]
- Peirano A, Cocito S, Banfi V, et al. 2011. Phenology of the Mediterranean seagrass Posidonia oceanica (L.) Delile: medium and long-term cycles and climate inferences. Aquatic Botany 94: 77–92. doi: 10.1016/j.aquabot.2010.11.007. [DOI] [Google Scholar]
- Pereira F, Piló D, Carvalho AN, et al. 2022. Epibiont assemblages on limpet shells: biodiversity drivers in intertidal rocky shores. Marine Environmental Research 174: 105556. doi: 10.1016/j.marenvres.2022.105556. [DOI] [PubMed] [Google Scholar]
- Pergent G, Boudouresque CF, Dumay O, Pergent-Martini C, Wyllie-Echeverria S.. 2008. Competition between the invasive macrophyte Caulerpa taxifolia and the seagrass Posidonia oceanica: contrasting strategies. BMC Ecology 8: 20. doi: 10.1186/1472-6785-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez LF. 2006. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biological Invasions 8: 927–939. doi: 10.1007/s10530-005-5103-3. [DOI] [Google Scholar]
- Solandt J-L. 2022. Cawsand Advanced Mooring Systems interim project results. [online] Available at: https://media.mcsuk.org/documents/Plymouth_Seagrass_-_Final_report_2022.pdf (14 March 2023, date last accessed).
- Stafford NB, Bell SS.. 2006. Space competition between seagrass and Caulerpa prolifera (Forsskaal) Lamouroux following simulated disturbances in Lassing Park, FL. Journal of Experimental Marine Biology and Ecology 333: 49–57. doi: 10.1016/j.jembe.2005.11.025. [DOI] [Google Scholar]
- Strong JA, Dring MJ, Maggs CA.. 2006. Colonisation and modification of soft substratum habitats by the invasive macroalga Sargassum muticum. Marine Ecology Progress Series 321: 87–97. doi: 10.3354/meps321087. [DOI] [Google Scholar]
- Tanner JE. 2006. Landscape ecology of interactions between seagrass and mobile epifauna: The matrix matters. Estuarine, Coastal and Shelf Science 68: 404–412. doi: 10.1016/j.ecss.2006.01.029. [DOI] [Google Scholar]
- Thomsen MS, McGlathery K.. 2006. Effects of accumulations of sediments and drift algae on recruitment of sessile organisms associated with oyster reefs. Journal of Experimental Marine Biology and Ecology 328: 22–34. doi: 10.1016/j.jembe.2005.06.016. [DOI] [Google Scholar]
- Thomsen MS. 2010. Experimental evidence for positive effects of invasive seaweed on native invertebrates via habitat-formation in a seagrass bed. Aquatic Invasions 5: 341–346. doi: 10.3391/ai.2010.5.4.02. [DOI] [Google Scholar]
- Thomsen MS, Wernberg T, Engelen AH, et al. 2012. A meta-analysis of seaweed impacts on seagrasses: generalities and knowledge gaps. PLoS One 7: e28595. doi: 10.1371/journal.pone.0028595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treneman NC, Borges L, Shipway R, Raupach MJ, Altermark B, Carlton JT.. 2018. A molecular phylogeny of wood-borers (Teredinidae) from Japanese tsunami marine debris. Aquatic Invasions 13: 101–112. doi: 10.3391/ai.2018.13.1.08. [DOI] [Google Scholar]
- Tweedley JR, Jackson EL, Attrill MJ.. 2008. Zostera marina seagrass beds enhance the attachment of the invasive alga Sargassum muticum in soft sediments. Marine Ecology Progress Series 354: 305–309. doi: 10.3354/meps07242. [DOI] [Google Scholar]
- Vergeer LHT, Aarts TL, Degroot JD.. 1995. The wasting disease and the effect of abiotic factors (light-intensity, temperature, salinity) and infection with Labyrinthula zosterae on the phenolic content of Zostera marina. Aquatic Botany 52: 35–44. [Google Scholar]
- Westera MB, Lavery PS.. 2006. A comparison of hole punch and needle punch methods for the measurement of seagrass productivity. Aquatic Botany 85: 267–269. doi: 10.1016/j.aquabot.2006.05.007. [DOI] [Google Scholar]
- Williams SL. 2007. Introduced species in seagrass ecosystems: status and concerns. Journal of Experimental Marine Biology and Ecology 350: 89–110. doi: 10.1016/j.jembe.2007.05.032. [DOI] [Google Scholar]
- With KA. 2002. The landscape ecology of invasive spread. Conservation Biology 16: 1192–1203. [Google Scholar]
- Wood LE, Dey K, Clubley C, Kennerley A, Guilder J, Smith ER, Trégarot E.. 2022. The impact of invasive aquatic animals on tourism, recreation and biological invasions. In Barrros A, Shackleton R, Rew L, Pizarro C, Pauchard A, eds. Tourism, recreation and biological invasions. Wallingford: CABI, 109–120. [Google Scholar]
- Wright JT, McKenzie LA, Gribben PE.. 2007. A decline in the abundance and condition of a native bivalve associated with Caulerpa taxifolia invasion. Marine and Freshwater Research 58: 263–272. doi: 10.1071/mf06150. [DOI] [Google Scholar]
- Zapata O, McMillan C.. 1979. Phenolic-acids in seagrasses. Aquatic Botany 7: 307–317. doi: 10.1016/0304-3770(79)90032-9. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


