Abstract
Background and Aims
Marine heatwaves (MHWs) are widely recognized as pervasive drivers of ecosystem change, yet our understanding of how different MHW properties mediate ecological responses remains largely unexplored. Understanding MHW impacts on foundation species is particularly important, given their structural role in communities and ecosystems.
Methods
We simulated a series of realistic MHWs with different levels of intensity (Control: 14 °C, Moderate: 18 °C, Extreme: 22 °C) and duration (14 or 28 d) and examined responses of two habitat-forming kelp species in the southwest UK. Here, Laminaria digitata reaches its trailing edge and is undergoing a range contraction, whereas Laminaria ochroleuca reaches its leading edge and is undergoing a range expansion.
Key Results
For both species, sub-lethal stress responses induced by moderate-intensity MHWs were exacerbated by longer duration. Extreme-intensity MHWs caused dramatic declines in growth and photosynthetic performance, and elevated bleaching, which were again exacerbated by longer MHW duration. Stress responses were most pronounced in L. ochroleuca, where almost complete tissue necrosis was observed by the end of the long-duration MHW. This was unexpected given the greater thermal safety margins assumed with leading edge populations. It is likely that prolonged exposure to sub-lethal thermal stress exceeded a physiological tipping point for L. ochroleuca, presumably due to depletion of internal reserves.
Conclusions
Overall, our study showed that exposure to MHW profiles projected to occur in the region in the coming decades can have significant deleterious effects on foundation kelp species, regardless of their thermal affinities and location within respective latitudinal ranges, which would probably have consequences for entire communities and ecosystems.
Keywords: Trailing edge, leading edge, range margins, climate change, seaweed
INTRODUCTION
Marine heatwaves (MHWs) are emerging as forceful agents of ecosystem change (Smith et al., 2022). Over the last 100 years they have become longer (Oliver et al., 2018) and more frequent (Holbrook et al., 2020; Thoral et al., 2022) with significant impacts on marine populations (Smale and Wernberg, 2013; Thomsen et al., 2019), communities (Wernberg et al., 2012; Arafeh-Dalmau et al., 2019), ecosystems (Wernberg et al., 2016; Suryan et al., 2021) and the services they provide for human societies (Smale et al., 2019; Smith et al., 2021). MHWs are projected to intensify in the coming decades (Frölicher et al., 2018; Oliver et al., 2019; Hayashida et al., 2020; Marin et al., 2021), which will probably have devastating impacts on coastal and oceanic ecosystems (Smith et al., 2022). As such, identifying species’ physiological tipping points is a fundamental step towards a predictive framework for adaptation and management in a warmer world.
MHWs are most likely to have negative impacts in summer, as it is here where temperatures outside the range of what is naturally experienced are exceeded. On top of this, their properties (e.g. intensity, duration) vary markedly between events (Hobday et al., 2018), which will also determine organismal responses. In terms of ecological impacts, acute but short-lived MHWs will probably invoke different responses compared to chronic, lower-intensity but long-lived MHWs (Smith et al., 2022). If upper critical thermal limits (i.e. CTmax) are exceeded for a given organism for a sufficient amount of time, and physiological thresholds are surpassed, then mortality may ensue with potential consequences for the wider population (Hochachka and Somero, 2002). However, prolonged exposure to sub-lethal temperatures can lead to unsustainable energy deficits, as energy acquisition cannot always keep pace with increasing respiratory demand (Kooijman and Kooijman, 2010; Pörtner, 2012). As reserves become depleted, overall fitness and resilience typically decline (Atkinson et al., 2020; Suryan et al., 2021; Straub et al., 2022). Indeed, such cumulative heat exposure is known to mediate susceptibility of corals to bleaching (Hughes et al., 2017) and affect the development of benthic invertebrates (Mann, 2009). Whilst research into the impacts of MHWs has expanded considerably in recent years (Bass et al., 2021), few studies have explicitly compared ecological responses to MHWs with different characteristics.
The distributions of marine ectotherms are strongly constrained by temperature (Sunday et al., 2012), so that populations at range margins are particularly responsive to warming trends (Smale et al., 2019; Harvey et al., 2022). At trailing range edges, exceedance of thermal maxima may reduce performance and ultimately lead to mortality, while at leading range edges increased temperature may be favourable, pushing organisms towards their thermal optima, increasing performance and underpinning population expansion (Sunday et al., 2014; Sanford et al., 2019). Where foundation species (e.g. macroalgae, seagrasses and corals) undergo population-level changes, widespread ecological changes may ensue due to their elevated importance in structuring communities and ecosystems (Wright and Jones, 2006; Wernberg et al., 2016). Kelps (i.e. large brown seaweeds primarily belonging to the order Laminariales) serve as foundation species along temperate and polar coastlines (Duarte et al., 2022), where they form highly productive (Pessarrodona et al., 2022) and diverse forests (Teagle et al., 2017). As the distribution and ecophysiological performance of kelp species are strongly constrained by temperature (Eggert, 2012), MHWs have recently been linked with impacts at population, community and ecosystem levels (Wernberg et al., 2012, 2016; Smale and Wernberg, 2013; Arafeh-Dalmau et al., 2019; Thomsen et al., 2019), which in turn has knock-on consequences for processes such as carbon cycling (Gao et al., 2021). A better understanding of the susceptibility of kelp species to MHW intensification is vital for improving ecological predictions and developing approaches to management and conservation.
In the northeast Atlantic, a number of kelp species have undergone range shifts or population-level changes in response to both gradual ocean-warming and MHW events (Smale et al., 2013; Filbee-Dexter et al., 2020; Smale, 2020). In SW England, the golden kelp Laminaria ochroleuca approaches its leading range edge, whereas L. digitata approaches its trailing range edge. These species have opposing thermal affinities, with L. ochroleuca tolerating temperatures up to 24 °C (Franco et al., 2018) compared to 21 °C for L. digitata (Simonson et al., 2015). These opposing affinities have led to L. ochroleuca proliferating here in recent years (Smale et al., 2015; Teagle and Smale, 2018), whereas L. digitata is anticipated to undergo a poleward range contraction (Simkanin et al., 2005; Raybaud et al., 2013). Sea surface temperatures experienced during summer MHWs in SW England can exceed 20 °C and projected intensification is likely to increase maximum intensities in the coming years and decades. Therefore, the contrasting thermal tolerances and responses to recent warming trends of these kelp species makes them useful models to examine responses to anticipated MHW events.
Here, we used these kelp species as a model system to test the following hypotheses: (1) high-intensity, long-duration MHWs are more detrimental to kelp performance than moderate-intensity and/or short-duration MHWs due to greater cumulative exposure to heat stress; and (2) warm-adapted kelp species found towards the leading range edge would be less susceptible to MHWs than more cold-adapted species found towards the trailing range edge, due to greater thermal safety margins.
MATERIALS AND METHODS
Plants were collected from Firestone Bay, Plymouth Sound, UK (50.360 872, −4.162 201) where dense stands of both L. digitata and L. ochroleuca are found in sympatry. Average temperatures here range from 9.4 °C in February to 16.6 °C in August but maximum temperatures of > 20 °C are occasionally experienced under summertime MHW conditions. Therefore, the experiment was conducted over June–August as this is the time of year most likely to surpass thermal thresholds and when ecological responses would be expected to be most severe. Mean sea temperatures recorded for the months that preceded the experiment, May and June (2022), were 13.03 and 14.7 °C, respectively, and no MHW events were identified during this time. The collection site is largely sheltered from wave action and representative of the wider region (in terms of coastal geomorphology and other key drivers).
Experimental MHW treatments comprised two intensities and two durations in a fully crossed design, in which durations were independent and ran in parallel. Our MHW definition followed Hobday et al. (2016), where temperatures must exceed the upper 90th percentile relative to a baseline climatology of the region for more than five consecutive days. MHW intensity categories were defined according to Hobday et al. (2018), which is based on multiples of the difference between the 90th percentile and the 30-year climatological mean. To determine this in our study region, we extracted MHW metrics for the period 1982–2022 for the area off the coast of southwest UK, in the Western English Channel (between 49°N to 51°3ʹN and 3°2ʹW to 6°W) using the r package ‘RmarineHeatWaves’ (Smith et al., 2018). This returned a climatological mean summertime (July–September) sea temperature of 16.0 °C, with an upper 90th percentile threshold for an MHW of 17.3 °C. Therefore, multiples of 1.3 °C above the climatological mean of 16.0 °C were used to assign MHW categories. Our intensities consisted of Control (14 °C: ambient, annual average seawater temperature), Moderate (18 °C: 1–2 × upper 90th percentile − climatological mean) and Extreme (22 °C: 4–5 × upper 90th percentile − climatological mean). Historical analysis of MHW events showed that mean duration was ~14 d, with a maximum recorded duration of 92 d. As such, we used 14 d for a ‘Short-MHW’ and 28 d to represent a ‘Long-MHW’.
Thirty-six mature (n = 6 per treatment) L. digitata and L. ochroleuca plants were haphazardly collected from June 2022 and transported in mesh bags to the laboratory. After 3 d in a 1000-L holding tank at ambient temperature (14 °C), a disc (diameter = 3.03 cm, area = 7.07 cm2) was excised from each plant (from the central portion of the blade, above the meristematic area) and placed in an independent 2-L tank. These tanks were placed in 64-L water baths that were used to control experimental temperatures. In the field, these species will experience a wide range of light intensities (up to full sunlight at low tide). Here, light was provided through LED lighting tiles (AquaRay) under a photosynthetic flux density of ~120 µmol m−2 s−1 and a 12:12 light–dark regime, which is in excess of the light compensation point for intertidal seaweeds (~2 to 7 µmol photons m−2 s−1; see Hurd et al. 2014). Each tank was continuously aerated with its own air stone, which also agitated the water to ensure nutrient delivery to the kelp surface. Water was changed every 3 d with freshly sourced seawater from the adjacent Plymouth Sound to prevent nutrient limitation. Salinity and temperature were checked daily and maintained between 33–35 ppt and ±0.5 °C.
Discs were maintained in their experimental 2-L tanks for 5 d, to allow healing of marginal wounds. Temperatures were then raised at a rate of 1 °C d−1, until the desired intensity (Moderate = 18 °C and Extreme = 22 °C) was reached. The MHW then progressed for either 14 d (Short MHW) or 28 d (Long MHW). At the end of the MHW, temperatures were lowered at a rate of 1 °C d−1 until they returned to control conditions (14 °C). Recovery was monitored after 5 d at 14 °C (see Supplementary Data Fig. S1 for treatment schematic). Responses were measured at three time points: (1) start of the MHW (St-MHW), (2) end of the MHW (End-MHW) and (3) at the end of the recovery period (End-Rec) (Fig. S1). At each of these time points, photosynthetic performance, relative growth rate (RGR) and bleaching were measured.
Photosynthetic performance was determined by the quantum efficiency of photosystem II (PSII) (Fv/Fm) and measured after 15 min dark acclimation using a Pocket PEA chlorophyll fluorimeter (Hansatech Instruments) (Butler, 1978). Fv/Fm is defined as the relationship between the ratio of photochemical quenching (Fv) and the total fluorescence when the reaction centres of PSII are closed (Fm) and has been shown to provide an efficient indicator of stress in macroalgae (Jueterbock et al., 2014; King et al., 2018; Hereward et al., 2020). Fv/Fm values between 0.7 and 0.8 are considered healthy, whereas decreasing values below 0.7 indicate increasing stress due to reduced functionality of photosynthetic apparatus and potential photoinhibition (Maxwell and Johnson, 2000; Bischof et al., 2008).
RGR (% d−1) was used to determine growth or tissue loss due to senescence, using the following equation:
where F = final weight at a given time point, I = initial weight at the start of the MHW and t = number of days since St-MHW. Finally, a semi-quantitative bleaching severity index was used to quantify tissue bleaching. This consisted of six categories ranging from 1 (no bleaching) to 6 (discs completely bleached) (Supplementary Data Table S1).
Statistical analysis
Responses of L. digitata and L. ochroleuca to simulated MHWs were analysed separately before informal comparisons between species were made. Differences in all response variables were examined with a repeated-measures analysis of variance (ANOVA), using the ‘anova_test’ function in the ‘rstatix’ package (Kassambra, 2021). The model had three factors: Duration (two levels, Short-MHW and Long-MHW); Intensity (three levels, Control: 14 °C, Moderate: 18 °C, and Extreme: 22 °C); and Time point (Fv/Fm and bleaching responses had three levels, St-MHW, End-MHW and End-Rec, whereas RGR had two levels, End-MHW and End-Rec). Where sphericity was violated (Mauchly’s test), degrees of freedom were corrected using the Greenhouse–Geisser correction. Where significant interactions were detected (P < 0.05), post hoc pair-wise comparisons were conducted to identify where differences lay.
RESULTS
Photosynthetic capacity (Fv/Fm)
For L. digitata, we recorded a significant Intensity × Time point interaction (Table 1). Across both durations, significant reductions in Fv/Fm values were observed in the Extreme MHW treatment over time (i.e. End-MHW and End-Rec compared with St-MHW) but not for the Control and Moderate MHW treatments (Fig. 1). In the Extreme treatment for the Short-MHW, Fv/Fm values decreased from 0.73 ± 0.07 at St-MHW to 0.40 ± 0.08 at End-MHW. A slight recovery to 0.47 ± 0.11 was observed at End-Rec but values remained depressed compared to St-MHW (Supplementary Data Table S2). Similar trends were observed for the Extreme treatment and Long-MHW (Fig. 1). Whilst no interaction with Duration was observed, indicating patterns were consistent across both Long and Short-MHWs, Fv/Fm values in the Moderate intensity treatment were lower at End-MHW in the Long-MHW compared to the Short-MHW, before recovering to similar values by End-Rec. Values of Fv/Fm under Control conditions were largely consistent throughout the experiment, ranging from 0.73 ± 0.01 at St-MHW to 0.71 ± 0.01 at End-Rec.
Table 1.
Results of repeated-measures ANOVA to test for differences in Fv/Fm, relative growth rate and bleaching index for the kelp species Laminaria digitata and Laminaria ochroleuca in response to simulated marine heatwave experiments. Significant values (P < 0.05) are highlighted in bold.
| Laminaria digitata | Laminaria ochroleuca | |||||
|---|---|---|---|---|---|---|
| Effect | d.f. | F | P | d.f. | F | P |
| F v /F m | ||||||
| Duration | 1 | 0.5 | 0.480 | 1 | 47.6 | <0.001 |
| Intensity | 1.02 | 8.6 | 0.030 | 2 | 28.9 | <0.001 |
| Timepoint | 2 | 8.5 | 0.007 | 2 | 32.5 | <0.001 |
| Duration:Intensity | 1.1 | 0.2 | 0.680 | 2 | 8.3 | 0.009 |
| Duration:Timepoint | 2 | 0.1 | 0.900 | 2 | 3.01 | 0.09 |
| Intensity:Timepoint | 1.66 | 4.8 | 0.045 | 4 | 12.8 | 0.002 |
| Duration:Intensity:Timepoint | 4 | 0.4 | 0.770 | 4 | 1.5 | 0.200 |
| Relative growth rate | ||||||
| Duration | 1 | 2 | 0.240 | 1 | 3.6 | 0.160 |
| Intensity | 2 | 8.2 | 0.008 | 2 | 26.9 | <0.001 |
| Timepoint | 1 | 12.8 | 0.016 | 2 | 35 | <0.001 |
| Duration:Intensity | 2 | 2 | 0.182 | 2 | 13.7 | <0.001 |
| Duration:Timepoint | 1 | 12.7 | 0.160 | 1.09 | 9.1 | <0.003 |
| Intensity:Timepoint | 2 | 12.7 | 0.002 | 1.24 | 20.98 | <0.001 |
| Duration:Intensity:Timepoint | 2 | 12.7 | 0.002 | 4 | 7.2 | <0.001 |
| Bleaching | ||||||
| Duration | 1 | 2.02 | 0.210 | 1 | 3.6 | 0.110 |
| Intensity | 1.01 | 8.2 | 0.030 | 2 | 26.9 | <0.001 |
| Timepoint | 2 | 9.5 | 0.005 | 2 | 35 | <0.001 |
| Duration:Intensity | 1 | 2.01 | 0.210 | 2 | 13.7 | 0.001 |
| Duration:Timepoint | 2 | 4.9 | 0.03 | 1.09 | 9.2 | 0.002 |
| Intensity:Timepoint | 4 | 9.34 | <0.001 | 1.24 | 20.1 | 0.003 |
| Duration:Intensity:Timepoint | 4 | 5.7 | 0.003 | 4 | 7.2 | <0.001 |
Fig. 1.
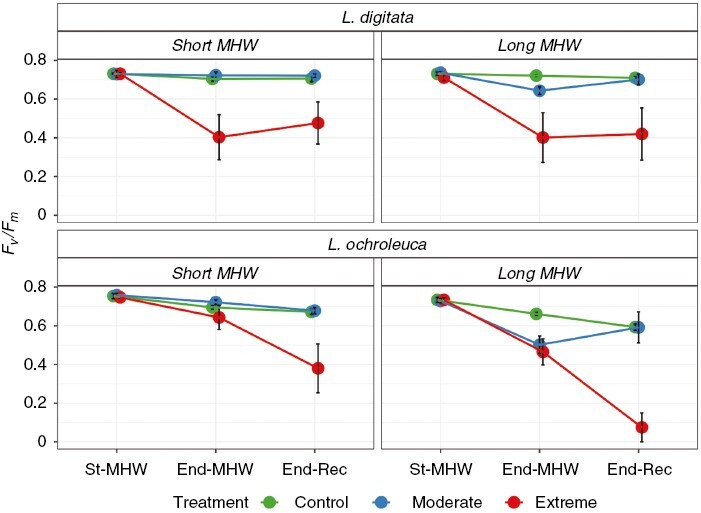
Mean ± s.e. Fv/Fm values for Laminaria digitata (top) and Laminaria ochroleuca (bottom) in response to Short (14 d, left) or Long (28 d, right) simulated MHWs of different intensities. St-MHW = start of MHW, End-MHW = end of MHW period, End-Rec = end of recovery phase; n = 6 per treatment.
For L. ochroleuca, we also detected a significant Intensity × Time interaction (Table 1). Post hoc tests showed significant reductions in Fv/Fm values were observed at the End-MHW in the Extreme intensity compared to the Control (Fig. 1; Supplementary Data Table S2). Here, in the Short-MHW, Fv/Fm values decreased from 0.74 ± 0.01 at St-MHW to 0.64 ± 0.07 at End-MHW and continued to decline to 0.38 ± 0.12 by End-Rec. Patterns in the Long-MHW were more pronounced, with Fv/Fm values under Extreme MHW conditions declining from 0.73 ± 0.01 at St-MHW to 0.46 ± 0.07 by End-MHW and 0.07 ± 0.07 by End-Rec. Whilst no interaction between Duration and Time point was observed, indicating patterns were consistent across both the Long and Short-MHWs, Fv/Fm values in the Moderate MHW intensity were lower at End-MHW in the Long-MHW compared to the Short-MHW, before recovering to control values by End-Rec. We did, however, record a highly significant Duration × Intensity interaction (Table 1), indicating that the overall effect of increasing intensity was greater in the Long-MHWs (Fig. 1). Under Control conditions, slight declines in Fv/Fm values were observed, from 0.74 ± 0.01 at St-MHW to 0.67 ± 0.01 at End-MHW and 0.63 ± 0.01 at End-Rec.
Relative growth rate
For L. digitata, there was a significant Duration × Intensity × Time interaction (Table 1). By End-MHW, the Extreme intensity caused a negative RGR of −0.81 ± 1.28 % d−1 in the Short-MHW and −1.17 ± 0.8 % d−1 in the Long-MHW (Fig. 2). By End-Rec this declined further to −1.53 ± 0.52 and −1.28 ± 0.44 % d−1 in the Short-MHW and Long-MHW respectively, by which point they were significantly different to the other treatments (Supplementary Data Table S3). No significant differences between durations were observed at either time point in the Extreme MHW intensity but differences were observed in the Moderate intensity (Table S3). In the Long-MHW, RGR under Moderate MHW conditions was −0.33 ± 0.41 % d−1 at End-MHW, which was significantly lower than 1.1 ± 0.37 % d−1 at the same point in the Short-MHW. At this point in the Long-MHW, differences were also evident between intensity treatments, with lower values for Moderate and Extreme MHWs compared with the Control (Fig. 2). Such differences were not observed between Control and Moderate treatments at End-MHW, in the Short-MHW. By End-Rec, positive RGR values were observed under Moderate MHW intensity for both Short-MHW and Long-MHW, whereas RGR remained negative for the Extreme treatment, suggesting limited recovery.
Fig. 2.
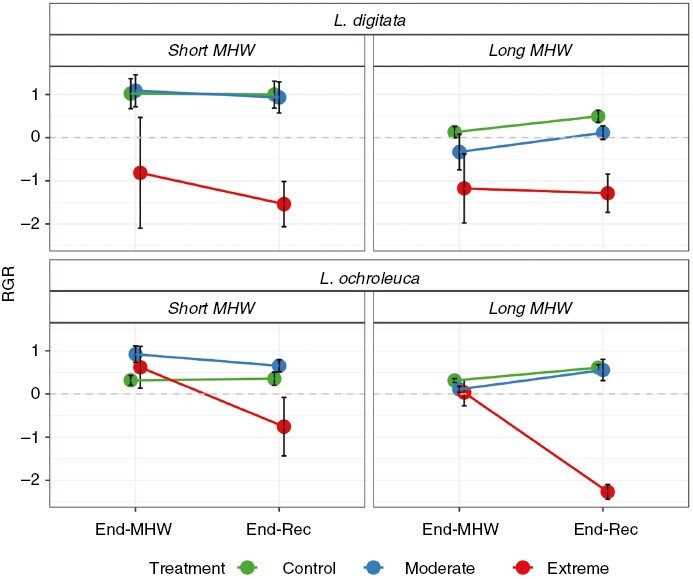
Mean ± s.e. relative growth rate (RGR, % d−1) of Laminaria digitata (top) and Laminaria ochroleuca (bottom) in response to Short (14 d, left) or Long (28 d, right) simulated MHWs of different intensities. St-MHW = start of MHW, End-MHW = end of MHW period, End-Rec = end of recovery phase; n = 6 per treatment.
For L. ochroleuca, we also recorded a significant Duration × Intensity × Time interaction (Table 1). At End-MHW, RGR was similar between intensity treatments within each duration and also similar between intensities across durations (Fig. 2; Supplementary Data Table S3). In the Short-MHW, RGR ranged from 0.31 ± 0.11 % d−1 (Control) to 0.92 ± 0.19 % d−1 (Moderate) compared to 0.03 ± 0.31 % d−1 (Extreme) to 0.31 ± 0.04 % d−1 (Control) in the Long-MHW. By End-Rec, RGR in the Extreme intensity declined markedly to −0.76 ± 0.6 % d−1 in the Short-MHW and −2.26 ± 0.17 % d−1 in the Long-MHW. In comparison, RGR in the Control and Moderate MHW treatments remained consistent or increased, indicating positive growth. Differences between durations were only observed at End-MHW, in the Moderate treatment, where RGR was significantly higher in the Short-MHW (0.92 ± 0.19 % d−1) compared to the Long-MHW (0.11 ± 0.07 % d−1) (Table S2).
Bleaching
For both L. digitata and L. ochroleuca, there was a significant Duration × Intensity × Time interaction (Table 1), which was largely driven by bleaching trends observed under Extreme MHW treatments (Fig. 3). For L. digitata, some bleaching was observed in the Short-MHW at End-MHW and End-Rec but significant differences between treatments were only detected at End-Rec of the Long-MHW (Supplementary Data Table S4). Here, mean bleaching index was 4.6 ± 0.61, which was also significantly higher to the 1.8 ± 0.83 observed at End-Rec in the Short-MHW. For L. ochroleuca, some bleaching was evident in the Short-MHW, where mean bleaching was 2.6 ± 1.1 at End-Rec but significant differences between treatments were only observed at End-Rec of the Long-MHW (Table S4). Here, mean bleaching was 5.8 ± 0.16, which was also significantly higher than at the same time point in the Short-MHW.
Fig. 3.
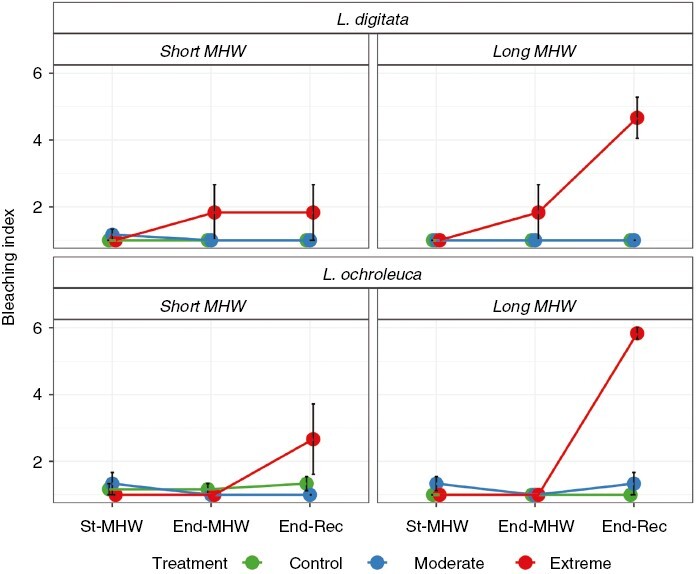
Mean ± s.e. bleaching index of Laminaria digitata (top) and Laminaria ochroleuca (bottom) in response to Short (14 d, left) or Long (28 d, right) simulated MHWs of different intensities. St-MHW = start of MHW, End-MHW = end of MHW period, End-Rec = end of recovery phase; n = 6 per treatment.
DISCUSSION
MHWs are increasingly recognized as forceful and pervasive drivers of ecological change, and are likely to intensify in coming decades as a consequence of anthropogenic climate change (Holbrook et al., 2020). However, our understanding of the relative importance of different MHW characteristics, such as duration, timing, and maximum or cumulative intensity, in driving ecological responses remains mostly theoretical with empirical studies largely lacking. Here, we examined the interactive effects of MHW intensity and duration on populations of two congeneric kelp species located towards opposing ends of their distributions. We found that both species were vulnerable to MHWs and, in line with our first prediction, that high-intensity long-duration warming events induced the most detrimental responses. Crucially, both species were severely impacted by high-intensity MHWs, which did not support our second prediction, that the more southerly distributed L. ochroleuca would exhibit greater resilience than the more northerly distributed L. digitata. This has implications not only for predicting responses to ocean warming in the study region but also for more general approaches to predicting population-level vulnerability.
Range margins represent the front line of climate change impacts, with trailing edge populations deemed at highest risk of decline or extinction and leading edges expanding or proliferating in newly suitable habitat (Poloczanska et al., 2013; Pinsky et al., 2022). Here, we show that while a trailing edge population (L. digitata) is vulnerable to an extreme MHW regardless of duration, the interactive effects of long duration and extreme intensity cause mortality within a leading edge population (L. ochroleuca). For L. digitata, stress responses observed at 22 °C (Extreme MHW) are not surprising given its optimal range for growth is 10–15 °C and widespread damage to the cellular structure can occur after just 1 week at 21 °C (Bolton and Luning, 1982; Simonson et al., 2015). Given populations of L. ochroleuca experience short-term temperature spikes of >22 °C at the trailing edge in Portugal and individuals have been shown to maintain positive growth during 2-week incubations at ~24 °C (Franco et al., 2018) and 4-week exposures to 22 °C (Bass et al., 2023), the complete tissue necrosis we observed by the end of the recovery period in our long-duration, extreme-intensity MHW treatment was unexpected. It seems that while L. ochroleuca is resistant to thermal stress for short periods, a physiological tipping point is exceeded after extended exposures (i.e. 28 d), probably driven by a depletion of internal reserves. Whilst L. digitata is known to produce storage compounds throughout summer following cessation of the peak growth phase, L. ochroleuca continues fast growth into summer rather than laying down storage compounds (Schiener et al., 2015; Pessarrodona et al., 2019). As such, the relative lack of internal reserves, combined with increased thermal stress and metabolic rate, may have underpinned the negative responses of L. ochroleuca to prolonged MHW exposure. This may have been exacerbated by the use of excised tissue in our experiments that limits access to wider storage reserves (Gevaert et al. 2008). Importantly, the largest decline in performance of L. ochroleuca was observed in the recovery period, suggesting carry-over effects of MHW stress or microbial activity (Minuti et al., 2021), which have also previously been observed in the kelp Sacharina latissima (Nepper-Davidsen et al., 2019). This probably explains why comparable declines in performance of L. ochroleuca were not observed in a similar study that used similar MHW treatments (22 °C for 28 d) (Bass et al., 2023) and highlights the importance of post-MHW monitoring. Alternatively, decomposition of different kelp tissue by bacteria and fungi under elevated temperatures could be an important driver of differences in rates of necrosis, as has been suggested previously with these species (Wright et al., 2022), and this warrants further study.
Increasing MHW duration also exacerbated sub-lethal stress responses in both species. The moderate MHW (18 °C) was not stressful for either species during or after the short-duration MHW, as has been observed previously for these species following a 16-d simulated MHW at 18 °C (Hargrave et al., 2017). However, clear declines in Fv/Fm values were evident for both species at the end of the long-duration MHW. Mediating sub-lethal responses to thermal stress requires the reallocation of energy to protection and repair at the cost of other aspects of performance (Davison, 1991). This in turn may reduce resilience to other perturbations such as disease outbreaks (Qiu et al., 2019) or reduce ecological competitiveness and alter interactions that influence population dynamics (Provost et al., 2019; Vergés et al., 2019). Therefore, although both species recovered following the moderate MHW, indicating that any damage sustained was reversible, this could still have implications for organism performance and population stability. Alternatively, such sub-lethal exposures can increase the resistance of a population to subsequent MHWs through activation and the legacy of a number of thermoregulatory mechanisms (Jueterbock et al., 2021).
Summertime seawater temperatures regularly exceed 18 °C in the study region, whilst under MHW conditions they can reach ~20 °C. Our data show that these temperatures are high enough to invoke sub-lethal stress in both species but as warming trends progress and MHW intensities approach 22 °C (extreme conditions) both species may be vulnerable to severe physiological damage. Whilst it was surprising that L. digitata was more resilient to extreme MHW stress, given its cooler thermal affinity, it is important to note that it is not just exceedance of physiological tipping points associated with extreme temperatures that constrain latitudinal distributions. For L. digitata, reproduction peaks over summer but is inhibited by temperatures >18 °C (Bartsch et al., 2013) and, as such, it is likely that a range contraction may manifest through reproductive failure before physiological crashes associated with MHWs. For L. ochroleuca, expansion at its leading edge seems to be limited by reproductive failure below 10 °C. Therefore, as warming progresses, proliferation will probably continue, as it is released from its winter reproduction cold-limit but this may be curtailed to some extent by physiological stress associated with future intensification of summer MHWs. Regardless, the anticipated vulnerability of both L. digitata and L. ochroleuca to current and future MHWs will probably have ramifications for wider community structure and the functioning of temperate reef ecosystems in the study region. In particular, if both species exhibit reduced performance and population declines under MHW intensification, the wider ecological consequences may be significant, given the lack of functionally similar species that could potentially replace them (King et al., 2018). Ultimately, understanding the longer-term consequences of exposure to intensifying MHWs for individual performance and population structure requires further experimentation and sustained monitoring.
Our findings have implications for anticipating and predicting future impacts of MHWs more generally. Increasingly, species vulnerability assessments are based on mechanistic processes rather than correlative approaches (Evans et al., 2015) and one such technique is to determine CTmax and project suitable thermal habitat in future space (Sunday et al., 2012; Franco et al., 2018; Marbà et al., 2022). Typically, estimates of CTmax are acquired from either historical literature or contemporary short-term incubations. For example, Franco et al. (2018) exposed L. ochroleuca to 2-week temperature incubations to inform models that predicted an overall range expansion into northern latitudes. Whilst it is unquestionable that L. ochroleuca is proliferating towards its leading range edge in the UK and Ireland (Smale et al., 2015; Teagle and Smale, 2018; Schoenrock et al., 2021) such models may perform poorly or overestimate the pace of expansion of L. ochroleuca.
In conclusion, our experimental approach showed that duration and intensity are important characteristics that mediate MHW impacts on foundation kelp species at opposing ends of their distributions. This has implications both directly for the current study system and more broadly for assessing population vulnerability to increasing MHW activity. In particular, exposure to long-duration extreme-intensity events severely compromised the ecophysiological performance of both kelp species, which could have community- and ecosystem-level implications as natural systems are subjected to MHW intensification in the coming decades. It is also important to note that duration and intensity are not the only characteristics that differ between MHW events. The rate of onset, variability and frequency of events themselves may also interact to impact performance in a number of ways (Genin et al., 2020). Whilst our study is a critical first step to accurately assessing the impacts of MHWs, a complete understanding will require much more detailed systematic manipulative experimentation combined with sustained observations of populations in natural systems.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: Timeline of the MHW experiment on Laminaria digitata and Laminaria ochroleuca. Table S1: Semi-quantitative bleaching severity index used to quantify the presence and severity of senescence in Laminaria digitata and Laminaria ochroleuca in response to marine heatwave simulations. Table S2: Post hoc pairwise comparisons of repeated-measures ANOVA to test for differences in Fv/Fm for the kelps Laminaria digitata and Laminaria ochroleuca in response to simulated marine heatwave experiments. Table S3: Post hoc pairwise comparisons of repeated-measures ANOVA to test for differences in relative growth rate for the kelps Laminaria digitata and Laminaria ochroleuca in response to simulated marine heatwave experiments. Table S4: Post hoc pairwise comparisons of repeated-measures ANOVA to test for differences in bleached tissue for the kelps Laminaria digitata and Laminaria ochroleuca in response to simulated marine heatwave experiments.
ACKNOWLEDGEMENTS
We would like to thank the editorial staff and two anonymous reviewers for their constructive feedback.
Contributor Information
Tayla Leathers, Marine Biological Association of the United Kingdom, The Laboratory, Plymouth PL1 2PB, UK.
Nathan G King, Marine Biological Association of the United Kingdom, The Laboratory, Plymouth PL1 2PB, UK.
Andy Foggo, School of Biological and Marine Sciences, University of Plymouth, Plymouth PL4 8AA, UK.
Dan A Smale, Marine Biological Association of the United Kingdom, The Laboratory, Plymouth PL1 2PB, UK.
FUNDING
D.S., T.L. and N.K. were funded by a UKRI Future Leaders Fellowship awarded to D.S. (MR/S032827/1).
REFERENCES
- Arafeh-Dalmau N, Montano-Moctezuma G, Martinez J, Beas-Luna R, Schoeman D, Torres-Moye G.. 2019. Extreme marine heatwaves alter kelp forest community near its equatorward distribution limit. Frontiers in Marine Science 6: 499. [Google Scholar]
- Atkinson J, King NG, Wilmes SB, Moore PJ.. 2020. Summer and winter marine heatwaves favor an invasive over native seaweeds. Journal of Phycology 56: 1591–1600. [DOI] [PubMed] [Google Scholar]
- Bartsch I, Vogt J, Pehlke C, Hanelt D.. 2013. Prevailing sea surface temperatures inhibit summer reproduction of the kelp Laminaria digitata at Helgoland (North Sea). Journal of Phycology 49: 1061–1073. [DOI] [PubMed] [Google Scholar]
- Bass A, Wernberg T, Thomsen M, Smale D.. 2021. Another decade of marine climate change experiments: trends, progress and knowledge gaps. Frontiers in Marine Science 8: 1–11.35685121 [Google Scholar]
- Bass AV, Smith KE, Smale DA.. 2023. Marine heatwaves and decreased light availability interact to erode the ecophysiological performance of habitat‐forming kelp species. Journal of Phycology 59: 481–495. [DOI] [PubMed] [Google Scholar]
- Bischof K, Hanelt D, Wiencke C.. 2008. Acclimation of maximal quantum yield of photosynthesis in the brown alga Alaria esculenta under high light and UV radiation. Plant Biology 1: 435–444. [Google Scholar]
- Bolton J, Luning K.. 1982. Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Marine Biology 66: 89–94. [Google Scholar]
- Butler W. 1978. Energy distribution in the photochemical apparatus of photosynthesis. Annual Review of Plant Physiology 23: 345–378. [Google Scholar]
- Davison I. 1991. Environmental effects on algal photosynthesis: temperature. Journal of Phycology 27: 2–8. [Google Scholar]
- Duarte C, Gattuso J, Hancke K, et al. 2022. Global estimates of the extent and production of macroalgal forests. Global Ecology and Biogeography 31: 1422–1439. [Google Scholar]
- Eggert A. 2012. Seaweed responses to temperature. In: Wiencke C, Bischof K. eds.. Seaweed biology: Novel insights into ecophysiology, ecology and utilization. Berlin, Germany: Springer, 47–66. [Google Scholar]
- Evans TG, Diamond SE, Kelly MW.. 2015. Mechanistic species distribution modelling as a link between physiology and conservation. Conservation Physiology 3: cov056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbee-Dexter K, Wernberg T, Grace S, et al. 2020. Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Scientific Reports 10: 13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco J, Tuya F, Bertocci I, et al. 2018. The ‘golden kelp’ Laminaria ochroleuca under global change: Integrating multiple eco-physiological responses with species distribution models. Journal of Ecology 106: 47–58. [Google Scholar]
- Frölicher TL, Fischer EM, Gruber N.. 2018. Marine heatwaves under global warming. Nature 560: 360–364. [DOI] [PubMed] [Google Scholar]
- Gao G, Zhao X, Jiang M, Gao L.. 2021. Impacts of marine heatwaves on algal structure and carbon sequestration in conjunction with ocean warming and acidification. Frontiers in Marine Science 8: 758651. [Google Scholar]
- Genin A, Levy L, Sharon G, Raitsos DE, Diamant A.. 2020. Rapid onsets of warming events trigger mass mortality of coral reef fish. Proceedings of the National Academy of Sciences of the United States of America 117: 25378–25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert F, Janquin M-A, Davoult D.. 2008. Biometrics in Laminaria digitata: a useful tool to assess biomass, carbon and nitrogen contents. Journal of Sea Research 60: 215–219. [Google Scholar]
- Hargrave M, Foggo A, Pessarrodona A, Smale D.. 2017. The effects of warming on the ecophysiology of two co-existing kelp species with contrasting distributions. Oecologia 183: 531–543. [DOI] [PubMed] [Google Scholar]
- Harvey B, Marshall K, Harley C, Russel B.. 2022. Predicting responses to marine heatwaves using functional traits. Trends in Ecology and Evolution 37: 20–29. [DOI] [PubMed] [Google Scholar]
- Hayashida H, Matear R, Strutton P, Zhang X.. 2020. Insights into projected changes in marine heatwaves from a high-resolution ocean circulation model. Nature Communications 11: 4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereward HFR, King NG, Smale DA.. 2020. Intra-annual variability in responses of a canopy forming kelp to cumulative low tide heat stress: implications for populations at the trailing range edge. Journal of Phycology 56: 146–158. [DOI] [PubMed] [Google Scholar]
- Hobday A, Alexander L, Perkins-Kirkpatrick S, et al. 2016. A hierarchical approach to defining marine heatwaves. Progress in Oceanography 141: 227–238. [Google Scholar]
- Hobday A, Oliver E, Sen Gupta A, et al. 2018. Categorising and naming marine heatwaves. Oceanography 31: 162–173. [Google Scholar]
- Hochachka P, Somero G.. 2002. Biochemical adaptaion: mechanism and process in physiological evolution. Oxford: Oxford University Press. [Google Scholar]
- Holbrook N, Sen Gupta A, Oliver E, et al. 2020. Keeping pace with marine heatwaves. Nature Reviews Earth & Environment 1: 482–493. [Google Scholar]
- Hughes T, Kerry J, Álvarez-Noriega M, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543: 373–377. [DOI] [PubMed] [Google Scholar]
- Hurd CL, Harrison PJ, Bischof K, Lobban CS.. 2014. Seaweed ecology and physiology. Cambridge: Cambridge University Press. [Google Scholar]
- Jueterbock A, Kollias S, Smolina I, et al. 2014. Thermal stress resistance of the brown alga Fucus serratus along the North-Atlantic coast: Acclimatisation potential to climate change. Marine Genomics 13: 27–36. [DOI] [PubMed] [Google Scholar]
- Jueterbock A, Minne A, Cock J, et al. 2021. Priming of marine marcophytes for enhanced restoration success and food security in future oceans. Frontiers in Marine Science 8: 658485. [Google Scholar]
- Kassambra A. 2021. rstatix: pipe-friendly framework for basic statistical tests. R Package. https://cran.r-project.org/web/packages/rstatix/index.html. [Google Scholar]
- King N, Wilcockson D, Webster R, Smale D, Hoelters L, Moore P.. 2018. Cumulative stress restricts niche filling potential of habitat-forming kelps in a future climate. Functional Ecology 32: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman B, Kooijman S.. 2010. Dynamic energy budget theory for metabolic organisation. Cambridge: Cambridge University Press. [Google Scholar]
- Mann R. 2009. Some biochemical and physiological aspects of growth and gametogenesis in Crassostrea gigas and Ostrea edulis grown at sustained elevated temperatures. Journal of the Marine Biological Association of the United Kingdom 59: 95–110. [Google Scholar]
- Marbà N, Jordà G, Bennett S, Duarte C.. 2022. Seagrass thermal limits and vulnerability to future warming. Frontiers in Marine Science 9: 860826. [Google Scholar]
- Marin M, Feng M, Phillips H, Bindoff N.. 2021. A global, multiproduct analysis of coastal marine heatwaves: distribution, characteristics, and long-term trends. Journal of Geophysical Research, Oceans 126: e2020JC016708. [Google Scholar]
- Maxwell K, Johnson G.. 2000. Chlorophyll fluorescence – a pratical guide. Journal of Experimental Botany 51: 659–668. [DOI] [PubMed] [Google Scholar]
- Minuti JJ, Byrne M, Hemraj DA, Russell BD.. 2021. Capacity of an ecologically key urchin to recover from extreme events: Physiological impacts of heatwaves and the road to recovery. The Science of the Total Environment 785: 147281. [DOI] [PubMed] [Google Scholar]
- Nepper-Davidsen J, Andersen DT, Pedersen MF.. 2019. Exposure to simulated heatwave scenarios causes long-term reductions in performance in Saccharina latissima. Marine Ecology Progress Series 630: 25–39. [Google Scholar]
- Oliver E, Donat M, Burrows M, et al. 2018. Longer and more frequent marine heatwaves over the past century. Nature Communications 9: 1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver E, Burrows M, Donat M, et al. 2019. Projected marine heatwaves in the 21st century and the potential for ecological impact. Frontiers in Marine Science 6: 1–12.36817748 [Google Scholar]
- Pessarrodona A, Foggo A, Smale DA.. 2019. Can ecosystem functioning be maintained despite climate‐driven shifts in species composition? Insights from novel marine forests. Journal of Ecology 107: 91–104. [Google Scholar]
- Pessarrodona A, Assis J, Filbee-Dexter K, et al. 2022. Global seaweed productivity. Science Advances 8: eabn2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky ML, Comte L, Sax DF.. 2022. Unifying climate change biology across realms and taxa. Trends in Ecology & Evolution 37: 672–682. [DOI] [PubMed] [Google Scholar]
- Poloczanska ES, Brown CJ, Sydeman WJ, et al. 2013. Global imprint of climate change on marine life. Nature Climate Change 3: 919–925. [Google Scholar]
- Pörtner H. 2012. Integrating climate-related stressor effects on marine organisms: unifying principles linking molecule to ecosystem level changes. Marine Ecology Progress Series 470: 273–290. [Google Scholar]
- Provost E, Kelaher BP, Dworianyn S, Russel B, Connell S, Ghedini G.. 2019. Climate-driven disparities among ecological interactions threaten kelp forest persistence. Global Change Biology 23: 353–361. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Coleman MA, Provost E, et al. 2019. Future climate change is predicted to affect the microbiome and condition of habitat-forming kelp. Proceedings Biological Sciences 286: 20181887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybaud V, Beaugrand G, Goberville E, et al. 2013. Decline in kelp in west Europe and climate. PLoS One 8: e66044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford E, Sones J, García-Reyes M, Goddard J, Largier J.. 2019. Widespread shifts in the coastal biota of northern California during the 2014–2016 marine heatwaves. Scientific Reports 9: 4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiener P, Black KD, Stanley MS, Green DH.. 2015. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. Journal of Applied Phycology 27: 363–373. [Google Scholar]
- Schoenrock KM, O’Callaghan R, O’Callaghan T, O’Connor A, Stengel DB.. 2021. An ecological baseline for Laminaria hyperborea forests in western Ireland. Limnology and Oceanography 66: 3439–3454. [Google Scholar]
- Simkanin C, Power AM, Myers A, et al. 2005. Using historical data to detect temporal changes in the abundances of intertidal species on Irish shores. Journal of the Marine Biological Association of the United Kingdom 85: 1329–1340. [Google Scholar]
- Simonson E, Scheibling R, Metaxas A.. 2015. Kelp in hot water: I Warming seawater temperature induces weakening and loss of kelp tissue. Marine Ecology Progress Series 537: 89–104. [Google Scholar]
- Smale DA. 2020. Impacts of ocean warming on kelp forest ecosystems. The New Phytologist 225: 1447–1454. [DOI] [PubMed] [Google Scholar]
- Smale D, Wernberg T.. 2013. Extreme climatic event drives range contraction of a habitat-forming species. Proceedings of the Royal Society B 280: 20122829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ.. 2013. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecology and Evolution 3: 4016–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale D, Wernberg T, Yunnie A, Vance T.. 2015. The rise of Laminaria ochroleuca in the Western English Channel (UK) and comparisons with its competitor and assemblage dominant Laminaria hyperborea. Marine Ecology 36: 1033–1044. [Google Scholar]
- Smale DA, Wernberg T, Oliver ECJ, et al. 2019. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nature Climate Change 9: 306–312. [Google Scholar]
- Smith A, Oliver E, Schlegal R.. 2018. Detect marine heat waves amd marine cold spells- package ‘RMarineHeatWaves’. https://cran.r-project.org/web/packages/RmarineHeatWaves/index.html. [Google Scholar]
- Smith KE, Burrows MT, Hobday AJ, et al. 2021. Socioeconomic impacts of marine heatwaves: global issues and opportunities. Science 374: eabj3593. [DOI] [PubMed] [Google Scholar]
- Smith KE, Burrows MT, Hobday AJ, et al. 2022. Biological impacts of marine heatwaves. Annual Review of Marine Science 15: 119–145. [DOI] [PubMed] [Google Scholar]
- Straub S, Wernberg T, Marzinelli E, Vergés A, Kelaher B, Coleman M.. 2022. Persistence of seaweed forests in the Anthropocene will depend on warming and marine heatwave profiles. Journal of Phycology 58: 22–35. [DOI] [PubMed] [Google Scholar]
- Sunday J, Bates A, Dulvy N.. 2012. Thermal tolerance and the global redistribution of animals. Nature Climate Change 2: 686–690. [Google Scholar]
- Sunday J, Bates A, Kearney M, et al. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proceedings of the National Academy of Sciences 111: 5610–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryan RM, Arimitsu ML, Coletti HA, et al. 2021. Ecosystem response persists after a prolonged marine heatwave. Scientific Reports 11: 6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teagle H, Smale DA.. 2018. Climate‐driven substitution of habitat‐forming species leads to reduced biodiversity within a temperate marine community. Diversity and Distributions 24: 1367–1380. [Google Scholar]
- Teagle H, Hawkins SJ, Moore PJ, Smale DA.. 2017. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. Journal of Experimental Marine Biology and Ecology 492: 81–98. [Google Scholar]
- Thomsen M, Mondardini L, Alestra T, et al. 2019. Local extinction of bull kelp (Durvillaea spp) due to a marine heatwave. Frontiers in Marine Science 6: 1–10.36817748 [Google Scholar]
- Thoral F, Montie S, Thomsen M, Tait L, Pinkerton M, Schiel D.. 2022. Unravelling seasonal trends in coastal marine heatwave metrics across global biogeographical realms. Scientific Reports 12: 7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergés A, McCosker E, Mayer-Pinto M, et al. 2019. Tropicalisation of temperate reefs: Implications for ecosystem functions and management actions. Functional Ecology 33: 1000–1013. [Google Scholar]
- Wernberg T, Smale D, Tuya F, et al. 2012. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nature Climate Change 3: 78–82. [Google Scholar]
- Wernberg T, Bennett S, Babcock R, et al. 2016. Climate-driven regime shift of a temperate marine ecosystem. Science 353: 169–172. [DOI] [PubMed] [Google Scholar]
- Wright J, Jones C.. 2006. The concept of organisms as ecosystem engineers ten years on: progress, limitations and challenges. BioScience 56: 203–209. [Google Scholar]
- Wright LS, Pessarrodona A, Foggo A.. 2022. Climate-driven shifts in kelp forest composition reduce carbon sequestration potential. Global Change Biology 28: 5514–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


