Abstract
Background and Aims
Large brown macroalgae serve as foundation organisms along temperate and polar coastlines, providing a range of ecosystem services. Saccorhiza polyschides is a warm-temperate kelp-like species found in the northeast Atlantic, which is suggested to have proliferated in recent decades across the southern UK, possibly in response to increasing temperatures, physical disturbance and reduced competition. However, little is known about S. polyschides with regard to ecological functioning and population dynamics across its geographical range. Here we examined the population demography of S. polyschides populations in southwest UK, located within the species’ range centre, to address a regional knowledge gap and to provide a baseline against which to detect future changes.
Methods
Intertidal surveys were conducted during spring low tides at three sites along a gradient of wave exposure in Plymouth Sound (Western English Channel) over a period of 15 months. Density, cover, age, biomass and morphology of S. polyschides were quantified. Additionally, less frequent sampling of shallow subtidal reefs was conducted to compare intertidal and subtidal populations.
Key Results
We recorded pronounced seasonality, with fairly consistent demographic patterns across sites and depths. By late summer, S. polyschides was a dominant habitat-former on both intertidal and subtidal reefs, with maximum standing stock exceeding 13 000 g wet weight m−2.
Conclusions
Saccorhiza polyschides is a conspicuous and abundant member of rocky reef assemblages in the region, providing complex and abundant biogenic habitat for associated organisms and high rates of primary productivity. However, its short-lived pseudo-annual life strategy is in stark contrast to dominant long-lived perennial laminarian kelps. As such, any replacement or reconfiguration of habitat-forming macroalgae due to ocean warming will probably have implications for local biodiversity and community composition. More broadly, our study demonstrates the importance of high-resolution cross-habitat surveys to generate robust baselines of kelp population demography, against which the ecological impacts of climate change and other stressors can be reliably detected.
Keywords: Saccorhiza polyschides, Furbelows, kelp, marine forests, seaweeds, macroalgae, foundation species, habitat-former, temperate rocky reefs, seasonality, wave exposure, ocean warming
INTRODUCTION
Kelps – large brown macroalgae – are widely distributed across the world’s temperate and polar coastlines (Jayathilake and Costello, 2021), where they form vital ‘marine forest’ habitats (Duarte et al., 2022). These forests typically exhibit high levels of biodiversity and primary productivity (Steneck et al., 2002; Teagle et al., 2017; King et al., 2021; Smale et al., 2021; Pessarrodona et al., 2022a; United Nations (UN) Environment Programme, 2023), and provide a range of ecosystem services with direct and indirect socioeconomic value (Smale et al., 2013; Eger et al., 2023). While the ecological structure and function of kelp species vary to some extent across regions and habitats, they generally serve as foundation organisms within coastal ecosystems (Lüning, 1985; Steneck et al., 2002; Smale et al., 2013).
Kelp species dominate shallow rocky habitats along the extensive coastline of the northeast Atlantic (Smale et al., 2013). Whereas some kelp species have been intensively studied (e.g. Laminaria hyperborea, L. digitata, Saccharina latissima) (e.g. Kain, 1979; Bartsch et al., 2008; Diehl et al., 2023 and references therein), information on the distribution and demography for other species (e.g. Saccorhiza polyschides, Laminaria ochroleuca, Alaria esculenta) remains more spatially limited or lacking for many areas. Furthermore, species and populations inhabiting intertidal and/or wave-sheltered environments are generally better studied than those occupying subtidal and/or wave-exposed habitats (Smale et al., 2013; Araújo et al., 2016), due to their restricted accessibility.
Impacts of climate change on kelp forests
Marine forests are currently threatened by a range of human-mediated stressors, including climate change factors (e.g. ocean warming and marine heatwaves, ocean acidification, increased storminess, rising sea levels), the spread of invasive species, fishing impacts (e.g. mechanical damage and sedimentation from trawling, and trophic cascades) and decreased coastal water quality (, Harley et al., 2006; Filbee-Dexter et al., 2020; Norderhaug et al., 2020; Smith et al., 2021b; IPCC, 2022). Ocean warming, in particular, is emerging as a pervasive driver of ecosystem change, causing species range shifts, local and regional extirpations, species replacements, altered ecological interactions, changes in population demography and community composition, and even reconfigurations of entire ecosystems (Hawkins et al., 2009; Merzouk and Johnson, 2011; Harley et al., 2012; Koch et al., 2013; Araújo et al., 2016; Assis et al., 2018; Smale, 2020). In some regions, the ‘tropicalization’ of former kelp beds, the rise of turf algae and shifts to barren grounds caused by increased herbivory (from sea urchins, for example) have been observed and attributed to ocean warming and related environmental changes (Vergés et al., 2014; Pessarrodona et al., 2021, 2022b). Ecological responses of marine forests to anthropogenic climate change can also have substantial socioeconomic impacts (Smith et al., 2021a and references therein).
Species range shifts in the NE Atlantic related to ocean warming, and long-term monitoring efforts
In the northeast Atlantic, recent ocean warming trends have affected several forest-forming macroalgal species across multiple regions, most often manifesting as species range shifts or changes in population demography (Assis et al., 2018; Smale, 2020). For example, populations of Laminaria hyperborea, L. ochroleuca and Saccorhiza polyschides have declined towards their southern (trailing) range edges and, conversely, proliferated and expanded towards their northern (leading) edges (Müller et al., 2009; Fernández, 2011; Smale et al., 2013; Casado-Amezúa et al., 2019). In addition, local extinctions of populations found towards species’ trailing range edges have also been observed (Fernández, 2011; Díez et al., 2012; Casado-Amezúa et al., 2019).
Along parts of the coastline of the UK and Ireland, warm-tolerant species such as L. ochroleuca and the non-native kelp Undaria pinnatifida have proliferated and expanded their distributions (Epstein and Smale, 2017b; Teagle and Smale, 2018; Schoenrock et al., 2019), whereas some more northerly distributed cold-adapted species, such as Alaria esculenta, have undergone population declines and probable range contractions (Simkanin et al., 2005; Birchenough and Bremner, 2010).
It is likely, however, that other impacts of ocean warming on marine forests have gone undetected and under-reported, due to a lack of baseline data and monitoring efforts in many areas. That said, in some well-studied regions, long-term monitoring of kelp forests using a range of approaches, such as snorkelling and diving, towed video, remotely operated vehicles, satellite-derived imagery, and citizen/community science initiatives, has reliably quantified temporal trends (Thompson, 2021; Reshitnyk et al., 2023). Even in regions or countries with a strong track record of marine monitoring, however, tropical ecosystems have received far more resources and attention than their temperature counterparts, which are typically dominated by kelp forests (e.g. Australia, see Bennett et al., 2015). Historically, the NE Atlantic is a relatively data-poor region of macroalgae research, particularly concerning sustained observation and monitoring (Smale et al., 2013) when compared to well-studied ecosystems such as the Giant Kelp forests (Macrocystis pyrifera) along the Californian coast (Schiel and Foster, 2015).
A deeper understanding of spatiotemporal variability patterns in kelp population demographics persisting under a range of environmental conditions and stressors is needed to monitor and predict responses to environmental changes. Given that kelp populations exhibit high levels of intra- and inter-annual variability due to, for example, temporal growth strategies and winter storm disturbances (Kain, 1979; Seymour et al., 1989; Smale and Vance, 2015), it is imperative that any efforts to address kelp demography capture seasonal changes. Similarly, kelp species and populations are highly variable across spatial gradients, as morphology, density and standing stock are strongly influenced by key abiotic and biotic variables (e.g. wave exposure, light availability, grazing pressure). As such, spatial variability should be considered in any baseline survey. While sustained observations through long-term monitoring represent the optimal approach for detecting climate change impacts, monitoring programmes are generally costly and logistically challenging and are lacking for most kelp species, habitats and regions. Citizen or community science initiatives represent a useful low-cost tool to collect long-term data while engaging the local stakeholders in marine research and drawing attention to the health of marine species, habitats and ecosystems (Sandahl and Tøttrup, 2020; Fraisl et al., 2022). Community science projects that involve kelp forest observations are, for example, currently undertaken by Reef Check in California, Reef Life Survey in temperate Australia and Seasearch along parts of the British coast (Bull et al., 2013; Freiwald et al., 2021; Lucrezi, 2021; Edgar et al., 2023). However, in the absence of long-term monitoring or well-established community science initiatives, appropriately designed focused surveys that target key or indicator species can fill knowledge gaps, provide a baseline against which to detect changes and inform about the general condition of the ecosystem.
We focus on one kelp species, Saccorhiza polyschides, which is expected to proliferate under ongoing ocean warming and other environmental changes, in a biogeographical transition zone in the Western English Channel (Dinter, 2001; Dauvin, 2019). We examine spatiotemporal variability in demography to generate a robust baseline on range-centre populations that can be used to predict, detect and test future changes of a habitat-forming kelp species under climate change.
Saccorhiza polyschides in the NE Atlantic
Saccorhiza polyschides (Lightfoot) Batters 1902, commonly known as ‘Furbelows’, is a large canopy-forming brown macroalga belonging to the order Tilopteridales. Although not a ‘true kelp’ in the traditional taxonomic definition (i.e. belonging to the order Laminariales), it serves a similar ecological function as other marine forest formers and is commonly included within kelp assemblages (Smale et al., 2013). Saccorhiza polyschides sporophytes become fertile and reproduce in late summer/early autumn, with new recruits appearing in the following spring. Recruits exhibit rapid growth through summer and autumn to mature sporophytes before the onset of senescence (Norton and Burrows, 1969). As the overall lifespan of the macroscopic sporophyte does not exceed 12–18 months (Sauvageau, 1915; Norton and Burrows, 1969), S. polyschides is a so-called ‘pseudo-annual’ species, whereas most ‘true’ kelp species in the NE Atlantic are longer-lived, multi-year perennials (i.e. Laminaria sp.). Due to its fast growth and high productivity within one season, S. polyschides has been described as an opportunistic pioneer species (Norton and Burrows, 1969; Kain and Jones, 1975; Assis et al., 2013; Smale et al., 2013; Pereira, 2014; Fernández et al., 2022).
As with other kelp species, sporophytes of S. polyschides comprise three main morphological components: a digitated blade (i.e. lamina), stipe and holdfast. Unlike true kelps, however, S. polyschides exhibits a distinctive hollow bulbous holdfast with many haptera, converging into a flattened stipe, which is twisted at its base just above the holdfast (see Barber, 1889). Kelp sporophytes, and especially their holdfasts, provide an important habitat for faunal assemblages by offering shelter, nursery grounds and increased food supply for invertebrates (McKenzie and Moore, 1981; Thiel and Vásquez, 2000; Feehan et al., 2014; Teagle et al., 2017, 2018) and coastal fishes (McKenzie and Moore, 1981; Gordon, 1983; Salland and Smale, 2021; Christie et al., 2022). The large bulbous holdfast of S. polyschides offers plentiful living space for fauna (Norton, 1971), and it can persist for much of the year even following the senescence of the rest of the sporophyte, representing important habitat for over-wintering assemblages (Salland and Smale, 2021).
Saccorhiza polyschides extends from the low intertidal zone into subtidal habitats to depths of 25 m or more (Arnold et al., 2016; Smale and Moore, 2017; D. A. Smale, unpubl. data) and is distributed in the NE Atlantic from the Mediterranean and Morocco polewards to Norway and the Faroes (Norton, 1977; Lüning, 1985; Smale et al., 2013). In warmer, more southerly regions within its latitudinal distribution, S. polyschides is often the dominant habitat-former and exhibits high population densities (Fernández and Niell, 1982; Fernández, 2011), while in cooler, more northerly regions it tends to be outcompeted by Laminaria species and is generally found in mixed stands or restricted to disturbed or marginal habitats (Hawkins and Harkin, 1985; Smale and Moore, 2017). Limited evidence suggests that this species has increased in abundance and spatial extent along the southern coastline of the UK in recent decades (Birchenough and Bremner, 2010; Smale et al., 2013). This is perhaps in response to rising temperatures becoming more favourable for its performance, as well as increased disturbance to Laminaria forests reducing competition and other environmental changes (Kain and Jones, 1975). It has therefore been described as a potential ‘winner’ of ocean warming (Smale et al., 2013), particularly for populations found towards the range centre or leading range edge, such as those in UK waters. However, only limited data on local S. polyschides populations in SW England are available to confirm this assumption and the empirical evidence base remains limited. Notwithstanding some early natural history work on its morphology, demography and ecological role within the ecosystem (Barber, 1889; Burrows, 1958; Norton, 1969; Norton and Burrows, 1969), the only recent study focused on S. polyschides in the UK and across the wider central area of its latitudinal distribution examined spatial variability in sporophyte density and morphology from a single sampling event (Salland and Smale, 2021).
Aim of baseline study on S. polyschides populations in a biogeographical transition zone
Given the role of S. polyschides as a habitat-forming foundation species and the rapid growth rates observed for sporophytes through spring and summer, this species may be important for local biodiversity and primary productivity, yet robust information on population dynamics within a biogeographical transition zone (i.e. the Western English Channel, see Dinter, 2001; Southward et al., 2005; Cox et al., 2016; Dauvin, 2019) and its range-centre is lacking. Without this knowledge, it is not possible to detect nor assess the wider ecological consequences of future changes. We conducted surveys at three intertidal rocky shores over 15 months, as well as a less frequent survey of three subtidal reefs, to quantify the structure of S. polyschides populations within a region of probable climate-driven expansion, to address this pressing knowledge gap.
MATERIALS AND METHODS
Survey design
Surveys of intertidal S. polyschides populations were conducted every month during periods of spring low tides at a tidal height of +0.5–0.8 m (relative to chart datum) at three rocky shore sites in and around Plymouth Sound (50°N), southwest UK. A mean tidal range of 4.7 m influences Plymouth Sound and sea temperature typically ranges between 8 and 18 °C throughout the year (Pessarrodona et al., 2018b). The three survey sites were situated along a gradient of wave exposure (Supplementary Data S1) from semi-sheltered Mount Batten (MB-sh; protected by the coastline of the Sound and a man-made breakwater structure) to moderately sheltered/exposed Bovisand (BS-mod; open towards the Western English Channel, partly sheltered from prevailing swell by an adjacent headland) to fully exposed Heybrook Bay (HY-exp; open to the Western English Channel with minimal protection) (Fig. 1) (Salland and Smale, 2021). All sites were deemed representative of the wider region (i.e. shallow rocky reef with silt/sandy patches and deeper gullies; see Supplementary Data S1) and were without obvious local anthropogenic impacts (i.e. pollution, trawling).
Fig. 1.
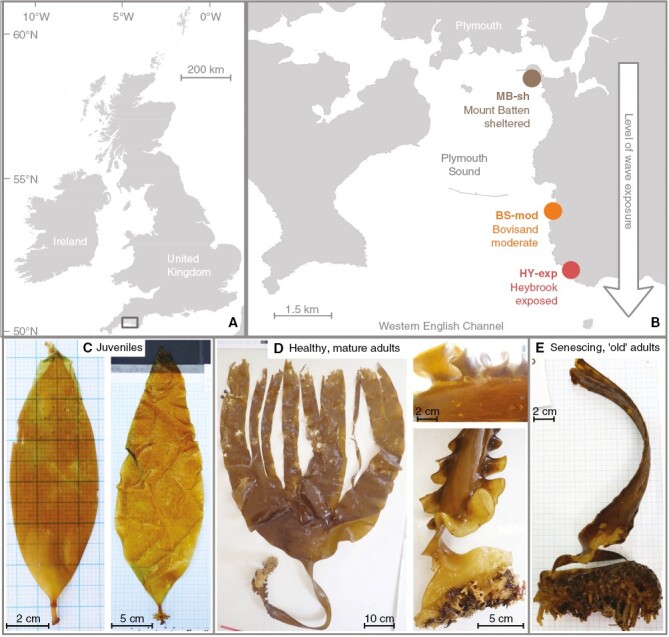
Survey location and morphological differences between sporophyte age classes of S. polyschides. (A) Map indicating region of study area in southwest England (grey box) and (B) location of three sites along a wave exposure gradient in Plymouth Sound. (C–E) Representative examples of the three age/size classes of S. polyschides: (C) juvenile recruits with a total length < 30 cm and incomplete development of bulbous holdfast; (D) healthy adult ‘plants’ with total length > 30 cm, complete development of closed, bulbous holdfast (bottom right), possible presence of fertile sporophyll tissue (top right) and some limited tissue loss at the distal tips of blades caused by grazing and wave action; (E) senescing, ‘old’ adults exhibiting partial or complete decay of blade, stipe and/or holdfast.
Surveys commenced in February 2020 (survey month 1) and continued until April 2021 (survey month 15). Due to adverse weather conditions, surveys were not feasible in January 2021 (survey month 12) and therefore data were not collected in this month. Additionally, S. polyschides populations on adjacent shallow subtidal reefs (2–4 m below chart datum) at all three sites were surveyed by SCUBA diving on three occasions (June, August and October 2020; survey months 5, 7 and 9, respectively) to examine differences in population demography and morphology with increasing water depth.
Density and cover
During each survey, ten 1-m2 quadrats were haphazardly placed in areas of appropriate habitat (stratified for emergent rocky substrate rather than rock pools or sandy patches) within the appropriate tidal height, positioned at least 2 m apart from one another. The density (number of individuals) and cover (visually estimated as a percentage) of S. polyschides sporophytes of each age class were recorded in situ. Age classes were defined as either juveniles, healthy adults, or senescent adults based on a modified classification scheme from Norton and Burrows (1969) (detailed further in Fig. 1 and Supplementary Data S2). The cover of ‘other kelps’ (i.e. L. hyperborea, L. ochroleuca, L. digitata, Saccharina latissima and U. pinnatifida) was also recorded (from May 2020 onwards; visual estimation). Due to the three-dimensional multi-layered structure within the water column in a marine forest, estimates of total percentage cover often exceeded 100 % since different species and size classes overlap within the quadrat (Smale and Moore, 2017).
Sporophyte biomass and morphology
During each survey, ten representative sporophytes per site and month were randomly collected by carefully removing each one from the substratum and transferring it into a cotton bag. These samples were taken from a separate part of the shore to the density quadrats. On return to the laboratory, individuals were assigned to one of the three age classes, photographed, measured and weighed. Measurements of length and fresh weight biomass (blotted tissue dry) were obtained separately for the different sporophyte components (i.e. holdfast, stipe and blade), while the weight of the sporophyll (reproductive frills) and mature sorus tissue was obtained where present. Sorus tissue forms ‘a palisade-like cell layer at the surface of the thallus’ (Norton and Burrows, 1969), which permits visible detection of the mature tissue.
Estimation of standing stock and biomass accumulation
To estimate the standing stock of S. polyschides, mean density values were multiplied by mean sporophyte biomass for each month and site, to yield g wet weight m−2 (g WW m−2). Maximal biomass accumulation (maximal standing stock), the gain of biomass from a juvenile recruit to a fully grown mature sporophyte, was used to estimate total biomass accumulation during a growing season, as a proxy for primary productivity.
Statistical analysis
Between-site and between-month variation in response variables was examined with univariate permutational analysis of variance (PERMANOVA), conducted with R version 4.0.0 (R Core Team, 2020), R Studio and PRIMER 7.0.21 (Anderson et al., 2008; Clarke et al., 2014). If not stated otherwise, results are given as monthly means with standard error (± s.e.) per site and depth. Data (untransformed) were used to construct similarity matrices based on Euclidean distances (dummy value of ‘1’). Intertidal (three sites, 14 months) and subtidal (three sites, three months) datasets were analysed separately with ‘site’ and ‘month’ as fixed factors (9999 permutations under a reduced model with Monte Carlo correction). Pairwise post-hoc tests were conducted where significant effects were detected.
RESULTS
Density and cover
The density of S. polyschides sporophytes and the composition of age classes across the year in the intertidal zone exhibited high variability between months, following a seasonal pattern of recruitment and growth (Fig. 2A, B). Mean total density ranged from 0 ± 0 to 118 ± 44 individuals m−2.
Fig. 2.
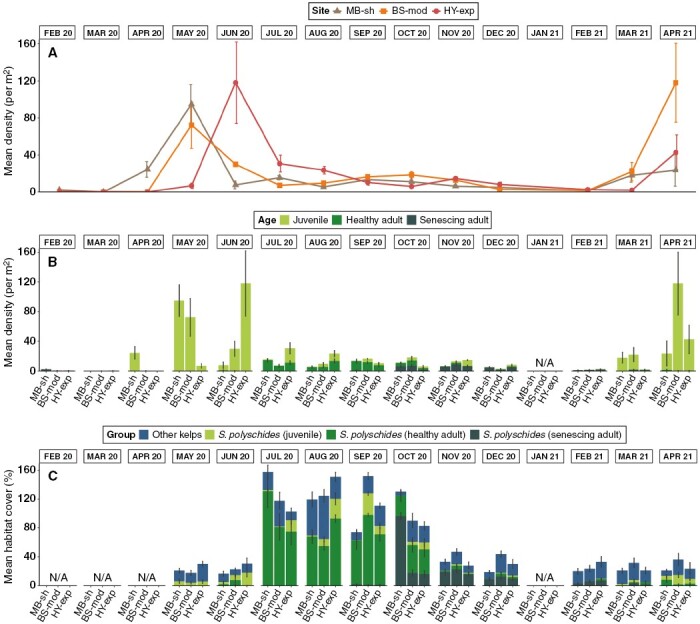
Spatiotemporal variability in density and cover of S. polyschides populations in intertidal habitats at three sites (with increasing levels of wave exposure from left, MB-sh, to right, HY-exp) in Plymouth Sound over 15 months. (A) Mean total density (± s.e.) and (B) mean density of each age class at each site and month during the intertidal survey. (C) Mean habitat percentage cover (± s.e.) of S. polyschides (differentiated between three age classes) and other kelps (Laminaria spp., Saccharina latissima, U. pinnatifida) recorded at each site and month.
Juvenile sporophytes dominated in spring and early summer (March/April until June), with mean density increasing more than 100-fold over 2–3 months. Maximum mean density of juveniles was observed at the more wave-exposed sites HY-exp in June 2020 and at BS-mod in April 2021, at 118.1 ± 44 and 118 ± 43 individuals m−2, respectively. Following rapid growth of recruits, populations were dominated by an ‘older’, mature adult sporophyte cohort in summer and autumn (July–October), with a maximum mean density of 14.4 ± 2.4 individuals m−2 recorded at MB-sh in July 2020. In the winter months (November–February/March), populations comprised senescing ‘old’ adults. The maximum mean density of senescing sporophytes (8.8 ± 1.7 individuals m−2) was recorded at BS-mod in November 2020. We observed a smaller cohort of late recruits, appearing in late summer, which did not become fertile until the following year. We also observed an overlap between decaying holdfasts, late recruits and newly observed recruits in late winter 2020 and early spring 2021.
Kelps (i.e. S. polyschides and Laminariales) dominated the intertidal habitat between July and October, covering more than half of the surveyed shore (Fig. 2C). The cover of S. polyschides increased markedly through summer into autumn, becoming the dominant space occupier between July and October (ranging from 132.6 ± 7.9 % at MB-sh in July to 60 ± 11.4 % at HY-exp in October).
For all response variables, univariate PERMANOVA detected a highly significant effect of the ‘site × month’ interaction term, as well as the main effect of ‘month’ (Table 1). However, there were no significant differences between the term ‘site’ in density and cover. Post-hoc comparisons within levels of the interaction term showed that the magnitude of differences between ‘month’ were not always consistent between sites and therefore along the wave exposure gradient (Supplementary Data S3).
Table 1.
Results of a univariate PERMANOVA to test for differences in intertidal kelp density, cover and biometric measurements between sites and sampling months of intertidal S. polyschides populations. PERMANOVAs (9999 permutations) are based on Euclidean distances with a dummy value of 1, under a reduced model with Monte Carlo (MC) correction. ‘Site’ and ‘month’ as fixed factors. Significant values are indicated in bold (P ≤ 0.05). Post-hoc pairwise test followed PERMANOVAs (Supplementary Data S3).
| Response variable | Site | Month | Site × month | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intertidally | d.f. | F | P (MC) | d.f. | F | P (MC) | d.f. | F | P (MC) | Res d.f. |
| Density of S. polyschides per m 2 | ||||||||||
| S. polyschides (sum) | 2 | 0.48 | 0.6223 | 13 | 8.9854 | 0.0001 | 26 | 4.9851 | 0.0001 | 378 |
| Cover of S. polyschides per m 2 | ||||||||||
| S. polyschides (sum) | 2 | 0.152 | 0.8539 | 11 | 68.28 | 0.0001 | 22 | 5.5141 | 0.0001 | 324 |
| Biometrics per S. polyschides individual | ||||||||||
| Total biomass/WW | 2 | 8.6256 | 0.0004 | 13 | 51.211 | 0.0001 | 26 | 2.2436 | 0.0005 | 378 |
| Total length | 2 | 13.501 | 0.0001 | 13 | 134.7 | 0.0001 | 26 | 2.4561 | 0.0001 | 378 |
| Sorus biomass/WW | 2 | 21.049 | 0.0001 | 13 | 22.738 | 0.0001 | 26 | 8.3645 | 0.0001 | 378 |
| Standing stock | ||||||||||
| g WW m−2 | 2 | 2.197 | 0.1116 | 13 | 15.872 | 0.0001 | 26 | 3.275 | 0.0001 | 378 |
For subtidal populations (sampled only in June, August and October), maximum mean density was observed in June (67 ± 27.4 individuals m−2 at HY-exp), dominated by juvenile recruits. This was followed by high adult density in August (22.6 ± 6.2 individuals m−2 at BS-mod), and a mixed stand of healthy and senescing adults in October (14.7 ± 6.2 individuals m−2 at BS-mod) (Fig. 3A, B). We recorded juvenile recruits at all subtidal sampling events with the exception of MB-sh in October. The coverage of S. polyschides contributed to more than half of all subtidal habitat-forming seaweeds at BS-mod and HY-exp in August, and at MB-sh and BS-mod in October (Fig. 3C). In August, S. polyschides was the most dominant kelp at all sites, but in June and October, the contribution of other kelps exceeded that of S. polyschides. Univariate PERMANOVA (Table 2) detected a significant ‘site × month’ interaction for both density and cover, but a main effect of ‘site’ was only detected for density and a main effect of ‘month’ was only detected for cover. Post-hoc comparisons within levels of the interaction term for density showed that the magnitude of differences between ‘month’ was not always consistent between sites (Supplementary Data S4), but for coverage, we could detect no differences between sites in June and October (Supplementary Data S4). In general, the density of S. polyschides in subtidal habitats was lower than that observed in intertidal habitats for corresponding months, but the cover of S. polyschides was broadly comparable.
Fig. 3.
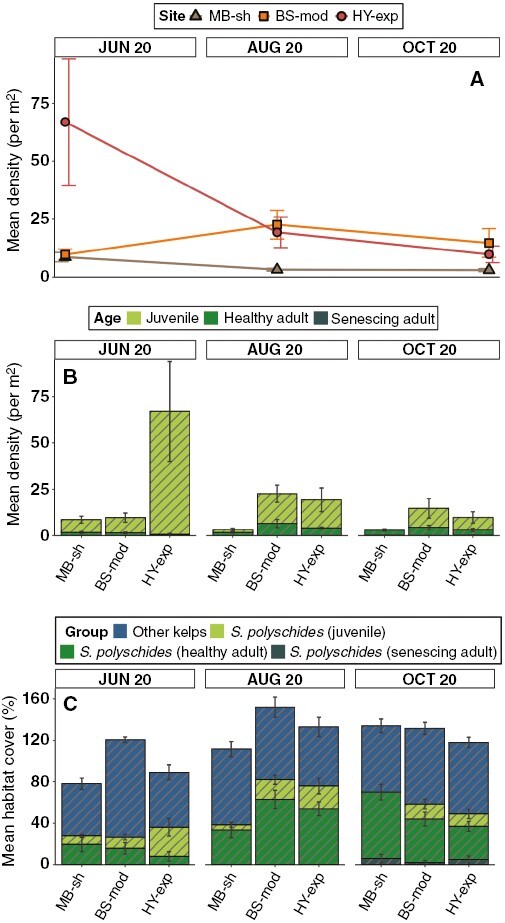
Spatiotemporal variability in density and cover of S. polyschides populations in subtidal habitats. (A) Mean total density (± s.e.), and (B) mean density of age classes at each site (along a gradient of wave exposure) and month. (C) Mean habitat percentage cover (± s.e.) of S. polyschides (differentiated between three age classes) and other kelps (Laminaria spp., Saccharina latissima, U. pinnatifida). Note that subtidal survey months are not consecutive.
Table 2.
Results of univariate PERMANOVA for subtidal kelps in June, August and October 2020. PERMANOVAs (9999 permutations) are based on Euclidean distances with a dummy value of 1, under a reduced model with Monte Carlo (MC) correction. ‘Site’ and ‘month’ as fixed factors. Significant values are indicated in bold (P ≤ 0.05). Post-hoc pairwise test followed PERMANOVAs (Supplementary Data S4).
| Response variable | Site | Month | Site × month | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| subtidally | d.f. | F | P (MC) | d.f. | F | P (MC) | d.f. | F | P (MC) | Res d.f. |
| Density of S. polyschides per m 2 | ||||||||||
| S. polyschides (sum) | 2 | 5.6284 | 0.0047 | 2 | 2.9474 | 0.0568 | 4 | 3.5225 | 0.0114 | 81 |
| Cover of S. polyschides per m 2 | ||||||||||
| S. polyschides (sum) | 2 | 1.0583 | 0.3504 | 2 | 12.785 | 0.0001 | 4 | 3.6592 | 0.0078 | 81 |
| Biometrics per S. polyschides individual | ||||||||||
| Total biomass/WW | 2 | 0.3 | 0.7434 | 2 | 13.556 | 0.0001 | 4 | 4.7573 | 0.0015 | 81 |
| Total length | 2 | 5.2642 | 0.0066 | 2 | 7.2153 | 0.001 | 4 | 1.4334 | 0.2223 | 81 |
| Sorus biomass/WW | 2 | 0.2506 | 0.78 | 2 | 7.2847 | 0.0012 | 4 | 4.4964 | 0.0027 | 81 |
| Standing stock | ||||||||||
| g WW m−2 | 2 | 3.9109 | 0.0231 | 2 | 0.3802 | 0.6829 | 4 | 0.9631 | 0.4316 | 81 |
Biomass and morphology
At all intertidal sites, sporophyte size and biomass exhibited pronounced seasonality, increasing throughout the development period from a juvenile recruit to full maturity, followed by a decrease in biomass and size during the period of decay and senescence (Fig. 4). Maximum mean biomass (Fig. 4A, B) of 598.1 ± 97.8 g was recorded in September at BS-mod, whereas maximum mean length (Fig. 4C, D) of 182.95 ± 12.8 cm was recorded in July 2020 at BS-mod. During periods of peak biomass (i.e. summer months), the structural blade compartment consisted of ~50 % of the total sporophyte weight, whereas the relative contribution of holdfasts to total weight was greater in other periods (Fig. 4B). With regard to total length, the relative contribution of blade structures was generally greater than that of stipes and holdfasts (Fig. 4D).
Fig. 4.
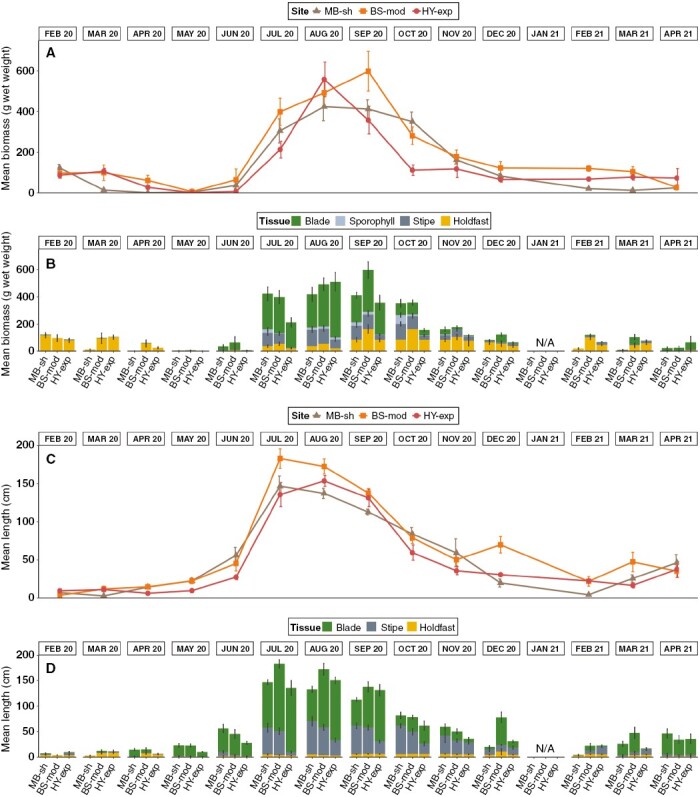
Spatiotemporal variability in biometric measurements from individuals sampled from intertidal S. polyschides populations. Mean total biomass (A, B) and total length (C, D) (± s.e.). Stacked bar plots indicate values for each structural tissue component.
Development of mature sorus was recorded between August and December 2020 (Fig. 5). Additionally, minor sorus tissue production outside the fertile summer months was recorded in March and April 2021 in some individuals. We recorded clear differences between sites in the weight of sorus tissue (Fig. 5A), with the highest values at the least exposed/most sheltered site (MB-sh) and lowest sorus production at the most exposed site (HY-exp). Sorus tissue was recorded on all structural compartments of the sporophyte (Fig. 5B), not only on the sporophyll. The most prominent fertile sporophyll tissue and highest sporophyll biomass were observed in September and October (Fig. 5B). Univariate PERMANOVA detected significant differences in all response variables between the main factors of ‘site’ and ‘month’, as well as the interaction term (Table 1). Post-hoc tests within the interaction term of main factors (Supplementary Data S3) showed that the magnitude of differences between sites varied across months, but did not exhibit a clear seasonal pattern.
Fig. 5.
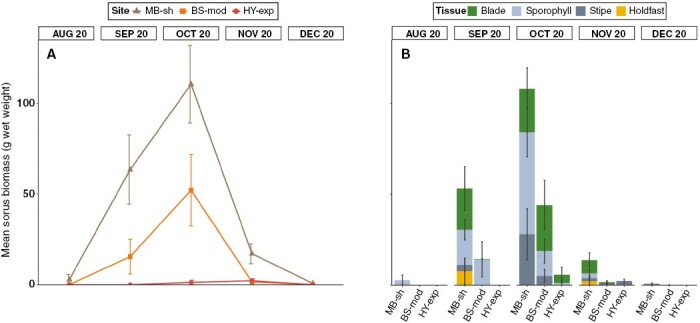
Spatiotemporal variability in sorus biomass from intertidal S. polyschides populations. (A) Mean biomass (± s.e.) of fertile sorus tissue (wet weight) and (B) mean biomass of sorus associated with each structural component during main reproductive months.
For subtidal populations, sporophyte biomass and length increased from June to August at all sites (to varying degrees), while trends from August to October varied between sites (Fig. 6). Maximum mean sporophyte biomass was observed at MB-sh in October (655.3 ± 114.4 g), with blades comprising ~50 % of total biomass (Fig. 6A, B). Maximum mean length was recorded at BS-mod in October (134.3 ± 12.3 cm) and was more consistent between sites and sampling events (Fig. 6C, D). Univariate PERMANOVA detected a significant ‘site’ × ‘month’ interaction term for biomass (Table 2) with post-hoc tests indicating that sites did not differ in August and October but did in June (Supplementary Data S4). A significant ‘site × month’ interaction term for sorus biomass, however, indicated differences in August and October, but not in June. Total sporophyte length varied between the main factors of site (pairwise post-hoc test: BS-mod > HY-exp = MB-sh) and month (pairwise post-hoc test: June < August = October). In general, sporophyte biomass and length in subtidal habitats was greater than that observed in intertidal habitats for the corresponding months of June and October, but less in the intermediate month (August).
Fig. 6.
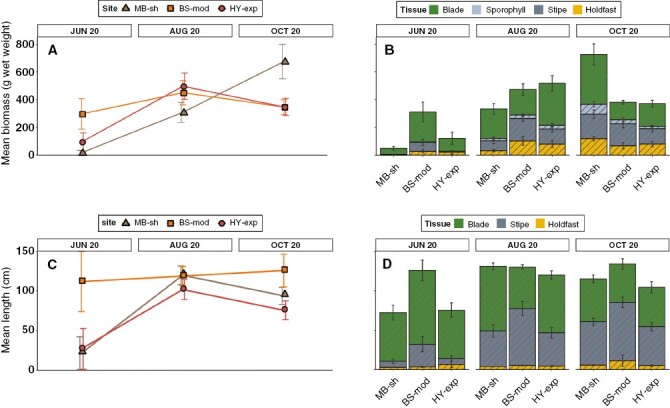
Spatiotemporal variability in biometric measurements from individuals sampled from subtidal S. polyschides populations. Mean total biomass (A, B) and total length (C, D) (± s.e.). Stacked bar plots indicate values for each structural tissue component. Note that subtidal survey months are not consecutive.
Standing stock
Estimates of standing stock ranged from 0 to over 13 000 g WW m−2 and exhibited high variability between sites and months (Fig. 7). For intertidal populations, standing stock peaked at HY-exp in August (13 176 g WW m−2), and at BS-mod (9809 g WW m−2) and MB-sh (5565 g WW m−2) in September, following rapid increases from June onwards (Fig. 7). Standing stock declined rapidly after September, returning to negligible values through winter when holdfasts represented the majority of the biomass. Univariate PERMANOVA detected a significant interaction term (Table 1), with post-hoc tests indicating that differences between sites did not persist in all months (Supplementary Data S3).
Fig. 7.
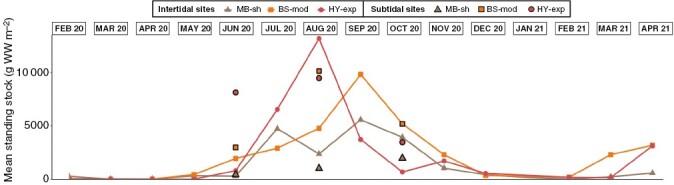
Spatiotemporal variability of estimated mean standing stock biomass for S. polyschides populations in both intertidal (lines and symbols) and subtidal (bold symbols only) habitats.
For subtidal populations, estimates of standing stock were greatest at BS-mod (10 132 g WW m−2) and HY-exp (9476 g WW m−2) in August, and peaked in October at MB-sh (1966 g WW m−2) (Fig. 7). On average, standing stock at HY-exp (more exposed site) was about six times greater than at MB-sh (more sheltered site). Indeed, univariate PERMANOVA detected a significant main effect of ‘site’ (Table 2), with higher standing stock values recorded at HY-exp and BS-mod compared with least exposed MB-sh. In general, standing stock estimates were lower in the sheltered/moderate sites than at the more exposed sites in the intertidal as well as in the subtidal zone (MB-sh < BS-mod < HY-exp). In June, standing stock estimates for subtidal populations were four-fold higher than those of intertidal populations, but estimates were more comparable between habitats by August and October.
Due to the pseudo-annual growth strategy of S. polyschides, maximum standing stock values can be considered proxies for annual biomass accumulation, as almost all organic matter fixed later senesces and is released into the environment. As such, estimates of biomass accumulation across both depths (mean of intertidal and subtidal measurements) were lowest at the most wave-sheltered site (3765 g WW m−2 year−1 at MB-sh) and markedly higher at the more wave-exposed sites (9970 g WW m−2 year−1 at BS-mod; 11 326 g WW m−2 yr−1 at HY-exp).
DISCUSSION
We present the first study on population demography of S. polyschides situated within the central area of the species’ range, in the Western English Channel (southwest UK). Previous research on the population dynamics of S. polyschides and their associated communities in the UK was conducted 40–60 years ago (Burrows, 1958; Norton, 1968, 1969, 1971, 1977; Norton and Burrows, 1969), towards the (former) leading range edge on the Isle of Man (54°N).
In our study, we observed marked seasonality in the density, coverage, biomass, morphology and standing stock of S. polyschides, with relatively minimal variation between sites situated along a gradient of wave exposure, and depths. Density and cover data show an expected structural development of individuals and an ‘ageing’ population through several sporophyte life stages during one growth season: progressing from juvenile to healthy adult sporophytes in spring and summer, followed by a senescing population in late autumn and winter.
Along the shores of the Western English Channel, S. polyschides usually forms mixed stands with ‘true’ Laminarian kelps. Intertidal stands are typically characterized by L. digitata and to a lesser extent Saccharina latissima and U. pinnatifida (Yesson et al., 2015a; Epstein et al., 2019), while subtidal habitats are typically dominated by L. hyperborea and, to a lesser extent, L. ochroleuca (Smale and Moore, 2017; Smale et al., 2022). Our study showed that these mixed kelp stands are, in fact, dominated by S. polyschides in summer, which provides substantial habitat and covers over 50 % of the rocky substrate. Our estimates of the standing stock of S. polyschides peaked at ~13 000 g WW m−2 in late summer. We recorded peak production of fertile sorus tissue in late summer/early autumn. In winter, no fertile tissue was recorded, and population density and standing stock were drastically reduced. Only a small component of the population remained as remnant holdfasts throughout winter, persisting until the beginning of the following recruitment season. The marked contrast from dense space-occupying S. polyschides populations in summer to collapsed, remnant populations in winter is driven by the pseudo-annual life cycle of the species as described by Norton and Burrows (1969).
We recorded some variation in population dynamics across our survey sites, which were situated along a gradient of wave exposure. It is well established that variation in wave exposure can influence kelp population demography, by affecting settlement, recruitment, sporophyte density and morphology, productivity, and fitness (Smale et al., 2011; Burrows, 2012; Pedersen et al., 2012). For example, positive responses to increasing wave exposure have been reported for Sargassum muticum in Ireland (Baer and Stengel, 2010), whereas negative responses to increasing wave exposure have been recorded for both the non-native kelp U. pinnatifida in SW England (Epstein and Smale, 2017a) and the giant kelp Macrocystis pyrifera in California (Graham et al., 1997). As such, site-level variability between populations of S. polyschides may have been driven, at least in part, by differences in wave exposure, although other abiotic and biotic factors can drive variability at this spatial scale, including competition (Epstein et al., 2019), grazing pressure (Plouguerné et al., 2006), habitat topography (Harries et al., 2007a, b) and oceanographic features (e.g. upwelling, freshwater input, turbidity) (Fernandez et al., 1988; Fernández, 2011; Pereira et al., 2015; Bermejo et al., 2016).
We recorded greater production of sorus material under sheltered rather than exposed conditions (MB-sh > BS-mod > HY-exp), whereas peak standing stock, and therefore annual productivity, was greater under exposed rather than sheltered conditions (HY-exp > BS-mod > MB-sh), in both intertidal and subtidal habitats. This suggests that wave exposure could influence growth and energy strategies, as has been shown for other kelp species (Buschmann et al., 2006; Pedersen et al., 2012). However, the causative effect of wave exposure on the production of fertile material by S. polyschides sporophytes remains speculative at this point.
We recorded a high density of late-season (October) recruits in subtidal populations, similar to observations by Norton and Burrows (1969), who recorded recruitment and the presence of mature sporophytes in subtidal populations throughout the whole year at the Isle of Man (~54°N). Reduced wave action and lower thermal stress in subtidal, compared with intertidal, habitats might favour year-round development of recruits, increase sporophyte longevity and lead to greater standing stock in the late season. For example, at the end of the peak-growth season (October), we recorded greater biomass and total length of sporophytes in subtidal compared with intertidal habitats, where sporophytes showed increased shredding of blade tissue and signs of decay. That said, the maximum recorded standing stock of S. polyschides did not differ significantly between depths.
Saccorhiza polyschides sporophytes were shorter-lived than perennial Laminaria species, and, as such, offered less stable and persistent habitat for associated species, such as epiphytes, benthic invertebrates but also small fishes (Salland and Smale, 2021). Similarly, standing stock within S. polyschides populations was strongly seasonal, with maximum values in late summer/autumn, followed by a period of release of organic matter into the environment as detritus. This intense late-season pulse of detritus release is in contrast to dominant laminarial kelp species in the northeast Atlantic, which either release a pulse of detritus in spring or release detritus more gradually through the year (Pessarrodona et al., 2018a; Gilson et al., 2023). Differences in biomass accumulation and detritus production between dominant species may have implications for local carbon cycling or supply for detrital foodwebs (Gilson et al., 2021; Guerrero-Meseguer et al., 2023). The mean standing stock of S. polyschides, across all sites and surveys, was ~2300 g WW m−2, which is about one-third of the average standing stock values reported for the dominant kelp L. hyperborea in the southwest of the UK (Smale et al., 2016, 2020). However, due to the pronounced seasonality in the productivity of S. polyschides, maximum observed standing stock values exceeded 13 000 g WW m−2. This is more than double the annual mean standing stock values reported for L. hyperborea populations in the UK.
Saccorhiza polyschides is an opportunistic kelp that is thought to be thriving and proliferating along the Western English Channel (Birchenough and Bremner, 2010; Smale et al., 2013, 2015). To date, several studies on S. polyschides have been conducted towards its southern distribution range (Iberia–Morocco), where this species can be (or has been) locally dominant and a key habitat-former (Fernández, 2011; Díez et al., 2012; Tuya et al., 2012; Voerman et al., 2013; Pereira, 2014; Assis et al., 2017; Casado-Amezúa et al., 2019). Along the coasts of Morocco and the Iberian Peninsula, population declines have been reported (Fernández, 2011; Díez et al., 2012; Assis et al., 2017; Casado-Amezúa et al., 2019), probably as a result of ocean warming leading to unfavourable thermal conditions, as well as shifts in nutrient availability related to upwelling events (Lüning, 1985; Fernandez et al., 1988; Fernández, 2011; Pereira et al., 2015). In a comparable study on seasonal dynamics of intertidal S. polyschides populations, Pereira et al. (2015) found contrasting patterns between populations from different latitudes. Populations persisting at lower latitudes (~41°N) exhibited shorter seasonal life cycles with sporophytes only present between April and September, whereas populations found at higher latitudes (~48°N) exhibited year-round presence and recruitment of sporophytes. In the current study (~50°N), we also observed the presence of sporophytes throughout the year but recruitment of sporophytes into intertidal populations exhibited seasonality, with the highest rates in spring and summer. Clearly, population dynamics differ across the distribution of S. polyschides, probably due to variation in environmental conditions and/or species interactions, and further research on patterns of spatiotemporal variability of S. polyschides populations across its wider biogeographical range in the northeast Atlantic is warranted.
CONCLUSION
Our study showed that S. polyschides is a conspicuous and seasonally dominant component of macroalgal assemblages on rocky reefs towards the centre of its range (i.e. the Western English Channel), where it probably contributes considerably to ecosystem functioning through habitat provisioning and biomass accumulation and release. We observed distinct seasonal variability, characterized by high recruitment in spring, peak sporophyte biomass and high standing stock in summer, followed by declines in density, cover and biometric measurements during winter. These patterns exhibited a reasonable level of consistency across sites and depths. By late summer, S. polyschides was a dominant habitat-former with maximum mean standing stock > 13 000 g WW m−2, providing a complex and abundant biogenic habitat for associated organisms.
Saccorhiza polyschides is a warm-tolerant, opportunistic species with high local population densities. It has probably proliferated in recent years across the southwest of the UK (Smale et al., 2013), and has extended, or is predicted to extend, its distribution polewards (Yesson et al., 2015b; Assis et al., 2017, 2020). As such, we expect this species to become increasingly dominant under projected climate change, particularly in response to ocean warming, increased storminess and other environmental changes. Our study provides a robust baseline on the population demographics of S. polyschides against which to detect future changes in the Western English Channel, although more information on interannual variability and long-term trends is needed. More generally, it offers a useful case study and approach and provides a foundation for further research on the ecological role of the species within the wider temperate reef ecosystem.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. SI2: Detailed method description/age classification of S. polyschides. SI3: Post-hoc table. SI4: Post-hoc table.
ACKNOWLEDGEMENTS
Thanks to all members of the BEECH group and volunteers for their support during fieldwork and in the lab, especially Alissa Bass, Katie Smith, Nadia Frontier, Miguel Richmond, Emma Stuart, Sophie Corrigan and Larisa Lewis. We would like to thank two anonymous reviewers for their thoughtful feedback which significantly improved the manuscript.
Contributor Information
Nora Salland, The Marine Biological Association of the United Kingdom, The Laboratory, Citadel Hill, Plymouth PL1 2PB, UK; School of Ocean and Earth Science, University of Southampton, European Way, Southampton SO14 3ZH, UK.
Catherine Wilding, The Marine Biological Association of the United Kingdom, The Laboratory, Citadel Hill, Plymouth PL1 2PB, UK.
Antony Jensen, School of Ocean and Earth Science, University of Southampton, European Way, Southampton SO14 3ZH, UK.
Dan A Smale, The Marine Biological Association of the United Kingdom, The Laboratory, Citadel Hill, Plymouth PL1 2PB, UK.
FUNDING
This work was supported by the Natural Environmental Research Council as part of the INSPIRE DTP [NE/S007210/1 to N.S.] and UK Research and Innovation Future Leaders Fellowship [MR/S032827/1 to D.S.].
REFERENCES
- Anderson MJ, Gorley RN, Clarke KR.. 2008. Permanova+ for primer: guide to software and statistical methods, Plymouth, UK: PRIMER-E Ltd. [Google Scholar]
- Araújo RM, Assis J, Aguillar R, et al. 2016. Status, trends and drivers of kelp forests in Europe: an expert assessment. Biodiversity and Conservation 25: 1319–1348. [Google Scholar]
- Arnold M, Teagle H, Brown MP, Smale DA.. 2016. The structure of biogenic habitat and epibiotic assemblages associated with the global invasive kelp Undaria pinnatifida in comparison to native macroalgae. Biological Invasions 18: 661–676. [Google Scholar]
- Assis J, Castilho Coelho N, Alberto F, et al. 2013. High and distinct range-edge genetic diversity despite local bottlenecks. PLoS One 8: e68646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis J, Berecibar E, Claro B, et al. 2017. Major shifts at the range edge of marine forests: the combined effects of climate changes and limited dispersal. Scientific Reports 7: 44348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis J, Araújo MB, Serrão EA.. 2018. Projected climate changes threaten ancient refugia of kelp forests in the North Atlantic. Global Change Biology 24: e55–e66. [DOI] [PubMed] [Google Scholar]
- Assis J, Fragkopoulou E, Frade D, et al. 2020. A fine-tuned global distribution dataset of marine forests. Scientific Data 7: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer J, Stengel DB.. 2010. Variability in growth, development and reproduction of the non-native seaweed Sargassum muticum (Phaeophyceae) on the Irish west coast. Estuarine, Coastal and Shelf Science 90: 185–194. [Google Scholar]
- Barber CA. 1889. On the structure and development of the bulb in Laminaria bulbosa, Lamour. Annals of Botany os-3: 41–64. [Google Scholar]
- Bartsch I, Wiencke C, Bischof K, et al. 2008. The genus Laminaria sensu lato: recent insights and developments. European Journal of Phycology 43: 1–86. [Google Scholar]
- Bennett S, Wernberg T, Connell SD, Hobday AJ, Johnson CR, Poloczanska ES.. 2015. The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests. Marine and Freshwater Research 67: 47–56. [Google Scholar]
- Bermejo R, De La Fuente G, Ramírez-Romero E, Vergara JJ, Hernández I.. 2016. Spatial variability and response to anthropogenic pressures of assemblages dominated by a habitat forming seaweed sensitive to pollution (northern coast of Alboran Sea). Marine Pollution Bulletin 105: 255–264. [DOI] [PubMed] [Google Scholar]
- Birchenough S, Bremner J.. 2010. Shallow and shelf subtidal habitats and ecology. MCCIP Annual Report Card 2010-11. MCCIP Science Review: Marine Climate Change Impacts Partnership. www.mccip.org.uk/arc [Google Scholar]
- Bull JC, Mason S, Wood C, Price ARG.. 2013. Benthic marine biodiversity patterns across the United Kingdom and Ireland determined from recreational diver observations: a baseline for possible species range shifts induced by climate change. Aquatic Ecosystem Health & Management 16: 20–30. [Google Scholar]
- Burrows EM. 1958. Sublittoral algal population in Port Erin Bay, Isle of Man. Journal of the Marine Biological Association of the United Kingdom 37: 687–703. [Google Scholar]
- Burrows MT. 2012. Influences of wave fetch, tidal flow and ocean colour on subtidal rocky communities. Marine Ecology Progress Series 445: 193–207. [Google Scholar]
- Buschmann AH, Moreno C, Vásquez JA, Hernández-González MC.. 2006. Reproduction strategies of Macrocystis pyrifera (Phaeophyta) in Southern Chile: the importance of population dynamics. Journal of Applied Phycology 18: 575–582. [Google Scholar]
- Casado-Amezúa P, Araújo R, Bárbara I, et al. 2019. Distributional shifts of canopy-forming seaweeds from the Atlantic coast of Southern Europe. Biodiversity and Conservation 28: 1151–1172. [Google Scholar]
- Christie H, Andersen GS, Tveiten LA, Moy FE.. 2022. Macrophytes as habitat for fish. ICES Journal of Marine Science 79: 435–444. [Google Scholar]
- Clarke KR, Gorley RN, Somerfield PJ, Warwick RM.. 2014. Change in marine communities: an approach to statistical analysis and interpretation. Plymouth: PRIMER-E. [Google Scholar]
- Cox CB, Moore PD, Ladle RJ.. 2016. Biogeography: An ecological and evolutionary approach. Chichester: John Wiley & Sons. [Google Scholar]
- Dauvin J-C. 2019. The English Channel: La manche. In: Sheppard C. ed. World Seas: an environmental evaluation. 2 edn. New York: Academic Press. [Google Scholar]
- Diehl N, Li H, Scheschonk L, et al. 2023. The sugar kelp Saccharina latissima I: recent advances in a changing climate. EcoEcoRxiv. doi: 10.32942/X2W59T. https://ecoevorxiv.org/repository/view/5680/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díez I, Muguerza N, Santolaria A, Ganzedo U, Gorostiaga JM.. 2012. Seaweed assemblage changes in the eastern Cantabrian Sea and their potential relationship to climate change. Estuarine, Coastal and Shelf Science 99: 108–120. [Google Scholar]
- Dinter WP. 2001. Biogeography of the OSPAR Maritime Area. A synopsis and synthesis of biogeographical distribution patterns described for the North-East Atlantic. Bonn: BfN Federal Agency for Nature Conservation. [Google Scholar]
- Duarte CM, Gattuso J-P, Hancke K, et al. 2022. Global estimates of the extent and production of macroalgal forests. Global Ecology and Biogeography 31: 1422–1439. [Google Scholar]
- Edgar GJ, Stuart-Smith RD, Heather FJ, et al. 2023. Continent-wide declines in shallow reef life over a decade of ocean warming. Nature 615: 858–865. [DOI] [PubMed] [Google Scholar]
- Eger AM, Marzinelli EM, Beas-Luna R, et al. 2023. The value of ecosystem services in global marine kelp forests. Nature Communications 14: 1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein G, Smale D.. 2017a. Environmental and ecological factors influencing the spillover of the non-native kelp, Undaria pinnatifida, from marinas into natural rocky reef communities. Biological Invasions 20: 1049–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein G, Smale DA.. 2017b. Undaria pinnatifida: a case study to highlight challenges in marine invasion ecology and management. Ecology and Evolution 7: 8624–8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein G, Foggo A, Smale D.. 2019. Inconspicuous impacts: widespread marine invader causes subtle but significant changes in native macroalgal assemblages. Ecosphere 10: e02814. [Google Scholar]
- Feehan CJ, Francis FTY, Scheibling RE.. 2014. Harbouring the enemy: kelp holdfasts protect juvenile sea urchins from predatory crabs. Marine Ecology Progress Series 514: 149–161. [Google Scholar]
- Fernández C. 2011. The retreat of large brown seaweeds on the north coast of Spain: the case of Saccorhiza polyschides. European Journal of Phycology 46: 352–360. [Google Scholar]
- Fernández C, Niell FX.. 1982. Zonación de fitobentos intermareal de la región de Cabo Peñas (Asturias). Investigaciones pesqueras 46: 121–141. [Google Scholar]
- Fernandez JA, Perez-Celorrio B, Ibañez M.. 1988. Sobre la presencia de Saccorhiza polyschides (Light) Batt En la costa guipuzcoana. ¿Especie indicadora de cambios climáticos? Lurralde 11: 201–216. [Google Scholar]
- Fernández C, Piñeiro-Corbeira C, Barrientos S, Barreiro R.. 2022. Could the annual Saccorhiza polyschides replace a sympatric perennial kelp (Laminaria ochroleuca) when it comes to supporting the holdfast-associated fauna? Marine Environmental Research 182: 105772. [DOI] [PubMed] [Google Scholar]
- Filbee-Dexter K, Wernberg T, Grace SP, et al. 2020. Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Scientific Reports 10: 13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisl D, Hager G, Bedessem B, et al. 2022. Citizen science in environmental and ecological sciences. Nature Reviews Methods Primers, 2: 64. [Google Scholar]
- Freiwald J, Mcmillian SM, Abbott D.. 2021. Reef Check California instruction manual. A guide to monitoring California’s kelp forests. 10 ed. Marina del Rey, CA: Reef Check California. [Google Scholar]
- Gilson AR, Smale DA, Burrows MT, O’Connor NE.. 2021. Spatio-temporal variability in the deposition of beach-cast kelp (wrack) and inter-specific differences in degradation rates. Marine Ecology Progress Series 674: 89–102. [Google Scholar]
- Gilson A, Smale DA, Burrows M, White L, O’connor N.. 2023. Seasonal and spatial variability in rates of primary production and detritus release by intertidal stands of Laminaria digitata and Saccharina latissima on wave-exposed shores in the northeast Atlantic. Ecology and Evolution 13: e10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JCD. 1983. Some notes on small kelp forest fish collected from Saccorhiza polyschides bulbs on the Isle of Cumbrae Scotland. Ophelia 22: 173–183. [Google Scholar]
- Graham MH, Harrold C, Lisin S, Light K, Watanabe JM, Foster MS.. 1997. Population dynamics of Giant Kelp Macrocystis pyrifera along a wave exposure gradient. Marine Ecology Progress Series 148: 269–279. [Google Scholar]
- Guerrero-Meseguer L, Veiga P, Rubal M.. 2023. Spatio-temporal variability of wrack along the northern Portuguese sandy beaches. Estuaries and Coasts 46: 818–828. [Google Scholar]
- Harley CDG, Randall Hughes A, Hultgren KM, et al. 2006. The impacts of climate change in coastal marine systems. Ecology Letters 9: 228–241. [DOI] [PubMed] [Google Scholar]
- Harley CDG, Anderson KM, Demes KW, et al. 2012. Effects of climate change on global seaweed communities. Journal of Phycology 48: 1064–1078. [DOI] [PubMed] [Google Scholar]
- Harries DB, Cook E, Donnan DW, Mair JM, Harrow S, Wilson JR.. 2007a. The establishment of the invasive alga Sargassum muticum on the west coast of Scotland: rapid northwards spread and identification of potential new areas for colonisation. Aquatic Invasions 2: 367–377. [Google Scholar]
- Harries DB, Harrow S, Wilson JR, Mair JM, Donnan DW.. 2007b. The establishment of the invasive alga Sargassum muticum on the west coast of Scotland: a preliminary assessment of community effects. Journal of the Marine Biological Association of the United Kingdom 87: 1057–1067. [Google Scholar]
- Hawkins SJ, Harkin E.. 1985. Preliminary canopy removal experiments in algal dominated communities low on the shore and in the shallow subtidal on the Isle of Man. Botanica Marina 28: 223. [Google Scholar]
- Hawkins SJ, Sugden HE, Mieszkowska N., et al. 2009. Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Marine Ecology Progress Series 396: 245–259. [Google Scholar]
- IPCC 2022. Climate Change 2022: Impacts, adaptation and vulnerability. In: Pörtner H-O., Roberts DC, Tignor M, et al. eds. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Jayathilake DRM, Costello MJ.. 2021. Version 2 of the world map of Laminarian kelp benefits from more arctic data and makes it the largest marine biome. Biological Conservation 257: 109099. [Google Scholar]
- Kain J. 1979. A view of the genus Laminaria. Oceanographie and Marine Biology: An Annual Review 17: 101–161. [Google Scholar]
- Kain JM, Jones NS.. 1975. Algal recolonization of some cleared subtidal areas. Journal of Ecology 63: 739–765. [Google Scholar]
- King NG, Moore PJ, Wilding C, Jenkins HL, Smale DA.. 2021. Multiscale spatial variability in epibiont assemblage structure associated with stipes of kelp Laminaria hyperborea in the northeast Atlantic. Marine Ecology Progress Series 672: 33–44. [Google Scholar]
- Koch M, Bowes G, Ross C, Zhang X-H.. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology 19: 103–132. [DOI] [PubMed] [Google Scholar]
- Lucrezi S. 2021. Characterising potential participants in kelp monitoring in the recreational diving community: a comparative study of South Africa and New Zealand. Global Ecology and Conservation 28: e01649. [Google Scholar]
- Lüning, K. 1985. Seaweeds - their Environment, Biogeography, and Ecophysiology, Chichester: John Wiley & Sons, Inc. [Google Scholar]
- Mckenzie JD, Moore PG.. 1981. The microdistribution of animals associated with the bulbous holdfasts of Saccorhiza polyschides (Phaeophyta). Ophelia 20: 201–213. [Google Scholar]
- Merzouk A, Johnson LE.. 2011. Kelp distribution in the northwest Atlantic Ocean under a changing climate. Journal of Experimental Marine Biology and Ecology 400: 90–98. [Google Scholar]
- Müller R, Laepple T, Bartsch I, Wiencke C.. 2009. Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters. Botanica Marina 52: 617. [Google Scholar]
- Norderhaug KM, Filbee-Dexter K, Freitas C, et al. 2020. Ecosystem-level effects of large-scale disturbance in kelp forests. Marine Ecology Progress Series 656: 163–180. [Google Scholar]
- Norton TA. 1968. Underwater observations on the vertical distribution of algae at St Mary’s, Isles of Scilly. British Phycological Bulletin 3: 585–588. [Google Scholar]
- Norton TA. 1969. Growth form and environment in Saccorhiza polyschides. Journal of the Marine Biological Association of the United Kingdom 49: 1025–1045. [Google Scholar]
- Norton TA. 1971. An ecological study of the fauna inhabiting the sublittoral marine alga Saccorhiza polyschides (Lightf) Batt. Hydrobiologia 37: 215–231. [Google Scholar]
- Norton TA. 1977. Experiments on the factors influencing the geographical distribution of Saccorhiza polyschides and Saccorhiza dermatodea. New Phytologist 78: 625–635. [Google Scholar]
- Norton TA, Burrows EM.. 1969. Studies on marine algae of the British Isles. 7. Saccorhiza polyschides (Lightf) Batt. British Phycological Journal 4: 19–53. [Google Scholar]
- Pedersen MF, Nejrup LB, Fredriksen S, Christie H, Norderhaug KM.. 2012. Effects of wave exposure on population structure, demography, biomass and productivity of the kelp Laminaria hyperborea. Marine Ecology Progress Series 451: 45–60. [Google Scholar]
- Pereira, T. 2014. Ecology of west Iberian kelps: Laminaria ochroleuca and Saccorhiza polyschides living near their distributional limits. PhD Thesis, Universidade do Algarve. [Google Scholar]
- Pereira TR, Engelen AH, Pearson GA, Valero M, Serrão EA.. 2015. Contrasting timing of life stages across latitudes – a case study of a marine forest-forming species. European Journal of Phycology 50: 361–369. [Google Scholar]
- Pessarrodona A, Foggo A, Smale DA.. 2018a. Can ecosystem functioning be maintained despite climate-driven shifts in species composition? Insights from novel marine forests. Journal of Ecology 107: 91–104. [Google Scholar]
- Pessarrodona A, Moore PJ, Sayer MDJ, Smale DA.. 2018b. Carbon assimilation and transfer through kelp forests in the NE Atlantic is diminished under a warmer ocean climate. Global Change Biology 24: 4386–4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessarrodona A, Filbee-Dexter K, Alcoverro T, et al. 2021. Homogenization and miniaturization of habitat structure in temperate marine forests. Global Change Biology 27: 5262–5275. [DOI] [PubMed] [Google Scholar]
- Pessarrodona A, Assis J, Filbee-Dexter K, et al. 2022a. Global seaweed productivity. Science Advances 8: eabn2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessarrodona A, Vergés A, Bosch NE, et al. 2022b. Tropicalization unlocks novel trophic pathways and enhances secondary productivity in temperate reefs. Functional Ecology 36: 659–673. [Google Scholar]
- Plouguerné E, Le Lann K, Connan S, Jechoux G, Deslandes E, Stiger-Pouvreau V.. 2006. Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalga Sargassum muticum (Yendo) Fensholt along the coast of Western Brittany (France). Aquatic Botany 85: 337–344. [Google Scholar]
- R Core Team 2020. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Reshitnyk L, Saccomanno V, Bell TW, Cavanaugh KC.. 2023. Mapping canopy-forming kelps in the northeast Pacific: a guidebook for decision-makers and practitioners. Heriot Bay, BC: The Hakai Institute. [Google Scholar]
- Salland N, Smale D.. 2021. Spatial variation in the structure of overwintering, remnant Saccorhiza polyschides sporophytes and their associated assemblages. Journal of the Marine Biological Association of the United Kingdom 101: 639–648. [Google Scholar]
- Sandahl A, Tøttrup AP.. 2020. Marine citizen science: recent developments and future recommendations. Citizen Science: Theory and Practice 5: 24. [Google Scholar]
- Sauvageau C. 1915. Sur le développement et la biologie d’une Laminaire (Saccorhiza bulbosa). Comptes rendus hebdomadaires des séances de l’Académie des Sciences, 160: 445–448. [Google Scholar]
- Schiel DR, Foster MS.. 2015. The biology and ecology of Giant Kelp forests. Los Angeles: University of California Press. [Google Scholar]
- Schoenrock KM, O’callaghan T, O’callaghan R, Krueger-Hadfield SA.. 2019. First record of Laminaria ochroleuca Bachelot de la Pylaie in Ireland in Béal an Mhuirthead, county Mayo. Marine Biodiversity Records 12: 9. [Google Scholar]
- Seymour RJ, Tegner MJ, Dayton PK, Parnell PE.. 1989. Storm wave induced mortality of giant kelp, Macrocystis pyrifera, in Southern California. Estuarine, Coastal and Shelf Science 28: 277–292. [Google Scholar]
- Simkanin C, Power AM, Myers A, et al. 2005. Using historical data to detect temporal changes in the abundances of intertidal species on Irish shores. Journal of the Marine Biological Association of the United Kingdom 85: 1329–1340. [Google Scholar]
- Smale DA. 2020. Impacts of ocean warming on kelp forest ecosystems. The New Phytologist 225: 1447–1454. [DOI] [PubMed] [Google Scholar]
- Smale DA, Moore PJ.. 2017. Variability in kelp forest structure along a latitudinal gradient in ocean temperature. Journal of Experimental Marine Biology and Ecology 486: 255–264. [Google Scholar]
- Smale DA, Vance T.. 2015. Climate-driven shifts in species’ distributions may exacerbate the impacts of storm disturbances on North-east Atlantic kelp forests. Marine and Freshwater Research 67: 65–74. [Google Scholar]
- Smale DA, Wernberg T, Vance T.. 2011. Community development on subtidal temperate reefs: the influences of wave energy and the stochastic recruitment of a dominant kelp. Marine Biology 158: 1757–1766. [Google Scholar]
- Smale DA, Burrows MT, Moore P, O’connor N, Hawkins SJ.. 2013. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecology and Evolution 3: 4016–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale DA, Wernberg T, Yunnie ALE, Vance T.. 2015. The rise of Laminaria ochroleuca in the Western English Channel (UK) and comparisons with its competitor and assemblage dominant Laminaria hyperborea. Marine Ecology 36: 1033–1044. [Google Scholar]
- Smale DA, Burrows MT, Evans AJ, et al. 2016. Linking environmental variables with regional-scale variability in ecological structure and standing stock of carbon within UK kelp forests. Marine Ecology Progress Series 542: 79–95. [Google Scholar]
- Smale DA, Pessarrodona A, King N, et al. 2020. Environmental factors influencing primary productivity of the forest-forming kelp Laminaria hyperborea in the northeast Atlantic. Scientific Reports 10: 12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale DA, Pessarrodona A, King N, Moore PJ.. 2021. Examining the production, export, and immediate fate of kelp detritus on open-coast subtidal reefs in the Northeast Atlantic. Limnology and Oceanography 67: S36–S49. doi: 10.1002/lno.11970 [DOI] [Google Scholar]
- Smale DA, Teagle H, Hawkins SJ, et al. 2022. Climate-driven substitution of foundation species causes breakdown of a facilitation cascade with potential implications for higher trophic levels. Journal of Ecology 110: 2132–2144. [Google Scholar]
- Smith KE, Burrows MT, Hobday AJ, et al. 2021a. Socioeconomic impacts of marine heatwaves: global issues and opportunities. Science 374: eabj3593. [DOI] [PubMed] [Google Scholar]
- Smith KE, Moore PJ, King NG, Smale DA.. 2021b. Examining the influence of regional-scale variability in temperature and light availability on the depth distribution of subtidal kelp forests. Limnology and Oceanography 67: 314–328. [Google Scholar]
- Southward AJ, Langmead O, Hardman-Mountford NJ, et al. 2005. Long-term oceanographic and ecological research in the Western English Channel. Advances in Marine Biology 47: 1–105. [DOI] [PubMed] [Google Scholar]
- Steneck RS, Graham MH, Bourque BJ, et al. 2002. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation 29: 436–459. [Google Scholar]
- Teagle H, Smale DA.. 2018. Climate-driven substitution of habitat-forming species leads to reduced biodiversity within a temperate marine community. Diversity and Distributions 24: 1367–1380. [Google Scholar]
- Teagle H, Hawkins SJ, Moore PJ, Smale DA.. 2017. The role of kelp species as biogenic habitat formers in coastal marine ecosystems. Journal of Experimental Marine Biology and Ecology 492: 81–98. [Google Scholar]
- Teagle H, Moore PJ, Jenkins H, Smale DA.. 2018. Spatial variability in the diversity and structure of faunal assemblages associated with kelp holdfasts (Laminaria hyperborea) in the northeast Atlantic. PLoS One 13: e0200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel M, Vásquez JA.. Are kelp holdfasts islands on the ocean floor? — Indication for temporarily closed aggregations of peracarid crustaceans. In: Jones MB, Azevedo JM, Neto AI, Costa AC, Martins AMF, eds. Island, ocean and deep-sea biology. Dordrecht: Springer Netherlands, 2000, 45–54. [Google Scholar]
- Thompson, M. 2021. MaPP kelp monitoring protocol. In: Marine Plan Partnership for the North Pacific Coast (MAPP), M. P. P. F. T. N. P. C. (ed.).
- Tuya F, Cacabelos E, Duarte P, et al. 2012. Patterns of landscape and assemblage structure along a latitudinal gradient in ocean climate. Marine Ecology Progress Series 466: 9–19. [Google Scholar]
- United Nations (UN) Environment Programme. 2023. Into the Blue: securing a sustainable future for kelp forests. Nairobi: UN Environment Programme. [Google Scholar]
- Vergés A, Steinberg PD, Hay ME, et al. 2014. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proceedings of the Royal Society B: Biological Sciences 281: 20140846. 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voerman SE, Llera E, Rico JM.. 2013. Climate driven changes in subtidal kelp forest communities in NW Spain. Marine Environmental Research 90: 119–127. [DOI] [PubMed] [Google Scholar]
- Yesson C, Bush LE, Davies AJ, Maggs CA, Brodie J.. 2015a. The distribution and environmental requirements of large brown seaweeds in the British Isles. Journal of the Marine Biological Association of the United Kingdom 95: 669–680. [Google Scholar]
- Yesson C, Bush LE, Davies AJ, Maggs CA, Brodie J.. 2015b. Large brown seaweeds of the British Isles: evidence of changes in abundance over four decades. Estuarine, Coastal and Shelf Science 155: 167–175. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


