Abstract
Objective.
Several advanced therapies have been licensed across the related conditions of psoriatic arthritis (PsA), Crohn disease (CD), ulcerative colitis (UC), and noninfectious uveitis. We sought to summarize results from randomized controlled trials (RCTs) investigating the efficacy and safety of advanced therapies for these related conditions in patients with PsA.
Methods.
We updated the previous systematic search conducted in 2013 with literature reviews of MEDLINE, Embase, and the Cochrane Library (from February 2013 to August 2020) on this subject; only those new studies are presented here. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework.
Results.
The number of RCTs meeting eligibility criteria were 12 for CD, 15 for UC, and 5 for uveitis. The tumor necrosis factor inhibitor (TNFi) class appears to be efficacious and safe across CD, UC, and uveitis, with the exception of etanercept. Interleukin 12/23 inhibitors (IL-12/23i) are efficacious for CD and UC. Phase II and III RCTs of Janus kinase inhibitors ( JAKi) and IL-23i in CD and UC are promising in terms of efficacy and safety. IL-17i must be used with great caution in patients with PsA at high risk of inflammatory bowel disease (IBD). RCTs in uveitis have mainly studied adalimumab.
Conclusion.
We have identified 32 recent RCTs in IBD and uveitis and updated recommendations for managing patients with PsA and these related conditions. A multispecialty approach is essential to effectively, safely, and holistically manage such patients. Advanced therapies are not equally efficacious across these related conditions, with dosing regimens and safety varying.
Keywords: GRAPPA, psoriasis, psoriatic arthritis
Psoriatic arthritis (PsA) is known to have a shared pathogenesis with inflammatory bowel diseases (IBD), such as Crohn disease (CD), ulcerative colitis (UC), and IBD-unclassified (IBD-U), and different forms of inflammatory eye disease. The evidence for this is derived from epidemiological and genetic studies showing shared heritability and familial clustering.1–4
Cohort studies and metaanalyses estimate a lifetime risk of incident IBD in patients with spondyloarthritis (SpA) to be 4% to 14%, and perhaps higher in axial compared with peripheral SpA.1,3,4 Macroscopic intestinal inflammation is estimated to affect 30–44%2,5 and microscopic inflammation 46–66%2,6 of patients with SpA in general, but especially those with axial predominant SpA (axSpA).
Uveitis is characterized by inflammation of the uvea and is anatomically classified into anterior, intermediate, posterior, and panuveitic eye inflammation types. Approximately 30% to 40% of patients with uveitis have an associated immune-mediated inflammatory disease (IMID),7,8 while other infectious etiologies (viral, fungal, or bacterial) or injuries exist. A large number of uveitis cases do not fit into any well-defined diagnostic category and are labeled idiopathic. One of the differences between PsA and axSpA is that in PsA, the acute anterior form of uveitis is less common.9 IMID has therefore been proposed as a more precise term for these and other overlapping conditions.10
Our objectives were to summarize results from recent RCTs in patients with IBD and/or uveitis and investigate the efficacy and safety of advanced therapies, which have also been tested in patients with PsA, to inform treatment choices in patients with PsA.
METHODS
Literature search.
A systematic search was conducted in 2013 to inform the 2014 GRAPPA treatment recommendations for PsA.11 We conducted an update of the 2013 systematic review to inform the 2021 update of the GRAPPA treatment recommendations regarding related conditions.12 These related conditions included CD, UC, and uveitis (including noninfectious etiologies of acute and chronic anterior uveitis, posterior uveitis, and panuveitis). In the present paper, we present only the results of studies published since February 2013 until August 2020.
Inclusion/exclusion criteria.
We sought to identify RCTs in patients with IBD or uveitis who were treated with pharmaceutical drugs recognized as treatments for PsA and that had a placebo comparator arm. Eligibility criteria are detailed in Table 1. Comprehensive searches were conducted of 3 bibliographic databases (MEDLINE, Embase, and the Cochrane Library; see Supplementary Table 1 for MEDLINE search, available with the online version of this article) from February 19, 2013, to August 28, 2020. Open-label extension (OLE) and long-term extension (LTE) studies meeting eligibility criteria, but found to no longer have a control treatment arm, were excluded from further analysis.
Table 1.
Eligibility criteria for searches of RCTs in patients with IBD or uveitis treated with pharmaceutical drugs recognized as treatments for PsA.
| Factor | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
|
| ||
| Study design | RCTs of any design (individual or cluster randomization, step-wedge design). Secondary, post hoc, and subgroup analyses of individual RCTs. Extension studies of RCTs. |
Secondary evidence (ie, systematic reviews, guidelines/ recommendations, evidence-based synopses). Quantitative primary studies that are not RCTs (eg, nonrandomized controlled studies, before-and-after studies, cohort studies, case studies, case series). Qualitative studies. Conference abstracts. Editorials, commentaries, trial protocols, letters, etc. |
| Participants and conditions of interest | Adult populations (age ≥ 18 yrs; if mixed ages, include if results for adults are reported separately or if most participants are adults). Diagnosis of IBD (UC, CD, subclinical colitis) or uveitis. | Pediatric only focus (< 18 yrs). |
| Interventions or exposures | Any DMARD, targeted synthetic or biologic drug or combination used in the treatment of PsA (oral, injection, IV). | Nonpharmacological interventions. NSAIDs only. Steroids only. |
| Comparisons or control groups | Any comparator (active, sham, PBO). | NA. |
| Outcomes of interest | Symptoms and signs including outcomes relating to disease activity and impact. Disease progression. Safety outcomes (AEs, side effects). Any length of follow-up will be considered. |
|
| Setting | Any. | |
AE: adverse event; CD: Crohn disease; DMARD: disease-modifying antirheumatic drug; IBD: inflammatory bowel disease; IV: intravenous; NA: not applicable; NSAID: nonsteroidal antiinflammatory drug; PBO: placebo; PsA: psoriatic arthritis; RCT: randomized controlled trial; UC: ulcerative colitis.
Data extraction.
Unique article titles and abstracts were screened by a single coauthor against predefined eligibility criteria (Table 1). Full-text articles of those remaining were independently assessed by pairs of coauthors (formed based upon volunteering for this duty) for eligibility, with a third reviewer (DRJ or MEH) consulted in the case of disagreements. No significant disagreements were encountered. Included studies underwent data extraction and assessment for risk of bias using the Cochrane risk of bias tool by 1 coauthor and were independently checked by a second coauthor (NC), with a third reviewer (DRJ or MEH) consulted in the case of disagreements.13 No significant disagreements were encountered.
GRADE rating.
Each eligible trial was assessed using the GRADE-level assessment of quality of evidence.14 Several coauthor group meetings were undertaken to reach a consensus on recommendation for (strong/weak), recommendation against (strong/weak), or no recommendation (no, insufficient, or conflicting evidence) for each agent. The GRADE recommendations were made based on prior reviews and the updated RCTs.12
Ethics.
This paper does not require ethical or institutional review board approval.
RESULTS
We screened 311 full-text articles and reviewed 72 potential RCTs (Figure); 40 were excluded because of lack of controls or missing outcome data. We included 32 eligible RCTs for review: 12 RCTs for CD (Table 2), 15 RCTs for UC (Table 3), and 5 RCTs for uveitis (Table 4).
Figure.
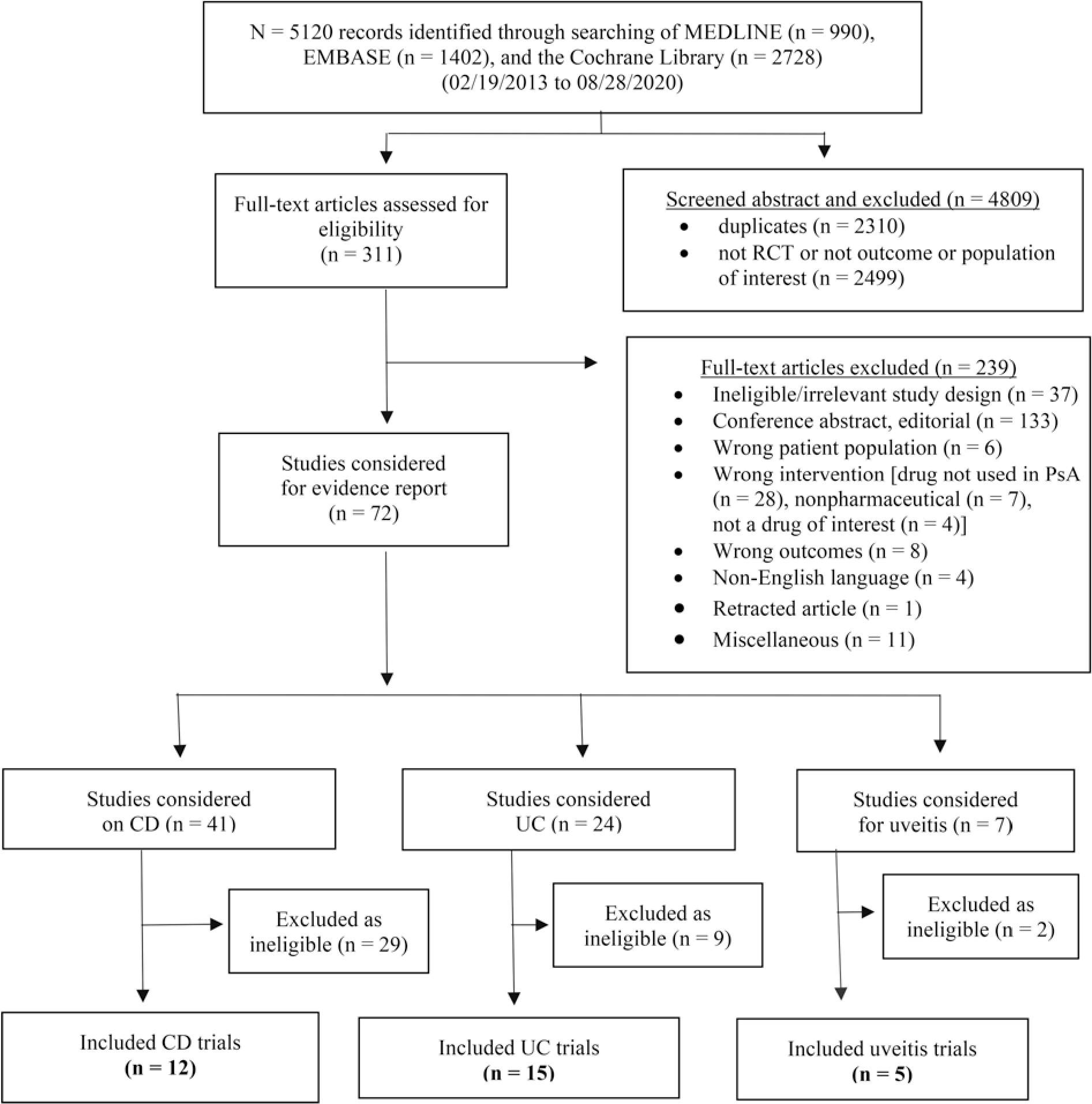
Flowchart of the study selection process for RCTs of CD, UC, and uveitis with treatments used in PsA. CD: Crohn disease; PsA: psoriatic arthritis; RCT: randomized controlled trial; UC: ulcerative colitis.
Table 2.
Advanced therapy PBO-controlled RCTs in Crohn disease. Efficacy and safety results for new studies from February 2013 to August 2020
| Medication Class vs PBO | Author, Year | N | (1) Primary and (2) Key Secondary Endpoints | Endpoints Met | Study Limitations |
|---|---|---|---|---|---|
|
| |||||
| IFX | Regueiro 201615 | 297 | (1) Clinical recurrence at wk 76 (2) Endoscopic recurrence at wk 76 (2) Clinical recurrence at wk 104 (2) CDAI change at wk 104 |
IFX = PBO IFX > PBO IFX = PBO IFX = PBO |
Entry restricted to CD cases who had had ileocolonic resection with ileocolonic anastomosis. Moderate-high risk of bias as domains poorly reported in the study. |
| UST | Feagan 201616 | 761 | (1) CDAI-100p or CDAI < 150 at wk 6 (2) CDAI remission at wk 8 (2) CDAI-70p at wk 8 (2) CRP decrease at wk 8 (2) Calprotectin decrease at wk 6 |
Both UST > PBO (UST 130 mg = UST 6 mg/kg) Both UST > PBO Both UST > PBO Both UST > PBO Both UST > PBO |
- |
| Sands 201817 | 761 | (2) IBDQ-MCID at wk 8 (2) SF-36 PCS at wk 8 (2) SF-36 MCS at wk 8 |
Both UST > PBO No difference UST 6 mg/kg > PBO |
A paper focused on PROMs. | |
| Feagan 201615 | 397 | (1) CDAI < 150 at wk 44 (2) CDAI-70p at wk 44 (especially if remission after induction) (2) IBDQ decrease at wk 44 (2) IBDQ-MCID at wk 44 |
Both UST > PBO (UST Q8W = UST Q12W) Both UST > PBO UST Q8W = UST Q12W Both UST > PBO (UST Q8W = UST Q12W) UST Q8W > PBO |
TNFi-IR mandated; otherwise, low risk of bias in the study. | |
| Sands 201816 | 397 | (2) SF-36 PCS-MCID at wk 44 (2) SF-36 MCS-MCID at wk 44 |
UST Q8W > PBO Both UST > PBO |
A paper focused on PROMs. | |
| RZB | Feagan 201718 | 121 | (1) CDAI ≤ 150 at wk 12 (2) CDAI-100p at wk 12 (2) CDEIS ≤ 4 at wk 12 (2) IBDQ at wk 12 (2) HRQOL at wk 12 |
600 mg > PBO (not 200 mg) 600 mg > PBO (not 200 mg) 600 mg > PBO (not 200 mg) 600 mg > PBO (not 200 mg) 600 mg > PBO (not 200 mg) |
Low risk of bias in the study. |
| MEDI2070 | Sands 201719 | 121 | CDAI-100p at wk 8 (1) CDAI ≤ 150 at wk 8 CRP decrease Calprotectin decrease |
700 > PBO No difference 700 > PBO 700 > PBO |
TNFi-IR mandated; otherwise, low risk of bias in the study. |
| BRO | Targan 201620 | 130 | (1) CDAI ≤ 150 at wk 6 (2) CDAI-100p at wk 6 (2) CDAI-Δ at wk 6 (2) CRP (2) Calprotectin |
PBO > all BRO groups No difference No difference No difference No difference |
Several exclusions compared with other studies. Therefore, likely milder severity CD cohort than in clinical practice, consequently contributing to the risk of bias in the study. |
| PF-04236921 | Danese 201921 | 247 | (1) CDAI-70p at wk 12 (2) CDAI remission (2) CRP decrease (2) Calprotectin decrease (2) IBDQ score (2) Change in EQ-5D |
50 mg > PBO 50 mg > PBO All doses > PBO No difference No difference No difference |
TNFi-IR mandated; otherwise, low risk of bias in the study. |
| TOF | Sandborn 201422 | 139 | (1) CDAI-70p at wk 4 (2) CDAI-100p at wk 4 (2) CRP decrease at wk 4 (2) Calprotectin decrease at wk 4 (2) IBDQ-10 at wk 4 |
No difference No difference 15 mg > PBO 15 mg > PBO No difference |
Moderate risk of bias: high screening fail rate (41%); very high PBO response rate leading to inadvertent selection bias. |
| Panes 201723 | 180 | (1) CDAI-100p or CDAI < 150 at wk 24 (2) CRP decrease at wk 24 |
No difference (either dose) TOF 10 > PBO |
– | |
| FILGO | Vermeire 201724 | 174 | (1) CDAI at wk 10 (2) Histopathology (2) SES-CD (2) IBDQ-QoL (2) PRO2 |
FILGO 200 > PBO FILGO 200 > PBO No difference FILGO 200 > PBO FILGO 200 > PBO |
Low risk of bias. |
| UPA | Mohamed 202025 | 220 | (1) Dose-response at wk 16 (1) Safety |
Dose-related response, especially 24 mg QID > PBO No dose-safety association observed |
High attrition of 27% in PBO compared with UPA groups, contributing to risk of bias in the study. |
| Sandborn 202026 | 220 | (1) Clinical remission at wk 16 (1) Endoscopic remission at wk 12/16 |
All UPA doses = PBO Higher UPA doses > PBO |
||
BRO: brodalumab; CD: Crohn disease; CDAI: Crohn’s Disease Activity Index; CDEIS: Crohn’s Disease Endoscopic Index of Severity; CRP: C-reactive protein; EQ-5D: EuroQol 5-dimension questionnaire; FILGO: filgotinib; HRQOL: health-related quality of life; IBDQ-10: 10-item Inflammatory Bowel Disease Questionnaire; IBDQ-MCID: Inflammatory Bowel Disease Questionnaire minimal clinically important difference; IBDQ-QoL: Inflammatory Bowel Disease Questionnaire quality of life; IFX: infliximab; MCS: mental component summary; NR: not reported; p: point; PBO: placebo; PCS: physical component summary; PRO2: patient-reported composite score; PROM: patient-reported outcome measure; Q12W: every 12 weeks; Q8W: every 8 weeks; QID: 4 times daily; RZB: risankizumab; SES-CD: Simplified Endoscopy Score for Crohn’s Disease; SF-36: 36-item Short Form Health Survey; TNFi-IR: inadequate response to tumor necrosis factor inhibitor; TOF: tofacitinib; UPA: upadacitinib; UST: ustekinumab.
Table 3.
Advanced therapy PBO-controlled RCTs in UC. Efficacy and safety for new studies from February 2013 to August 2020.
| Medication Class vs PBO | Author, Year | N | (1) Primary and (2) Key Secondary Endpoints |
Endpoints Met | Study Limitations and Population |
|---|---|---|---|---|---|
|
| |||||
| ADA | Suzuki 201427 | 273 | (1) Clinical response, mucosal healing, and remission at wks 8 and 52 | Induction with ADA 160/80 mg led to early response and mucosal healing. Maintenance ADA had greater rates of long-term response (31%), remission (23%), and mucosal healing (29%) vs PBO. |
Efficacy and safety of ADA in Japanese patients with moderately to severely active UC. ADA 80/40 (80 mg at wk 0 then 40 mg every other wk) vs ADA 160/80 (160/80 mg at wk 0/2 then 40 mg every other wk) vs PBO. |
| Reinisch 201328 | 576 | (1) Clinical remission, clinical response, and mucosal healing at wk 52 for ITT-A3 and ITT-E groups | Rates of remission, response, and healing similar for both groups. ADA effective for maintaining clinical remission. | 52-week efficacy of ADA in patients with moderately to severely active UC who failed CS and/or immunosuppressants. Results of 52 wk open-label follow-up of patients with moderate to severe UC who participated in ULTRA 1. ITT-A3 is ITT amended protocol. Originally 2 arms, now 3 arms: ADA 160/80 mg (160 mg at wk 0, 80 mg at wk 2) and 40 mg at wks 4 and 6, vs ADA 80/40 (80 mg at wk 0, 40 mg at wk 2, 4, and 6), vs PBO. ITT-E is any version of protocol. Patients who received ≥ 1 injection of study drug enrolled at any time. |
|
| Sandborn 201329 | ULTRA 2: 248 | (1) Clinical response, remission, and mucosal healing at wk 52 (2) Steroid-free remission and steroid discontinuation rates |
49.6% achieved clinical response, 30.9% clinical remission, and 43.1% mucosal healing at wk 52. 21.1% achieved steroid-free remission and 37.8% were steroid-free. |
1-yr maintenance outcomes among patients with moderately to severely active UC who responded to induction therapy with ADA: subgroup analyses from ULTRA 2. | |
| Colombel 201430 | ULTRA 1: 600 ULTRA 2: 1094 |
(1) Remission, mucosal healing, and improved QOL assessed in ULTRA 1 and 2 up to wk 208 (2) Maintenance of remission and mucosal healing in ULTRA 3 |
ADA more effective than PBO in maintaining remission rates, mucosal healing, and improved QOL up to 4 yrs. | 4-yr maintenance treatment with ADA in patients with moderately to severely active UC: data from ULTRA 1, 2, and 3. | |
| GOL | Gibson 201631 | 1240 | (1) Assess safety and maintenance of efficacy from end of main study through the first year | Patients on SC GOL every 4 wks through 2 yrs maintained clinical benefits and reduced CS use. No new safety signals observed. |
Maintenance of efficacy and continuing safety of GOL for active UC: PURSUIT-SC maintenance study extension through 1 yr. |
| Hibi 201732 | 144 | (1) Clinical response through maintenance at wk 54 (2) Clinical remission and mucosal healing at maintenance wk 30 and 54 |
Patients on SC GOL maintained clinical response at wk 54 (56.3%) vs PBO (19.4%). At wk 30 and 54, 50% achieved clinical remission vs PBO (6.5%), and 59.4% experienced mucosal healing vs PBO (16.1%). |
Efficacy and safety of GOL 52-week maintenance therapy in Japanese patients with moderate to severely active UC: a phase III, double-blind, randomized, PBO-controlled study (PURSUIT-J study). Induction phase was 200 mg at wk 0 and 100 mg at wk 2 through 6 wks. Then entered maintenance phase at 100 mg vs PBO every 4 wks for 52 wks. | |
| Sandborn 201433 | 1064 | (1) Phase III endpoint clinical response at wk 6 (2) Clinical remission, mucosal healing, and change in IBDQ scores |
Rates of clinical response at wk 6 were 51.0% and 54.9% for patients given 200 mg/100 mg and 400 mg/ 200 mg GOL vs 30.3% PBO. Rates of remission, healing, and change in IBDQ greater for both GOL groups vs PBO (P < 0.05). |
SC GOL induces clinical response and remission in patients with moderate to severe UC. PURSUIT-SC study Phase II: dose-finding to evaluate dose-response relationship and select IV GOL induction regimens for further evaluation. Phase III: dose-confirming to evaluate safety and efficacy of selected regimens. Phase II: 1:1:1:1 at GOL doses 100/50 mg, 200/100 mg or 400/200 mg. After phase II dose-finding data analyses, 200/100 mg and 400/200 mg doses selected for further evaluation. In phase III: 1:1:1. |
|
| Sandborn 201434 | 464 | (1) Clinical response/remission at wk 54 (2) Clinical remission and mucosal healing at wk 30 and 54 |
Clinical remission and had mucosal healing (27.8% and 42.4%) than patients given PBO (15.6% and 26.6%; P = 0.004 and P = 0.002, respectively) or 50 mg GOL (23.2% and 41.7%, respectively). | Not powered to detect a statistical difference between the GOL and PBO groups for clinical remission. | |
| Rutgeerts 201535 | 291 | (1) Dose-response relationship (2) Clinical remission and mucosal healing |
No dose-response was observed in Phase II. Efficacy with single-dose GOL IV induction was lower than expected. No difference between receiving GOL vs PBO. |
RCT: a PBO-controlled study of IV GOL induction therapy for UC. PURSUIT-IV study: Phase II: 1:1:1:1 at 1, 2, or 4 mg/kg Phase III: 1:1:1 at 2 or 4 mg/kg |
|
| MTX | Carbonnel 201536 | 111 | (1) Steroid-free remission at wk 16 (2) Clinical remission and endoscopic healing without steroids at wk 16 and/or wk 24 |
MTX not superior to PBO. No difference. |
MTX is not superior to PBO in inducing steroid-free remission but induces steroid-free clinical remission in a larger proportion of patients with UC. |
| Herfarth 201837 | 179 | (1) Patients who remained relapse free and in remission at wk 48 without use of steroids/other medication | MTX not superior to PBO in preventing relapses, maintaining of steroid-free response, or remission in UC. | ||
| APR | Danese 202038 | 170 | (1) Clinical remission at wk 12 (defined by total Mayo score < 2) | Not met. 30 mg = 31.6% 40 mg = 21.8% PBO = 12.1% |
APR: 30 mg (n = 57) APR: 40 mg (n = 55) PBO: (n = 58) |
| TOF | Panes 201539 | 194 | (1) Effect of TOF on PROs (IBDQ) and (IBD PRITI) at wk 8 | IBDQ score: improvement significantly greater for TOF 15 mg BID vs PBO. On IBD PRITI, most patients reported satisfaction for 15 mg BID. |
0.5 mg or 3 mg or 10 mg or 15 mg or PBO BID. |
| Sandborn 201740 | 1 and 2: 598 and 541 Sustain: 593 |
(1) OCTAVE Induction 1 and 2: Remission at wk 8 (2) OCTAVE Sustain: Remission at wk 52 |
OCTAVE 1: remission in 18.5% patients vs 8.2% PBO OCTAVE 2: remission in 16.6% patients vs 3.6% PBO Remission 34.3% for 5 mg patients and 40.6% for 10 mg vs 11.1% PBO |
TOF as induction and maintenance therapy for UC. 3 phase III trials: OCTAVE Induction 1 and 2, OCTAVE Sustain. OCTAVE Induction 1 and 2: 10 mg BID vs PBO for 8 wks. OCTAVE Sustain: 5 or 10 mg vs PBO for 52 wks. |
|
| UST | Sands 201941 | Induction: 961 Maintenance: 523 |
(1) Clinical remission at wk 8 (Induction) (2) Clinical remission at wk 44 (Maintenance) |
Remission at wk 8 higher for patients who received 130 mg (15.6%) or 6 mg/kg (15.5%) than PBO (5.3%). Remission at wk 44 higher for patients given 90 mg every 12 wks (38.4%) or every 8 wks (43.8%) than PBO (24%). |
UST as induction and maintenance therapy for UC. 8-wk induction trial: 130 mg IV vs weight-range-based dose (6 mg/kg) vs PBO. 44-wk maintenance trial: 90 mg every 12 wks or 8 wks vs PBO. |
ADA: adalimumab; APR: apremilast; BID: 2 times daily; CS: corticosteroid; CUCQ: Crohn’s and Ulcerative Colitis Questionnaire; GOL: golimumab; IBD PRITI: Inflammatory Bowel Disease Patient-Reported Treatment Impact survey; IBDQ: Inflammatory Bowel Disease Questionnaire; ITT: intent-to-treat analysis; IV: intravenous; MTX: methotrexate; OCTAVE: Oral Clinical Trials for Tofacitinib in Ulcerative Colitis; PBO: placebo; PRO: patient-reported outcome; QOL: quality of life; SC: subcutaneous; TOF: tofacitinib; UC: ulcerative colitis; ULTRA: Ulcerative Colitis Long-Term Remission and Maintenance with Adalimumab; UST: ustekinumab.
Table 4.
Advanced therapy PBO-controlled RCTs in noninfectious uveitis. Efficacy and safety in new studies from February 2013 to August 2020.
| Medication Class vs PBO | Author, Year | n | (1) Primary and (2) Key Secondary Endpoints |
Endpoints Met | Study Limitations and Population |
|---|---|---|---|---|---|
|
| |||||
| ADA | Jaffe 201642 | 117 | (1) Time to treatment failure occurring at or after wk 6 (2) Change in anterior chamber cell grade, vitreous haze grade, and BCVA (2) AEs and SAEs |
Patients on ADA less likely to have treatment failure than PBO (24 wks vs 13 wks). Change better in ADA group than PBO. More AEs and SAEs for ADA vs PBO. |
Patients with active noninfectious uveitis. Patients assigned to receive ADA (a loading dose of 80 mg followed by a dose of 40 mg every 2 wks) or matched PBO. All patients received a mandatory prednisone burst followed by tapering of prednisone over the course of 15 wks. |
| Nguyen 201643 | 226 | (1) Time to treatment failure (2) Risk of uveitis flare and loss of visual acuity |
Treatment failure in 39% patients in the ADA group vs 55% patients in the PBO group Time to treatment failure: ADA > 18 months vs 8.3 months in PBO. ADA significantly lowered risk of uveitic flare or loss of visual acuity. |
Patients with inactive noninfectious uveitis controlled by corticosteroids (VISUAL II). ADA: n = 115 Control: n = 111 |
|
| Mackensen 201844 | 25 | (1) Improved BCVA (> 2 lines) at 3 months | ADA superior over PBO in severe ocular inflammation. | Patients with different forms of refractory uveitis. ADA: n = 10 Control: n = 15 |
|
| SEC | Letko 201545 | 37 | (1) % of patients with treatment response (2) % of patients with remission |
30 mg/kg + 10 mg/kg produced higher response and remission rates than 300 mg + 30 mg/kg IV dose. Statistically and clinically superior to 300 mg SC dose. | Patients with noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. SEC 300 mg SC vs 30 mg/kg IV vs 10 mg/kg IV vs saline IV/SC (PBO). |
| MTX vs MMFa | Niemeyer 201746 | 80 | (1) Treatment success (BCVA) (2) QOL (IND-VFQ, SF-36) |
No significant difference between 2 arms for change in BCVA. Significant overall improvement in visual acuity and function in patients for both arms. However, mental health score of SF-36 decreased. |
Patients with intermediate, posterior, and panuveitis. |
No PBO arm. ADA: adalimumab; AE: adverse event; BCVA: best corrected visual acuity; IND-VFQ: Indian Vision Function Questionnaire; IV: intravenous; MMF: myco- phenolate mofetil; MTX: methotrexate; PBO: placebo; QOL: quality of life; RCT: randomized controlled trials; SAE: serious adverse events; SC: subcutaneous; SEC: secukinumab; SF-36: 36-item Short Form Health Survey.
RCTs of CD.
Twelve RCTs met eligibility criteria for final reporting, as shown in Table 2.15–26 Since 2013, no new primary studies comparing adalimumab (ADA) or golimumab (GOL) with placebo have been published. Several treatments had OLE or LTE studies without a placebo arm and were excluded. No study reported if the subjects had concomitant PsA, SpA, inflammatory arthritis, psoriasis, or uveitis.
TNFi.
The PREVENT RCT15 studied 297 biologic-experienced cases with ileocolonic resection and anastomosis (Table 2). Participants randomized to infliximab (IFX) vs placebo were no more likely to attain the study’s primary endpoint of no clinical recurrence at week 76, nor was efficacy found for most secondary endpoints. IFX was only statistically significantly better than placebo as measured by the probability of endoscopic recurrence.
IL-12/23i: Ustekinumab.
The phase III UNITI portfolio of RCTs testing ustekinumab (UST) induction and maintenance therapy in patients with CD who are TNFi-naïve (n = 761) and TNFi-inadequate responders (IR; n = 397) showed consistent and statistically significant efficacy of UST (p40-specific subunit inhibitors of IL-23) over placebo for the primary and most secondary endpoints, without new safety signals, both at week 6 and week 44 (Table 2).16,17
IL-23i: Risankizumab.
A phase II RCT of risankizumab (RZB; p19-specific subunit inhibitors of IL-23) enrolled 121 CD cases and stratified by steroid-IR, conventional synthetic-IR, and TNFi-IR.18 RZB at 600 mg (but not 200 mg) was significantly more efficacious than placebo across all primary and secondary endpoints, with no new safety signals.18 A phase III study for this agent is in progress.
IL-23i: MEDI2070.
A phase IIa RCT of MEDI2070 (IL-23i, subsequently called brazikumab) enrolled 121 TNFi-IR cases and stratified by lines of TNFi previously used.19 MEDI2070 was significantly more likely than placebo to attain the primary endpoint (100-point improvement in the Crohn’s Disease Activity Index [CDAI] at week 8) and efficacy was also found for several secondary endpoints. Phase III studies for this agent are in progress.
IL-17i: Brodalumab.
Targan et al demonstrated a detrimental effect of brodalumab (BRO; IL-17A receptor antagonist) on CD in a study of 130 steroid-IR, conventional synthetic-IR, and biologic-naïve CD cases (Table 2).20 Despite eligibility criteria only permitting the recruitment of patients with mild severity CD, patients treated with placebo were far more likely to achieve the primary endpoint (150 point improvement in CDAI at week 6) than all BRO dose groups. Placebo and BRO groups were not statistically different for secondary endpoints.
IL-6i: PF-04236921.
PF-04236921 (IL-6i) was tested in a dose-ranging phase II RCT of 247 TNFi-IR cases with CD.21 The 50 mg dose was more likely than placebo to attain the primary endpoint (70-point improvement in CDAI at week 12), but few secondary endpoints showed efficacy (Table 2).
JAKi: Tofacitinib.
The JAKi tofacitinib (TOF), has been tested in 1 phase II RCT22,23 reported at week 4 and at week 24, as shown in Table 2. The 139 steroid-IR, conventional synthetic-IR, and/or biologic-IR cases were stratified by baseline CDAI, then randomized to TOF 1 mg/day, 5 mg/day, 15 mg/day, or placebo. The primary endpoint (70-point improvement in CDAI at week 4) was statistically no different in the TOF arms vs the placebo arm. The secondary endpoints (100-point improvement in CDAI and 10-item IBD Questionnaire) did not show efficacy.22 TOF has not attained regulatory approvals for CD, and no phase III studies are in progress.
JAKi: Filgotinib.
A phase II RCT of another JAKi, filgotinib (FILGO), recruited 174 conventional synthetic-IR cases with CD and randomized participants to either FILGO (100 mg 4 times daily [QID] or 200 mg QID) or placebo.24 FILGO (200 mg) was found to be significantly more likely than placebo to attain the primary endpoint of CDAI improvement at week 10 and most secondary endpoints. The safety profile was clinically acceptable and risk of bias was low.24 Phase III RCTs are in progress.
JAKi: Upadacitinib.
A phase II dose-ranging RCT tested 5 doses of upadacitinib (UPA; JAKi) in 220 steroid-IR, conventional synthetic-IR, and/or biologic-IR cases (Table 2).25 During the 16-week induction, the higher doses of UPA were most efficacious, without altering safety profiles. However, by week 52 there was no significant difference in the primary endpoint (clinical remission) between the UPA arms and placebo.26 Endoscopic remission was statistically more likely with higher-dose UPA than placebo.26 Phase III RCTs are in progress.
Summary of treatments for CD.
Coauthor consensus meetings reviewed the several large high-quality RCTs of TNFi and 1 large RCT of UST and made a strong recommendation for both (Table 5). For IL-23i, good efficacy was seen for RZB and MEDI2070, but this was only supported by 1 RCT for each; thus, the group made a weak recommendation for IL-23i in CD, pending the publication of further results. JAKi treatments (UPA and FILGO) have weak recommendations for, whereas TOF, which did not show efficacy in CD, was given a weak recommendation against use. The group agreed on a strong recommendation against IL-17i in CD, given the lack of improvement in CD seen with BRO compared with placebo. As there was only 1 medium-sized RCT of IL-6i, there was insufficient evidence to make a recommendation. No recent studies were found for GOL or etanercept (ETN).
Table 5.
Summary of GRADE recommendations for advanced treatments for CD, UC, and uveitis.
| Indication | Strong Recommendation for | Weak Recommendation for | Weak Recommendation Against | Strong Recommendation Against | No Recommendationa |
|---|---|---|---|---|---|
|
| |||||
| CD | TNFi (ADA, IFX, CZP) IL-12/23i (UST) |
IL-23i (RZB, MEDI2070) JAKi (UPA, FILGO) csDMARD (MTX) |
TNFi (ETN) JAKi (TOF) |
IL-17i (SEC) | IL-6i (insufficient evidence) GOL (no study) |
| UC | TNFi (ADA, IFX, GOL) IL-12/23i (UST) JAKi (TOF) |
PDE4i (APR) | csDMARD (MTX) | CZP (no study) ETN (no recent study) IL-17i (insufficient evidence) |
|
| Uveitis | TNFi (ADA) TNFi (non-RCT for IFX, CZP, GOL) csDMARD (MTX) |
TNFi (ETN) | IL-17i (insufficient evidence) | ||
Recommendations were based on available evidence from reviews and the current updated review.
No recommendation: no RCTs or insufficient or conflicting evidence. ADA: adali- mumab; APR: apremilast; CD: Crohn disease; csDMARD: conventional synthetic disease-modifying antirheumatic drug; CZP: certolizumab pegol; ETN: etanercept; FILGO: filgotinib; GOL: golimumab; GRADE: Grading of Recommendations Assessment, Development, and Evaluation; IFX: infliximab; IL-12/23i: interleukin 12/23 inhibitor; IL-17i: interleukin 17 inhibitor; IL-23i: interleukin 23 inhibitor; IL-6i: interleukin 6 inhibitor; JAKi: Janus kinase inhibitor; MTX: methotrexate; PDE4i: phosphodiesterase-4 inhibitor; RCT: randomized controlled trial; RZB: risankizumab; TNFi: tumor necrosis factor inhibitor; TOF: tofacitinib; UC: ulcerative colitis; UPA: upadacitinib; UST: ustekinumab.
RCTs of UC
A total of 23 studies were screened and 15 were eligible for review, as shown in Table 3,27–41 with 8 studies excluded because of long-term maintenance or a lack of control group.
ADA.
The efficacy and safety of ADA compared to placebo has been reported in active UC in 4 RCTs.27–30 Two trials, Ulcerative Colitis Long-Term Remission and Maintenance with Adalimumab (ULTRA) 1 (N = 576) and ULTRA 2 (N = 248), evaluated an 8-week induction therapy with ADA and demonstrated better remission, mucosal healing, and quality of life (QOL) compared to placebo.28,29 During the ULTRA 3 trial, an additional trial focusing on TNFi-experienced patients, lower response rates compared to TNFi-naïve patients were observed,29 with similar efficacy and safety seen at year 4.30 A RCT performed in Japan found 23.2% of patients treated with ADA achieved remission by week 52, and 32.5% of the patients were able to taper down corticosteroids.27
GOL.
GOL, another TNFi biologic, was studied in 5 RCTs.31–35 The PURSUIT trials included 2 6-week inductions trials, a maintenance study and a study in a Japanese cohort.31,33–35 The PURSUIT-M trial demonstrated early clinical response to GOL treatment.31 The phase III trial, PURSUIT-J (N = 144) demonstrated that subcutaneous GOL maintained clinical efficacy to week 54 among induction responders.32 More patients randomized to GOL in PURSUIT-SC achieved a clinical response at 6 weeks and were more likely to achieve remission and mucosal healing.33 In PURSUIT-IV (N = 291), a single-dose IV administration of GOL in patients with moderate-to-severe UC did not lead to significant improvements in clinical outcomes.35
Methotrexate.
Two RCTs determined that methotrexate (MTX) was not superior to placebo in induction of steroid-free remission among patients with UC who are steroid-dependent.36,37 Further, the prevention of UC relapse was not significantly different between groups during the 48-week maintenance part of this trial.37
Apremilast.
An oral inhibitor of phosphodiesterase 4, apremilast (APR), was evaluated in a phase II RCT in patients with active UC, but showed no efficacy compared to placebo.38
TOF.
The Oral Clinical Trials for Tofacitinib in Ulcerative Colitis (OCTAVE) portfolio of trials studied TOF in adults with active UC.39,40 In the phase II trial (N = 194), patients on TOF (15 mg) reported a significant improvement of symptoms from baseline compared with placebo.39 In the induction trials, TOF (10 mg) twice daily achieved clinical remission in 18.5% of OCTAVE 1 patients and 16.6% of OCTAVE 2 patients.40 The OCTAVE Sustain (N = 593) maintenance phase further confirmed the efficacy of TOF, with 40.6% of patients taking 10 mg twice daily and 34.3% of patients taking 5 mg twice daily achieving clinical remission, compared with only 11.1% of patients taking placebo.40 In terms of safety, there was similar increased risk of herpes zoster as was seen in rheumatoid arthritis and psoriasis trials, with a higher rate of serious infection compared with placebo.40
UST.
A large RCT of UST (N = 961) with an 8-week induction and 44-week maintenance found UST was more effective than placebo for reducing UC remissions.41
Summary of treatments for UC.
Based on our review and our coauthor consensus meetings (Table 5), a strong recommendation was made for TNFi (ADA and GOL), JAKi (TOF), and IL-12/23i (UST), all with a low risk of bias. For the phosphodiesterase-4 inhibitor (APR), there is a single small study that did not show efficacy in UC and so the group made a weak recommendation against. For MTX, as both RCTs did not show efficacy in UC, a strong recommendation against was given. No recent RCTs for ETN or certolizumab pegol were found.
RCTs of uveitis
A total of 7 RCTs were screened and 5 RCTs (Table 4) were eligible.42–46 Corticosteroids have long been the standard treatment for patients with ocular inflammation; however, their long-term use confers risks to patients.47,48 We sought to summarize the RCTs of uveitis treatments, other than corticosteroids, that are also commonly used to treat patients with PsA.
ADA.
Three RCTs assessed the efficacy of ADA in treating flares of uveitis and improvement in visual acuity scores.42–44 In 2 trials, patients received prednisone at baseline along with ADA treatment, which was then tapered and stopped during the trial.42,43 ADA demonstrated steroid-sparing effects and flares of uveitis were delayed compared with the placebo group.42,43 A small RCT in cases of refractory noninfectious uveitis showed significant reduction in ocular inflammation in the ADA group.44
Secukinumab.
Secukinumab (SEC; IL-17i) demonstrated efficacy in a small trial of acute-on-chronic noninfectious uveitis.45 Patients with uveitis receiving intravenous (at significantly higher doses than are used in clinical practice) vs subcutaneous SEC responded faster and with greater likelihood of remission.45 Perhaps subcutaneous SEC did not attain sufficient concentrations for uveitis treatment in this trial.
MTX.
One RCT evaluated the QOL in patients with uveitis treated with either MTX or mycophenolate mofetil.46 Although the visual symptoms improved, the overall physical health scores did not show improvement and mental health-related QOL scores declined.
Summary of treatments for uveitis.
Uveitis presents a challenge to make definitive recommendations, as studies were done in a uveitis cohort and extrapolated to PsA. The 2 large RCTs and 1 smaller RCT of ADA in uveitis, with low risk of bias, allowed the group to make a weak recommendation for TNFi (Table 5), except for ETN, with a weak recommendation against. A small comparison trial led to a weak recommendation for MTX based on improved QOL indicators. There was only 1 small trial in SEC that met our criteria, and the consensus was that there was insufficient evidence to make a recommendation from this single study.
DISCUSSION
In keeping with our eligibility criteria, in this review we have only reported on RCTs in the Results section. In the forthcoming Discussion, we will highlight and signpost the reader to notable non-RCT studies for further reading.
Our review of the literature demonstrated that not all treatments used for PsA are also effective for IBD and/or uveitis, dosing regimens can vary, there can be safety considerations, and reimbursement depends upon the indication. We propose that the outcomes of these trials may be extrapolated to patients with PsA with comorbid IBD or uveitis, and thus be used to personalize their treatment, keeping in mind that we are currently lacking RCTs conducted in people with PsA and these related conditions.
Given the varying clinical phenotypes and natural histories that our patients with IMIDs can manifest, a multispecialty approach is essential to effectively, safely, and holistically manage these patients. As a result, therapeutic algorithms are becoming more complex, with an increasing proportion of patients needing a more personalized approach, independent of algorithms.49 This is an approach increasingly advocated by international recommendations, including GRAPPA 2021,12 the American College of Rheumatology,50 and the European Alliance of Associations for Rheumatology 2019 treatment recommendations for PsA.51 The aim is to more effectively diagnose different IMID manifestations, intervene early to prevent clinical sequelae — especially those that are irreversible, reduce disease activity in multiple domains to prevent morbidity and irreversible damage, prevent disease related complications, and improve prognosis and QOL.
Treatment choices for PsA may be affected by treatments for comorbid conditions. For example, IL-17i has been shown to exacerbate known CD. There is now strong evidence based upon 2 independent phase II RCTs that IL-17 antagonists exacerbate CD20,52 and would therefore be contraindicated in patients with PsA and active CD. The same may be applied to IL-17i use in UC.
There are numerous high-quality studies supporting TNFi use (except ETN) in UC, both as monotherapy and combined with conventional synthetic agents. The JAKi TOF has proven effective in UC, albeit not in CD. Further studies of other JAKi (UPA and FILGO) and IL-23i are in progress for UC and CD. Although MTX has been widely used in clinical practice for UC, only recently have there been well-designed RCTs evaluating MTX in UC. Surprisingly, both RCTs did not support MTX to induce steroid-free remission or prevent relapses, compared to placebo.36
The RCTs of uveitis discussed in this review should serve to inform treatment choices in patients with PsA suffering with uveitis in the absence of specific studies in PsA. In severe or untreated cases of uveitis, for example, one must initiate prompt treatment in order to prevent vision loss, which still accounts for 10% to 15% causes of legal blindness in the United States and carries significant personal and societal impact.7,47 ADA is the first TNFi approved for intermediate, posterior, and panuveitis. However, there is still a major need for more RCTs to better inform treatment recommendations. In particular, there are few/no RCTs in the various subsets of uveitis and no studies of prognosis of uveitis in patients with PsA. ETN use is not recommended in patients with PsA with concomitant uveitis because of its poor efficacy for uveitis and the risk uveitis poses for irreversible eye damage, including blindness. Some studies (not eligible for our review) found anti-TNF agents in ankylosing spondylitis and juvenile idiopathic arthritis, and azathioprine in Behcet disease were effective for uveitis.53,54 Efforts to convene international expert consensus are underway to develop guidance on biologic therapy for noninfectious uveitis.47
In conclusion, we have identified recent RCTs in IBD and uveitis that should be considered when managing patients with PsA and these related conditions. For some classes of treatment there is consistent efficacy, whereas for other classes there appears to be differential efficacy across IMID domains. One must be cognizant of differences in safety profiles between different biologics, and the emerging small-molecule therapies. Small-molecule therapies might be more prone to off-target effects that may make their efficacy and safety more difficult to handle as a class. As our therapeutic armamentarium for IMIDs is increasing, we are entering an exciting era of greater multispecialty collaboration, which will also pose unique challenges.
Supplementary Material
Acknowledgments
DRJ acknowledges research support from the Cambridge Arthritis Research Endeavour and the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014).
DRJ has received research grants, education grants, and/or speaker/advisory board honoraria from pharmaceutical companies, including from AbbVie, Amgen, Biogen, Celgene, Lilly, Ferris, Fresenius Kabi, Galapagos/Gilead, GSK, Celltrion, Janssen, Merck, Novartis, Pfizer, Roche, Sandoz, and UCB. ERS has participated in advisory boards, given conferences, or received grants from AbbVie, Amgen, BMS, Lilly, GSK, Janssen, Novartis, Pfizer, Sandoz, Roche, and UCB. AK has been a consultant to AbbVie, Amgen, Lilly, Janssen, Novartis, and UCB. TR has received research/educational grants and/or speaker/consultation fees from AbbVie, Arena, Aslan, AstraZeneca, BI, BMS, Celgene, Ferring, Galapagos, Gilead, GSK, Heptares, LabGenius, Janssen, Mylan, MSD, Novartis, Pfizer, Sandoz, Takeda, and UCB. FR has provided consulting or been on the advisory board for Adnovate, Agomab, Allergan, AbbVie, Arena, BI, Celgene/BMS, CDISC, Cowen, Ferring, Galapagos, Galmed, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Horizon Therapeutics, Image Analysis, Index, Jannsen, Koutif, Mestag, Metacrine, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surmodics, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB, Ysios, and 89Bio. S. Siebert has received institutional research funding from Amgen (previously Celgene), BI, BMS, Lilly, Janssen, and UCB; and honoraria/speaker fees from AbbVie, Biogen, Celgene, GSK, Janssen, Novartis, and UCB. MZ has been on the research/speaker/advisory boards for AbbVie, Amgen, Janssen, Lilly, Merck, Novartis, Sandoz, and Pfizer. S. Schwartzman has been a speaker for AbbVie, Jannssen, Lilly, Pfizer, Novartis, and UCB; a consultant for AbbVie, Janssen, Lilly, Myriad, Novarits, Regeneron, Sanofi, UCB, Stelexis, Jubilant, and Teijin; and is a board member of the National Psoriasis Foundation and is on the scientific advisory board of Myriad. JTR has provided consulting for Gilead, AbbVie, Novartis, Revolo, Affibody, Neoluekin, Corvus, Horizon, Roivant, and Priovant; received research support from Horizon, Pfizer, and the Malassezia Foundation; received royalties from UpToDate; and been on the data monitoring committee for Celgene/BMS and Clinical Endpoints Committee for Lilly. BM received a research grant from Novartis. RL received a research grant, an education grant, and has been a speaker for Lilly, AbbVie, and Novartis. MML has received research grants, education grants, and/or speaker/advisory board honoraria from pharmaceutical companies, including AbbVie, Amgen, Lilly, Galapagos/Gilead, Janssen, Novartis, and Pfizer. MS has been a consultant for Novartis. JAS has received consultant fees from Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two Labs, Adept Field Solutions, Clinical Care Options, Clearview Healthcare Partners, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, MedIQ, Jupiter Life Science, UBM, Trio Health, Medscape, WebMD, Practice Point Communications, the National Institutes of Health, and the American College of Rheumatology; received institutional research support from Zimmer Biomet; food and beverage payments from Intuitive Surgical/Philips Electronics North America; owns stock options in TPT Global Tech, Vaxart, Atyu BioPharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris, Enzolytics, Seres, Tonix, Charlotte’s Web; and is on the speaker’s bureau of Simply Speaking. JR received support for attending meetings or travel from AbbVie, Novartis, and UCB. MEH received speaker and advisory fees from AbbVie, BMS, Pfizer, Novartis, Lilly, Janssen, and UCB. The remaining authors declare no conflicts of interest relevant to this article.
Footnotes
ONLINE SUPPLEMENT
Supplementary material accompanies the online version of this article.
REFERENCES
- 1.Fragoulis GE, Liava C, Daoussis D, Akriviadis E, Garyfallos A, Dimitroulas T. Inflammatory bowel diseases and spondyloarthropathies: From pathogenesis to treatment. World J Gastroenterol 2019;25:2162–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee YH, Ji JD, Kim JS, et al. Ileocolonoscopic and histologic studies of Korean patients with ankylosing spondylitis. Scand J Rheumatol 1997;26:473–6. [DOI] [PubMed] [Google Scholar]
- 3.Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 2015;74:65–73. [DOI] [PubMed] [Google Scholar]
- 4.de Winter JJ, van Mens LJ, van der Heijde D, Landewé R, Baeten DL. Prevalence of peripheral and extra-articular disease in ankylosing spondylitis versus non-radiographic axial spondyloarthritis: a meta-analysis. Arthritis Res Ther 2016;18:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopylov U, Starr M, Watts C, Dionne S, Girardin M, Seidman EG. Detection of Crohn disease in patients with spondyloarthropathy: The SpACE capsule study. J Rheumatol 2018;45:498–505. [DOI] [PubMed] [Google Scholar]
- 6.Van Praet L, Van den Bosch FE, Jacques P, et al. Microscopic gut inflammation in axial spondyloarthritis: A multiparametric predictive model. Ann Rheum Dis 2013;72:414–7. [DOI] [PubMed] [Google Scholar]
- 7.González MM, Solano MM, Porco TC, et al. Epidemiology of uveitis in a US population-based study. J Ophthalmic Inflamm Infect 2018;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Chada LM, Merola JF. Comorbidities associated with psoriatic arthritis: Review and update. Clin Immunol 2020;214:108397. [DOI] [PubMed] [Google Scholar]
- 9.Egeberg A, Khalid U, Gislason GH, Mallbris L, Skov L, Hansen PR. Association of Psoriatic Disease With Uveitis: A Danish Nationwide Cohort Study. JAMA Dermatol 2015;151:1200–5. [DOI] [PubMed] [Google Scholar]
- 10.Jadon DR, Stober C, Pennington SR, FitzGerald O. Applying precision medicine to unmet clinical needs in psoriatic disease. Nat Rev Rheumatol 2020;16:609–27. [DOI] [PubMed] [Google Scholar]
- 11.Coates LC, Kavanaugh A, Ritchlin CT; GRAPPA Treatment Guideline Committee. Systematic review of treatments for psoriatic arthritis: 2014 update for the GRAPPA. J Rheumatol 2014;41:2273–6. [DOI] [PubMed] [Google Scholar]
- 12.Coates LC, Corp N, van der Windt DA, Soriano ER, Kavanaugh A. GRAPPA treatment recommendations: An update from the 2020 GRAPPA meeting. J Rheum Supp 2021;48:65–6. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- 15.Regueiro M, Feagan BG, Zou B, et al. ; PREVENT Study Group. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterol 2016;150:1568–78. [DOI] [PubMed] [Google Scholar]
- 16.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016;375:1946–60. [DOI] [PubMed] [Google Scholar]
- 17.Sands BE, Han C, Gasink C, et al. The effects of ustekinumab on health-related quality of life in patients with moderate to severe Crohn’s disease. J Crohns Colitis 2018;12:883–95. [DOI] [PubMed] [Google Scholar]
- 18.Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: A randomised, double-blind, placebo-controlled phase 2 study. Lancet 2017;389:1699–1709. [DOI] [PubMed] [Google Scholar]
- 19.Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: A phase 2a study. Gastroenterol 2017;153:77–86.e6. [DOI] [PubMed] [Google Scholar]
- 20.Targan SR, Feagan B, Vermeire S, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn’s disease. Am J Gastroenterol 2016;111:1599–1607. [DOI] [PubMed] [Google Scholar]
- 21.Danese S, Vermeire S, Hellstern P, et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut 2019;68:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandborn WJ, Ghosh S, Panes J, et al. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12:1485–93.e2. [DOI] [PubMed] [Google Scholar]
- 23.Panés J, Sandborn WJ, Schreiber S, et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: Results of two phase IIb randomised placebo-controlled trials. Gut 2017;66:1049–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeire S, Schriber S, Petryka R, et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017;389:266–75. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed MF, Klünder B, Lacerda AP, Othman AA. Exposure-response analyses for upadacitinib efficacy and safety in the Crohn’s disease CELEST study and bridging to the extended-release formulation. Clin Pharmacol Ther 2020;107:639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandborn WJ, Feagan BG, Loftus EV Jr, et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterol 2020;158:2123–38.e8. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki Y, Motoya S, Hanai H, et al. Efficacy and safety of adalimumab in Japanese patients with moderately to severely active ulcerative colitis. J Gastroenterol 2014;49:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reinisch W, Sandborn WJ, Panaccione R, et al. 52-week efficacy of adalimumab in patients with moderately to severely active ulcerative colitis who failed corticosteroids and/or immunosuppressants. Inflamm Bowel Dis 2013;19:1700–9. [DOI] [PubMed] [Google Scholar]
- 29.Sandborn WJ, Colombel JF, D’Haens G, et al. One-year maintenance outcomes among patients with moderately-to-severely active ulcerative colitis who responded to induction therapy with adalimumab: subgroup analyses from ULTRA 2. Aliment Pharmacol Ther 2013;37:204–13. [DOI] [PubMed] [Google Scholar]
- 30.Colombel JF, Sandborn WJ, Ghosh S, et al. Four-year maintenance treatment with adalimumab in patients with moderately to severely active ulcerative colitis: Data from ULTRA 1, 2, and 3. Am J Gastroenterol 2014;109:1771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson PR, Feagan BG, Sandborn WJ, et al. Maintenance of efficacy and continuing safety of golimumab for active ulcerative colitis: PURSUIT-SC maintenance study extension through 1 Year. Clin Transl Gastroenterol 2016;7:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hibi T, Imai Y, Senoo A, Ohta K, Ukyo Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: a phase 3, double-blind, randomized, placebo-controlled study-(PURSUIT-J study). J Gastroenterol 2017;52:1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandborn WJ, Feagan BG, Marano C, et al. ; PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterol 2014;146:85–e15. [DOI] [PubMed] [Google Scholar]
- 34.Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterol 2014;146:96–109.e1. [DOI] [PubMed] [Google Scholar]
- 35.Rutgeerts P, Feagan BG, Marano CW, et al. Randomised clinical trial: a placebo-controlled study of intravenous golimumab induction therapy for ulcerative colitis. Aliment Pharmacol Ther 2015;42:504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carbonnel F, Colombel JF, Filippi J, et al. Methotrexate is not superior to placebo for inducing steroid-free remission, but induces steroid-free clinical remission in a larger proportion of patients with ulcerative colitis. Gastroenterol 2016;150:380–8.e4. [DOI] [PubMed] [Google Scholar]
- 37.Herfarth H, Barnes EL, Valentine JF, et al. ; Clinical Research Alliance of the Crohn’s and Colitis Foundation. Methotrexate is not superior to placebo in maintaining steroid-free response or remission in ulcerative colitis. Gastroenterol 2018;155:1098–108.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danese S, Neurath MF, Kopoń A, et al. Effects of apremilast, an oral inhibitor of phosphodiesterase 4, in a randomized trial of patients with active ulcerative colitis. Clin Gastroenterol Hepatol 2020;18:2526–34.e9. [DOI] [PubMed] [Google Scholar]
- 39.Panés J, Su C, Bushmakin AG, Cappelleri JC, Mamolo C, Healey P. Randomized trial of tofacitinib in active ulcerative colitis: analysis of efficacy based on patient-reported outcomes. BMC Gastroenterol 2015;15:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandborn WJ, Su C, Panes J. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:496–7. [DOI] [PubMed] [Google Scholar]
- 41.Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 42.Jaffe GJ, Dick AD, Brézin AP, et al. Adalimumab in patients with active noninfectious uveitis. N Engl J Med 2016;375:932–43. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen QD, Merrill PT, Jaffe GJ, et al. Adalimumab for prevention of uveitic flare in patients with inactive non-infectious uveitis controlled by corticosteroids (VISUAL II): a multicentre, double-masked, randomised, placebo-controlled phase 3 trial. Lancet 2016;388:1183–92. [DOI] [PubMed] [Google Scholar]
- 44.Mackensen F, Heinz C, Jakob E, et al. Randomized controlled study to evaluate the efficacy of adalimumab in patients with different forms of refractory uveitis. Ocul Immunol Inflamm 2018;26:1015–22. [DOI] [PubMed] [Google Scholar]
- 45.Letko E, Yeh S, Foster CS, et al. ; AIN457A2208 Study Group. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology 2015;122:939–48. [DOI] [PubMed] [Google Scholar]
- 46.Niemeyer KM, Gonzales JA, Rathinam SR, et al. Quality-of-life outcomes from a randomized clinical trial comparing antimetabolites for intermediate, posterior, and panuveitis. Am J Ophthalmol 2017;179:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dick AD, Rosenbaum JT, Al-Dhibi HA, et al. ; Fundamentals of Care for Uveitis International Consensus Group. Guidance on noncorticosteroid systemic immunomodulatory therapy in noninfectious uveitis: Fundamentals of care for uveitis (FOCUS) initiative. Ophthalmol 2018;125:757–73. [DOI] [PubMed] [Google Scholar]
- 48.Yasir M, Goyal A, Sonthalia S, ed. Corticosteroid adverse effects. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [Internet. Accessed October 6, 2022.] Available from: https://www.ncbi.nlm.nih.gov/books/NBK531462 [PubMed] [Google Scholar]
- 49.Gudu T, Jadon DR. Multidisciplinary working in the management of axial and peripheral spondyloarthritis. Ther Adv Musculoskelet Dis 2020;12:1759720X20975888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh JA, Guyatt G, Ogdie A, et al. Special article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019; 71:5–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: Unexpected results of a randomised, double-blind placebo-controlled trial. Gut 2012;61:1693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramanan AV, Dick AD, Jones AP, et al. ; SYCAMORE Study Group. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med 2017;376:1637–46. [DOI] [PubMed] [Google Scholar]
- 54.Yazici H, Pazarli H, Barnes CG, et al. A controlled trial of azathioprine in Behçet’s syndrome. N Engl J Med 1990;322:281–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


