Abstract
Background
The sugar kelp Saccharina latissima is a Laminariales species widely distributed in the Northern Hemisphere. Its physiology and ecology have been studied since the 1960s, given its ecological relevance on western temperate coasts. However, research interest has been rising recently, driven mainly by reports of negative impacts of anthropogenically induced environmental change and by the increased commercial interest in cultivating the species, with several industrial applications for the resulting biomass.
Scope
We used a variety of sources published between 2009 to May 2023 (but including some earlier literature where required), to provide a comprehensive review of the ecology, physiology, biochemical and molecular biology of S. latissima. In so doing we aimed to better understand the species’ response to stressors in natural communities, but also inform the sustainable cultivation of the species.
Conclusion
Due to its wide distribution, S. latissima has developed a variety of physiological and biochemical mechanisms to adjust to environmental changes, including adjustments in photosynthetic parameters, modulation of osmolytes and antioxidants, reprogramming of gene expression and epigenetic modifications, among others summarized in this review. This is particularly important because massive changes in the abundance and distribution of S. latissima have already been observed. Namely, presence and abundance of S. latissima has significantly decreased at the rear edges on both sides of the Atlantic, and increased in abundance at the polar regions. These changes were mainly caused by climate change and will therefore be increasingly evident in the future. Recent developments in genomics, transcriptomics and epigenomics have clarified the existence of genetic differentiation along its distributional range with implications in the fitness at some locations. The complex biotic and abiotic interactions unraveled here demonstrated the cascading effects the disappearance of a kelp forest can have in a marine ecosystem. We show how S. latissima is an excellent model to study acclimation and adaptation to environmental variability and how to predict future distribution and persistence under climate change.
Keywords: acclimation, biogeography, climate change, local adaptation, macroalgae, marine ecology, metabolites, molecular biology, omics, physiology, seaweed, warming
INTRODUCTION
Kelps, in the strict sense including only representatives of the order Laminariales, are brown macroalgae (Phaeophyceae) growing on shallow rocky shores along the Atlantic, Pacific and Indian Oceans (Wernberg et al., 2019). In the Northern Hemisphere, kelps are represented mainly by the genera Alaria, Laminaria and Saccharina (Bolton, 2010; Wernberg et al., 2019). Kelps have received considerable attention, given their ecological roles, the several ecosystem services they provide and the several commercial applications of their extracts (e.g. Bartsch et al., 2008; Smale et al., 2013). Recently, threats to kelp persistence around the globe have been reviewed, and the need for conservation measures has been reiterated (e.g. Filbee-Dexter et al., 2019; Smale, 2020; Filbee-Dexter and Wernberg, 2018).
Among kelps, Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders (Lane et al., 2006) is one of the most well studied, especially in more recent years. Saccharina latissima is a boreal–temperate kelp widely distributed across the Northern Hemisphere, from polar to temperate regions (Fig. 1). Given its wide distribution range covering highly distinct climatic regions, this species is a brilliant model to understand environmental and adaptation. Moreover, given that it contains several valuable metabolites for the industry, research on its biochemical composition is well developed and provides an understanding on how mechanisms work at the metabolome level for kelps. Recently, ‘-omics’ tools have been developed and applied in S. latissima as researchers try to understand the genetic diversity that underlies the adaptation of S. latissima to different temperature, salinity and light regimes. This, in the context of climate change, which is precipitating the retreat and local extinction of several kelps, results in S. latissima being an excellent model to understand resilience and adaptation in brown algae.
Fig. 1.
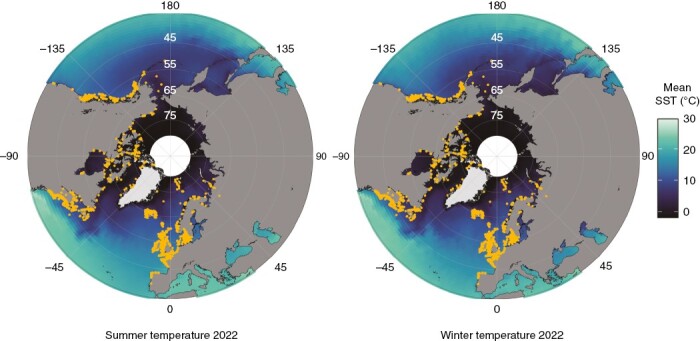
The worldwide distribution of Saccharina latissima. Occurrence data of S. latissima (orange dots) were collected from databases [Global Biodiversity Information Facility (www.gbif.org) and the Ocean Biogeographic Information System (http://iobis.org)]. Occurrence data cover the time frame between 1903 and 2020. Note that the points size is increased to allow visualization at this large scale and does not display the real areal extent Sea surface temperature data (colour gradient) from 2022 [left panel, summer temperature (21 March to 21 September 2022); and right panel, winter temperature (1 January to 21 March 2022 and 21 September to 31 December 2022)] were downloaded from the NOAA database (https://coastwatch.pfeg.noaa.gov/erddap/). The maps integrate the monthly temperature mean with latitude and longitude averaged as integers. There are white areas around the North Pole, where the projection makes data interpolation impossible. Maps were created with the R package ggOceanMaps (Vihtakari, 2022).
This review (part I) focuses on knowledge generated over the past ~15 years, particularly on recent developments that provide new insights into the physiology and ecology of S. latissima. It is divided into six main themes, with a final ‘Conclusions’ section highlighting the needs for future research. For a review of previous work, we refer the reader to Bartsch et al. (2008). The second part of the review (The sugar kelp Saccharina latissima II: recent advances in farming and applications) focuses on the latest applied research, farming and applications for S. latissima (Saether et al. 2023).
Life cycle and phenology
Saccharina latissima, like all Laminariales, is characterized by a haplo-diplontic (haploid–diploid) heteromorphic life cycle (Fig. 2; Schiel and Foster, 2006; Bartsch et al., 2008). Sessile macroscopic sporophytes (2n) of S. latissima usually grow up to 4 m (White and Marshall, 2007) and vary greatly in their morphological appearance (Fig. 3; Diehl et al., 2023). Bigger specimens can be found in Arctic regions (~7 m and larger; T. Vonnahme & S. Niedzwiedz, pers. comm., June 2023). The species grows typically on rocky shores in the upper subtidal zone to depths of 15–30 m, attached to hard rock using a branched claw-like holdfast (Pehlke and Bartsch, 2008; Bekkby and Moy, 2011; Bischof et al., 2019). It has also been reported growing on fine sediment attached to sparse gravel, pebbles (Bluhm et al., 2022; Filbee-Dexter et al., 2022b) and on tubeworms (Bracken, 2018). The sporophyte of S. latissima changes greatly in morphology depending on exposure and environmental factors (Fig. 3; Lüning, 1990; Van den Hoek et al., 1995). In general, the phylloid is elongate, undivided and without a midrib, but may have bullations (wrinkled surface) and wavy rims (White and Marshall, 2007). Under moderate wave exposure, S. latissima develops narrow fronds and solid cauloids (Lüning, 1990; Van den Hoek et al., 1995). In addition, sporophytes tend to develop longer and heavier stipes at greater depths to enhance light capture (Ronowicz et al., 2022). This morphological plasticity has led to misidentification and taxonomic confusion. For example, Saccharina angustissima has only recently been elevated to species level, being until then considered a morphotype of S. latissima (Augyte et al., 2018), whereas both Saccharina longicruris and Saccharina groenlandica were synonymized with S. latissima (McDevit and Saunders, 2010; Longtin and Saunders, 2015). The adult sporophyte exhibits basal meristematic growth. Sporophytes normally have a lifespan of 3 years, reaching their maximum size in the second growing season (Lee, 1989). However, specimens in the intertidal zone are annuals (Lee, 1989).
Fig. 2.
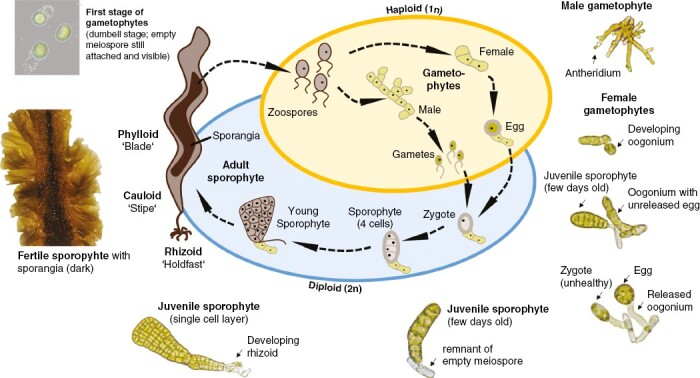
Life cycle of Saccharina latissima. The life cycle of S. latissima can be split into a diploid phase (blue) and a haploid phase (yellow). Adult sporophytes (2n) release zoospores, which grow into either female or male gametophytes (1n). Female gametophytes release eggs (1n); male gametophytes release gametes (1n). The egg and gamete fuse to form a zygote (2n), which grows into a sporophyte (2n). Sporophyte photograph: S. Forbord. Microscopic photographs and description (I. Bartsch) are included to provide additional information about the variety and diversity of gametophytes.
Fig. 3.

Morphological variability of European Saccharina latissima sporophytes. The white bars represent 20 cm. (A) Ny-Ålesund, Spitsbergen; collected from the Old Pier, 10 m depth, moderate exposure (photograph: N. Diehl). (B) Ansnas, Norway; collected in a small bay, 1–2 m depth, protected (photograph: N. Diehl). (C) Runde, Norway; collected from rocks surrounded by sand, 1–2 m depth, moderate exposure (photograph: N. Diehl). (D) Runde, Norway; collected in a Laminaria digitata forest, 1–3 m depth, exposed (photograph: N. Diehl). (E) Locmariaquer, France; collected from rocky shores, high tidal range, 3–5 m depth, moderate exposure (photograph: L. Fouqueau). (F) Helgoland, Germany; collected from rocky shores, 5 m depth, exposed (photograph: A. Wagner). Figure modified from Diehl et al. (2023).
When mature, sporangia accumulate into easily recognizable sori on sporophytes of S. latissima and produce microscopic spores (n) (Fig. 2; Forbord et al., 2012). As free-living stages, spores and gametes are the phases that allow for dispersal, albeit usually limited to a few metres in kelps. Therefore, spores tend to settle near parent sporophytes (Schiel and Foster, 2006). Sex is expressed at the haploid stage, and gametes and gametophytes present sexually dimorphic traits. Female gametophyte cells and nuclei are larger and rounder, whereas male gametophytes cells are smaller and tend to form filaments with more cells (Lüning and Neushul, 1978; Goecke et al., 2022) which allows for identification and separation of sexes in the laboratory.
After the seminal work in the 1970s and 1980s by Lüning in Europe and by Lee and Brinkhuis in North America (e.g. Lüning, 1980; Bolton and Lüning, 1982; Lee and Brinkhuis, 1988), research targeting the sexual reproductive stages of S. latissima stalled. Recently, the research interest has risen again, driven by the need to manipulate the sexual life cycle in aquaculture. Hence, recent advances have enabled researchers to control the reproductive period artificially in the laboratory at several stages, allowing for scientific experimentation and improving the economic sustainability of seaweed aquaculture (Charrier et al., 2017). Also, methodological advances have allowed better examination of the development of embryos to study cellular interactions in the embryo (Clerc et al., 2022), quantify DNA content in different cell types (Goecke et al., 2022) and improve protocols for studying embryogenesis (Theodorou et al., 2021).
At the spore stage, sporogenesis (production of spores) in the wild typically peaks during winter, being negligible in summer; however, the extent of the sorus formation period is dependent on the geographical region (Bartsch et al., 2008; Andersen et al., 2011; Boderskov et al., 2021). In the laboratory, sporogenesis is commonly induced by applying short-day light treatments, mimicking the light conditions of autumn/winter, and by removing the basal blade of the meristem, to remove inhibitors, ensuring year-round spore availability for farmers and researchers (Forbord et al., 2012). In turn, a recent study reported higher and faster induction of sporulation in tissues under complete darkness than in short-day treatments (Boderskov et al., 2021).
At the gametophyte stage, gametogenesis (maturation) can be induced or prevented by manipulating both biotic and abiotic conditions (see below on the next paragraph). When gametogenesis is prevented, gametophytes remain vegetative and continue to grow, remaining viable for several years [≥1 year reported in S. latissima (Ebbing et al., 2021b); ≤30 years in several Laminaria sp. (Druehl et al., 2005; Martins et al., 2019)], also referred to as delayed gametophytes. Cultures of delayed gametophytes can function as genetic diversity reservoirs if conserved by cryopreservation, which has been applied successfully to the gametophytes of S. latissima (Visch et al., 2019). In parallel, vegetative growth of gametophyte cultures can be boosted to produce enough biomass for cultivation facilities. In the wild, delayed gametophytes might represent a marine resource analogous of terrestrial seed banks, preserving the algae in a resting stage during harsh environmental conditions and allowing for a rapid recovery once the conditions improve (Schiel and Foster, 2006). However, the high levels of gene expression reported in vegetative gametophytes indicate that these gametophytes are metabolically active and not resting stages where growth is stopped, calling for more research on the topic (Monteiro et al., 2019a). Recent methodological advances, such as the use of flow cytometry to isolate gametophytes of S. latissima, will allow for a more cost-effective gametophyte control at a larger scale (Augyte et al., 2020). For more information on aquacultural approaches, see Review II (Saether et al., 2023).
The maturation of female gametophytes depends on the interaction of temperature, light quality and intensity, nutrients and biotic factors. Blue light is required for female gametophytes to mature, and as temperature rises, more blue light is required until an inhibitory species-specific threshold: 20 °C in S. latissima (Lüning and Dring, 1972; Lee and Brinkhuis, 1988). Therefore, in laboratory conditions, if only exposed to red light, gametophytes will tend to grow vegetatively, because growth is unaffected by light quality (Lüning and Dring, 1975). Recently, a study revealed that light quality was significant only at lower intensities; at higher intensities, both red and blue light induced maturation (Ebbing et al., 2021b).
Concerning nutrients, it has been shown that iron is necessary for oogenesis in kelps; hence, iron is typically excluded from nutrient solutions given to stock culture meant to grow vegetatively (Motomura and Sakai, 1981; Lewis et al., 2013). Also, nutrient enrichment favours gametophyte growth; however, caution must be taken with the proliferation of diatoms, the growth of which is inhibited by addition of germanium dioxide (Kerrison et al., 2016; Nielsen et al., 2016a).
Concerning biotic factors, concentrations above an optimal initial density of gametophytes inhibit fertilization, regardless of temperature and light intensities (Ebbing et al., 2020). The authors ruled out reduced nutrients or light intensity as the cause of inhibition of fertilization at high concentrations; hence, the underlying mechanism remains unknown. Another relevant biotic factor is the sex ratio of cultures, with a higher proportion of female gametophytes decreasing the reproductive yield, most relevant at high culture densities (Ebbing et al., 2021a).
Concerning phenology, in the wild, the maturation process of S. latissima typically peaks in winter, with sporophytes growing at the highest rate over spring, after which they often senesce over summer owing to high temperatures. However, in some sites, the species produces sori throughout the year (Boderskov et al., 2021). Although reproduction can occur over several months, reproductive success and sporophyte growth depend on the month when sporogenesis occurs. In Denmark (temperate Atlantic), the percentage of fertile sporophytes (with visible sorus formation) varies markedly over the year, peaking in November and December and reaching null values in July and September. The number of viable spores released also varies monthly, decreasing steadily from a maximum in November to February, with a surge in March and April (Boderskov et al., 2021). Meiospores of S. latissima (from Alaska, USA; Arctic Pacific) released in July resulted in larger gametophytes but smaller sporophytes when compared with spores released in August (Raymond and Stekoll, 2021), whereas for spores originated from S. latissima collected in April (from Ireland, temperate Atlantic), growth rates of gametophytes were five to ten times higher than from spores originated in February (Nielsen et al., 2016a).
Concerning sporophyte growth, seasonal variation in growth rates is notable along the coast of Norway, with sporophytes from northern Norway reaching their maximum frond length and biomass ~2 months earlier than sporophytes occurring in the south of the country (Forbord et al., 2020).
The fact that recent studies (Ebbing et al., 2020; Boderskov et al., 2021) sometimes contradict previous findings and/or show a more complex control of life-cycle transitions highlights the need for further research on this topic, testing for more single and interacting drivers and accounting for possible site-specific responses.
ADVANCES IN ‘OMICS’
Genomics
The decrease in sequencing costs has led to an increase in genomic resources for non-model species, such as brown algae, that have been severely understudied until recently. Nuclear genomes are now available for some Phaeophyta species [e.g. Ectocarpus sp. (Cock et al., 2010), Saccharina japonica (Ye et al., 2015; Liu et al., 2019), Undaria pinnatifida (Shan et al., 2020; Graf et al., 2021)], and plastid and mitochondrial genomes are also mounting (e.g. Oudot-Le Secq et al., 2006; Chen et al., 2019; Rana et al., 2021). For S. latissima, a mitochondrial genome is available (Wang et al., 2016) but not a nuclear genome, although efforts are underway (M. Cock, pers. comm.; https://phaeoexplorer.sb-roscoff.fr/home/). Based on genetic data, a taxonomic re-organization was proposed in 2006 that reassigned the previously Laminaria saccharina to Saccharina latissima, the currently accepted species name (Lane et al., 2006). Since then, other species have been synonymized with S. latissima (Neiva et al., 2018), highlighting the need for more extensive sampling across described and possible sites where S. latissima occurs to assess the intraspecific diversity better. The availability of validated DNA barcodes for the species [mitochondrial cytochrome c oxidase I (COI) and ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL) (Ratnasingham and Hebert, 2007)] is important to confirm the identity of S. latissima samples. Moreover, it allows for the species to be detected in environmental DNA surveys, which allow for identification and quantification of several species from a unique sample using metabarcoding techniques (Deiner et al., 2017). Population structure, connectivity and genetic diversity in S. latissima have been studied using microsatellites (e.g. Guzinski et al., 2016; Luttikhuizen et al., 2018; Mooney et al., 2018), COI (Neiva et al., 2018) and, more recently, double digest restriction site-associated DNA sequencing, and the results are discussed in the section entitled ‘Biogeographical patterns’. Using a genomic selection approach, breeding values of the gametophytes of S. latissima were estimated and correlated with phenotypic traits of sporophytes, especially wet and dry weight per metre; however, low genetic correlations among different years are concerning and need to be explored further (Huang et al., 2023). These approaches inform current attempts to establish breeding programmes and, in the future, to domesticate S. latissima (Yarish et al., 2017; Umanzor et al., 2021).
Transcriptomics
Responses of organisms to stress are often measured by physiological parameters, such as survival, reproductive success or growth, which are extremely relevant because they underlie the success of species. However, the underlying molecular mechanism often remains unknown even when significant physiological responses are found after exposure to a stressor (Bischof et al., 2019). Transcriptomics approaches focus on the expression of mRNA following a stimulus. Given the nature of mRNA, this approach measures a transient response that can be encoded at the DNA level or via epigenetic mechanisms (Stark et al., 2019). The application of this approach to non-model organisms has been rising in recent years, and methods have improved considerably in a short period. Nonetheless, the use of transcriptomics in brown algae is lagging and has been applied to only a few species [e.g. Laminaria digitata (Liesner et al., 2022); Undaria pinnatifida (Graf et al., 2022); and mostly on the brown algal model Ectocarpus (e.g. Ahmed et al., 2014; Mignerot et al., 2019) and the commercially important Saccharina japonica (e.g. Liu et al., 2014; Zhang et al., 2021)]. Although access to transcriptomic data in brown algae has been made easier by advances in (higher) model plants, namely Arabidopsis thaliana (e.g. Zhang et al., 2017), the evolutionary distance between Phaeophyceae and plants and other algae creates challenges. There is still a very low annotation rate of expressed genes in brown algae because few functional studies have been conducted in this group, given that approaches such as reverse genetics are not available (Kroth, 2013; Bringloe et al., 2020). However, promising advances have been made recently, and the use of CRISPR/Cas9 technology might enable a better understanding of the function of each gene in the metabolism of this group (Badis et al., 2021).
Gene-expression patterns in S. latissima were investigated initially using microarrays (Heinrich et al., 2012a, b; Heinrich et al., 2015, 2016), but more recently, RNA-sequencing has been applied (Monteiro et al., 2019a, b; Pearson et al., 2019; Li et al., 2020a; Li et al., 2020b), and reference genes for real-time quantitative PCR have been developed (Xing et al., 2021). Transcriptomic studies in S. latissima have revealed an intricate metabolism-wide programming of gene expression in the species in response to environmental drivers, discussed in the section ‘Responses to environmental drivers’.
Epigenomics
Epigenomics have been shown to play a crucial role in defining a phenotype (Moore et al., 2013; Anastasiadi et al., 2021). Given its sessile lifestyle and often low dispersal distances, S. latissima is likely to rely on epigenetic mechanisms and epigenetic variation. Epigenetic mechanisms play an essential role in the adaptation of a population, and in the coping mechanisms of an individual in reaction to local conditions, ecotype differentiation (eco-phenotype) or rapid changes in local conditions (= local).
The known, non-exclusive epigenetic mechanisms encompass non-coding RNA, histone modification and DNA methylation (Boquete et al., 2021). They have been shown to play a role in establishment, maintenance and control of gene expression without changes to the DNA sequence (Anastasiadi et al., 2021); hence, they play a key role in the eco-evolutionary dynamics of a species (Calosi et al., 2016; Anastasiadi et al., 2021). Research on epigenetic modulation and variation thereof is well established in plant biology (Richards et al., 2017). However, in kelp, the study of epigenetics has recently gained momentum, with only a handful of studies to date (Phaeophyceae; Cock et al., 2010; Liu et al., 2019; Fan et al., 2020a; Teng et al., 2021; Scheschonk et al., 2022).
Regarding epigenetic mechanisms in the genus Saccharina, only DNA cytosine methylation has been investigated so far (Liu et al., 2019; Fan et al., 2020a; Teng et al., 2021; Scheschonk et al., 2022). ‘DNA methylation’ in plants and algae describes the methylation of a cytosine in the DNA (5ʹ-methylcytosine). DNA cytosine methylation can occur within and outside genes in the sequence context of CG, CHG or CHH (with ‘H’ being any base except G; Bewick et al., 2017). Genes are typically methylated in the CG context in animals (Schmitz et al., 2019), and methylation of the CG context in gene bodies of nuclear DNA is between 2 and 86 % across Viridiplantae (Bewick et al., 2017). Methylations in the CG, CHG and CHH contexts were found to act in silencing transposable elements in and outside of genes (Zhou et al., 2020) or to act in regulation of transcript expression (Dubin et al., 2015; L. Zhang et al., 2018a; Boquete et al., 2021). With this, they are important to consider as aspects of and adaptation processes. Moreover, it has been proposed in plants that CG methylation regulates the inheritance of other types of epigenetic information (Mathieu et al., 2007).
Within brown algae, there seem to be group-specific occurrences regarding the types of epigenetic mechanisms. Histone modification has been observed in Ectocarpus siliculosus (Cock et al., 2010; Bourdareau et al., 2021), whereas DNA cytosine methylation was found to be negligible, which led to the assumption that DNA methylation is negligible in brown algae (Cock et al., 2010). However, in the kelps S. latissima and S. japonica, it has recently been established that methylation plays a significant role in gene expression, for both the nuclear genome and the chloroplast genome (e.g. Fan et al., 2020a, b; Yang et al., 2021; Scheschonk et al., 2022). Hence, it is likely that the totality of epigenetic modifications of importance in S. latissima can be assessed only by testing for the respective mechanism in the species, or possibly the congener species (S. japonica), but cannot be implied per se by findings from other genera within the group of Phaeophyceae. The studies focusing on Saccharina spp. investigated the impact of cytosine methylation on both life-cycle stages at the transcriptomic level (S. japonica; Liu et al., 2019; Fan et al., 2020; Teng et al., 2021) and differences in cytosine methylation attributable to cultivation and latitudinal location (possibly heritable traits) observable at the sporophyte stage (S. latissima; Scheschonk et al., 2022; L. Scheschonk, unpubl. res.). Cytosine methylation was shown to influence gene expression in both life-cycle stages (predominantly, the non-heritable methylation variant CHH; ~56 %; Yang et al., 2021), with higher methylations found in the gametophyte stage for both nuclear and chloroplast genome (Fan et al., 2020b; Teng et al., 2021). In both life-cycle stages and genomes (nuclear and chloroplast), high levels of cytosine DNA methylation led to the silencing of the respective DNA sequence, acting as an additional control mechanism in gene expression (Fan et al., 2020a). At the population level, differences in cytosine methylation were observed between latitudes in populations regardless of cultivation status (laboratory and wild; Scheschonk et al., 2022; L. Scheschonk, unpubl. res.). This implies hereditary additional control imposed via cytosine methylation. As in other sequences, regions became methylated only during the cultivation process in both origins, and DNA cytosine methylations are likely to be a mechanism of rapid adaptation, because changes in habitat (wild to cultivation) initiated epigenetic changes within a generation.
RESPONSES TO ENVIRONMENTAL DRIVERS
Temperature
The composition and biogeographical distribution patterns of macroalgal communities are largely determined by temperature (Lüning, 1984; Adey and Steneck, 2001; Wiencke and Bischof, 2012). Thus, climate change, particularly warming and marine heatwaves (MHWs), is a major threat to marine forests (e.g. Harley et al., 2012; Smale, 2020). Hobday et al. (2016) defined MHWs as a temperature increase above the 90th percentile of the 30-year mean for >5 days consecutively; however, several publications mention MHWs as prolonged anomalously warm water events. As the use of the term ‘MHW’ differs among studies, in this review, we refer to the wording of the individual studies.
Much is known about the general thermal characteristics of S. latissima, mainly in terms of survival, reproduction, photosynthesis and growth (Bartsch et al., 2008). Like other kelps, S. latissima is a cold-temperate organism (Araújo et al., 2016). The survival threshold of sporophytes has been shown to be location specific. Sporophytes from Helgoland presented optimal growth between 10 and 15 °C (Bolton and Lüning, 1982), although they tolerated an extensive range of temperature, from 0 to 23 °C, for shorter periods, with sharply increasing mortality rates at >20 °C (Fortes and Lüning, 1980; Lüning, 1984, 1990a). Sporophytes from Nova Scotia were found to have decreasing growth rates with increasing temperatures between 11 and 21 °C, high mortality at 18 °C and no survival at 21 °C after merely 2 weeks (Simonson et al., 2015a). On the contrary, S. latissima sporophytes from Brittany survive ≤25 °C for >1 week (Diehl et al., 2021). Susceptibility to high temperature was shown to vary with environmental thermal history, thus between seasons and years (Niedzwiedz et al., 2022). Gametophytes of S. latissima exhibited a broader thermal tolerance, surviving temperatures down to −1.5 °C and up to 23–25 °C (tom Dieck, 1993). Differences in temperature sensitivity were also found between laboratory cultures and field sporophytes (Heinrich et al., 2016) and between male and female gametophytes (Monteiro et al., 2019a). Consequently, generalizations about thermal limits of S. latissima based on limited spatial covering and without consideration of generational effects should be handled carefully.
Detrimental effects of suboptimal high temperatures on S. latissima often include compromised growth (e.g. Bolton and Lüning, 1982; Simonson et al., 2015b), but high temperature can also lead to weakening of the tissue structure (Simonson et al., 2015b), increasing blade erosion (Krumhansl et al., 2014; Simonson et al., 2015b), enhanced biofouling and epiphytism (Andersen et al., 2013; Forbord et al., 2020), complex modifications in photosynthetic mechanisms, lowered chlorophyll a and fucoxanthin concentrations (Andersen et al., 2013), a strongly increased de-epoxidation state of the xanthophyll cycle (Nepper-Davidsen et al., 2019; Diehl et al., 2021) and reduced kelp carbon decomposition (Filbee-Dexter et al., 2022a). Exposure to elevated, although not lethal, temperature is harmful in the long term for S. latissima (Andersen et al., 2013; Nepper-Davidsen et al., 2019). Warming in the Arctic, however, might promote kelp populations, with densities being higher in warmer areas than at comparable colder sites (Wiktor et al., 2022). At the warmer sites, S. latissima was also found at slightly greater depths.
It is increasingly relevant to look at the impact of MHWs on seaweeds (Straub et al., 2019). Strong correlations between MHW events over the last 60 years and loss of S. latissima forests in the East and West North Atlantic were found (Filbee-Dexter et al., 2020). Nevertheless, few studies simulating MHW scenarios have been conducted on S. latissima (see Nepper-Davidsen et al., 2019; Diehl et al., 2021; Niedzwiedz et al., 2022). After a simulated 3-week MHW event in Danish waters, most samples died within a few days at 24 °C, and impairing effects of high but sub-lethal temperatures (18 and 21 °C) were observed in a 2-week recovery phase (Nepper-Davidsen et al., 2019). Thereby, interrelationships were demonstrated between reduced growth, reduced photosynthetic performance, carbon uptake and pigment composition. At the same temperatures (11, 18 and 21 °C), no changes in C:N and phlorotannins were detected in specimens from Nova Scotia, Canada (Simonson et al., 2015b). The impact of local MHWs in the summer on five European S. latissima populations ranging from southern Brittany to Spitsbergen revealed strong physiological and biochemical divergences between the populations. Increased mortality and decreased photosynthetic performance at the higher temperature amplitude treatments were detected exclusively in the rear-edge populations from Helgoland (German Bight) and Brittany, while the Arctic population was unaffected (Diehl et al., 2021). In Norway, strong differences in the physiological condition of S. latissima were observed, showing, e.g. decreased growth and more erosion in a hot year compared with a cooler year (Armitage et al., 2017). The impact of MHWs also varies by year and season, as shown for field sporophytes from Helgoland (Niedzwiedz et al., 2022). Saccharina latissima was more sensitive to high temperatures at the end of summer and during an extremely warm year.
High and excessively low temperatures alter physiological and biochemical properties of S. latissima. Overall, wild S. latissima from Iceland revealed a positive correlation of carbohydrates and negative correlations of proteins with the environmental temperature (Coaten et al., 2023). Lower pigment concentrations were found at temperatures of <10 °C, whereas the de-epoxidation state of the xanthophyll cycle was significantly higher compared with higher temperature treatments (Olischläger et al., 2017; Monteiro et al., 2019b; Li et al., 2020a), and higher phosphorylation rates of mitogen-activated protein kinases were measured at 2 °C than at 7 °C (Parages et al., 2013). Additionally, strongly enhanced mannitol concentrations were detected in young sporophytes from Brittany after 0 °C treatment, indicating a strong anti-freezing response of the species (C Monteiro et al., 2020a). Consequently, S. latissima will most probably benefit from the predicted rising temperatures in subpolar and polar regions (Filbee-Dexter et al., 2019; Diehl and Bischof, 2021), because the physiological functions of S. latissima will be enhanced (Iñiguez et al., 2016). Yet, darkness during the polar night seems to outcompete the positive effects of warming (Scheschonk et al., 2019), and low water temperature is a requirement for survival (Gordillo et al., 2022). Warming in winter accelerated weight loss of young sporophytes over 4 months of darkness, with ~50 % at 8 °C and 40 % at 3 °C (Gordillo et al., 2022). Furthermore, dark respiration of Arctic S. latissima sporophytes increased with increasing temperatures (3, 7 and 11 °C) (Niedzwiedz and Bischof, 2023).
Arctic S. latissima gametophytes did not survive at 20 °C in the laboratory but grew at ≤15 °C, with higher growth rates between 10 and 15 °C than at 5 °C (measured as the length of both male and female gametophytes) (Park et al., 2017). Another laboratory study looking at Arctic gametophytes showed that they survive at 20 °C by heat stress mechanisms that were induced extensively at the transcriptomic level at this temperature, whereas this was not the case at 4 and 12 °C (Monteiro et al., 2019a).
Considering spore germination, a higher temperature of 9 °C increased the germination rate of spores compared with 5 °C for Arctic individuals (Zacher et al., 2016). In an experiment with individuals from North America, at temperatures between 4 and 12 °C, lower temperatures negatively influenced the size of gametophytes and sporophytes and the production of eggs and young sporophytes (Raymond and Stekoll, 2021). When looking at sexual reproduction, sex-biased responses to temperature were found, with male gametophytes being more resilient to higher temperatures than females; females grew at a slower rate, and pathways related to fecundity were repressed (Monteiro et al., 2019a). Likewise, higher temperatures increased the proportion of male gametophytes in an earlier study (Lee and Brinkhuis, 1988), but not more recently (Park et al., 2017).
Recently, the impact of increasing temperatures in the Arctic, in combination with decreased salinity (Monteiro et al., 2019b; Diehl and Bischof, 2021), increased partial pressure of CO2 (pCO2) (Olischläger et al., 2014, 2017; Iñiguez et al., 2016), ultraviolet (UV) radiation stress (Parages et al., 2013), increased sedimentation (Zacher et al., 2016) or increased nutrient conditions (Diehl and Bischof, 2021), were investigated. All these studies showed that growth, photosynthetic performance, biochemical composition and the transcriptomics of S. latissima were strongly affected by temperature. The species would benefit from higher temperatures in Arctic regions, whereas the impact of the other drivers was less pronounced or there was no impact at all. On the contrary, the early stages of S. latissima appear vulnerable to strong warming and interaction with other factors in the Arctic. Overall, strong interactions between light and temperature were also detected in different microstages, highlighting the impairing effect of UV-B radiation (Müller et al., 2008, 2012). Increased production of superoxide anion radicals was measured in gametophytes in increasing temperatures between 2 and 18 °C and slightly under UV radiation (Müller et al., 2012). Temperatures ≤21 °C combined with hyposalinity diminished the spore settlement of S. latissima from Alaska (Lind and Konar, 2017). Although higher temperatures generally lead to higher germination rates of Arctic S. latissima spores, temperature and grazing had an interactive effect (Zacher et al., 2016). At 5 °C, the germination rate was higher when grazers were present, whereas at 9 °C, the reverse happened. The same pattern holds for the density of juvenile sporophytes. The species-specific interactive effects revealed a differential response between co-occurring kelps in the Arctic.
Large ecosystem shifts from S. latissima canopies or dominance to turfs or barrens have been reported. Generally, the loss of S. latissima populations has been attributed to warming to a certain extent. In Norway, S. latissima communities were observed to be replaced by ephemeral, filamentous turf algae (Moy and Christie, 2012; Christie, Andersen et al., 2019). This ecosystem shift was proposed to have been driven mainly by extraordinarily high temperatures over summer, in combination with eutrophication (Moy and Christie, 2012). Loss of S. latissima beds and shifts to turf-dominated ecosystems were also observed in Nova Scotia, Canada, caused by increased temperature and diverse unbalanced multitrophic interactions (Filbee-Dexter et al., 2016).
The impacts of interactions between MHWs and biota on kelp forests appear to be extremely dynamic and complex. Although the severe declines of S. latissima in the eastern and western North Atlantic were attributable primarily to large increases in the frequency and cumulative intensity of MHWs, excluding alternative effects, such as turbidity or biological factors (Filbee-Dexter et al., 2020), kelp forest mortality and recovery in other regions were found also to be controlled in a top-down manner (Christie et al., 2019b; Norderhaug et al., 2021). Thus, multifactorial experimental set-ups are of major importance in identifying the complexity of reactions to climate change and local anthropogenic stressors (Strain et al., 2014). Overall, much research has been done on Arctic and Norwegian populations of S. latissima. In contrast, the knowledge about the potential of southern populations is scarce and should receive particular attention in future studies.
Hydro-optics
As photosynthetic organisms, seaweeds are dependent on light availability to survive. Irradiance effects on S. latissima have already been well studied for decades and have been summarized by Bartsch et al. (2008). Both extremely high and low photosynthetic active/available radiation (PAR) and mainly UV radiation (UVR) cause modifications in multiple biochemical and physiological processes in S. latissima, with early life stages and adult sporophytes showing differences in susceptibility.
More recent studies have demonstrated that reduced irradiance negatively affects the growth performance of sporophytes in situ (Spurkland and Iken, 2011; Forbord et al., 2020) without diminishing the photosynthetic performance (Spurkland and Iken, 2011) but still promoting biofouling (Forbord et al., 2020). The maximum modelled distribution depth of S. latissima in Arctic fjords followed the extent of the meltwater plume, being shallower close to the glaciers and deeper in outer fjord regions (Niedzwiedz and Bischof, 2023). Pronounced variability was found in different parts of the phylloid regarding the long-term storage of the carbohydrate laminarin in Arctic field sporophytes between October and early February (Scheschonk et al., 2019). Also, other biochemical components, such as mannitol or nitrogen, declined greatly during the dark season. Interestingly, darkness appeared to be optimal for artificial sporogenesis of Danish S. latissima compared with other light levels (20–120 μmol photons m−2 s−1) (Boderskov et al., 2021).
A few studies suggest that other response variables, beyond the main physiological and biochemical parameters, are involved in to variations in light in S. latissima. Enhanced release of organic iodine and reduced release of reactive organic bromine and chlorine were found after PAR (23 µmol photons m−2 s−1) + UVR exposure (Laturnus et al., 2010). The impacts of PAR (~10 µmol photons m−2 s−1) and UVR were also investigated in chloroplasts of vegetative (non-soral) and fertile (soral) tissue of S. latissima (Holzinger et al., 2011). The fertile tissue cells were not affected by PAR + UVR, whereas negative effects were found in vegetative parts. For instance, decreased optimal quantum yields of photosystem II Fv/Fm were measured under UVR treatment, and the chloroplast structure was altered, i.e. including more physodes. Another study revealed that the oxygen consumption rate of S. latissima was significantly higher in high light conditions (300 µmol photons m−2 s−1) compared with low light conditions (3 µmol photons m−2 s−1) (McDowell et al., 2015).
Sedimentation and epibiosis have a strong impact on light availability. Saccharina latissima can withstand short-term sediment cover (Roleda and Dethleff, 2011; Picard et al., 2022), whereas longer burial negatively affects its vitality and morphology (Roleda and Dethleff, 2011). Furthermore, it was shown that sediment from melting ice weakened the recruitment of S. latissima (Zacher et al., 2016). Overgrowth with epibionts, with consequent shading, can reduce growth and survival of the species (Andersen et al., 2018).
Polar night imposes very special conditions for Arctic S. latissima, especially when combined with future increases in winter temperatures. Treatments of light/dark or darkness alone seem to have a greater effect on S. latissima than the various temperatures applied (0, 4 or 8 °C) (Scheschonk et al., 2019). The lower laminarin content at elevated temperatures (8 °C) suggests that prolonged darkness might be a problem for S. latissima under future temperature trends.
In a comparable study on S. latissima sporophytes, low temperatures (2 °C) and PAR (10 µmol photons m−2 s−1) + UVR treatments activated the rapid phosphorylation of mitogen-activated protein kinases, while UVR generally impaired the photosynthetic performance (Parages et al., 2013). A study in juvenile Arctic sporophytes revealed that Fv/Fm remained unchanged in low PAR treatments (~24 µmol photons m−2 s−1), even with the addition of UVR, and that it decreased under high light stress (~110 µmol photons m−2 s−1), especially when combined with UVR (Heinrich et al., 2012b; Heinrich et al., 2015). Remarkably, the photosynthetic performance exhibited particularly severe reduction at high PAR + high temperatures (17 vs. 2 and 7 °C) (Heinrich et al., 2012b), whereas when UVR was included in a comparable set-up, the strongest inhibition occurred in the high PAR + UVR treatment at 2 °C, compared with 7 and 12 °C (Heinrich et al., 2015). Thus, high temperatures appear to mitigate the impairing effects of UVR on S. latissima sporophytes. However, these observations were more pronounced in laboratory cultures than in field sporophytes (Heinrich et al., 2015).
Investigation of the effects of irradiance (<10 and 30–50 μmol photons m−2 s−1), temperature (4, 8 or 12 °C) and season on gametophyte growth and reproduction of S. latissima revealed that the gametophyte length, sporophyte length, fraction of female gametophytes with eggs, and fraction of female gametophytes with sporophytes were all altered mainly by temperature and season (Raymond and Stekoll, 2021). Irradiance significantly affected all response parameters except for gametophyte length; however, interactions were found only for sporophyte length (irradiance × temperature).
In the last decade, transcriptomic responses of S. latissima to different light conditions have been investigated (Heinrich et al., 2012b; Heinrich et al., 2015, 2016; Li et al., 2020b; Xing et al., 2021). On the time scale of 24 h exposure, the combination of high temperature and high PAR induced more transcriptomic regulation than low temperature and low PAR. High PAR and high temperature widely downregulated genes involved in photosynthesis, including photosystem I/II components, thylakoid protein and light harvest complex proteins with strong folds (≤60-fold). Genes encoding reactive oxygen species (ROS) scavenging enzymes, oxygen heat shock proteins and proteins involved in proteolysis were upregulated under high PAR and high temperature conditions. In contrast, the combination of high PAR and low temperature generally upregulated genes encoding photosynthesis, ROS scavengers and heat shock proteins, whereas downregulated genes encoded proteolysis-related protein. Exposure to UVR for 24 h also induced a wide regulation of gene expression, mainly including photosynthetic components, DNA repair, vitamin B6 biosynthesis and ROS scavengers, which supported that UVR negatively affected photosynthesis and damaged DNA (Heinrich et al., 2012b). Long-term (14 days) exposure to PAR, UVR and temperature combinations resulted in large transcriptomic reprogramming, which did not cause physiological adjustments. The combination of high PAR and UVA caused more gene regulation than the single exposure to high PAR or UVR and mainly upregulated genes encoding photosynthetic components, pigment metabolism, glycine, serine and threonine metabolism and ROS scavenging enzymes. The transcriptomic responses of S. latissima to 14 days of darkness at two temperatures revealed that darkness induced more regulated genes than increased temperature (Li et al., 2020b). Darkness downregulated genes encoding enzymes involved in glycolysis and metabolite biosynthesis. Some energy-consuming processes, e.g. photosynthetic components and biosynthesis of transporters were also repressed. On the contrary, genes coding for the catabolism of lipid and laminarin, the glyoxylate cycle and signalling were upregulated in darkness, pointing out the possible energy source of S. latissima during the polar night.
Salinity
Coastal salinity frequently varies with tidal ranges, precipitation, freshwater plumes from rivers or terrestrial run-offs (Lüning, 1990), increasing with climate change (Holt et al., 2010; Masson-Delmotte et al., 2021). Variation in salinity is particularly relevant for the physiology of S. latissima in Arctic fjord systems owing to enhanced sea ice and glacier melting (Hanelt et al., 2001; Svendsen et al., 2002; Sundfjord et al., 2017). Fluctuations in salinity lead to osmotic stress, with consequences at the physiological and biochemical level, which is, overall, well studied for seaweeds (see Karsten, 2012 and references therein) but not for S. latissima. Although Laminaria sensu lato is considered a rather stenohaline genus (Bartsch et al., 2008), S. latissima is known physiologically to tolerate broad ranges of salinities between absolute salinities (SA) 5 and 60 (Karsten, 2007), although young sporophytes were shown to have a tolerance of down to SA 11 in laboratory conditions (Karsten, 2007; Peteiro and Sánchez, 2012), which allows the species to inhabit brackish waters (Nielsen et al., 2016c; Mortensen, 2017). Nevertheless, hyposalinity results in decreased growth (e.g. Spurkland and Iken, 2011; Marinho et al., 2015; Bruhn et al., 2016; Forbord et al., 2020), diminished photosynthetic performance (e.g. Karsten, 2007; Spurkland and Iken, 2011; Peteiro and Sánchez, 2012) and loss of pigmentation (Karsten, 2007; Peteiro and Sánchez, 2012). Furthermore, decreased carbon dioxide exchange rates were detected at low salinities (Mortensen, 2017). Generally, salinity has a strong effect on the biochemical composition of S. latissima. For instance, the content of sulfated fucose-rich polysaccharides, measured with fucoidan, generally increased at absolute salinities (SA 15–25) in the Baltic Sea; however, the pattern did not hold for all locations (Bruhn et al., 2017). Samples of S. latissima from an Atlantic population hold higher contents of fucose-containing sulfated polysaccharides than a Baltic population, which experiences lower salinity variation than the former population (Ehrig and Alban, 2015). Along the salinity gradient of the Baltic Sea’s, effects of salinity were observed in carbohydrates, proteins, pigments and nitrogen contents (Nielsen et al., 2016a). However, it should be noted that these observations were not necessarily consistent between different populations or experimental frameworks (Manns et al., 2017; Diehl et al., 2023).
Little is known about the interaction between salinity and other factors in S. latissima, with only salinity × temperature having been investigated so far. Recent studies revealed that potentially, hyposalinity is highly stressful for S. latissima in combination with temperature variation. In the Baltic Sea, low salinity in combination with high summer temperatures decreases the productivity of S. latissima owing to high physiological stress in cultivated seaweed (Nielsen et al., 2014). Arctic field adult sporophytes of S. latissima, however, were almost unaffected by an increase in temperature (from 4 to 10 °C) and hyposalinity (SA 25) in mimicked field conditions (Diehl et al., 2020), although slightly increased growth and photosynthetic performance (Fv/Fm) were detected at higher temperatures. In contrast to adult sporophytes, more pronounced effects of both parameters and some interaction of salinity and temperature are detectable in the early life stages of S. latissima. For instance, elevated temperatures and low salinities decreased spore settlement and gametophyte growth (Lind and Konar, 2017). The impact of temperature × salinity interaction was investigated in young sporophytes from Brittany and the Arctic by running comparable experiments on specimens from both locations (Monteiro et al., 2019b; C. Monteiro et al., 2020a; Li et al., 2020a). Remarkably, similar effects were observed in young sporophytes from the two regions. Lower salinities had little negative impact on growth and Fv/Fm and modified the xanthophyll-cycle pigment pool. The effects of different temperatures were more pronounced, revealing ameliorating effects of higher temperatures and diminishing effects of lower temperatures.
At the transcriptomic level, an ameliorating effect of high temperature was observed for algae from Brittany and Svalbard (Monteiro et al., 2019b; Li et al., 2020a). The treatments at low salinity (SA 20) at 0 and 8 °C elicited more differentially expressed genes than at 15 °C and low salinity. Geographical variation also played an important role, because the combination of low salinity and low temperature was especially stressful for sporophytes from Brittany (not exposed to 0 °C in their environment of origin) than from Svalbard. In response to low salinity, metabolic pathways such as photosynthesis and carbon assimilation were downregulated, and some gene coding enzymes contributed to the xanthophyll cycle and cell wall metabolism were also down-regulated. Moreover, genes coding for heat shock proteins and enzymes involved in the synthesis of mannitol and proline were not significantly regulated during this experiment, perhaps revealing that the stress was mild or that the regulation of salt stress is more intricate than expected, involving several other pathways than those already described for other environmental drivers.
Nutrients
The macronutrients nitrogen (N) and phosphorus (P) serve as essential elements for photosynthesis and growth, of which N is considered the main limiting resource for macroalgal productivity (Roleda and Hurd, 2019). An overview of nutrient physiology and factors affecting nutrient uptake in seaweeds is provided by Roleda and Hurd (2019). Effects of various nutrient regimes have been well investigated for Laminariales, including S. latissima (summarized by Bartsch et al., 2008). Laminariales can accumulate nutrient reserves over winter when nutrient conditions are favourable (Bartsch et al., 2008; Lubsch and Timmermans, 2019) and have an optimal environmental nitrate concentration of ~10 μm but also tolerate oligotrophic conditions (Kerrison et al., 2015). Nutrient depletion has long been known to have negative impacts on the physiological status of S. latissima, resulting, for instance, in lower growth rate and lower photosynthetic performance (Williams and Herbert, 1989; Gerard, 1997a, b; Korb and Gerard, 2000; Roleda and Hurd, 2019). A recent study revealed that the development, density and growth in length of young sporophytes were also diminished in nutrient-poor conditions (Raymond and Stekoll, 2021). Nitrate uptake rates are linearly related to the substrate concentrations for both N-limited and N-saturated young sporophytes, indicating that S. latissima requires high ambient nitrate concentrations in the environment to produce rapid growth. Sporophytes with deficient internal nitrogen pools exhibited higher uptake rates of nitrate than sporophytes with higher internal nitrogen pools (Forbord et al., 2021). As a result, the growth of S. latissima decreases significantly over the summer, although it can continue to grow for some time even in low nutrient conditions (Nielsen et al., 2014; Lubsch and Timmermans, 2019; Forbord et al., 2020). The ability of the species to store nutrients is also considered an advantage in direct competition for habitat with other seaweeds (Armitage et al., 2017). Several physiological parameters of S. latissima are also limited by bioavailable P (Bruhn et al., 2016). Comparing the effect of P enrichment on spores and gametophytes in February and April showed that growth was supported by elevated P levels (23–69 μm), and earlier gametophyte development appeared under P treatment in April (Nielsen et al., 2016a). Sufficient or slightly enhanced N supply is reported to have beneficial effects on the response of S. latissima with respect to several environmental stressors. For instance, it was found that UV damage in S. latissima can be mitigated or prevented by enriched (50 µm) N supply (Davison et al., 2007). Recent studies on nutrient × light interactions showed the high importance of nutrients (N + P). Specimens were not much altered overall by the different natural light intensities, but growth and intracellular N were positively affected by elevated nutrient conditions (Boderskov et al., 2016; Jevne et al., 2020). The contents of total carbon (C) decreased, and chlorophyll a and fucoxanthin increased in nutrient-rich conditions and varied between frond parts (Boderskov et al., 2016). No distinct interaction of light and nutrients was determined. However, interactions of nutrients and light were found regarding sterolic compounds (de Jong et al., 2021). Highest sterol content was measured at low nutrient and high light, although enhanced nutrient conditions combined with high light resulted in unchanged or even decreased concentrations. However, the authors attributed the results to reduced photosynthetic function rather than nutrient fluctuations.
A recent study on the interaction of nutrient availability and wave exposure revealed that fronds grow narrow under high wave exposure and in high nutrient concentrations and wider in low nutrient concentrations (Zhu et al., 2021). Additionally, the biomass, shape and C:N ratio of the frond surface were affected by waves, nutrients and their interaction. Thereby, specific morphological changes can compensate for nutrient-poor conditions.
Eutrophication has become a common phenomenon in coastal regions, triggered mainly by anthropogenic nutrient input (Skjoldal, 1993; Norderhaug et al., 2015). A moderately enhanced N (~3–20 µm) supply was reported to influence the physiology of S. latissima positively (e.g. Chapman et al., 1978; Conolly and Drew, 1985; Gerard, 1997a). However, severe eutrophication levels combined with high temperatures are detrimental (Moy and Christie, 2012). In contrast, Arctic primary production was reported to be limited by low nutrient availability (<1 µm), but nutrient concentrations are expected to increase and alter seasonal patterns as melting, and thus freshwater run-off, increases and occurs earlier (Zacher et al., 2010; Filbee-Dexter et al., 2019). Only marginal positive effects of nutrient enrichment on the physiological and biochemical status were reported in sporophytes of S. latisima in the Arctic (Gordillo, 2006; Diehl and Bischof, 2021). Temperature effects outcompeted nutrient supply, and no significant interactions of temperature and nutrients were determined (Diehl and Bischof, 2021).
Saccharina latissima can act as a bioremediator. In investigating the potential of S. latissima to remove nutrients from eutrophic brackish fjord systems and the parallel effects on several chemical compounds of the species, it was found to survive hyposalinity in elevated nutrient conditions (Mortensen, 2017). Higher protein and tissue N content and lower contents of β-glucans and iodine were found in young S. latissima maintained in brackish water with nutrient supplementation compared with conditions in seawater with adequate nutrient supply. Furthermore, the study revealed that the beneficial effects of increased nutrient levels were greater in young sporophytes than in older ones. The potential of algae to sequester nutrients poses great potential for establishing integrated multi-trophic aquaculture, which aims to reduce eutrophication caused by intensive fish farming (Kim et al., 2015; Marinho et al., 2015). While removing large amounts of N from the environment, S. latissima benefits from the elevated nutrient conditions by enhancing its growth by ≤50 % compared with a reference site (e.g. Sanderson et al., 2012; Broch et al., 2013; Wang et al., 2014; Fossberg et al., 2018). Different studies describe enhanced growth, photosynthetic activity, N (protein) concentration and pigment content, resulting in higher biomass quality of cultivated S. latissima (Sanderson et al., 2012; Wang et al., 2014; Rugiu et al., 2021; for further information, see Saether et al., 2023).
The effects of micronutrients on S. latissima are still largely unexplored. Trace metals are essential for various metabolic functions in seaweeds but can also be harmful at higher concentrations (Stengel et al., 2005 and references therein). The only studies on the effects of microelements, e.g. iodine or copper, on S. latissima were conducted >30 years ago (Hsiao and Druehl, 1973; Brinkhuis and Chung, 1986; Chung and Brinkhuis, 1986). However, for other Laminariales, iodine has been shown to support osmotic functions (Nitschke and Stengel, 2014), iron had a strong impact on gametogenesis (Raymond and Stekoll, 2021), and copper modified the transcriptomic profile (Zhang et al., 2019). The extent to which abiotic factors and distribution patterns affect the concentration of microelements in S. latissima is unknown. In addition, the fact that S. latissima accumulates micronutrients from the environment (e.g. Schiener et al., 2015; Bruhn et al., 2016; Nielsen et al., 2016b) is of high relevance to the food industry, because concentrations above certain thresholds can exclude S. latissima biomass from human consumption (e.g. Bruhn et al., 2019; Kim et al., 2019; Roleda et al., 2019).
pH
Ocean acidification (OA) refers to the ongoing decrease in seawater pH and variations in carbonate chemistry resulting from the substantial marine uptake of CO2 since the Industrial Revolution (Doney et al., 2020). Studies about the effects of OA on S. latissima have focused mainly on growth, photo-physiology and biochemistry. Ocean acidification has been reported to increase (Gordillo et al., 2015; Olischläger et al., 2017; Young and Doall, 2021), not affect (Iñiguez et al., 2016; Olischläger et al., 2017) or even decrease (Swanson and Fox, 2007) the growth rates of S. latissima, according to the duration of the experiment and the levels of pCO2 applied. Photophysiology, reflected by different parameters (e.g. pigments, photosynthetic O2 evolution and CO2 uptake, and chlorophyll a fluorescence), also showed various responses in OA conditions. For example, in some studies, it was shown that OA (~1000 and ~800 ppm, respectively) significantly increased the rates of photosynthetic CO2 uptake and O2 evolution rates (Longphuirt et al., 2013; Nunes et al., 2016), whereas another study failed to detect differences in net photosynthesis rates between ambient (390 ppm) and increased pCO2 levels (1200 ppm) (Iñiguez et al., 2016). Regarding the biochemistry, S. latissima was found to use more CO2 than bicarbonate (HCO3−) as the photosynthetic carbon source, revealed by the signatures of a stable carbon isotope (δ13C) (Young and Doall, 2021). The contents of soluble carbohydrates, nitrogen and lipids changed in sporophytes of a temperate population of S. latissima, whereas they remained stable in the Arctic samples when pCO2 increased alone (Olischläger et al., 2014). Saccharina latissima has been found to mitigate the negative effects of OA on farmed bivalves by increasing pH and the saturation state for aragonite (Young et al., 2022). Thereby, the co-cultivation of bivalves and S. latissima is likely to be a promising integrated multi-trophic aquaculture approach to generate synergistic benefits in future OA scenarios.
The effects of OA on S. latissima have been investigated in interaction with temperature (Olischläger et al., 2014, 2017; Iñiguez et al., 2016) and UVR (Gordillo et al., 2015). The effects of increased pCO2 on growth, biochemical composition and photosynthetic performances of S. latissima were generally less pronounced than those of increased temperature (Olischläger et al., 2017). Furthermore, Arctic S. latissima was more resilient to increased pCO2 and more likely to benefit from climate change than the temperate population, as reflected by its increased growth rates at elevated pCO2 and higher temperatures (Olischläger et al., 2014, 2017). The interactive effects of OA and UVR illustrated that OA increased the growth of S. latissima, meanwhile inhibiting a series of UVR-driven responses (e.g. pigments and photosynthetic electron transport) (Gordillo et al., 2015). Owing to the various responses of S. latissima to OA discussed above, more work is needed to understand how OA is affecting S. latissima and will continue do so in the future. Besides, no studies on the molecular mechanisms regulating responses of S. latissima to OA are available to date, hence transcriptomics and/or metabolomics could help to understand the gene regulation and related metabolic pathways of S. latissima in OA conditions.
BIOTIC INTERACTIONS
Microbiome
Macroalgal functioning must be considered to be a result of the interactions between the algal hosts and their associated microbiota, forming a singular entity, the algal holobiont (Egan et al., 2013). Algal microbial partners can be prokaryotes, such as viruses, Archaea or bacteria, and eukaryotes, such as fungi. Bacterial partners regulate and support macroalgal health and fitness (Goecke et al., 2010), pathogen resistance (Wiese et al., 2009), to a changing environment (Dittami et al., 2016), and metabolism (Burgunter-Delamare et al., 2020).
The S. latissima microbiota has become a subject of interest only in recent years (Vallet et al., 2018; Tourneroche et al., 2020; King et al., 2022; Liu et al., 2022; Burgunter-Delamare et al., 2023). Bacteria associated with S. latissima are also found classically in other brown macroalgae (Hollants et al., 2013) and belong predominantly to the Proteobacteria and Bacteroidota phyla (Tourneroche et al., 2020; Burgunter-Delamare et al., 2023). At the class level, Alphaproteobacteria and Gammaproteobacteria (Liu et al., 2022; Burgunter-Delamare et al., 2023), Deltaproteobacteria, Bacilli, Flavobacteriia, Planctomycetia and Verrucomicrobiae (Liu et al., 2022) have been found. Bacterial strain isolation experiments determined that strains were affiliated with Actinobacteria, Bacteroidetes, Firmicutes and Alpha-, Beta- and Gammaproteobacteria and belonged to 21 genera (Wiese et al., 2009). The genera Marinobacter, Psychromonas, Litorimonas and Aquimarina were also exclusively found attached to the blade of S. latissima and not in the surrounding seawater (Liu et al., 2022). The bacterial composition changes gradually along the blade, shifting from a lower to higher alpha-diversity from the meristem to the distal part, reflecting the age gradient (Staufenberger et al., 2008; Burgunter-Delamare et al., 2022, 2023). The degree of colonization is linked, in part, to the types of metabolites released by the algae (Tourneroche et al., 2020).
A bacterial core is found in S. latissima independent of the geographical origin, season or physiological state of the specimens. When looking at the meristematic part, a small core, comprising the four genera Granulosicoccus sp., Litorimonas sp., Hellea sp. and Blastopirellula sp., was found in two studies [8 of 13 Amplicon Sequence Variant (ASVs) and four of nine genera (King et al., 2022); four genera (Burgunter-Delamare et al., 2023)]. Five additional ASVs (Croceitalea sp., Robiginitomaculum sp., Gammaproteobacteria sp., OM190 sp. and KI89A_clade sp.) were also found in this blade region (King et al., 2022). The bacterial core composition also shows shifts from low to higher diversity along the blade at the genus level. The distal bacterial core comprises the four genera found in the meristem core plus the five genera Algitalea, Arenicella, Portibacter, Tenacibaculum and Bdellovibrio (Burgunter-Delamare et al., 2023). In addition, when looking at the core community and the ASVs found specifically attached to a particular tissue, particularly Granulosicoccus and Litorimonas, ecology and genome profiles suggest that they might be necessary functionally for the host (King et al., 2022; Burgunter-Delamare et al., 2023). For example, the Granulosicoccus genus might help its host thanks to key functions encoded in its genome (e.g. alginate metabolism, vitamin B12 biosynthesis, nitrogen reduction from nitrate to ammonium, or dissolved organic matter assimilation) and thus potentially providing the kelp with vitamins and available nitrogen (Kang et al., 2018; Capistrant‐Fossa et al., 2021; Weigel et al., 2022).
Fungi infect the blade more often than other parts, and fungal communities comprise principally Ascomycota and Basidiomycota (Vallet et al., 2018; Tourneroche et al., 2020), with a predominance of Dothideomycetes and Sordariomycetes (Vallet et al., 2018) or Psathyrellaceae (Tourneroche et al., 2020). Additionally, S. latissima is colonized by viruses classified as Phaeovirus [Saccharina latissima virus, SlatV, family Phycodnaviridae (Schroeder and Mckeown, 2021)]. They are latent double-stranded DNA viruses that insert their genome into that of their host (McKeown et al., 2017) and exist in three subgroups (A, B and C). Phaeoviruses are geographically widespread in the Laminariales (McKeown et al., 2018). In particular, Laminaria and Saccharina genera are infected by Phaeovirus from subgroup C (McKeown et al., 2017). Identifications of these viruses are supported by novel Phaeovirus major capsid protein (mcpl MCP) sequences found in kelp (by PCR) (McKeown et al., 2017, 2018; Schroeder and Mckeown, 2021).
Environmental factors can influence the composition of the microbiota in S. latissima (King et al., 2022). Several studies have compared the bacterial population from different geographical origins and found regional structuring in S. latissima [Baltic and North Sea (Staufenberger et al., 2008; Lachnit et al., 2009), North and West Scotland, Wales and South England (King et al., 2022); Brittany, Helgoland and Skagerrak (Burgunter-Delamare et al., 2023)]. The global epibacterial communities of S. latissima were differentiated between the Baltic and North Sea (Staufenberger et al., 2008; Lachnit et al., 2009). Differences regarding salinity, tidal range and bacterioplankton composition between sampling sites are likely to explain this. A regional structuring across British sites (North and West Scotland, Wales and South England) was also discovered, whereby bacterial communities in Wales differ from those in North and West Scotland. Here, the temperature is not the factor responsible, but rather the variable portion of the microbiota that reflects random and determinant processes within the host environment (King et al., 2022), because reef habitats are highly dynamic and influenced by several factors that vary across multiple scales (Kaiser, 2011; Lamy et al., 2018). In the same way, samples from Brittany, Helgoland and Skagerrak cluster according to their region of origin (Burgunter-Delamare et al., 2023). Abiotic factors can lead to cellular stress and senescence and will thus create a new ecological niche for specific bacterial groups (Burgunter-Delamare et al., 2023). Also, algal genotypes differ depending on the region (see ‘Biogeographical patterns’) (Guzinski et al., 2016, 2020) and can impact bacterial communities. The chemical and lipid content in membranes also varies with environmental factors (see ‘Responses to environmental drivers’), hence attractiveness for bacteria is influenced (Burgunter-Delamare et al., 2023). Furthermore, the associated microbial communities can vary with seasonality. Regardless of the mechanisms, seasonal changes can vary from site to site; therefore, any conclusions drawn about seasonality are valid only for the studied area. Differences between winter and spring were found at the blades and rhizoid levels of S. latissima from the Baltic Sea (Staufenberger et al., 2008). In Brittany (Roscoff, France), the abundances of Firmicutes, Actinobacteria and Alpha- and Gammaproteobacteria were impacted, with an increase in autumn for the Firmicutes and Alphaproteobacteria, in summer for the Actinobacteria and in spring for the Gammaproteobacteria. The seasonal changes were linked to the nutrient content of seawater and the chemical composition of the algae (Burgunter-Delamare et al., 2023).
Although the biological impact of viruses on their hosts is largely unknown, researchers are working on the microbial effects on the host regarding potential pathogens. By performing co-culture experiments with bacteria specifically isolated from S. latissima, it has been shown that a disruption in the microbiota composition (dysbiosis) is correlated with an increase in quorum sensing molecules (bacterial ability to detect and respond to cell population density through gene regulation) and a decrease in algal growth (Burgunter-Delamare, 2022). Also, Aquimarina, Parcubacteria and Peronosporomycetes were suggested as potential pathogens of S. latissima (Liu et al., 2022). Conversely, initial evidence that fungal partners of brown macroalgae might protect their host in vivo by producing molecules as an active chemical defence has been provided by Vallet et al. (2018). Thus, the algal microbiota might manage the infection rate of pathogenic microbes in the phycosphere.
Mobile biota
Kelps are essential coastal habitats for many commercially important fish and crustacean species (Seitz et al., 2014). However, specific associations between fish/crustaceans and S. latissima have been poorly assessed. One study found 358 individuals of fish and crustaceans associated with S. latissima communities in Southern Norway, higher than the number of individuals associated with eelgrass and turf algae but lower than the specimens caught in forests of Laminaria hyperborea (700). Regarding species richness and diversity, eelgrass beds held higher diversity than S. latissima and the other habitats (Christie et al., 2022). Habitat preferences of fish are species specific and vary with life stages. Young (<1-year-old) cod in Norwegian waters prefer red algae and eelgrass to habitats dominated by S. latissima, whereas cod >1 year old used all seaweed and seagrass habitats equally. In turn, the fishes Goldsinny wrasse (Ctenolabrus rupestris) and corkwing wrasse (Symphodus melops) preferred S. latissima and red algae over eelgrasses (Dunlop et al., 2022). In the Northwest (NW) Atlantic, the residential fish cunner (Tautogolabrus adspersus) uses S. latissima and other large-blade Phaeophyta for foraging and refuge (O’Brien et al., 2018). Saccharina latissima offers a better refuge for fish (>1 cm in length) but a lower-quality habitat for meso-invertebrates than other morphologically different macroalgae, such as turf (Ware et al., 2019). However, a decline of large predatory fish has cascading effects throughout the food web, ultimately reinforcing the decline of S. latissima in some regions (Eriksson et al., 2009).
Epi- and endobiota
Saccharina latissima, like other kelps, can serve as a substratum, allowing smaller algae and animals to grow on (epiphytes) or inside (endophytes) its thalli (Bartsch et al., 2008). Considering epiphytes, both macroalgae (e.g. Ectocarpus siliculosus, Ulva lactuca and Champia parvula) and microalgae (e.g. pennate diatoms, including genera Licmophora, Navicula and Nitzschia) were observed on the surface of S. latissima (Liu et al., 2022). Considering endophytes, microscopic brown algae with filamentous thalli, mostly Ectocarpales sensu lato, are common in kelps (reviewed by Bartsch et al., 2008) and in S. latissima (Bernard et al., 2018). A study revealed that 88 % of endophyte algae from kelps belonged to the genera Laminarionema and Laminariocolax, with two isolates belonging to the genera Ectocarpus (Bernard et al., 2019b). Furthermore, the most common endophyte in European S. latissima is Laminarioema elsbetiae (Bernard et al., 2019a). The infection rates of endophytic algae in wild S. latissima along the European coasts were found to be ≤100 % (Bernard et al., 2018). The occurrence and abundance of epi-/endophytic algae were affected both by environmental factors, such as seasons and locations, and by characteristics of S. latissima, such as age and position (Peteiro and Freire, 2013a; Bernard et al., 2019b; Corrigan et al., 2023). For example, the abundance of epiphytes on S. latissima was observed to be significantly higher for fronds growing in the sheltered area of the bay compared with those farmed at an exposed location, and the greatest quantities of epiphytes were on the apical parts of S. latissima blades (Peteiro and Freire, 2013a). Besides, cultivated S. latissima in Northern Brittany was not found to be affected by Laminarioema elsbetiae, which is highly prevalent in the wild populations of European S. latissima (Bernard et al., 2019a). The infection with epibionts can reduce the photosynthesis of S. latissima by hindering ≤90 % of available light, revealed in laboratory conditions (Andersen et al., 2018).
In addition to causing morphological changes, endophytic algae also adversely impact the physiological and biochemical traits of kelps, such as growth and reproduction. Transcriptomic analysis demonstrated that S. latissima upregulated many cell-wall modification-related genes and stress response-related genes during the infection of the endophyte Laminarioema elsbetiae, suggesting that endophytic algae damaged the cell wall and induced oxidative stresses in S. latissima (Xing et al., 2021). In Norway, cultivated S. latissima sustains a heavy load of epibionts, ≤90 % of available area, causing light deprivation driven mainly by epiphytic algae and ascidians and, to a lesser extent, by bryozoans (Andersen et al., 2018). The lack of S. latissima populations at the Skagerrak coast was suggested to be attributable to heavy epiphytism rather than the direct effect of abiotic factors on S. latissima, because transplanted sporophytes were able to grow and mature until the epiphyte load increased in the summer (Andersen et al., 2011). The reduced growth and survival of kelp populations in shallow waters are also driven by the heavy load of epibionts, driving S. latissima populations deeper down and reducing their vertical distribution. This impact is seasonal and site specific; hence, it probably interacts with other environmental factors to drive the ongoing decline of S. latissima populations (Andersen et al., 2018).
In the wild, the bryozoan Membranipora membranacea, which is an epiphyte on S. latissima, has negative effects on populations of S. latissima in the NW Atlantic, namely tissue weakening, breakage and, ultimately, loss of kelp biomass (Attridge et al., 2022). Populations of this bryozoan, invasive in the Northeast (NE) Atlantic, are expected to increase under climate change scenarios, further impacting S. latissima populations in the area (Denley et al., 2019). In the NE Atlantic, M. membranacea is a common native bryozoan, and although very little is known for natural populations, impacts of this species on cultivated S. latissima are already reported (e.g. Førde et al., 2016; Forbord et al., 2020). Another common bryozoan on kelps is Electra pilosa; however, this species has a slower growth rate and less substrate preference than M. membranacea and seems to have a more benign effect on kelps, including S. latissima; a pattern that holds on both sides of the Atlantic (Yorke and Metaxas, 2011; Førde et al., 2016).
Mobile and epiphytic communities associated with S. latissima farms in Norway were shown to be significantly different from those associated with wild stands, holding less biodiversity and a smaller number of individuals (Bekkby et al., 2023). The dominant species also differed between farmed and wild stands, with isopods being abundant in farmed S. latissima and nearly absent in the wild sporophytes. Also, kelp farms represent an additional, richer habitat than the surrounding water column (Bekkby et al., 2023). An S. latissima farm in Sweden had a significantly positive impact on the amount and diversity of benthic infauna and attracted a similar number of mobile taxa to the nearby wild sites (Visch et al., 2020). In a field study in Ireland comparing the associated biota of four macroalgae (S. latissima, Halydris siliquosa, Fucus serratus and Sargassum muticum), S. latissima held the lowest biomass of epiphytic algae of the four species (Strong et al., 2009). Saccharina latissima supported a broad epiphytic faunal community (significantly different from the other macroalgae), with the species Gibbula umbilicalis, Corophium volutator and Ischyrocarus anguipes being characteristic of the thallus of S. latissima. In turn, the grazer amphipod Dexamine spinosa was considerably more abundant in Sargassum muticum than in S. latissima and had no significant effect on the growth of S. latissima. Saccharina latissima also showed more resilience to fouling (with only 9 % of biomass loss) when compared with the invasive Sargassum muticum (with mean losses of 70 %) (Strong et al., 2009).
The biota associated with S. latissima in Kongsfjorden, a high Arctic fjord on the west coast of Spitsbergen, was assessed (Shunatova et al., 2018). One hundred and eleven sessile taxa were reported in S. latissima individuals attached to stone in 2018: 80 animals (of these, 56 were Bryozoa) and 30 algae taxa (of these, 36 were Phaeophyceae and 11 Florideophyceae) (Shunatova et al., 2018). Species richness associated with S. latissima was higher than in nearby sediment substrates. Both species richness and biomass varied with microhabitat and season, being considerably higher on the holdfast compared with blades and stipes and in January compared with May and September.
Grazers
Although S. latissima contains high levels of phlorotannins that decrease the digestibility of the species, several animals can still graze directly on it. Among them is the snail Lacuna vincta (O’Brien and Scheibling, 2016; Young and Doall, 2021). A comparative study revealed that S. latissima is one of the preferred food sources for L. vincta and the macroalgae that elicits a higher growth rate of the snail (Chavanich and Harris, 2002). This snail prefers reproductive over vegetative tissue, probably owing to lower levels of phlorotannins in the former, compromising the reproductive success of S. latissima (O’Brien and Scheibling, 2016). Lacuna vincta also consumes S. latissima at higher rates when pretreated with high temperatures (21 °C), probably because the tissue is easier to consume (weaker and more fragile at higher temperatures) (Simonson et al., 2015a). The grazing rate of L. vincta appeared to be unaffected by changing temperatures (Simonson et al., 2015a) but decreased in OA conditions (Young and Doall, 2021).
A significant group in the coastal food web are sea urchins. Across the globe, events of mass grazing by sea urchins have decimated kelp forests and given rise to sea urchin barrens (Filbee-Dexter and Scheibling, 2014b). Several studies have shown that grazing pressure of the green sea urchin Strongylocentrotus droebachiensis led to the decline of L. hyperborea (e.g. Rinde et al., 2014) in several areas in the NE Atlantic and of Saccharina longicruris, now S. latissima, in the NW Atlantic. Although field studies investigating the direct link between S. droebachiensis and S. latissima are rare, laboratory experiments show that S. droebachiensis indeed feeds on S. latissima (Daggett et al., 2010; Eddy et al., 2012), and growth rates of the sea urchins fed S. latissima or other species of macroalgae is similar (Carrier et al., 2017).
The growth and survival of S. droebachiensis are, in turn, controlled by its predators (Norderhaug et al., 2021) and by disease outbreaks (Feehan, 2014). A field and laboratory study in Nova Scotia showed that the presence of the crab Cancer borealis did not change the foraging behaviour of the sea urchin on S. latissima. A greater proportion of sea urchins around cages with S. latissima than without was also determined, revealing some response to a food cue (Harding and Scheibling, 2015). Another study revealed that juveniles of S. droebachiensis inhabiting S. latissima holdfasts are 20–30 % less likely to be predated by the crabs C. borealis and Cancer irroratus when compared with treatments with no refuge (Feehan et al., 2019). Also, there was a correlation between S. latissima volume and the size of sea urchin juveniles, showing that S. latissima serves as food, habitat and refuge for S. droebachiensis (Feehan and Francis, 2014). Moreover, S. latissima detritus remains a main food source even for deep-living sea urchins (60 m) that can maintain a good reproductive status (Filbee-Dexter, 2014). In a laboratory experiment with samples of S. latissima from Alaska, a high sediment load (as in a land-terminating glacier) led to a sharp decrease in grazing rates of S. droebachiensis on S. latissima. In the same experiment, increasing temperature had no effect on grazing rates (Traiger, 2019).
Other species of sea urchin feed on S. latissima, such as Arbacia punctulata, although they prefer turf algae to S. latissima (Hamel, 2022). The purple sea urchin Paracentrotus lividus also feeds on S. latissima (Castilla-Gavilán et al., 2019), although the best growth performance is achieved when fed on the red alga Palmaria palmata. A set of mesocosm experiments compared respiration and consumption rates of several grazers under medium and increased temperatures (Gilson et al., 2021). The common sea urchin Echinus esculentus preferred the combination of S. latissima and L. digitata over Laminaria ochroleuca and Saccorhiza polyschides, the gastropod Steromphala umbilicalis consumed more of the latter, and the amphipod Gammarus spp. did not exhibit a preference. In addition, both E. esculentus and Gammarus spp. increased their respiration rates under warming, but only Gammarus spp. increased their consumption rates. In turn, S. umbilicalis increased growth with warming, but the other two species did not. Another animal group feeding on S. latissima are fish, such as wrasses, although S. latissima represents only a small percentage of their diet (Bourlat et al., 2021). However, more studies looking at the gut content of fish are necessary to understand better the pressure exerted by this group of grazers.
A recent study revealed that kelp forests have recovered (L. hyperborea and S. latissima considered together) along the northern Norwegian coast (Christie et al., 2019b). The recovery was suggested as the result of complex interactive effects of temperature on the food web. In the southern part of the previous sea urchin barren, the recovery of kelp is attributable to a decline in sea urchins following direct and indirect effects of increasing temperature (Christie et al., 2019b), whereas in the northernmost regions of Norway, the recovery seems to be driven by top-down control. Overfishing of cod leads to a increase in predatory crustaceans, hence an decrease in sea urchin abundance, which results in a decreased grazing pressure on kelp (Christie et al., 2019b; Norderhaug et al., 2021). Given that this region is monitored closely (Moy and Christie, 2012; Christie et al., 2019a, b), this could be an ideal opportunity to understand shifts between phases and determine what actions are successful in recovering S. latissima populations. Such knowledge can then be applied to less studied regions. Considering the diversity of animals feeding on S. latissima and the unknowns related to their interactions with other species and physical factors, more work is necessary to clarify the impact of grazing on S. latissima.
Algal competitors
Saccharina latissima disappeared in the early 2000s from several sites in Norway and has been replaced by turf algae (Moy and Christie, 2012). Since then, several studies have tried to understand the underlying mechanisms and monitor any changes (e.g. Andersen et al., 2018; Christie et al., 2019a, b). Although some studies have reported that a regime shift has occurred (S. latissima was no longer able to recover and had been replaced by turf algae), recent monitoring efforts have revealed some recovery, although temporally and spatially variable. A similar regime shift has occurred in the NW Atlantic. Off Nova Scotia, Canada’s kelp biomass (mainly composed of L. digitata and S. latissima) was recently found to have decreased by 85–99 % when compared with the first monitoring campaigns in 1949 (Filbee-Dexter et al., 2016). In the Gulf of Maine, a phase shift from canopy algae (including S. latissima) to ephemeral turf algae has occurred, and now 50–90 % of the bottom is dominated by red and green algae that were not common in the 1980s (Dijkstra et al., 2017). Associated biota was found in lower numbers in S. latissima and other canopy species than in highly branched and filamentous algae. Nevertheless, high numbers of several gastropods were associated with S. latissima, including Lacuna vincta, Margarite helicinus and Mitrella (Dijkstra et al., 2017). The presence of turf algae reduced S. latissima populations further by competing for space. Saccharina latissima is increasingly recruiting from turf algae, but the individuals are smaller, the survival rate lower, and they are more likely to be dislodged by wave action than sporophytes attached to rocky reefs, hence decreasing the health of the populations (Burek et al., 2018; Feehan et al., 2019). It was suggested that individuals are smaller because energy is diverted to larger holdfasts required to stabilize sporophytes in a more unstable substratum (turfs compared with rocks). Detachment rates of turf-attached S. latissima are more pronounced at high wave-action sites or after storm events. This pattern was consistent throughout the distributional range of S. latissima in the NW Atlantic.
A field study in Northern Ireland revealed that the invasive Sargassum muticum did not compete with S. latissima stands (Strong and Dring, 2011). Another potential competing species is the invasive green alga Codium fragile ssp. fragile. A study in Nova Scotia compared C. fragile with S. latissima in terms of the composition of its detritus and contribution to the detrital food chain (Krumhansl, 2012), revealing that degradation in S. latissima was faster and resulted in greater mass loss than C. fragile. The C:N ratio was higher in S. latissima than in C. fragile throughout decomposition, resulting in a lower nutritional value of S. latissima than of C. fragile. This resulted in associated macrofauna that was more abundant but less diverse on S. latissima than on C. fragile.
BIOGEOGRAPHICAL PATTERNS
Population differentiation at the genetic level
The population structure, genetic diversity and connectivity of populations of S. latissima have been explored in recent years (Guzinski et al., 2016, 2020; Nielsen et al., 2016c; Luttikhuizen et al., 2018; Mooney et al., 2018; Neiva et al., 2018; Grant and Chenoweth, 2021). Overall, population differentiation, low within-population genetic diversity and low connectivity have been observed, although regional and local patterns can differ.
Only one study compared samples across oceans, identifying four differentiated phylogroups: (1) including specimens from NW Pacific (Japan, as Saccharina coriacea), NE (British Columbia) Pacific and Greenland and Hudson Bay in NW Atlantic; (2) NE Atlantic; (3) NW Atlantic; and (4) samples from Russia previously identified as Saccharina cichorioides (Neiva et al., 2018). Together with recent findings on individuals in the NE Pacific and Bering Sea (Grant and Chenoweth, 2021), the hypothesis of a northern refugium during the Last Glacial Maximum for the species is gaining support, in contrast to the previous hypothesis of recolonization from southern European populations, as has been suggested for other seaweed species (Bringloe et al., 2020). Further differentiation of S. latissima populations exists within the NE Atlantic phylogroup, with distinct ‘northern’ and ‘southern’ clusters (Neiva et al., 2018). Those authors suggest that speciation might be in progress within these phylogroups, in accordance with another study determining population differentiation between seven European populations (Luttikhuizen et al., 2018). Furthermore, it was shown that within-population genetic diversity is lowest for the southern populations (Spain and Portugal) and the isolated island population on Helgoland, German Bight, and highest in Spitsbergen (Guzinski et al., 2016). This was also confirmed by a more recent study using both microsatellites and double digest restriction site-associated DNA sequencing, to explore the genetic diversity of 11 populations in the NE Atlantic (Guzinski et al., 2020).
At smaller scales, populations of S. latissima revealed low genetic diversity within a brackish population (Denmark), while significant differences were observed between brackish and marine populations (Denmark vs. Norway and Sweden) (Nielsen et al., 2016c). In the Irish Sea, populations from Scotland, the Isle of Man and Northern Ireland were also shown to be differentiated (Mooney et al., 2018). In Norway, isolation-by-distance has been observed in S. latissima; however, the grouping seems to differ according to the method of analysis owing to the use of different genetic markers and sampling sites and sizes. In general, northern populations (Svalbard and Lofoten) are observed to be genetically distinct, suggesting that a physical barrier (islands) drives genetic differentiation. Overall, along the Norwegian coastline, results range from three different genetic groups (Evankow et al., 2019) to generally connected populations (Ribeiro et al., 2022). Local adaptation has been discussed for the general connection, because including a locus under positive selection altered the results of the genetic structure, even in the face of gene flow (Ribeiro et al., 2022). Like European populations, a differentiation in ‘cold’ and ‘temperate’ clusters was found in the NW Atlantic phylogroup, although less pronounced (Neiva et al., 2018). Fine-scale genetic structure and low within-population genetic diversity have been found for populations along the eastern Maine region in the NW Atlantic (Breton et al., 2018). However, comparing the same markers, lower allelic richness and heterozygosity were reported in NW Atlantic than in NE populations (Guzinski et al., 2016). Lower genetic diversity in the NW Atlantic compared with the NE has been reported for other benthic taxa (Wares and Cunningham, 2001). A recent study in S. latissima with more sampling sites revealed a biogeographical barrier at Cape Cod separating the populations in the Gulf of Maine and southern New England (Mao et al., 2020).
Despite the apparent wealth of studies targeting the population structure of S. latissima, they differ in the locations studied and methods applied, preventing a wide comparison and global conclusions. All studies generally show that within-population genetic diversity is low, which is concerning because it indicates that populations might not have the adaptive potential to face increasing environmental change at sites where it is most extreme. Moreover, they report low connectivity that could result from stretches of land, waves and currents and variation in salinity depending on the site that restricts colonization of disturbed populations. For a successful conservation and/or restoration plan for the species, more data are needed on population differentiation, covering a large number of locations across the geographical distribution but also spatial heterogeneity at smaller scales (e.g. islands or other isolated populations). Different markers and sequencing depth provide slightly different results, which should be taken into account when choosing the methods.
However, most studies on population differentiation have neglected the epigenetic component of local adaptation, which is strong in S. latissima across latitudes (Scheschonk et al., 2022). The epigenetic component might explain the general capacity of this species to adjust to rapid changes and colonize very different habitats. Hence, even with the apparent low genetic diversity, epigenetic differences might be high, and therefore it is crucial that they are considered in future studies.
Phenotypic plasticity and local adaptation
Phenotypic plasticity refers to the ability of a single genotype to modify its phenotype in response to changing conditions (Nicotra et al., 2010; King et al., 2018). In contrast, ecotypes are locally adapted populations that are phenotypically and genetically differentiated for adaptive traits, meaning that they perform better in the local conditions than another population from a distant location with other local environmental factors (Kawecki and Ebert, 2004; Nicotra et al., 2010). Ecotypes can emerge by long-term exposure to selective environmental pressures (Nicotra et al., 2010), such as temperature ecotypes in different climate zones. For example, stress responses and recovery from ocean warming and heat waves were shown to differ between organisms and across latitudes (Winters et al., 2011; Liesner et al., 2020a). By local adaptation and acclimation mechanisms, species can vary in tolerance and performance to biotic and abiotic factors.
In models or simulations, broadly distributed species are usually treated as single homogeneous physiological units (Reed et al., 2011). However, seaweeds such as S. latissima can exhibit different specific responses to distinct environmental conditions, of which temperature is a key factor (Lüning, 1990; Adey and Steneck, 2001; see also ‘Responses to environmental drivers’). Overall, the influences of various abiotic factors on the morphology, physiology and biochemical composition of S. latissima have been studied extensively, and a high degree of capacity for has been found. Little is known about how geographical patterns influence the capacity of the species.
Morphological plasticity is linked with adjustments to local conditions in different sites (Lüning, 1990; Peteiro and Freire, 2013b; Visch et al., 2020; Zhu et al., 2021; Diehl et al., 2023). Effects of wave exposure on the frond length and width of S. latissima have been described in the field (Chapman, 1973) and in laboratory conditions (Gerard, 1987; Zhu et al., 2021). Sporophytes typically form narrow blades with solid stipes in more wave-exposed habitats, whereas blades are broader with hollow stipes in sheltered habitats (Lüning, 1990). Specimens with hollow stipes will float when detached, possibly impacting the fate of detritus. Controlled laboratory experiments revealed an interaction between wave action and nutrient availability (Zhu et al., 2021). Under wave action, S. latissima sporophytes developed a rough, more intricate frond surface that allowed for a higher nutrient and light uptake, resulting in high biomass and frond length even in low nutrient conditions (Zhu et al., 2021). Additionally, sporophytes from a glacier-influenced area in Alaska have been described as narrower and longer than oceanic individuals (Spurkland and Iken, 2012), while in Svalbard (European Arctic), the biomass and size of S. latissima were lower in glacier-influenced sites. In the same fjord, sporophytes of S. latissima were longer and heavier at greater depths (Ronowicz et al., 2022). For laboratory-grown individuals (from the gametophyte stage), sporophytes from the Arctic were narrower and longer than sporophytes from Brittany (Monteiro et al., 2019b), indicating eco-phenotypes (see below). Morphological plasticity is very common in S. latissima and has led to misidentifications. For example, S. angustissima, formerly considered a morphotype of S. latissima (Augyte et al., 2018), is endemic to Maine (USA). Very exposed conditions result in narrow blades; otherwise, it is morphologically very similar to S. latissima but shows genetic divergence.
Recent studies investigated the biochemical plasticity of field-grown sporophytes of S. latissima. By comparing the lipidomic composition and other parameters such as total carbon, lipid, protein, and carbohydrate contents of S. latissima, it was possible to distinguish populations from France, Norway and the UK (Monteiro et al., 2020b). High intraspecific variability and habitat-specific phenotypes in morphology and biochemical composition were also found in field sporophytes of S. latissima across its entire distribution range in Europe, although without apparent geographical patterns (Diehl et al., 2023).
In addition, different populations of S. latissima were shown to vary in sensitivity to environmental factors, such as temperature (Olischläger et al., 2014, 2017; Monteiro et al., 2019b; Diehl et al., 2021, 2023). The existence of ecotypes regarding specific local parameters, such as temperature, salinity, pCO2 and light, have been postulated for the NE and NW Atlantic (Lüning and Dring, 1975; Gerard, 1987, 1988, 1990; Gerard and Du Bois, 1988; Müller et al., 2008; Spurkland and Iken, 2012; Olischläger et al., 2014, 2017). In contrast, other studies did not find evidence for ecotypic differentiation and instead suggested high phenotypic plasticity in S. latissima (Bolton and Lüning, 1982; Spurkland and Iken, 2011). Several studies have proposed ecological differentiation between populations from Spitsbergen and Helgoland (Müller et al., 2008; Olischläger et al., 2014, 2017). Differences in biochemical composition and physiological performance were reported under different temperature and CO2 treatments (Olischläger et al., 2014, 2017). In a multiple-stressor experiment on laboratory cultures of S. latissima from Brittany and the Arctic, the results suggested the existence of ecotypes in S. latissima (Monteiro et al., 2019b; Li et al., 2020a). Responses to salinity and temperature variation diverged between Brittany and the Arctic, resulting in variations in morphology and in differences in growth rate, pigment content and gene-expression profiles. At the transcriptomic level, short-term responses differed between sporophytes from the two sites in magnitude and in the metabolic pathways involved, which were correlated to some degree with the local conditions (Monteiro et al., 2019b).
Along the Norwegian coast (58–69°N), populations of cultivated S. latissima display higher blade length and biomass in central and northern regions that peak later in the season than for individuals in the south (Forbord et al., 2020). Increased growth in north and central populations was coupled with higher protein content and delayed onset of biofouling.
Concerning vertical distribution, cultivated S. latissima sporophytes in Norway display higher biomass yields and frond length at 1–2 m depth compared with 8–9 m depth (Forbord et al., 2020). However, this is not the case for the Baltic coast of Denmark, where frond size and dry matter reached the highest values at depths of >11 m (Nielsen et al., 2016b).
To date, it has been shown that S. latissima is adapted to local conditions throughout its wide geographical distribution. Several studies focused on regional differences, however intra-regional, among-sites differences have also been shown (e.g. Smale and Moore, 2017; Wang et al., 2021; Diehl et al., 2023), which complicates the analysis of latitudinal effects on S. latissima but reveals its ability to acclimatize. Adjustments to abiotic drivers are site specific and, therefore, cannot be generalized from one population to the entire species complex. Nevertheless, definite ecotypes cannot yet be confirmed, and the question of whether S. latissima exhibits ecotypes or not is not fully resolved. In addition, most studies conducted on ecotypes so far have been focused on the genetic level as an explanation for the intraspecific variability (phenotypes as local expression of a genotype).
However, adaptation can also be powered by epigenetic mechanisms, which have been demonstrated recently in S. latissima (Scheschonk et al., 2022). These findings show that, like the concept of phenotypic plasticity, the epigenome of S. latissima is likely to play a vital role in local and adaptation in this species. To highlight the importance of non-genetic gene control for local adaptation/processes, the term ‘eco-phenotype’ has been suggested (Scheschonk et al., 2022). It indicates epigenetic mechanisms (within and across generations; see ‘Epigenomics’) to be involved in the variation of the phenotype in response to local parameters.
Phylogeographical differentiation of S. latissima populations has been reported across the Northern Hemisphere, also over small geographical distances (see ‘Population differentiation at the genetic level’). Although it is hypothesized that the European S. latissima species complex has not reached an equilibrium, the emergence of ecotypes could occur and eventually lead to different species (Luttikhuizen et al., 2018; Neiva et al., 2018). However, this might be precluded by the rapid changes in its habitats attributable to climate change. The fact that there is evidence that divergence between different populations is expressed at transcriptomic and epigenetic levels (Monteiro et al., 2019b; Scheschonk et al., 2022) suggests that ecotypes might emerge at the phenotypic level (or as more pronounced eco-phenotypes) in future or might be revealed with more extreme environmental pressure or testing of different parameters.
The variability in phenotypic plasticity and formation of ecotypes in S. latissima described above is based on different approaches (various laboratory experiments, in situ measurements and reciprocal transplants), environmental criteria (temperature, salinity and irradiance) and response parameters (growth, survival, fitness and biochemical composition). These differences complicate a systematic comparison of results and warrant a discussion of which parameters are most helpful in assessing phenotypic plasticity or local adaptation. ‘Common garden experiments’ or reciprocal transplants of field specimens from distinct populations are widely accepted methods to assess ecotypic differentiation (Kawecki and Ebert, 2004). However, reciprocal transplants cannot be applied in protected areas, such as Spitsbergen (Ministry of Climate and Environment Norway, 2001), and concerns regarding genetic contamination are warranted (Guzinski et al., 2016; Luttikhuizen et al., 2018). Hence, a combination of methodologies, both experimental work and omics tools, could provide a better picture of the existence of ecotypes in S. latissima.
Ecological forecast
Climate change, especially global warming, has affected the distribution and abundance of many kelps (Smale, 2020; Fragkopoulou et al., 2022). Kelps are projected to shift continuously northwards in the future (Wilson et al., 2019; Krause-Jensen et al., 2020). Saccharina latissima has already been observed and estimated to decrease in Nova Scotia (Filbee-Dexter et al., 2016), the Gulf of Maine (Witman and Lamb, 2018), Rhode Island (Feehan et al., 2019), Norway (Bekkby and Moy, 2011; Moy and Christie, 2012), Sweden (Eriksson et al., 2002), Helgoland (Pehlke and Bartsch, 2008), the Iberian Coast (Casado-Amezúa et al., 2019) and the eastern English Channel and Strait of Dover (Araújo et al., 2016 and references therein), whereas it is increasing in biomass in Greenland (Krause-Jensen et al., 2012, 2020) and Svalbard (Bartsch et al., 2016) (see Fig. 1).
Species distribution models (SDMs) have been regarded as an effective tool for predicting marine species distribution shifts, using the species occurrence data and environmental variables available (Robinson et al., 2011). In the last decade, SDMs have been applied to evaluate the distribution of S. latissima in Norway (Bekkby and Moy, 2011) and the British Isles (Yesson et al., 2015). Furthermore, other models have considered the effect of climate change on the distribution of S. latissima and projected its future distribution trends (Müller et al., 2009; Assis et al., 2018; Goldsmit et al., 2021). The northward shift of S. latissima was first projected by relating the temperature requirements of S. latissima and the modelling of sea surface temperature isotherms in 2080–2099 (Müller et al., 2009). By constructing SDMs of kelp forests in the year 2100 under a future scenario (RCP 8.5), S. latissima was projected to extend to higher latitudes and inhabit the entire Arctic coast, while retreating from its southern limits in Nova Scotia, NW Iberia and Brittany towards Newfoundland and southwest Ireland (Assis et al., 2018). In the Eastern Canadian Arctic, under RCP 8.5, S. latissima was projected to have the largest gain (64 000 km2) of suitable habitats in 2050 and second largest gain (17 000 km2) in 2100 of the kelps studied (Goldsmit et al., 2021). However, some areas were projected to be lost in 2100, such as north of Baffin Bay, Foxe Basin and Hudson Bay (Goldsmit et al., 2021).
Although SDM is a powerful tool to predict the potential distribution of species under future climate scenarios, the accuracy of predictions is often disputed. For example, few studies have taken into account in SDMs the physiological limits of seaweeds, although this has proved useful for modelling macroalgal distribution (Martínez et al., 2015). Besides, the discrepancy between model predictions and long-term field observations of the abundance of Arctic kelps suggests that SDMs might overestimate the potential of kelps for northern expansion in the short term (Filbee-Dexter et al., 2019). The possible reasons might be the extensive gaps between available substrates, the limited dispersal ability of kelps, and other abiotic factors, such as turbidity and light penetration (Filbee-Dexter et al., 2019; Smale, 2020). Hence, it is crucial to track the occurrence and absence of S. latissima throughout the whole distributional limit in the future to improve the precision of model predictions. Modelling exercises that include physiological data generated from experiments and that account for possible local adaptation are also worth considering. To achieve more accurate predictions, it is also essential to improve the spatial resolution of environmental data layers available to consider the variable physical landscape of the intertidal and shallow subtidal zones where S. latissima occurs and to account for regional patterns that might override large-scale warming patterns, e.g. upwelling (Potter et al., 2013; Meneghesso et al., 2020).
CONSERVATION AND RESTORATION
Given the severe decline of kelp forests globally, action is needed to protect these important ecosystems in the future. Threats to S. latissima have been discussed in previous sections (effects of abiotic and biotic factors largely driven by climate change). Evidence of the impacts of other anthropogenic activities, such as pollution, on S. latissima is scarce. The rare examples include hydrogen peroxide on salmon farms that induced significant mortality and reduced photosynthetic efficiency of nearby S. latissima juveniles (Haugland et al., 2019). In contrast, S. latissima juveniles at sites impacted by the Exxon Valdez oil spill presented higher densities than reference sites 2 years after the spill, and populations recovered 10 years later (Dean and Jewett, 2001).
Kelp forests have been included in conventions aiming to protect habitats, namely the Convention of Bern and the Habitats Directive, both at the European level, and in the list of threatened species and habitats of the Convention for the Protection of the Marine Environment of the NE Atlantic (OSPAR) (de Bettignies et al., 2021). Nevertheless, specific measures targeting conservation of kelps and, more specifically, S. latissima are rare. Marine Protected Areas (MPAs) in the Atlantic have not yet been designed to protect kelp forests, but many include areas with kelp forests, providing some protection because harvest is forbidden. This is the case in some MPAs in Norway, France, the UK and Germany. However, the effects of these measures have not been evaluated, and little is known about the efficiency of MPAs in conserving kelps (de Bettignies et al., 2021). A study in California, USA, revealed that after 15 years, the abundance of sea urchins inside the MPA remained unchanged and giant kelp populations did not differ between inside and outside the MPA (Malakhoff and Miller, 2021). However, another study in a 30-year-old marine reserve in New Zealand demonstrated that the MPA effectively conserves populations of the kelp Ecklonia radiata. Outside MPAs, where fishing still occurred, sites were dominated by sea urchins and turf algae, whereas inside the MPA, healthy populations of E. radiata were present (Peleg et al., 2023). Marine Protected Areas in Chile have successfully preserved intertidal populations of the commercially harvested Lessonia spp. (González-Roca et al., 2021). These are encouraging results and call for similar actions for S. latissima if aiming for the protection and/or restoration of its populations. Considerable baseline information will be required to evaluate the effect of MPAs and other conservation measures, such as reducing local pollution inputs or limiting coastal construction, on the conservation of S. latissima.
If conservation actions fail, restoration might be the way to go. One strategy to recover populations is to plant new individuals where they have been lost/decreased, aiming to restore the populations. A few studies aiming to find the best techniques for restoration have been performed on S. latissima (Fredriksen et al., 2020; Tsiamis et al., 2020; Le François et al., 2023). In a trial in Quebec, Canada, the production of S. latissima sporophytes was successful and worked best on artificial substrate and using a binder-based method for spraying gametophytes (Le François et al., 2023). In contrast, a study in Scotland revealed that the abundance of S. latissima and other kelps in an artificial reef was low, and in turn, turf seaweeds were abundant (Tsiamis et al., 2020). This is in accordance with a review on artificial seaweed reefs that concluded that the success of reforesting macroalgae is variable and depends on the scale, structural composition, materials used and surface complexity (Jung et al., 2022). A trial in Norway was also successful using the ‘green gravel’ method, in which stones are seeded in the laboratory and are planted in the field only when sporophytes reach 2–3 cm (Fredriksen et al., 2020). Another strategy for restoration of kelps is grazer control. A study in Norway showed that sea urchin decline following treatment with quicklime allowed for kelp forest recovery, including S. latissima (Strand et al., 2020). Other strategies not yet tested for S. latissima include the harvest of grazers and destructive hammering of sea urchin populations (Eger et al., 2022). Up to now, research on restoration practices in S. latissima is scarce, and no large-scale restoration plan has been attempted.
Scientific debate is ongoing on whether assisted evolution (or assisted adaptation) is warranted when restoring degraded and vulnerable populations. Assisted evolution entails that the genetic diversity of populations is increased artificially, by moving new genotypes to a population, boosting genetic diversity within, using intraspecific hybrid vigour or heterosis or genome editing (Coleman et al., 2020; van Oppen and Coleman, 2022). These methods raise important ethical questions that might limit their use (Filbee-Dexter and Smajdor, 2019). Overall, this is an area of research that we expect to attract a lot of attention in the near future as the need to restore degraded habitats becomes evident, and best practices need to be discussed.
CONCLUSIONS
All in all, S. latissima has been studied intensively over the last 15 years, and important new insights have been gained (Fig. 4). Nevertheless, new findings usually raise new questions, and here we highlight the most current research priorities.
Fig. 4.
Research values of Saccharina latissima sporophytes: ecosystem services, economic values and drivers. Schematic display of the manifold ecosystem services and economic application. Saccharina latissima is represented as a bicycle chain powering many ecosystem services: providing habitat, feed and a nursery ground for the associated micro- and macrofauna (see main text, section ‘Biotic interactions’); improving the water quality by accumulating high concentrations of harmful elements; improving the air quality by releasing oxygen; and sequestering carbon. These ecological values lead to a multitude of economic values. In nature, S. latissima provides coastal protection by reducing wave energy, increasing fishing and diving tourism, and enhancing fisheries by serving as a nursery ground for economically important fish species (‘Biotic interactions’). Harvested S. latissima is used for: food; feed; extraction of bioactive compounds, with applications in pharmaceutical, medical, cosmetics, paper and processed food industries, among others (see more in Saether et al., 2023); and development of biofuels and biomaterials (see more in Saether et al., 2023). The main drivers of S. latissima survival and growth are temperature, light availability, salinity, nutrients (see ‘Response to environmental drivers’) and biotic factors (‘Biotic interactions’) that significantly modify the ecological and economic services provided. Ongoing research leads the way for a deeper understanding of kelp ecosystems and new applications (‘Conclusion’).
Generally, as already stated in the review of the genus Laminaria by Bartsch et al. (2008), microscopic life-history phases have received considerably less research attention than the sporophyte stage. Spores, stages of gametophyte development, gametes and microscopic sporophytes should all be studied more intensely. Direct comparisons between life-history stages have to be included in future studies to identify phase-specific responses to environmental drivers. Knowledge is lacking on demographic patterns, life span and the spatial and temporal variability of life-cycle stages. Also, studies on differences in gametophyte sexes and sporophyte maturity are largely underrepresented. Only by examining the sensitivity throughout the entire life cycle and across the geographical distribution of S. latissima will it be possible to gain a comprehensive understanding of the resilience of the species to climate change, which is an important component for management of conservation and cultivation. Regarding climate change, most attention has been given to the impact of warming and marine heat waves. However, other weather extremes, such as marine cold spells (Schlegel et al., 2021) or climate change-related increases in storm surges, can have a huge impact and should be considered in future studies. Furthermore, to date, studies investigating the impact of irradiation on S. latissima have focused mainly on changes in PAR and the effect of UVR. However, increased sediment input along all coastal regions (meltwater run-off, river outflows and precipitation) not only leads to a reduction of PAR but also affects the spectral composition of the water column. Especially in Arctic regions, the environmental light spectrum changes drastically owing to accelerating glacial melt and permafrost thaw, reducing the photosynthetically available radiation (Niedzwiedz and Bischof, 2023). Therefore, in further experimental and modelling research on S. latissima, the spectral composition of radiation should be incorporated.
The strongest impact of climate change on marine life has been observed in the Arctic (Masson-Delmotte et al., 2021), where pronounced seasonal light conditions exist. Overall, seaweeds in Arctic regions have been studied intensively (Lebrun et al., 2022). Nevertheless, the adaptive responses to polar day, polar night and the respective transitions are poorly investigated. Furthermore, melting sea ice and glaciers change salinity or result in coastal darkening (Konik et al., 2021), which can result in additional stress for Arctic S. latissima and should be analysed further. In addition, increasing temperatures are especially pronounced during Arctic winters, with significant environmental consequences (Maturilli et al., 2015). However, only very few winter data for Arctic S. latissima are available. In this context, transgenerational effects in cold have been shown for L. digitata (Liesner et al., 2020b), and the same might hold for S. latissima. Data on growth rates, stress responses and biotic interactions for the rear-edge populations of S. latissima are also lacking. The uneven distribution of studies across the distributional range of the species (focusing on central populations in Germany, the UK and mainland Norway) limits our understanding of its potential for to various environmental conditions. To date, the question of whether S. latissima exhibits different ecotypes remains unanswered and requires further research.
When testing the consequences of climate change, an important and very complex topic is the interaction of drivers. Hence, multifactorial approaches are being applied increasingly but are still a minority, despite their high ecological relevance. The interplay of various altering factors might have synergistic or antagonistic impacts on the resilience and susceptibility of S. latissima, hence these factors are key to understanding survival and success in the future. Experiments testing the impact of ongoing climate change mostly use average values over large scales, e.g. average sea surface temperature increase, and fail to include relevant temporal and spatial variability at different scales (Seabra et al., 2015; Bates et al., 2018). Different intensities, durations and recovery periods in marine heatwave experiments result in different responses of S. latissima. Moreover, inter-annual and seasonal variability in the thermal stresses of S. latissima has been shown (Niedzwiedz et al., 2022). In general, seasonality strongly impacts the physiological and biochemical parameters of S. latissima; however, little is known about how phenology changes across the distributional range and how it is affected by climate change. Future research needs to include more intricate experimental designs that address more variability and how it might affect the survival of S. latissima.
The application of ‘omics’ to S. latissima is expected soon to increase sharply, as costs decrease and technologies quickly improve. Nonetheless, ‘omics’ approaches to S. latissima and other kelps lag behind other major taxonomic groups, and there is still much to be explored. Recent work on the transcriptomic responses in S. latissima should be expanded to include more abiotic and biotic drivers and complex interactive responses to climate change. In addition, transcriptomic studies should be combined with metabolomics and proteomics to understand how regulation occurs fully. However, a major caveat to these approaches is the lack of functional annotation, which limits our interpretation of results. More efforts in the molecular and biochemical characterization of genes are necessary, and knowledge generated for S. japonica (a closely related species) will help to streamline progress for S. latissima (e.g. Zhang et al., 2018b).
Another severe knowledge gap is how epigenetic mechanisms modulate responses in S. latissima. The modulation of DNA methylation in response to an environmental stimulus has recently been demonstrated in S. latissima (Scheschonk et al., 2022), but whether non-coding RNAs and histone modifications are also involved has not yet been tested. Given that these last two mechanisms have been demonstrated in other brown algae (Bourdareau et al., 2021; Bai et al., 2023), studies examining these patterns in S. latissima will surely follow. In addition, active gene modulation would be required to assess the definite impact of any given epigenetic modulation on the gene expression.
Regarding the microbiome, most microbiota studies for S. latissima have focused on describing the microbial partners. Consequently, there is a need to expand the research on co-cultures to investigate causal relationships. Specific isolates of interest, such as bacterial core, specialized metabolizers and pathogens, can be used to study their impact on algal growth and morphology (Burgunter-Delamare, 2022). Furthermore, more research is needed on the impact of potential pathogens on the physiological state of S. latissima and the composition of its entire microbiota. In silico predictions of beneficial metabolic network complementarity are a way to identify specific interactions between S. latissima and its microbiota. There is also a need to start cataloguing genes and their functions for both the microbiome and the host, which will require a combination of metagenomic and metatranscriptomic studies linking microbial and host gene expression. Viruses have been described recently in Laminariales and reported to infect two-thirds of the host populations (McKeown et al., 2017), highlighting the importance of incorporating viruses in studies on algal microbiota.
All the ‘omic’ data recently generated are being used to improve breeding of macroalgae, which still lags far behind plant crops. Several of these land crop techniques are expected to be applied to S. latissima as investment in aquaculture facilities is rising on both sides of the North Atlantic. However, these techniques might raise social and ethical issues that will need to be discussed with society in the next decades (for a discussion on the topic, see Charrier et al., 2020).
Although the distribution of S. latissima is fairly well documented in some regions, repeated monitoring and detailed distribution data are lacking in other regions, e.g. south of Europe and Russian waters. New technologies, such as remote sensing, drone imagery, video by underwater vehicles and environmental DNA approaches can assist greatly in monitoring the occurrence of S. latissima (e.g. De Pooter et al., 2017; Douay et al., 2022). Studies across the biogeographical distribution range of S. latissima will help to distinguish between present phenotypic plasticity and adaptation patterns present in the species and how it might be affected by climate change scenarios.
Despite overwhelming evidence that S. latissima populations are declining and that this compromises the ecosystem services they provide, there are still few management actions in place. Moreover, if present, these are country or region specific, without international perspective and guidance. Hence, the effectiveness of management actions already applied to other macroalgae has not been tested for S. latissima. It is imperative that this is put into action if we aim to maintain the remaining populations and restore some of the others. Management actions tested in other seaweeds that might also prove successful with S. latissima include improving water quality (by decreasing nutrient load, for example), Marine Protected Areas and grazer control (Strain et al., 2015; Eger et al., 2022; Peleg et al., 2023). As political interest and societal benefits in recovering kelp populations are increasing, securing the financial and logistical means to undergo large-scale restoration efforts might become more feasible (Eger et al., 2020; Filbee-Dexter et al., 2022c).
Dedicated to Christian Wiencke on the occasion of his 75th birthday
Contributor Information
Nora Diehl, Marine Botany, Faculty of Biology and Chemistry, University of Bremen, 28359 Bremen, Germany.
Huiru Li, Key Laboratory of Mariculture (Ministry of Education), Fisheries College, Ocean University of China, Qingdao 266003, China.
Lydia Scheschonk, Independent Researcher.
Bertille Burgunter-Delamare, Matthias Schleiden Institute of Genetics, Bioinformatics and Molecular Botany, Friedrich Schiller University Jena, 07743 Jena, Germany.
Sarina Niedzwiedz, Marine Botany, Faculty of Biology and Chemistry, University of Bremen, 28359 Bremen, Germany.
Silje Forbord, Department of Fisheries and New Biomarine Industry, SINTEF Ocean AS, 7465 Trondheim, Norway.
Maren Sæther, Seaweed Solutions AS, Bynesveien 50C, 7018 Trondheim, Norway.
Kai Bischof, Marine Botany, Faculty of Biology and Chemistry, University of Bremen, 28359 Bremen, Germany.
Catia Monteiro, CIBIO, Research Centre in Biodiversity and Genetic Resources – InBIO Associate Laboratory, Campus of Vairão, University of Porto, Vairão, Portugal; BIOPOLIS Program in Genomics, Biodiversity and Land Planning, CIBIO, Campus of Vairão, Vairão, Portugal.
Funding
C.M. is supported by FutureMARES (grant number 869300) and previously by ThermalBuffer (PTDC/BIA-BMA/31088/2017 and PTDC/BIA-BMA/31088/2017). The contributions of N.D. and S.N. were conducted in the framework of the project FACE-IT (The Future of Arctic Coastal Ecosystems—Identifying Transitions in Fjord Systems and Adjacent Coastal Areas). FACE-IT has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement number 869154. B.B.D. received funding from the collaborative research centre SFB1127/2/3 ChemBioSys (Deutsche Forschungsgemeinschaft, project ID 239748522) and previously a joint PhD scholarship from the Brittany region (Project HOSALA) and Sorbonne University (ED227). Work supported by National Funds through FCT-Fundação para a Ciência e Tecnologia in the scope of the project UIDP/50027/2020.
Data availability
This article has been submitted to the preprint server EcoEvoRxiv (https://doi.org/10.32942/X2W59T).
LITERATURE CITED
- Adey WH, Steneck RS.. 2001. Thermogeography over time creates biogeographic regions: a temperature/space/time-integrated model and an abundance-weighted test for benthic marine algae. Journal of Phycology 37: 677–698. [Google Scholar]
- Ahmed S, Cock JM, Pessia E, et al. 2014. A haploid system of sex determination in the brown alga Ectocarpus sp. Current Biology: CB 24: 1945–1957. [DOI] [PubMed] [Google Scholar]
- Anastasiadi D, Venney CJ, Bernatchez L, Wellenreuther M.. 2021. Epigenetic inheritance and reproductive mode in plants and animals. Trends in Ecology & Evolution 36: 1124–1140. [DOI] [PubMed] [Google Scholar]
- Andersen GS, Steen H, Christie H, Fredriksen S, Moy FE.. 2011. Seasonal patterns of sporophyte growth, fertility, fouling, and mortality of Saccharina latissima in Skagerrak, Norway: implications for forest recovery. Journal of Marine Biology 2011: 690375. [Google Scholar]
- Andersen GS, Pedersen MF, Nielsen SL.. 2013. Temperature acclimation and heat tolerance of photosynthesis in Norwegian Saccharina latissima (Laminariales, Phaeophyceae). Journal of Phycology 49: 689–700. [DOI] [PubMed] [Google Scholar]
- Andersen GS, Moy FE, Christie H.. 2018. In a squeeze: epibiosis may affect the distribution of kelp forests. Ecology and Evolution 9: 2883–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo RM, Assis J, Aguillar R, et al. 2016. Status, trends and drivers of kelp forests in Europe: an expert assessment. Biodiversity and Conservation 25: 1319–1348. [Google Scholar]
- Armitage CS, Husa V, Petelenz-Kurdziel EA, Sjøtun K.. 2017. Growth and competition in a warmer ocean: a field experiment with a non-native and two native habitat-building seaweeds. Marine Ecology Progress Series 573: 85–99. [Google Scholar]
- Assis J, Araújo MB, Serrão EA.. 2018. Projected climate changes threaten ancient refugia of kelp forests in the North Atlantic. Global Change Biology 24: e55–e66. [DOI] [PubMed] [Google Scholar]
- Attridge C, Metaxas A, Denley D.. 2022. Wave exposure affects the persistence of kelp beds amidst outbreaks of the invasive bryozoan Membranipora membranacea. Marine Ecology Progress Series 702: 39–56. [Google Scholar]
- Augyte S, Lewis L, Lin S, Neefus CD, Yarish C.. 2018. Speciation in the exposed intertidal zone: the case of Saccharina angustissima comb. nov. & stat. nov. (Laminariales, Phaeophyceae). Phycologia 57: 100–112. [Google Scholar]
- Augyte S, Wikfors GH, Pitchford S, et al. 2020. The application of flow cytometry for kelp meiospore isolation. Algal Research 46: 101810. [Google Scholar]
- Badis Y, Scornet D, Harada M, et al. 2021. Targeted CRISPR-Cas9-based gene knockouts in the model brown alga Ectocarpus. The New Phytologist 231: 2077–2091. [DOI] [PubMed] [Google Scholar]
- Bai M, Yue S, Wang W, et al. 2023. Identification and characterization of long non-coding RNAs involved in sex-related gene regulation in kelp Saccharina japonica. Journal of Ocean University of China 22: 755–765. [Google Scholar]
- Bartsch I, Wiencke C, Bischof K, et al. 2008. The genus Laminaria sensu lato: recent insights and developments. European Journal of Phycology 43: 1–86. [Google Scholar]
- Bartsch I, Paar M, Fredriksen S, et al. 2016. Changes in kelp forest biomass and depth distribution in Kongsfjorden, Svalbard, between 1996–1998 and 2012–2014 reflect Arctic warming. Polar Biology 39: 2021–2036. [Google Scholar]
- Bates AE, Helmuth B, Burrows MT, et al. 2018. Biologists ignore ocean weather at their peril. Nature 560: 299–301. [DOI] [PubMed] [Google Scholar]
- Bekkby T, Moy FE.. 2011. Developing spatial models of sugar kelp (Saccharina latissima) potential distribution under natural conditions and areas of its disappearance in Skagerrak. Estuarine, Coastal and Shelf Science 95: 477–483. [Google Scholar]
- Bekkby T, Torstensen RRG, Grünfeld LAH, et al. 2023. ‘Hanging gardens’ – comparing fauna communities in kelp farms and wild kelp forests. Frontiers in Marine Science 10: 1066101. [Google Scholar]
- Bernard M, Rousvoal S, Jacquemin B, Ballenghien M, Peters AF, Leblanc C.. 2018. qPCR-based relative quantification of the brown algal endophyte Laminarionema elsbetiae in Saccharina latissima: variation and dynamics of host—endophyte interactions. Journal of Applied Phycology 30: 2901–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M, Rousvoal S, Collet N, et al. 2019a. A highly prevalent filamentous algal endophyte in natural populations of the sugar kelp Saccharina latissima is not detected during cultivation in Northern Brittany. Aquatic Living Resources 32: 21. [Google Scholar]
- Bernard MS, Strittmatter M, Murúa P, et al. 2019b. Diversity, biogeography and host specificity of kelp endophytes with a focus on the genera Laminarionema and Laminariocolax (Ectocarpales, Phaeophyceae). European Journal of Phycology 54: 39–51. [Google Scholar]
- Bewick AJ, Niederhuth CE, Ji L, et al. 2017. The evolution of CHROMOMETHYLASES and gene body DNA methylation in plants. Genome Biology 18: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof K, Buschbaum C, Fredriksen S, et al. 2019. Kelps and environmental changes in Kongsfjorden: stress perception and responses. In Hop H, Wiencke C, eds.: The ecosystem of Kongsfjorden, Svalbard. Cham: Springer Nature Switzerland AG, 373–422. [Google Scholar]
- Bluhm BA, Brown K, Rotermund L, Williams W, Danielsen S, Carmack EC.. 2022. New distribution records of kelp in the Kitikmeot Region, Northwest Passage, Canada, fill a pan-Arctic gap. Polar Biology 45: 719–736. [Google Scholar]
- Boderskov T, Schmedes PS, Bruhn A, Rasmussen MB, Nielsen MM, Pedersen MF.. 2016. The effect of light and nutrient availability on growth, nitrogen, and pigment contents of Saccharina latissima (Phaeophyceae) grown in outdoor tanks, under natural variation of sunlight and temperature, during autumn and early winter in Denmark. Journal of Applied Phycology 28: 1153–1165. [Google Scholar]
- Boderskov T, Rasmussen MB, Bruhn A.. 2021. Obtaining spores for the production of Saccharina latissima: seasonal limitations in nature, and induction of sporogenesis in darkness. Journal of Applied Phycology 33: 1035–1046. [Google Scholar]
- Bolton JJ. 2010. The biogeography of kelps (Laminariales, Phaeophyceae): a global analysis with new insights from recent advances in molecular phylogenetics. Helgoland Marine Research 64: 263–279. [Google Scholar]
- Bolton JJ, Lüning K.. 1982. Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Marine Biology 66: 89–94. [Google Scholar]
- Boquete MT, Muyle A, Alonso C.. 2021. Plant epigenetics: phenotypic and functional diversity beyond the DNA sequence. American Journal of Botany 108: 553–558. [DOI] [PubMed] [Google Scholar]
- Bourdareau S, Tirichine L, Lombard B, et al. 2021. Histone modifications during the life cycle of the brown alga Ectocarpus. Genome Biology 22: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourlat SJ, Faust E, Wennhage H, et al. 2021. Wrasse fishery on the Swedish West Coast: towards ecosystem-based management. ICES Journal of Marine Science 78: 1386–1397. [Google Scholar]
- Bracken MES. 2018. When one foundation species supports another: tubeworms facilitate an extensive kelp bed in a soft-sediment habitat. Ecosphere 9: e02429. [Google Scholar]
- Breton TS, Nettleton JC, O’Connell B, Bertocci M.. 2018. Fine-scale population genetic structure of sugar kelp, Saccharina latissima (Laminariales, Phaeophyceae), in eastern Maine, USA. Phycologia 57: 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringloe T, Starko S, Wade R, et al. 2020. Phylogeny and evolution of the brown algae. Critical Reviews in Plant Sciences 39: 281–321. [Google Scholar]
- Brinkhuis BH, Chung IK.. 1986. The effects of copper on the fine structure of the kelp Laminaria saccharina (L) Lamour. Marine Environmental Research 19: 205–223. [Google Scholar]
- Broch O, Ellingsen I, Forbord S, et al. 2013. Modelling the cultivation and bioremediation potential of the kelp Saccharina latissima in close proximity to an exposed salmon farm in Norway. Aquaculture Environment Interactions 4: 187–206. [Google Scholar]
- Bruhn A, Tørring DB, Thomsen M, et al. 2016. Impact of environmental conditions on biomass yield, quality, and bio-mitigation capacity of Saccharina latissima. Aquaculture Environment Interactions 8: 619–636. [Google Scholar]
- Bruhn A, Janicek T, Manns D, et al. 2017. Crude fucoidan content in two North Atlantic kelp species, Saccharina latissima and Laminaria digitata—seasonal variation and impact of environmental factors. Journal of Applied Phycology 29: 3121–3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruhn A, Brynning G, Johansen A, et al. 2019. Fermentation of sugar kelp (Saccharina latissima)—effects on sensory properties, and content of minerals and metals. Journal of Applied Phycology 31: 3175–3187. [Google Scholar]
- Burek KE, O’Brien JM, Scheibling RE.. 2018. Wasted effort: recruitment and persistence of kelp on algal turf. Marine Ecology Progress Series 600: 3–19. [Google Scholar]
- Burgunter-Delamare B. 2022. Etudes des interactions hôte-microbiote chez l’algue brune Saccharina latissima. PhD Thesis, Sorbone Université, Paris. [Google Scholar]
- Burgunter-Delamare B, KleinJan H, Frioux C, et al. 2020. Metabolic complementarity between a brown alga and associated cultivable bacteria provide indications of beneficial interactions. Frontiers in Marine Science 7: 85. [Google Scholar]
- Burgunter-Delamare B, Tanguy G, Legeay E, Boyen C, Dittami SM.. 2022. Effects of sampling and storage procedures on 16S rDNA amplicon sequencing results of kelp microbiomes. Marine Genomics 63: 100944. [DOI] [PubMed] [Google Scholar]
- Burgunter-Delamare B, Rousvoal S, Legeay E, et al. 2023. The Saccharina latissima microbiome: effects of region, season, and physiology. Frontiers in Microbiology 13: 1050939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calosi P, De Wit P, Thor P, Dupont S.. 2016. Will life find a way? Evolution of marine species under global change. Evolutionary Applications 9: 1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capistrant‐Fossa KA, Morrison HG, Engelen AH, et al. 2021. The microbiome of the habitat‐forming brown alga Fucus vesiculosus (Phaeophyceae) has similar cross‐Atlantic structure that reflects past and present drivers. Journal of Phycology 57: 1681–1698. [DOI] [PubMed] [Google Scholar]
- Carrier TJ, Eddy SD, Redmond S.. 2017. Solar-dried kelp as potential feed in sea urchin aquaculture. Aquaculture International 25: 355–366. [Google Scholar]
- Casado-Amezúa P, Araújo R, Bárbara I, et al. 2019. Distributional shifts of canopy-forming seaweeds from the Atlantic coast of Southern Europe. Biodiversity and Conservation 28: 1151–1172. [Google Scholar]
- Castilla-Gavilán M, Cognie B, Ragueneau E, Turpin V, Decottignies P.. 2019. Evaluation of dried macrophytes as an alternative diet for the rearing of the sea urchin Paracentrotus lividus (Lamarck, 1816). Aquaculture Research 50: 1762–1769. [Google Scholar]
- Chapman ARO. 1973. Phenetic variability of stipe morphology in relation to season, exposure, and depth in the non-digitate complex of Laminaria Lamour (Phaeophyta, Laminariales) in Nova Scotia. Phycologia 12: 53–57. [Google Scholar]
- Chapman ARO, Markham JW, Lüning K. 1978. Effects of nitrate concentration on the growth and physiology of Laminaria saccharina (Phaeophyta) in culture. Journal of phycology 14: 195–198. [Google Scholar]
- Charrier B, Abreu MH, Araujo R, et al. 2017. Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. The New Phytologist 216: 967–975. [DOI] [PubMed] [Google Scholar]
- Charrier B, Barbier M, Araújo R, Holdt S, Jacquemin B, Rebours C.. 2020. Development and objectives of the PHYCOMORPH European Guidelines for the Sustainable Aquaculture of Seaweeds (PEGASUS). Botanica Marina 63: 5–16. [Google Scholar]
- Chavanich S, Harris LG.. 2002. The influence of macroalgae on seasonal abundance and feeding preference of a subtidal snail, Lacuna vincta (Montagu) (Littorinidae) in the Gulf of Maine. Journal of Molluscan Studies 68: 73–78. [Google Scholar]
- Chen J, Zang Y, Shang S, Tang X.. 2019. The complete mitochondrial genome of the brown alga Macrocystis integrifolia (Laminariales, Phaeophyceae). Mitochondrial DNA Part B 4: 635–636. [Google Scholar]
- Christie H, Andersen GS, Bekkby T, et al. 2019a. Shifts between sugar kelp and turf algae in Norway: regime shifts or fluctuations between different opportunistic seaweed species? Frontiers in Marine Science 6: 72. [Google Scholar]
- Christie H, Gundersen H, Rinde E, et al. 2019b. Can multitrophic interactions and ocean warming influence large-scale kelp recovery? Ecology and Evolution 9: 2847–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie H, Andersen GS, Tveiten LA, Moy FE.. 2022. Macrophytes as habitat for fish. ICES Journal of Marine Science 79: 435–444. [Google Scholar]
- Chung IK, Brinkhuis BH.. 1986. Copper effects in early stages of the kelp, Laminaria saccharina. Marine Pollution Bulletin 17: 213–218. [Google Scholar]
- Clerc T, Boscq S, Attia R, Kaminski Schierle GS, Charrier B, Läubli NF.. 2022. Cultivation and imaging of S. latissima embryo monolayered cell sheets inside microfluidic devices. Bioengineering (Basel, Switzerland) 9: 718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coaten DJ, Guls HD, Þorsteinsdóttir M, Halldórsson HP.. 2023. Effect of environmental physico-chemical parameters on the biochemical composition of wild Icelandic Laminaria digitata and Saccharina latissima (Laminariaceae, Phaeophyceae). Regional Studies in Marine Science 60: 102839. [Google Scholar]
- Cock JM, Sterck L, Rouze P, et al. 2010. The Ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 465: 617–621. [DOI] [PubMed] [Google Scholar]
- Coleman MA, Wood G, Filbee-Dexter K, et al. 2020. Restore or redefine: future trajectories for restoration. Frontiers in Marine Science 7: 237. [Google Scholar]
- Conolly NJ, Drew EA. 1985. Physiology of Laminaria: III. Effect of a Coastal Eutrophication Gradient on Seasonal Patterns of Growth and Tissue Composition in L digitata LAMOUR. and L. saccharina (L) LAMOUR. Marine Ecology 6: 181–195. [Google Scholar]
- Corrigan S, Brown AR, Tyler CR, et al. 2023. Development and diversity of epibiont assemblages on cultivated sugar kelp (Saccharina latissima) in relation to farming schedules and harvesting techniques. Life (Basel, Switzerland) 13: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daggett TL, Pearce CM, Robinson SMC, Chopin T.. 2010. Does method of kelp (Saccharina latissima) storage affect its food value for promoting somatic growth of juvenile green sea urchins (Strongylocentrotus droebachiensis)? Journal of Shellfish Research 29: 247–252. [Google Scholar]
- Davison IR, Jordan TL, Fegley JC, Grobe CW.. 2007. Response of Laminaria saccharina (Phaeophyta) growth and photosynthesis to simultaneous ultraviolet radiation and nitrogen limitation. Journal of Phycology 43: 636–646. [Google Scholar]
- Dean TA, Jewett SC.. 2001. Habitat-specific recovery of shallow subtidal communities following the Exxon Valdez oil spill. Ecological Applications 11: 1456–1471. [Google Scholar]
- de Bettignies T, de Bettignies F, Bartsch I, et al. 2021. Background document for kelp forests habitat. OSPAR 66: 1–67. [Google Scholar]
- de Jong DLC, Timmermans KR, de Winter JM, Derksen GCH. 2021. Effects of nutrient availability and light intensity on the sterol content of Saccharina latissima (Laminariales, Phaeophyceae). Journal of Applied Phycology 33: 1101–1113. [Google Scholar]
- Deiner K, Bik HM, Mächler E, et al. 2017. Environmental DNA metabarcoding: transforming how we survey animal and plant communities. Molecular Ecology 26: 5872–5895. [DOI] [PubMed] [Google Scholar]
- Denley D, Metaxas A, Fennel K.. 2019. Community composition influences the population growth and ecological impact of invasive species in response to climate change. Oecologia 189: 537–548. [DOI] [PubMed] [Google Scholar]
- De Pooter D, Appeltans W, Bailly N, et al. 2017. Toward a new data standard for combined marine biological and environmental datasets - expanding OBIS beyond species occurrences. Biodiversity Data Journal 5: e10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl N, Bischof K.. 2021. Coping with a changing Arctic: mechanisms of acclimation in the brown seaweed Saccharina latissima from Spitsbergen. Marine Ecology Progress Series 657: 43–57. [Google Scholar]
- Diehl N, Karsten U, Bischof K.. 2020. Impacts of combined temperature and salinity stress on the endemic Arctic brown seaweed Laminaria solidungula J. Agardh. Polar Biology 43: 647–656. [Google Scholar]
- Diehl N, Roleda MY, Bartsch I, Karsten U, Bischof K.. 2021. Summer heatwave impacts on the European kelp Saccharina latissima across its latitudinal distribution gradient. Frontiers in Marine Science 8: 1433. [Google Scholar]
- Diehl N, Steiner N, Bischof K, Karsten U, Heesch S.. 2023. Exploring intraspecific variability – biochemical and morphological traits of the sugar kelp Saccharina latissima along latitudinal and salinity gradients in Europe. Frontiers in Marine Science 10: 1042. [Google Scholar]
- Dijkstra J, Harris L, Mello K, Litterer A, Wells C, Ware C.. 2017. Invasive seaweeds transform habitat structure and increase biodiversity of associated species. Journal of Ecology 105: 1668–1678. [Google Scholar]
- Dittami SM, Duboscq-Bidot L, Perennou M, et al. 2016. Host–microbe interactions as a driver of acclimation to salinity gradients in brown algal cultures. The ISME Journal 10: 51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doney SC, Busch DS, Cooley SR, Kroeker KJ.. 2020. The impacts of ocean acidification on marine ecosystems and reliant human communities. Annual Review of Environment and Resources 45: 83–112. [Google Scholar]
- Douay F, Verpoorter C, Duong G, Spilmont N, Gevaert F.. 2022. New hyperspectral procedure to discriminate intertidal macroalgae. Remote Sensing 14: 346. [Google Scholar]
- Druehl LD, Collins JD, Lane CE, Saunders GW.. 2005. An evaluation of methods used to assess intergeneric hybridization in kelps using Pacific laminariales (Phaeophyceae). Journal of Phycology 41: 250–262. [Google Scholar]
- Dubin MJ, Zhang P, Meng D, et al. 2015. DNA methylation in Arabidopsis has a genetic basis and shows evidence of local adaptation. eLife 4: e05255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K, Staby A, van der Meeren T, et al. 2022. Habitat associations of juvenile Atlantic cod (Gadus morhua L) and sympatric demersal fish communities within shallow inshore nursery grounds. Estuarine, Coastal and Shelf Science 279: 108111. [Google Scholar]
- Ebbing A, Pierik R, Bouma T, Kromkamp JC, Timmermans K.. 2020. How light and biomass density influence the reproduction of delayed Saccharina latissima gametophytes (Phaeophyceae). Journal of Phycology 56: 709–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbing APJ, Fivash GS, Martin NB, et al. 2021a. In-culture selection and the potential effects of changing sex ratios on the reproductive success of multiannual delayed gametophytes of Saccharina latissima and Alaria esculenta. Journal of Marine Science and Engineering 9: 1250. [Google Scholar]
- Ebbing APJ, Pierik R, Fivash G, et al. 2021b. The role of seasonality in reproduction of multiannual delayed gametophytes of Saccharina latissima. Journal of Phycology 57: 1580–1589. [DOI] [PubMed] [Google Scholar]
- Eddy SD, Brown NP, Kling AL, Watts SA, Lawrence A.. 2012. Growth of juvenile green sea urchins, Strongylocentrotus droebachiensis, fed formulated feeds with varying protein levels compared with a macroalgal diet and a commercial abalone feed. Journal of the World Aquaculture Society 43: 159–173. [Google Scholar]
- Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T.. 2013. The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiology Reviews 37: 462–476. [DOI] [PubMed] [Google Scholar]
- Eger AM, Vergés A, Choi CG, et al. 2020. Financial and institutional support are important for large-scale kelp forest restoration. Frontiers in Marine Science 7: 535277. [Google Scholar]
- Eger AM, Layton C, McHugh TA, Gleason M, Eddy N.. 2022. Kelp restoration guidebook: lessons learned from kelp projects around the world. Arlington: The Nature Conservancy. [Google Scholar]
- Ehrig K, Alban S.. 2015. Sulfated galactofucan from the brown alga Saccharina latissima—variability of yield, structural composition and bioactivity. Marine Drugs 13: 76–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson BK, Johansson G, Snoeijs P.. 2002. Long-term changes in the macroagal vegetation of the inner Gullmar Fjord, Swedish Skagerrak coast. Journal of Phycology 38: 284–296. [Google Scholar]
- Eriksson BK, Ljunggren L, Sandström A, et al. 2009. Declines in predatory fish promote bloom‐forming macroalgae. Ecological Applications 19: 1975–1988. [DOI] [PubMed] [Google Scholar]
- Evankow A, Christie H, Hancke K, et al. 2019. Genetic heterogeneity of two bioeconomically important kelp species along the Norwegian coast. Conservation Genetics 20: 615–628. [Google Scholar]
- Fan X, Han W, Teng L, et al. 2020a. Single-base methylome profiling of the giant kelp Saccharina japonica reveals significant differences in DNA methylation to microalgae and plants. The New Phytologist 225: 234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Xie W, Wang Y, Xu D, Zhang X, Ye N.. 2020b. The complete chloroplast genome of Saccharina latissima. Mitochondrial DNA. Part B, Resources 5: 3481–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feehan C, Scheibling RE.. 2014. Disease as a control of sea urchin populations in Nova Scotian kelp beds. Marine Ecology Progress Series 500: 149–158. [Google Scholar]
- Feehan C, Francis F, Scheibling RE.. 2014. Harbouring the enemy: kelp holdfasts protect juvenile sea urchins from predatory crabs. Marine Ecology Progress Series 514: 149–161. [Google Scholar]
- Feehan CJ, Grace SP, Narvaez CA.. 2019. Ecological feedbacks stabilize a turf-dominated ecosystem at the southern extent of kelp forests in the Northwest Atlantic. Scientific Reports 9: 7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbee-Dexter K, Scheibling RE.. 2014a. Detrital kelp subsidy supports high reproductive condition of deep-living sea urchins in a sedimentary basin. Aquatic Biology 23: 71–86. [Google Scholar]
- Filbee-Dexter K, Scheibling RE.. 2014b. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Marine Ecology Progress Series 495: 1–25. [Google Scholar]
- Filbee-Dexter K, Smajdor A.. 2019. Ethics of assisted evolution in marine conservation. Frontiers in Marine Science 6: 20. [Google Scholar]
- Filbee-Dexter K, Feehan CJ, Scheibling RE.. 2016. Large-scale degradation of a kelp ecosystem in an ocean warming hotspot. Marine Ecology Progress Series 543: 141–152. [Google Scholar]
- Filbee-Dexter K, Wernberg T. 2018. Rise of turfs: A new battlefront for globally declining kelp forests. BioScience 68: 67–76. [Google Scholar]
- Filbee-Dexter K, Wernberg T, Fredriksen S, Norderhaug KM, Pedersen MF.. 2019. Arctic kelp forests: diversity, resilience and future. Global and Planetary Change 172: 1–14. [Google Scholar]
- Filbee-Dexter K, Wernberg T, Grace SP, et al. 2020. Marine heatwaves and the collapse of marginal North Atlantic kelp forests. Scientific Reports 10: 13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbee-Dexter K, Feehan CJ, Smale DA, et al. 2022a. Kelp carbon sink potential decreases with warming due to accelerating decomposition. PLoS Biology 20: e3001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbee-Dexter K, MacGregor KA, Lavoie C, et al. 2022b. Sea ice and substratum shape extensive kelp forests in the Canadian Arctic. Frontiers in Marine Science 9: 754074. [Google Scholar]
- Filbee-Dexter K, Wernberg T, Barreiro R, et al. 2022c. Leveraging the blue economy to transform marine forest restoration. Journal of Phycology 58: 198–207. [DOI] [PubMed] [Google Scholar]
- Forbord S, Skjermo J, Arff J, et al. 2012. Development of Saccharina latissima (Phaeophyceae) kelp hatcheries with year-round production of zoospores and juvenile sporophytes on culture ropes for kelp aquaculture. Journal of Applied Phycology 24: 393–399. [Google Scholar]
- Forbord S, Matsson S, Brodahl GE, et al. 2020. Latitudinal, seasonal and depth-dependent variation in growth, chemical composition and biofouling of cultivated Saccharina latissima (Phaeophyceae) along the Norwegian coast. Journal of Applied Phycology 32: 2215–2232. [Google Scholar]
- Forbord S, Etter SA, Broch OJ, Dahlen VR, Olsen Y.. 2021. Initial short-term nitrate uptake in juvenile, cultivated Saccharina latissima (Phaeophyceae) of variable nutritional state. Aquatic Botany 168: 103306. [Google Scholar]
- Førde H, Forbord S, Handå A, et al. 2016. Development of bryozoan fouling on cultivated kelp (Saccharina latissima) in Norway. Journal of Applied Phycology 28: 1225–1234. [Google Scholar]
- Fortes MD, Lüning K.. 1980. Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgoländer Meeresuntersuchungen 34: 15–29. [Google Scholar]
- Fossberg J, Forbord S, Broch OJ, et al. 2018. The potential for upscaling kelp (Saccharina latissima) cultivation in salmon-driven integrated multi-trophic aquaculture (IMTA). Frontiers in Marine Science 5: 418. [Google Scholar]
- Fragkopoulou E, Serrão EA, De Clerck O, et al. 2022. Global biodiversity patterns of marine forests of brown macroalgae. Global Ecology and Biogeography 31: 636–648. [Google Scholar]
- Fredriksen S, Filbee-Dexter K, Norderhaug KM, et al. 2020. Green gravel: a novel restoration tool to combat kelp forest decline. Scientific Reports 10: 3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard VA. 1987. Hydrodynamic streamlining of Laminaria saccharina Lamour in response to mechanical stress. Journal of Experimental Marine Biology and Ecology 107: 237–244. [Google Scholar]
- Gerard VA. 1988. Ecotypic differentiation in light-related traits of the kelp Laminaria saccharina. Marine Biology 97: 25–36. [Google Scholar]
- Gerard VA. 1990. Ecotypic differentiation in the kelp Laminaria saccharina: phase-specific adaptation in a complex life cycle. Marine Biology 107: 519–528. [Google Scholar]
- Gerard VA. 1997a. The role of nitrogen nutrition in high‐temperature tolerance of the kelp, Laminaria Saccharina (Chromophyta). Journal of Phycology 33: 800–810. [Google Scholar]
- Gerard VA. 1997b. Environmental stress during early development of kelp sporophytes (Laminaria saccharina): how long do effects persist? Journal of Applied Phycology 9: 5–9. [Google Scholar]
- Gerard VA, Du Bois KR.. 1988. Temperature ecotypes near the southern boundary of the kelp Laminaria saccharina. Marine Biology 97: 575–580. [Google Scholar]
- Gilson AR, Smale DA, O’Connor N.. 2021. Ocean warming and species range shifts affect rates of ecosystem functioning by altering consumer–resource interactions. Ecology 102: e03341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goecke F, Labes A, Wiese J, Imhoff JF.. 2010. Chemical interactions between marine macroalgae and bacteria. Marine Ecology Progress Series 409: 267–299. [Google Scholar]
- Goecke F, Gómez Garreta A, Martín–Martín R, Rull Lluch J, Skjermo J, Ergon A.. 2022. Nuclear DNA content variation in different life cycle stages of sugar kelp, Saccharina latissima. Marine Biotechnology 24: 706–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmit J, Schlegel RW, Filbee-Dexter K, et al. 2021. Kelp in the Eastern Canadian Arctic: current and future predictions of habitat suitability and cover. Frontiers in Marine Science 18: 742209. [Google Scholar]
- González-Roca F, Gelcich S, Pérez-Ruzafa A, Vega JMA, Vásquez JA.. 2021. Exploring the role of access regimes over an economically important intertidal kelp species. Ocean & Coastal Management 212: 105811. [Google Scholar]
- Gordillo FJL, Aguilera J, Wiencke C, Jiménez C.. 2015. Ocean acidification modulates the response of two Arctic kelps to ultraviolet radiation. Journal of Plant Physiology 173: 41–50. [DOI] [PubMed] [Google Scholar]
- Gordillo FJL, Carmona R, Jiménez C.. 2022. A warmer Arctic compromises winter survival of habitat-forming seaweeds. Frontiers in Marine Science 8:750209. [Google Scholar]
- Gordillo FJL, Aguilera J, Jiménez C. 2006. The response of nutrient assimilation and biochemical composition of Arctic seaweeds to a nutrient input in summer. Journal of Experimental Botany 57: 2661–2671. [DOI] [PubMed] [Google Scholar]
- Graf L, Shin Y, Yang JH, et al. 2021. A genome-wide investigation of the effect of farming and human-mediated introduction on the ubiquitous seaweed Undaria pinnatifida. Nature Ecology & Evolution 5: 360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf L, Shin Y, Yang JH, Hwang IK, Yoon HS.. 2022. Transcriptome analysis reveals the spatial and temporal differentiation of gene expression in the sporophyte of Undaria pinnatifida. Algal Research 68: 102883. [Google Scholar]
- Grant WS, Chenoweth E.. 2021. Phylogeography of sugar kelp: northern ice-age refugia in the Gulf of Alaska. Ecology and Evolution 11: 4670–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzinski J, Mauger S, Cock JM, Valero M.. 2016. Characterization of newly developed expressed sequence tag-derived microsatellite markers revealed low genetic diversity within and low connectivity between European Saccharina latissima populations. Journal of Applied Phycology 28: 3057–3070. [Google Scholar]
- Guzinski J, Ruggeri P, Ballenghien M, et al. 2020. Seascape genomics of the sugar kelp Saccharina latissima along the North Eastern Atlantic latitudinal gradient. Genes 11: 1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel KJ. 2022. Feeding preference of the sea urchin Arbacia punctulata for algal turf over kelp in a degraded kelp forest ecosystem. MSc Thesis, Montclair State University, Montclair.
- Hanelt D, Tüg H, Bischof K, et al. 2001. Light regime in an Arctic fjord: a study related to stratospheric ozone depletion as a basis for determination of UV effects on algal growth. Marine Biology 138: 649–658. [Google Scholar]
- Harding APC, Scheibling RE.. 2015. Feed or flee: effect of a predation-risk cue on sea urchin foraging activity. Journal of Experimental Marine Biology and Ecology 466: 59–69. [Google Scholar]
- Harley CDG, Anderson KM, Demes KW, et al. 2012. Effects of climate change on global seaweed communities. Journal of Phycology 48: 1064–1078. [DOI] [PubMed] [Google Scholar]
- Haugland B, Rastrick S, Agnalt A, Husa V, Kutti T, Samuelsen BO.. 2019. Mortality and reduced photosynthetic performance in sugar kelp Saccharina latissima caused by the salmon-lice therapeutant hydrogen peroxide. Aquaculture Environment Interactions 11: 1–17. [Google Scholar]
- Heinrich S, Frickenhaus S, Glöckner G, Valentin K.. 2012a. A comprehensive cDNA library of light- and temperature-stressed Saccharina latissima (Phaeophyceae). European Journal of Phycology 47: 83–94. [Google Scholar]
- Heinrich S, Valentin K, Frickenhaus S, John U, Wiencke C.. 2012b. Transcriptomic analysis of acclimation to temperature and light stress in Saccharina latissima (Phaeophyceae). PLoS One 7: e44342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich S, Valentin K, Frickenhaus S, Wiencke C.. 2015. Temperature and light interactively modulate gene expression in Saccharina latissima (Phaeophyceae). Journal of Phycology 51: 93–108. [DOI] [PubMed] [Google Scholar]
- Heinrich S, Valentin K, Frickenhaus S, Wiencke C.. 2016. Origin matters — comparative transcriptomics in Saccharina latissima (Phaeophyceae). Journal of Experimental Marine Biology and Ecology 476: 22–30. [Google Scholar]
- Hobday AJ, Alexander LV, Perkins SE, et al. 2016. A hierarchical approach to defining marine heatwaves. Progress in Oceanography 141: 227–238. [Google Scholar]
- Hollants J, Leliaert F, De Clerck O, Willems A.. 2013. What we can learn from sushi: a review on seaweed–bacterial associations. FEMS Microbiology Ecology 83: 1–16. [DOI] [PubMed] [Google Scholar]
- Holt J, Wakelin S, Lowe J, Tinker J.. 2010. The potential impacts of climate change on the hydrography of the northwest European continental shelf. Progress in Oceanography 86: 361–379. [Google Scholar]
- Holzinger A, Di Piazza L, Lütz C, Roleda MY.. 2011. Sporogenic and vegetative tissues of Saccharina latissima (Laminariales, Phaeophyceae) exhibit distinctive sensitivity to experimentally enhanced ultraviolet radiation:photosynthetically active radiation ratio. Phycological Research 59: 221–235. [Google Scholar]
- Hsiao SIC, Druehl LD.. 1973. Environmental control of gametogenesis in Laminaria saccharina. IV. In situ development of gametophytes and young sporophytes. Journal of Phycology 9: 160–165. [Google Scholar]
- Huang M, Robbins KR, Li Y, et al. 2023. Genomic selection in algae with biphasic lifecycles: a Saccharina latissima (sugar kelp) case study. Frontiers in Marine Science 10: 1040979. [Google Scholar]
- Iñiguez C, Carmona R, Lorenzo MR, Niell FX, Wiencke C, Gordillo FJL.. 2016. Increased temperature, rather than elevated CO2, modulates the carbon assimilation of the Arctic kelps Saccharina latissima and Laminaria solidungula. Marine Biology 163: 248. [Google Scholar]
- Jevne LS, Forbord S, Olsen Y.. 2020. The effect of nutrient availability and light conditions on the growth and intracellular nitrogen components of land-based cultivated Saccharina latissima (Phaeophyta). Frontiers in Marine Science 7: 557460. [Google Scholar]
- Jung S, Chau TV, Kim M, Na W-B.. 2022. Artificial seaweed reefs that support the establishment of submerged aquatic vegetation beds and facilitate ocean macroalgal afforestation: a review. Journal of Marine Science and Engineering 10: 1184. [Google Scholar]
- Kaiser MJ. 2011. Marine ecology: processes, systems, and impacts. New York: Oxford University Press. [Google Scholar]
- Kang I, Lim Y, Cho J-C.. 2018. Complete genome sequence of Granulosicoccus antarcticus type strain IMCC3135T, a marine gammaproteobacterium with a putative dimethylsulfoniopropionate demethylase gene. Marine Genomics 37: 176–181. [DOI] [PubMed] [Google Scholar]
- Karsten U. 2007. Salinity tolerance of Arctic kelps from Spitsbergen. Phycological Research 55: 257–262. [Google Scholar]
- Karsten U. 2012. Seaweed acclimation to salinity and desiccation stress. In: Wiencke C, Bischof K, eds. Seaweed biology: novel insights into ecophysiology, ecology and utilization. Ecological Studies, Vol. 219. Berlin, Heidelberg: Springer, 87–107. [Google Scholar]
- Kawecki TJ, Ebert D.. 2004. Conceptual issues in local adaptation. Ecology Letters 7: 1225–1241. [Google Scholar]
- Kerrison PD, Stanley MS, Edwards MD, Black KD, Hughes AD.. 2015. The cultivation of European kelp for bioenergy: site and species selection. Biomass and Bioenergy 80: 229–242. [Google Scholar]
- Kerrison PD, Stanley MS, Kelly M, MacLeod A, Black KD, Hughes AD.. 2016. Optimising the settlement and hatchery culture of Saccharina latissima (Phaeophyta) by manipulation of growth medium and substrate surface condition. Journal of Applied Phycology 28: 1181–1191. [Google Scholar]
- Kim JK, Kraemer GP, Yarish C.. 2015. Use of sugar kelp aquaculture in Long Island Sound and the Bronx River Estuary for nutrient extraction. Marine Ecology Progress Series 531: 155–166. [Google Scholar]
- Kim JK, Kraemer G, Yarish C.. 2019. Evaluation of the metal content of farm grown Gracilaria tikvahiae and Saccharina latissima from Long Island Sound and New York Estuaries. Algal Research 40: 101484. [Google Scholar]
- King NG, McKeown NJ, Smale DA, Moore PJ.. 2018. The importance of phenotypic plasticity and local adaptation in driving intraspecific variability in thermal niches of marine macrophytes. Ecography 41: 1469–1484. [Google Scholar]
- King NG, Moore PJ, Thorpe JM, Smale DA.. 2022. Consistency and variation in the kelp microbiota: patterns of bacterial community structure across spatial scales. Microbial Ecology 85: 1265–1275. [DOI] [PubMed] [Google Scholar]
- Konik M, Darecki M, Pavlov AK, Sagan S, Kowalczuk P.. 2021. Darkening of the Svalbard fjords waters observed with satellite ocean color imagery in 1997–2019. Frontiers in Marine Science 8: 699318. [Google Scholar]
- Korb RE, Gerard VA.. 2000. Effects of concurrent low temperature and low nitrogen supply on polar and temperate seaweeds. Marine Ecology Progress Series 198: 73–82. [Google Scholar]
- Krause-Jensen D, Marbà N, Olesen B, et al. 2012. Seasonal sea ice cover as principal driver of spatial and temporal variation in depth extension and annual production of kelp in Greenland. Global Change Biology 18: 2981–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause-Jensen D, Archambault P, Assis J, et al. 2020. Imprint of climate change on Pan-Arctic marine vegetation. Frontiers in Marine Science 7: 617324. [Google Scholar]
- Kroth PG. 2013. Getting a grip on genetic modification in brown algae. Journal of Phycology 49: 816–818. [DOI] [PubMed] [Google Scholar]
- Krumhansl K, Scheibling RE.. 2012. Detrital subsidy from subtidal kelp beds is altered by the invasive green alga Codium fragile ssp fragile. Marine Ecology Progress Series 456: 73–85. [Google Scholar]
- Krumhansl KA, Lauzon-Guay J-S, Scheibling RE.. 2014. Modeling effects of climate change and phase shifts on detrital production of a kelp bed. Ecology 95: 763–774. [DOI] [PubMed] [Google Scholar]
- Lachnit T, Blümel M, Imhoff JF, Wahl M.. 2009. Specific epibacterial communities on macroalgae: phylogeny matters more than habitat. Aquatic Biology 5: 181–186. [Google Scholar]
- Lamy T, Reed DC, Rassweiler A, et al. 2018. Scale-specific drivers of kelp forest communities. Oecologia 186: 217–233. [DOI] [PubMed] [Google Scholar]
- Lane CE, Mayes C, Druehl LD, Saunders GW.. 2006. A multi‐gene molecular investigation of the kelp (Laminariales, Phaeophyceae) supports substantial taxonomic re‐organization. Journal of Phycology 42: 962–962. [Google Scholar]
- Laturnus F, Svensson T, Wiencke C.. 2010. Release of reactive organic halogens by the brown macroalga Saccharina latissima after exposure to ultraviolet radiation. Polar Research 29: 379–384. [Google Scholar]
- Lebrun A, Comeau S, Gazeau F, Gattuso J-P.. 2022. Impact of climate change on Arctic macroalgal communities. Global and Planetary Change 219: 103980. [Google Scholar]
- Lee JA, Brinkhuis BH.. 1988. Seasonal light and temperature interaction effects on development of Laminaria saccharina (Phaeophyta) gametophytes and juvenile sporophytes. Journal of Phycology 24: 181–191. [Google Scholar]
- Lee RE. 1989. Phycology. Cambridge: Cambridge University Press. [Google Scholar]
- Le François NR, Tremblay-Gratton A, Drouin-Johnson C, et al. 2023. Nature-based coastal restoration: development of an early-rearing production protocol of sugar kelp (Saccharina latissima Linnaeus) for bottom planting activities in the Gulf of St-Lawrence (Québec, Canada). Frontiers in Marine Science 10: 1135417. [Google Scholar]
- Lewis RJ, Green MK, Afzal ME.. 2013. Effects of chelated iron on oogenesis and vegetative growth of kelp gametophytes (Phaeophyceae). Phycological Research 61: 46–51. [Google Scholar]
- Li H, Monteiro C, Heinrich S, et al. 2020a. Responses of the kelp Saccharina latissima (Phaeophyceae) to the warming Arctic: from physiology to transcriptomics. Physiologia Plantarum 168: 5–26. [DOI] [PubMed] [Google Scholar]
- Li H, Scheschonk L, Heinrich S, et al. 2020b. Transcriptomic responses to darkness and the survival strategy of the kelp Saccharina latissima in the early Polar night. Frontiers in Marine Science 7: 592033. [Google Scholar]
- Liesner D, Fouqueau L, Valero M, et al. 2020a. Heat stress responses and population genetics of the kelp Laminaria digitata (Phaeophyceae) across latitudes reveal differentiation among North Atlantic populations. Ecology and Evolution 10: 9144–9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesner D, Shama LNS, Diehl N, Valentin K, Bartsch I.. 2020b. Thermal plasticity of the kelp Laminaria digitata (Phaeophyceae) across life cycle stages reveals the importance of cold seasons for marine forests. Frontiers in Marine Science 7: 456. [Google Scholar]
- Liesner D, Pearson GA, Bartsch I, et al. 2022. Increased heat resilience of intraspecific outbred compared to inbred lineages in the kelp Laminaria digitata: Physiology and Transcriptomics. Frontiers in Marine Science 9: 838793. [Google Scholar]
- Lind AC, Konar B.. 2017. Effects of abiotic stressors on kelp early life-history stages. Algae 32: 223–233. [Google Scholar]
- Liu F, Wang W, Sun X, Liang Z, Wang F.. 2014. RNA-Seq revealed complex response to heat stress on transcriptomic level in Saccharina japonica (Laminariales, Phaeophyta). Journal of Applied Phycology 26: 1585–1596. [Google Scholar]
- Liu T, Wang X, Wang G, et al. 2019. Evolution of complex thallus alga: genome sequencing of Saccharina japonica. Frontiers in Genetics 10: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wikfors GH, Clark P, et al. 2022. A deep dive into the epibiotic communities on aquacultured sugar kelp Saccharina latissima in Southern New England. Algal Research 63: 102654. [Google Scholar]
- Longphuirt SN, Eschmann C, Russell C.. 2013. Seasonal and species-specific response of five brown macroalgae to high atmospheric CO2. Marine Ecology Progress Series 493: 91–102. [Google Scholar]
- Longtin CM, Saunders GW.. 2015. On the utility of mucilage ducts as a taxonomic character in Laminaria and Saccharina (Phaeophyceae) – the conundrum of S. groenlandica. Phycologia 54: 440–450. [Google Scholar]
- Lubsch A, Timmermans KR.. 2019. Uptake kinetics and storage capacity of dissolved inorganic phosphorus and corresponding dissolved inorganic nitrate uptake in Saccharina latissima and Laminaria digitata (Phaeophyceae). Journal of Phycology 55: 637–650. [DOI] [PubMed] [Google Scholar]
- Lüning K. 1980. Critical levels of light and temperature regulating the gametogenesis of three Laminaria species (Phaeophyceae). Journal of Phycology 16: 1–15. [Google Scholar]
- Lüning K. 1984. Temperature tolerance and biogeography of seaweeds: the marine algal flora of Helgoland (North Sea) as an example. Helgoländer Meeresuntersuchungen 38: 305–317. [Google Scholar]
- Lüning K. 1990. Seaweeds: their environment, biogeography, and ecophysiology. Stuttgart: John Wiley & Sons, Inc. [Google Scholar]
- Lüning K, Dring MJ.. 1972. Reproduction induced by blue light in female gametophytes of Laminaria saccharina. Planta 104: 252–256. [DOI] [PubMed] [Google Scholar]
- Lüning K, Dring MJ.. 1975. Reproduction, growth and photosynthesis of gametophytes of Laminaria saccharina grown in blue and red light. Marine Biology 29: 195–200. [Google Scholar]
- Lüning K, Neushul M.. 1978. Light and temperature demands for growth and reproduction of laminarian gametophytes in southern and central California. Marine Biology 45: 297–309. [Google Scholar]
- Luttikhuizen PC, van den Heuvel FHM, Rebours C, Witte HJ, van Bleijswijk JDL, Timmermans K.. 2018. Strong population structure but no equilibrium yet: genetic connectivity and phylogeography in the kelp Saccharina latissima (Laminariales, Phaeophyta). Ecology and Evolution 8: 4265–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malakhoff KD, Miller RJ.. 2021. After 15 years, no evidence for trophic cascades in marine protected areas. Proceedings Biological Sciences 288: 20203061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns D, Nielsen MM, Bruhn A, Saake B, Meyer AS.. 2017. Compositional variations of brown seaweeds Laminaria digitata and Saccharina latissima in Danish waters. Journal of Applied Phycology 29: 1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Augyte S, Huang M, et al. 2020. Population genetics of sugar kelp throughout the northeastern United States using genome-wide markers. Frontiers in Marine Science 7: 694. [Google Scholar]
- Marinho GS, Holdt SL, Birkeland MJ, Angelidaki I.. 2015. Commercial cultivation and bioremediation potential of sugar kelp, Saccharina latissima, in Danish waters. Journal of Applied Phycology 27: 1963–1973. [Google Scholar]
- Martínez B, Arenas F, Trilla A, Viejo RM, Carreño F.. 2015. Combining physiological threshold knowledge to species distribution models is key to improving forecasts of the future niche for macroalgae. Global Change Biology 21: 1422–1433. [DOI] [PubMed] [Google Scholar]
- Martins N, Pearson GA, Gouveia L, Tavares AI, Serrão EA, Bartsch I.. 2019. Hybrid vigour for thermal tolerance in hybrids between the allopatric kelps Laminaria digitata and L. pallida (Laminariales, Phaeophyceae) with contrasting thermal affinities. European Journal of Phycology 54: 548–561. [Google Scholar]
- Masson-Delmotte V, Zhai P, Pirani A, et al. 2021. IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: IPCC. [Google Scholar]
- Mathieu O, Reinders J, Čaikovski M, Smathajitt C, Paszkowski J.. 2007. Transgenerational stability of the Arabidopsis epigenome is coordinated by CG methylation. Cell 130: 851–862. [DOI] [PubMed] [Google Scholar]
- Maturilli M, Herber A, König-Langlo G.. 2015. Surface radiation climatology for Ny-Ålesund, Svalbard (789°N), basic observations for trend detection. Theoretical and Applied Climatology 120: 331–339. [Google Scholar]
- McDevit DC, Saunders GW.. 2010. A DNA barcode examination of the Laminariaceae (Phaeophyceae) in Canada reveals novel biogeographical and evolutionary insights. Phycologia 49: 235–248. [Google Scholar]
- McDowell RE, Amsler MO, Li Q, Lancaster JR Jr, Amsler CD.. 2015. The immediate wound-induced oxidative burst of Saccharina latissima depends on light via photosynthetic electron transport. Journal of Phycology 51: 431–441. [DOI] [PubMed] [Google Scholar]
- McKeown DA, Stevens K, Peters AF, et al. 2017. Phaeoviruses discovered in kelp (Laminariales). The ISME Journal 11: 2869–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown DA, Schroeder JL, Stevens K, et al. 2018. Phaeoviral infections are present in Macrocystis, Ecklonia and Undaria (Laminariales) and are influenced by wave exposure in Ectocarpales. Viruses 10: 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneghesso C, Seabra R, Broitman BR, et al. 2020. Remotely-sensed L4 SST underestimates the thermal fingerprint of coastal upwelling. Remote Sensing of Environment 237: 111588. [Google Scholar]
- Mignerot L, Avia K, Luthringer R, et al. 2019. A key role for sex chromosomes in the regulation of parthenogenesis in the brown alga Ectocarpus. PLoS Genetics 15: e1008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Climate and Environment Norway. 2001. Svalbard Environmental Protection Act. Norway: Ministry of Climate and Environment Norway. (Ministry of Climate and Environment Norway, Ed.). [Google Scholar]
- Monteiro C, Heinrich S, Bartsch I, et al. 2019a. Temperature modulates sex-biased gene expression in the gametophytes of the kelp Saccharina latissima. Frontiers in Marine Science 6: 769. [Google Scholar]
- Monteiro C, Li H, Bischof K, et al. 2019b. Is geographical variation driving the transcriptomic responses to multiple stressors in the kelp Saccharina latissima? BMC Plant Biology 19: 513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro C, Li H, Diehl N, et al. 2020a. Modulation of physiological performance by temperature and salinity in the sugar kelp Saccharina latissima. Phycological Research 69: 48–57. [Google Scholar]
- Monteiro J, Rey F, Melo T, et al. 2020b. The unique lipidomic signatures of Saccharina latissima can be used to pinpoint their geographic origin. Biomolecules 10: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney KM, Beatty GE, Elsäßer B, et al. 2018. Hierarchical structuring of genetic variation at differing geographic scales in the cultivated sugar kelp Saccharina latissima. Marine Environmental Research 142: 108–115. [DOI] [PubMed] [Google Scholar]
- Moore LD, Le T, Fan G.. 2013. DNA methylation and its basic function. Neuropsychopharmacology 38: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen LM. 2017. Diurnal carbon dioxide exchange rates of Saccharina latissima and Laminaria digitata as affected by salinity levels in Norwegian fjords. Journal of Applied Phycology 29: 3067–3075. [Google Scholar]
- Motomura T, Sakai Y.. 1981. Effect of chelated iron in culture media on oogenesis in Laminaria angustata. Nippon Suisan Gakkaishi 47: 1535–1540. [Google Scholar]
- Moy FE, Christie H.. 2012. Large-scale shift from sugar kelp (Saccharina latissima) to ephemeral algae along the south and west coast of Norway. Marine Biology Research 8: 309–321. [Google Scholar]
- Müller R, Wiencke C, Bischof K.. 2008. Interactive effects of UV radiation and temperature on microstages of Laminariales (Phaeophyceae) from the Arctic and North Sea. Climate Research 37: 203–213. [Google Scholar]
- Müller R, Laepple T, Bartsch I, Wiencke C.. 2009. Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters. Botanica Marina 52: 617–638. [Google Scholar]
- Müller R, Desel C, Steinhoff FS, Wiencke C, Bischof K.. 2012. UV‐radiation and elevated temperatures induce formation of reactive oxygen species in gametophytes of cold‐temperate/Arctic kelps (Laminariales, Phaeophyceae). Phycological Research 60: 27–36. [Google Scholar]
- Neiva J, Paulino C, Nielsen MM, et al. 2018. Glacial vicariance drives phylogeographic diversification in the amphi-boreal kelp Saccharina latissima. Scientific Reports 8: 1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepper-Davidsen J, Andersen DT, Pedersen MF.. 2019. Exposure to simulated heatwave scenarios causes long-term reductions in performance in Saccharina latissima. Marine Ecology Progress Series 630: 25–39. [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. 2010. Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15: 684–692. [DOI] [PubMed] [Google Scholar]
- Niedzwiedz S, Bischof K.. 2023. Glacial retreat and rising temperatures are limiting the expansion of temperate kelp species in the future Arctic. Limnology and Oceanography 68: 816–830. [Google Scholar]
- Niedzwiedz S, Diehl N, Fischer P, Bischof K.. 2022. Seasonal and inter-annual variability in the heatwave tolerance of the kelp Saccharina latissima (Laminariales, Phaeophyceae). Phycological Research 70: 212–222. [Google Scholar]
- Nielsen MM, Krause-Jensen D, Olesen B, Thinggaard R, Christensen PB, Bruhn A.. 2014. Growth dynamics of Saccharina latissima (Laminariales, Phaeophyceae) in Aarhus Bay, Denmark, and along the species’ distribution range. Marine Biology 161: 2011–2022. [Google Scholar]
- Nielsen MM, Kumar JP, Soler-Vila A, Johnson MP, Bruhn A.. 2016a. Early stage growth responses of Saccharina latissima spores and gametophytes. Part 1: inclusion of different phosphorus regimes. Journal of Applied Phycology 28: 387–393. [Google Scholar]
- Nielsen MM, Manns D, D’Este M, et al. 2016b. Variation in biochemical composition of Saccharina latissima and Laminaria digitata along an estuarine salinity gradient in inner Danish waters. Algal Research 13: 235–245. [Google Scholar]
- Nielsen MM, Paulino C, Neiva J, Krause-Jensen D, Bruhn A, Serrão EA.. 2016c. Genetic diversity of Saccharina latissima (Phaeophyceae) along a salinity gradient in the North Sea–Baltic Sea transition zone. Journal of Phycology 52: 523–531. [DOI] [PubMed] [Google Scholar]
- Nitschke U, Stengel DB.. 2014. Iodine contributes to osmotic acclimatisation in the kelp Laminaria digitata (Phaeophyceae). Planta 239: 521–530. [DOI] [PubMed] [Google Scholar]
- Norderhaug KM, Gundersen H, Pedersen A, et al. 2015. Effects of climate and eutrophication on the diversity of hard bottom communities on the Skagerrak coast 1990–2010. Marine Ecology Progress Series 530: 29–46. [Google Scholar]
- Norderhaug KM, Nedreaas K, Huserbråten M, Moland E.. 2021. Depletion of coastal predatory fish sub-stocks coincided with the largest sea urchin grazing event observed in the NE Atlantic. Ambio 50: 163–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes J, McCoy SJ, Findlay HS, et al. 2016. Two intertidal, non-calcifying macroalgae (Palmaria palmata and Saccharina latissima) show complex and variable responses to short-term CO2 acidification. ICES Journal of Marine Science 73: 887–896. [Google Scholar]
- O’Brien JM, Scheibling RE.. 2016. Nipped in the bud: mesograzer feeding preference contributes to kelp decline. Ecology 97: 1873–1886. [DOI] [PubMed] [Google Scholar]
- O’Brien BS, Mello K, Litterer A, Dijkstra JA.. 2018. Seaweed structure shapes trophic interactions: a case study using a mid-trophic level fish species. Journal of Experimental Marine Biology and Ecology 506: 1–8. [Google Scholar]
- Olischläger M, Iñiguez C, Gordillo FJL, Wiencke C.. 2014. Biochemical composition of temperate and Arctic populations of Saccharina latissima after exposure to increased pCO2 and temperature reveals ecotypic variation. Planta 240: 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olischläger M, Iñiguez C, Koch K, Wiencke C, Gordillo FJL.. 2017. Increased pCO2 and temperature reveal ecotypic differences in growth and photosynthetic performance of temperate and Arctic populations of Saccharina latissima. Planta 245: 119–136. [DOI] [PubMed] [Google Scholar]
- Oudot-Le Secq M-P, Loiseaux-de Goer S, Stam WT, Olsen JL.. 2006. Complete mitochondrial genomes of the three brown algae (Heterokonta: Phaeophyceae) Dictyota dichotoma, Fucus vesiculosus and Desmarestia viridis. Current Genetics 49: 47–58. [DOI] [PubMed] [Google Scholar]
- Parages ML, Heinrich S, Wiencke C, Jiménez C.. 2013. Rapid phosphorylation of MAP kinase-like proteins in two species of Arctic kelps in response to temperature and UV radiation stress. Environmental and Experimental Botany 91: 30–37. [Google Scholar]
- Park J, Kim JK, Kong J-A, Depuydt S, Brown MT, Han T.. 2017. Implications of rising temperatures for gametophyte performance of two kelp species from Arctic waters. Botanica Marina 60: 39–48. [Google Scholar]
- Pearson GA, Martins N, Madeira P, Serrão EA, Bartsch I.. 2019. Sex-dependent and -independent transcriptional changes during haploid phase gametogenesis in the sugar kelp Saccharina latissima. PLoS One 14: e0219723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehlke C, Bartsch I.. 2008. Changes in depth distribution and biomass of sublittoral seaweeds at Helgoland (North Sea) between 1970 and 2005. Climate Research 37: 135–147. [Google Scholar]
- Peleg O, Blain C, Shears N.. 2023. Long‐term marine protection enhances kelp forest ecosystem stability. Ecological Applications 33: e2895. [DOI] [PubMed] [Google Scholar]
- Peteiro C, Freire O.. 2013a. Epiphytism on blades of the edible kelps Undaria pinnatifida and Saccharina latissima farmed under different abiotic conditions. Journal of the World Aquaculture Society 44: 706–715. [Google Scholar]
- Peteiro C, Freire O.. 2013b. Biomass yield and morphological features of the seaweed Saccharina latissima cultivated at two different sites in a coastal bay in the Atlantic coast of Spain. Journal of Applied Phycology 25: 205–213. [Google Scholar]
- Peteiro C, Sánchez N.. 2012. Comparing salinity tolerance in early stages of the sporophytes of a non-indigenous kelp (Undaria pinnatifida) and a native kelp (Saccharina latissima). Russian Journal of Marine Biology 38: 197–200. [Google Scholar]
- Picard MMM, Johnson LE, Ferrario F, et al. 2022. Drivers of kelp distribution in the Gulf of St Lawrence: insights from a transplant experiment. Marine Biology 169: 50. [Google Scholar]
- Potter KA, Woods HA, Pincebourde S.. 2013. Microclimatic challenges in global change biology. Global Change Biology 19: 2932–2939. [DOI] [PubMed] [Google Scholar]
- Rana S, Valentin K, Riehl J, et al. 2021. Analysis of organellar genomes in brown algae reveals an independent introduction of similar foreign sequences into the mitochondrial genome. Genomics 113: 646–654. [DOI] [PubMed] [Google Scholar]
- Ratnasingham S, Hebert PDN.. 2007. Bold: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular Ecology Notes 7: 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AET, Stekoll MS.. 2021. Conditions for staggering and delaying outplantings of the kelps Saccharina latissima and Alaria marginata for mariculture. Journal of the World Aquaculture Society 52: 1135–1157. [Google Scholar]
- Reed TE, Schindler DE, Waples RS.. 2011. Interacting effects of phenotypic plasticity and evolution on population persistence in a changing climate. Conservation Biology 25: 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro PA, Næss T, Dahle G, et al. 2022. Going with the flow – population genetics of the kelp Saccharina latissima (Phaeophyceae, Laminariales). Frontiers in Marine Science 9: 876420. [Google Scholar]
- Richards CL, Alonso C, Becker C, et al. 2017. Ecological plant epigenetics: evidence from model and non‐model species, and the way forward. Ecology Letters 20: 1576–1590. [DOI] [PubMed] [Google Scholar]
- Rinde E, Christie H, Fagerli CW, et al. 2014. The influence of physical factors on kelp and sea urchin distribution in previously and still grazed areas in the NE Atlantic. PLoS One 9: e100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson LM, Elith J, Hobday AJ, et al. 2011. Pushing the limits in marine species distribution modelling: lessons from the land present challenges and opportunities. Global Ecology and Biogeography 20: 789–802. [Google Scholar]
- Roleda MY, Dethleff D.. 2011. Storm-generated sediment deposition on rocky shores: simulating burial effects on the physiology and morphology of Saccharina latissima sporophytes. Marine Biology Research 7: 213–223. [Google Scholar]
- Roleda MY, Hurd CL.. 2019. Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia 58: 552–562. [Google Scholar]
- Roleda MY, Marfaing H, Desnica N, et al. 2019. Variations in polyphenol and heavy metal contents of wild-harvested and cultivated seaweed bulk biomass: health risk assessment and implication for food applications. Food Control 95: 121–134. [Google Scholar]
- Ronowicz M, Kukliński P, Włodarska-Kowalczuk M.. 2022. Morphological variation of kelps (Alaria esculenta, cf. Laminaria digitata, and Saccharina latissima) in an Arctic glacial fjord. Estuarine, Coastal and Shelf Science 268: 107802. [Google Scholar]
- Rugiu L, Hargrave MS, Enge S, Sterner M, Nylund GM, Pavia H.. 2021. Kelp in IMTAs: small variations in inorganic nitrogen concentrations drive different physiological responses of Saccharina latissima. Journal of Applied Phycology 33: 1021–1034. [Google Scholar]
- Saether S, Diehl N, Monteiro C, Niedzwiedz S, Burgunter-Delamare B, Bischof K, Scheschonk L, Li H, Forbord S. 2023. The sugar kelp Saccharina latissima II: Recent advances in farming and applications, preprint: 10.32942/X2SG77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JC, Dring MJ, Davidson K, Kelly MS.. 2012. Culture, yield and bioremediation potential of Palmaria palmata (Linnaeus) Weber & Mohr and Saccharina latissima (Linnaeus) C.E. Lane, C. Mayes, Druehl & G.W. Saunders adjacent to fish farm cages in northwest Scotland. Aquaculture 354–355: 128–135. [Google Scholar]
- Scheschonk L, Becker S, Hehemann JH, Diehl N, Karsten U, Bischof K.. 2019. Arctic kelp eco-physiology during the polar night in the face of global warming: a crucial role for laminarin. Marine Ecology Progress Series 611: 59–74. [Google Scholar]
- Scheschonk L, Bischof K, Kopp MEL, Jueterbock A.. 2022. Differences by origin in methylome suggest eco-phenotypes in the kelp Saccharina latissima. Evolutionary Applications 16: 262–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel DR, Foster MS.. 2006. The population biology of large brown seaweeds: ecological consequences of multiphase life histories in dynamic coastal environments. Annual Review of Ecology, Evolution, and Systematics 37: 343–372. [Google Scholar]
- Schiener P, Black KD, Stanley MS, Green DH.. 2015. The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. Journal of Applied Phycology 27: 363–373. [Google Scholar]
- Schlegel RW, Darmaraki S, Benthuysen JA, Filbee-Dexter K, Oliver ECJ.. 2021. Marine cold-spells. Progress in Oceanography 198: 102684. [Google Scholar]
- Schmitz RJ, Lewis ZA, Goll MG.. 2019. DNA methylation: shared and divergent features across eukaryotes. Trends in Genetics 35: 818–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder DC, Mckeown DA. 2021. Chapter 4. Viruses of seaweeds. In: Hurst CJ, ed. Studies in viral ecology, 2nd edn. Chichester: Wiley, 121–138. [Google Scholar]
- Seabra R, Wethey DS, Santos AM, Lima FP.. 2015. Understanding complex biogeographic responses to climate change. Scientific Reports 5: 12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz RD, Wennhage H, Bergström U, Lipcius RN, Ysebaert T.. 2014. Ecological value of coastal habitats for commercially and ecologically important species. ICES Journal of Marine Science 71: 648–665. [Google Scholar]
- Shan T, Yuan J, Su L, et al. 2020. First genome of the brown alga Undaria pinnatifida: chromosome-level assembly using PacBio and Hi-C technologies. Frontiers in Genetics 11: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shunatova N, Nikishina D, Ivanov M, et al. 2018. The longer the better: the effect of substrate on sessile biota in Arctic kelp forests. Polar Biology 41: 993–1011. [Google Scholar]
- Simonson EJ, Metaxas A, Scheibling RE.. 2015a. Kelp in hot water: II. Effects of warming seawater temperature on kelp quality as a food source and settlement substrate. Marine Ecology Progress Series 537: 105–119. [Google Scholar]
- Simonson EJ, Scheibling RE, Metaxas A.. 2015b. Kelp in hot water: I. Warming seawater temperature induces weakening and loss of kelp tissue. Marine Ecology Progress Series 537: 89–104. [Google Scholar]
- Skjoldal HR. 1993. Eutrophication and algal growth in the North Sea. In: Symposium Mediterranean Seas 2000: 445-489. [Google Scholar]
- Smale DA. 2020. Impacts of ocean warming on kelp forest ecosystems. The New Phytologist 225: 1447–1454. [DOI] [PubMed] [Google Scholar]
- Smale DA, Moore PJ.. 2017. Variability in kelp forest structure along a latitudinal gradient in ocean temperature. Journal of Experimental Marine Biology and Ecology 486: 255–264. [Google Scholar]
- Smale DA, Burrows MT, Moore P, O’Connor N, Hawkins SJ.. 2013. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecology and Evolution 3: 4016–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurkland T, Iken K.. 2011. Salinity and irradiance effects on growth and maximum photosynthetic quantum yield in subarctic Saccharina latissima (Laminariales, Laminariaceae). Botanica Marina 54: 355–365. [Google Scholar]
- Spurkland T, Iken K.. 2012. Seasonal growth patterns of Saccharina latissima (Phaeophyceae, Ochrophyta) in a glacially‐influenced subarctic estuary. Phycological Research 60: 261–275. [Google Scholar]
- Stark R, Grzelak M, Hadfield J.. 2019. RNA sequencing: the teenage years. Nature Reviews Genetics 20: 631–656. [DOI] [PubMed] [Google Scholar]
- Staufenberger T, Thiel V, Wiese J, Imhoff JF.. 2008. Phylogenetic analysis of bacteria associated with Laminaria saccharina. FEMS Microbiology Ecology 64: 65–77. [DOI] [PubMed] [Google Scholar]
- Stengel DB, McGrath H, Morrison LJ.. 2005. Tissue Cu, Fe and Mn concentrations in different-aged and different functional thallus regions of three brown algae from western Ireland. Estuarine, Coastal and Shelf Science 65: 687–696. [Google Scholar]
- Strain EMA, Thomson RJ, Micheli F, Mancuso FP, Airoldi L.. 2014. Identifying the interacting roles of stressors in driving the global loss of canopy-forming to mat-forming algae in marine ecosystems. Global Change Biology 20: 3300–3312. [DOI] [PubMed] [Google Scholar]
- Strain EMA, van Belzen J, van Dalen J, Bouma TJ, Airoldi L.. 2015. Management of local stressors can improve the resilience of marine canopy algae to global stressors. PLoS One 10: e0120837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand HK, Christie H, Fagerli CW, Mengede M, Moy F.. 2020. Optimizing the use of quicklime (CaO) for sea urchin management — a lab and field study. Ecological Engineering 143: 100018. [Google Scholar]
- Straub SC, Wernberg T, Thomsen MS, et al. 2019. Resistance to obliteration; responses of seaweeds to marine heatwaves. Frontiers in Marine Science 6: 763. [Google Scholar]
- Strong JA, Dring MJ.. 2011. Macroalgal competition and invasive success: testing competition in mixed canopies of Sargassum muticum and Saccharina latissima. Botanica Marina 54: 223–229. [Google Scholar]
- Strong JA, Maggs CA, Johnson MP.. 2009. The extent of grazing release from epiphytism for Sargassum muticum (Phaeophyceae) within the invaded range. Journal of the Marine Biological Association of the United Kingdom 89: 303–314. [Google Scholar]
- Sundfjord A, Albretsen J, Kasajima Y, et al. 2017. Effects of glacier runoff and wind on surface layer dynamics and Atlantic Water exchange in Kongsfjorden, Svalbard; a model study. Estuarine, Coastal and Shelf Science 187: 260–272. [Google Scholar]
- Svendsen H, Beszczynska-Møller A, Hagen JO, et al. 2002. The physical environment of Kongsfjorden–Krossfjorden, an Arctic fjord system in Svalbard. Polar Research 21: 133–166. [Google Scholar]
- Swanson AK, Fox CH.. 2007. Altered kelp (Laminariales) phlorotannins and growth under elevated carbon dioxide and ultraviolet-B treatments can influence associated intertidal food webs. Global Change Biology 13: 1696–1709. [Google Scholar]
- Teng L, Han W, Fan X, et al. 2021. Integrative analysis of chloroplast DNA methylation in a marine alga—Saccharina japonica. Plant Molecular Biology 105: 611–623. [DOI] [PubMed] [Google Scholar]
- Theodorou I, Opsahl-Sorteberg H-G, Charrier B.. 2021. Preparation of zygotes and embryos of the kelp Saccharina latissima for cell biology approaches. Bio-protocol 101: e4132. [Google Scholar]
- tom Dieck I. 1993. Temperature tolerance and survival in darkness of kelp gametophytes (Laminariales, Phaeophyta): ecological and biogeographical implications. Marine Ecology Progress Series 100: 253–264. [Google Scholar]
- Tourneroche A, Lami R, Burgaud G, et al. 2020. The bacterial and fungal microbiota of Saccharina latissima (Laminariales, Phaeophyceae). Frontiers in Marine Science 7: 587566. [Google Scholar]
- Traiger SB. 2019. Effects of elevated temperature and sedimentation on grazing rates of the green sea urchin: implications for kelp forests exposed to increased sedimentation with climate change. Helgoland Marine Research 73: 5. [Google Scholar]
- Tsiamis K, Salomidi M, Gerakaris V, et al. 2020. Macroalgal vegetation on a north European artificial reef (Loch Linnhe, Scotland): biodiversity, community types and role of abiotic factors. Journal of Applied Phycology 32: 1353–1363. [Google Scholar]
- Umanzor S, Li Y, Bailey D, et al. 2021. Comparative analysis of morphometric traits of farmed sugar kelp and skinny kelp, Saccharina spp., strains from the Northwest Atlantic. Journal of the World Aquaculture Society 52: 1059–1068. [Google Scholar]
- Vallet M, Strittmatter M, Murúa P, et al. 2018. Chemically-mediated interactions between macroalgae, their fungal endophytes, and protistan pathogens. Frontiers in Microbiology 9: 3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hoek C, Mann D, Jahns H.. 1995. Algae: an introduction to phycology. Cambridge: Cambridge University Press. [Google Scholar]
- van Oppen MJH, Coleman MA.. 2022. Advancing the protection of marine life through genomics. PLoS Biology 20: e3001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtakari M. 2022. ggOceanMaps: Plot Data on Oceanographic Maps using “ggplot2”, R package version 1.3.4. https://CRAN.R-project.org/package=ggOceanMaps (March 2023, date last accessed).
- Visch W, Rad-Menéndez C, Nylund GM, Pavia H, Ryan MJ, Day J.. 2019. Underpinning the development of seaweed biotechnology: cryopreservation of brown algae (Saccharina latissima) gametophytes. Biopreservation and Biobanking 17: 378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visch W, Kononets M, Hall POJ, Nylund GM, Pavia H.. 2020. Environmental impact of kelp (Saccharina latissima) aquaculture. Marine Pollution Bulletin 155: 110962. [DOI] [PubMed] [Google Scholar]
- Wang S, Fan X, Guan Z, et al. 2016. Sequencing of complete mitochondrial genome of Saccharina latissima ye-C14. Mitochondrial DNA. Part A, DNA Mapping, Sequencing, and Analysis 27: 4037–4038. [DOI] [PubMed] [Google Scholar]
- Wang X, Broch OJ, Forbord S, et al. 2014. Assimilation of inorganic nutrients from salmon (Salmo salar) farming by the macroalgae (Saccharina latissima) in an exposed coastal environment: implications for integrated multi-trophic aquaculture. Journal of Applied Phycology 26: 1869–1878. [Google Scholar]
- Wang X, Blikra MJ, Evensen TH, Skipnes D, James P.. 2021. Effects of site, depth and sori origin on the growth and minerals composition of cultivated Saccharina latissima (Phaeophyceae) in the north of Norway. Journal of Applied Phycology 34: 529–541. [Google Scholar]
- Ware C, Dijkstra J, Mello K, Stevens A, O’Brien B, Ikedo W.. 2019. A novel three dimensional analysis of functional-architecture that describes the properties of macroalgae as refuge. Marine Ecology Progress Series 608: 93–103. [Google Scholar]
- Wares JP, Cunningham CW.. 2001. Phylogeography and historical ecology of the North Atlantic intertidal. Evolution 55: 2455–2469. [DOI] [PubMed] [Google Scholar]
- Weigel BL, Miranda KK, Fogarty EC, Watson AR, Pfister CA.. 2022. Functional insights into the kelp microbiome from metagenome-assembled genomes. mSystems 7: e0142221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernberg T, Krumhansl K, Filbee-Dexter K, Pedersen MF. 2019. Chapter 3 - Status and Trends for the World’s Kelp Forests In: Sheppard C, ed. World Seas: an Environmental Evaluation (Second Edition). United States of America: Academic Press, 57–78. [Google Scholar]
- White N, Marshall CE.. 2007. Saccharina latissima - Sugar kelp. https://www.marlin.ac.uk./species/detail/1375 (14 January 2022, date last accessed).
- Wiencke C, Bischof K.. 2012. Seaweed biology – novel insights into ecophysiology, ecology and utilization. Heidelberg, New York, Dodrecht, London: Springer. [Google Scholar]
- Wiese J, Thiel V, Nagel K, Staufenberger T, Imhoff JF.. 2009. Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic Sea. Marine Biotechnology (New York, N.Y.) 11: 287–300. [DOI] [PubMed] [Google Scholar]
- Wiktor JM, Tatarek A, Kruss A, Singh RK, Wiktor JM, Søreide JE.. 2022. Comparison of macroalgae meadows in warm Atlantic versus cold Arctic regimes in the high-Arctic Svalbard. Frontiers in Marine Science 9: 1021675. [Google Scholar]
- Williams SL, Herbert SK.. 1989. Transient photosynthetic responses of nitrogen‐deprived Petalonia fascia and Laminaria saccharina (Phaeophyta) to ammonium resupply. Journal of Phycology 25: 515–522. [Google Scholar]
- Wilson KL, Skinner MA, Lotze HK.. 2019. Projected 21st‐century distribution of canopy‐forming seaweeds in the Northwest Atlantic with climate change. Diversity and Distributions 25: 582–602. [Google Scholar]
- Winters G, Nelle P, Fricke B, Rauch G, Reusch T.. 2011. Effects of a simulated heat wave on photophysiology and gene expression of high- and low-latitude populations of Zostera marina. Marine Ecology Progress Series 435: 83–95. [Google Scholar]
- Witman JD, Lamb RW.. 2018. Persistent differences between coastal and offshore kelp forest communities in a warming Gulf of Maine. PLoS One 13: e0189388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Q, Rousvoal S, Leblanc C.. 2021. Transcriptome-wide identification and evaluation of optimal reference genes for RT-qPCR expression analysis of Saccharina latissima responses to biotic and abiotic stress. Journal of Applied Phycology 33: 617–627. [Google Scholar]
- Yang X, Wang X, Yao J, Duan D.. 2021. Genome-wide mapping of cytosine methylation revealed dynamic DNA methylation patterns associated with sporophyte development of Saccharina japonica. International Journal of Molecular Sciences 22: 9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarish C, Kim JK, Lindell S, Kite-Powell H. 2017. Developing an environmentally and economically sustainable sugar kelp aquaculture industry in southern New England: from seed to market. Department of Marine Sciences. 4.https://digitalcommons.lib.uconn.edu/marine_sci/4/
- Ye N, Zhang X, Miao M, et al. 2015. Saccharina genomes provide novel insight into kelp biology. Nature Communications 6: 6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesson C, Bush LE, Davies AJ, Maggs CA, Brodie J.. 2015. Large brown seaweeds of the British Isles: evidence of changes in abundance over four decades. Estuarine, Coastal and Shelf Science 155: 167–175. [Google Scholar]
- Yorke AF, Metaxas A.. 2011. Interactions between an invasive and a native bryozoan (Membranipora membranacea and Electra pilosa) species on kelp and Fucus substrates in Nova Scotia, Canada. Marine Biology 158: 2299–2311. [Google Scholar]
- Young C, Doall M, Gobler CJ.. 2021. Dual benefit of ocean acidification for the laminarialean kelp Saccharina latissima: enhanced growth and reduced herbivory. Marine Ecology Progress Series 664: 87–102. [Google Scholar]
- Young CS, Sylvers LH, Tomasetti SJ, et al. 2022. Kelp (Saccharina latissima) mitigates coastal ocean acidification and increases the growth of North Atlantic bivalves in lab experiments and on an oyster farm. Frontiers in Marine Science 9: 881254. [Google Scholar]
- Zacher K, Rautenberger R, Hanelt D, Wulff A, Wiencke C. 2009. The abiotic environment of polar marine benthic algae In: Biology of Polar Benthic Algae. Walter de Gruyter, 9–22. [Google Scholar]
- Zacher K, Bernard M, Bartsch I, Wiencke C.. 2016. Survival of early life history stages of Arctic kelps (Kongsfjorden, Svalbard) under multifactorial global change scenarios. Polar Biology 39: 2009–2020. [Google Scholar]
- Zhang J, Li Y, Luo S, Cao M, Zhang L, Li X.. 2021. Differential gene expression patterns during gametophyte development provide insights into sex differentiation in the dioicous kelp Saccharina japonica. BMC Plant Biology 21: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cui C, Li Y, Wu H, Li X.. 2018a. A genome screen for the development of sex-specific DNA markers in Saccharina japonica. Journal of Applied Phycology 30: 1239–1246. [Google Scholar]
- Zhang P, Shao Z, Li L, Liu S, Yao J, Duan D.. 2018b. Molecular characterisation and biochemical properties of phosphomannomutase/phosphoglucomutase (PMM/PGM) in the brown seaweed Saccharina japonica. Journal of Applied Phycology 30: 2687–2696. [Google Scholar]
- Zhang S-S, Yang H, Ding L, et al. 2017. Tissue-specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. The Plant Cell 29: 1007–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Shan T, Pang S, Xu N.. 2019. Transcriptome profiling of the meristem tissue of Saccharina japonica (Phaeophyceae, Laminariales) under severe stress of copper. Marine Genomics 47: 100671. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liang G, Molloy PL, Jones PA.. 2020. DNA methylation enables transposable element-driven genome expansion. Proceedings of the National Academy of Sciences of the United States of America 117: 19359–19366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Ebbing A, Bouma TJ, Timmermans KR.. 2021. Morphological and physiological plasticity of Saccharina latissima (Phaeophyceae) in response to different hydrodynamic conditions and nutrient availability. Journal of Applied Phycology 33: 2471–2483. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has been submitted to the preprint server EcoEvoRxiv (https://doi.org/10.32942/X2W59T).


