Summary
Background
Depression is three to four times more prevalent in patients with neurological and inflammatory disorders than in the general population. For example, in patients with multiple sclerosis, the 12-month prevalence of major depressive disorder is around 25% and it is associated with a lower quality of life, faster disease progression, and higher morbidity and mortality. Despite its clinical relevance, there are few treatment options for depression associated with multiple sclerosis and confirmatory trials are scarce. We aimed to evaluate the safety and efficacy of a multiple sclerosis-specific, internet-based cognitive behavioural therapy (iCBT) programme for the treatment of depressive symptoms associated with the disease.
Methods
This parallel-group, randomised, controlled, phase 3 trial of an iCBT programme to reduce depressive symptoms in patients with multiple sclerosis was carried out at five academic centres with large outpatient care units in Germany and the USA. Patients with a neurologist-confirmed diagnosis of multiple sclerosis and depressive symptoms were randomly assigned (1:1:1; automated assignment, concealed allocation, no stratification, no blocking) to receive treatment as usual plus one of two versions of the iCBT programme Amiria (stand-alone or therapist-guided) or to a control condition, in which participants received treatment as usual and were offered access to the iCBT programme after 6 months. Masking of participants to group assignment between active treatment and control was not possible, although raters were masked to group assignment. The predefined primary endpoint, which was analysed in the intention-to-treat population, was severity of depressive symptoms as measured by the Beck Depression Inventory-II (BDI-II) at week 12 after randomisation. This trial is registered at ClinicalTrials.gov, NCT02740361, and is complete.
Findings
Between May 3, 2017, and Nov 4, 2020, we screened 485 patients for eligibility. 279 participants were enrolled, of whom 101 were allocated to receive stand-alone iCBT, 85 to receive guided iCBT, and 93 to the control condition. The dropout rate at week 12 was 18% (50 participants). Both versions of the iCBT programme significantly reduced depressive symptoms compared with the control group (BDI-II between-group mean differences: control vs stand-alone iCBT 6·32 points [95% CI 3·37–9·27], p<0·0001, effect size d=0·97 [95% CI 0·64–1·30]; control vs guided iCBT 5·80 points [2·71–8·88], p<0·0001, effect size d=0·96 [0·62–1·30]). Clinically relevant worsening of depressive symptoms was observed in three participants in the control group, one in the stand-alone iCBT group, and none in the guided iCBT group. No occurrences of suicidality were observed during the trial and there were no deaths.
Interpretation
This trial provides evidence for the safety and efficacy of a multiple sclerosis-specific iCBT tool to reduce depressive symptoms in patients with the disease. This remote-access, scalable intervention increases the therapeutic options in this patient group and could help to overcome treatment barriers.
Funding
National Multiple Sclerosis Society (USA).
Introduction
Compared with the general population, depression is around three to four times more prevalent in patients who have a chronic illness.1 In patients with multiple sclerosis, depression is the most common comorbidity,2 with a 12-month prevalence of major depressive disorder estimated at approximately 25%3 and a lifetime prevalence of up to 50%.4 Depression in this population is associated with cognitive impairment5 and reduced adherence to disease-modifying therapies.6 Depression also contributes substantially to the psychosocial burden of multiple sclerosis.7 Notably, depression could be one of the earliest manifestations of multiple sclerosis,8 and patients with comorbid depression are at risk of faster disability progression than those without.9,10 Depression in multiple sclerosis is also linked to higher cardiovascular morbidity and all-cause mortality,11,12 highlighting the effect of this comorbidity.
Despite its clinical importance, therapeutic options for depression in patients with multiple sclerosis remain scarce. Evidence for the efficacy of antidepressants is insufficient.13,14 By contrast, there is increasing evidence for the benefits of cognitive behavioural therapy (CBT) from numerous randomised controlled trials, as supported by meta-analyses.15,16
However, therapists who can deliver CBT and other psychological treatments that are tailored to the needs of patients with multiple sclerosis are not widely available. Common multiple sclerosis symptoms—such as mobility impairments, fatigue, or cognitive difficulties—can be additional barriers. To overcome these issues, remote-access options—such as CBT delivered via telephone—can be effective for the treatment of depression associated with multiple sclerosis;17 however, such interventions still require specialised therapists and are not available at scale. Effective, stand-alone, and scalable remote-access treatment options could therefore substantially improve clinical care. Online psychological interventions could help to reduce symptoms of depression in patients who do not also have a chronic medical illness18 as well as in patients with underlying medical conditions, including multiple sclerosis.19 Several small, single-centre, randomised controlled trials have been conducted using psychological internet-based tools to treat depression in patients with multiple sclerosis, yielding mixed results.20–22 In one of these trials, conducted in Germany, efficacy to reduce depressive symptoms in multiple sclerosis was shown for the unguided, generic internet-based CBT (iCBT) programme known as Deprexis.20 However, large confirmatory trials for any treatments of multiple sclerosis-associated depression that could inform clinical practice are scarce. To address this gap, we conducted a multicentre phase 3 trial to test the efficacy of the multiple sclerosis-specific iCBT tool Amiria, developed from the Deprexis programme, to reduce depressive symptoms in patients with multiple sclerosis.
Methods
Study design
This was a three-arm, parallel-group, multicentre, randomised, controlled, phase 3 trial conducted at five academic centres in Germany and the USA with large outpatient multiple sclerosis care units (Charité–Universitätsmedizin Berlin, Berlin, Germany; Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany; Cedars Sinai Medical Center, Los Angeles, CA, USA; Penn State University, University Park, PA, USA; and University of Missouri–Kansas City School of Medicine, Kansas City, KS, USA).
The study was reviewed and approved by the appropriate ethics review boards at each trial site before enrolling their first participant (Charité Institutional Review Board [EA1/102/16]; Ethics Board of the Chamber of Physicians Hamburg [PMC-137/16V]; UMKC Institutional Review Board [16–205]; Penn State University Institutional Review Board [#00004660]; and Cedars-Sinai Medical Center Institutional Review Board [Pro00045146]). The study protocol is available in the appendix (pp 39–67).
Participants
Inclusion criteria were age at least 18 years; neurologist-confirmed diagnosis of multiple sclerosis according to McDonald criteria;23 self-reported depressive symptoms (BDI-Fastscreen>4); fluency in German or English (depending on study site); willingness to engage in self-administration of an iCBT intervention for 12 weeks and complete follow-up; ability to travel to the outpatient centre for two clinical assessments (baseline and week 12); internet access at home; and provision of informed consent. Exclusion criteria were unwillingness or inability to consent; a diagnosis of bipolar disorder or psychosis (as established in the clinical interview); substantial neurocognitive impairments, dementia or autism (based on medical history and clinical judgement by the physician at the recruitment site); moderate or high risk of suicide by clinical impression; very severe depression that would interfere with the ability to participate in the study (based on clinical judgement by the physician at the recruitment site, patients with very severe depression were referred to local psychiatric services for immediate treatment); current psychotherapy or behavioural treatments for depression (defined as regular face-to-face sessions with a qualified psychotherapist, at study intake, either in individual or group settings, at a frequency of at least two sessions per month, started within the past 6 months before study intake); having started pharmacotherapy for depression within the past 2 months; a multiple sclerosis relapse or steroid treatment in the past 4 weeks; concurrent participation in another interventional clinical trial; and refusal to consent to the saving, processing and forwarding of pseudonymised data.
Participants were recruited from the respective outpatient units of the participating centres, via referrals from collaborating neurologists, and through self-referrals from online recruitment announcements in electronic newsletters sent out by the National Multiple Sclerosis Society in the USA and the German Multiple Sclerosis Society during the recruitment period. All participants provided written informed consent before enrolment and were financially compensated for their time and effort of clinical visits and outcome assessments.
Randomisation and masking
Participants were randomly assigned in a 1:1:1 ratio to one of the three study groups (stand-alone iCBT, guided iCBT, or control) by a fully automated random-allocation sequence built into the study platform (no blocking or stratification). To ensure concealed allocation, eligibility was established and all baseline assessments completed before executing fully automated randomisation via the study platform, in compliance with CONSORT guidelines. After leaving the study centre, participants received an automated email sent from the study platform to their registered email address informing them to log into the platform, where they would find a message regarding their group assignment and instructions to access the multiple sclerosis iCBT programme (if assigned to one of the active groups) or how long they would have to wait until access would be available (if assigned to the control group).
For patient-reported outcomes, masking of participants to group assignment between active treatment and control was not possible. However, for the clinician-reported outcomes (Montgomery-Åsberg Depression Rating Scale [MADRS] and clinical interviews), arrangements were made to keep raters masked to group assignment—participants were contacted to schedule study visits by staff who were not involved in the clinical assessments and participants were specifically instructed not to reveal their assigned group to the examiner during the visits.
Procedures
All participants were examined by a neurologist to confirm their multiple sclerosis diagnosis and to record relevant clinical information, such as current use of disease-modifying therapies. At baseline and at week 12, we also obtained patient-reported (Patient-Determined Disease Steps [PDDS]24) and clinician-rated (Expanded Disability Status Scale [EDSS]25) disability scores. Neuropsychological function was examined by a trained rater using the components of the Brief International Cognitive Assessment for Multiple Sclerosis:26 the Symbol Digit Modalities Test, the California Verbal Learning Test II, and the Brief Visuospatial Memory Test-Revised.
We used the Amiria iCBT programme either as stand-alone iCBT or with added standardised email support by a clinical psychologist (guided iCBT), and compared the results with those from the control group.
This multiple sclerosis-specific iCBT programme is based on principles and techniques used in CBT. The programme consists of ten sequential modules plus a summary module. Like Deprexis, it uses a simulated-dialogue approach by presenting brief, conversational text passages followed by multiple response options from which users can select. Subsequent content is then tailored to the patient’s individual responses. The user’s responses therefore determine the specific path through each module, and a simulated conversational flow—albeit in text rather than spoken format—is created. Depending on factors such as the user’s reading speed, response choices, or decisions to listen to optional audio recordings, each module can be completed in about 30–60 min. Contents are psychoeducation; behavioural activation; cognitive modification; mindfulness and acceptance; interpersonal skills; relaxation, physical exercise, and lifestyle modification; problem solving; expressive writing and forgiveness; positive psychology; and emotion-focused interventions. The iCBT used in this trial contained several elements specific to multiple sclerosis, which were developed in close collaboration with advisers living with the disease and experienced clinicians including neurologists, neuropsychologists, and psychotherapists with extensive experience in the care of patients with multiple sclerosis (appendix pp 5–14). Detailed descriptions of the content and functionalities of Deprexis, the generic version of this programme from which the multiple sclerosis-specific intervention was developed, can also be found in previous publications.27,28
Participants in the guided iCBT group received the multiple sclerosis-specific iCBT programme plus scheduled email contact with a therapist. The basic structure of the email support was based on our previous work29 and is described in detail in the manual (appendix pp 15–24). Three therapists with qualifications in clinical psychology or behavioural therapy were responsible for email support in the trial (AL and LI for patients enrolled in the German study sites and JH for patients enrolled in the USA). Supervision by an experienced, licensed psychotherapist (BM) and a registered psychologist with CBT qualifications (RM-M) was provided monthly for the study therapists.
The iCBT programme (both stand-alone and guided versions) tracks several indicators of usage, including days with activity in the programme and the number of modules participants completed. In addition, we tracked minutes with activity—a metric that uses 5-min blocks and excludes each block of inactivity—so that the logged usage times are a good estimate of time actually spent working with the programme.
Participants assigned to the control group continued to receive treatment as usual. After 6 months, participants in this group were offered access to the iCBT programme (unguided version).
In the primary trial phase, patients were randomly assigned to one of the three trial groups for 12 weeks. The trial also included a controlled extension phase (12 weeks–6 months after inclusion), during which participants who were randomly assigned to either stand-alone or guided iCBT were given continued access to the programme and could continue to work with it. The trial further included a non-controlled maintenance phase (6–12 months). At that time, participants who were originally assigned to the control group were offered access to the stand-alone iCBT programme. Participants in the other two groups, who had received guided iCBT and stand-alone iCBT, were randomly assigned to receive access to iCBT booster sessions (additional CBT content that is compatible with but goes beyond content already covered in previous programme modules) or not to receive such booster sessions. The booster sessions provided access to advanced CBT content and exercises as well as continued access to the previous content. A full overview of the trial design is provided in the appendix (p 43).
Outcomes
The predefined primary endpoint was the total score of the Beck Depression Inventory-II (BDI-II) at week 12 after randomisation (end of the primary trial phase). The BDI-II is a 21-item self-report depression questionnaire that has been found to be reliable, valid, and sensitive for assessing depression in the context of multiple sclerosis30 and produces similar results when administered in paper or online formats.31 The BDI-II score was obtained during the primary trial phase (baseline and week 12) and the extension phase (month 6 and month 12). During the primary trial phase, the BDI-II was also obtained online via the study platform at two interim time points (after 4 weeks and 8 weeks).
Preregistered secondary endpoints of this trial were patient-reported outcomes of quality of life using a generic questionnaire (the WHO Quality of Life-Brief Version [WHOQOL-BREF],32 consisting of four domains) and a multiple sclerosis-specific questionnaire (Multiple Sclerosis Impact Scale-29 [MSIS-29]33) and patient-reported outcomes for fatigue (the Fatigue Scale for Motor and Cognitive Functions [FSMC],34 with two domains, and the Chalder Fatigue Scale [CFS]35). All secondary endpoints were predefined and preregistered as change from baseline to week 12. Additional endpoints included clinician ratings of depressive symptoms (using the MADRS36) and caseness of major depressive disorder based on Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnostic criteria. These endpoints were predefined in the trial protocol. We also explored the effect of added therapist support (by comparing stand-alone iCBT and guided iCBT), stability of treatment effects at 6 months post baseline, and the effect of booster sessions versus no booster sessions at month 12 post baseline as additional outcome analyses of interest.
Predefined safety measures focused on new occurrence of suicidal ideation or intent. Self-report data on suicidal ideation and behaviour (from the Suicide Behaviors Questionnaire-Revised [SBQ-R]37) and the BDI-II were used (acute suicidality as indicated by response 3a or 3b on SBQ-R item 3 plus a score of 5 or 6 on SBQ-R item 4 or a score of 3 on BDI-II item 9 at any assessment). These responses would automatically be flagged by the study platform and responded to by the responsible study centre staff within 24 h, following the trial’s standard operating procedure for suicidality. Additional predefined safety measures were hospitalisation due to psychiatric disorder classified according to International Classification of Diseases (ICD) or DSM, suicidality detected during the clinical interviews or message exchange with the study therapists, or any lethal or life-threatening event (including suicide or suicide attempt). As an additional post-hoc safety measure, we analysed clinically relevant worsening of depression during the trial defined as change in BDI-II scores from below to above the cutoff for caseness (BDI-II>13).
Statistical analysis
A sample size of 100 patients per intervention group gives a conjunctive power (probability of rejecting both null hypotheses comparing stand-alone iCBT and guided iCBT to control) of 90% for a Dunnett’s test at the usual one-sided significance level of 2·5% assuming standardised mean differences of 0·5 for stand-alone iCBT versus control and 0·8 for guided iCBT versus control in the primary outcome change in BDI-II from baseline to week 12. The standardised mean difference for the stand-alone iCBT was informed by the effect observed in our previous phase 2 trial20 and the minimal clinically important difference of the BDI-II.38 On the basis of the dropout rates observed in our previous trial,20 the sample size was adjusted for 20% dropout: we aimed to recruit 125 patients per group, resulting in a total sample size of 375 patients. The power was simulated with 10 000 replications using East version 6.3.
The full analysis set was based on intention-to-treat principles: all randomly assigned patients with at least one post-baseline assessment were included in the analysis. A modified intention-to-treat population was analysed in a sensitivity analysis, including all patients who had registered in the iCBT programme. The safety set was defined as all participants who registered in the iCBT programme.
All analyses were predefined in the statistical analysis plan before unmasking (appendix pp 25–38) and follow relevant regulatory guidelines for the statistical analysis of randomised trials, including CPMP/ICH/363/96 E9 on statistical principles for clinical trials and guidance EMA/CHMP/295050/2013 from the European Medicines Agency on adjustment for baseline covariates in clinical trials. In line with these recommendations, all analyses are adjusted for baseline levels of the respective outcome measure.
The primary outcome (change in BDI-II from baseline to week 12) was analysed by means of linear mixed-effects models for repeated measures adjusted for baseline measurements with fixed effects for intervention, region (USA or Germany), time and baseline BDI-II score, and random subject effects for individual patients including all patients with at least one post-baseline measurement.39 Least-squares means (with 95% CI) are reported for the intervention groups as well as the difference between the least-squares group means (with 95% CI). Stand-alone iCBT versus control and guided iCBT versus control were assessed by a Dunnett’s test controlling the familywise type I error rate at the level of 2·5% (one-sided). In a secondary step, the added value of therapist email support iCBT versus stand-alone iCBT was tested at a two-sided level of 5%, if the effectiveness of stand-alone iCBT and guided iCBT for reducing depressive symptoms in multiple sclerosis was shown.
Standardised effect sizes are reported as Cohen’s d40 with corresponding 95% CIs (R package effsize, version 0.8.1). Effect size variances were computed as previously described.41 Pooled effect sizes after multiple imputation were computed on the basis of Rubin’s rules.42 As sensitivity analyses, last observation carried forward, multiple imputations, complete case, and analysis of the modified intention-to-treat population were used to deal with missing values for the BDI-II score (missing visits), and for each method an ANCOVA for the BDI-II score after 12 weeks was carried out with BDI-II score at baseline as covariate.
The analyses of secondary endpoints followed the same approach as for the primary endpoint. The number of patients with a clinical diagnosis of current major depressive disorder was analysed using a logistic regression model with the variables treatment group and baseline score of BDI-II. Statistical programming was done by the trial statisticians (TF, A-MK, and JW) using R version 4.0.0 and SAS version 9.4.
An independent data monitoring committee was established before the start of the trial to be consulted in case any predefined safety concerns were registered in the study platform.
This trial is registered at ClinicalTrials.gov, NCT02740361.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Participants were enrolled between May 3, 2017, and Nov 4, 2020; the last patient completed the primary trial phase on Jan 31, 2021. The original target sample size was 375 with a recruitment period of 36 months. Recruitment was temporarily halted owing to the COVID-19 pandemic and remained difficult during 2020 for all centres; the recruitment phase was therefore extended and was stopped in November, 2020, when the available funding was exhausted. We screened 485 patients, of whom 205 were excluded, 280 were enrolled and randomly assigned, and 279 were allocated to the trial groups with one excluded owing to a screening error (Charité Universitätsmedizin Berlin n=35, Universitätsklinikum Hamburg-Eppendorf n=102, Penn State University n=47, University of Missouri n=51, Cedars Sinai Medical Center n=44). The dropout rate, defined as the proportion of participants who did not complete outcome measures, for the primary trial phase (12 weeks) was 18% (50 of 279 participants; figure 1).
Figure 1: Trial profile.
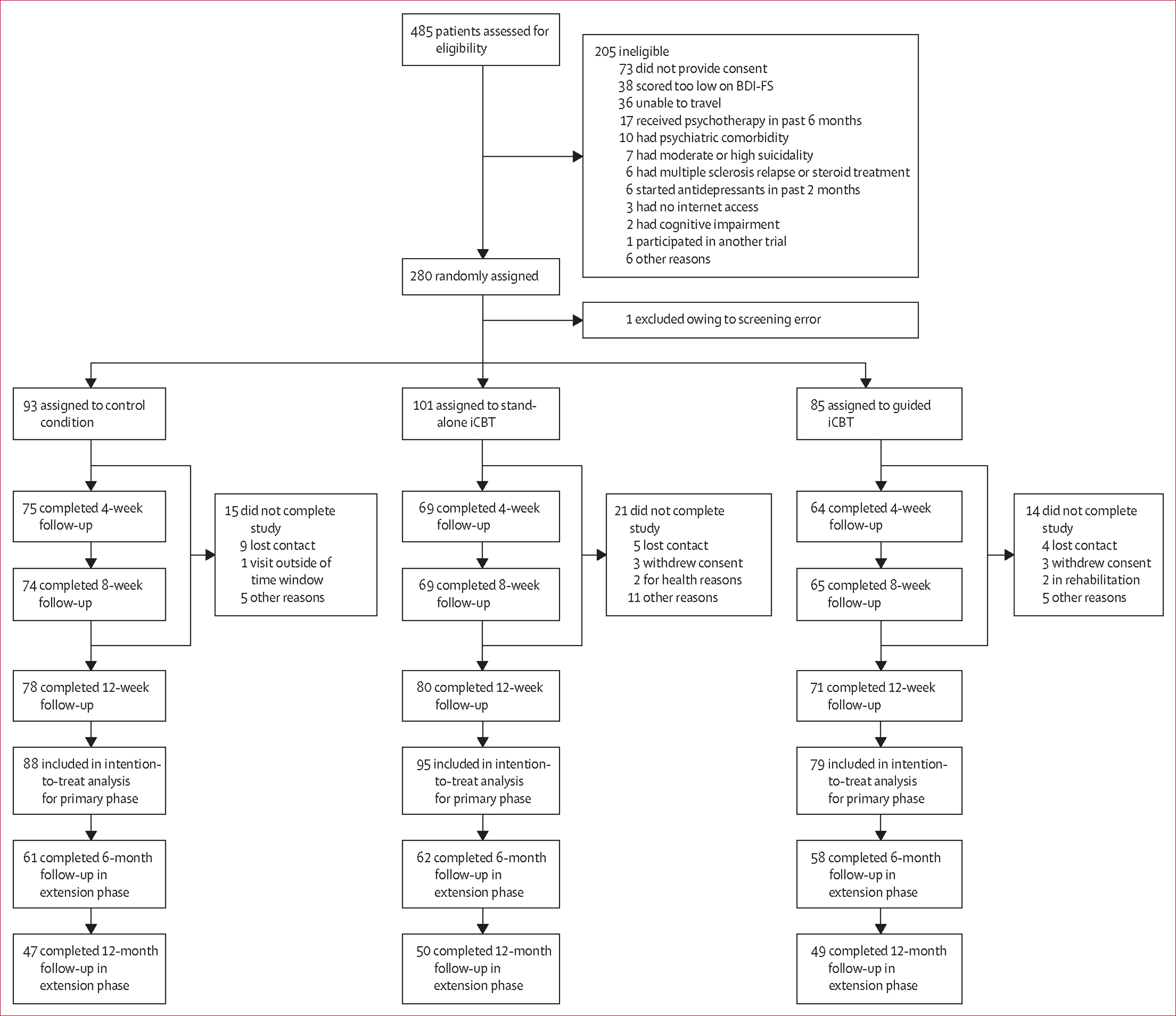
The intention-to-treat population included all randomly assigned patients who completed at least one post-baseline assessment (ie, any or all of the 4-week, 8-week, or 12-week follow-up assessments).
Baseline sociodemographic and clinical characteristics are shown in tables 1, 2. 44% of the participants were treated with antidepressants at baseline. PDDS and EDSS scores indicated moderate disability on average, but the sample included a wide range from no or mild disability to severe disability (range 0–8 for both EDSS and PDDS scores).
Table 1:
Baseline characteristics
| Control (n=88) | Stand-alone iCBT (n=95) | Guided iCBT (n=79) | |
|---|---|---|---|
|
| |||
| Age | 47·3 (11·1) | 46·5 (11·9) | 47·1 (12·1) |
| Sex | |||
| Female | 69 (78%) | 72 (76%) | 59 (75%) |
| Male | 19 (22%) | 23 (24%) | 20 (25%) |
| Multiple sclerosis disease course | |||
| Relapsing–remitting | 65 (74%) | 61 (64%) | 59 (75%) |
| Primary progressive | 8 (9%) | 17 (18%) | 9 (11%) |
| Secondary progressive | 14 (16%) | 15 (16%) | 8 (10%) |
| PDDS score, points | 3 (1–4) | 3 (1–4) | 3 (2–4) |
| EDSS score, points | 4·0 (2·7–5·3) | 4·0 (2·5–5·5) | 3·5 (2·5–5) |
| Time since diagnosis, years | 11·1 (7·7) | 10·0 (8·1) | 11·1 (9·7) |
| Time since first symptoms, years | 13·8 (8·3) | 14·3 (9·6) | 15·7 (13·0) |
| Disease-modifying therapies | |||
| Yes | 58 (66%) | 59 (62%) | 48 (61%) |
| No | 30 (34%) | 36 (38%) | 31 (39%) |
| Antidepressant treatment | |||
| None | 47 (53%) | 49 (52%) | 48 (61%) |
| Selective serotonin reuptake inhibitor | 25 (28%) | 22 (23%) | 20 (25%) |
| Serotonin noradrenaline reuptake inhibitor | 10 (11%) | 11 (12%) | 6 (8%) |
| Tricyclic antidepressant | 2 (2%) | 5 (5%) | 2 (3%) |
| Other | 7 (8%) | 13 (14%) | 4 (5%) |
| Psychotherapy | |||
| Yes | 4 (5%) | 5 (5%) | 8 (10%) |
| No | 84 (95%) | 90 (95%) | 71 (90%) |
| BICAMS | |||
| SDMT score, points | 50·3 (10·6) | 48·7 (12·2) | 49·7 (11·5) |
| CVLT-II score, points | 55·3 (10·5) | 53·5 (10·9) | 52·6 (10·2) |
| BVMT-R score, points | 25·5 (5·7) | 25·3 (6·4) | 24·5 (7·3) |
| Ethnicity | |||
| White | 61 (69%) | 70 (74%) | 45 (57%) |
| African American or Black | 0 | 1 (1%) | 4 (5%) |
| Hispanic or Latino/a | 0 | 1 (1%) | 0 |
| Other | 0 | 0 | 2 (3%) |
| Not provided | 27 (31%) | 23 (24%) | 28 (35%) |
Data are mean (SD), median (IQR), or n (%). BICAMS=Brief International Cognitive Assessment for Multiple Sclerosis. BVMT-R=Brief Visuospatial Memory Test-Revised. CVLT-II=California Verbal Learning Test-II. EDSS=Expanded Disability Status Scale. iCBT=internet-based cognitive behavioural therapy. PDDS=Patient-Determined Disease Steps. SDMT=Symbol Digit Modalities Test.
Table 2:
Baseline values of outcome measures
| Control (n=88) | Stand-alone iCBT (n=95) | Guided iCBT (n=79) | |
|---|---|---|---|
|
| |||
| BDI-II score, points | 21·3 (8·2) | 23·9 (7·2) | 24·6 (8·3) |
| FSMC score, points | 70·6 (17·9) | 71·1 (16·7) | 72·1 (16·0) |
| WHOQOL-BREF score, points | |||
| Psychological domain | 47·1 (13·6) | 42·6 (14·5) | 41·7 (14·3) |
| Physical domain | 51·4 (17·7) | 49·3 (18·4) | 47·4 (18·0) |
| Social relationships domain | 50·9 (21·2) | 49·0 (22·3) | 50·0 (19·4) |
| Environment domain | 70·0 (14·7) | 64·0 (16·4) | 65·2 (15·1) |
| CFS score, points | 21·1 (5·3) | 21·6 (5·9) | 21·5 (5·4) |
| MADRS score, points | 17·7 (6·5) | 19·6 (6·1) | 20·5 (7·8) |
| MSIS psychological domain score, points | 47·3 (19·0) | 54·2 (16·2) | 53·4 (19·1) |
| Diagnosis of major depressive disorder | |||
| Yes | 51 (58%) | 60 (63%) | 59 (75%) |
| No | 37 (42%) | 35 (37%) | 20 (25%) |
Data are mean (SD) or n (%). BDI-II=Beck Depression Inventory-II. CFS=Chalder Fatigue Scale. FSMC=Fatigue Scale for Motor and Cognitive Functions.
MADRS=Montgomery-Åsberg Depression Rating Scale. MSIS=Multiple Sclerosis Impact Scale. WHOQOL-BREF=WHO Quality of Life-Brief Version.
Participants assigned to the iCBT groups used the programme for a median of 11·0 days (IQR 6·0–18·2) of the 12-week primary trial phase (median for stand-alone iCBT 10·0 [6·0–18·5]; guided iCBT 12·0 [8·0–18·0]). The mean number of modules (stand-alone iCBT 6·9 [SD 6·0]; guided iCBT 9·2 [6·3]) and the mean number of hours worked with the programme (stand-alone iCBT 7·5 [10·2]; guided iCBT 9·8 [11·1]) were higher in the guided iCBT group than in the stand-alone iCBT group.
As shown by the difference in BDI-II score compared with control, significantly reduced depressive symptoms were seen in the stand-alone iCBT (difference 6·32 points [95% CI 3·37–9·27]; p<0·0001; effect size d=0·97 [95% CI 0·64–1·30]) and the guided iCBT (5·80 points [2·71–8·88]; p<0·0001; effect size d=0·96 [0·62–1·30]) groups at week 12 (figure 2, table 3). However, no significant difference was found between the stand-alone iCBT and guided iCBT groups at week 12.
Figure 2: Effects of treatment on the severity of depression symptoms.
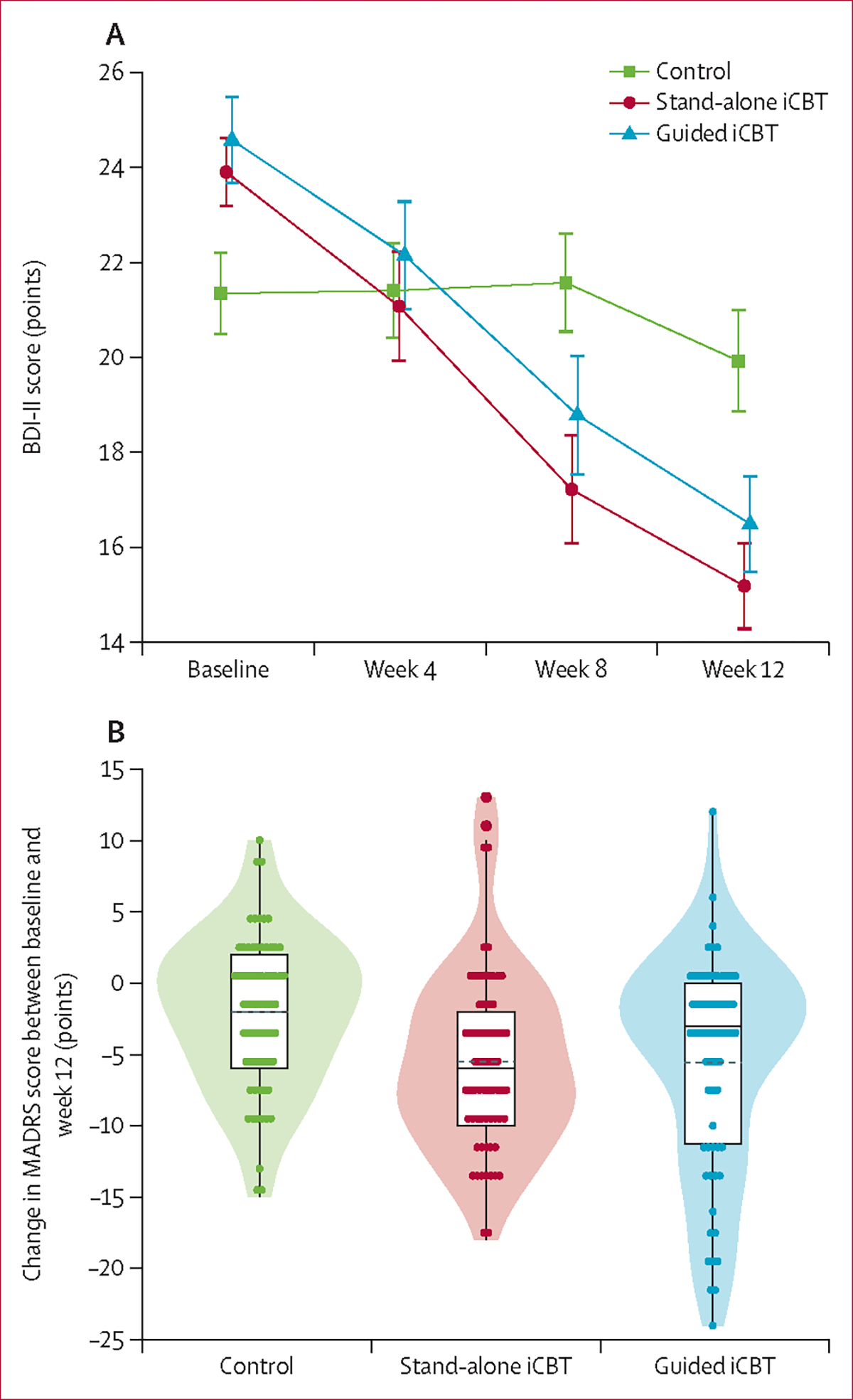
(A) BDI-II score for control and iCBT groups at baseline, week 4, week 8, and week 12. Data are mean (SE). (B) Violin plots showing the change in MADRS score between baseline and end of treatment at week 12. Data shown are IQR (boxes), mean (dotted line), median (solid line), and individual data points (dots). BDI-II=Beck Depression Inventory-II. MADRS=Montgomery-Åsberg Depression Rating Scale.
Table 3:
Effects of treatment on depressive symptoms at week 12 (primary endpoint)
| Estimate (95% CI) | p value | Cohen’s d (95% CI) | |
|---|---|---|---|
|
| |||
| Linear mixed model for repeated measures (ITT population) * | |||
| Control vs stand-alone iCBT | 6·32 (3·37–9·27) | <0·0001 | 0·97 (0·64–1·30) |
| Control vs guided iCBT | 5·80 (2·71–8·88) | <0·0001 | 0·96 (0·62–1·30) |
| ANCOVA with last observation carried forward (ITT population) † | |||
| Control vs stand-alone iCBT | 5·81 (3·38–8·24) | <0·0001 | 0·90 (0·59–1·20) |
| Control vs guided iCBT | 5·58 (3·02–8·13) | <0·0001 | 0·89 (0·57–1·21) |
| ANCOVA with multiple imputations (ITT population) † | |||
| Control vs stand-alone iCBT | 6·32 (4·20–8·45) | <0·0001 | 0·99 (0·67–1·31) |
| Control vs guided iCBT | 5·71 (3·48–7·94) | <0·0001 | 0·94 (0·60–1·27) |
| ANCOVA with complete cases † | |||
| Control vs stand-alone iCBT | 6·36 (3·85–8·86) | <0·0001 | 0·99 (0·66–1·33) |
| Control vs guided iCBT | 5·93 (3·33–8·54) | <0·0001 | 0·97 (0·63–1·32) |
| Linear mixed model for repeated measures (mITT population) † | |||
| Control vs stand-alone iCBT | 6·41 (3·37–9·46) | <0·0001 | 0·95 (0·61–1·30) |
| Control vs guided iCBT | 5·97 (2·88–9·07) | <0·0001 | 0·94 (0·60–1·29) |
Data are the difference in BDI-II score (points) between the two groups specified at week 12. BDI-II=Beck Depression Inventory-II. iCBT=internet-based cognitive behavioural therapy. ITT=intention to treat. mITT=modified intention to treat.
Prespecified primary analysis.
Prespecified sensitivity analysis.
We observed significant improvements in the generic measure of quality of life (WHOQOL-BREF, psychological, physical, and environmental domains) in both iCBT groups compared with the control group (table 4). Similarly, improvements were seen in multiple sclerosis-specific psychological quality-of life assessment (MSIS-29, psychological domain) in both iCBT groups compared with the control group. However, no consistently significant improvements were observed for other domains of generic or multiple sclerosis-specific quality of life in iCBT groups, and no robust effects were seen on measures of fatigue (FSMC and CFS).
Table 4:
Secondary and exploratory endpoints
| Estimate (95% CI) | p value | Cohen’s d (95% CI) | |
|---|---|---|---|
|
| |||
| WHOQOL-BREF psychological domain | |||
| Control vs stand-alone iCBT | −8·29 (−12·50 to −4·08) | <0·0001 | −0·78 (−1·10 to −0·45) |
| Control vs guided iCBT | −7·95 (−12·32 to −3·59) | <0·0001 | −0·76 (−1·10 to −0·42) |
| WHOQOL-BREF physical domain | |||
| Control vs stand-alone iCBT | −6·99 (−11·18 to −2·82) | 0·0002 | −0·62 (−0·94 to −0·29) |
| Control vs guided iCBT | −6·05 (−10·37 to −1·72) | 0·0021 | −0·59 (−0·92 to −0·25) |
| WHOQOL-BREF social relationships domain | |||
| Control vs stand-alone iCBT | −5·31 (−10·26 to −0·37) | 0·0164 | −0·41 (−0·73 to −0·09) |
| Control vs guided iCBT | −2·32 (−7·41 to 2·77) | 0·25 | −0·19 (−0·52 to 0·13) |
| WHOQOL-BREF environmental domain | |||
| Control vs stand-alone iCBT | −4·46 (−7·84 to −1·08) | 0·0035 | −0·57 (−0·89 to −0·25) |
| Control vs guided iCBT | −4·08 (−7·55 to −0·61) | 0·0091 | −0·52 (−0·85 to −0·19) |
| MSIS psychological domain | |||
| Control vs stand-alone iCBT | 9·02 (3·61 to 14·43) | 0·0003 | 0·76 (0·43 to 1·09) |
| Control vs guided iCBT | 7·77 (2·21 to 13·33) | 0·0021 | 0·62 (0·29 to 0·95) |
| MSIS physical domain | |||
| Control vs stand-alone iCBT | 3·70 (0·15 to 7·24) | 0·0197 | 0·39 (0·07 to 0·71) |
| Control vs guided iCBT | 2·57 (1·09 to 6·22) | 0·10 | 0·30 (−0·03 to 0·63) |
| FSMC | |||
| Control vs stand-alone iCBT | 2·93 (−0·57 to 6·42) | 0·0574 | 0·30 (−0·02 to 0·62) |
| Control vs guided iCBT | 1·94 (−1·66 to 5·54) | 0·19 | 0·22 (−0·11 to 0·55) |
| CFS | |||
| Control vs stand-alone iCBT | 1·49 (−0·23 to 3·02) | 0·0495 | 0·29 (−0·03 to 0·61) |
| Control vs guided iCBT | 0·20 (−1·56 to 1·97) | 0·56 | 0·03 (−0·30 to 0·35) |
| MADRS | |||
| Control vs stand-alone iCBT | 3·16 (0·84 to 5·48) | 0·0026 | 0·59 (0·25 to 0·94) |
| Control vs guided iCBT | 2·98 (0·61 to 5·35) | 0·0053 | 0·54 (0·19 to 0·90) |
Data are the difference in score (points) between the two groups specified on the stated scales at week 12. CFS=Chalder Fatigue Scale. FSMC=Fatigue Scale for Motor and Cognitive Functions. iCBT=internet-based cognitive behavioural therapy. MADRS=Montgomery-Åsberg Depression Rating Scale. MSIS=Multiple Sclerosis Impact Scale. WHOQOL-BREF=WHO Quality of Life-Brief Version.
We detected no concerns in the predefined safety measures regarding new occurrences of suicidality, as no participant registered a response of 3 on item 9 of the BDI-II at any time point (week 4, week 8, week 12, month 6, or month 12) nor any responses of 3a or 3b on SBQ-R item 3 plus a score of 5 or 6 on SBQ-R item 4 during any of the clinical visits. Accordingly, no concerns for suicidality in the enrolled patients were registered by the study platform during the entire study duration. We also did not detect any hospitalisations due to a psychiatric disorder, suicidality during the clinical interviews, suicidal thoughts mentioned in the message exchange with the study therapists, or any lethal or life-threatening events (including suicide or suicide attempt). Worsening of depressive symptoms during the trial from below to above the cutoff for caseness (BDI-II>13, baseline compared to week 12) was observed in three patients in the control group, one patient in the stand-alone iCBT group, and no patients in the guided iCBT group. Some additional adverse events were recorded during clinical examinations (including pain, urinary retention, diarrhoea, and others), none of which were deemed to be related to the intervention. A detailed list of adverse events is provided in the appendix (p 2).
Treatment effects for the primary endpoint, BDI-II score at week 12, were supported by four sensitivity analyses: ANCOVA with last observation carried forward, ANCOVA with multiple imputations, ANCOVA with complete cases, and linear mixed model for repeated measures using the modified intention-to-treat population. These analyses yielded similar point estimates and effect sizes to the primary analysis (table 3).
Treatment effects on depressive symptoms were substantiated by the clinician-rated MADRS, with significant improvements in both iCBT groups compared with control (table 4, figure 2B). No significant treatment effects were observed on major depressive disorder diagnosis (odds ratio based on logistic regression: iCBT vs control 0·62 [95% CI 0·29–13·1], p=0·21; guided iCBT vs control 0·62 [0·29–1·32], p=0·22).
Effects on the BDI-II score remained significant at 6 months, as shown by the differences compared with control in stand-alone iCBT (difference 6·79 points [95% CI 2·97–10·60]; p<0·0001; effect size d=0·88 [0·50–1·26]) and guided iCBT (4·99 points [1·04–8·94]; p=0·0030; effect size d=0·68 [0·31–1·05]) groups (appendix p 3), with no significant differences between the two iCBT groups.
The estimated mean difference in BDI-II score after 12 months between non-booster and booster groups was 0·266 points (95% CI −0·851 to 1·380; p=0·64). Both groups exhibited stable BDI-II scores up to month 12 and remained significantly below their baseline levels (all values p<0·0001; appendix p 4).
Discussion
This trial met its primary endpoint and provides evidence for the efficacy of this multiple sclerosis-specific online depression management tool as a stand-alone or guided application to reduce depressive symptoms in patients with multiple sclerosis over a 12-week period. Both versions were safe and improved domains of quality of life.
Despite the robust treatment effects on depressive symptoms as measured by the BDI-II and the MADRS, we did not observe significant differences in the proportions of participants meeting diagnostic criteria for major depressive disorder. This could in part be due to the fact that our trial was not powered to detect effects in this dichotomous endpoint. Previous work found that affective symptoms (eg, depressed mood and anhedonia) are more likely to remit with treatment, whereas other symptoms (eg, cognitive symptoms) tend to persist.43 As such, substantially larger sample sizes might be required to show an effect on the diagnosis of major depressive disorder.
The therapist support provided to participants in the guided iCBT group did not add to the treatment effect in any of the outcome measures investigated. In part, this finding could be attributed to the nature of the therapist support, which was largely geared towards motivation to work with the programme and did not include any actual therapeutic interventions on the therapists’ part. In addition, the proportion of participants receiving psychotherapy at baseline was slightly higher in the guided iCBT group (10%) than in the stand-alone iCBT group (5%), potentially attenuating the effects of iCBT in the guided group. A related question would be if a particular subgroup can be identified in which the therapist support did help substantially (eg, in patients with more severe depression, those with more severe multiple sclerosis, or those who more actively engaged in the exchange with the therapist). Such secondary analyses of our dataset will be conducted in subsequent work.
We were unable to reach the planned sample size of 375 participants (279 were enrolled), at least in part due to the difficulties associated with conducting clinical trials during the COVID-19 pandemic, particularly in a potentially vulnerable group.44 Given the robustness of the treatment effect (as supported by several sensitivity analyses of the primary endpoint and similar effects using a clinician-based rating), we are confident that our trial was still sufficiently powered. Further reassurance is provided by the larger effect sizes observed in this phase 3 trial than in our previous single-centre, phase 2 trial.20
We observed some differences in the baseline values of the BDI-II scores between the trial groups. Although all statistical analyses accounted for baseline levels of the respective outcome measure, future trials using iCBT in multiple sclerosis could consider using stratified randomisation to minimise the probability of baseline differences. Moreover, average residual BDI-II scores at the end of treatment remained higher than the clinical threshold for depression. This finding indicates that full remission is difficult to reach with an internet-based tool in many cases.
Masking is a key challenge for trials of behavioural interventions, particularly those with patient-reported outcomes. We therefore caution that the observed effects from our study cannot be directly compared with the effects observed in placebo-controlled drug trials for multiple sclerosis-associated depression.14 Regardless, results from the patient-reported BDI-II were supported by MADRS scores obtained by masked raters, providing some reassurance that unmasking of participants is unlikely to systematically bias our estimates of treatment effects.
The outcome of a randomised controlled trial depends on the choice of the control condition as much as the experimental treatment.45 The literature suggests that a treatment-as-usual or waitlist control condition in behavioural trials is associated with larger effect sizes relative to trials with active comparators.45 However, owing to the paucity of evidence for the efficacy of any treatment strategy for multiple sclerosis-associated depression,13 we believe that the use of a treatment-as-usual waitlist control group is appropriate.
This trial provides evidence for safety and efficacy of this multiple sclerosis-specific online tool as a stand-alone or guided application to reduce depressive symptoms in multiple sclerosis over a 12-week period. This remote-access, scalable intervention increases the therapeutic options in this patient group and could help to overcome treatment barriers.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed, PsycINFO, the Cochrane library, and Embase from database inception to March, 2018, using keywords “Multiple Sclerosis”, “psychological distress”, “stress reduction”, “distress”, “depressive symptoms”, and “anxiety symptoms”, yielding 15 trials of cognitive behavioural therapy (CBT) or mindfulness-based therapy for the treatment of depressive symptoms in multiple sclerosis. The analysis indicated that behavioural interventions such as CBT can be effective to reduce depression when delivered in person or remotely with moderate effect size, compared with treatment-as-usual, waitlist, or active-treatment control conditions. However, the overall quality of the trials was low, sample sizes were small, and considerable heterogeneity was present. We previously conducted one single-centre trial in Germany of internet-based CBT (iCBT) for reducing depressive symptoms in multiple sclerosis that showed good safety and efficacy. These findings support the potential of iCBT for the treatment of depression associated with multiple sclerosis but also highlight the need for large confirmatory multicentre trials that could inform clinical practice.
Added value of this study
We conducted a multicentre randomised controlled trial in two countries to test the efficacy of a multiple sclerosis-specific online depression-management tool. Compared with treatment as usual, we found a significant reduction in depressive symptoms after 12 weeks of treatment with either a stand-alone or a guided version of the tool, added to treatment as usual. The trial also showed beneficial effects on generic as well as multiple sclerosis-specific quality-of-life measures. Treatment effects were maintained at 6 months and at 12 months. No safety signals for increased risk of suicidality or other adverse events were detected.
Implications of all the available evidence
The available evidence supports the use of CBT-based treatments for depression associated with multiple sclerosis and suggests that scalable, fully automated CBT programmes, delivered using the internet, are effective in reducing depressive symptoms in these patients. Such programmes could be particularly helpful to facilitate access for patients who are unable to receive CBT in person.
Acknowledgments
This investigation was supported by a research grant (RG-15070–5418) from the NMSS. RM-M is in part funded by the UK Department of Health via the National Institute for Health Research Specialist Biomedical Research Centre for Mental Health award to the South London and Maudsley NHS Foundation and the Institute of Psychiatry at King’s College London, London, UK. We thank Bernhard Wellhöfer, Katharina Stößlein, Stefanie Märschenz, Luisa Kammler, Josephine Pora, and Gesa Pust for their contribution to the logistics of the trial, data acquisition, quality control, and data management. We also greatly appreciate the administrative support of the NeuroCure Clinical Research Center, Charité–Universitätsmedizin Berlin, Berlin, Germany.
Footnotes
Declaration of interests
SMG reports honoraria from Hexal and research grants from Biogen, the German Ministry for Education and Research, the German Ministry of Health, Deutsche Forschungsgemeinschaft, and the National Multiple Sclerosis Society (NMSS). TF reports personnel fees for consultancies (including data monitoring committees and advisory boards) from Bayer, Bristol Myers Squibb, CSL Behring, Enanta Pharmaceuticals, Fresenius Kabi, Galapagos, Johnson & Johnson, Janssen Pharmaceuticals, LivaNova, Minoryx Therapeutics, Novartis, Roche, Vifor Pharma, Immunic Therapeutics, and Kyowa Kirin. BM is an employee of the GAIA Group, the owner and distributor of commercial digital health interventions including Deprexis and the multiple sclerosis-specific internet-delivered cognitive behavioural therapy Amiria. RM-M reports personal fees from training in cognitive behavioural therapy for irritable bowel syndrome for Central and North West London NHS Foundation Trust and University of Southampton, outside the submitted work. She receives payment for consultancy to Mahana Therapeutics, has share options in Mahana Therapeutics, and is a beneficiary of a licence agreement between Mahana Therapeutics and King’s College London. SGL has participated in numerous clinical trials, receiving research support from Roche, Biogen, TG Therapeutics, Novartis, Bristol-Myers Squibb, Atara Biotherapeutics, Anokion, Immunic Therapeutics, Sanofi, and the Patient-Centered Outcomes Research Institute; has received support from NMSS-Investigator initiated studies on multiple sclerosis wellness and rehabilitation; and has unpaid board positions for the Friends of the Multiple Sclerosis Achievement Center and the Mid America Chapter of the NMSS. CR reports a stipend from Förderfond der Medizinischen Fakultät, Universitätsklinikum Hamburg Eppendorf. I-KP declares grants from Deutsche Multiple Sklerose Gesellschaft, Novartis, Teva Pharmaceuticals, and Roche; payment or honoraria from Almirall, Bayer Pharma, Biogen, BMS, Celgene, Genzyme, Janssen Pharmaceuticals, Merck, Novartis, Roche, and Teva Pharmaceuticals; and participation on data safety monitoring boards or advisory boards for Biogen, BMS, Janssen Pharmaceuticals, Merck, Novartis, and Roche. FP reports grants from the German Ministry for Education and Research, Deutsche Forschungsgemeinschaft, the Einstein Foundation, the Guthy Jackson Charitable Foundation, the EU FP7 Framework Program, Biogen, Genzyme, Merck Serono, Novartis, Bayer, Roche, Parexel, and Almirall; payment or honoraria from the Guthy Jackson Foundation, Bayer, Biogen, Merck Serono, Sanofi Genzyme, Novartis, Viela Bio, Roche, Union Chimique Belge (UCB), Mitsubishi Tanabe, and Celgene; support for attending meetings or travel from Guthy Jackson Foundation, Bayer, Biogen, Merck Serono, Sanofi Genzyme, Novartis, Alexion, Viela Bio, Roche, UCB, Mitsubishi Tanabe, and Celgene; participation on data safety monitoring boards or advisory boards for Celgene, Roche, UCB, and Merck; and unpaid editor positions for PLoS One and Neurology Neuroimmunology & Neuroinflammation. NLS receives research grants from the National Institutes of Health National Institute of Neurological Disorders and Stroke and a leadership role as Chair of the National Medical Advisory Committee of the NMSS. JMB has served on the Novartis unbranded speakers’ bureau and has received grant support from Genzyme. PAA has served on the EMD Serono speakers’ bureau and served as a consultant for Biogen and Roche Pharmaceuticals. CH reports payment or honoraria for presentations from Roche. All other authors declare no competing interests.
Contributor Information
Stefan M Gold, Klinik für Psychiatrie und Psychotherapie, Campus Benjamin Franklin, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany; Medizinische Klinik mS Psychosomatik, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany; German Center for Mental Health (DZPG), Berlin, Germany; Institut für Neuroimmunologie und Multiple Sklerose, Universitätklinikum Hamburg-Eppendorf, Hamburg, Germany.
Tim Friede, Department of Medical Statistics, University Medical Center Göttingen, Göttingen, Germany.
Björn Meyer, Research Department, GAIA AG, Hamburg, Germany.
Rona Moss-Morris, Psychology Department, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, UK.
Joanna Hudson, Psychology Department, Institute of Psychiatry, Psychology, and Neuroscience, King’s College London, London, UK.
Susanna Asseyer, NeuroCure Clinical Research Center, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Judith Bellmann-Strobl, NeuroCure Clinical Research Center, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany; Experimental and Clinical Research Center, Max Delbrück Center for Molecular Medicine and Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Andreas Leisdon, Klinik für Psychiatrie und Psychotherapie, Campus Benjamin Franklin, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Leonie Ißels, Klinik für Psychiatrie und Psychotherapie, Campus Benjamin Franklin, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Kristin Ritter, Klinik für Psychiatrie und Psychotherapie, Campus Benjamin Franklin, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
David Schymainski, Klinik für Psychiatrie und Psychotherapie, Campus Benjamin Franklin, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Hayley Pomeroy, Department of Neurology, Cedars Sinai Medical Center, Los Angeles, CA, USA.
Sharon G Lynch, Department of Neurology, University of Kansas School of Medicine, Kansas City, KS, USA.
Julia S Cozart, Department of Psychology, University of Missouri–Kansas City, Kansas City, MO, USA.
Joan Thelen, Department of Psychology, University of Missouri–Kansas City, Kansas City, MO, USA.
Cristina A F Román, Kessler Foundation, Rutgers New Jersey Medical School, Newark, NJ, USA; Department of Physical Medicine & Rehabilitation, Rutgers New Jersey Medical School, Newark, NJ, USA; Department of Neurology, Rutgers New Jersey Medical School, Newark, NJ, USA.
Margaret Cadden, Harvard Medical School, Massachusetts General Hospital/Brigham and Women’s Hospital, Boston, MA, USA.
Erin Guty, Department of Psychology, Penn State University, University Park, PA, USA.
Stephanie Lau, Institut für Neuroimmunologie und Multiple Sklerose, Universitätklinikum Hamburg-Eppendorf, Hamburg, Germany.
Jana Pöttgen, Institut für Neuroimmunologie und Multiple Sklerose, Universitätklinikum Hamburg-Eppendorf, Hamburg, Germany.
Caren Ramien, Institut für Neuroimmunologie und Multiple Sklerose, Universitätklinikum Hamburg-Eppendorf, Hamburg, Germany.
Susan Seddiq-Zai, Institut für Neuroimmunologie und Multiple Sklerose, Universitätklinikum Hamburg-Eppendorf, Hamburg, Germany.
Anna-Maria Kloidt, Department of Medical Statistics, University Medical Center Göttingen, Göttingen, Germany.
Johannes Wieditz, Department of Medical Statistics, University Medical Center Göttingen, Göttingen, Germany.
Iris-Katharina Penner, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Friedemann Paul, NeuroCure Clinical Research Center, Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany; Experimental and Clinical Research Center, Max Delbrück Center for Molecular Medicine and Charité–Universitätsmedizin Berlin, Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany.
Nancy L Sicotte, Department of Neurology, Cedars Sinai Medical Center, Los Angeles, CA, USA.
Jared M Bruce, Department of Biomedical and Health Informatics, School of Medicine, University of Missouri–Kansas City, Kansas City, MO, USA.
Peter A Arnett, Department of Psychology, Penn State University, University Park, PA, USA.
Christoph Heesen, Institut für Neuroimmunologie und Multiple Sklerose, Universitätklinikum Hamburg-Eppendorf, Hamburg, Germany.
Data sharing
Anonymised individual patient data from the primary trial phase—including data on treatment group, region (Germany or the USA), and measurements of primary and secondary endpoints—are available on request at https://zenodo.org/record/7965979.
References
- 1.Gold SM, Köhler-Forsberg O, Moss-Morris R, et al. Comorbid depression in medical diseases. Nat Rev Dis Primers 2020; 6: 69. [DOI] [PubMed] [Google Scholar]
- 2.Marrie RA. Comorbidity in multiple sclerosis: implications for patient care. Nat Rev Neurol 2017; 13: 375–82. [DOI] [PubMed] [Google Scholar]
- 3.Boeschoten RE, Braamse AMJ, Beekman ATF, et al. Prevalence of depression and anxiety in multiple sclerosis: a systematic review and meta-analysis. J Neurol Sci 2017; 372: 331–41. [DOI] [PubMed] [Google Scholar]
- 4.Siegert RJ, Abernethy DA. Depression in multiple sclerosis: a review. J Neurol Neurosurg Psychiatry 2005; 76: 469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitehouse CE, Fisk JD, Bernstein CN, et al. Comorbid anxiety, depression, and cognition in MS and other immune-mediated disorders. Neurology 2019; 92: e406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Washington F, Langdon D. Factors affecting adherence to disease-modifying therapies in multiple sclerosis: systematic review. J Neurol 2022; 269: 1861–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prisnie JC, Sajobi TT, Wang M, et al. Effects of depression and anxiety on quality of life in five common neurological disorders. Gen Hosp Psychiatry 2018; 52: 58–63. [DOI] [PubMed] [Google Scholar]
- 8.Disanto G, Zecca C, MacLachlan S, et al. Prodromal symptoms of multiple sclerosis in primary care. Ann Neurol 2018; 83: 1162–73. [DOI] [PubMed] [Google Scholar]
- 9.Binzer S, McKay KA, Brenner P, Hillert J, Manouchehrinia A. Disability worsening among persons with multiple sclerosis and depression: a Swedish cohort study. Neurology 2019; 93: e2216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKay KA, Tremlett H, Fisk JD, et al. Psychiatric comorbidity is associated with disability progression in multiple sclerosis. Neurology 2018; 90: e1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marrie RA, Walld R, Bolton JM, et al. Psychiatric comorbidity increases mortality in immune-mediated inflammatory diseases. Gen Hosp Psychiatry 2018; 53: 65–72. [DOI] [PubMed] [Google Scholar]
- 12.Palladino R, Chataway J, Majeed A, Marrie RA. Interface of multiple sclerosis, depression, vascular disease, and mortality: a population-based matched cohort study. Neurology 2021; 97: e1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minden SL, Feinstein A, Kalb RC, et al. Evidence-based guideline: assessment and management of psychiatric disorders in individuals with MS: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2014; 82: 174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch MW, Glazenborg A, Uyttenboogaart M, Mostert J, De Keyser J. Pharmacologic treatment of depression in multiple sclerosis. Cochrane Database Syst Rev 2011; 2: CD007295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hind D, Cotter J, Thake A, et al. Cognitive behavioural therapy for the treatment of depression in people with multiple sclerosis: a systematic review and meta-analysis. BMC Psychiatry 2014; 14: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghielen I, Rutten S, Boeschoten RE, et al. The effects of cognitive behavioral and mindfulness-based therapies on psychological distress in patients with multiple sclerosis, Parkinson’s disease and Huntington’s disease: two meta-analyses. J Psychosom Res 2019; 122: 43–51. [DOI] [PubMed] [Google Scholar]
- 17.Mohr DC, Hart SL, Julian L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry 2005; 62: 1007–14. [DOI] [PubMed] [Google Scholar]
- 18.Karyotaki E, Efthimiou O, Miguel C, et al. Internet-based cognitive behavioral therapy for depression: a systematic review and individual patient data network meta-analysis. JAMA Psychiatry 2021; 78: 361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White V, Linardon J, Stone JE, et al. Online psychological interventions to reduce symptoms of depression, anxiety, and general distress in those with chronic health conditions: a systematic review and meta-analysis of randomized controlled trials. Psychol Med 2022; 52: 548–73. [DOI] [PubMed] [Google Scholar]
- 20.Fischer A, Schröder J, Vettorazzi E, et al. An online programme to reduce depression in patients with multiple sclerosis: a randomised controlled trial. Lancet Psychiatry 2015; 2: 217–23. [DOI] [PubMed] [Google Scholar]
- 21.Boeschoten RE, Dekker J, Uitdehaag BM, et al. Internet-based treatment for depression in multiple sclerosis: a randomized controlled trial. Mult Scler 2017; 23: 1112–22. [DOI] [PubMed] [Google Scholar]
- 22.Sesel A-L, Sharpe L, Beadnall HN, Barnett MH, Szabo M, Naismith SL. A randomized controlled trial of a web-based mindfulness programme for people with MS with and without a history of recurrent depression. Mult Scler 2022; 28: 1392–401. [DOI] [PubMed] [Google Scholar]
- 23.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hohol MJ, Orav EJ, Weiner HL. Disease steps in multiple sclerosis: a simple approach to evaluate disease progression. Neurology 1995; 45: 251–55. [DOI] [PubMed] [Google Scholar]
- 25.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–52. [DOI] [PubMed] [Google Scholar]
- 26.Langdon DW, Amato MP, Boringa J, et al. Recommendations for a Brief International Cognitive Assessment for Multiple Sclerosis (BICAMS). Mult Scler 2012; 18: 891–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Twomey C, O’Reilly G, Meyer B. Effectiveness of an individually-tailored computerised CBT programme (Deprexis) for depression: a meta-analysis. Psychiatry Res 2017; 256: 371–77. [DOI] [PubMed] [Google Scholar]
- 28.Meyer B, Berger T, Caspar F, Beevers CG, Andersson G, Weiss M. Effectiveness of a novel integrative online treatment for depression (Deprexis): randomized controlled trial. J Med Internet Res 2009; 11: e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger T, Hämmerli K, Gubser N, Andersson G, Caspar F. Internet-based treatment of depression: a randomized controlled trial comparing guided with unguided self-help. Cogn Behav Ther 2011; 40: 251–66. [DOI] [PubMed] [Google Scholar]
- 30.Watson TM, Ford E, Worthington E, Lincoln NB. Validation of mood measures for people with multiple sclerosis. Int J MS Care 2014; 16: 105–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holländare F, Andersson G, Engström I. A comparison of psychometric properties between internet and paper versions of two depression instruments (BDI-II and MADRS-S) administered to clinic patients. J Med Internet Res 2010; 12: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skevington SM, Lotfy M, O’Connell KA. The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res 2004; 13: 299–310. [DOI] [PubMed] [Google Scholar]
- 33.Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain 2001; 124: 962–73. [DOI] [PubMed] [Google Scholar]
- 34.Penner IK, Raselli C, Stöcklin M, Opwis K, Kappos L, Calabrese P. The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue. Mult Scler 2009; 15: 1509–17. [DOI] [PubMed] [Google Scholar]
- 35.Cella M, Chalder T. Measuring fatigue in clinical and community settings. J Psychosom Res 2010; 69: 17–22. [DOI] [PubMed] [Google Scholar]
- 36.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–89. [DOI] [PubMed] [Google Scholar]
- 37.Osman A, Bagge CL, Gutierrez PM, Konick LC, Kopper BA, Barrios FX. The Suicidal Behaviors Questionnaire-Revised (SBQ-R): validation with clinical and nonclinical samples. Assessment 2001; 8: 443–54. [DOI] [PubMed] [Google Scholar]
- 38.Button KS, Kounali D, Thomas L, et al. Minimal clinically important difference on the Beck Depression Inventory—II according to the patient’s perspective. Psychol Med 2015; 45: 3269–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mallinckrodt CH, Clark SWS, Carroll RJ, Molenbergh G. Assessing response profiles from incomplete longitudinal clinical trial data under regulatory considerations. J Biopharm Stat 2003; 13: 179–90. [DOI] [PubMed] [Google Scholar]
- 40.Cohen J Statistical power analysis for the behavioral sciences, 2nd edn. Hillsdale, NJ: L Erlbaum Associates, 1988. [Google Scholar]
- 41.Cooper HM, Hedges LV, Valentine JC, eds. The handbook of research synthesis and meta-analysis, 2nd edn. New York, NY: Russell Sage Foundation, 2009. [Google Scholar]
- 42.Rubins rules. In: Heymans MW, Eekhout I. Applied missing data analysis with SPSS and (R) studio. https://bookdown.org/mwheymans/bookmi/rubins-rules.html (accessed July 29, 2022). [Google Scholar]
- 43.Gold SM, Otte C. Differential impact of affective and cognitive symptoms on remission of major depression. Lancet Psychiatry 2019; 6: 980. [DOI] [PubMed] [Google Scholar]
- 44.Garjani A, Middleton RM, Nicholas R, Evangelou N. Recovery from COVID-19 in multiple sclerosis: a prospective and longitudinal cohort study of the United Kingdom Multiple Sclerosis Register. Neurol Neuroimmunol Neuroinflamm 2021; 9: e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gold SM, Enck P, Hasselmann H, et al. Control conditions for randomised trials of behavioural interventions in psychiatry: a decision framework. Lancet Psychiatry 2017; 4: 725–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised individual patient data from the primary trial phase—including data on treatment group, region (Germany or the USA), and measurements of primary and secondary endpoints—are available on request at https://zenodo.org/record/7965979.


