Abstract
Multiple sclerosis (MS) is a chronic neuro-inflammatory disease resulting in disabilities that negatively impact patients’ life quality. While current treatment options do not reverse the course of the disease, treatment using mesenchymal stromal/stem cells (MSC) is promising. There has yet to be a consensus on the type and dose of MSC to be used in MS. This work aims to study the safety and efficacy of two treatment protocols of MSCs derived from the umbilical cord (UC-MSCs) and their secretome. The study included two groups of MS patients; Group A received two intrathecal doses of UC-MSCs, and Group B received a single dose. Both groups received UC-MSCs conditioned media 3 months post-treatment. Adverse events in the form of a clinical checklist and extensive laboratory tests were performed. Whole transcriptome analysis was performed on patients’ cells at baseline and post-treatment. Results showed that all patients tolerated the cellular therapy without serious adverse events. The general disability scale improved significantly in both groups at 6 months post-treatment. Examining specific aspects of the disease revealed more parameters that improved in Group A compared to Group B patients, including a significant increase in the (CD3+CD4+) expressing lymphocytes at 12 months post-treatment. In addition, better outcomes were noted regarding lesion load, cortical thickness, manual dexterity, and information processing speed. Both protocols impacted the transcriptome of treated participants with genes, transcription factors, and microRNAs (miRNAs) differentially expressed compared to baseline. Inflammation-related and antigen-presenting (HLA-B) genes were downregulated in both groups. In contrast, TNF-alpha, TAP-1, and miR142 were downregulated only in Group A. The data presented indicate that both protocols are safe. Furthermore, it suggests that administering two doses of stem cells can be more beneficial to MS patients. Larger multisite studies should be initiated to further examine similar or higher doses of MSCs.
Keywords: mesenchymal stem cells, multiple sclerosis, dose finding, conditioned medium, umbilical cord
Introduction
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease that affects young adults, with a higher prevalence in women1,2. MS patients suffer from motor and non-motor clinical symptoms that negatively impact their quality of life3,4. The pathological mechanisms responsible for the initiation and progression of MS are multifactorial and not entirely known5,6. Current treatment options are available for the relapsing–remitting form of the disease while treating progressive forms possesses limitations 7 . The available medication reduces the frequency of relapses and the incidence of relapse-related disabilities but does not stop disease progression or directly promote the repair of pre-existing damage in the central nervous system (CNS) 8 . Moreover, all approved therapies have reported adverse events9,10. Accordingly, there is an utmost need for new treatments that are safer, more effective in preventing disease progression, and can reverse disabilities.
Cell-based therapies hold great potential in addressing the abovementioned limitations of current treatments. Mesenchymal stromal/stem cells (MSCs) are the most commonly used type in clinical trials due to their relative genetic stability and safety record. MSCs were found to have the potential to modify the immune reaction and enhance the endogenous repair mechanisms. They are an emerging therapeutic option due to promising preclinical and clinical results 11 . MSCs are isolated from different tissues, including bone marrow, adipose tissue, and the umbilical cord. While bone marrow MSCs were considered a gold standard for clinical use, umbilical cord MSCs are attracting much attention and becoming the new standard for allogenic stem cell treatments 12 .
Umbilical cord–derived mesenchymal stem cells (UC-MSCs) have a unique combination of prenatal and postnatal stem cell properties 13 . They exhibit a higher proliferation rate and a more stable karyotype at later passages than Bone Marrow Derived MSCs (BM-MSCs) 13 . In addition, UC-MSCs have lower immunogenicity than BM-MSCs as they express fewer MHC-II molecules (HLA-DMA, HLA-DRA, and HLA-DPB1) and therefore are less likely to elicit an immune response when used in an allogenic setting 14 . UC-MSCs were found to have inherent therapeutic potential, attributed to a higher secretion of neurotrophic factors such as basic fibroblast growth factor (bFGF), nerve growth factor (NGF), neurotrophin 3 (NT3), neurotrophin 4 (NT4), and glial-derived neurotrophic factor (GDNF) compared to BM-MSCs 15 . Thus, UC-MSCs have a higher precommitment to an ectodermal and neural lineage than BM-MSCs. Furthermore, GDNF has been associated with axonal regeneration and myelination, which becomes deficient in areas of scars in MS patients’ CNS 16 .
The rationale for using UC-MSCs, an allogenic cell source in a clinical trial protocol for MS, would be to unify the therapeutic agent, which is reflected in the anti-inflammatory cytokines it produces and its neuroprotective efficacy 17 . In addition, donor age has been shown to have a negative impact on the quality of MSCs; UC-MSCs are considered a perinatal source explaining their higher ability to provide neuroprotection, immunomodulation, and remyelination 18 . The age-related therapeutic benefits were validated by recent findings that biological aging occurs in stem cells. The stem cells of older donors showed increased apoptosis, altered methylation patterns, altered secretomes, and excessive activation of Wnt/b-catenin signaling 18 . Importantly, many preclinical studies on allogeneic UC-MSCs to treat animal models for MS, followed by a case report on an MS woman and later by a small number of clinical studies, pointed to the safety and encouraging clinical outcomes19–22.
Although the intravenous (IV) administration of stem cells is the most common, a combined route of UC-MSCs injection could be an important efficacy element, as tracking studies have shown that MSCs injected intrathecally (IT) migrate to the site of injury in the white matter of the Experimental autoimmune encephalomyelitis (EAE) model, promoting neuroprotection 23 . This route of injection resulted in safe outcomes in a previous report 24 . In addition, few tracking studies have been conducted on human subjects by imaging techniques for up to 2 months post-IT injection, clearly indicating MSCs homing to the site of lesion in the CNS25,26. The migration of allogenic MSCs injected IT was found to be through the cerebrospinal fluid (CSF), reaching different locations of the CNS where they would persist without the need for immune suppressants 27 . Similarly, tracking IV -injected MSCs has shown their persistence in the tissue samples from patients of graft versus host disease (GVHD) for at least 50 days 28 .
UC-MSCs are thought to exert their benefit, at least in part, through their secretome, which comprises extracellular vesicles, growth factors, cytokines, and chemokines. Many of the soluble factors secreted by MSCs have been found to influence immune players in the EAE model. The role of EVs is being heavily investigated as a substitute for the MSCs although the heterogeneity of their content is still an obstacle. The MSCs’ secretome composition varies according to many factors, including donor, source of MSCs, and the culture conditions29,30. In a previous study, our group compared the immune-related analyte composition of BM-MSCs secretome content from seven MS patients, interleukins (IL-6, IL-8), vascular endothelial growth factor (VEGF), and monocyte chemoattractant protein-1 (MCP-1) were the biomarkers produced with the highest concentrations. Ten more cytokines were detected in varying and lower concentrations of less than 300 pg/ml, including IL-10, IL-12, IL-15 IL-2, FGF, and tumor necrosis factor-alpha (TNF-alpha) 24 . A recent study comparing the immune-related secretome effect reported a more potent anti-inflammatory effect of UC-MSCs compared to BM-MSCs and other MSCs sub-types examined 31 .
There is continued work to reach a consensus on safe and efficient protocols for stem cell preparation and administration to allow clinical studies to move toward an approved clinical use of stem cell therapy to treat MS. The exact dose needed for maximum benefit for MS patients from UC-MSCs and their secretome is yet to be established. In this study, we aimed to assess the safety and compare the efficacy of two cellular therapy protocols of escalating doses and one dose of their conditioned media (CM) containing the cell’s secretome. MSCs were injected in a combination of IT and IV routes. This investigation combined different tests to capture changes that might occur in one but not the other aspect of MS, thus considering the individual differences in the presentation of the disease.
Materials and Methods
This study is an open-label, comparative clinical trial, the protocol of which is part of a more extensive study combining stem cell treatment and physical therapy, the results of which are presented in a different manuscript. The detailed methodology of the assessment tools has previously been published 32 . Below is a summary of the patient recruitment procedure, stem cell preparation and administration protocol, safety and efficacy assessments, outcomes correlations, and gene expression microarray results comparing pre-treatment and post-treatment peripheral blood transcriptome profiles of participants.
Study Design
The Cell Therapy Center (CTC) Institutional Review Board (IRB) reviewed and approved the study protocol. All the laboratory and clinical steps were performed at the CTC at Jordan University. Participants were recruited from the MS registry list at the CTC facility, Jordanian-MS society, personal referral, local physicians, study personnel, and advertisements on social media.
Participants were presented to the study, signed an informed consent form, and were screened for inclusion and exclusion criteria. They underwent the Mini-Mental Status Examination (MMSE) to assess global cognitive function and the Expanded Disability Status Scale (EDSS) to determine disease severity. A screening form was used to fill in demographics, general history of the disease, past and current medication intake, and relevant clinical aspects. Eligible patients were enrolled, and their privacy and free will to withdraw at any time were respected according to the Helsinki Declaration. Patients were then allocated to one of the two arms of the study (Fig. 1).
Figure 1.

Flowchart of the study protocol used. MS: multiple sclerosis; UC-MSCs: umbilical cord-mesenchymal stromal/stem cells.
Stem Cell Preparation and Characterization
Umbilical cord MSCs (UC-MSCs) were isolated from a single umbilical cord donor obtained from Amal Hospital, Amman, Jordan. They were expanded as part of a cellular bank generated under clinical-grade conditions using xenogeneic-free media and in-house-prepared and pooled defibrinated platelet lysate. Individual doses of 150 × 106 MSCs at passage 4 were prepared from cryopreserved cell packs and then expanded under clinical-grade conditions. UC-MSCs were characterized in accordance with the International Society for Cellular Therapy (ISCT) recommendations 33 . Cells were observed microscopically for the spindle shape and attachment to tissue culture flasks. Flow cytometry was performed to confirm the expression of CD73, CD90, and CD105 surface molecules at a percentage above 90% and the absence of CD34, CD45, CD14, and CD3 surface markers at less than 5% (Fig. 2). In addition, the differentiation assessment into adipogenic, osteogenic, and chondrogenic lineages was performed according to the differentiation media manufacturing recommendations (StemPro differentiation media GIBCO; Thermo Fisher, Waltham, MA, USA). The quality of stem cells released for treatment was verified throughout the expansion. Mycoplasma contamination tests were performed, and chromosomal aberrations were tested using G banding karyotyping to ensure their safety.
Figure 2.
Flow cytometry analysis of surface marker expression for umbilical cord–derived MSCs (UC-MSCs). (A) Percentages of MSCs’ positive surface markers expression including CD73, CD90, CD44, and CD105. (B) Percentages of MSCs’ negative surface markers expression including CD45, CD34, CD11b, CD19, and HLA-DR. (Blue histograms represent isotype control, and red histograms represent stained samples). MSCs: mesenchymal stromal/stem cells.
Treatment Protocol
Patients were divided into two groups: Group A comprised 20 MS patients who received two doses, each with a total of 150 × 106 UC-MSCs divided into two injections, 50 × 106 administered through the IV route, and 100 × 106 administered through IT route. A month later, another similar dose was administered. At 3 months (±1 week) post-first-injection, 8 to 10 ml of UC-MSCs CM was delivered IT. On the other hand, Group B comprised 15 MS patients who received one dose of 150 × 106 UC-MSCs divided into 50 × 106 administered through the IV route and 100 × 106 administered through IT route. At 3 months, 8 to 10 ml UC-MSCs CM was delivered IT. Prior to each IT treatment, the same volume injected was withdrawn as CSF to maintain the CNS pressure and decrease the potential post-IT injection headache.
All participants were closely monitored at the CTC for 3 h and then through the phone 24 h post-treatment. Immediate and long-term emerging treatment-related adverse events (ETAE) were noted using a comprehensive questionnaire in addition to self-reported symptoms.
Before treatment (within a week), baseline assessments were conducted to establish a comparison reference of the efficacy of treatment. The tests were related to the various symptoms in MS patients, including motor, cognitive, and ophthalmologic measures at 3, 6, and 12 months post-treatment.
Post-treatment Assessments
Patient safety assessment
In addition to the self-reported and questioned ETAE, laboratory tests were performed in a CAPA-accredited outsourced laboratory at baseline and monitored at 3, 6, and 12 months. Tests included (1) blood leukocytes, erythrocytes, C3, C4, total serum protein, IgG, IgA, IgM; (2) CSF leukocytes, erythrocytes, neutrophil, glucose, protein, chloride, and gram-positive stain; and (3) liver and kidney function tests. In addition, flow cytometry was used to test for blood natural killer (NK), B, and the different T-cell lymphocyte percentages at baseline and the three follow-up time points according to the manufacturer protocol (Multitest TBNK; BD Biosciences, USA).
Treatment efficacy assessments
All patients were evaluated clinically using qualitative and quantitative tests. Below is the summary of the detailed protocol published earlier by our group 32 .
Disability assessment and general health
The EDSS was used to assess change in disability status post-treatment compared to baseline. The EDSS is the MS golden standard that evaluates the degree of disability 34 . In addition, the Barthel Index (BI), a self-report questionnaire that evaluates the ability of individuals to perform daily living and mobility activities, was used. Also, the Arabic-validated version of the 36-item Short Form Survey (SF-36 version 1), a health-related quality of life measure, was used 35 .
Motor functions assessments
Several motor function tests were performed with every follow-up visit for all 35 patients as follows: (1) submaximal testing: this seated recumbent stepper provides simultaneous movement of the upper and lower extremities in a continuous stepping motion 36 ; (2) 6-Minute Walk Test (6MWT): this test measures physical endurance capacity 37 ; (3) Grip strength: a frequently used method to measure hand grip strength 38 ; (4) 9-Hole Peg Test (9HPT): a specific test for manual dexterity 39 ; (4) Arabic version of the Dynamic Gait Index (DGI), a tool that was used to evaluate gait performance and to assess the likelihood of falling 40 ; (5) Arabic version of the Berg Balance Scale (BBS), a performance-based balance assessment tool, consisting of 14 common functional activities that occur in everyday life; (6) Timed-Up and go test, a test for assessing balance, walking ability and risk of fall, in which time in seconds is calculated when the individual stands up from a chair, walks 3 m, turns around, and sits back on the chair 41 ; (7) Arabic version of the Activities-Specific Balance Confidence (A-ABC) scale, a 16 item self-administered questionnaire designed to detect balance confidence, in various daily activities 42 ; (8) Arabic version of the Falls Efficacy Scale—International (FES-I), a self-report questionnaire that assesses fear of falling 43 .
Visual assessment
For each patient, the electrophysiology test, the Visual Evoked Potential (VEP) latency-100, which measures the speed of signal transmission in milliseconds (ms), was performed. For the optical coherence tomography (OCT), retinal nerve fiber layer thickness in micrometers (μm) was measured, with 100 μm being normal.
Radiological evaluation
All patients underwent a baseline magnetic resonance imaging (MRI) scanning less than a week before stem cell injection and repeated at 12 months post-treatment. Scanning was performed on a 3T Philips Ingenia MRI system (Philips, Netherlands). The imaging protocol included the following imaging sequences: high-resolution 3D-T1-weighted Fast-Field Echo with 1-mm three isotropic spatial resolution; reconstruction matrix = 240 × 240; inversion time = 1,000 ms; flip angle = 8°; TR = 8.08 ms; TE = 3.7 ms; turbo factor =148; Shot-to-Shot Interval = 3,000 ms; scan time = 5:86 min. 3D-T2-weighted, Turbo-Spin Echo, fluid-attenuated inversion recovery (FLAIR) with 0.74 × 0.74 × 3 mm3 spatial resolution; reconstruction matrix = 336 × 336; inversion time = 1,650 ms; flip angle = 90°; TR = 4,800 ms; TE = 309 ms; turbo factor = 177; scan time = 5:32 min.
White matter T2 lesion segmentation was performed as described by Al-Radaideh et al. 44 White matter T2-hyperintense lesion volume measurements for each patient were obtained using a semi-automated segmentation technique based on the Fuzzy Connectedness algorithm in Jim software (Jim version 7; Xinapse Systems, Northants, England) 45 .
Psychological and cognitive function assessments
Several tests were performed to cover different cognitive and psychological aspects of the MS disease, including (1) the Arabic version of the Beck Depression Inventory-II (BDI-II), a measure used to detect and grade levels of depression manifested in behavior 46 ; (2) the Arabic version of the Modified Fatigue Impact Scale (A-MFIS), a 21 item self-administered questionnaire that assesses multidimensional aspects of fatigue in terms of physical, cognitive, and psychosocial fatigue 47 ; (3) Hopkins Verbal Learning Test (HVLT) a verbal memory assessment 48 ; and (4) Symbol Digit Modalities Test (SDMT) measured information processing speed 49 .
Sleep quality assessment
Sleep disturbances were assessed using two tests: (1) The Arabic version of the Pittsburgh Sleep Quality Index (PSQI), the gold standard self-report assessment of global sleep quality, in which a higher score indicates poor sleep quality 50 and (2) the Arabic version of the Epworth Sleepiness Scale (ESS), a self-report measure for daytime sleepiness 51 .
Immunological assessments
Patients’ cellular components in blood were analyzed using the appropriate surface marker antibodies for T cells in addition to B cells and NK cells (Multitest 6-color TBNK reagent BD) using the IVD-certified FACS-CANTO, BD flow cytometer, and analyzed using the clinical software.
Affymetrix microarray analysis of gene expression
Mononuclear cells were isolated from the buffy coat of blood samples collected from all patients at baseline and 3 months later. RNA isolation was performed using TRIZOL reagent and consequent enzymes and buffers (QIAamp RNA Blood Mini Kit; QIAGEN, Germany).
For all RNA samples, an input of 100 ng RNA was used. A high purity ratio of >1.8 was set as the cut-off in the 260/280 nm absorbance ratio using a NanoDrop spectrophotometer (Thermo Fisher Scientific, USA). The integrity of the input RNA was measured by electrophoresis using 2100 bio-analyzer (Agilent, with a cut-off of RIN ≥7.
The gene expression profiling analysis was conducted on three representative participants from Group A and Group B. “GeneChip Human Transcriptome Array 2 (HTA 2)” was used with more than 6.0 million probes covering coding and non-coding transcripts. TAC 4.1 software was used for the analysis and graph generation.
Statistical Analysis
SPSS 23.0 was used to perform statistical analyses with alpha values set at 0.05 and 0.01 where noted. A two-sided, paired t test was used to test the effect of the standard penetration test (SPT). Pearson’s correlations were utilized to assess the relationship between the outcome measures of interest.
Changes in individual indicators measures (factors) were taken for each subject, subtracting the baseline level of the indicator from the level measured after 3, 6, and 12 months. Changes in lesion levels were compared with the 12-month mark only. These changes were averaged for subjects in each group and plotted with error bars representing the standard error. A t test was performed on these changes, and changes that differed from a baseline with a P < 0.05 were deemed significant. We also used the t test to determine significantly different group responses for each indicator (factor), P < 0.05.
For the gene expression analysis, statistical comparisons were made using the paired t tests or two-way analysis of variance (ANOVA) with Bonferroni corrections where appropriate. Values of P < 0.05 were considered significant.
Results
Baseline Characteristics
A total of 35 participants took part in this study: 20 participants in Group A and 15 in Group B. Table 1 shows participants’ demographics and clinical baseline characteristics. The average age and EDSS score at baseline match between the two groups, limiting the bias in the treatment outcome. On the other hand, male to female ratio and type of MS do not match, with Group B having a female majority and more relapsing–remitting patients. In contrast, Group A has more males and consists of more secondary-progressive patients.
Table 1.
Baseline Characteristics of Study Participants in Group A and Group B.
| Average age | Sex F:M | MS type | EDSS | Disease duration | MMSE | |
|---|---|---|---|---|---|---|
| Group A | 37.25 | 8F:12M | 6RR:12SP:2PP | 3.85 | 10.35 | 28.7 |
| G B | 35.8 | 12F:3M | 12RR:2SP:1PP | 3.7 | 7.9 | 29.3 |
MS: Multiple sclerosis; EDSS: Expanded Disability Severity Scale; MMSE: Mini-Mental Status Examination; RR: relapsing–remitting; PP: primary-progressive; SP: secondary-progressive.
Safety Outcomes Analysis
No serious ETAEs were noted, with headaches, slight fever, and local lumbar pain being the common symptoms that resolved before the first-month assessment. Blood and CSF tests performed for both groups showed no significant change compared to baseline. None of the treated patients reported high fever (>40°C). Gram-negative tests of all CSF samples were negative, reflecting the absence of bacterial meningitis.
Efficacy Outcome Analysis
Analysis of the individual changes pointed to an improvement in more than one aspect for most enrolled patients. Treatment outcome analysis was compared between the two groups using a variety of assessments spanning different clinical, biological, motor, cognitive, and radiological aspects of MS. Two patients were excluded from the efficacy analysis: subject A45 from Group A and subject D15 from Group B due to patient withdrawal. However, patient A45 blood sample was still used in the microarray experiment as it compared the 3 months post-injection effect prior to withdrawal. MATLAB version 2020A was used to analyze the data below.
Disability assessment and general health outcomes
Both groups showed an improvement trend at 3 months post-treatment, which became significant at 6 months post-treatment, reaching a decrease of one point on the 10-point EDSS scale that primarily assesses mobility. Only Group B patients maintained a significant improvement at 12 months, with an average improvement of two points on the EDSS scale (Fig. 3). In contrast, Group A patients showed a significant improvement in general health (SF-36) at 3- and 6-month time points, while Group B self-reported a positive trend at 12 months. This positive outcome in Group A is in parallel with a significant improvement in the energy and fatigue component of the SF-36 test 3 months post-treatment, as indicated in Fig. 3.
Figure 3.
Disability assessment and general health outcomes. EDSS and SF-36 score changes at 3, 6, and 12 months compared to baseline in both groups. EDSS’ overall self-reported disability scale decrease reflects improvement. SF-36 self-reported general health and energy and fatigue components in both groups, a scale where an increase reflects improvement. Group A (n = 19) and Group B (n = 14). (Significance <0.05 represented by the black dot). EDSS: Expanded Disability Status Scale; SF-36: 36-item Short Form Survey.
Motor function outcomes
Upper extremities’ fine motor skills examined by the 9HPT showed a favorable outcome by a decrease in the time required to fill the holes using the dominant and non-dominant hand for Group A (Fig. 4). Although changes are minor, statistical significance was reached in the dominant hand of Group A patients at 6 and 12 months post-treatment. In contrast, less improvement in Group B was recorded, with slight, significant improvement in the dominant hand at 12 months. Furthermore, Group A patients had a higher but more variable positive outcome on the fine motor skill of the non-dominant hand, while Group B did not. In contrast, the 6MWT pointed to a linear improvement trend in Group B and a linear worsening trend in Group A without reaching significance. Furthermore, the Berg balance test that assessed objectively change in static balance and fall risk between the two groups at 3-, 6-, and 12-month time points showed an improvement in Group B only, though insignificant. Group A patients showed a decreased trend at 3 and 6 months which became smaller at 12 months compared to 3 and 6 months, with a significant difference between the two groups’ balance outcomes (data not shown).
Figure 4.
Motor outcomes. Manual dexterity measure change using 9-HPT at 3,6 and 12 months compared to baseline in both groups where a decrease in time reflects improvement. Walking ability measure change (6-Minute Walk Test) at 3, 6, and 12 months compared to baseline in both groups, where an increase reflects improvement. Balance measure change (Berg balance test) at 3, 6, and 12 months compared to baseline in both groups, where an increase reflects improvement. (Significance <0.05 represented by the black dot). Group A (n = 19) and Group B (n = 14). 9-HPT: 9-Hole Peg Test.
Ophthalmology outcomes
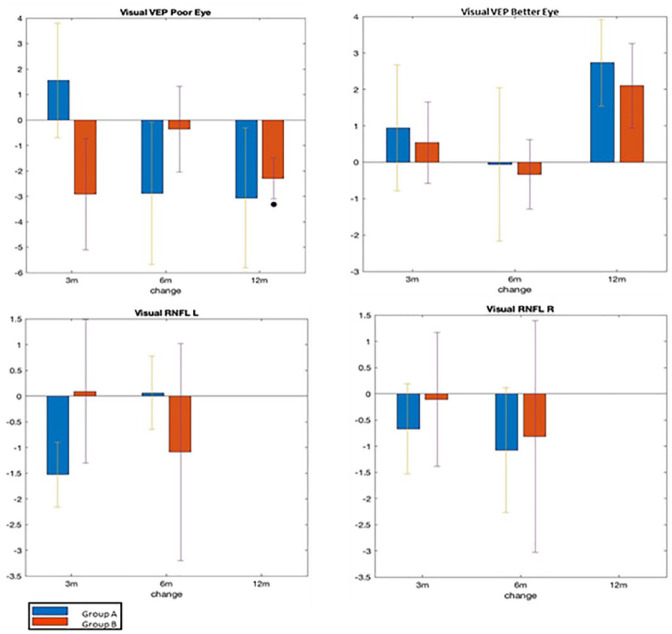
Fig. 5 presents the main ophthalmological results comparing outcomes in both groups for the relatively poor and better eye. The VEP examination of the poor eye showed a significant improvement in signal transmission latency at 12 months in both groups, with a significant outcome in Group B. There was no positive outcome for the better eye, and a slight deterioration at 12 months compared to 3 and 6 was detected in both groups.
Figure 5.
Ophthalmology outcomes. VEP measures in milliseconds the latency of signal transduction change at 3, 6, and 12 months compared to baseline in both groups, where a decrease reflects improvement in latency. Nerve fiber thickness (mm) measures change at 3, 6, and 12 months compared to baseline in both groups, where an increase reflects improvement. (Significance <0.05 represented by the black dot.) Group A (n = 19) and Group B (n = 14). VEP: Visual Evoked Potential; RNFL: Retinal Nerve Fiber Layer.
RNFL changes for the right and left eye were insignificant for both groups. Nevertheless, an interesting improvement trend in the optic nerve’s thickness in the left eye was found in Group A at 6 and 12 months compared to the decrease in nerve fiber thickness observed at 3 months.
Radiology outcomes
Results of the MRI scans for the cortical and subcortical gray and white matter as well as for the lesion load and ventricular system were recorded and compared to baseline scan results. Group A results show no significant morphological changes between the baseline and 12 months, but positive trends were nevertheless depicted. One year from the baseline, the volumetric assessment of the cortical thickness showed an increase in the cerebral cortical thickness (5) and a decrease in cortical area and volume (data not shown). Except for the left globus pallidus, all deep gray matter structures showed an insignificant volume decrease (data not shown). Examining the trend in lesion load caused by the MS disease, it was found to be positive for Group A as the lesion decreased at 12 months, while the opposite trend is shown for Group B, though both did not reach significance. On the other hand, significant adverse changes were reported in Group B in terms of a significantly decreased cortical thickness (Fig. 6), total cortical volume, and right thalamus volume (data not shown).
Figure 6.
Radiology outcomes. Total lesion load (mm3) in the brain and cervical spine at 12 months compared to baseline in both groups, a decrease reflects improvement. Left cortical thickness measure change (mm) at 12 months compared to baseline in both groups, where an increase reflects improvement. (Significance <0.05 represented by the black dot.) Group A (n = 19) and Group B (n = 14).
Cognitive and psychological outcomes
As indicated in Fig. 7, patients’ speed of information processing tested using the SDMT shows a significant maintained improvement in the speed of information processing in Group A with an average of 10 points (4 points suggested responder definition in clinical trials). Group B results fluctuated as they showed no change at 3 months, a trend of improvement at 6 months, and a trend of worsening at 12 months. Data from the BDI point to a similar improvement trend in the depression score at 3 months in both groups. Group A maintained this significant amelioration at 6 and 12 months, while Group B did not follow the same trend. It is worth mentioning that the baseline scores according to the BDI results varied between groups; the mean for Group A is 17.3 and Group B 11.1 with respective mean SD values of 11.3 and 7.4.
Figure 7.
Cognitive and psychological outcomes. Cognitive processing speed using Symbol Digit Modalities Test (SDMT) measures change at 3, 6, and 12 months compared to a baseline in each group, where an increase reflects improvement. Depression level change using the Beck Depression Inventory (BDI) at 3, 6, and 12 months compared to baseline, where a decrease reflects improvement. Group A (n = 19) and Group B (n = 14). (Significance <0.05 represented by the black dot.) Group A (n = 19) and Group B (n = 14).
Sleep quality outcomes
Changes in sleep disturbances examined using the PSQI questionnaire showed a significant improvement for Group A patients and a trend of improvement in Group B at 12 months. A smaller positive outcome was found in both groups at 3 and 6 months post-treatment with more variability among Group B patients. The positive outcome of this component was reflected in the global sleep quality with high variability between patients in Group B at all time points. This component of the PSQI test impacted the overall sleep quality of patients in both groups as shown in Fig. 8.
Figure 8.
Sleep quality and the sleep disturbances component outcome measures change at 3, 6, and 12 months compared to baseline using the PSQI test, where a decrease reflects improvement. (Significance <0.05 represented by the black dot.) Group A (n = 19) and Group B (n = 14). PSQI: Pittsburgh Sleep Quality Index.
Immunological outcomes
Flow cytometry results on participants’ peripheral blood cells assessed changes in the main immune players (T, B, and NK cells) during the study (Fig. 9). Results indicated a significant increase in CD4+ T cells, the subset of T lymphocytes comprising regulatory T cells (Treg) at 12 months, with a positive trend at 3 and 6 months in Group A. Group B, on the other hand, showed an overall positive trend at 12 months only. Moreover, total T lymphocytes, including Treg and the effector subset of T lymphocytes, were maintained at the same level as the baseline in Group A and did not increase during the 12-month study period. In Group B, the baseline level was maintained up to 6 months, after which an increase was detected at 12 months. NK cell response to therapy was positive in Group A, with a trend of decreased percentage of (CD16+, CD56+) cells at 3 months. A maintained expression compared to baseline was observed in Group B at 3 months, with a linear increase at 6 and 12 months (Fig. 9). B cells expressing CD19+ results indicated a decrease in Group B only with no negligible change in Group A at three time points.
Figure 9.
Immunological assessment of the change in T, B, and natural killer (NK) cell percentage in the peripheral blood of patients in each group. Flow cytometry measures of total T cells (CD3+CD4+CD8+), Treg containing subset (CD3+CD4+), B cells (CD19+), and NK cells (CD16+CD56+). A decrease in the total T, B, and NK cells is considered positive, while an increase in the Treg subset is positive. (Significance <0.05 represented by the black dot.) Group A (n = 19) and Group B (n = 14).
Gene expression change
Circulating cells in MS patients’ blood from both groups differentially expressed 47 genes, transcription factors, and regulatory elements post-UC-MSCs treatments compared to pretreatment. Thirty-nine transcripts were upregulated, and eight were downregulated at a cut-off of twofold change. Fig. 10A summarizes the results, while the complete list can be accessed online at the Gene Expression Omnibus (GEO) archives.
Figure 10.
Volcano plots of paired transcriptome analysis (A) at 3 months post-treatment compared to baseline. Combined samples from Groups A and B (n = 12). Analysis of Group A transcriptome (n = 3). Analysis of Group B transcriptome (n = 6). TNF-alpha: tumor necrosis factor-alpha. Differentially expressed transcripts (B) related to anti-inflammatory processes. EGR1 is upregulated at 3 months compared to baseline, while HLA-B and K channel inward rectifier are downregulated in the combined groups analysis (n = 6). TNF-alpha, miRNA-142, and TAP-1 downregulated in Group A only analysis (n = 3). Fold change >1.6, P < 0.05. (Dots represent individual samples.)
The generated paired analysis presented in the volcano plot differentiates between the transcripts that were either upregulated or downregulated in patients’ blood samples of both groups combined and each group separately. Fig. 10B illustrates the expression change at 3 months compared to baseline in both groups. Representative of those genes with expression changes are HLA-B, EGR1, and KCNJ15. Representative transcripts in Group A only include TNF-alpha, TAP-1, and miRNA142.
Outcome correlations
A total of 80 correlations between outcome measures for Group A and 72 for Group B are indicated in the confusion matrices (Fig. 11). Of total correlation, those with higher significance P < 0.01 point to the following correlations of Group B: (1) blood C4 with ABC scale, (2) CD3+CD4+ with SF-36-Energy/Fatigue, (3) visual OCT for the left eye with blood C4, (4) blood serum creatinine with SF-36-Energy/Fatigue, (5) blood C4 with SF-36-Physical functioning, (6) poor eye VEP with the non-dominant hand grip. Those with highly significant correlations P < 0.01 between the outcome measures of Group A are (1) right eye RNFL with the third-ventricle volume, (2) PSQI sleep quality with the right-lateral-ventricle, (3) left eye RNFL with left-cerebral white matter volume, (4) PSQI sleep quality with left-lateral-ventricle, (5) right eye RNFL with PSQI sleep latency, (6) PSQI sleep latency with left thalamus volume, (7) right-cerebral white matter volume with MS disease duration, (8) SF-36 pain with third-ventricle volume, (9) SF-36 (social functioning) with PSQI sleep quality, (10) PSQI sleep medications with MS disease duration.
Figure 11.
Correlation analysis. Correlations between the outcome measures of Group A and Group B. Yellow represents the high correlation between any two measures. CSF: cerebrospinal fluid; MFIS: Modified Fatigue Impact Scale; PSQI: Pittsburgh Sleep Quality Index; EDSS: Expanded Disability Status Scale; MMSE: Mini-Mental Status Examination.
Discussion
One of the critical questions in the field of MSC therapy for MS is the optimal dose of stem cells to be used for a safe and most effective outcome. A limited number of trials have compared different doses of MSCs in the same patient population. The current dose-finding study examined the safety and efficacy of using expanded human UC-MSCs and their CM to treat MS. An extensive examination of the different aspects of MS was conducted to reach a comprehensive, evidence-based conclusion. The execution of such a complex assessment protocol resulted from a collaborative effort between CTC staff, stem cell experts, and specialized clinicians from the Jordan University Hospital and valuable input from the MRI, physiotherapy, and immunology specialists from different academic institutions in Jordan.
Participants in this study were matched in terms of age, duration of disease, and overall disability status. Furthermore, using an in-house-generated UC-MSC cell bank expanded from a single umbilical cord sample eliminated the source of variability in stem cell potency. According to our data and other groups’ data, MSCs were found to release different cytokines and possess variable transcriptome profiles, depending on the donor and stem cell tissue source24,52.
Only a limited number of clinical studies have recently used allogenic UC-MSCs for MS patients and other neurological diseases 19 . The first encouraging case of using an allogenic source of MSCs to treat MS was in 2009; a woman of 55 years showed an improvement of 2 points on the EDSS score and a decrease in the CNS lesion after 50 days post-treatment with UC-MSCs 19 . Four years later, another case report was published using a protocol that combined two stem cell subtypes and two routes. IV UC-MCSs and IT BM-MSCs were administered repeatedly; patients were followed up for 4 years with no serious adverse events or clinical improvement but no new MRI lesions 20 . Another study described the IV infusion of UC-MSCs and reported a change from Th1 to Th2 immunity in patients treated with UC-MSCs, suggesting an immune-modulating impact 21 . The most recent study investigated a protocol of IV injection of 20 × 106 UC-MSCs over 7 days, with a total of 140 × 106 cells. The observed improvements were in the EDSS, bladder, bowel, and sexual dysfunction, non-dominant hand average scores, walk durations, and a general perception of a better quality of life. After 1 year, 15 of 18 (83.3%) participants had inactive lesions on their brain and cervical spinal cord MRI images 22 .
The present study reiterates the safety of using an allogenic source of stem cells at the highest dose to date with up to 300 × 106 UC-MSCs to treat MS. The follow-up questionnaires and clinical evaluation of patients, including the biological samples (blood, CSF, urine), showed no changes in the two groups of patients. Thus, administering one dose or two doses of 150 × 106 UC-MSCs and CM did not lead to any ETAE in the 45 treated patients. In contrast, safety reports of some monoclonal antibody treatments (biologics), usually well-tolerated by MS patients, reported cases of severe and fatal adverse events, including progressive multifocal leukoencephalopathy (PML)53,54.
Regarding efficacy assessments, it can be appreciated that the combination of clinical and cognitive, sleep, and psychological tests included in this study to the commonly used disability and motor assessments is a more comprehensive approach to detect improvements post stem cell and CM treatments. The results indicated that both stem cell treatment protocols halted the overall progressive deterioration in the EDSS during the 12-month follow-up period. An improvement at all time points for both groups, except for the 3 months for Group A, was observed (Fig. 3). The walking and balance results are in line with the EDSS outcomes in Group B, while a gradual deterioration was noted in Group A (Fig. 4) 55 . Meanwhile, the fine motor assessment showed more improvement in both the dominant and non-dominant hands in Group A compared to Group B at all time points (Fig. 4). Interestingly, the perception of physical improvement depicted in the self-reported general health assessment (SF-36) is higher in Group A than in Group B. The results show a complementarity rather than a correlation with the EDSS, as reported earlier 56 .
MRI results at 12 months pointed to two positive outcomes in Group A despite the lack of significance, especially when compared to the significant negative outcome in Group B at 12 months, echoing the progressive nature of MS (Fig. 6). Only Group A showed a decrease in lesion load, indicating a positive trend of recovery from focal damage. In addition, at the 12-month follow-up period, a significant reduction in total cortical volume observed in Group B points to a progression of neural degeneration related to the nature of the disease. This phenomenon was prevented in Group A, where no significant cortical volume loss was noted.
Cortical thickness diminution and thalamic gray matter atrophy have been found in RRMS patients compared to controls and were linked to cognitive deficits, anxiety, and depression 57 . Thus, the improved MRI imaging aspects in Group A reflected on some cognitive and psychological aspects examined in this study (Fig. 7). The results showed a sustained improvement throughout the three-time points in Group A compared to Group B, where a positive trend was noted at 3 months only. The positive significant results on the BDI depression scale for Group A were noted even though this group had average mild depression at baseline, while Group B patients had minimal depression. Similarly, the results of the SDMT evaluating cognitive changes most affected in MS patients, relating to the speed of information processing (memory and language), showed a significant amelioration in Group A only at 3 months, sustained at 6 and 12 months.
An important aspect of MS is sleep quality, which affects around 50% of patients 58 . Our data point to an overall positive outcome of cellular therapy on this aspect of the disease. Furthermore, the group receiving two doses of stem cells reached a significant improvement at 12 months in one of the most affected aspects of sleep in MS (especially in women), the sleep disturbances component of the PSQI test 58 . A recent study that found that interferon (IFN)-β injection was associated with less restful sleep in MS patients 59 .
An essential cellular indicator of MS remission is the shift toward CD4+ T cells containing the Tregs responsible for hampering the inflammatory process in MS. UC-MSCs treatment, either with one or two doses, has this beneficial effect in both groups. The increase in CD4+ T cells was significant at 12 months, with a positive trend as early as 3 months and maintained at 6 months. On the other hand, The CD8+ T-effector lymphocyte subset increased at 12 months in Group B only, pointing to the inflammatory process, which had been modulated at 3- and 6-month post-treatment and possible loss of therapeutic effect on the cellular level at 12 months. NK cells decreased further in Group A patients, reflecting the immune suppression. At the same time, B cells in Group B decreased more, suggesting a downregulation in the humoral response in the group of patients with more RRMS ratio and a more active humoral response. The stabilization of B-cell response despite the repeated injections of UC-MSCs in Group A also indicates the lack of immunogenicity of MSCs. Thus, the use of flow cytometry to test individualized patients’ immune responses in general and by the type of MS needs to be further investigated in future cellular therapy protocols.
A drawback of this clinical trial that could be overcome in future studies is the bias in the type of MS of participants, with an RRMS ratio bias in Group B and an SPMS bias in Group A. This might have impacted the differences in response to stem cell therapy regarding the different aspects of MS assessed. A clear distinction exists in terms of the course of disease and response to other types of therapy. However, in the study plan, MS patients were invited to participate regardless of the relapses and progression of symptoms. Similarities between the relapsing–remitting type and the progressive types in terms of CNS morphometric measurements have been reported 54 . In addition, there was a clear female bias in both groups with more females in Group B (12 F:3 M), and fewer females in Group A (8 F:12M). This bias also prevented the independent assessment of results according to gender in each group. According to both previous Atlas editions, women are twice as likely as men to have MS globally and as high as 4:1 in certain regions 60 . Furthermore, individual physical characteristics could also be considered in the future for optimizing the therapy dose. Body weight could affect the availability of MSCs administered through the IV route, while spine length and abdominal girth, which affect the CSF volume, could impact the availability of MSCs administered IT 61 .
The correlation analysis executed in this study aimed to examine associations between the different tests, especially between non-motor and motor functions or invasive and non-invasive measures. Fewer correlations were found between the different outcome measures in Group B than in Group A at 12 months post-treatment. Some outcomes are intuitive and open the door for future larger investigations, such as correlations between CD4+ T cells and the energy and fatigue levels, the visual outcome and the hand grip and the sleep quality, and left-ventricle and right-ventricle MRI volume.
The microarray gene expression analysis provides insight into molecular changes and mechanisms of action of stem cell treatment leading to the positive results observed in both groups (GEO Accession number: GSE 235689). Comparing gene expression at baseline and 3 months post-treatment for three patients from Group A and three from Group B resulted in a change in 47 transcripts (Fig. 8). Among the differentially expressed genes is the regulatory transcription factor EGR1, with the highest increase in expression of 5.14-fold change. EGR1 plays an important role in brain plasticity and neuropsychiatric disorders. It was also found in a previous study to be differentially expressed in MS brain and blood compared to controls and was suggested to be a candidate biomarker for MS 62 . In addition, HLA-B decreased expression (−1.75) in both groups indicates immune modulation. As HLA-B, and not HLA-A or HLA-C, was found to present peptides following cytokine stimulation 63 . Another interesting gene expression change is the 1.86-fold decrease in KCNJ15 expression, an inward rectifier potassium channel, that has been linked to diabetes, Alzheimer’s disease, epilepsy, and Parkinson’s disease, suggesting a broader role in the pathogenesis of the CNS64,65. MSC therapy in both groups also induced a significant 2.24-fold increase in the expression of the antioxidative gene SESN3, a member of the stress-responsive protein family Sestrins (SESNs). Serum levels of its relative SESN2 were found to be significantly lower in MS patients than in controls 66 . The function of Sesn3 in MS is to be verified, as SESN2 was found to control ROS-dependent neuropathic pain signaling after peripheral nerve injury 67 . Another interesting finding of this microarray analysis is the increased expression of NELL2 with a 1.78-fold change. NELL2 is expressed primarily in the brain and plays an important role in neuron homeostasis and axon development during neuronal differentiation. Counterintuitively, the analysis pointed to abundant and increased expression of CD69, a previously described surface marker on T-effector cells, that seems to have a suppressive effect on Tregs through IL-10 suppression 68 . Furthermore, CD40LG was found to be increased at 3 months post-treatment compared to baseline, indicating ongoing inflammation and autoantibody production.
The exact function of treatment on small nuclear RNAs is also to be examined, as the increased expression of SNORA38B points to an effect on the RNA processing at the nucleolus and Cajal body of cells in patients’ peripheral blood after 3 months. These results are evidence of the variety of molecular pathways used by MSCs to alleviate MS inflammatory milieu regardless of the dose used to treat MS patients.
To examine whether the dose of UC-MSCs produced different effects on the transcriptome of patients’ blood cells, each group was analyzed separately. The analysis showed a differential expression at 3 months post-treatment compared to baseline in Group A only of TNF-alpha (−2.07), TAP-1 (−2.23), and miR142 (2.87), all well described and linked to relieved inflammation in patients69–71. This points to an advantage of two UC-MSCs doses as it brought in more immune players, which can directly be linked to more positive treatment outcomes obtained in the current trial. These transcriptome results open the door for more clinically relevant in vitro functional tests to study the MSCs and their paracrine effects on the different blood and CNS cellular components.
In summary, this study demonstrates the safety and efficacy of both treatment protocols with comprehensive assessment tools, using an allogenic stem cell source originating from a single umbilical cord donor and expanded in vitro. The various aspects studied point to halting and reversing MS symptoms using stem cell therapy with parallel effects on the cellular and gene expression levels. There is an advantage of administering two doses compared to one, which warrants more extensive studies on larger numbers of MS patients. Examining the addition of more doses and a more extended follow-up period is recommended for future studies.
The findings of this study emphasize the critical role of regenerative medicine in managing MS. Optimizing the dose of treatment is an essential milestone for the standardization of protocols to attain a safe cellular treatment of MS symptoms. The many aspects studied detected a reversal of some MS symptoms and stabilization of others. The clear advantage of administering two doses of UC-MSCs instead of one in this study, in addition to one dose of CM, is encouraging.
These results help guide clinicians to initiate trials based on the current protocol and encourage patients to enroll in similar studies. Multisite collaborative efforts would expedite the use of this potentially effective therapy to ease the socioeconomic burden of such a chronic, devastating disease. The findings of gene expression changes post stem cell treatment are an essential insight that relates the difference in stem cell dose to changes in specific immune players.
Acknowledgments
The study team thanks the participating MS patients for their time and dedication to completing this study. Appreciations go to “Nimat Shifa” MS-Patients’ Society for logistics assistance. They extend their gratitude to Dr Maher Al-Sarraf and Al-Amal Hospital staff for donating the umbilical cord sample. Gratitude goes to CTC nurses for patients’ care and safety outcomes follow-up. They also thank Ms Mayada Moumani and Ms Manal Azzouni for their administrative help. Valued assistance in data collection and documentation was provided by Ms Rimaa Abu Saadeh, Dr Nada Asem, and Malak Alhajahjeh.
Footnotes
Author Contributions: AAW, FA, SD, MA and AAG contributed to the study design. SA, FA, MA overall data analysis and interpretation. FJ, DA, RR and BH stem cell preparation, characterization and dosage. RR and DA flow cytometry results and figure generation. SD, HB and FJ stem cell transplantation, neurological assessments and data analysis. AAR, SA radiological assessment and data generation and interpretation, OA and SA ophthalmological assessment and data generation. MD and JA motor, sleep, cognitive and psychological assessment and data interpretation. MRH, HK and FJ immunological and whole transcriptome assessments, data generation and interpretation.
Clinical Trial Registration: Registry: ClinicalTrials.gov, Identifier: NCT03326505.
Data Availability: Human transcriptome analysis results are uploaded online at the NCBI Gene Expression Omnibus (GEO) archive GEOarchive in a spreadsheet format as a batch deposit. Accession number GSE 235689. The corresponding author may be contacted for data not presented.
Ethical Approval: The Cell Therapy Center (CTC) IRB reviewed and approved the study protocol number IRB/4/2017.
Permission to Reproduce Material From Other Sources: No materials were reproduced from other sources in this manuscript.
Statement of Human and Animal Rights: The clinical trial was conducted in agreement with international human rights. This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: Participants presented and met the criteria of the study and signed an informed consent.
Research Ethics and Patient Consent: The Cell Therapy Center (CTC) IRB reviewed and approved the study protocol number IRB/4/2017. Participants presented and met the criteria of the study and signed an informed consent.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Scientific Research Fund, Ministry of Higher Education in the Hashemite Kingdom of Jordan (grant number: MPH/1/38/2017).
ORCID iD: Dana Alhattab  https://orcid.org/0000-0002-0509-6182
https://orcid.org/0000-0002-0509-6182
References
- 1. Koch-Henriksen N, Sørensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9(5):520–32. [DOI] [PubMed] [Google Scholar]
- 2. Zeydan B, Atkinson EJ, Weis DM, Smith CY, Gazzuola Rocca L, Rocca WA, Keegan BM, Weinshenker BG, Kantarci K, Kantarci OH. Reproductive history and progressive multiple sclerosis risk in women. Brain Commun. 2020;2(2):fcaa185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams AE, Vietri JT, Isherwood G, Flor A. Symptoms and association with health outcomes in relapsing-remitting multiple sclerosis: results of a US patient survey. Mult Scler Int. 2014;2014:203183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zwibel HL, Smrtka J. Improving quality of life in multiple sclerosis: an unmet need. Am J Manag Care. 2011;17:S139. [PubMed] [Google Scholar]
- 5. Lassmann H. Multiple sclerosis pathology. Cold Spring Harb Perspect Med. 2018;8:a028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545–58. [DOI] [PubMed] [Google Scholar]
- 7. Ingwersen J, Aktas O, Hartung HP. Advances in and algorithms for the treatment of relapsing-remitting multiple sclerosis. Neurotherapeutics. 2016;13(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shirani A, Okuda DT, Stüve O. Therapeutic advances and future prospects in progressive forms of multiple sclerosis. Neurotherapeutics. 2016;13(1):58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prosperini L, Haggiag S, Tortorella C, Galgani S, Gasperini C. Age-related adverse events of disease-modifying treatments for multiple sclerosis: a meta-regression. Mult Scler. 2021;27(9):1391–402. [DOI] [PubMed] [Google Scholar]
- 10. Kolb-Mäurer A, Goebeler M, Mäurer M. Cutaneous adverse events associated with interferon-β treatment of multiple sclerosis. Int J Mol Sci. 2015;16:14951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scolding NJ, Pasquini M, Reingold SC, Cohen JA. Cell-based therapeutic strategies for multiple sclerosis. Brain. 2017;140:2776–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alanazi A, Alassiri M, Jawdat D, Almalik Y. Mesenchymal stem cell therapy: a review of clinical trials for multiple sclerosis. Regen Ther. 2022;21:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical cord as prospective source for mesenchymal stem cell-based therapy. Stem Cells Int. 2016;2016:6901286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balasubramanian S, Thej C, Venugopal P, Priya N, Zakaria Z, Sundarraj S, Majumdar AS. Higher propensity of Wharton’s jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol Int. 2013;37:507–15. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57:1178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alhattab D, Jamali F, Ali D, Hammad H, Adwan S, Rahmeh R, Samarah O, Salah B, Hamdan M, Awidi A. An insight into the whole transcriptome profile of four tissue-specific human mesenchymal stem cells. Regen Med. 2019;14:841–65. [DOI] [PubMed] [Google Scholar]
- 18. Scruggs BA, Semon JA, Zhang X, Zhang S, Bowles AC, Pandey AC, Imhof KM, Kalueff AV, Gimble JM, Bunnell BA. Age of the donor reduces the ability of human adipose-derived stem cells to alleviate symptoms in the experimental autoimmune encephalomyelitis mouse model. Stem Cells Transl Med. 2013;2(10):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liang J, Zhang H, Hua B, Wang H, Wang J, Han Z, Sun L. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler. 2009;15(5):644–46. [DOI] [PubMed] [Google Scholar]
- 20. Hou ZL, Liu Y, Mao XH, Wei CY, Meng MY, Liu YH, Zhuyun Yang Z, Zhu H, Short M, Bernard C, Xiao ZC. Transplantation of umbilical cord and bone marrow-derived mesenchymal stem cells in a patient with relapsing-remitting multiple sclerosis. Cell Adh Migr. 2013;7:404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, Zhang YZ, Lou JY, Cao B, Wang YL. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014;23:S113–22. [DOI] [PubMed] [Google Scholar]
- 22. Riordan NH, Morales I, Fernández G, Allen N, Fearnot NE, Leckrone ME, Markovich DJ, Mansfield D, Avila D, Patel AN, Kesari S, et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med. 2018;16:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gordon D, Pavlovska G, Uney JB, Wraith DC, Scolding NJ. Human mesenchymal stem cells infiltrate the spinal cord, reduce demyelination, and localize to white matter lesions in experimental autoimmune encephalomyelitis. J Neuropathol Exp Neurol. 2010;69:1087–95. [DOI] [PubMed] [Google Scholar]
- 24. Dahbour S, Jamali F, Alhattab D, Al-Radaideh A, Ababneh O, Al-Ryalat N, Al-Bdour M, Hourani B, Msallam M, Rasheed M, Huneiti A, et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci Ther. 2017;23:866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007;16:461–66. [DOI] [PubMed] [Google Scholar]
- 26. Chotivichit A, Ruangchainikom M, Chiewvit P, Wongkajornsilp A, Sujirattanawimol K. Chronic spinal cord injury treated with transplanted autologous bone marrow-derived mesenchymal stem cells tracked by magnetic resonance imaging: a case report. J Med Case Rep. 2015;9:79. doi: 10.1186/s13256-015-0535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. [DOI] [PubMed] [Google Scholar]
- 28. von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–78. [DOI] [PubMed] [Google Scholar]
- 29. Chouw A, Facicilia G, Sartika CR, Faried A, Milanda T. Factors influencing the therapeutic potential of the MSC-derived secretome. Regen Eng Transl Med. 2022;8:384–93. [Google Scholar]
- 30. Chouw A, Sartika CR, Milanda T, Faried A. Interleukins profiling in umbilical cord mesenchymal stem cell-derived secretome. Stem Cells Cloning. 2022;15:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peshkova M, Korneev A, Suleimanov S, Vlasova II, Svistunov A, Kosheleva N, Timashev P. MSCs’ conditioned media cytokine and growth factor profiles and their impact on macrophage polarization. Stem Cell Res Ther. 2023;14:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alghwiri AA, Jamali F, Aldughmi M, Khalil H, Al-Sharman A, Alhattab D, Al-Radaideh A, Awidi A. The effect of stem cell therapy and comprehensive physical therapy in motor and non-motor symptoms in patients with multiple sclerosis: a comparative study. Medicine. 2020;99:e21646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–17. [DOI] [PubMed] [Google Scholar]
- 34. Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol. 2014;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey. Manual and interpretation guide; 1993. https://www.researchgate.Net/profile/John-Ware-6/publication/313050850_SF-36_Health_Survey_Manual_Interpretation_Guide/links/594a5b83aca2723195de5c3d/SF-36-Health-Survey-Manual-Interpretation-Guide.pdf [accessed 2024 February 13].
- 36. Billinger SA, Van Swearingen E, McClain M, Lentz AA, Good MB. Recumbent stepper submaximal exercise test to predict peak oxygen uptake. Med Sci Sports Exerc. 2012;44:1539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldman MD, Marrie RA, Cohen JA. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult Scler. 2008;14:383–90. [DOI] [PubMed] [Google Scholar]
- 38. Bohannon RW, Peolsson A, Massy-Westropp N, Desrosiers J, Bear-Lehman J. Reference values for adult grip strength measured with a Jamar dynamometer: a descriptive meta-analysis. Physiotherapy. 2006;92:11–15. [Google Scholar]
- 39. Feys P, Lamers I, Francis G, Benedict R, Phillips G, LaRocca N, Hudson LD, Rudick R. Multiple Sclerosis Outcome Assessments Consortium. The Nine-Hole Peg Test as a manual dexterity performance measure for multiple sclerosis. Mult Scler. 2017;23:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McConvey J, Bennett SE. Reliability of the Dynamic Gait Index in individuals with multiple sclerosis. Arch Phys Med Rehabil. 2005;86:130–33. [DOI] [PubMed] [Google Scholar]
- 41. Herman T, Giladi N, Hausdorff JM. Properties of the “timed up and go” test: more than meets the eye. Gerontology. 2011;57:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Powell LE, Myers AM. The Activities-Specific Balance Confidence (ABC) scale. J Gerontol A Biol Sci Med Sci. 1995;50:M28–34. [DOI] [PubMed] [Google Scholar]
- 43. Yardley L, Beyer N, Hauer K, Kempen G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing. 2005;34:614–19. [DOI] [PubMed] [Google Scholar]
- 44. Al-Radaideh A, Athamneh I, Alabadi H, Hbahbih M. Cortical and subcortical morphometric and iron changes in relapsing-remitting multiple sclerosis and their association with white matter T2 lesion load: a 3-tesla magnetic resonance imaging study. Clin Neuroradiol. 2019;29:51–64. [DOI] [PubMed] [Google Scholar]
- 45. Udupa JK, Samarasekera S. Fuzzy connectedness and object definition: theory, algorithms, and applications in image segmentation. Graph Models Image Process. 1996;58246–61. [Google Scholar]
- 46. Sacco R, Santangelo G, Stamenova S, Bisecco A, Bonavita S, Lavorgna L, Trojano L, D’Ambrosio A, Tedeschi G, Gallo A. Psychometric properties and validity of Beck Depression Inventory II in multiple sclerosis. Eur J Neurol. 2016;23:744–50. [DOI] [PubMed] [Google Scholar]
- 47. Téllez N, Río J, Tintoré M, Nos C, Galán I, Montalban X. Does the Modified Fatigue Impact Scale offer a more comprehensive assessment of fatigue in MS? Mult Scler. 2005;11:198–202. [DOI] [PubMed] [Google Scholar]
- 48. Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol. 1991;5:125–42. [Google Scholar]
- 49. Parmenter BA, Weinstock-Guttman B, Garg N, Munschauer F, Benedict RH. Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modalities Test. Mult Scler. 2007;13(1):52–57. [DOI] [PubMed] [Google Scholar]
- 50. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. [DOI] [PubMed] [Google Scholar]
- 51. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–45. [DOI] [PubMed] [Google Scholar]
- 52. Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14:141–45. [DOI] [PubMed] [Google Scholar]
- 53. Krajnc N, Bsteh G, Berger T, Mares J, Hartung HP. Monoclonal antibodies in the treatment of relapsing multiple sclerosis: an overview with emphasis on pregnancy, vaccination, and risk management. Neurotherapeutics. 2022;19:753–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Crimi A, Menze B, Maier O, Reyes M, Handels H, eds. Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries: First International Workshop, Brainles 2015, Held in Conjunction with MICCAI 2015; Munich, Germany, October 5, 2015, Revised Selected Papers (Vol. 9556). Springer; 2016. https://books.google.ae/books?hl=en&lr=&id=cwvNCwAAQBAJ&oi=fnd&pg=PR5&dq=Brusini+L,+et+al.+In:+Brainlesion:+glioma,+multiple+sclerosis,+stroke+and+traumatic+brain+injuries:+6th+international+workshop,+BrainLes+2020,+held+in+conjunction+with+MICCAI+2020+(Revised+selected+papers,+part+I+6)%3B+2020+Oct+4%3B+Lima,+Peru.&ots=Y1sGaZu10e&sig=2dQQ5E2eMrMXNwpSaFGP3CepmmI&redir_esc=y#v=onepage&q&f=false [Google Scholar]
- 55. Jallouli S, Ghroubi S, Dhia IB, Yahia A, Elleuch MH, Sakka S, Mhiri C, Hammouda O. Effect of melatonin intake on postural balance, functional mobility and fall risk in persons with multiple sclerosis: a pilot study. Int J Neurosci. 2024:134:137–47. [DOI] [PubMed] [Google Scholar]
- 56. Miller A, Dishon S. Health-related quality of life in multiple sclerosis: the impact of disability, gender and employment status. Qual Life Res. 2006;15:259–71. [DOI] [PubMed] [Google Scholar]
- 57. Cruz-Gomez ÁJ, Forero L, Lozano-Soto E, Cano-Cano F, Sanmartino F, Rashid-López R, Paz-Expósito J, Gómez Ramirez JD, Espinosa-Rosso R, González-Rosa JJ. Cortical thickness and serum NfL explain cognitive dysfunction in newly diagnosed patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8:e1074. doi: 10.1212/nxi.0000000000001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jerković A, Mikac U, Matijaca M, Košta V, Ćurković Katić A, Dolić K, Vujović I, Šoda J, Đogaš Z, Pavelin S, Rogić Vidaković M. Psychometric properties of the Pittsburgh Sleep Quality Index (PSQI) in patients with multiple sclerosis: factor structure, reliability, correlates, and discrimination. J Clin Med. 2022;11:2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rocchi C, Pulcini A, Vesprini C, Totaro V, Viticchi G, Falsetti L, Danni MC, Bartolini M, Silvestrini M, Buratti L. Sleep in multiple sclerosis patients treated with interferon beta: an actigraphic study. Neurol Res. 2020;42:744–48. [DOI] [PubMed] [Google Scholar]
- 60. Walton C, King R, Rechtman L, Kaye W, Leray E, Marrie RA, Robertson N, La Rocca N, Uitdehaag B, van der Mei I, Wallin M, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS. Mult Scler. 2020;26:1816–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Liu H, Zhou HM, Wu XW, Zhou QH. Correlation between individual physical characteristics and spinal cerebrospinal fluid volume. Clin Anat. 2023;36:420–25. [DOI] [PubMed] [Google Scholar]
- 62. Islam T, Rahman MR, Karim MR, Huq F, Quinn JMW, Moni MA. Detection of multiple sclerosis using blood and brain cells transcript profiles: insights from comprehensive bioinformatics approach. Inform Med Unlocked. 2019;16:100201. [Google Scholar]
- 63. Javitt A, Barnea E, Kramer MP, Wolf-Levy H, Levin Y, Admon A, Merbl Y. Pro-inflammatory cytokines alter the immunopeptidome landscape by modulation of HLA-B expression. Front Immunol. 2019;10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Okamoto K, Iwasaki N, Doi K, Noiri E, Iwamoto Y, Uchigata Y, Fujita T, Tokunaga K. Inhibition of glucose-stimulated insulin secretion by KCNJ15, a newly identified susceptibility gene for type 2 diabetes. Diabetes. 2012;61:1734–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang S, Li Z, Ding X, Zhao Z, Zhang M, Xu H, Lu J, Dai L. Integrative analyses identify KCNJ15 as a candidate gene in patients with epilepsy. Neurol Ther. 2022;11:1767–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Odabas FO, Uca AU, Akdag T, Demirdögen F, Altas M, Tokgoz OS. Possible roles of sestrin2 in multiple sclerosis and its relationships with clinical outcomes. Arq Neuropsiquiatr. 2022;80:399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kallenborn-Gerhardt W, Lu R, Syhr KM, Heidler J, von Melchner H, Geisslinger G, Bangsow T, Schmidtko A. Antioxidant activity of sestrin 2 controls neuropathic pain after peripheral nerve injury. Antioxid Redox Signal. 2013;19:2013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yu L, Yang F, Zhang F, Guo D, Li L, Wang X, Liang T, Wang J, Cai Z, Jin H. CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production. Cell Death Dis. 2018;9:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci. 2021;22:2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Talebi F, Ghorbani S, Chan WF, Boghozian R, Masoumi F, Ghasemi S, Vojgani M, Power C, Noorbakhsh F. MicroRNA-142 regulates inflammation and T cell differentiation in an animal model of multiple sclerosis. J Neuroinflammation. 2017;14:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cui D, Wang J, Zeng Y, Rao L, Chen H, Li W, Li Y, Li H, Cui C, Xiao L. Generating hESCs with reduced immunogenicity by disrupting TAP1 or TAPBP. Biosci Biotechnol Biochem. 2016;80:1484–91. [DOI] [PubMed] [Google Scholar]