Abstract
Sepsis is a disorder of host response caused by severe infection that can lead to life-threatening organ dysfunction. There is no specific treatment for sepsis. Although there are many different pathogens that can cause sepsis, endothelial dysfunction is a frequent mechanism resulting in vascular leakage and coagulation problem. Recent studies on the regulatory pathways of vascular endothelium have shown that the disturbance of angiopoietin (Ang) /Tie2 axis can induce endothelial cell activation, which is the core pathogenesis of sepsis. In this review, we aim to discuss the regulation of Ang/Tie2 axis and the biomarkers involved in the context of sepsis. Also, we attempt to explore the prospective and feasibility of Ang/Tie2 axis as a potential target for sepsis intervention to improve clinical outcomes.
Keywords: sepsis, coagulation, endothelium, angiopoietin, Tie2
Introduction
Sepsis, as a dysregulated host response caused by severe infection, accounts for a large proportion of critically ill patients and is a major public health concern worldwide. 1 As a heterogeneous syndrome of physiologic, pathologic, and biochemical abnormalities, sepsis leads to microvascular leakage and irregular coagulation process, thus resulting in multiple organ dysfunction. 1 Despite its high morbidity and mortality, current treatment for sepsis is still based on symptomatic supports and there is no specific therapy. 2 In the development of sepsis, the endothelium is recognized as an intact and independent organ that plays its role. 3 With the interaction of inflammatory factors, endothelial cells are activated to promote the secretion of adhesion molecules and coagulation factors in order to resist the invasion of pathogens. 4 However, a persistent and severe inflammatory reaction may lead to the destruction of blood vessel barrier and trigger vascular leakage, which manifests as acute vascular dysfunction, resembling the overwhelming syndrome of sepsis. 5 Recent researches have shown that the angiopoietin (Ang)/Tie2 axis, together with its downstream signaling pathways, plays a significant role in the regulation of vascular maturation, stability and integrality during angiogenesis. 6 Furthermore, the specific axis is involved in quiescence and permeability alteration of endothelial cells, the disruption of which is closely associated with endothelial injury and vasculature dysfunction in sepsis.7,8 The inflammatory state in sepsis activates endothelial cells and regulates coagulation function through the Ang/Tie2 axis, leading to microthrombosis and vessel barrier damage, which in turn causes multiple organ failure. 9 Ang/Tie2 is the core of inflammation, coagulation and endothelial function in sepsis.
In this review, we aim to discuss the regulatory role of the Ang/Tie2 axis in sepsis, its use as a biomarker, and explore the potential value of the Ang/Tie2 axis in the treatment of sepsis.
The Ang/Tie2 Axis
Angiopoietin
Angiopoietin, a family of oligomeric-secreted glycoprotein, comprising Ang-1, Ang-2, Ang-3 and Ang-4.10–12 Each member of Ang family has a similar protein structure, which consists of three parts: superclustering domain, α-helix-rich coiled-coil domain on the N-terminal, and fibrinogen-like domain on the C-terminal. The coiled-coil domain promotes the multimerization of Ang molecules, while the fibrinogen-like domain is the most conserved part, containing a receptor-binding region that determines whether a particular Ang is activated. 13 Ang-1 and Ang-2 are the two most characterized types, which bind to the corresponding receptor Tie2 on endothelial cells and regulate the permeability and stability through intercellular junctions.
Ang-1
Ang-1 is produced predominantly by vascular smooth muscle cells, pericytes and platelet α-granules.10,14,15 Composed of 498 amino acids, the presence of superclustering domain along with the coiled-coil domain arrays the single molecule Ang-1 into a homotetramer and mediates its oligomerization. The polymeric form of Ang-1 facilitates the aggregation and cross-phosphorylation of Tie2 on the endothelial cell surface, which is necessary for the following signaling transformation. 13 The fibrinogen-like domain on the C-terminal is used to bind the immunoglobulin (Ig)-epidermal growth factor (EGF) region of Tie2 ectodomain. 16 Fact has been observed that the phenotype of Ang-1-knockout mice die at about 12.5 days of embryo, with embryos edema, hemorrhage and vascular hypoplasia, largely resembles the phenotype of Tie2-knockout ones. 10 Under physiological conditions, Ang-1 binds to and phosphorylates Tie2 receptor, providing a continuous signaling for Tie2 activation. The downstream signaling activates the second messenger Akt, which further leads to decreased endothelial apoptosis and promotes vascular stability. It also triggers angiogenesis, but does not directly stimulate the growth process. 17
Ang-2
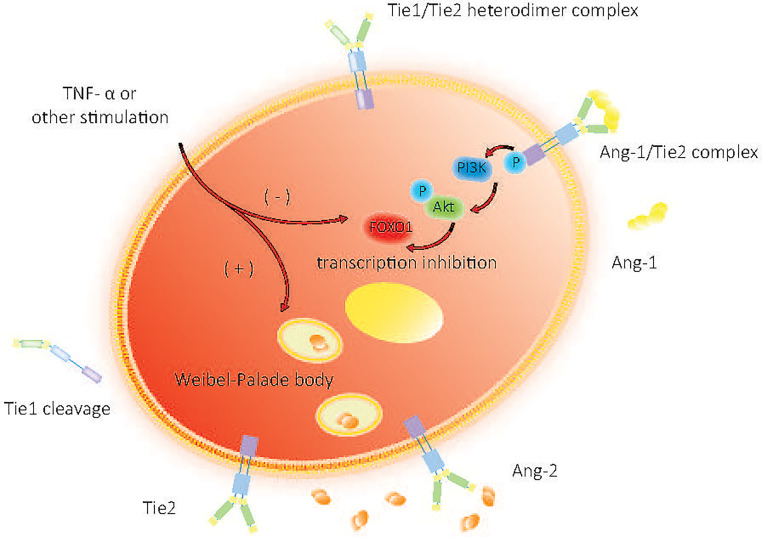
Expressed mainly by endothelial cells, Ang-2 is composed of 496 amino acids and has 60% homology with Ang-1. The high homology in- between makes Ang-2 a natural competitive inhibitor of Ang-1, and its expression is highly regulated. 11 Unlike Ang-1, Ang-2 is released by endothelial cells in an autocrine manner. Evidence suggests that Ang-2 is expressed in a distinct granular manner and is found to get pre-produced in Weibel-Palade bodies of endothelial cells. 18 In a quiescent state, endothelial cells secrete Ang-2 in the pattern of autocrine, maintaining low levels of Tie2 receptor phosphorylation. When the autocrine process is blocked, the basic activation levels of Tie2 are reduced along with its downstream PI3K/Akt pathways, which further attenuates the resistance of endothelial cells to apoptosis. 19 Upon stimulation, such as TNF-α and other inflammatory cytokines, Ang-2 is rapidly released and participates immediately in the suppression of Tie2 signaling, 20 as outlined in figure 1. Researches have shown that Ang-2 expression level is increased in the site of frequent vascular remodeling, acting as a destabilizing factor of blood vessels. 11 Compared with Ang-1, Ang-2 has a lower affinity in binding with Tie2. In the presence of the more potent agonist Ang-1, Ang-2 exhibits inhibition of the Tie2 signaling. This indicates that Ang-2 is an context-dependent partial agonist of endothelial cells.19,21 Under inflammation status, Ang-2 competitively binds to Tie2, blocks the Ang-1/Tie2 signaling pathway, suppresses Tie2 phosphorylation and inhibits vascular stabilization. This alters intercellular junctions, compromises vascular integrity, weakens the vascular barriers, and increases vascular permeability, making endothelial cells vulnerable to inflammatory cytokines. 22 The complex interaction between Ang/Tie2 axis suggests that this signaling pathway may be a potential diagnostic and therapeutic target for sepsis and its associated complications. 23
Figure 1.
Quiescent and activated state of endothelial cells.
Ang-1 phosphorylates the Tie2 receptor and provides a continuous downstream signaling, activating the second messenger Akt. Upon stimulation, Ang-2 is rapidly released and participates in Tie2 signal suppression. Tie2 phosphorylation is shut down by increased levels of Ang-2 while level of FOXO1 transcription gets enhanced, continuously generating more Ang-2 molecules.
Tie
Tie molecules were named for the tyrosine kinases with homologous domains of immunoglobulin and EGF. 24 Both Tie1 and Tie2 have the ectodomains structure of Ig domain, EGF homology repeats and fibronectin type III (FNIII) repeats. 16 They are members of Tie family, serving as receptors of Ang to promote angiogenesis as well as maintain vascular homeostasis. 25
Tie1
Tie1, an orphan receptor, is crucial for the Ang-mediated processes of angiogenesis, vascular remodeling and endothelial cell proliferation. 26 Tie1 is not directly involved in the binding of Ang. When Ang-1 and Ang-2 bind to Tie2, Tie1-Tie2 interaction increases to form heterodimer complexes through β1 integrin. 27 Deficiency of Tie1 attenuates Tie2 phosphorylation as well as its downstream Akt phosphorylation and FOXO1 inactivation. 28 In the pathological conditions such as infection and inflammation shown in figure 1., Tie1 cleavage occurs, which activates Ang-2 from Tie2 agonism to antagonism, and increases FOXO1 activation by inhibiting the phosphorylation of Tie2 and AKT, leading to the reduction of the activity of Ang-1 and Ang-2 agonist. 29
Tie2
Tie2 is a transmembrane tyrosine kinase nearly exclusively produced by endothelial cells. As an Ang receptor, 30 Tie2 is expressed in both quiescent and activated endothelial cells, and is essential for angiogenesis and the stabilization of vascular barrier. The FNIII domain of Tie2 extracellular region (ECR) can get self-associated to form an FNIII domain-mediated dimer, one primarily involving loops in the FNIIIb/FNIIIc boundary region, and the other mediated entirely by the interaction of two FNIIIc domains. The former is acquired by soluble Tie2 (sTie2) dimerization in solution, while both of them can get engaged in Ang-1-induced Tie2 activation. 31 At quiescence state, Ang-1 binds to the ectodomain of Tie2 in a trans-configuration to induce its activation. Tie2 dimerizes and gets phosphorylated, and its downstream intracellular pathway Akt is phosphorylated by PI3K, followed by FOXO1. Nucleus exclusion inhibits the transcription of FOXO1 so as to maintain cell homeostasis by inhibiting its binding with Ang-2. 29 As Ang-2 levels rise in sepsis, the phosphorylation of Tie2 is inhibited, and the transcription of downstream FOXO1 gets enhanced, producing more Ang-2 molecules, and vascular endothelial stability is disrupted. 7 We can find a brief process description in figure 1.
The Ang/Tie2 Axis in Sepsis
As a clinical syndrome with high heterogeneity and mortality, early warning of sepsis is of great clinical significance. Although SOFA score can be used to assess the extent of organ dysfunction in septic patients, 1 there is still no specific or well-defined approach for their early identification and diagnosis. We seek to identify an effective indicator playing its role in early warning and severity stratification in sepsis, so as to enhance the early identification and diagnosis of septic patients, guide the prognostic assessment and promote the rational allocation of medical resources.
Ang-1/Ang-2 as a Biomarker in Sepsis
The vascular endothelium is an important organ involved in the development of sepsis. As endothelial cell activation usually precedes its dysfunction, biomarkers of activated endothelial cells in serum or plasma may be detected before classical biomarkers. 32 Therefore, it may be used as a biomarker for early diagnosis, evaluation of severity and prognosis of systemic infectious diseases. 33 In the Ang/Tie2 axis, Ang-1 maintains the endothelium quiescence, whereas Ang-2 breaks down endothelial intercellular junctions and promotes the process of angiogenesis. 34 The changes in Ang-2-triggered vascular instability and Ang-1 as a vascular stabilizer can act as a potent predictor of severe infection in the early stages of sepsis. Studies have shown that the expression of Ang-2 is up-regulated in septic patients and is closely related to the severity. 9 The ratio of Ang-2/Ang-1 is expected to indicate a state of endothelial destabilization and destruction. In a study of 440 consecutive patients with sepsis, patients were divided into SIRS, sepsis, severe sepsis and septic shock. 35 Ang-2/Ang-1 was increased in septic patients, and its level was further elevated in patients with severe sepsis and septic shock, respectively. Sepsis non-survivors had a higher Ang-2/Ang-1 ratio than survivors. And for survivors, their Ang-2/Ang-1 got a lower level at the end of the study than that of the first day. Therefore, conclusion received is that serum Ang-2/Ang-1 ratio is associated with the severity of sepsis and reflects the prognosis.
In addition, there are still other diagnostic biomarkers besides Ang-2/Ang-1, such as Ang-1/Tie2 35 and thrombomodulin (TM) 36 . For the complicated infectious condition, it could be more precise to compare multiple markers at one time and comprehensively monitor their fluctuation.
The Role of Ang-2 in Sepsis: A Double-Edged Sword
The expression of Ang-2 is increased in sepsis, and the Ang balance is biased towards Ang-2. The increased binding of Ang-2/Tie2 blocks Tie2 activation, leading to endothelial instability. Therefore, the vast majority of studies suggest that therapies targeting Ang-2 are beneficial. However, several studies have also shown a beneficial effect of Ang-2 in sepsis. Tzepi et al 37 showed that injection of recombinant Ang-2 had a protective effect on septic shock caused by inactivated Pseudomonas aeruginosa or Escherichia coli in mice. In contrast, Ang-2 had no protective effect against lipopolysaccharide (LPS)-induced septic shock, suggesting a key difference in the sepsis models that have been used. Safioleas et al 38 reported improved survival after injection of recombinant Ang-2 in an experimental model of septic pyelonephritis in rabbits. The protective effect was associated with the reversal of sepsis-induced immunosuppression. Therefore, Ang-2 seems to have a dual role in sepsis. Ang-2 may play a protective role against local infections, such as promoting the recruitment of inflammatory cells. However, when this initial local response transforms into a diffuse overwhelming systemic immune response, the effects of Ang-2 may become harmful. 6 The multifaceted roles of Ang-2 in sepsis remain to be investigated.
The Prospective of Ang/Tie2 Axis as a Therapeutic Target in Sepsis
Due to the significant role of the Ang/Tie2 signaling pathway in the process of sepsis, relevant studies on its targeted therapies are being explored.39,40 Ang-2-deficient mice are more resistant to inflammation, high cellular permeability and cell apoptosis. Efforts to reduce plasma Ang-2 or block its signaling pathways may prevent and treat multiple organ dysfunction caused by sepsis to some extent. 41 Ang-2-binding and Tie2-activating antibody (ABTAA), an uncommon Ang-2 antibody, can trigger Ang-2 aggregation upon binding to it. 42 ABTAA/Ang-2 complexes forms and subsequently Tie2 gets activated and is used as an intervention for sepsis-associated microvascular dysfunction, resulting in less cell injuries and higher survival compared with conventional Ang-2-blocking antibody. 42 Activated protein C (APC) is a kind of natural anticoagulant approved for the treatment of severe sepsis, which has the function of anti-inflammation, anti-apoptosis and stabilizing vascular barrier. 43 APC can promote vascular endothelial stabilization, maintain its barrier function and exert anticoagulant effects through regulation of the Ang/Tie2 signaling axis. 43 It competes with Ang-2 and binds directly to Tie2 with a stronger binding affinity, which further rapidly promotes the expression and activation of Tie2, increases the Ang-1/Ang-2 ratio and enhances endothelial barrier function as well as improves the integrity. 44 Administration of Ang-1 can improve the inhibition of Ang-1 and the elevation of Ang-2 induced by sepsis, thus improving the prognosis and reducing mortality. 45 Although blocking the Ang/Tie2 pathway may be an effective way to treat sepsis, multiple signaling pathways co-regulate the process of endothelial activation in sepsis such as NF-kB and VEGF/VEGFR2. Thus, drugs that inhibit only one single pathway may not fully achieve the expected therapeutic efficacy. Co-inhibitors of multiple pathways may be the future research direction. 46
Treatment Targeted the Ang/Tie2 Axis in Sepsis-Induced ARDS
Acute respiratory distress syndrome (ARDS) is one of the major complications of septic patients, characterized by lung inflammation and vascular leakage with poor prognosis. 47 Researches have shown that the process of vascular leakage in sepsis-induced ARDS may be mediated by the Ang/Tie2 signaling axis. 48 The level of circulating Ang-2 can be elevated in septic patients with poor gas exchange and impaired oxidation. 49 Excessive Ang-2 plays an important role in the destruction of lung endothelial cells, resulting in suppression of Tie2 signaling, disruption of vessel barrier function and excessive permeability of the pulmonary capillary, which can be reversed by Ang-1. 49 Fluid leakage from the pulmonary vessels into the alveolar septum impairs gas exchange and reduces lung compliance, which further contributes to ARDS. 50 Either Ang-2 or Tie2 knockout alone can replicate the state of endothelial barrier disruption in septic patients, an effect that can be restored in the clinical rehabilitation phase or get reversed by Ang-1. Ang/Tie2 signaling pathway mediates sepsis-induced acute lung injury (ALI) or ARDS by regulating the inflammatory response and permeability of vascular endothelium. 51 It is therefore regarded as a biomarker of endothelial injury and coagulation dysfunction with the significance of clinical diagnosis during ARDS. 52
Treatment Targeted the Ang/Tie2 Axis in Sepsis-Induced Coagulopathy
Sepsis-induced coagulopathy (SIC) is the activation of intravascular coagulation caused by endothelial dysfunction in sepsis.53,54 The Ang/Tie2 axis, which maintains endothelial homeostasis, is thought to play a significant role in SIC, resisting systemic inflammation and thrombosis. 7 Disruption of the Ang/Tie2 axis is a sentinel event in SIC that occurs before the onset of significant SIC in sepsis. It can be observed that vascular leakage, leukocyte recruitment and formation of thrombosis occur simultaneously as shown in figure 2. The levels of its biomarker can promote the early diagnosis of SIC and predict the mortality more accurately. 8 The levels of serum Ang-1 and sTie2 in SIC patients were lower than those in patients without SIC, while the levels of Ang-2 and the ratio of Ang-2/Ang-1 were shown to be higher. 55 Tie2 knockout alone can replicate sepsis-associated microthrombotic reaction. Activation of Tie2 inhibits the prothrombotic response of LPS to endothelial cells and normalizes the thrombosis process in sepsis. 8 This effect does not increase the risk of bleeding and may provide better strategies for SIC treatment.
Figure 2.
Endothelium activation, vascular barrier disruption and thrombosis formation in sepsis.
Disruption of the Ang/Tie2 axis is a sentinel event in SIC which occurs early in sepsis. It can be observed that vascular leakage, leukocyte recruitment and thrombosis occur simultaneously.
Treatment Targeted the Ang/Tie2 Axis in Sepsis-Induced AKI
Acute kidney injury (AKI) is caused by acute decline of renal function, with the pathophysiological changes including apoptosis of glomerular and tubular endothelial cells, release of inflammatory factors and dysfunction of the immune system. 56 It is one of the most common complications of ICU patients who are suffering from sepsis. 57 Currently, serum creatinine level is regarded as an indicator of acute kidney injury, but it lacks specificity and cannot prospectively predict the risk of renal injury in patients with sepsis. 58 Recent evidence suggests that systemic inflammation and endothelial activation contribute to the process of AKI.59,60 Oxidation damage and microcirculation disorders may have been present prior to the onset of overt kidney damage, which induces Ang-2 elevation and increases the risk of AKI. The level of serum Ang-2 and Ang-2/Ang-1 ratio gets increased along with severity of AKI grows higher. 61 Additionally, as an indicator of the severity of microvascular leakage in sepsis, the level of Ang-2 is also independently associated with the development of sepsis-associated AKI. Ang-2 was superior to other endothelial biomarkers in severe sepsis-associated AKI, and its serum level can be significantly elevated. Changes in Ang-2 levels correlated with the severity of AKI, 62 which may provide a new approach for the diagnosis and monitoring the severity progression of the disease.
Treatment Targeted the Ang/Tie2 Axis in Sepsis-Induced Other Organ Dysfunction
Sepsis can also lead to other organ dysfunction, such as sepsis-induced liver injury, sepsis-induced cardiomyopathy, sepsis-induced encephalopathy and so on. Elias et al 63 reported that Ang-1 supplementation restored liver function and reduced mortality in sepsis patients. Preclinical studies have shown that elevated Ang-2 levels and decreased Ang-1 levels contribute to myocardial depression during inflammation. 64 Richter et al 65 demonstrated that sepsis patients with ongoing cardiovascular dysfunction manifested sustained increase in Ang-2/Ang-1 ratios. Gurnik et al 66 reported that the endothelial glycocalyx in brain microvessels was disrupted and blood–brain barrier permeability was increased in Ang-2-overexpressing mice. The above results suggested that Ang/Tie2 axis is involved in sepsis-induced liver injury, cardiomyopathy, encephalopathy and so on. Therefore, treatment targeting Ang/Tie2 axis may be beneficial for sepsis-induced organ dysfunction.
Conclusion
Ang/Tie2 signaling axis not only participates in angiogenesis in normal physiological context, but also plays a significant role in the regulation of vascular barrier and coagulation activation in the process of sepsis. The axis maintains the stability of the endothelium, and its disruption in sepsis leads to multiple organ dysfunction, and even death. The pathophysiological mechanism underlying Tie2-mediated microvascular dysfunction in sepsis remains to be fully understood. Clinical studies on the Ang/Tie2 axis include the evaluation of Ang-1, Ang-2, sTie2 and Ang-2/Ang-1 levels, which are expected to be early biomarkers for diagnosis and predicting severity stratification in septic patients. At present, the treatment of sepsis mainly relies on appropriate antibiotic administration and symptomatic supporting therapy. Ang/Tie2 axis may be important therapeutic targets in sepsis. Further researches are being widely carried out with the aim at developing Tie2-targeted therapy to improve the prognosis of sepsis. We expect that the study of Ang/Tie2 signaling axis will bring new breakthroughs for diagnosis, severity stratification and treatment of septic patients.
Footnotes
Author Contributions: Conceptualization and writing-original draft by Yawen Chi; Investigation and assisting in content by Sihan Yu, Jia Yin, Danyan Liu, Mengke Zhuo; Writing-review and editing, funding acquisition, resources and supervision by Xu Li. All authors reviewed the manuscript and all approved of the final version.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Key Research and Development Program of China (grant number 2022YFC2304605), National Natural Science Foundation of China (grant number 82272195).
ORCID iD: Xu Li https://orcid.org/0000-0001-6750-9539
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J, Vincent JL, Adhikari NKJ, et al. Sepsis: A roadmap for future research. Lancet Infect Dis. 2015;15(5):581-614. [DOI] [PubMed] [Google Scholar]
- 3.Ait-Oufella H, Maury E, Lehoux S, et al. The endothelium: Physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 2010;36(8):1286-1298. [DOI] [PubMed] [Google Scholar]
- 4.Joffre J, Hellman J, Ince C, et al. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020;202(3):361-370. [DOI] [PubMed] [Google Scholar]
- 5.Dolmatova EV, Wang KK, Mandavilli R, et al. The effects of sepsis on endothelium and clinical implications. Cardiovasc Res. 2021;117(1):60-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholz A, Plate KH, Reiss Y. Angiopoietin-2: A multifaceted cytokine that functions in both angiogenesis and inflammation. Ann N Y Acad Sci. 2015;1347(1):45-51. [DOI] [PubMed] [Google Scholar]
- 7.Sack KD, Kellum JA, Parikh SM. The angiopoietin-Tie2 pathway in critical illness. Crit Care Clin. 2020;36(2):201-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higgins SJ, De Ceunynck K, Kellum JA, et al. Tie2 protects the vasculature against thrombus formation in systemic inflammation. J Clin Invest. 2018;128(4):1471-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David S, Mukherjee A, Ghosh CC, et al. Angiopoietin-2 may contribute to multiple organ dysfunction and death in sepsis. Crit Care Med. 2012;40(11):3034-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7):1161-1169. [DOI] [PubMed] [Google Scholar]
- 11.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55-60. [DOI] [PubMed] [Google Scholar]
- 12.Valenzuela DM, Griffiths JA, Rojas J, et al. Angiopoietins 3 and 4: Diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A. 1999;96(5):1904-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87(7):1171-1180. [DOI] [PubMed] [Google Scholar]
- 14.Hirschberg R, Wang S, Mitu GM. Functional symbiosis between endothelium and epithelial cells in glomeruli. Cell Tissue Res. 2008;331(2):485-493. [DOI] [PubMed] [Google Scholar]
- 15.Li JJ, Huang YQ, Basch R, et al. Thrombin induces the release of angiopoietin-1 from platelets. Thromb Haemost. 2001;85(2):204-206. [PubMed] [Google Scholar]
- 16.Barton WA, Tzvetkova-Robev D, Miranda EP, et al. Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat Struct Mol Biol. 2006;13(6):524-532. [DOI] [PubMed] [Google Scholar]
- 17.Akwii RG, Sajib MS, Zahra FT, et al. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8(5):471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiedler U, Scharpfenecker M, Koidl S, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150-4156. [DOI] [PubMed] [Google Scholar]
- 19.Yuan HT, Khankin EV, Karumanchi SA, et al. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29(8):2011-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le CTK, Laidlaw G, Morehouse CA, et al. Synergistic actions of blocking angiopoietin-2 and tumor necrosis factor-alpha in suppressing remodeling of blood vessels and lymphatics in airway inflammation. Am J Pathol. 2015;185(11):2949-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thamm K, David S. Role of angiopoietin-2 in infection - A double-edged sword? Cytokine. 2016. Jul;83:61-63. [DOI] [PubMed] [Google Scholar]
- 22.Thurston G, Daly C. The Complex role of angiopoietin-2 in the angiopoietin-Tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2(9):a006550. doi: 10.1101/cshperspect.a006650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss A. The angiopoietin: Tie 2 interaction: A potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013;24(6):579-592. [DOI] [PubMed] [Google Scholar]
- 24.Partanen J, Armstrong E, Mäkelä TP, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12(4):1698-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376(6535):70-74. [DOI] [PubMed] [Google Scholar]
- 26.Korhonen EA, Lampinen A, Giri H, et al. Tie1 controls angiopoietin function in vascular remodeling and inflammation. J Clin Invest. 2016;126(9):3495-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller SB, Kontos CD. Tie1: An orphan receptor provides context for angiopoietin-2/Tie2 signaling. J Clin Invest. 2016;126(9):3188-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potente M, Urbich C, Sasaki K, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115(9):2382-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim M, Allen B, Korhonen EA, et al. Opposing actions of angiopoietin-2 on Tie2 signaling and FOXO1 activation. J Clin Invest. 2016;126(9):3511-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puri MC, Rossant J, Alitalo K, et al. The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 1995;14(23):5884-5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore JO, Lemmon MA, Ferguson KM. Dimerization of Tie2 mediated by its membrane-proximal FNIII domains. Proc Natl Acad Sci U S A. 2017;114(17):4382-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bate N, Lodge J, Brindle NPJ. Intrinsic differences in the mechanisms of Tie2 binding to angiopoietins exploited by directed evolution to create an Ang2-selective ligand trap. J Biol Chem. 2021;297(2):100888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang YY, Li CS, Shao R, et al. The role of biomarkers of endothelial activation in predicting morbidity and mortality in patients with severe sepsis and septic shock in intensive care: A prospective observational study. Thromb Res. 2018 Nov;171:149-154. [DOI] [PubMed] [Google Scholar]
- 34.Leligdowicz A, Richard-Greenblatt M, Wright J, et al. Endothelial activation: The Ang/Tie axis in sepsis. Front Immunol. 2018;24(9):838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang YY, Li CS, Shao R, et al. Prognostic significance of the angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for early sepsis in an emergency department. Crit Care. 2015;19(1):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daly C, Qian XZ, Castanaro C, et al. Angiopoietins bind thrombomodulin and inhibit its function as a thrombin cofactor. Sci Rep. 2018;8(1):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tzepi M, Giamarellos-Bourboulis EJ, Carrer DP, et al. Angiopoietin-2 enhances survival in experimental sepsis induced by multidrug-resistant Pseudomonas aeruginosa. J Pharmacol Exp Ther. 2012;343(2):278-287. [DOI] [PubMed] [Google Scholar]
- 38.Safioleas K, Giamarellos-Bourboulis EJ, Carrer DP, et al. Reverse kinetics of angiopoietin-2 and endotoxins in acute pyelonephritis: Implications for anti-inflammatory treatment? Cytokine. 2016 May;81:28-34. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Kontos CD, Annex BH, et al. A systems biology model of junctional localization and downstream signaling of the Ang-Tie signaling pathway. NPJ Syst Biol Appl. 2021;7(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Disc. 2017;16(9):635-661. [DOI] [PubMed] [Google Scholar]
- 41.Reilly JP, Wang F, Jones TK, et al. Plasma angiopoietin-2 as a potential causal marker in sepsis-associated ARDS development: Evidence from Mendelian randomization and mediation analysis. Intensive Care Med. 2018;44(11):1849-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han S, Lee SJ, Kim KE, et al. Amelioration of sepsis by TIE2 activation-induced vascular protection. Sci Transl Med. 2016;8(335):335ra55. doi: 10.1126/scitranslmed.aad9260. [DOI] [PubMed] [Google Scholar]
- 43.Minhas N, Xue ML, Fukudome K, et al. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2010;24(3):873-881. [DOI] [PubMed] [Google Scholar]
- 44.Minhas N, Xue ML, Jackson CJ. Activated protein C binds directly to Tie2: Possible beneficial effects on endothelial barrier function. Cell Mol Life Sci. 2017;74(10):1895-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.David S, Park J-K, Meurs M, et al. Acute administration of recombinant angiopoietin-1 ameliorates multiple-organ dysfunction syndrome and improves survival in murine sepsis. Cytokine. 2011;55(2):251-259. [DOI] [PubMed] [Google Scholar]
- 46.Luxen M, van Meurs M, Molema G. Unlocking the untapped potential of endothelial kinase and phosphatase involvement in sepsis for drug treatment design. Front Immunol. 2022;13(13):867625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. New Engl J Med. 2005;353(16):1685-1693. [DOI] [PubMed] [Google Scholar]
- 48.Kumpers P, Lukasz A. The curse of angiopoietin-2 in ARDS: On stranger TI(E)des. Crit Care. 2018;22(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3(3):e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parikh SM. Dysregulation of the angiopoietin-Tie-2 axis in sepsis and ARDS. Virulence. 2013;4(6):517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van der Heijden M, Amerongen GPV, Chedamni S, et al. The angiopoietin-Tie2 system as a therapeutic target in sepsis and acute lung injury. Expert Opin Ther Targets. 2009;13(1):39-53. [DOI] [PubMed] [Google Scholar]
- 52.Sivapalan P, Bonnesen B, Jensen JU. Novel perspectives regarding the pathology, inflammation, and biomarkers of acute respiratory distress syndrome. Int J Mol Sci. 2020;22(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2(1):16037. [DOI] [PubMed] [Google Scholar]
- 54.Iba T, Levy JH. Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology. 2020;132(5):1238-1245. [DOI] [PubMed] [Google Scholar]
- 55.Wada T, Jesmin S, Gando S, et al. Using angiogenic factors and their soluble receptors to predict organ dysfunction in patients with disseminated intravascular coagulation associated with severe trauma. Crit Care. 2012;16(2):R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koza Y. Acute kidney injury: Current concepts and new insights. J Inj Violence Res. 2016;8(1):58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peerapornratana S, Manrique-Caballero CL, Gomez H, et al. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96(5):1083-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380(9843):756-766. [DOI] [PubMed] [Google Scholar]
- 59.Jongman RM, van Klarenbosch J, Molema G, et al. Angiopoietin/Tie2 dysbalance is associated with acute kidney injury after cardiac surgery assisted by cardiopulmonary bypass. PLoS One. 2015;10(8):e0136205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumpers P, Hafer C, David S, et al. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: Relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med. 2010;36(3):462-470. [DOI] [PubMed] [Google Scholar]
- 61.Robinson-Cohen C, Katz R, Price BL, et al. Association of markers of endothelial dysregulation Ang1 and Ang2 with acute kidney injury in critically ill patients. Crit Care. 2016;20(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu WK, McNeil JB, Wickersham NE, et al. Angiopoietin-2 outperforms other endothelial biomarkers associated with severe acute kidney injury in patients with severe sepsis and respiratory failure. Crit Care. 2021;25(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elias G, Schonfeld M, Saleh S, et al. Sepsis-induced endothelial dysfunction drives acute-on-chronic liver failure through angiopoietin-2-HGF-C/EBPβ pathway. Hepatology. 2023;78(3):803-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dallabrida SM, Ismail N, Oberle JR, et al. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circ Res. 2005;96(4):e8-e24. [DOI] [PubMed] [Google Scholar]
- 65.Richter RP, Zheng L, Ashtekar AR, et al. Associations of plasma angiopoietins-1 and -2 and angiopoietin-2/-1 ratios with measures of organ injury and clinical outcomes in children with sepsis: A preliminary report. Pediatr Crit Care Med. 2020;21(9):e874-e878. [DOI] [PubMed] [Google Scholar]
- 66.Gurnik S, Devraj K, Macas J, et al. Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol. 2016;131(05):753-773. [DOI] [PMC free article] [PubMed] [Google Scholar]