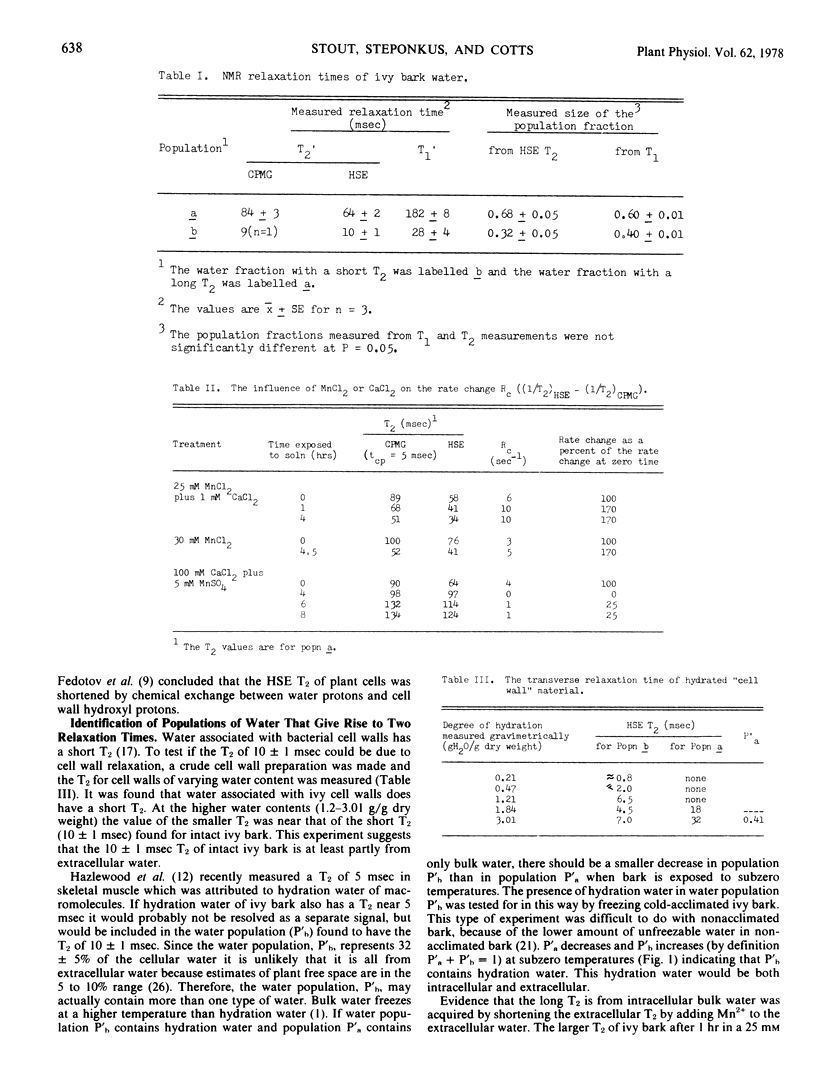
Abstract
Measurement of nuclear magnetic resonance (NMR) relaxation times (transverse [T2] and longitudinal [T1]) for Hedera helix L. cv. Thorndale (ivy) bark water indicates the presence of at least two populations of water with different relaxation characteristics. One population of water with short T2 and T1 was found to be composed of both hydration water and extracellular free water. The second population of water with long T2 and T1 was identified as intracellular bulk water.
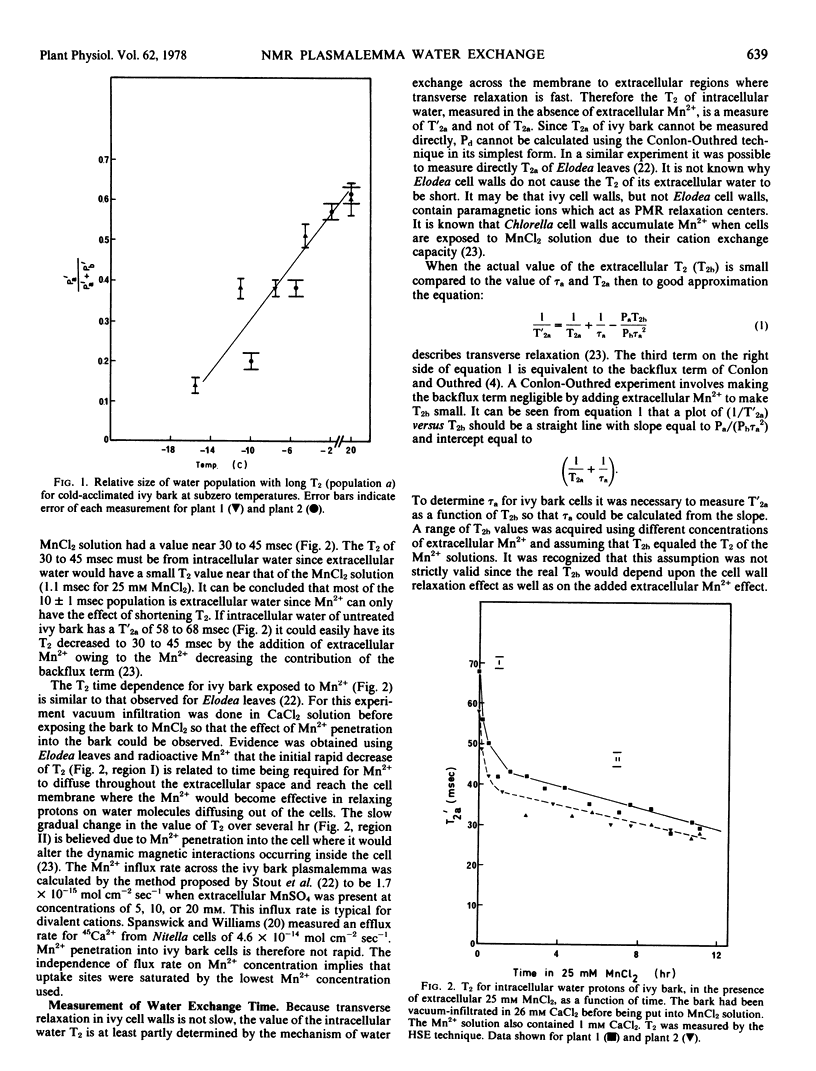
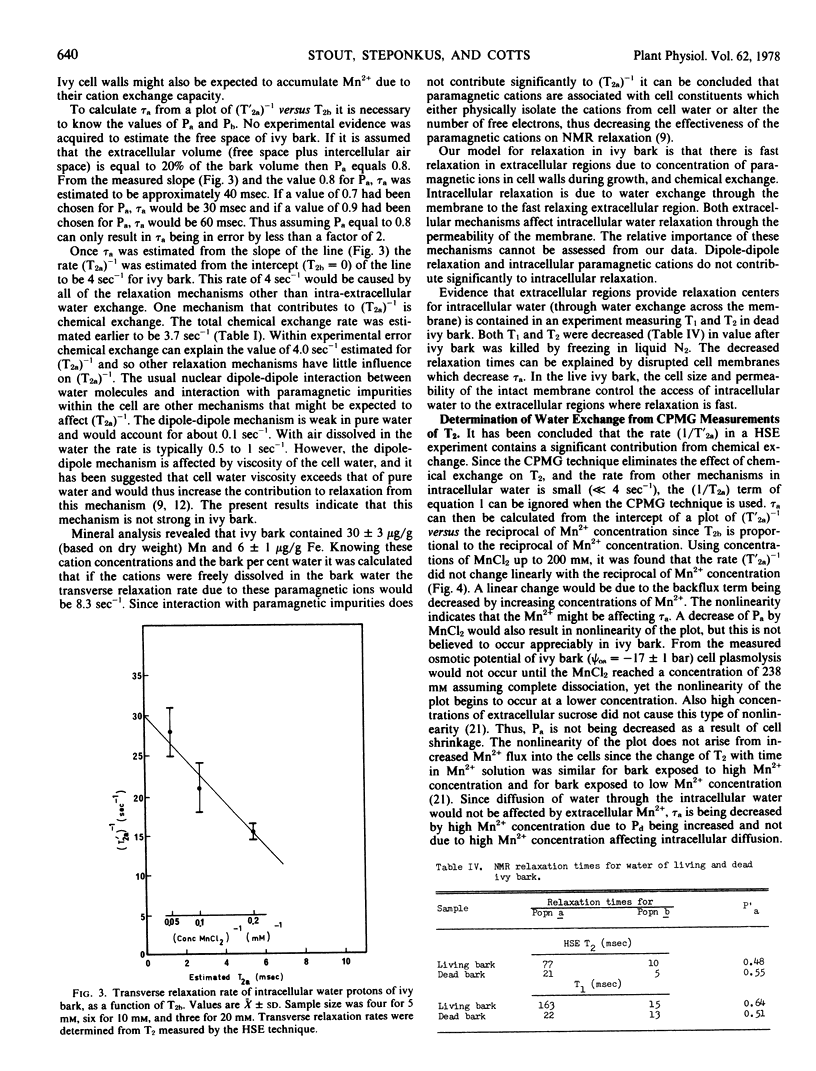
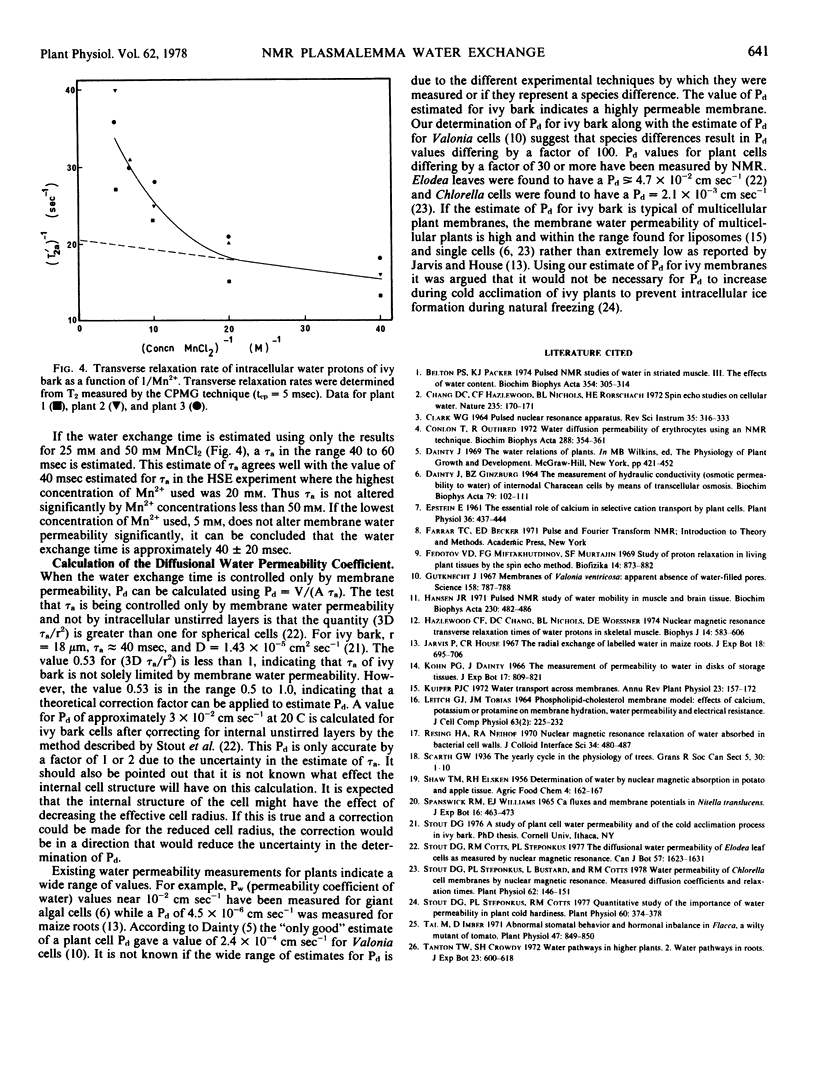
NMR relaxation of extracellular water protons is controlled by cell wall surface effects, possibly due to binding of paramagnetic cations by the cell walls. NMR relaxation of intracellular water protons is controlled by both water exchange to the extracellular environment and chemical exchange with a population of protons that is chemically shifted from that of the bulk water. The relaxation time of intracellular water is not measurably affected, either by intracellular paramagnetic ions or by increased viscosity of intracellular water. Manganese flux into the cells occurs at 1.7 × 10−15 moles cm−2 seconds−1 and is independent of extracellular Mn2+ concentration in the range 5 to 20 mm.
The intracellular-extracellular water exchange time of ivy bark was found to be predominantly limited by membrane water permeability. A diffusional water permeability coefficient (Pd) of approximately 3 × 10−2 cm seconds−1 was calculated for ivy cell membranes at 20 C.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belton P. S., Packer K. J. Pulsed NMR studies of water in striated muscle. 3. The effects of water content. Biochim Biophys Acta. 1974 Jul 4;354(2):305–314. doi: 10.1016/0304-4165(74)90015-4. [DOI] [PubMed] [Google Scholar]
- Chang D. C., Hazlewood C. F., Nichols B. L., Rorschach H. E. Spin echo studies on cellular water. Nature. 1972 Jan 21;235(5334):170–171. doi: 10.1038/235170a0. [DOI] [PubMed] [Google Scholar]
- Conlon T., Outhred R. Water diffusion permeability of erythrocytes using an NMR technique. Biochim Biophys Acta. 1972 Nov 2;288(2):354–361. doi: 10.1016/0005-2736(72)90256-8. [DOI] [PubMed] [Google Scholar]
- DAINTY J., GINZBURG B. Z. THE MEASUREMENT OF HYDRAULIC CONDUCTIVITY (OSMOTIC PERMEABILITY TO WATER) OF INTERNODAL CHARACEAN CELLS BY MEANS OF TRANSCELLULAR OSMOSIS. Biochim Biophys Acta. 1964 Jan 27;79:102–111. doi: 10.1016/0926-6577(64)90043-9. [DOI] [PubMed] [Google Scholar]
- Epstein E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol. 1961 Jul;36(4):437–444. doi: 10.1104/pp.36.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedotov V. D., Miftakhutdinova F. G., Murtazin Sh F. Issledovanie protonnoi relaksatsii v zhivykh rastitel'nykh tkaniakh metodom spinovogo ékha. Biofizika. 1969 Sep-Oct;14(5):873–882. [PubMed] [Google Scholar]
- Gutknecht J. Membranes of Valonia ventricosa: apparent absence of water-filled pores. Science. 1967 Nov 10;158(3802):787–788. doi: 10.1126/science.158.3802.787. [DOI] [PubMed] [Google Scholar]
- Hansen J. R. Pulsed NMR study of water mobility in muscle and brain tissue. Biochim Biophys Acta. 1971;230(3):482–486. doi: 10.1016/0304-4165(71)90177-2. [DOI] [PubMed] [Google Scholar]
- Hazlewood C. F., Chang D. C., Nichols B. L., Woessner D. E. Nuclear magnetic resonance transverse relaxation times of water protons in skeletal muscle. Biophys J. 1974 Aug;14(8):583–606. doi: 10.1016/S0006-3495(74)85937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITCH G. J., TOBIAS J. M. PHOSPHOLIPID-CHOLESTEROL MEMBRANE MODEL: EFFECTS OF CALCIUM, POTASSIUM OR PROTAMINE ON MEMBRANE HYDRATION, WATER PERMEABILITY AND ELECTRICAL RESISTANCE. J Cell Physiol. 1964 Apr;63:225–232. doi: 10.1002/jcp.1030630213. [DOI] [PubMed] [Google Scholar]
- Resing H. A., Neihof R. A. Nuclear magnetic resonance relaxation of water adsorbed on bacterial cell walls. J Colloid Interface Sci. 1970 Dec;34(4):480–487. doi: 10.1016/0021-9797(70)90209-2. [DOI] [PubMed] [Google Scholar]
- Stout D. G., Steponkus P. L. Quantitative study of the importance of water permeability in plant cold hardiness. Plant Physiol. 1977 Sep;60(3):374–378. doi: 10.1104/pp.60.3.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout D. G., Steponkus P. L. Water permeability of chlorella cell membranes by nuclear magnetic resonance: measured diffusion coefficients and relaxation times. Plant Physiol. 1978 Jul;62(1):146–151. doi: 10.1104/pp.62.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M., Imber D. Abnormal Stomatal Behavior and Hormonal Imbalance in Flacca, a Wilty Mutant of Tomato: III. Hormonal Effects on the Water Status in the Plant. Plant Physiol. 1971 Jun;47(6):849–850. doi: 10.1104/pp.47.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]


