Abstract
INTRODUCTION:
Surgical management of Crohn’s disease (CD) is common. Postoperative complications include anastomotic stricturing (AS). The natural history and risk factors for AS have not been elucidated.
METHODS:
A retrospective cohort study of patients with CD who underwent ileocolonic resection (ICR) with ≥1 postoperative ileocolonoscopy between 2009 and 2020. Postoperative ileocolonoscopies with corresponding cross-sectional imaging were evaluated for evidence of AS without neoterminal ileal extension. Severity of AS and endoscopic intervention at time of detection were collected. Primary outcome was development of AS. Secondary outcome was time to AS detection.
RESULTS:
A total of 602 adult patients with CD underwent ICR with postoperative ileocolonoscopy. Of these, 426 had primary anastomosis, and 136 had temporary diversion at time of ICR. Anastomotic configuration consisted of 308 side-to-side, 148 end-to-side, and 136 end-to-end. One hundred ten (18.3%) patients developed AS with median time of 3.2 years to AS detection. AS severity at time of detection was associated with need for repeat surgical resection for AS. On multivariable Cox proportional hazard regression, anastomotic configuration and temporary diversion were not associated with risk of or time to AS. Preoperative stricturing disease was associated with decreased time to AS (adjusted hazard ratio 1.8; P = 0.049). Endoscopic ileal recurrence before AS was not associated with subsequent AS detection.
DISCUSSION:
AS is a relatively common postoperative CD complication. Patients with previous stricturing disease behavior are at increased risk of AS. Anastomotic configuration, temporary diversion, and ileal CD recurrence do not increase risk of AS. Early detection and intervention for AS may help prevent progression to repeat ICR.
Keywords: anastomotic strictures, postoperative Crohn’s disease, natural history
Graphical Abstract
INTRODUCTION
Patients with Crohn’s disease (CD) most commonly present with inflammatory CD behavior at time of diagnosis; however, over time, upward of 30%–50% of patients develop fibrostenosing disease (1–5). Despite advances in therapeutic management, stricturing and penetrating disease often require surgical management, with approximately 20% of patients requiring an ileocolonic resection (ICR) within 10 years of diagnosis (6–9). However, most patients still develop endoscopic postoperative recurrence (POR), which commonly occurs at the level of or proximal to the anastomosis (8,10–12).
One potential postoperative complication commonly encountered clinically is anastomotic stricturing, which may occur rapidly after surgical intervention or develop more slowly over time. Although some anastomotic strictures (AS) may be related to chronic inflammation and fibrosis, AS may develop devoid of concurrent anastomotic inflammation or luminal extension. We hypothesized a priori that potential AS risk factors may include anastomotic configuration or temporary proximal diversion. Although no near-term differences in endoscopic POR by conventional anastomotic configuration have been consistently reported, long-term sequelae including anatomic distortions have been described (13–15). Furthermore, anastomotic configuration may differentially alter microvascular supply or have varying juxtaposed luminal diameters modifying AS risk (15–17). Surgical diversion with temporary cessation of fecal stream may increase likelihood of distal luminal narrowing like that which can be seen in chronically diverted individuals (18). Consequently, this study aims to describe the natural history and risk factors for development of AS in surgically managed CD.
METHODS
A multihospital, single healthcare system retrospective cohort study of adult patients with CD who underwent ICR between 2009 and 2020 was conducted. Inclusion criteria included (i) age ≥18 years (ii) CD diagnosis confirmed by ≥2 ICD-9 or ICD-10 codes entered by a gastroenterologist or colorectal surgeon; (iii) ICR indicated for CD management; (iv) restoration of intestinal continuity; and (v) ≥1 postoperative ileocolonoscopy after restoration of intestinal continuity.
Demographic and clinical data
All demographic, CD history, operative, and therapeutic management data were collected through manual chart review by 2 independent reviewers (S.P.B. and R.S.S.). Demographic data included sex, age at CD diagnosis and ICR, and tobacco use history. Preoperative CD clinical data included CD location and behavior, history of perianal disease, history of preoperative biologic exposure, and number of previous CD-indicated ICR. Operative data were obtained through the operative report and included creation of primary anastomosis, anastomosis configuration including side-to-side (STS), end-to-side (ETS), and end-to-end (ETE), creation and type of diverting ileostomy (loop or end), and date of bowel continuity restoration. Of note, during the period of the study, more recent novel anastomotic orientations (e.g., Kono-S) were not routinely used. Postoperative data included postoperative biologic prophylaxis defined as initiation of biologics (adalimumab, infliximab, certolizumab, vedolizumab, and ustekinumab) within 3 months of restoration of intestinal continuity, postoperative ileocolonoscopy reports, repeat ICR for CD management, and total postoperative follow-up time defined as time from bowel continuity restoration to date of most recent postoperative ileocolonoscopy.
Ileocolonoscopy data and outcomes
Ileocolonoscopies performed ≥3 months from date of surgery or bowel continuity restoration were captured for review. When not prospectively recorded by the endoscopist, endoscopic activity was retrospectively graded using the modified Rutgeerts score based on ileocolonoscopy images and procedural reports (19). A blinded, retrospective Rutgeerts score evaluator (S.P.B.) was trained by an inflammatory bowel disease gastroenterologist (B.H.C.) and validated (.90% accuracy) using a sample data set before data collection. Endoscopic POR was defined as modified Rutgeerts score ≥i2b disease. AS was defined as any degree of narrowing or stricturing confined to the ileocolonic anastomosis without extension into the neoterminal ileum as identified by the endoscopist. If there was any uncertainty regarding isolation of stricture to anastomosis, cross-sectional imaging studies in the 3 months on either side of the colonoscopy were reviewed and used to adjudicate location disputes, based on Society of Abdominal Radiology guidelines (20). If no imaging was available in such cases, consensus adjudication was performed by study team (S.P.B., M.Z.K., and B.H.C.). As there is no validated criterion to categorize endoscopic stricture severity, AS severity was graded by if AS was traversable at initial detection. Interventions at time of AS detection and in future ileocolonoscopies were collected based on procedural report text and included endoscopic balloon dilation, endoscopic stricturotomy, surgical stricturoplasty or resection, and no intervention. To determine the impact of ileal inflammation on AS, patients with i2a disease were reclassifies as i0 or i1 +/− AS based on their ileal luminal disease.
The primary outcome was defined as time to AS development.
Statistical analyses
Continuous and categorical variables were described by medians (interquartile range) and count (percentages) and compared by using Kruskal-Wallis test and the Wilcoxon rank-sum test, respectively. Statistical significance was defined as P < 0.05. Kaplan-Meier analysis and multivariable Cox proportional hazard regression modeling were performed to determine association of independent variables on time to AS development. Patients were censored at loss of follow-up, repeat ICR, or no AS development at 7.5 years of follow-up. The number of independent variables included in the regression model adhered to the rule of 10 to limit model overfitting (21). Subgroup analysis of primary outcome was conducted on patients who received primary anastomosis at time of ICR.
Ethical considerations
The institutional review board approved the study at study center. All ethical principles laid out in the Declaration of Helsinki were followed.
RESULTS
Study population
In our cohort, 870 adult patients with CD underwent ICR during the study period. Of these patients, 602 (69.2%) had ≥1 postoperative ileocolonoscopy after date of bowel continuity restoration and formed the study cohort. Anastomosis configuration at time of primary anastomosis or ileostomy reversal consisted of 308 STS (51.2%), 158 ETS (26.2%), and 136 ETE (22.6%). Of these patients, 426 (70.8%) underwent primary anastomosis (78 ETE, 118 ETS, and 230 STS), 136 (22.6%) had creation of loop ileostomy with subsequent reversal, and 42 (6.6%) had creation of end ileostomy with reversal. The median age at time of ICR was 35 (26–48) years. Patients primarily had stricturing (44.1%), penetrating (17.0%), or stricturing plus penetrating disease behavior (32.1%). Approximately a quarter of patients were actively smoking at time of ICR, 31.4% had a history of perianal disease, and 35.5% had at least 1 previous ICR. The median time to initial postoperative ileocolonoscopy and total postoperative follow-up time were 1.08 (0.66–2.13) and 4.17 (1.94–6.45) years, respectively.
There were differences in patient populations by anastomotic configuration (Table 1). Patients receiving ETE were younger (P = 0.002), more likely to have isolated colonic involvement of disease (P = 0.01), penetrating disease (57.3%; P = 0.02), preoperative biologic exposure (63.2%; P = 0.04), creation of ileostomy at time of ICR (42.6%; P < 0.001), postoperative biologic prophylaxis (28.7%; P = 0.02), and longer follow-up (P = 0.02).
Table 1.
Study population by anastomotic configuration at time of ICR
| Overall (N = 602) | End-to-end (N = 136) | End-to-side (N = 158) | Side-to-side (N = 308) | P value | |
|---|---|---|---|---|---|
| Age at CD diagnosis (yr), median (IQR) | 23.00 (17.00–30.00) | 20.00 (16.00–27.00) | 25.00 (18.00–33.00) | 24.00 (18.00–31.00) | 0.002 |
| Age at ICR (yr), median (IQR) | 35.00 (26.00–48.00) | 33.00 (25.00–46.25) | 37.00 (25.00–49.00) | 34.00 (27.00–48.25) | 0.254 |
| CD location, n (%) | 0.047 | ||||
| Colon | 21 (3.5) | 10 (7.4) | 6 (3.8) | 5 (1.6) | |
| Ileocolon | 325 (54.0) | 73 (53.7) | 86 (54.4) | 166 (53.9) | |
| TI | 256 (42.5) | 53 (39.0) | 66 (41.8) | 137 (44.5) | |
| CD behavior, n (%) | 0.003 | ||||
| Inflammatory | 41 (6.8) | 5 (3.7) | 18 (11.5) | 18 (5.8) | |
| Penetrating | 102 (17.0) | 35 (25.7) | 19 (12.1) | 48 (15.6) | |
| Stricturing | 265 (44.1) | 53 (39.0) | 63 (40.1) | 149 (48.4) | |
| Stricturing and penetrating | 193 (32.1) | 43 (31.6) | 57 (36.3) | 93 (30.2) | |
| Tobacco use history, n (%) | 0.393 | ||||
| Never | 355 (59.2) | 86 (63.2) | 98 (62.4) | 171 (55.7) | |
| Former | 105 (17.5) | 22 (16.2) | 22 (14.0) | 61 (19.9) | |
| Active | 140 (23.3) | 28 (20.6) | 37 (23.6) | 75 (24.4) | |
| History of previous ICR, n (%) | 0.931 | ||||
| 0 | 387 (64.5) | 82 (60.3) | 104 (65.8) | 201 (65.7) | |
| 1 | 122 (20.3) | 31 (22.8) | 33 (20.9) | 58 (19.0) | |
| 2 | 55 (9.2) | 14 (10.3) | 13 (8.2) | 28 (9.2) | |
| ≥3 | 36 (6.0) | 9 (6.6) | 8 (5.1) | 19 (6.2) | |
| Upper GI CD, n (%) | 103 (17.1) | 27 (19.9) | 26 (16.6) | 50 (16.2) | 0.631 |
| Sex (male), n (%) | 287 (47.7) | 72 (52.9) | 62 (39.2) | 153 (49.7) | 0.039 |
| History of perianaldisease, n (%) | 188 (31.4) | 51 (37.5) | 44 (28.0) | 93 (30.4) | 0.19 |
| Preoperative biologic exposure, n (%) | 321 (53.6) | 86 (63.2) | 79 (50.0) | 156 (51.1) | 0.036 |
| Stoma creation at time of ICR, n (%) | 176 (29.2) | 58 (42.6) | 40 (25.3) | 78 (25.3) | <0.001 |
| Ileostomy type, n (%) | 0.001 | ||||
| Primary anastomosis | 426 (70.8) | 78 (57.4) | 118 (74.7) | 230 (74.7) | |
| Loop | 136 (22.6) | 41 (30.1) | 32 (20.3) | 63 (20.5) | |
| End | 40 (6.6) | 17 (12.5) | 8 (5.1) | 15 (4.9) | |
| Postoperative biologic prophylaxis (≤3 mo), n (%) | 128 (21.3) | 39 (28.7) | 24 (15.2) | 65 (21.1) | 0.019 |
| Postoperative follow-up time (yr), median (IQR) | 4.17 (1.94–6.45) | 4.91 (2.29–6.49) | 3.23 (1.69–5.87) | 4.35 (2.01–6.77) | 0.019 |
CD, Crohn's disease; GI, gastrointestinal; ICR, ileocolonic resection; IQR, interquartile range
Natural history of anastomotic stricturing
In the study cohort, 110 (18.3%) patients developed AS at some point during their postoperative course. The median time to AS was 3.2 (1.4–5.0) years from time of bowel continuity restoration. In patients developing AS, 31 (28.2%) patients developed AS within 1.5 years of ICR, 51 (46.4%) within 1.5–5 years of ICR, and 28 (25.5%) after 5 years from ICR; with no differences in demographic or clinical characteristics (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/AJG/C986). The median number of postoperative ileocolonoscopies was (1–3) with no difference between patients who did or did not develop AS (P = 1).
The majority of AS (n = 77; 70%) on initial detection were traversable by either adult or pediatric colonoscope. Traversable AS (2.51 [1.08–4.45] years) was detected 1.4 years earlier than nontraversable AS(3.90 [2.12–4.95]years) (P = 0.048).At time of AS detection, 53 (48.2%) patients received balloon dilation, 6 (5.5%) had endoscopic stricturotomy, and 6 (5.5%) required surgical resection. The remaining patients (N = 45; 40.1%) did not receive any procedural intervention. Nontransversible AS at time of detection was associated with increased risk of surgical resection at any time during postoperative course (P < 0.01). In patients requiring AS intervention, patients with balloon dilation had AS detection 1.3 years earlier than those who needed endoscopic or surgical resection at time of detection (P = 0.28). After AS detection, an additional 14 of 83 (16.9%) patients who did not require endoscopic or surgical resection at initial AS and had clinical follow-up developed AS progression that required surgical resection because of intestinal obstruction.
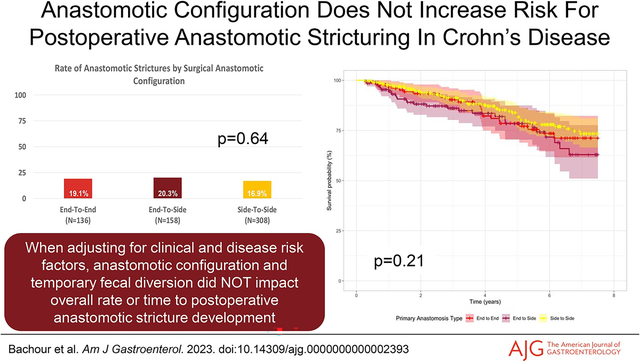
Of the 26 patients who required AS surgical resection, the distribution of initial endoscopic intervention in these patients consisted of 10 endoscopic balloon dilation, 4 endoscopic stricturotomy, 6 surgical resection, and 6 who received no intervention. In the 12 patients requiring endoscopic or surgical resection at time of AS detection, 11 (91.2%) had severe, nontraversable AS at initial detection. Median time to AS resection from time of ICR was4.0 years. In patients who did not require AS resection at time of detection, median time from AS detection to progression to resection was 1.4 years. The anastomotic configuration of patients developing AS consisted of 26 ETE, 32 ETS, and 52 STS (Figure 1a).
Figure 1.
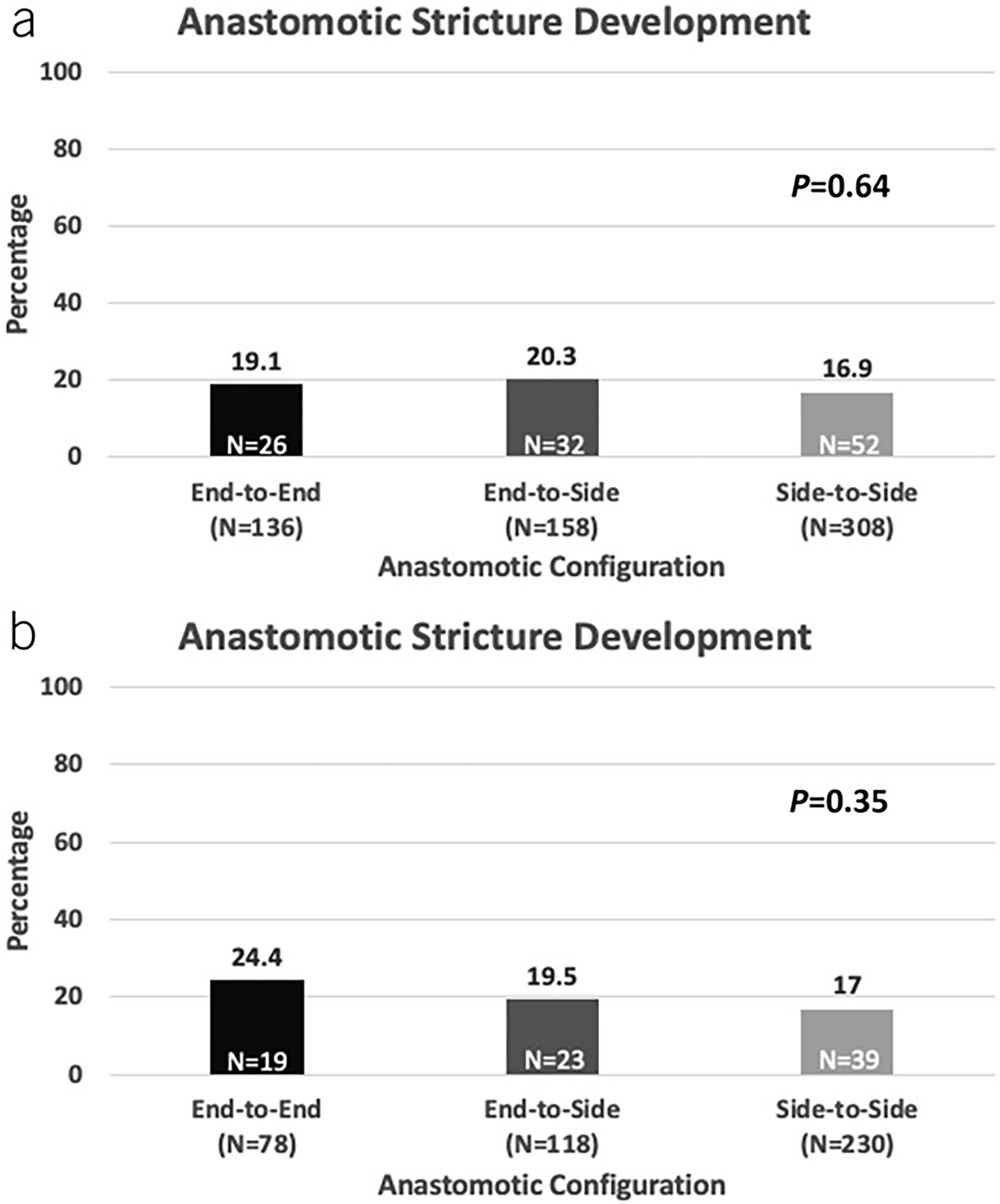
Rate of anastomotic stricture development in (a) entire study cohort and (b) patients receiving primary anastomosis only.
Most patients did not have coexisting ileal endoscopic POR at time of AS detection, with modified Rutgeerts score distribution of 64 i0/i1 (58.1%), 12 i2b (10.9%), 6 i3 (5.5%), and 28 i4 (25.5%). In patients with AS, 33 (30.0%) patients had endoscopic ileal POR (i ≥ i2b) detection on a previous ileocolonoscopy before AS detection.
Time to anastomotic stricturing detection analysis
On Kaplan-Meier survival analysis, anastomotic configuration was not associated with time to development of AS (P = 0.21) (Figure 2). On univariate analysis, age at CD diagnosis (P = 0.02) and preoperative stricturing CD behavior (P = 0.04) were associated with earlier AS development. On multivariable Cox proportional hazard regression modeling, neither anastomotic configuration, ileostomy creation at ICR, tobacco use, nor postoperative biologic prophylaxis were associated with time to AS (Table 2). Preoperative stricturing CD behavior was associated with decreased time to AS compared with patients without stricturing disease preoperatively (adjusted hazard ratio 1.78 [1.01–3.15]; P = 0.049) (Table 2).
Figure 2.
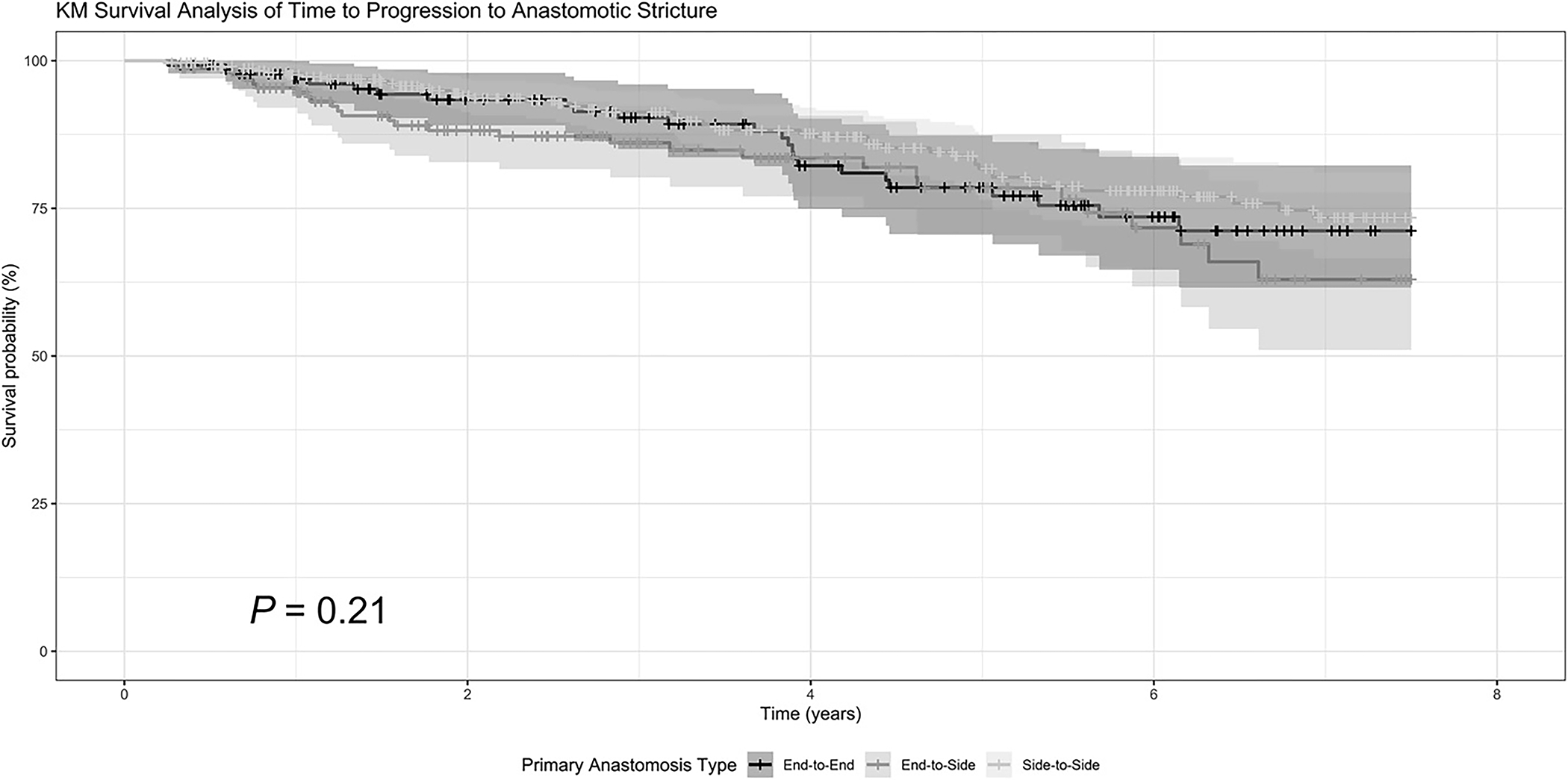
Kaplan-Meier (KM) survival curve of time to anastomotic stricture detection by anastomotic configuration. Statistical testing was performed using the log-rank test.
Table 2.
Multivariable Cox proportional hazard regression model of risk factors for time to AS
| aHR (95% CI) | P value | |
|---|---|---|
| Anastomosis (reference: end-to-end) | — | — |
| End-to-side | 1.57 (0.91–2.72) | 0.10 |
| Side-to-side | 0.90 (0.55–1.49) | 0.69 |
| Age at CD diagnosis (yr) | 0.98 (0.96–0.99) | 0.03 |
| Stricturing CD behavior | 1.78(1.003–3.15) | 0.049 |
| Postoperative biologic prophylaxis | 1.05 (0.63–1.75) | 0.85 |
| Ileostomy creation | 1.23(0.80–1.91) | 0.344 |
| Upper GI CD | 1.28 (0.79–2.05) | 0.32 |
| ≥2 ICR (including index ICR) | 1.31 (0.88–1.97) | 0.19 |
| Active smoking at time of ICR | 1.05 (0.67–1.67) | 0.82 |
aHR, adjusted hazard ratio; CD, Crohn's disease; CI, confidence interval; GI, gastrointestinal; ICR, ileocolonic resection.
Subgroup analysis of patients with primary anastomosis
In the present cohort, 426 patients received primary anastomosis at time of ICR. Patients who received primary anastomosis had less penetrating disease behavior (P < 0.001), upper gastrointestinal involvement of disease (P = 0.04), history of perianal disease (P < 0.001), and preoperative biologic exposure (P < 0.001) compared with patients who received a diverting ileostomy at time of ICR (see Supplementary Table 2, Supplementary Digital Content 1, http://links.lww.com/AJG/C986). Of the 426 patients, 81 (19.0%) developed AS (Figure 1b). The median time to AS was 3.4 (1.2–5.0) years.
On univariate time to AS analysis, patients with primary anastomosis who developed AS earlier were younger (P = 0.04), had preoperative ileocolonic disease (P = 0.03), and had a history of ICR (P = 0.02). However, on multivariable Cox proportional hazard regression modeling, no independent variables were associated with time to AS development (Table 3).
Table 3.
Multivariable Cox proportional hazard regression for time to AS in patients with primary anastomosis
| aOR (95% CI) | P value | |
|---|---|---|
| Anastomosis (reference: end-to-end) | — | — |
| End-to-side | 1.30 (0.69–2.44) | 0.41 |
| Side-to-side | 0.75 (0.43–1.31) | 0.32 |
| Age at CD diagnosis (yr) | 0.98 (0.96–1.0) | 0.07 |
| CD location (isolated ilealdisease) | 0.64 (0.39–1.03) | 0.07 |
| Stricturing CD behavior | 1.81 (0.89–3.65) | 0.10 |
| Postoperative biologic prophylaxis | 0.68 (0.36–1.31) | 0.25 |
| ≥2 ICR (including index ICR) | 1.51 (0.97–2.36) | 0.07 |
aHR, adjusted hazard ratio; CD, Crohn's disease; CI, confidence interval; ICR, ileocolonic resection.
DISCUSSION
In this retrospective cohort study, we found that approximately one-fifth of adult patients with CD who underwent ICR developed AS and nearly 20% of those required surgical resection. Neither anastomotic configuration nor temporary diversion was independently associated with time to AS, whereas preoperative stricturing disease behavior was associated with earlier AS. These data suggest that isolated AS development is relatively common in postoperative CD, has a significant associated morbidity, and that surgical anastomotic configuration and diversion decision-making do not impact the likelihood and time to AS development. To the best of our knowledge, this study is the first to evaluate risk factors for AS development.
In the current study, patients with previous known stricturing disease behavior and earlier age at CD diagnosis were at increased risk of earlier AS development. These findings were robust on multivariable modeling and were near significance on sensitivity analysis of patients receiving primary anastomosis—with non-significance most likely due to underpowering. These data suggest that AS may be part of the natural history of postoperative CD. After ICR, patients are considered to have surgical remission of their CD. Our study only found that 9% of patients had AS at time of index postoperative ileocolonoscopy. However, because patients were followed longitudinally, the prevalence of AS nearly doubled. This is consistent with the proposed pathophysiology of CD strictures, which suggests that over time, chronic transmural inflammation leads to a pleiotropic inflammatory marker response, resulting in increased extracellular matrix deposition and fibrosis (1,22). Similarly, this is what is commonly observed in preoperative CD because up to 30%–50% of patients develop stricturing disease overtime (1,3,23). Although a significant portion of patients develop stricturing disease during their CD course, to date, there are no known markers that directly predict stricturing disease; however, many clinical, serologic, microbiotic, and genetic markers have been established to predict more aggressive CD and increased risk of POR (12,23–29). Our data suggest that previous stricturing disease increases the risk of and decreases the time to AS, suggesting that luminal stricturing proclivities may also influence isolated anastomotic complications. In addition, our study did not find that ileal inflammation before or at time of AS, previous risk factors for endoscopic POR identified by our group (e.g., perioperative intraabdominal septic complications), nor indicators of treatment refractory patients (e.g., preoperative biologic exposure) were associated with AS. This suggests that AS development is driven by localized anastomotic inflammation independent of ileal inflammation.
In this study, we demonstrated that neither anastomotic configuration nor temporary diversion was associated with time to AS. Although patients receiving diverting ileostomy exhibited more aggressive CD disease (higher rates of penetrating disease, perianal disease, and biologic exposure), this did not translate to higher rates, increased severity, or decreased time to AS. Although temporary diversion of the fecal stream from the ileum and ileocolic anastomosis delays histologic and endoscopic recurrence in CD, there may be a concern that diversion may also promote a stricturing process, such as that seen in chronically diverted individuals (30,31). Previous studies have shown that fecal diversion reduces risk of recurrence while diverted; however, after stoma reversal, patients return to their baseline risk of POR (32–34). Although these studies did not note AS, our data are in concordance with this finding because patients who had temporary ileostomy did not have a different risk profile for AS. Similarly, although not specific to AS, previous randomized controlled trials and meta-analyses have shown that traditional anastomotic configurations (STS, STE, and ETE) have not been associated with increased risk of POR—which our group has previously confirmed (13,35–38). The current study extends this to suggest that anastomotic configuration is not associated with time to AS development. Of note, previous meta-analyses and our study do not include newer anastomotic configurations such as the Kono-S. In sum, surgical techniques and decision for temporary diversion do not impact the risk of AS.
Although this study suggests that AS is a relatively common phenomena in surgically managed CD, our data suggest that early detection and intervention of AS may help prevent repeat surgical resection for intestinal obstruction. We observed those whose initial AS was able to be treated with balloon dilation rather than resection had their AS detected over a year earlier. In patients who were treated with endoscopic balloon dilation at time of AS detection, a minority (20%) had progression of AS requiring surgical resection. These data are consistent with previous research that has shown that endoscopic balloon dilation is an effective intervention for traversable strictures (2,39,40). We admit the possibility of detection bias exists, with more mild strictures being detected earlier and amenable to balloon dilation and more severe, nontraversable strictures only amenable to surgical resection detected later. However, this would still argue for early and sustained postoperative monitoring to increase early AS detection, appropriate endoscopic intervention, to possibly mitigate the subsequent surgical risk. In addition, we were unable to classify how many patients had symptomatic AS, given the retrospective nature of this study and variability in clinical documentation.
This study is not without its limitations. ASs are commonly clinically encountered, but no validated endoscopic or radiographic definitions exist. Thus, we used a broad definition according to clinician interpretation. More stringent definitions may impact the observed prevalence and association. Clinical interventions along with indications and immediate outcomes were not standardized or universally documented to include for analysis. Given the number of observed outcomes, independent variables included in multivariable analyses, in addition to subgroup analyses aimed at evaluating AS interventions, were potentially underpowered for detection. However, to the best of our knowledge, this is the largest retrospective cohort specifically evaluating AS. Given this study was performed across a multihospital system including greater than 20 individual surgeons, surgeon data were unable to be included in association analyses because of concerns for overfitting. Histologic data regarding surgical resection at time of index ICR were not readily available. This may confound results because positive surgical margins for activity may predispose for AS development. Outside of postoperative biologic prophylaxis, other therapeutic data (e.g., nonsteroidal anti-inflammatory drugs) were not included in analyses, which may impact AS development and progression. Finally, the retrospective nature of the study and statistical modeling predispose to known limitations, possible residual confounding, and biases of design.
In conclusion, AS development is a relatively common complication of surgically managed CD. Patients with previous stricturing disease behavior are at increased risk of earlier AS. By contrast, anastomotic configuration or need for diverting ileostomy does not increase risk of AS. Increased endoscopic surveillance with early detection and intervention of AS may help prevent progression of AS severity and need for surgical resection. Prospective validation studies are needed to confirm these hypothesis-generating findings.
Supplementary Material
Study Highlights.
WHAT IS KNOWN
Anastomotic strictures not including the neoterminal ileum can occur in postoperative Crohn’s disease.
No known risk factors for development of anastomotic strictures have been reported.
WHAT IS NEW HERE
Anastomotic configuration and temporary diverting ileostomy do not increase risk of anastomotic stricture.
Anastomotic stricture severity can progress with time.
Previous stricturing disease behavior increases risk of anastomotic strictures.
Financial support:
S.P.B. received research funding from the Cleveland Clinic Lerner Research Institute Research Program Committee grant; however, all work was performed independent of this funding. There are no disclosures relevant to the data presented in this article.
Footnotes
CONFLICTS OF INTEREST
Potential competing interests: F.R. reports consulting and advisory boards for Adynovate, Agomab, Allergan, AbbVie, Boehringer Ingelheim, Celgene/BMS, CDISC, Cowen, Galmed, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Mestag, Metacrine, Morphic, Organovo, Origo, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Surmodics, Surrozen, Takeda, Techlab, Theravance, Thetis, UCB, Ysios, and 89Bio and research funding from the NIH, Helmsley Charitable Trust, Crohn’s and Colitis Foundation, UCB, Pliant, BMS, AbbVie, Pfizer, Boehringer Ingelheim, Morphic, and Kenneth Rainin Foundation. E.B. reports consulting for AbbVie, Gilead, Pfizer, and TARGET-RWE. J.A. reports receiving research grants from BioFire Diagnostics; consultancy fees or honorarium from BioFire Diagnostics and Janssen; and holds US patent 2012/0052124A1. S.D.H. reports consulting fees for Shionogi, Takeda, and Guidepoint and research grant support Crohn’s & Colitis Foundation. M.R. reports serving on the advisory board or consultant for AbbVie, Janssen, UCB, Takeda, Pfizer, Miraca Labs, Amgen, Celgene, Seres, Allergan, Genentech, Gilead, Salix, Prometheus, Lilly, TARGET Pharma Solutions, ALFASIGMA, S.p.A., and Bristol Myers Squibb (BMS). B.H.C. reports consulting fees for TARGET-RWE, AbbVie, Janssen, and Takeda. B.L.C. receives financial support for advisory boards and consultant for AbbVie, Celgene-Bristol Myers Squibb, Lilly, Pfizer, Sublimity Therapeutics, Takeda, and TARGET-RWE; CME Companies: Academy for Continued Healthcare Learning, Cornerstones, Medscape, PTCE, and Vindico; and Speaking: AbbVie. All other authors report no conflicts of interests.
Guarantor of the article: Benjamin H. Click, MD, MS.
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/AJG/C986.
REFERENCES
- 1.Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology 2017;152:340–50.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan WPW, Mourad F, Leong RW. Crohn’s disease associated strictures. J Gastroenterol Hepatol 2018;33:998–1008. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 4.Rieder F, Lawrance IC, Leite A, et al. Predictors of fibrostenotic Crohn’s disease. Inflamm Bowel Dis 2011;17:2000–7. [DOI] [PubMed] [Google Scholar]
- 5.Rieder F, de Bruyn JR, Pham BT, et al. Results of the 4th scientific workshop of the ECCO (Group II): Markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis 2014;8:1166–78. [DOI] [PubMed] [Google Scholar]
- 6.Tsai L, Ma C, Dulai PS, et al. Contemporary risk of surgery in patients with ulcerative colitis and Crohn’s disease: A meta-analysis of population-based cohorts. Clin Gastroenterol Hepatol 2021;19:2031–45.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murthy SK, Begum J, Benchimol EI, et al. Introduction of anti-TNF therapy has not yielded expected declines in hospitalisation and intestinal resection rates in inflammatory bowel diseases: A population-based interrupted time series study. Gut 2020;69:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frolkis AD, Dykeman J, Negrón ME, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: A systematic review and meta-analysis of population-based studies. Gastroenterology 2013;145:996–1006. [DOI] [PubMed] [Google Scholar]
- 9.Ramadas AV, Gunesh S, Thomas GAO, et al. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): A study of changes in medical treatment and surgical resection rates. Gut 2010;59:1200–6. [DOI] [PubMed] [Google Scholar]
- 10.Rutgeerts P, Geboes K, Vantrappen G, et al. Natural history of recurrent Crohn’s disease at the ileocolonic anastomosis after curative surgery. Gut 1984;25:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.e3. [DOI] [PubMed] [Google Scholar]
- 12.De Cruz P, Kamm MA, Prideaux L, et al. Postoperative recurrent luminal Crohn’s disease: A systematic review. Inflamm Bowel Dis 2012;18:758–77. [DOI] [PubMed] [Google Scholar]
- 13.McLeod RS, Wolff BG, Ross S, et al. Recurrence of Crohn’s disease after ileocolic resection is not affected by anastomotic type: Results of a multicenter, randomized, controlled trial. Dis Colon Rectum 2009;52:919–27. [DOI] [PubMed] [Google Scholar]
- 14.Luglio G, Kono T. Surgical techniques and risk of postoperative recurrence in CD: A game changer? Inflamm Intest Dis 2021;7:21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kono T, Fichera A. Surgical treatment for Crohn’s disease: A role of Kono-S anastomosis in the west. Clin Colon Rectal Surg 2020;33:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr ND, Pullan BR, Schofield PF. Microvascular studies in non-specific inflammatory bowel disease. Gut 1986;27:542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown SR,Fearnhead NS,Faiz OD,et al.TheAssociationofColoproctologyof Great Britain and Ireland consensus guidelines in surgery for inflammatory bowel disease. Colorectal Dis 2018;20(Suppl 8):3–117. [DOI] [PubMed] [Google Scholar]
- 18.Tominaga K, Kamimura K, Takahashi K, et al. Diversion colitis and pouchitis: A mini-review. World J Gastroenterol 2018;24:1734–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990;99:956–63. [DOI] [PubMed] [Google Scholar]
- 20.Bruining DH, Zimmermann EM, Loftus EV, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology 2018;286:776–99. [DOI] [PubMed] [Google Scholar]
- 21.Peduzzi P, Concato J, Kemper E,et al. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 1996; 49:1373–9. [DOI] [PubMed] [Google Scholar]
- 22.Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: A systematic review. Gut 2013;62:1072–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louis E, Michel V, Hugot JP, et al. Early development of stricturing or penetrating pattern in Crohn’s disease is influenced by disease location, number of flares, and smoking but not by NOD2/CARD15 genotype. Gut 2003;52:552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beaugerie L, Seksik P, Nion-Larmurier I, et al. Predictors of Crohn’s disease. Gastroenterology 2006;130:650–6. [DOI] [PubMed] [Google Scholar]
- 25.Crespi M, Dulbecco P, De Ceglie A, et al. Strictures in Crohn’s disease: From pathophysiology to treatment. Dig Dis Sci 2020;65:1904–16. [DOI] [PubMed] [Google Scholar]
- 26.Rieder F, Schleder S, Wolf A, et al. Serum anti-glycan antibodies predict complicated Crohn’s disease behavior: A cohort study. Inflamm Bowel Dis 2010;16:1367–75. [DOI] [PubMed] [Google Scholar]
- 27.Bachour SP, Shah RS, Rieder F, et al. Intra-abdominal septic complications after ileocolic resection increases risk for endoscopic and surgical postoperative Crohn’s disease recurrence. J Crohns Colitis 2022; 16:1696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherland LR, Ramcharan S, Bryant H, et al. Effect of cigarette smoking on recurrence of Crohn’s disease. Gastroenterology 1990;98:1123–8. [DOI] [PubMed] [Google Scholar]
- 29.Vasiliauskas EA, Kam LY, Karp LC, et al. Marker antibody expression stratifies Crohn’s disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut 2000;47:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology 1998;114:262–7. [DOI] [PubMed] [Google Scholar]
- 31.Vaughn BP, Moss AC. Prevention of post-operative recurrence of Crohn’s disease. World J Gastroenterol 2014;20:1147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet 1991; 338:771–4. [DOI] [PubMed] [Google Scholar]
- 33.Mennigen R, Heptner B, Senninger N, et al. Temporary fecal diversion in the management of colorectal and perianal Crohn’s disease. Gastroenterol Res Pract 2015;2015:e286315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gklavas A. Risk factors for postoperative recurrence of Crohn’s disease with emphasis on surgical predictors. Ann Gastroenterol 2017;30:598–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Connelly TM, Messaris E. Predictors of recurrence of Crohn’s disease after ileocolectomy: A review. World J Gastroenterol 2014;20:14393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caprilli R, Corrao G, Taddei G, et al. Prognostic factors for postoperative recurrence of Crohn’s disease. Gruppo Italiano per lo Studio del Colon e del Retto (GISC). Dis Colon Rectum 1996;39:335–41. [DOI] [PubMed] [Google Scholar]
- 37.Simillis C, Yamamoto T, Reese GE, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol 2008;103:196–205. [DOI] [PubMed] [Google Scholar]
- 38.Bachour S, Shah R, Lyu R, et al. DOP17. In high-risk Crohn’s disease patients, anastomosis configuration types have similar rates of endoscopic recurrence. J Crohns Colitis 2021;15:S055–S056. [Google Scholar]
- 39.Lopes S, Rodrigues-Pinto E, Andrade P, et al. Endoscopic balloon dilation of Crohn’s disease strictures-safety, efficacy and clinical impact. World J Gastroenterol 2017;23:7397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirai F. Current status of endoscopic balloon dilation for Crohn’s disease. Intest Res 2017;15:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


