Abstract
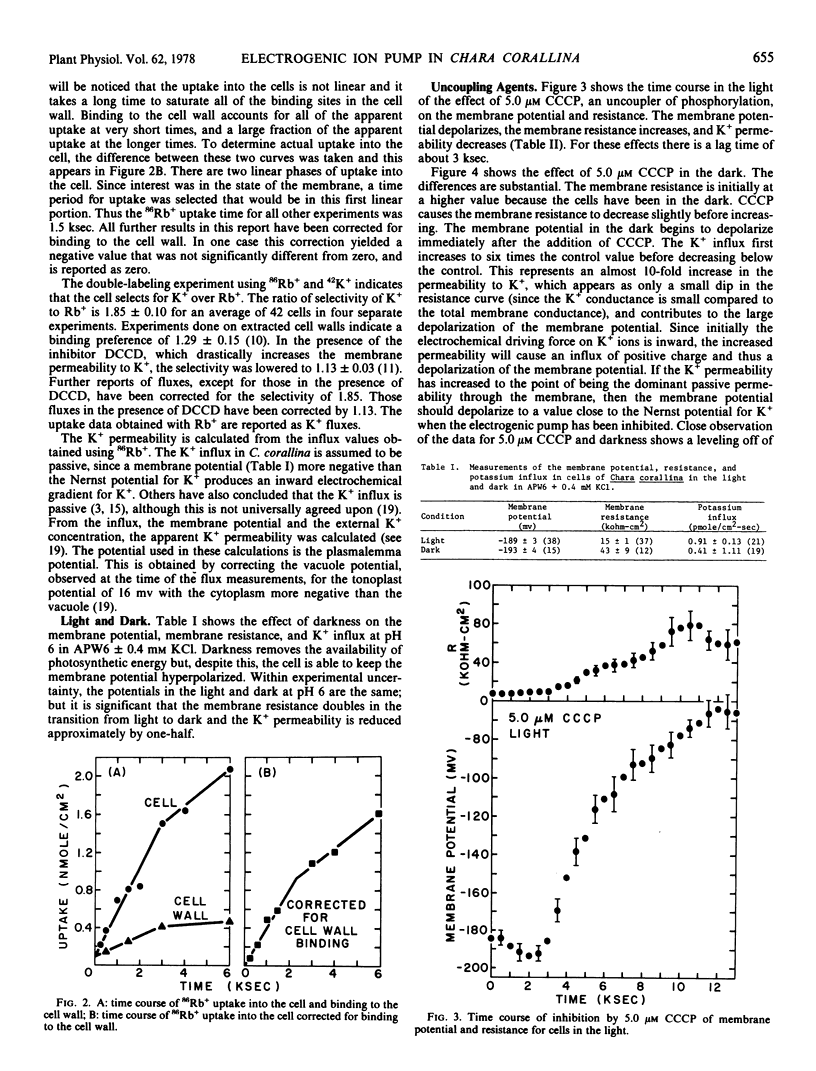
The effects of various inhibitors on the membrane potential, resistance, and K+ permeability of Chara corallina were measured, providing evidence that there is an electrogenic pump in the membrane. It was found that: (a) 5.0 μm carbonyl cyanide m-chlorophenyl hydrazone depolarizes the membrane potential and increases the membrane resistance. This inhibition is faster in the dark than in the light but the extent of inhibition is the same in both cases. (b) Fifty μm dicyclohexylcarbodiimide increases the resistance and the K+ permeability and depolarizes the membrane to a diffusion potential mainly controlled by K+. (c) Forty μm diethylstilbestrol and 0.1 mm 2,4-dinitrophenol increase the resistance and depolarize the potential to a value given by the Goldman diffusion equation. (d) Both 3-(3,4-dichlorophenyl)-1,1-dimethylurea and darkness (at pH 6) cause the membrane resistance to increase but neither has a large effect on the potential. 3-(3,4-dichlorophenyl)-1,1-Dimethylurea increases K+ permeability while darkness decreases it.
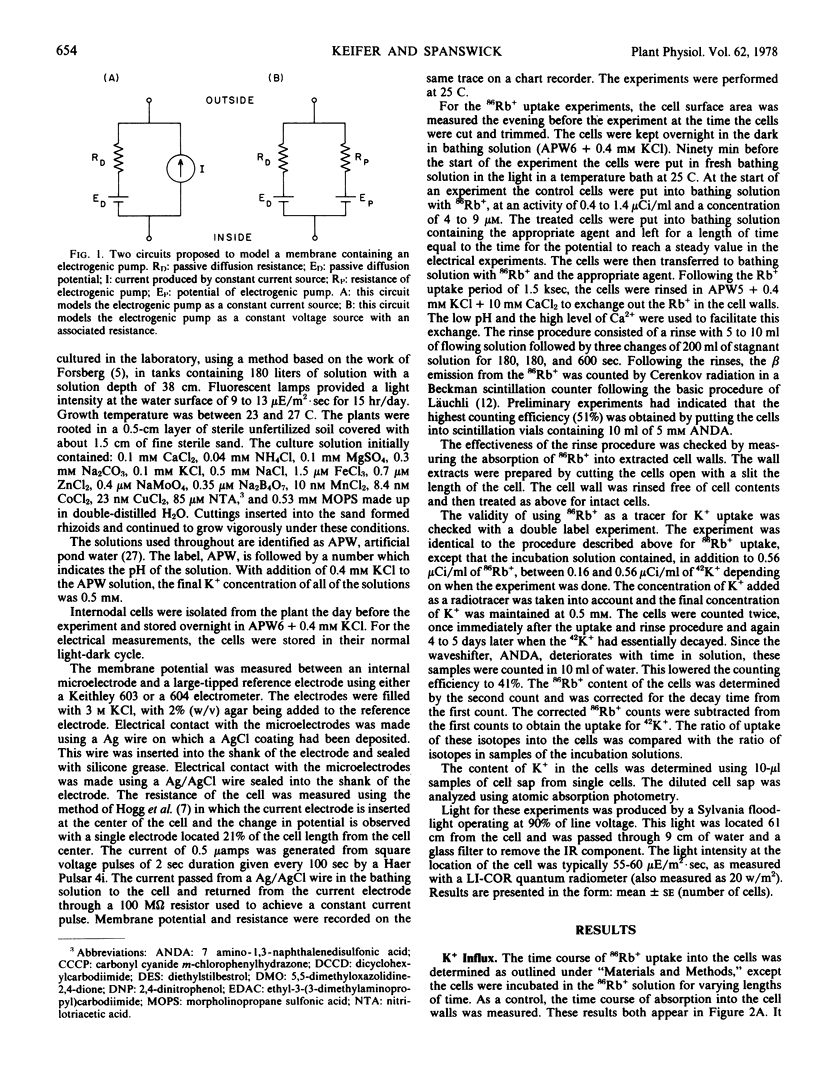
In all cases, the increase in resistance is interpreted as an inhibition of conductance through the electrogenic pump. As a consequence of this inhibition, the electrogenic component of the membrane potential is reduced, depolarizing the membrane. The electrogenic pump may be an H+-ATPase in the plasmalemma.
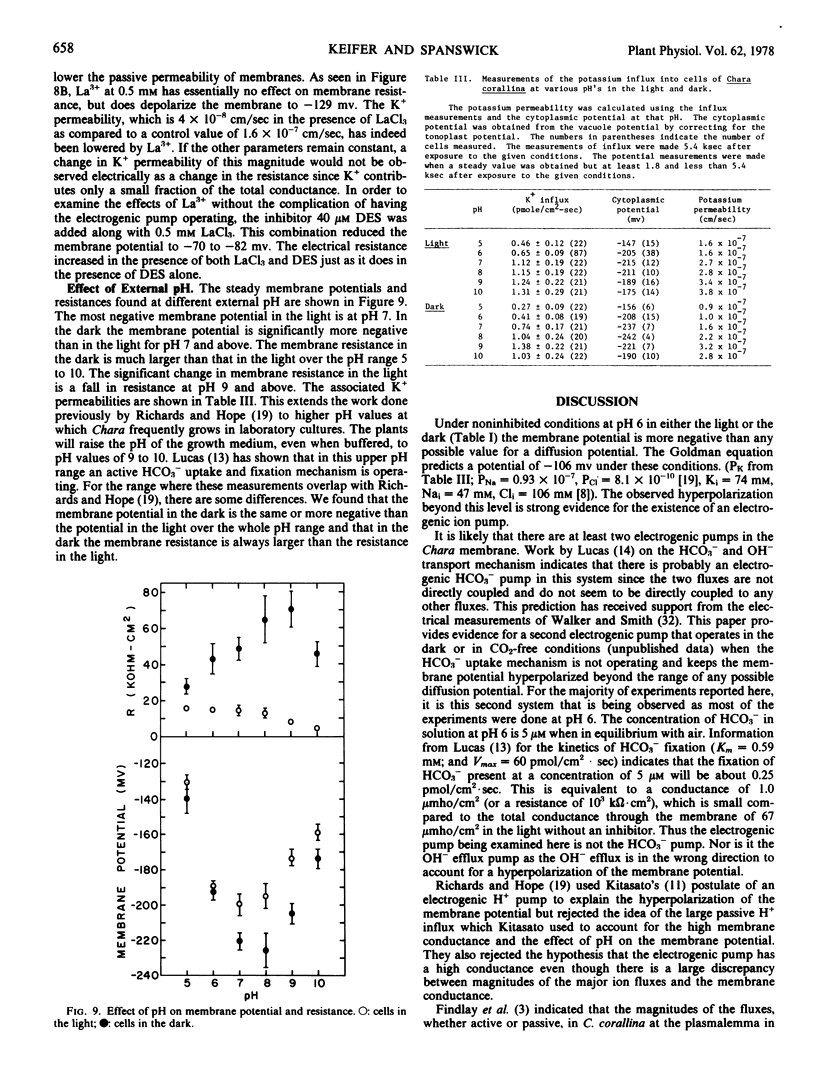
5,5-Dimethyloxazolidine-2,4-dione at 5.0 mm decreases the membrane resistance, by lowering the internal pH providing more substrate for the pump. La3+ decreased cation permeability and depolarized the membrane but, since it had little effect on the membrane resistance, it probably does not affect the electrogenic pump.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Findlay G. P., Hope A. B., Pitman M. G., Smith F. A., Walker N. A. Ionic fluxes in cells of Chara corallina. Biochim Biophys Acta. 1969;183(3):565–576. doi: 10.1016/0005-2736(69)90170-9. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Hogg J., Williams E. J., Johnston R. J. A simplified method for measuring membrane resistances in Nitella translucens. Biochim Biophys Acta. 1968 Apr 29;150(3):518–520. doi: 10.1016/0005-2736(68)90152-1. [DOI] [PubMed] [Google Scholar]
- Kitasato H. The influence of H+ on the membrane potential and ion fluxes of Nitella. J Gen Physiol. 1968 Jul;52(1):60–87. doi: 10.1085/jgp.52.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A. Radioassay for beta-emitters in biological materials using Cerenkov radiation. Int J Appl Radiat Isot. 1969 Apr;20(4):265–270. doi: 10.1016/0020-708x(69)90054-4. [DOI] [PubMed] [Google Scholar]
- Prochaska L. J., Gross E. L. The effect of 1-ethyl-3(3-dimethylaminopropyl)carbodiimide on calcium binding and associated changes in chloroplast structure and chlorophyll a fluorescence in spinach chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):126–135. doi: 10.1016/0005-2728(75)90211-x. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Effects of external cations on the intracellular potential. J Gen Physiol. 1965 Sep;49(1):69–92. doi: 10.1085/jgp.49.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slayman C. L., Long W. S., Lu C. Y. The relationship between ATP and an electrogenic pump in the plasma membrane of Neurospora crassa. J Membr Biol. 1973;14(4):305–338. doi: 10.1007/BF01868083. [DOI] [PubMed] [Google Scholar]
- Spanswick R. M. Evidence for an electrogenic ion pump in Nitella translucens. I. The effects of pH, K + , Na + , light and temperature on the membrane potential and resistance. Biochim Biophys Acta. 1972 Oct 23;288(1):73–89. doi: 10.1016/0005-2736(72)90224-6. [DOI] [PubMed] [Google Scholar]
- Takata M., Pickard W. F., Lettvin J. Y., Moore J. W. Ionic conductance changes in lobster axon membrane when lanthanum is substituted for calcium. J Gen Physiol. 1966 Nov;50(2):461–471. doi: 10.1085/jgp.50.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]


