ABSTRACT
Most Staphylococcus aureus isolates carry multiple bacteriophages in their genome, which provide the pathogen with traits important for niche adaptation. Such temperate S. aureus phages often encode a variety of accessory factors that influence virulence, immune evasion and host preference of the bacterial lysogen. Moreover, transducing phages are primary vehicles for horizontal gene transfer. Wall teichoic acid (WTA) acts as a common phage receptor for staphylococcal phages and structural variations of WTA govern phage-host specificity thereby shaping gene transfer across clonal lineages and even species. Thus, bacteriophages are central for the success of S. aureus as a human pathogen.
INTRODUCTION
The diversity of the Staphylococcus aureus species is mainly determined by mobile genetic elements, many of which are prophages or phage-related genomic islands. Strain evolution as a result of short- and long-term adaptation to diverse environments is tightly linked to phages. Many phages carry accessory genes coding for staphylococcal virulence factors, which are important for the success of certain S. aureus clonal complexes (CCs). Second, phages support the induction, packaging, and transfer of genomic islands (1, 2). This topic is reviewed elsewhere. Third, phage-mediated transduction is an efficient way to transfer not only extrachromosomal mobile elements, such as plasmids, but also chromosomal markers (albeit with lower efficiency). S. aureus is thought not to be naturally competent, so that recombination and horizontal gene transfer are mostly phage mediated and, to a lesser extent, conjugative. Here, we will first give a brief overview of previously used methods to classify S. aureus phages. Then we will mainly focus on the impact of temperate phages on the evolution of the bacterial host.
CLASSIFICATION OF S. AUREUS PHAGES
All known S. aureus phages belong to the order Caudovirales (tailed phages) and are composed of an icosahedral capsid filled with double-stranded DNA and a thin filamentous tail. Based on the complete genomes of 27 phages (3), S. aureus phages were assigned to three size classes: siphoviruses, with a genome size of 39 to 43 kb, podoviruses, with a smaller genome size of 16 to 18 kb, and myoviruses, with a genome size of 120 to 140 kb. This capsid and genome size-based classification correlates with the tail morphology as observed by electron microscopy: Podoviridae have a very short tail, Siphoviridae, a long noncontractile tail, and Myoviridae, a long contractile, double-sheathed tail (for recent reviews, see 4–6). All of the known temperate staphylococcal phages belong to the Siphoviridae, which are the primary focus of this chapter.
The siphovirus genomes are usually organized into six functional modules: lysogeny, DNA replication, packaging, head, tail, and lysis (7–9). The evolution of phage lineages was driven by the lateral gene transfer of interchangeable genetic elements (modules), which consist of functionally related genes. A functional module found in one phage can be replaced in another phage by a sequence-unrelated module that fulfils the same or related functions; often, genes within such modules travel together (10). Thus, multiple alignments of S. aureus phage genomes reveal a chimeric and mosaic structure resulting from horizontal transfer and recombination (3, 5, 9, 11–13).
Due to this modular structure, phage nomenclature and classification of the Siphoviridae are challenging and are a matter of debate. In Table 1 the different designations and properties of a representative set of S. aureus Siphoviridae are illustrated. In one approach phages were classified based on protein repertoire relatedness (5, 7). Thereby, Siphoviridae from S. aureus were clustered into six major clades. Clades 4 to 6 (Sfi21-like phages) share characteristic features with the capsid region of Escherichia coli HK97 phage and use the cos-site strategy for DNA packaging. Clades 1 to 3 (Sfi11-like pac-type phages) are related to the Bacillus subtilis SPP1 phage (7). The Sfi11/Sfi21-like grouping of Siphoviridae is based on genome analysis of lactococcal phages. Gene clusters extending from the DNA packaging genes to the tail genes were found to be represented by two unrelated configurations: one is characteristic of cos-site phages (prototype: phage Sfi21), and the other is characteristic of pac-site phages (prototype: phage Sfi11).
TABLE 1.
Classification and properties of selected S. aureus Siphoviridaee
| Phage name | Sfi typea | Cladea | Intb | Serob | Holinc | Virulenced | cos/pac | attB | Phage or attB in 8325c |
|---|---|---|---|---|---|---|---|---|---|
| Φ55 | Sfi11 | II | Sa1 | B | 438 | SAOUHSC_00851: 823130-823148 | |||
| ΦMu50B | Sfi11 | Sa1 | B | 438 | pac | TTCGAAATGGAAGGTAGTA | |||
| ΦETA | Sfi11 | 2 | Sa1 | B | 276 | eta | |||
| ΦSa1JH1 | Sa1 | B | 276 | ||||||
| ΦETA2 | Sa1 | B | 276 | eta | |||||
| Φ252A | Sa2 | A | 303 | SAOUHSC_01583: Φ12, 1463618-1508581 | |||||
| ΦSa2mw | Sfi21 | Sa2 | A | 303 | lukFS-PV | cos: CGGCGGGGGC | ACCATCACATTATGATGATATGTTTATTT | ||
| ΦPVL108 | Sfi21 | 5 | Sa2 | Fb | 303 | lukFS-PV | |||
| Φ2958PVL | Sfi21 | 4 | Sa2 | A | 303 | lukFS-PV | |||
| ΦPVL | Sfi21 | 5 | Sa2 | Fb | 303 | lukFS-PV | |||
| ΦSa2USA300 | Sfi21 | Sa2 | A | 303 | lukFS-PV | ||||
| ΦSLT | Sfi21 | 4 | Sa2 | A | 303 | lukFS-PV | |||
| Φ12 | Sfi11 | 4 | Sa2 | A | 303 | ||||
| Φ13 | Sfi21 | 5 | Sa3 | Fb | 255a | sak, chp, scn | cos: CGGAGCAGA | TGTATCCAAACTGG | hlb: Φ13, 2031923-2074632- |
| ΦN315 | Sfi21 | 6 | Sa3 | Fa | 255a | sep, sak, chp, scn | |||
| ΦSa3mw | Sfi21 | Sa3 | Fb | 255a | sek, seq, sea, sak, scn | ||||
| ΦNM3 | 6 | Sa3 | Fa | 255a | sea, sak, chp, scn | ||||
| ΦMu50A | Sfi21 | ND | Sa3 | Fa | 255a | sea, sak, scn | |||
| ΦSa3USA300 | Sa3 | Fa | 255b | sak, chp, scn | |||||
| ΦSa3JH9 | Sa3 | Fa | 255b | sak, chp, scn | |||||
| Φ42E | Sfi21 | IV | Sa3 | A | 255a | ||||
| ΦSa4JH1 | Sa4 | A | 438 | CATGTAATTCC | SAOUHSC_00958 (htrA): 933127-933137 | ||||
| ΦSa4ms | Sa4 | A | 303 | ||||||
| Φ11 | Sfi11 | 1 | Sa5 | B | 438 | pac | CTTCCCATGG | SAOUHSC_02090: Φ11, 1923398- 1967013 | |
| ΦPV83 | Sa5 | Fb | 255a | lukM, lukF-PV | |||||
| Φ187 | Sfi11 | I | Sa5 | L | 255a | ||||
| Φ29 | Sfi11 | II | Sa5 | B | 438 | ||||
| ΦNM1 | ND | 1 | Sa5 | B | 303 | ||||
| Φ88 | Sfi11 | II | Sa5 | B | 438 | ||||
| Φ52A | Sfi11 | II | Sa6 | B | 438 | SAOUHSC_00300 (geh): 316250-316257 | |||
| Φ80 | Sfi11 | 2 | Sa6 | B | 438 | ||||
| ΦNM4 | 2 | Sa6 | B | 303 | ATCATACAAGGATGGGAT | ||||
| ΦSa6JH9 | Sa6 | B | 438 | ||||||
| ΦCOL | Sa6 | A | 303 | ||||||
| L54a | Sa6 | A | ? | ||||||
| Φtp310-2 | Sa6 | A | 303 | ||||||
| Φ53 | Sfi11 | I | Sa7 | B | 303 | Intergenic, downstream SAOUHSC_01079 (isdB): 1042159-1042167 | |||
| Φ80alpha | Sfi11 | 1 | Sa7 | B | 303 | pac | AGGTATCTG | ||
| Φ85 | Sfi11 | I | Sa7 | B | 303 | ||||
| ΦNM2 | Sa7 | B | 438 | AGGTATCTG | |||||
| Φ6390 | Sa7 | B | 255a | sak | |||||
| Φ92 | Sfi11 | II | Sa7 | B | 438 | ||||
| ɸSaov2 | Sa7 | F | |||||||
| ΦRF122 | Sa8 | B | 438 | pac | CGGAAGGTAAGGGA | SAOUHSC_T00050 (trnaS): 1864312-1864325 | |||
| Φ96 | Sfi11 | 2 | Sa9 | B | 303 | pac | ND | tmRNA, 788659 | |
| ΦEW | Sfi11 | 3 | Sa11 | ND | 435 | pac | ND | SAOUHSC_00581: 584240 |
Classification based on reference 5.
Classification based on reference 12.
Location of the native phage or the attB site in the genome of reference strain 8325 (GenBank accession number NC_007795.1).
virulence genes
Abbreviations: ND, Not determined; tmRNA, transfer messenger RNA.
In another approach, S. aureus Siphoviridae were classified according to polymorphism of the int gene and the modular composition of lysogeny, regulation, replication, structural, and lytic modules (9, 12) (Table 1). The modules can be defined by multiplex PCR or sequence analyses of selected genes within the modules. S. aureus prophages were primarily classified on the basis of int gene homology (9, 12, 13). By including information on the allelic variation of other modules, a further meaningful subdivision can be achieved. A similar approach to the general classification of bacteriophages has been proposed previously (14, 15). Kahánková et al. (9) established a mulitplex PCR assay to distinguish between different types of integrase (10 types), antirepressor (five types), replication proteins (four types), dUTPase (four types), portal protein (eight types), tail appendices (four types), and endolysin (four types). The proposed extended typing scheme covering seven major genomic modules enables not only the differentiation of phages but also the design of a classification system. For the basic description and rapid differentiation of a phage strain, the serological group type, the int type, and the endolysin type are the most relevant (located at the left side, the middle, and the right side of the prophage genomes). Using a simplified scheme, e.g., phage ɸ11 can be classified as Sa5int-Ba-ami1 or phage ɸ13 as Sa3int-Fb-ami3 (9). Despite the multiple mosaic variants, there are close links between particular modules; e.g., all phages using polA for replication are serogroup A.
There are several reasons to classify S. aureus prophages primarily based on int polymorphism. First, nucleotide sequences are well conserved within int groups, making the gene an ideal target for PCR amplification. The int grouping has good discriminatory power, reflecting the diversity of the S. aureus phage population. Recombination and exchange of certain modules seem to occur more often between phages of the same Sa-int group than between phages of different Sa-int groups (16). Second, the int typing allows for prediction of the chromosomal location of the prophage. Last, the int type is closely linked to the virulence gene content of the prophage and can therefore convey information about the pathogenic potential of the bacterial lysogen (12). Most of the S. aureus phages can be assigned to one of the major Sa-int types 1 to 8.
PHAGE INTEGRATION/EXCISION
All prophages integrate to position the int gene in close proximity to the bidirectional origin of chromosome replication. Based on amino acid sequence homology and catalytic residues, most integrases belong to the tyrosine recombinase type family. Only the integrases of Sa7int phages were found to belong to the serine recombinase type family (12). Despite the usually strong association of the int type with the integration site, there are also events where a phage may integrate in an illegitimate attachment site. This phenomenon was described to occur for Sa3int phages during chronic lung infections of cystic fibrosis patients (17). Under these conditions, the reconstitution of the phage-interrupted hlb gene may be an advantage. When these mislocated phages were induced and used to reinfect S. aureus in vitro, the phages reintegrated at their dedicated attachment site within hlb. Sa3 phages are only rarely found in livestock-associated S. aureus strains of CC398. In these strains the canonical attB site within hlb is altered, and therefore Sa3int phages integrate at diverse alternative attB sites (18, 19). The mechanism for excision of S. aureus Siphoviridae is much less well understood. Excision followed by replication may result not only in bacterial lysis and phage release but also in a process termed “active lysogeny.” This term was recently introduced to describe instances where prophages temporally and reversibly excise from the chromosome without lysing the bacterial host (20). This might be seen as a form of bacterial gene regulation that possibly improves bacterial fitness. Evidence for active lysogeny in S. aureus comes from a recent sequencing approach to detect extrachromosomal phages in S. aureus (21). Furthermore, it could be demonstrated that phages are readily induced under infectious conditions (17, 22–25). Analysis of isolates from cystic fibrosis patients revealed that translocation of the Sa3int phages often leads to a splitting of the bacterial population (17) into Hlb-positive (phage-cured) and phage-positive fractions. Both the phage-encoded virulence factors and Hlb are secreted factors; thus, functional complementation can be assumed.
PHAGE-BACTERIAL RECOGNITION
Adsorption to the bacterial host is the first critical step within the phage life cycle. Interaction of the receptor-binding protein (RBP) of the phage and its receptor initiates the infection cycle and, importantly, determines the host range and specificity (26). Phage receptors have to satisfy the following distinct requirements to be suitable for viral attachment. (i) Accessibility: phage receptors have to be accessible to the phage by random Brownian motion, flow, or diffusion. (ii) Abundance: with attachment being a stochastic process, the abundance of the receptor must be high enough to permit a sufficient probability of RBP-receptor contact. (iii) Constancy: the chemical composition of the receptor must be stable enough to allow evolutionary adaption of the phage to possible changes in the receptor appearance. Wall teichoic acid (WTA) of S. aureus fulfills all three requirements imposed on a proper phage receptor and was shown to act as the primary phage receptor. It is a major component of the cell wall and is expressed by all S. aureus strains. Most S. aureus strains produce WTA comprised of ribitol-phosphate (RboP) repeats. The complex biosynthesis of WTA is reviewed elsewhere (27, 28). WTA is covalently attached to the peptidoglycan of the cell wall (29, 30). Further derivatization of WTA is achieved in two ways. Attachment of N-acetyl-glucosamine residues (GlcNAc) occurs at the C4-position of the RboP unit by two glycosyltransferases, TarS and TarM, which attach GlcNAc in the β-position and α-position, respectively (31, 32). Recently, a third prophage-encoded glycosyltransferase, named TarP, was identified (33). Unlike TarS, TarP catalyzes the attachment of GlcNAc in the β-1,3-position.
The second modification of WTA is attachment of d-alanine residues at the C2-position by the Dlt-machinery (34). In contrast, a completely different type of WTA, composed of a glycerol-phosphate (GroP) repeating unit modified with α-N-acetyl-galactosamine (GalNAc) residues is found in S. aureus strains belonging to CC395 (35). This GroP WTA resembles the WTA structure found in many coagulase-negative staphylococci (36). Early studies identified B. subtilis mutants lacking WTA or WTA glycosylation. Those mutants appeared to be resistant against certain B. subtilis bacteriophages (37, 38). It was subsequently shown that the sugar moiety of WTA is also important for S. aureus phage interaction. Treatment of S. aureus with exo-β-acetylglucosaminidase or use of WTA glycosylation mutants leads to a decrease in phage binding (39, 40).
Although a majority of publications describe only carbohydrate-based phage interactions (WTA in combination with peptidoglycan structures), work by Nordström et al. (41) suggested that additional proteinaceous factors, prominent among them, surface protein A, interfere with phage adsorption. To our knowledge, this is the only account of surface protein phage interactions reported for S. aureus. Interestingly, a secondary protein receptor besides the primary receptor WTA has been described for the Gram-positive model organism B. subtilis. The lytic phage Spp1 adsorbs first in a reversible manner to WTA, which accelerates an irreversible interaction with the membrane receptor YueB and injection of viral DNA (42). A similar phenomenon was described for the membrane protein PIP, which serves in lactococcal species as a secondary phage receptor (43). However, PIP homologues in S. aureus have not been shown to be necessary for phage adsorption (44). Thus, so far, there is no clear evidence for a secondary proteinaceous receptor in S. aureus.
The advent of bacterial genetics allowed further insights into the molecular basis of bacteria-phage interactions. An early study suggested that besides WTA, a different anionic polymer of the S. aureus cell envelope, lipoteichoic acid, might serve as a receptor for the tail protein ORF636 of S. aureus phage ΦSLT (45). Lipoteichoic acids (LTA) consist of d-alanylated glycerol phosphate repeating units attached to the cell membrane of S. aureus (46). However, using an LTA-deficient mutant strain of RN4220, no difference in the infectivity of phages expressing homologues of the tail protein of ΦSLT could be observed (47). In contrast to the LTA-deficient mutant, gene deletion of tagO, the first gene of the WTA biosynthesis pathway, leads to a phage-resistant phenotype (47, 48). By creating a transposon mutant library, Xia et al. (32) were able to isolate the RN4220 mutant K6, which is deficient in WTA glycosylation. K6 carries a transposon interrupting the gene coding for the α-glycosyltransferase TarM. K6 additionally carries a premature stop codon in tarS coding for the β-glycosyltransferase (31). K6 was resistant to a wide range of siphoviruses (47). Interestingly, as reviewed later, lytic myoviruses (Φ812 and ɸK) were able to infect K6 despite the lack of WTA glycosylation.
By using defined knockouts of the two identified ribitol-phosphate glycosyltransferases, tarM and tarS, it could be demonstrated that siphoviruses of serogroup B do not seem to differentiate between α- or β-GlcNAcylation at the ribitol C4 position (31, 44) (Fig. 1A). The receptor promiscuity of siphoviruses might reflect their role as the main vectors of horizontal gene transfer. Of note, Winstel et al. (49) demonstrated that S. aureus pathogenicity islands (SaPIs) can be transferred between strains expressing α- or β-GlcNAcylated WTA. Notably, β-1,3-GlcNAcylation by TarP seemed to reduce the infection and SaPI-transfer capacity of serogroup B siphoviruses (33) (Fig. 1A). More importantly, the Winstel and coworkers publication established the compatibility of phage and host receptors as a key driver of cross-species horizontal gene transfer (49). Even distantly related bacterial species such as Listeria monocytogenes can engage in horizontal gene transfer with S. aureus (49, 50), as long as they express a compatible phage receptor. In contrast, lack of RboP-GlcNAc WTA excludes bacteria from the exchange of genetic information. For instance, S. aureus PS187 (CC395), equipped with GroP-type WTA, is cut off from horizontal gene transfer with RboP-WTA-expressing S. aureus, but is able to engage in exchange with coagulase-negative species that share a similar GroP-type WTA (35, 49). Interestingly, ectopic expression of RboP-type biosynthesis genes in coagulase-negative staphylococci with GroP-WTA rendered them susceptible to S. aureus phages (49). Hence, it can be speculated that RBP-receptor incompatibility is a major hindrance for horizontal gene transfer between S. aureus and coagulase-negative staphylococci. The other two described classes of morphological groups, namely Myoviridae and Podoviridae, show more diverse receptor specificities, that are discussed later in the article.
FIGURE 1.
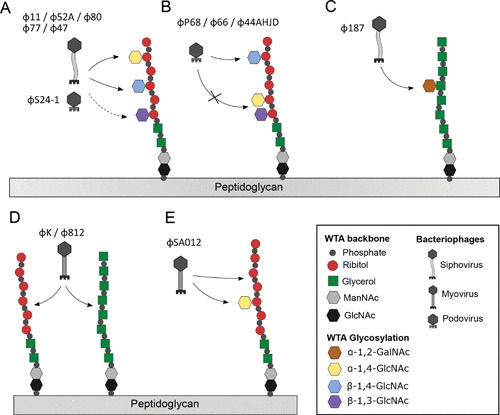
Receptor specificity of S. aureus phages. (A) Siphoviruses Φ11, Φ80, Φ52A, Φ47, and Φ77 and podovirus SA24-1 recognize α-or β-1,4-GlcNAc-RboP WTA. β-1,3-GlcNAc-WTA is adsorbed to less strongly by Φ80, Φ52A, and Φ11. (B) Podoviruses ΦP68, Φ44AHJD, and Φ66 bind to β-1,4-GlcNAc-RboP WTA and are blocked by β-1,3-GlcNAc or α-1,4-GlcNAc modifications. (C) Siphovirus Φ187 binds to α-GalNAC-GroP. (D) Myovirus ΦK, Φ812, attache to the backbone of GroP and/or RboP. (E) ΦSA012 recognizes both the RboP WTA backbone and α-1,4-GlcNAc-RboP by two different RBPs.
Returning to the postulated requirements for a phage receptor (accessibility, abundance, and constancy), one can audit whether they hold true for WTA as the phage receptor of S. aureus. WTA is an exposed cell surface molecule attached to the peptidoglycan (51), which would allow easy access to phages. The negative charge of WTA, conferred by phosphate residues, might allow polar or ionic interaction with RBPs. LTA, due to its presence in the more internal cell membrane of S. aureus, might therefore be of less utility as a receptor for bacteriophages. Additionally, WTA is a highly abundant glycopolymer that constitutes up to 60% of the cell wall mass in many Gram-positive bacteria. It is a key component of the cell wall, and no natural isolates of S. aureus without WTA have been reported so far. The indispensability and abundance of WTA ensure a high probability of phage-receptor contact, which is crucial for the adsorption process. Coagulase-negative bacteria, especially Staphylococcus epidermidis, show an increased diversity of WTA glycosylation patterns (36, 52). S. aureus appears to encode only one housekeeping glycosyltransferase, TarS. The tarS gene is found in almost all S. aureus genomes (53). The second glycosyltransferase, TarM, appears to have been acquired very early in S. aureus evolution (54). However, certain clonal lineages, such as CC5 and CC398, seem to have deleted tarM during their emergence or may have acquired phage-encoded tarP. The introduction of more sophisticated WTA glycosylation patterns might lead to less phage interaction and therefore to a decrease in the ability to participate in horizontal gene transfer. The described receptor constancy of WTA together with the widespread absence of clustered regularly interspaced short palindromic repeat (CRISPR)/Cas systems allows rapid acquisition and exchange of genetic elements by phages or phage-like particles (SaPIs), which ensures the role of S. aureus as a major human pathogen.
OCCURRENCE AND ROLE OF ACCESSORY PHAGE GENES
Although phages may be regarded as selfish elements, bacteria have learned to use them for their own purposes, and lysogeny can be regarded as a motor for short-term evolution. In many pathogens, phages provide the bacteria with additional genes that enable them to establish a new lifestyle. In S. aureus, several such phage-encoded virulence factors have been described, an observation originally described as phage conversion. Genes coding for Panton-Valentine leukocidin (lukSF) exfoliative toxin A (eta) (55), the cell-wall anchored protein SasX (56), and the immune evasion cluster (IEC) composed of enterotoxin S (sea), staphylokinase (sak), the chemotaxis inhibitory protein (chp), and the staphylococcal complement inhibitor (scn) (57) are the best-characterized phage-encoded virulence factors in S. aureus. A transposon mutant library was screened for virulence genes, and phage-encoded virulence genes were detected on all four prophages from strain Newman (58). Moreover, small RNAs involved in gene regulation (SprD = teg14) (59) or coding for the type I toxin-antitoxin system (SprF1/SprG1) (60) are encoded on Sa3int phages. With the availability of new phage and S. aureus genomes, new putative phage-encoded virulence genes (e.g., those coding for putative Clp protease or phospholipase) are being discovered and are awaiting functional analysis (18, 61–63).
Interestingly, accessory genes are strongly associated with phages of certain int groups and are localized at the left or (more frequently) right ends of the phage. There is a link between the encoded virulence factors, the int module, and the lytic module (holin and amidase genes), which are localized at the opposite end of the prophages (9). One may assume that it is evolutionarily beneficial to interchange this whole unit. Of note, these modules are in close proximity after phage excision in the circular and/or concatamer form of the phage. The close link of the lytic module and the inserted virulence factors is perhaps favored to optimize the phage control of the expression of the virulence genes (64). For instance, the expression of the virulence genes becomes cotranscribed with the late phage genes upon phage induction (64–66).
Sa1int Phages Carrying eta
The exfoliative toxins are virulence factors of S. aureus that cause bullous impetigo and its disseminated form, staphylococcal scalded-skin syndrome. The clinical symptoms vary from blisters anywhere on the body to multiple lesions complicated by conjunctivitis and staphylococcal scalded-skin syndrome (67). The exfoliative toxin A gene (eta) is carried in the genomes of Sa1int phages (9, 12, 13). An eta homolog is also carried by SaPI2 but is not known to be functional (1). However, these phages can be differentiated into at least six types due to variation in different modules (68). These eta-phages were associated with outbreaks of methicillin-resistant S. aureus (MRSA) and methicillin-susceptible S. aureus strains of various CCs in Japan and the Czech Republic (67, 69, 70) and are also present in a subpopulation of CC121 strains associated with superficial infections (25, 71).
Sa2int Phages Carrying lukSF
lukSF encodes the bi-component leucotoxin PVL, which targets human phagocytes through interaction with the complement receptors C5aR1 and C5aR2 (72). lukSF-encoding phages are strongly associated with skin and soft tissue infection and necrotizing pneumonia, which can also affect young, immunocompetent people (73–76). The majority of lukSF-carrying phages are Sa2int phages (66, 77–82). These phages are integrated within a conserved ORF which is surrounded by a cluster of tandemly repeated genes. In CC80 strains, phage induction led to the acquisition of host DNA into the phage genome probably due to a homologous recombination event between direct repeats of the two paralogous genes adjacent to the phage integration site (83). Phage excision was accompanied by an additional chromosomal deletion in this region. PCR-based typing schemes were established and modified to further subtype the lukSF-encoding phages (78, 84, 85). These studies confirm the high mosaicism of phage genomes and show that different S. aureus lineages have acquired a diverse set of lukSF phages. The combination of lukSF phages with methicillin resistance is characteristic of community-associated MRSA strains that are spreading in various continents. Strains of the most prominent USA300 lineage (CC8 isolates) are mainly spreading in North America, whereas in Europe CC80 strains are spreading, in Asia, CC59 strains, in the Asia Pacific region, CC30 strains, and in Australia, CC93 strains are more prevalent. The global spread of different S. aureus clones containing different lukSF-carrying phages supports the idea that PVL provides some fitness advantage to the bacterial host. The most common feature of all these strains is the strong association of lukSF phages and superficial, recurrent skin infections (75).
Sa3int Phages Carrying the IEC
Sa3int phages are by far the most prevalent S. aureus phages. Up to 96% of human nasal isolates were found to carry Sa3int phages integrated into the hlb gene (17, 86). Hlb can modify endothelial cells and platelets by both toxin sphingomyelinase and biofilm ligase activities, thereby increasing infection severity (87). The hlb-converting phages carry genes representing the IEC (sea, sak, chp, and scn) (57), coding for highly human-specific virulence genes. SEA has been described to modulate the function of chemokine receptors such as CCR1, CCR2, and CCR5 (76). CHIPS blocks two G-protein coupled receptors involved in chemotaxis and phagocyte activation (C5a and the formylated peptide receptor) (88). SAK is a potent plasminogen activator with pleiotropic function including fibrinolysis and antiopsonic activity. The latter occurs through degradation of immunoglobulin G and C3b/C3bi on the surface of staphylococci (89). SCIN is a specific inhibitor of the C3 convertase of the complement system (90). Seven IEC variants were discovered, carrying different combinations of sea (or sep), sak, chp, and scn. The genes’ order was conserved and always in the same 5′ to 3′ orientation. Scn was the only gene present in all IECs analyzed, whereas, e.g., sea was detectable in only 27% of the IECs (57). Interestingly, genes coding for a type I toxin-antitoxin system (SprF1/SprG1) are also localized within the IEC gene cluster (60).
Sa7int Phages Carrying sak
As an exception to the rule, sak could be detected not only on Sa3int phages but also occasionally on Sa7int phages. Curiously, such a sak-encoding Sa7int phage is found in derivatives of the laboratory strain 8325-4 (17). The widely used phage-cured strain 8325-4 was somehow lysogenized with such a phage and has since been distributed to laboratories worldwide (designated strain RN6390 or ISP479C). A similar phage was detected in most of the prevalent hospital-associated MRSA clones (belonging to the ST5 lineage) isolated in southern Germany (91). These strains were highly successful over the years and were quickly diversifying, as illustrated by differences in pulsed-field gel electrophoresis patterns and antibiotic susceptibility. Notably, these isolates can be clearly discriminated from other CC5 isolates (ST225, a single-locus variant of ST5) circulating in Germany, which are characterized by the high prevalence of a typical Sa1int phage (91, 92).
SasX-Carrying Phage in MRSA
The assumption that mobile genetic elements promote the spread of bacterial clones was emphasized by the emergence of highly epidemic MRSA strains carrying a phage harboring a newly identified cell wall-anchored virulence factor, SasX (56). SasX promotes nasal colonization, bacterial aggregation, and virulence. sasX, similar to other phage-carried virulence genes, is located as an accessory gene at the right end of a phiSPβ-like prophage. The genome size of 127 kb is significantly larger than that of a typical S. aureus siphovirus and highly similar to a prophage found in S. epidermidis strain RP62A (93), indicating that the phage and thus a new virulence trait was acquired from S. epidermidis. This phage is spreading between S. aureus strains and is also found in MRSA strains of the CC5 lineage (56). It is unclear whether this phage and other genetic elements managed to cross the species barrier and, if so, how.
TarP-Carrying Phages
Recently, the phage-associated WTA-glycosyltransferase TarP was found to glycosylate the WTA in an alternative way (33). The tarP gene is located in the lysogenic module of three different S. aureus prophages with integrase groups, Sa1int, Sa3int, and Sa9int. The tarP-Sa3int phage, ΦN315, additionally encodes the IEC discussed above. tarP-encoding phages can be found in clonal lineages CC5 (hospital-associated clones with Sa3int TarP-phages) and CC398 (livestock-associated clones with Sa1int and Sa9int TarP-phages). Modification of WTA by TarP lead to reduced opsonization by IgG in human sera. The immune evasion capabilities of TarP were reflected by lower immunogenicity of TarP-modified WTA in comparison with TarS-modified WTA.
ROLE OF PHAGES IN HUMAN ADAPTATION OF ANIMAL ISOLATES
S. aureus colonizes a variety of animal species and adapts to particular species through changes in the core genome as well as potential phage-encoded virulence genes (94). However, transmission of strains between human and animal reservoirs also occurs. Since the early 2000s MRSA strains with the sequence type ST398 were described as colonizing pigs and also causing infections in humans living in close contact with livestock (95, 96). Strains belonging to the CC398 lineage are commonly found in livestock and are resistant to multiple antibiotics but lack several important virulence factors (97–99). More recently, human to human transmission of CC398 strains was reported. Critical in this jump are Sa3int phages that generally are absent in CC398 strains but are present in most livestock strains infecting humans without livestock contact (61, 100). The importance of Sa3int for virulence of CC398 strains to humans has also been demonstrated more directly, because the presence of Sa3int decreased phagocytosis by human but not by pig polymorphonuclear neutrophils, while β-hemolysin production was abolished due to integration of the phage in the hlb gene (101).
Interestingly, the livestock-associated MRSA CC398 strains originated in humans as a methicillin-susceptible S. aureus strain, and upon introduction into livestock they lost Sa3int and acquired methicillin resistance and resistance to tetracycline, which is commonly used in livestock production (102, 103). The recent reintroduction of Sa3int into the livestock-associated MRSA strain population appears to be restricted by mutations in the 14-base pair phage attachment site of many CC398 strains (104) that leave the hlb reading frame intact but reduce integration of Sa3int (18, 19, 105). In these strains Sa3int integrates elsewhere in the chromosome, and the majority of those integration sites contain a 4-nucleotide sequence (5′-CTGG-3′) that is shared with the bona fide integration site in hlb (19). Importantly, the location of the integration appears to influence the stability of the Sa3int prophage in the livestock strains (18, 19), indicating that the success of the livestock MRSA strains in humans may depend on the location of the prophage. Interestingly, Sa3int also can promote animal adaptation, because some of the avian isolates carry a Sa3int-like phage with two putative avian-niche-specific genes (102).
The loss of Sa3int in the original jump of methicillin-susceptible S. aureus strains to livestock and the observation that the hlb gene of livestock-associated CC398 strains is mutated such that the phage integration site is eliminated but the hlb gene is kept intact underscores the role of the phages in lysogenic conversion. It also underscores the probability that Hlb is important for livestock but not for human pathogenesis. Also, the human to bovine jump of CC8 strains has been associated with the loss of Sa3int, similar to the human to livestock jump of CC398 (102, 106).
Phages other than Sa3int have also been associated with the human adaptation of CC398. Several studies document the presence of a phiMR11-like phage in these strains, and further analysis suggested that it is a defective phage that may act as a helper phage which interacts with a coresiding Sa3int phage to promote the expression of phage gene products (61, 107, 108). In another subset of CC398 strains associated with human to human transmission, Sa7int phages were detected (103). Little is known about this phage, but its association with strains being transmitted between humans suggests that it may be important for human adaptation.
DUAL CONTROL OF PHAGE-ENCODED VIRULENCE GENES: LINK TO HOST REGULATORY SYSTEMS AND PHAGE LIFE CYCLE
Phage-encoded virulence genes are integrated into the regulatory mechanism of the bacterial host and modulated in a manner surprisingly similar to bacterial chromosome-encoded virulence factors. The alternative sigma factor B seems to inhibit the expression of most, if not all, of the currently analyzed phage-encoded virulence factors (109–111). Furthermore, the two-component regulatory system saeRS and, to a lesser extent, the quorum-sensing system agr (66, 109, 110, 112) are required for the activation of most of the phage-encoded virulence factors, such as eta, pvl, scn, and chp. Interestingly, as an exception to this observation, sak was not, or was only marginally, influenced by sae and/or agr (66, 110). Both the sae and agr regulatory systems are essential for the coordinated expression of many bacterial chromosome-encoded virulence factors, and mutants deficient in these factors are clearly less virulent as shown in different animal models of infection. Thus, the phage-encoded virulence factors are integrated into different regulatory circuits employed by the bacteria. It is likely that the prophages acquired these virulence genes along with their pre-existing chromosomally determined regulatory features. Moreover, the expression of phage-encoded virulence genes is also influenced by subinhibitory concentrations of certain antibiotics. β-Lactam antibiotics, for instance, enhance PVL production presumably via the transcriptional factors SarA and Rot (112).
The expression of these virulence factors is also tightly linked to the phage life cycle. Prophages are induced by environmental conditions that lead to DNA damage, including exposure to reactive oxygen species generated by leukocytes or exposure to exogenous agents such as antibiotics (65, 113, 114). It has been demonstrated that under such phage-inducing conditions, the transcription of the virulence factors that are localized in close proximity to the lysis module of the phage genome is increased (64–66). This phenomenon is partially due to a multicopy effect caused by phage replication. However, it has also been shown that transcription becomes intimately linked to the phage genes through cotranscription with the now derepressed lysis genes (65, 66). In this regard, the use of antibiotics that induce the SOS response, such as quinolones or β-lactam antibiotics, is a special concern. Antibiotic-induced expression of phage-encoded toxin genes is well documented for E. coli prophages harboring Shiga-toxin-encoding genes (stx). Quinolones enhance stx transcription, Stx production, and toxin release from the bacterial cells via phage-mediated lysis and death in mice (115).
MYOIRIDAE AND PODOVIRIDAE
Although more is known about the siphoviruses, the phages belonging to the Myoviridae and Podoviridae are receiving increasing attention because of their therapeutic potential in combating S. aureus infections. In this context, their nontemperate and lytic nature does not carry the risk of enabling the spread of virulence or antibiotic-resistance genes upon phage treatment (116).
The receptor interaction of Myoviridae and Podoviridae demonstrates more diversity in comparison with the discussed Siphoviridae. The well-described Myovirus ΦK and Φ812 only require either a GroP or RboP backbone of WTA (Fig. 1D) (35, 47). Structural elucidation of the RBP of Myovirus ɸ812 demonstrates a sophisticated conformational change of the double-layered baseplate upon binding of the bacterial cell wall (116). Hence, their high receptor promiscuity allows infection of many coagulase-negative staphylococci expressing GroP-WTA (49, 117). Alternatively, Twortlikevirus ΦSA012 appears to distinguish between α- and β-GlcNAcylation (118). At least two RBPs are encoded by ΦSA012: one interacting with the RboP backbone and the other, with α-1,4-GlcNAc residues (Fig. 1E). Interestingly, Podovirus ΦP68, Φ44AHJD, and Φ66, requiring β-1,4-GlcNAcylated WTA, fail to adsorb and infect strains carrying WTA modified with α-GlcNAc or β-1,3-GlcNAc residues in a dominant manner (33, 54). This finding demonstrates that certain WTA glycosylation patterns are able to protect S. aureus from viral predation by preventing proper phage adsorption. Furthermore, it shows that the alternative WTA glycosyltransferase, TarM and TarP, play an antiviral role. Obtaining phage resistance by blocking the host receptor is an often-observed feature (119, 120). However, TarM-mediated glycosylation does not block all S. aureus podoviruses from infection. Certain podoviruses, such as S24-1, appear to have evolved an RBP that allows adsorption to α- or β-GlcNAcylated WTA (121). Noticeably, this RBP shares key amino acids with the RBP of siphovirus ɸ11, GP45, also shown to facilitate binding to α- or β-GlcNAcylated WTAs (44).
The application of phages to limit S. aureus both in vitro and in vivo has been tested extensively, and there seems to be some therapeutic potential (122, 123). In the following, we mention just a few examples of how phages may be used to combat S. aureus. Additionally, the therapeutic use of phage lytic proteins in S. aureus is yielding promising results, showing good efficacy without apparent side effects (124). During dairy production a phage cocktail consisting of Myoviridae and Podoviridae as well as lytic Siphoviridae eradiated a 106 CFU/g S. aureus population after 14 days in Cheddar cheese curd during ripening at 4°C (125). Also, in biofilms, phages appear to reduce S. aureus numbers as exemplified in two studies using members of the Myoviridae family (126, 127). However, if present in sublethal doses, the action of the lytic phages may promote DNA release and collectively enhance biofilm formation, suggesting that caution should be used when considering phage therapy for eradication of S. aureus biofilms (128). The potency of phage therapy in vivo has also been evaluated. In a mouse model of bovine mastitis a phage cocktail significantly reduced infection with a clinical bovine mastitis when applied 4 h postinoculation as demonstrated by improved pathology and decreased bacterial counts. Importantly, phage quantification indicated that the phage cocktail maintained high intramammary phage titers without spreading systemically (129). Future studies are likely to further address the therapeutic potential of targeting S. aureus with phages.
PHAGE DYNAMICS: MOVEMENT OF THE PHAGE WITHIN AND BETWEEN STAPHYLOCOCCAL SPECIES
Whereas most S. aureus isolates harbor multiple phages, less is known about the prevalence and nature of phages in coagulase-negative staphylococci. Analyses of available phage genome sequences of coagulase-negative staphylococci revealed a modular structure similar to that of S. aureus phages. The transfer of phages between different staphylococci is also supported by cluster analyses of phages from different staphylococcal species (130). CRISPR/Cas loci are present in some S. epidermidis strains but are lacking in most S. aureus isolates. These loci are involved in the recognition and cleavage of foreign DNA. Therefore, it was postulated that the gene flow is uni-directional (131), as indicated by several instances in which genetic material was presumably transferred from S. epidermidis to the more pathogenic S. aureus species. For example, the staphylococcal cassette chromosome expressing methicillin resistance (SCCmec) genomic islands that carry the mecA gene conferring resistance to methicillin at least occasionally originate from S. epidermidis (132). Genetic exchange might be possible between different staphylococcal species because they live in similar environments, such as on the skin or in the nose. Additionally, phages might persist in a specific environment even though the bacterial host is already eliminated through the action of the immune system or antibiotics. Such phages or transducing particles may then infect coinhabitants, providing them with new properties.
PHAGES MEDIATE HORIZONTAL GENE TRANSFER
Horizontal gene transfer is commonly observed in S. aureus, and phages are believed to be major contributors (6, 16). In 1959, transduction was described for a staphylococcal phage (133), and soon thereafter, several phages were shown to be transducing (134), particularly those belonging to serological group B (135), such as Φ11, Φ80a, and Φ80 (136, 137). Transduction is the process by which bacterial DNA, during lytic replication of the phage, is mis-packaged into phage capsids forming transducing particles and upon release can be taken up by bacterial cells. In generalized transduction, the phage machinery recognizes pseudo pac-sites that mimic the sequence from where phage DNA packaging is initiated during lytic growth, and thus, in principle, any bacterial DNA can be transferred by this process. Normally, it is thought that the recipient strain should be susceptible to the transducing phage, but this appears not to be the case, because plasmid DNA is effectively transduced into recipient S. aureus strains by ɸ29, ɸ52A, and ɸ80α as well as by prophage ɸ53 in spite of their insensitivity to the lytic action of the transducing phage (138). In S. aureus, the transfer of antibiotic resistance genes has mostly been studied, but metabolic traits have also been transduced, as have been various mobile genetic elements (139, 140). The size of the DNA transferred by a transducing particle is limited to that of the S. aureus siphophage genome (approximately 43 kbp) (8, 141), and molecules of smaller sizes such as plasmids are transferred as linear multimers (142). The size limitation of the phage capsid prevents transfer of larger chromosomal elements such as the SCCmec. The SCCmec cassettes vary in size from 20 kbp to 60 kbp, so only the smaller SCCmecs are expected to transfer through generalized transduction, as demonstrated for SCCmec type IV and SCCmec type I (143, 144). Even then, transfer of methicillin resistance is a rare event, and other factors are likely required, such as lysogeny prior to transduction and the presence of a penicillinase in the recipient strain (145). The need for penicillinase activity was confirmed and explained by the transcriptional regulation of the mec gene by the plasmid-borne blaR1-blaI regulatory genes (143). Thus, transduction is possibly responsible for the transfer of SCCmec elements, although additional factors are required.
In the laboratory, the phages Φ11 and Φ80a in particular have been used extensively for genetic manipulations of S. aureus (146). However, outside of the lab a diverse range of transducing phages are being reported (49, 147, 148). One example is Φ187, which only binds and transduces ST395 strains expressing an unusual WTA (Fig. 1C) that, surprisingly, is also present in S. epidermidis and L. monocytogenes, which are also transducible (49). Another phage, S6, isolated from sewage, proved to be a giant myophage that is able to transduce plasmid and methicillin resistance between S. aureus and non-aureus staphylococcal species, including S. epidermidis, S. felis, S. sciuri, and S. pseudintermedius (148).
Although some phages are able to readily transduce between staphylococcal species, horizontal gene transfer appears generally limited within the species through restriction-modification (R-M) systems. The principle function of these R-M systems is to protect the cell by degrading foreign DNA. If the phage is derived from a host with the same R-M system, the phage DNA becomes methylated at the cognate restriction site and thus is protected. Strains of the major CCs were shown to differ in their R-M specificity genes (149). Thus, mobile genetic elements present in one strain move to a strain of the same lineage at a higher frequency than to strains of other lineages. Consequently, S. aureus lineages carry a unique combination of core variable genes, suggesting only vertical transmission of these genes (149). It has also been shown that prophage prevalence is associated with the clonal background of S. aureus, indicating that the spread of the phages in the bacterial population is at least partially restricted (12, 13). In certain CCs, some phage groups are completely absent, whereas others are significantly less or significantly more frequent. The most prominent disequilibrium was the finding that CC15 strains do not carry Sa3int phages, although this is the most common phage group found in S. aureus, with a prevalence of up to 90% (17, 57, 150). In addition, many isolates from the CC15 complex carried none of the seven prophage groups, suggesting that this lineage is particularly restrictive to the uptake of foreign DNA. From these studies, it is clear that restriction barriers are important in limiting phage transmission and likely also transduction. Other barriers may also be present, such as the CRISPR/Cas system, but their biological importance is unknown because the system has only been reported for a few S. aureus strains (151).
Although it is well accepted that transduction is central for horizontal gene transfer in staphylococci, surprisingly little is known of the process under biologically relevant conditions. Recently, we observed that phages spontaneously released from a subpopulation of lysogenic cells can infect, lyse, and efficiently transfer DNA from a phage-susceptible bacterial population back to the intact lysogenic population, which itself survives because of immunity to phage killing (152). The process, which we termed “auto-transduction,” is driven by the spontaneous release of phages from the lysogenic population. Upon infection of susceptible cells, phages are formed together with transducing particles containing bacterial DNA and mobile genetic elements such as plasmids or SaPIs. These elements are effectively introduced in the original population, which due to lysogeny resists phage killing (152). Even though the phenomenon of transducing particles entering lysogens was observed in the first transduction experiments (153), we still do not know the extent to which it has biological impact. What, for example, remains to be studied are the conditions that promote the spontaneous release of prophages and those that influence the formation of transducing particles. A recent study showed that the ratio of transducing particles to phages is affected by antibiotics (154), thus stressing the need for in vivo studies that assess the potential impact of antimicrobial therapy on transduction.
Recently, another study investigated transduction and found that packaging of bacterial DNA in transducing particles differs depending on whether they arise from an induced, temperate phage or from an infection (155). Interestingly, while infection with a transducing phage leads even to packaging of the bacterial chromosome in transducing particles, the induction of a temperate transducing phage leads to preferential packaging of bacterial DNA downstream of the integration site in a process termed “lateral transduction.” By lateral transduction, several hundred kilobases of S. aureus DNA is packaged with high frequency, leading to hypermobility of this chromosomal region (155). With these findings in mind, we are beginning to understand how bacteriophages can be the main vehicle of staphylococcal horizontal gene transfer.
ACKNOWLEDGMENTS
We thank the Deutsche Forschungsgemeinschaft (DFG) and Danish National Research Foundation, grant DNRF120, for funding.
We thank Carina Rohmer for help gathering the information summarized in Table 1. Part of this chapter is based on a previous review (6).
Contributor Information
Hanne Ingmer, Department of Veterinary and Animal Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
David Gerlach, Interfaculty Institute of Microbiology and Infection Medicine, University of Tübingen, Tübingen, Germany.
Christiane Wolz, Interfaculty Institute of Microbiology and Infection Medicine, University of Tübingen, Tübingen, Germany.
Vincent A. Fischetti, The Rockefeller University, New York, NY
Richard P. Novick, Skirball Institute for Molecular Medicine, NYU Medical Center, New York, NY
Joseph J. Ferretti, Department of Microbiology & Immunology, University of Oklahoma Health Science Center, Oklahoma City, OK
Daniel A. Portnoy, Department of Molecular and Cellular Microbiology, University of California, Berkeley, Berkeley, CA
Miriam Braunstein, Department of Microbiology and Immunology, University of North Carolina-Chapel Hill, Chapel Hill, NC.
Julian I. Rood, Infection and Immunity Program, Monash Biomedicine Discovery Institute, Monash University, Melbourne, Australia
REFERENCES
- 1.Novick RP, Christie GE, Penadés JR. 2010. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol 8:541–551 10.1038/nrmicro2393. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penadés JR, Chen J, Quiles-Puchalt N, Carpena N, Novick RP. 2015. Bacteriophage-mediated spread of bacterial virulence genes. Curr Opin Microbiol 23:171–178 10.1016/j.mib.2014.11.019. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Kwan T, Liu J, DuBow M, Gros P, Pelletier J. 2005. The complete genomes and proteomes of 27 Staphylococcus aureus bacteriophages. Proc Natl Acad Sci U S A 102:5174–5179 10.1073/pnas.0501140102. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Łobocka M, Hejnowicz MS, Dąbrowski K, Gozdek A, Kosakowski J, Witkowska M, Ulatowska MI, Weber-Dąbrowska B, Kwiatek M, Parasion S, Gawor J, Kosowska H, Głowacka A. 2012. Genomics of staphylococcal Twort-like phages: potential therapeutics of the post-antibiotic era. Adv Virus Res 83:143–216 10.1016/B978-0-12-394438-2.00005-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Deghorain M, Van Melderen L. 2012. The staphylococci phages family: an overview. Viruses 4:3316–3335 10.3390/v4123316. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia G, Wolz C. 2013. Phages of Staphylococcus aureus and their impact on host evolution. Infect Genet Evol 21:593–601. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Brüssow H, Desiere F. 2001. Comparative phage genomics and the evolution of siphoviridae: insights from dairy phages. Mol Microbiol 39:213–222 10.1046/j.1365-2958.2001.02228.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Iandolo JJ, Worrell V, Groicher KH, Qian Y, Tian R, Kenton S, Dorman A, Ji H, Lin S, Loh P, Qi S, Zhu H, Roe BA. 2002. Comparative analysis of the genomes of the temperate bacteriophages phi 11, phi 12 and phi 13 of Staphylococcus aureus 8325. Gene 289:109–118 10.1016/S0378-1119(02)00481-X. [DOI] [PubMed] [Google Scholar]
- 9.Kahánková J, Pantůček R, Goerke C, Růžičková V, Holochová P, Doškař J. 2010. Multilocus PCR typing strategy for differentiation of Staphylococcus aureus siphoviruses reflecting their modular genome structure. Environ Microbiol 12:2527–2538 10.1111/j.1462-2920.2010.02226.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Hatfull GF, Hendrix RW. 2011. Bacteriophages and their genomes. Curr Opin Virol 1:298–303 10.1016/j.coviro.2011.06.009. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canchaya C, Proux C, Fournous G, Bruttin A, Brüssow H. 2003. Prophage genomics. Microbiol Mol Biol Rev 67:238–276 10.1128/MMBR.67.2.238-276.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goerke C, Pantucek R, Holtfreter S, Schulte B, Zink M, Grumann D, Bröker BM, Doskar J, Wolz C. 2009. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J Bacteriol 191:3462–3468 10.1128/JB.01804-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarthy AJ, Witney AA, Lindsay JA. 2012. Staphylococcus aureus temperate bacteriophage: carriage and horizontal gene transfer is lineage associated. Front Cell Infect Microbiol 2:6 10.3389/fcimb.2012.00006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrence JG, Hatfull GF, Hendrix RW. 2002. Imbroglios of viral taxonomy: genetic exchange and failings of phenetic approaches. J Bacteriol 184:4891–4905 10.1128/JB.184.17.4891-4905.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lima-Mendez G, Van Helden J, Toussaint A, Leplae R. 2008. Reticulate representation of evolutionary and functional relationships between phage genomes. Mol Biol Evol 25:762–777 10.1093/molbev/msn023. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.McCarthy AJ, Loeffler A, Witney AA, Gould KA, Lloyd DH, Lindsay JA. 2014. Extensive horizontal gene transfer during Staphylococcus aureus co-colonization in vivo. Genome Biol Evol 6:2697–2708 10.1093/gbe/evu214. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goerke C, Wirtz C, Flückiger U, Wolz C. 2006. Extensive phage dynamics in Staphylococcus aureus contributes to adaptation to the human host during infection. Mol Microbiol 61:1673–1685 10.1111/j.1365-2958.2006.05354.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Kraushaar B, Hammerl JA, Kienöl M, Heinig ML, Sperling N, Dinh Thanh M, Reetz J, Jäckel C, Fetsch A, Hertwig S. 2017. Acquisition of virulence factors in livestock-associated MRSA: lysogenic conversion of CC398 strains by virulence gene-containing phages. Sci Rep 7:2004 10.1038/s41598-017-02175-4. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Y, Nielsen LN, Hvitved A, Haaber JK, Wirtz C, Andersen PS, Larsen J, Wolz C, Ingmer H. 2017. Commercial biocides induce transfer of prophage Φ13 from human strains of Staphylococcus aureus to livestock CC398. Front Microbiol 8:2418 10.3389/fmicb.2017.02418. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feiner R, Argov T, Rabinovich L, Sigal N, Borovok I, Herskovits AA. 2015. A new perspective on lysogeny: prophages as active regulatory switches of bacteria. Nat Rev Microbiol 13:641–650 10.1038/nrmicro3527. [PubMed] [DOI] [PubMed] [Google Scholar]
- 21.Utter B, Deutsch DR, Schuch R, Winer BY, Verratti K, Bishop-Lilly K, Sozhamannan S, Fischetti VA. 2014. Beyond the chromosome: the prevalence of unique extra-chromosomal bacteriophages with integrated virulence genes in pathogenic Staphylococcus aureus. PLoS One 9:e100502 10.1371/journal.pone.0100502. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin T, Bokarewa M, McIntyre L, Tarkowski A, Corey GR, Reller LB, Fowler VG Jr. 2003. Fatal outcome of bacteraemic patients caused by infection with staphylokinase-deficient Staphylococcus aureus strains. J Med Microbiol 52:919–923 10.1099/jmm.0.05145-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 23.Peacock SJ, Moore CE, Justice A, Kantzanou M, Story L, Mackie K, O’Neill G, Day NP. 2002. Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect Immun 70:4987–4996 10.1128/IAI.70.9.4987-4996.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle-Vavra S, Jones M, Gourley BL, Holmes M, Ruf R, Balsam AR, Boulware DR, Kline S, Jawahir S, Devries A, Peterson SN, Daum RS. 2011. Comparative genome sequencing of an isogenic pair of USA800 clinical methicillin-resistant Staphylococcus aureus isolates obtained before and after daptomycin treatment failure. Antimicrob Agents Chemother 55:2018–2025 10.1128/AAC.01593-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goerke C, Matias y Papenberg S, Dasbach S, Dietz K, Ziebach R, Kahl BC, Wolz C. 2004. Increased frequency of genomic alterations in Staphylococcus aureus during chronic infection is in part due to phage mobilization. J Infect Dis 189:724–734 10.1086/381502. [PubMed] [DOI] [PubMed] [Google Scholar]
- 26.Bertozzi Silva J, Storms Z, Sauvageau D. 2016. Host receptors for bacteriophage adsorption. FEMS Microbiol Lett 363:fnw002 10.1093/femsle/fnw002. [PubMed] [DOI] [PubMed] [Google Scholar]
- 27.Brown S, Santa Maria JP Jr, Walker S. 2013. Wall teichoic acids of Gram-positive bacteria. Annu Rev Microbiol 67:313–336 10.1146/annurev-micro-092412-155620. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weidenmaier C, Lee JC. 2016. Structure and function of surface polysaccharides of Staphylococcus aureus. Curr Top Microbiol Immunol 409:57–93. [PubMed] [DOI] [PubMed] [Google Scholar]
- 29.Dengler V, Meier PS, Heusser R, Kupferschmied P, Fazekas J, Friebe S, Staufer SB, Majcherczyk PA, Moreillon P, Berger-Bächi B, McCallum N. 2012. Deletion of hypothetical wall teichoic acid ligases in Staphylococcus aureus activates the cell wall stress response. FEMS Microbiol Lett 333:109–120 10.1111/j.1574-6968.2012.02603.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 30.Schaefer K, Matano LM, Qiao Y, Kahne D, Walker S. 2017. In vitro reconstitution demonstrates the cell wall ligase activity of LCP proteins. Nat Chem Biol 13:396–401 10.1038/nchembio.2302. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown S, Xia G, Luhachack LG, Campbell J, Meredith TC, Chen C, Winstel V, Gekeler C, Irazoqui JE, Peschel A, Walker S. 2012. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc Natl Acad Sci U S A 109:18909–18914 10.1073/pnas.1209126109. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia G, Maier L, Sanchez-Carballo P, Li M, Otto M, Holst O, Peschel A. 2010. Glycosylation of wall teichoic acid in Staphylococcus aureus by TarM. J Biol Chem 285:13405–13415 10.1074/jbc.M109.096172. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerlach D, Guo Y, De Castro C, Kim SH, Schlatterer K, Xu FF, Pereira C, Seeberger PH, Ali S, Codée J, Sirisarn W, Schulte B, Wolz C, Larsen J, Molinaro A, Lee BL, Xia G, Stehle T, Peschel A. 2018. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature 563:705–709 10.1038/s41586-018-0730-x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 34.Reichmann NT, Cassona CP, Gründling A. 2013. Revised mechanism of d-alanine incorporation into cell wall polymers in Gram-positive bacteria. Microbiology 159:1868–1877 10.1099/mic.0.069898-0. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winstel V, Sanchez-Carballo P, Holst O, Xia G, Peschel A. 2014. Biosynthesis of the unique wall teichoic acid of Staphylococcus aureus lineage ST395. MBio 5:e00869 10.1128/mBio.00869-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endl J, Seidl PH, Fiedler F, Schleifer KH. 1984. Determination of cell wall teichoic acid structure of staphylococci by rapid chemical and serological screening methods. Arch Microbiol 137:272–280 10.1007/BF00414557. [PubMed] [DOI] [PubMed] [Google Scholar]
- 37.Young FE. 1967. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A 58:2377–2384 10.1073/pnas.58.6.2377. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glaser L, Ionesco H, Schaeffer P. 1966. Teichoic acids as components of a specific phage receptor in Bacillus subtilis. Biochim Biophys Acta 124:415–417 10.1016/0304-4165(66)90211-X. [DOI] [PubMed] [Google Scholar]
- 39.Coyette J, Ghuysen JM. 1968. Structure of the cell wall of Staphylococcus aureus, strain Copenhagen. IX. Teichoic acid and phage adsorption. Biochemistry 7:2385–2389 10.1021/bi00846a048. [PubMed] [DOI] [PubMed] [Google Scholar]
- 40.Chatterjee AN. 1969. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol 98:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nordström K, Forsgren A, Cox P. 1974. Prevention of bacteriophage adsorption to Staphylococcus aureus by immunoglobulin G. J Virol 14:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baptista C, Santos MA, São-José C. 2008. Phage SPP1 reversible adsorption to Bacillus subtilis cell wall teichoic acids accelerates virus recognition of membrane receptor YueB. J Bacteriol 190:4989–4996 10.1128/JB.00349-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monteville MR, Ardestani B, Geller BL. 1994. Lactococcal bacteriophages require a host cell wall carbohydrate and a plasma membrane protein for adsorption and ejection of DNA. Appl Environ Microbiol 60:3204–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Koç C, Kühner P, Stierhof YD, Krismer B, Enright MC, Penadés JR, Wolz C, Stehle T, Cambillau C, Peschel A, Xia G. 2016. An essential role for the baseplate protein Gp45 in phage adsorption to Staphylococcus aureus. Sci Rep 6:26455 10.1038/srep26455. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaneko J, Narita-Yamada S, Wakabayashi Y, Kamio Y. 2009. Identification of ORF636 in phage phiSLT carrying Panton-Valentine leukocidin genes, acting as an adhesion protein for a poly(glycerophosphate) chain of lipoteichoic acid on the cell surface of Staphylococcus aureus. J Bacteriol 191:4674–4680 10.1128/JB.01793-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Percy MG, Gründling A. 2014. Lipoteichoic acid synthesis and function in Gram-positive bacteria. Annu Rev Microbiol 68:81–100 10.1146/annurev-micro-091213-112949. [PubMed] [DOI] [PubMed] [Google Scholar]
- 47.Xia G, Corrigan RM, Winstel V, Goerke C, Gründling A, Peschel A. 2011. Wall teichoic acid-dependent adsorption of staphylococcal siphovirus and myovirus. J Bacteriol 193:4006–4009 10.1128/JB.01412-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bae T, Baba T, Hiramatsu K, Schneewind O. 2006. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol Microbiol 62:1035–1047 10.1111/j.1365-2958.2006.05441.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 49.Winstel V, Liang C, Sanchez-Carballo P, Steglich M, Munar M, Bröker BM, Penadés JR, Nübel U, Holst O, Dandekar T, Peschel A, Xia G. 2013. Wall teichoic acid structure governs horizontal gene transfer between major bacterial pathogens. Nat Commun 4:2345 10.1038/ncomms3345. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Novick RP. 2009. Phage-mediated intergeneric transfer of toxin genes. Science 323:139–141 10.1126/science.1164783. [PubMed] [DOI] [PubMed] [Google Scholar]
- 51.Umeda A, Yokoyama S, Arizono T, Amako K. 1992. Location of peptidoglycan and teichoic acid on the cell wall surface of Staphylococcus aureus as determined by immunoelectron microscopy. J Electron Microsc (Tokyo) 41:46–52. [PubMed] [Google Scholar]
- 52.Sadovskaya I, Vinogradov E, Li J, Jabbouri S. 2004. Structural elucidation of the extracellular and cell-wall teichoic acids of Staphylococcus epidermidis RP62A, a reference biofilm-positive strain. Carbohydr Res 339:1467–1473 10.1016/j.carres.2004.03.017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 53.Winstel V, Xia G, Peschel A. 2014. Pathways and roles of wall teichoic acid glycosylation in Staphylococcus aureus. Int J Med Microbiol 304:215–221 10.1016/j.ijmm.2013.10.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 54.Li X, Gerlach D, Du X, Larsen J, Stegger M, Kühner P, Peschel A, Xia G, Winstel V. 2015. An accessory wall teichoic acid glycosyltransferase protects Staphylococcus aureus from the lytic activity of Podoviridae. Sci Rep 5:17219 10.1038/srep17219. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi T, Hayashi T, Takami H, Ohnishi M, Murata T, Nakayama K, Asakawa K, Ohara M, Komatsuzawa H, Sugai M. 2001. Complete nucleotide sequence of a Staphylococcus aureus exfoliative toxin B plasmid and identification of a novel ADP-ribosyltransferase, EDIN-C. Infect Immun 69:7760–7771 10.1128/IAI.69.12.7760-7771.2001. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li M, Du X, Villaruz AE, Diep BA, Wang D, Song Y, Tian Y, Hu J, Yu F, Lu Y, Otto M. 2012. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med 18:816–819 10.1038/nm.2692. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. 2006. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol 188:1310–1315 10.1128/JB.188.4.1310-1315.2006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bae T, Banger AK, Wallace A, Glass EM, Aslund F, Schneewind O, Missiakas DM. 2004. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci USA 101:12312–12317 10.1073/pnas.0404728101. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chabelskaya S, Gaillot O, Felden B. 2010. A Staphylococcus aureus small RNA is required for bacterial virulence and regulates the expression of an immune-evasion molecule. PLoS Pathog 6:e1000927 10.1371/journal.ppat.1000927. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinel-Marie ML, Brielle R, Felden B. 2014. Dual toxic-peptide-coding Staphylococcus aureus RNA under antisense regulation targets host cells and bacterial rivals unequally. Cell Reports 7:424–435 10.1016/j.celrep.2014.03.012. [PubMed] [DOI] [PubMed] [Google Scholar]
- 61.Diene SM, Corvaglia AR, François P, van der Mee-Marquet N, Regional Infection Control Group of the Centre Region. 2017. Prophages and adaptation of Staphylococcus aureus ST398 to the human clinic. BMC Genomics 18:133 10.1186/s12864-017-3516-x. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deutsch DR, Utter B, Fischetti VA. 2016. Uncovering novel mobile genetic elements and their dynamics through an extra-chromosomal sequencing approach. Mob Genet Elements 6:e1189987 10.1080/2159256X.2016.1189987. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeman M, Mašlaňová I, Indráková A, Šiborová M, Mikulášek K, Bendíčková K, Plevka P, Vrbovská V, Zdráhal Z, Doškař J, Pantůček R. 2017. Staphylococcus sciuri bacteriophages double-convert for staphylokinase and phospholipase, mediate interspecies plasmid transduction, and package mecA gene. Sci Rep 7:46319 10.1038/srep46319. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sumby P, Waldor MK. 2003. Transcription of the toxin genes present within the Staphylococcal phage phiSa3ms is intimately linked with the phage’s life cycle. J Bacteriol 185:6841–6851 10.1128/JB.185.23.6841-6851.2003. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goerke C, Köller J, Wolz C. 2006. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob Agents Chemother 50:171–177 10.1128/AAC.50.1.171-177.2006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wirtz C, Witte W, Wolz C, Goerke C. 2009. Transcription of the phage-encoded Panton-Valentine leukocidin of Staphylococcus aureus is dependent on the phage life-cycle and on the host background. Microbiology 155:3491–3499 10.1099/mic.0.032466-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 67.Růžičková V, Pantůček R, Petráš P, Machová I, Kostýlková K, Doškař J. 2012. Major clonal lineages in impetigo Staphylococcus aureus strains isolated in Czech and Slovak maternity hospitals. Int J Med Microbiol 302:237–241 10.1016/j.ijmm.2012.04.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 68.Holochová P, Růzicková V, Dostálová L, Pantůcek R, Petrás P, Doskar J. 2010. Rapid detection and differentiation of the exfoliative toxin A-producing Staphylococcus aureus strains based on phiETA prophage polymorphisms. Diagn Microbiol Infect Dis 66:248–252 10.1016/j.diagmicrobio.2009.10.008. [PubMed] [DOI] [PubMed] [Google Scholar]
- 69.Shi D, Higuchi W, Takano T, Saito K, Ozaki K, Takano M, Nitahara Y, Yamamoto T. 2011. Bullous impetigo in children infected with methicillin-resistant Staphylococcus aureus alone or in combination with methicillin-susceptible S. aureus: analysis of genetic characteristics, including assessment of exfoliative toxin gene carriage. J Clin Microbiol 49:1972–1974 10.1128/JCM.01742-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamaguchi T, Nishifuji K, Sasaki M, Fudaba Y, Aepfelbacher M, Takata T, Ohara M, Komatsuzawa H, Amagai M, Sugai M. 2002. Identification of the Staphylococcus aureusetd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect Immun 70:5835–5845 10.1128/IAI.70.10.5835-5845.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kurt K, Rasigade JP, Laurent F, Goering RV, Žemličková H, Machova I, Struelens MJ, Zautner AE, Holtfreter S, Bröker B, Ritchie S, Reaksmey S, Limmathurotsakul D, Peacock SJ, Cuny C, Layer F, Witte W, Nübel U. 2013. Subpopulations of Staphylococcus aureus clonal complex 121 are associated with distinct clinical entities. PLoS One 8:e58155 10.1371/journal.pone.0058155. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spaan AN, Henry T, van Rooijen WJM, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJC, van Kessel KPM, Vandenesch F, Lina G, van Strijp JAG. 2013. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13:584–594 10.1016/j.chom.2013.04.006. [PubMed] [DOI] [PubMed] [Google Scholar]
- 73.Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, Liassine N, Bes M, Greenland T, Reverdy ME, Etienne J. 2003. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9:978–984 10.3201/eid0908.030089. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zanger P, Nurjadi D, Schleucher R, Scherbaum H, Wolz C, Kremsner PG, Schulte B. 2012. Import and spread of Panton-Valentine leukocidin-positive Staphylococcus aureus through nasal carriage and skin infections in travelers returning from the tropics and subtropics. Clin Infect Dis 54:483–492 10.1093/cid/cir822. [PubMed] [DOI] [PubMed] [Google Scholar]
- 75.Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC. 2013. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis 13:43–54 10.1016/S1473-3099(12)70238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saeed K, Gould I, Espositio S, Ahmad-Saeed N, Ahmed SS, Alp E, Bal AM, Bassetti M, Bonnet E, Chan M, Coombs G, Dancer S, David MZ, De Simone G, Dryden M, Guardabassi L, Hanitsch LG, Hijazi K, Kruger R, Lee A, Leistner R, Pagliano P, Righi E, Schneider-Burrus S, Skov RL, Tattevin P, Van Wamel W, Vos MC, Voss A. 2017. Panton-Valentine leucocidin (PVL) Staphylococcus aureus a position statement from the international society of chemotherapy. Int J Antimicrob Agents 215:52–125. [PubMed] [Google Scholar]
- 77.Kaneko J, Kimura T, Narita S, Tomita T, Kamio Y. 1998. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene 215:57–67 10.1016/S0378-1119(98)00278-9. [DOI] [PubMed] [Google Scholar]
- 78.Ma XX, Ito T, Kondo Y, Cho M, Yoshizawa Y, Kaneko J, Katai A, Higashiide M, Li S, Hiramatsu K. 2008. Two different Panton-Valentine leukocidin phage lineages predominate in Japan. J Clin Microbiol 46:3246–3258 10.1128/JCM.00136-08. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Narita S, Kaneko J, Chiba J, Piémont Y, Jarraud S, Etienne J, Kamio Y. 2001. Phage conversion of Panton-Valentine leukocidin in Staphylococcus aureus: molecular analysis of a PVL-converting phage, phiSLT. Gene 268:195–206 10.1016/S0378-1119(01)00390-0. [DOI] [PubMed] [Google Scholar]
- 80.Boakes E, Kearns AM, Ganner M, Perry C, Hill RL, Ellington MJ. 2011. Distinct bacteriophages encoding Panton-Valentine leukocidin (PVL) among international methicillin-resistant Staphylococcus aureus clones harboring PVL. J Clin Microbiol 49:684–692 10.1128/JCM.01917-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L, Shopsin B, Zhao Y, Smyth D, Wasserman GA, Fang C, Liu L, Kreiswirth BN. 2012. Real-time nucleic acid sequence-based amplification assay for rapid detection and quantification of agr functionality in clinical Staphylococcus aureus isolates. J Clin Microbiol 50:657–661 10.1128/JCM.06253-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Otter JA, Kearns AM, French GL, Ellington MJ. 2010. Panton-Valentine leukocidin-encoding bacteriophage and gene sequence variation in community-associated methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect 16:68–73 10.1111/j.1469-0691.2009.02925.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 83.Wirtz C, Witte W, Wolz C, Goerke C. 2010. Insertion of host DNA into PVL-encoding phages of the Staphylococcus aureus lineage ST80 by intra-chromosomal recombination. Virology 406:322–327 10.1016/j.virol.2010.07.017. [PubMed] [DOI] [PubMed] [Google Scholar]
- 84.Sanchini A, Del Grosso M, Villa L, Ammendolia MG, Superti F, Monaco M, Pantosti A. 2014. Typing of Panton-Valentine leukocidin-encoding phages carried by methicillin-susceptible and methicillin-resistant Staphylococcus aureus from Italy. Clin Microbiol Infect 20:O840–O846 10.1111/1469-0691.12679. [PubMed] [DOI] [PubMed] [Google Scholar]
- 85.Zhao H, Hu F, Jin S, Xu X, Zou Y, Ding B, He C, Gong F, Liu Q. 2016. Typing of Panton-Valentine leukocidin-encoding phages and lukSF-PV gene sequence variation in Staphylococcus aureus from China. Front Microbiol 7:1200 10.3389/fmicb.2016.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verkaik NJ, Benard M, Boelens HA, de Vogel CP, Nouwen JL, Verbrugh HA, Melles DC, van Belkum A, van Wamel WJ. 2011. Immune evasion cluster-positive bacteriophages are highly prevalent among human Staphylococcus aureus strains, but they are not essential in the first stages of nasal colonization. Clin Microbiol Infect 17:343–348 10.1111/j.1469-0691.2010.03227.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 87.Herrera A, Kulhankova K, Sonkar VK, Dayal S, Klingelhutz AJ, Salgado-Pabón W, Schlievert PM. 2017. Staphylococcal β-toxin modulates human aortic endothelial cell and platelet function through sphingomyelinase and biofilm ligase activities. MBio 8:e00273-17 10.1128/mBio.00273-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Haas CJ, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJ, Heezius EC, Poppelier MJ, Van Kessel KP, van Strijp JA. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med 199:687–695 10.1084/jem.20031636. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peetermans M, Vanassche T, Liesenborghs L, Lijnen RH, Verhamme P. 2016. Bacterial pathogens activate plasminogen to breach tissue barriers and escape from innate immunity. Crit Rev Microbiol 42:866–882 10.3109/1040841X.2015.1080214. [PubMed] [DOI] [PubMed] [Google Scholar]
- 90.Rooijakkers SH, Ruyken M, Roos A, Daha MR, Presanis JS, Sim RB, van Wamel WJ, van Kessel KP, van Strijp JA. 2005. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol 6:920–927 10.1038/ni1235. [PubMed] [DOI] [PubMed] [Google Scholar]
- 91.Schulte B, Bierbaum G, Pohl K, Goerke C, Wolz C. 2013. Diversification of clonal complex 5 methicillin-resistant Staphylococcus aureus strains (Rhine-Hesse clone) within Germany. J Clin Microbiol 51:212–216 10.1128/JCM.01967-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nübel U, Dordel J, Kurt K, Strommenger B, Westh H, Shukla SK, Zemlicková H, Leblois R, Wirth T, Jombart T, Balloux F, Witte W. 2010. A timescale for evolution, population expansion, and spatial spread of an emerging clone of methicillin-resistant Staphylococcus aureus. PLoS Pathog 6:e1000855 10.1371/journal.ppat.1000855. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holden MT, Lindsay JA, Corton C, Quail MA, Cockfield JD, Pathak S, Batra R, Parkhill J, Bentley SD, Edgeworth JD. 2010. Genome sequence of a recently emerged, highly transmissible, multi-antibiotic- and antiseptic-resistant variant of methicillin-resistant Staphylococcus aureus, sequence type 239 (TW). J Bacteriol 192:888–892 10.1128/JB.01255-09. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guinane CM, Penadés JR, Fitzgerald JR. 2011. The role of horizontal gene transfer in Staphylococcus aureus host adaptation. Virulence 2:241–243 10.4161/viru.2.3.16193. [PubMed] [DOI] [PubMed] [Google Scholar]
- 95.Huijsdens XW, van Dijke BJ, Spalburg E, van Santen-Verheuvel MG, Heck ME, Pluister GN, Voss A, Wannet WJ, de Neeling AJ. 2006. Community-acquired MRSA and pig-farming. Ann Clin Microbiol Antimicrob 5:26 10.1186/1476-0711-5-26. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schijffelen MJ, Boel CH, van Strijp JA, Fluit AC. 2010. Whole genome analysis of a livestock-associated methicillin-resistant Staphylococcus aureus ST398 isolate from a case of human endocarditis. BMC Genomics 11:376 10.1186/1471-2164-11-376. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Argudín MA, Tenhagen BA, Fetsch A, Sachsenröder J, Käsbohrer A, Schroeter A, Hammerl JA, Hertwig S, Helmuth R, Bräunig J, Mendoza MC, Appel B, Rodicio MR, Guerra B. 2011. Virulence and resistance determinants of German Staphylococcus aureus ST398 isolates from nonhuman sources. Appl Environ Microbiol 77:3052–3060 10.1128/AEM.02260-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valentin-Domelier AS, Girard M, Bertrand X, Violette J, François P, Donnio PY, Talon D, Quentin R, Schrenzel J, van der Mee-Marquet N, Bloodstream Infection Study Group of the Réseau des Hygiénistes du Centre (RHC). 2011. Methicillin-susceptible ST398 Staphylococcus aureus responsible for bloodstream infections: an emerging human-adapted subclone? PLoS One 6:e28369 10.1371/journal.pone.0028369. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Price-Whelan A, Poon CK, Benson MA, Eidem TT, Roux CM, Boyd JM, Dunman PM, Torres VJ, Krulwich TA. 2013. Transcriptional profiling of Staphylococcus aureus during growth in 2 M NaCl leads to clarification of physiological roles for Kdp and Ktr K+ uptake systems. MBio 4:e00407-13 10.1128/mBio.00407-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCarthy AJ, van Wamel W, Vandendriessche S, Larsen J, Denis O, Garcia-Graells C, Uhlemann AC, Lowy FD, Skov R, Lindsay JA. 2012. Staphylococcus aureus CC398 clade associated with human-to-human transmission. Appl Environ Microbiol 78:8845–8848 10.1128/AEM.02398-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jung P, Abdelbary MM, Kraushaar B, Fetsch A, Geisel J, Herrmann M, Witte W, Cuny C, Bischoff M. 2017. Impact of bacteriophage Saint3 carriage on the immune evasion capacity and hemolytic potential of Staphylococcus aureus CC398. Vet Microbiol 200:46–51 10.1016/j.vetmic.2016.02.015. [PubMed] [DOI] [PubMed] [Google Scholar]
- 102.Price LB, Stegger M, Hasman H, Aziz M, Larsen J, Andersen PS, Pearson T, Waters AE, Foster JT, Schupp J, Gillece J, Driebe E, Liu CM, Springer B, Zdovc I, Battisti A, Franco A, Zmudzki J, Schwarz S, Butaye P, Jouy E, Pomba C, Porrero MC, Ruimy R, Smith TC, Robinson DA, Weese JS, Arriola CS, Yu F, Laurent F, Keim P, Skov R, Aarestrup FM. 2012. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. MBio 3:e00305-11 10.1128/mBio.00305-11. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uhlemann AC, Porcella SF, Trivedi S, Sullivan SB, Hafer C, Kennedy AD, Barbian KD, McCarthy AJ, Street C, Hirschberg DL, Lipkin WI, Lindsay JA, DeLeo FR, Lowy FD. 2012. Identification of a highly transmissible animal-independent Staphylococcus aureus ST398 clone with distinct genomic and cell adhesion properties. MBio 3:e00027-12 10.1128/mBio.00027-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coleman D, Knights J, Russell R, Shanley D, Birkbeck TH, Dougan G, Charles I. 1991. Insertional inactivation of the Staphylococcus aureus beta-toxin by bacteriophage phi 13 occurs by site- and orientation-specific integration of the phi 13 genome. Mol Microbiol 5:933–939 10.1111/j.1365-2958.1991.tb00768.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 105.van Alen S, Ballhausen B, Kaspar U, Köck R, Becker K. 2018. Prevalence and genomic structure of bacteriophage phi3 in human-derived livestock-associated methicillin-resistant Staphylococcus aureus isolates from 2000 to 2015. J Clin Microbiol 56:e00140-18 10.1128/JCM.00140-18. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Resch G, François P, Morisset D, Stojanov M, Bonetti EJ, Schrenzel J, Sakwinska O, Moreillon P. 2013. Human-to-bovine jump of Staphylococcus aureus CC8 is associated with the loss of a β-hemolysin converting prophage and the acquisition of a new staphylococcal cassette chromosome. PLoS One 8:e58187 10.1371/journal.pone.0058187. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van der Mee-Marquet NL, Corvaglia A, Haenni M, Bertrand X, Franck JB, Kluytmans J, Girard M, Quentin R, François P. 2014. Emergence of a novel subpopulation of CC398 Staphylococcus aureus infecting animals is a serious hazard for humans. Front Microbiol 5:652 10.3389/fmicb.2014.00652. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Mee-Marquet N, Corvaglia AR, Valentin AS, Hernandez D, Bertrand X, Girard M, Kluytmans J, Donnio PY, Quentin R, François P. 2013. Analysis of prophages harbored by the human-adapted subpopulation of Staphylococcus aureus CC398. Infect Genet Evol 18:299–308 10.1016/j.meegid.2013.06.009. [PubMed] [DOI] [PubMed] [Google Scholar]
- 109.Kato F, Kadomoto N, Iwamoto Y, Bunai K, Komatsuzawa H, Sugai M. 2011. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun 79:1660–1670 10.1128/IAI.00872-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rooijakkers SH, Ruyken M, van Roon J, van Kessel KP, van Strijp JA, van Wamel WJ. 2006. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol 8:1282–1293 10.1111/j.1462-5822.2006.00709.x. [PubMed] [DOI] [PubMed] [Google Scholar]
- 111.Bronner S, Stoessel P, Gravet A, Monteil H, Prévost G. 2000. Variable expressions of Staphylococcus aureus bicomponent leucotoxins semiquantified by competitive reverse transcription-PCR. Appl Environ Microbiol 66:3931–3938 10.1128/AEM.66.9.3931-3938.2000. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dumitrescu O, Choudhury P, Boisset S, Badiou C, Bes M, Benito Y, Wolz C, Vandenesch F, Etienne J, Cheung AL, Bowden MG, Lina G. 2011. Beta-lactams interfering with PBP1 induce Panton-Valentine leukocidin expression by triggering sarA and rot global regulators of Staphylococcus aureus. Antimicrob Agents Chemother 55:3261–3271 10.1128/AAC.01401-10. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wagner PL, Waldor MK. 2002. Bacteriophage control of bacterial virulence. Infect Immun 70:3985–3993 10.1128/IAI.70.8.3985-3993.2002. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maiques E, Ubeda C, Campoy S, Salvador N, Lasa I, Novick RP, Barbé J, Penadés JR. 2006. beta-Lactam antibiotics induce the SOS response and horizontal transfer of virulence factors in Staphylococcus aureus. J Bacteriol 188:2726–2729 10.1128/JB.188.7.2726-2729.2006. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang X, McDaniel AD, Wolf LE, Keusch GT, Waldor MK, Acheson DW. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis 181:664–670 10.1086/315239. [PubMed] [DOI] [PubMed] [Google Scholar]
- 116.Nováček J, Šiborová M, Benešík M, Pantůček R, Doškař J, Plevka P. 2016. Structure and genome release of Twort-like Myoviridae phage with a double-layered baseplate. Proc Natl Acad Sci U S A 113:9351–9356 10.1073/pnas.1605883113. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pantůcek R, Rosypalová A, Doskar J, Kailerová J, Růzicková V, Borecká P, Snopková S, Horváth R, Götz F, Rosypal S. 1998. The polyvalent staphylococcal phage phi 812: its host-range mutants and related phages. Virology 246:241–252 10.1006/viro.1998.9203. [PubMed] [DOI] [PubMed] [Google Scholar]
- 118.Takeuchi I, Osada K, Azam AH, Asakawa H, Miyanaga K, Tanji Y. 2016. The presence of two receptor-binding proteins contributes to the wide host range of staphylococcal Twort-like phages. Appl Environ Microbiol 82:5763–5774 10.1128/AEM.01385-16. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat Rev Microbiol 8:317–327 10.1038/nrmicro2315. [PubMed] [DOI] [PubMed] [Google Scholar]
- 120.Seed KD. 2015. Battling phages: how bacteria defend against viral attack. PLoS Pathog 11:e1004847 10.1371/journal.ppat.1004847. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uchiyama J, Taniguchi M, Kurokawa K, Takemura-Uchiyama I, Ujihara T, Shimakura H, Sakaguchi Y, Murakami H, Sakaguchi M, Matsuzaki S. 2017. Adsorption of Staphylococcus viruses S13′ and S24-1 on Staphylococcus aureus strains with different glycosidic linkage patterns of wall teichoic acids. J Gen Virol 98:2171–2180 10.1099/jgv.0.000865. [PubMed] [DOI] [PubMed] [Google Scholar]
- 122.Kutter EM, Kuhl SJ, Abedon ST. 2015. Re-establishing a place for phage therapy in Western medicine. Future Microbiol 10:685–688 10.2217/fmb.15.28. [PubMed] [DOI] [PubMed] [Google Scholar]
- 123.Górski A, Międzybrodzki R, Weber-Dąbrowska B, Fortuna W, Letkiewicz S, Rogóż P, Jończyk-Matysiak E, Dąbrowska K, Majewska J, Borysowski J. 2016. Phage therapy: combating infections with potential for evolving from merely a treatment for complications to targeting diseases. Front Microbiol 7:1515 10.3389/fmicb.2016.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gutierrez D, Fernandez L, Rodriguez A. 2018. Are phage lytic proteins the secret weapon to kill Staphylococcus aureus? MBio 9:e01923-17. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.El Haddad L, Roy JP, Khalil GE, St-Gelais D, Champagne CP, Labrie S, Moineau S. 2016. Efficacy of two Staphylococcus aureus phage cocktails in cheese production. Int J Food Microbiol 217:7–13 10.1016/j.ijfoodmicro.2015.10.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 126.Alves DR, Gaudion A, Bean JE, Perez Esteban P, Arnot TC, Harper DR, Kot W, Hansen LH, Enright MC, Jenkins AT. 2014. Combined use of bacteriophage K and a novel bacteriophage to reduce Staphylococcus aureus biofilm formation. Appl Environ Microbiol 80:6694–6703 10.1128/AEM.01789-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gutiérrez D, Vandenheuvel D, Martínez B, Rodríguez A, Lavigne R, García P. 2015. Two Phages, phiIPLA-RODI and phiIPLA-C1C, Lyse Mono- and Dual-Species Staphylococcal Biofilms. Appl Environ Microbiol 81:3336–3348 10.1128/AEM.03560-14. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fernández L, González S, Campelo AB, Martínez B, Rodríguez A, García P. 2017. Low-level predation by lytic phage phiIPLA-RODI promotes biofilm formation and triggers the stringent response in Staphylococcus aureus. Sci Rep 7:40965 10.1038/srep40965. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Breyne K, Honaker RW, Hobbs Z, Richter M, Żaczek M, Spangler T, Steenbrugge J, Lu R, Kinkhabwala A, Marchon B, Meyer E, Mokres L. 2017. Efficacy and Safety of a Bovine-Associated Staphylococcus aureus Phage Cocktail in a Murine Model of Mastitis. Front Microbiol 8:2348 10.3389/fmicb.2017.02348. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Deghorain M, Bobay LM, Smeesters PR, Bousbata S, Vermeersch M, Perez-Morga D, Drèze PA, Rocha EP, Touchon M, Van Melderen L. 2012. Characterization of novel phages isolated in coagulase-negative staphylococci reveals evolutionary relationships with Staphylococcus aureus phages. J Bacteriol 194:5829–5839 10.1128/JB.01085-12. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Otto M. 2013. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. BioEssays 35:4–11 10.1002/bies.201200112. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wielders CL, Vriens MR, Brisse S, de Graaf-Miltenburg LA, Troelstra A, Fleer A, Schmitz FJ, Verhoef J, Fluit AC. 2001. In-vivo transfer of mecA DNA to Staphylococcus aureus [corrected]. Lancet 357:1674–1675 10.1016/S0140-6736(00)04832-7. [DOI] [PubMed] [Google Scholar]
- 133.Morse ML. 1959. Transduction by staphylococcal bacteriophage. Proc Natl Acad Sci USA 45:722–727 10.1073/pnas.45.5.722. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pattee PA, Baldwin JN. 1961. Transduction of resistance to chlortetracycline and novobiocin in Staphylococcus aureus. J Bacteriol 82:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dowell CE, Rosenblum ED. 1962. Serology and transductin in staphylococcal phage. J Bacteriol 84:1071–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Novick R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155–166 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 137.Kayser FH, Wüst J, Corrodi P. 1972. Transduction and elimination of resistance determinants in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2:217–223 10.1128/AAC.2.3.217. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mašlaňová I, Stříbná S, Doškař J, Pantůček R. 2016. Efficient plasmid transduction to Staphylococcus aureus strains insensitive to the lytic action of transducing phage. FEMS Microbiol Lett 363:363 10.1093/femsle/fnw211. [PubMed] [DOI] [PubMed] [Google Scholar]
- 139.Murphey WH, Rosenblum ED. 1964. Selective medium for carbohydrate-utilizing transductants of Staphylococcus aureus. J Bacteriol 87:1198–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ubelaker MH, Rosenblum ED. 1978. Transduction of plasmid determinants in Staphylococcus aureus and Escherichia coli. J Bacteriol 133:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Christie GE, Matthews AM, King DG, Lane KD, Olivarez NP, Tallent SM, Gill SR, Novick RP. 2010. The complete genomes of Staphylococcus aureus bacteriophages 80 and 80α--implications for the specificity of SaPI mobilization. Virology 407:381–390 10.1016/j.virol.2010.08.036. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Novick RP, Edelman I, Lofdahl S. 1986. Small Staphylococcus aureus plasmids are transduced as linear multimers that are formed and resolved by replicative processes. J Mol Biol 192:209–220 10.1016/0022-2836(86)90360-8. [DOI] [PubMed] [Google Scholar]
- 143.Chlebowicz MA, Mašlaňová I, Kuntová L, Grundmann H, Pantůček R, Doškař J, van Dijl JM, Buist G. 2014. The Staphylococcal Cassette Chromosome mec type V from Staphylococcus aureus ST398 is packaged into bacteriophage capsids. Int J Med Microbiol 304:764–774 10.1016/j.ijmm.2014.05.010. [PubMed] [DOI] [PubMed] [Google Scholar]
- 144.Scharn CR, Tenover FC, Goering RV. 2013. Transduction of staphylococcal cassette chromosome mec elements between strains of Staphylococcus aureus. Antimicrob Agents Chemother 57:5233–5238 10.1128/AAC.01058-13. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cohen S, Sweeney HM. 1973. Effect of the prophage and penicillinase plasmid of the recipient strain upon the transduction and the stability of methicillin resistance in Staphylococcus aureus. J Bacteriol 116:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krausz KL, Bose JL. 2016. Bacteriophage Transduction in Staphylococcus aureus: Broth-Based Method. Methods Mol Biol 1373:63–68 10.1007/7651_2014_185. [PubMed] [DOI] [PubMed] [Google Scholar]
- 147.Varga M, Pantůček R, Růžičková V, Doškař J. 2016. Molecular characterization of a new efficiently transducing bacteriophage identified in meticillin-resistant Staphylococcus aureus. J Gen Virol 97:258–268 10.1099/jgv.0.000329. [PubMed] [DOI] [PubMed] [Google Scholar]
- 148.Uchiyama J, Takemura-Uchiyama I, Sakaguchi Y, Gamoh K, Kato S, Daibata M, Ujihara T, Misawa N, Matsuzaki S. 2014. Intragenus generalized transduction in Staphylococcus spp. by a novel giant phage. ISME J 8:1949–1952 10.1038/ismej.2014.29. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Waldron DE, Lindsay JA. 2006. Sau1: a novel lineage-specific type I restriction-modification system that blocks horizontal gene transfer into Staphylococcus aureus and between S. aureus isolates of different lineages. J Bacteriol 188:5578–5585 10.1128/JB.00418-06. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Matthews AM, Novick RP. 2005. Staphylococcal phages, p 297–318. In Waldor MK, Friedman DI, Adhya SL (ed), Phages Their Role in Bacterial Pathogenesis and Biotechnology American Society for Microbiology Press. Washington, DC. [Google Scholar]
- 151.Marraffini LA, Sontheimer EJ. 2008. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322:1843–1845 10.1126/science.1165771. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haaber J, Leisner JJ, Cohn MT, Catalan-Moreno A, Nielsen JB, Westh H, Penadés JR, Ingmer H. 2016. Bacterial viruses enable their host to acquire antibiotic resistance genes from neighbouring cells. Nat Commun 7:13333 10.1038/ncomms13333. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zinder ND, Lederberg J. 1952. Genetic exchange in Salmonella. J Bacteriol 64:679–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Stanczak-Mrozek KI, Laing KG, Lindsay JA. 2017. Resistance gene transfer: induction of transducing phage by sub-inhibitory concentrations of antimicrobials is not correlated to induction of lytic phage. J Antimicrob Chemother 72:1624–1631 10.1093/jac/dkx056. [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen J, Quiles-Puchalt N, Chiang YN, Bacigalupe R, Fillol-Salom A, Chee MSJ, Fitzgerald JR, Penadés JR. 2018. Genome hypermobility by lateral transduction. Science 362:207–212 10.1126/science.aat5867. [PubMed] [DOI] [PubMed] [Google Scholar]


