Abstract
Long-range chromatin interactions control metazoan gene transcription. However, the involvement of intra- and interchromosomal interactions in development and oncogenesis remains unclear. TAL1/SCL is a critical transcription factor required for the development of all hematopoietic lineages; yet, aberrant TAL1 transcription often occurs in T-cell acute lymphoblastic leukemia (T-ALL). Here, we report that oncogenic TAL1 expression is regulated by different intra- and interchromosomal loops in normal hematopoietic and leukemic cells, respectively. These intra- and interchromosomal loops alter the cell-type-specific enhancers that interact with the TAL1 promoter. We show that human SET1 (hSET1)-mediated H3K4 methylations promote a long-range chromatin loop, which brings the + 51 enhancer in close proximity to TAL1 promoter 1 in erythroid cells. The CCCTC-binding factor (CTCF) facilitates this long-range enhancer/promoter interaction of the TAL1 locus in erythroid cells while blocking the same enhancer/promoter interaction of the TAL1 locus in human T-cell leukemia. In human T-ALL, a T-cell-specific transcription factor c-Maf-mediated interchromosomal interaction brings the TAL1 promoter into close proximity with a T-cell-specific regulatory element located on chromosome 16, activating aberrant TAL1 oncogene expression. Thus, our study reveals a novel molecular mechanism involving changes in three-dimensional chromatin interactions that activate the TAL1 oncogene in human T-cell leukemia.
Keywords: TAL1/SCL oncogene, chromatin loops, CTCF insulator, T-cell leukemia, epigenetic regulation
INTRODUCTION
Tissue- and developmental stage-specific activation of enhancer elements regulates metazoan gene expression, which determines cell identity. Because genes are regulated by sequence-specific transcription factors and their corresponding enhancers, changes in these factors and interaction between elements influence gene expression patterns and subsequently cellular function. Therefore, perturbations in the activity of these transcriptional regulators compromise their function and may initiate malignant transformation. One such key regulator is the basic helix-loop-helix (bHLH) transcription factor TAL1/SCL (hereafter referred to as TAL1), originally identified by virtue of its involvement in a T-cell acute lymphoblastic leukemia (T-ALL)-specific chromosomal translocation.1–4 Its expression is required for the development of all hematopoietic cell lineages.5,6 Deletion of Tal1 in mice led to embryonic lethality in midgestation due to a complete loss of yolk sac hematopoiesis.7,8 In addition, the Tal1-null embryoid stem cells were unable to contribute to hematopoiesis in vivo in chimeric mice,7 indicating that TAL1 has a critical role in early hematopoietic lineage commitment and differentiation.
To act as a transcription regulator, TAL1 associates with coregulators that often possess histone-modifying and -remodeling activities to regulate the transcription of downstream target genes.9,10 Cooperation of GATA-1, TAL1 and chromatin-remodeling factor Brg1 shapes the erythroid-specific chromatin landscape and determines the erythroid transcription program.11 TAL1 also autoregulates itself by forming a complex with GATA-1 and by binding to consensus GATA-E-box motifs presented in its own enhancer and promoter.12,13 The human TAL1 gene is located on chromosome 1p32 and is tightly regulated by various cis-regulatory elements, which control TAL1 expression levels in different hematopoietic lineages and stages.14–16 Transgenic reporter knockin mouse studies and DNase I hypersensitive assays have revealed that the TAL1 locus contains distinct promoters and distal enhancer elements that together control temporal and spatial TAL1 expression patterns.14,17–19 Lineage-restricted Promoter 1a is utilized in erythroid, megakaryocytic and mast cells; promoter 1b is active in primitive myeloid and mast cells.1,20 Similar to the promoters, distinct TAL1 enhancers also associate with different TAL1 expression patterns. The −4 Kb and + 18/19 enhancers initiate TAL1 expression in mesoderm derivatives associated with early formation of endothelial and hematopoietic stem and progenitor cells, whereas the + 40 Kb enhancer (+ 51 Kb in human) is transcriptionally active in erythroid cells.13,16,21 Despite extensive studies on the identification of TAL1 regulatory elements, the detailed epigenetic mechanisms governing differential enhancer and promoter action to selectively activate TAL1 in different stages of hematopoietic differentiation remain to be illustrated.
Despite its role in normal hematopoiesis, ectopic transcriptional activation of TAL1 is the most frequent gain-of-function mutation observed in T-ALL patients. Aberrant activation of TAL1 was found in 40–60% of T-ALL patients, resulting from chromosomal translocation (4–5%), or interstitial chromosome deletion (25–30%), or by an undefined mechanism (60%).22–24 Ectopic activation of Tal1 transcription in T cells led to the development of leukemia and lymphoma in mice.25,26 In contrast, deletion of TAL1 in T-ALL leads to a loss of the leukemic phenotype and induces apoptosis27, implicating an important role of TAL1 activation in T-cell neoplastic disease. Surprisingly, it remains largely unknown how the TAL1 oncogene is activated in human T-cell acute leukemia. Moreover, knowledge of the molecular mechanisms governing ectopic TAL1 activation is scarce, especially in T-ALL cases lacking TAL1 locus chromosomal rearrangements.
Here, we report that, although TAL1 promoter 1 is active in all lineages expressing TAL1, the + 51 enhancer is selectively active in erythroid precursors and inactive in leukemic T cells. hSET1-mediated H3K4 methylation facilitates an erythroid-specific long-range chromatin interaction between the + 51 enhancer and TAL1 promoter 1, which activates TAL1 gene transcription in erythroid precursor cells. In contrast, in T-ALL cell lines and patients, the T-cell-specific proto-oncoprotein c-Maf mediates an interchromosomal interaction that brings TAL1 promoter 1 in close proximity to a T-cell-specific DNA regulatory element on chromosome 16. This interaction is critical for ectopic TAL1 expression and leukemic cell proliferation in human T-ALL. Further, we found that CTCF differentially reorganized the chromatin structure in normal erythroid cells and leukemic cells, keeping the + 51 enhancer in close proximity to the TAL1 promoter in erythroid cells while deflecting the + 51 enhancer from interacting with the promoter in T-ALL. Thus, our studies revealed novel molecular mechanisms by which the oncogenic transcription factor TAL1 is regulated by changes in chromatin loops in normal and malignant hematopoiesis.
MATERIALS AND METHODS
Cell lines, constructs and small hairpin RNA-mediated knockdown K562 and Jurkat cells were maintained as described.28 HL-60 was cultured in IMDM supplemented with 20% fetal bovine serum. Human cord blood-derived CD34+ cells were enriched through positive immune selection by flow cytometry and maintained as described previously.29 The cells were subjected to erythroid differentiation to CD36+ cells over a 7-day period and then sorted for CD36+ population for 3C and ChIP analyses. The hSET1 knockdown (KD) constructs were generated by subcloning small hairpin RNA (shRNA) oligonucleotides into pSuper.retro.puro vector (Clontech, Mountain View, CA, USA) as described previously.28 All stable KD cells were maintained in medium containing 1 μg/ml puromycin (Calbiotech, Spring Valley, CA, USA).
Native ChIP, formaldehyde cross-linked ChIP and ChIP-seq
Native ChIP assays for histone modifications and formaldehyde cross-linked ChIP for transcription factors and modifying enzymes were performed as described previously.30 The relative enrichment was determined by the following equation: 2Ct(IP)-Ct(ref). Primer sequences across the TAL1 locus and antibodies are listed in the Supplementary Information. ChIP-Seq assays in CD34+ and CD36+ cells were performed as outlined previously31 and are described in Supplementary Information.
Chromosome conformation capture (3C) and circular chromosome conformation capture (4C) assays
The 3C assay was performed as described previously with minor modifications.32 In brief, 2 × 107 cells were crosslinked with 2% formaldehyde for 10 min and lysed. Real-time PCR was performed to quantitate 3C interactions using SYBR after validation of each primer pair as described.33 Relative crosslinking frequencies were calculated and plotted after normalization to loading control and ERCC3 control.34
The 4C assay was performed as described previously,35 with minor modifications. In brief, 5 × 105 cells were crosslinked with 2% formaldehyde for 10 min and digested overnight. 4C-ligated DNA was prepared and amplified by nested PCR. The PCR products were cloned into pCR-TOPOII vector (Invitrogen, Grand Island, NY, USA) for sequencing.
Reverse transcription and quantitative PCR
Total RNA was prepared using the RNeasy mini isolation kit according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA). One microgram RNA was reverse transcribed using the Superscript II reverse Transcriptase (Invitrogen). complementary DNA was analyzed using real-time PCR (quantitative reverse transcription-PCR) with a MyiQ Single-Color real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). Primer sequences are listed in Supplementary Information (Supplementary Table S2).
Colony formation assay for CD34+ hematopoietic stem cells
The human colony formation assay was performed as described by StemCell Technologies (Vancouver, BC, Canada) using complete Methocult. Vector control (1 × 104) or hSET1 KD CD34+ cells were seeded in 4 ml SFEM medium supplemented with 10 ng/ml granulocyte colony-stimulating factor, 20 ng/ml stem cell factor, 10 ng/ml interleukin-3 (IL3), 10 ng/ml IL6 and 6U/ml erythropoietin. The cells were then plated in triplicate on methylcellulose cell culture plates and cultured for 18 days, with fresh medium supplemented with cytokines added every week. Different hematopoietic colonies including erythroid progenitors, colony-forming unit-erythroid (CFU-E) and blast-forming unit-erythroid, granulocyte/macrophage progenitors, CFU-granulocyte and macrophage, CFU-granulocyte, erythroid, macrophage and megakaryocyte were observed and counted following guidelines as described by StemCell Technologies.
RESULTS
Distinct patterns of histone modifications are associated with TAL1 enhancer/promoter activities
Recent genome-wide studies predict a correlation between the different levels of histone modifications, such as the methylation statuses of Lys 4 residue on histone H3 tails and enhancer/promoter activities.36,37 Given that TAL1 is tightly controlled by multiple cis-regulatory elements in different stages of hematopoiesis, we examined the H3K4me2, H3K4me3, H3K9/14ac and H3K27me3 patterns across the 166 Kb of the TAL1 locus in K562, Jurkat, Rex, HPB-ALL and HL-60 cells by using the ChIP-qPCR assay with antibodies specific to these modifications. K562 is a human erythroleukemia cell line and Jurkat is a T-ALL cell line in which TAL1 is highly activated. HL-60 was derived from an acute myeloid leukemia patient in whom the TAL1 gene was silenced. The pattern of active and repressive histone modifications associated with gene activity in these three cell types across the entire STIL1-TAL1-MAP17 locus is shown in Figure 1 and Supplementary Figure S1. There are marked enrichments of H3K4me2 and H3K9/14ac in K562 cells at TAL1 promoter 1, + 19, enhancer Map17 promoter and + 51 enhancer. H3K4me3 is particularly enriched at TAL1 promoter 1, but not at other regulatory elements (Figure 1a). In HL-60 and HPB-ALL cells in which TAL1 is inactive, there are large peaks of H3K27me3 over TAL1 promoter IV and promoter 1, marking the silenced TAL1 gene (Figure 1b and Supplementary Figure S1A). Although TAL1 is expressed in Jurkat and REX T-ALL cells, only promoters 1 and IV are marked by H3K9/14ac, H3K4me2 and H3K4me3. No active modifications are detected at + 19 and + 51 enhancers (Figure 1c and Supplementary Figure S1A). Moreover, no H3K27me3 is detected in TAL1-expressing K562 and Jurkat cells (Supplementary Figure S1B). This pattern of histone modifications suggests that TAL1 promoter 1 is differentially regulated by different regulatory elements in erythroid and leukemic cells. Our results show that the well-characterized enhancers required for normal hematopoietic expression of TAL1 in the locus13,14 are epigenetically inactive in T-ALL cells. Therefore, aberrant TAL1 activation in T-ALL cells may require distinct regulatory element(s).
Figure 1.
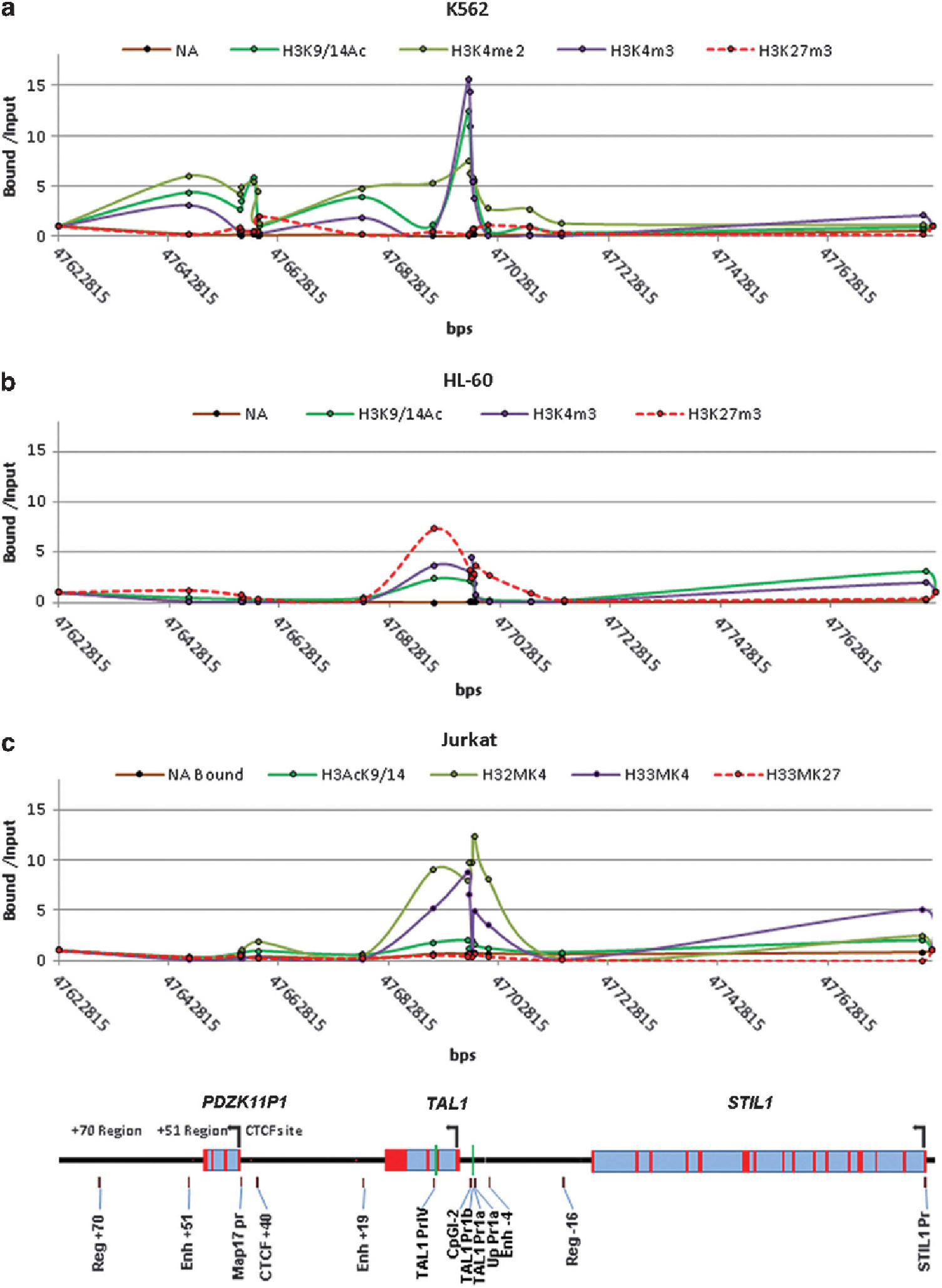
Distinct histone modification patterns are associated with cell-type-specific enhancer/promoter activities. ChIP analysis of histone modifications across the STIL-TAL1-MAP17 loci in K562 (a), HL-60 (b) and Jurkat (c) cells.
The recruitment of hSET1 correlates with TAL1 transcriptional activation during hematopoiesis hSET1 is a histone methyltransferase that specifically methylates Lys 4 at histone H3 tails. Given that TAL1 colocalizes with the hSET1 complex at the TAL1 target genes during erythroid differentiation38 and both + 51 enhancer and TAL1 promoter 1 contain composite E-box/GATA motifs,13 we reasoned that recruitment of the hSET1 complex by TAL1 may be responsible for high levels of H3K4 methylations in the TAL1 locus in erythroid cells. To test this possibility, we examined the global interactions between TAL1 and hSET1 at the human genome comparing human primary CD34+ hematopoietic stem cells (HSCs) and CD36+ erythroid precursors using unbiased ChIP-seq technologies. Approximately 50% of intergenic bound hSET1 colocalized with TAL1 in CD36+ erythroid precursors (Supplementary Figure S2A), suggesting that TAL1 recruits the hSET1 complex to regulate its genome-wide targets in erythroid cells. As we expected, TAL1 and hSET1 complexes bind to both the + 51 enhancer and promoter 1 in the TAL1 locus in primary hematopoietic cells. This binding correlates strongly with H3K4 methylations at these elements (Figures 2a and b). In particular, H3K4me3 was enriched around the transcription start site of the TAL1 gene (Figures 2a and b). Although the hSET1 complex is recruited to both the + 51 enhancer and promoter 1 of the TAL1 gene in K562 (Figure 2c) and primary hematopoietic cells (Figures 2a and b), both TAL1 and hSET1 are not bound to the TAL1 locus in T-ALL Jurkat cells (Figure 2c and Supplementary Figure S2B). Thus, our data suggest that recruitment of the hSET1 complex facilitates promoter H3K4 methylations and transcriptional activation of TAL1 during normal hematopoiesis, but not in malignant Jurkat cells.
Figure 2.
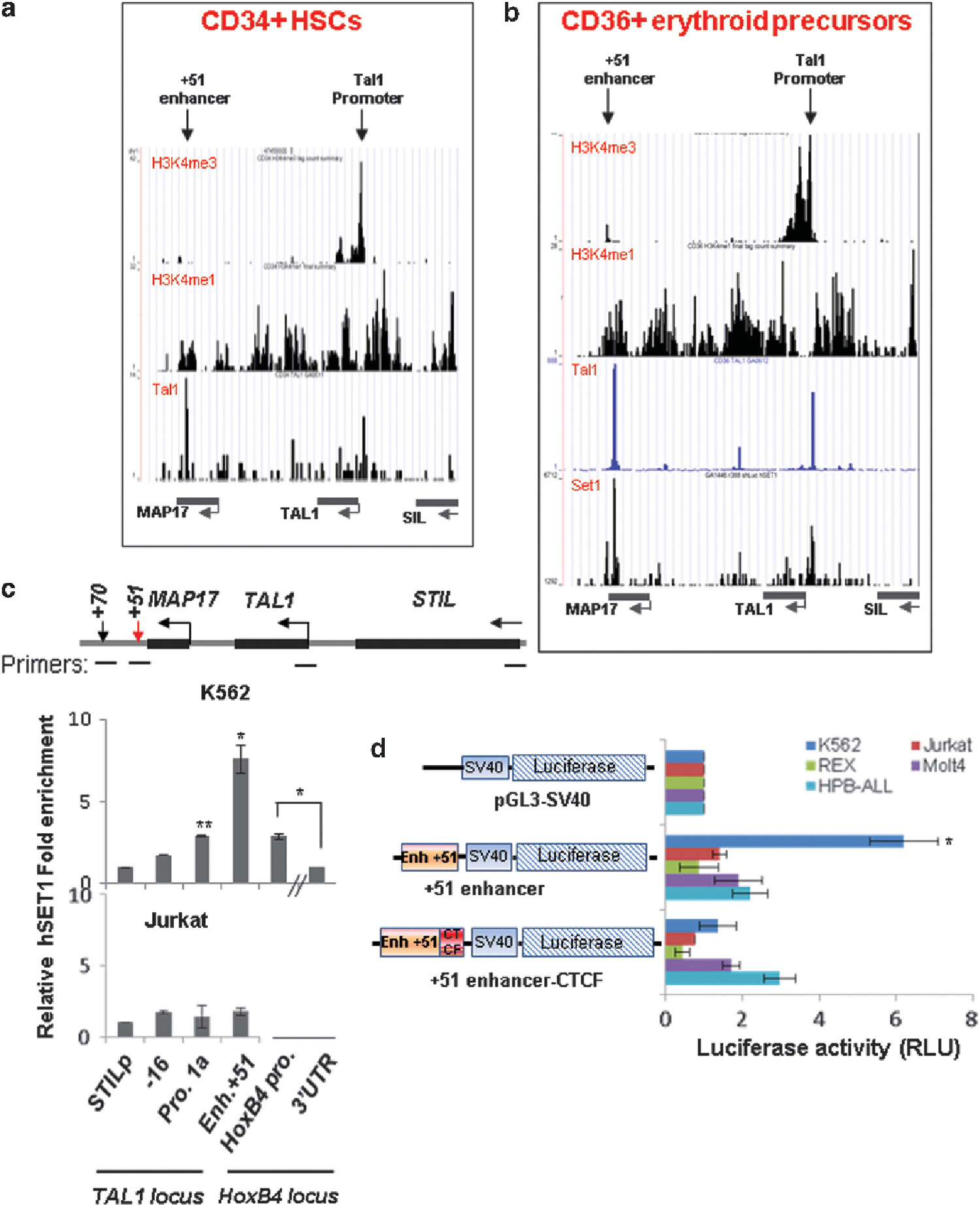
The + 51 enhancer activates TAL1 promoter 1 in the erythroid lineage by recruiting the hSET1 HMT complex. (a) ChIP-seq analyses of H3K4me1, H3K4me3 and TAL1 binding at the TAL1 locus in CD34+ HSCs. (b) ChIP-seq analyses of TAL1 binding, hSET1 recruitment, H3K4me1 and H3K4me3 at the TAL1 locus in CD36+ erythroid precursors. (c) ChIP analysis of hSET1 recruitment at the TAL1 locus in K562 cells (top) and T-ALL Jurkat (bottom) cells. Data are shown as mean ± s.d. *P < 0.05; **P < 0.01. (d) K562 and several T-ALL cells were transfected with a control pGL3-SV40 luciferase reporter, a + 51 enhancer-driven pGL3-SV40 luciferase reporter, or a + 51 enhancer-driven pGL3-SV40 luciferase reporter containing a + 53 CTCF site inserted between the + 51 enhancer and the SV40 promoter. A CMV-driven renilla luciferase plasmid was used as a transfection control. Transfected cells were cultured for 48 h and lysed for the measurement of luciferase activity (RLU). Data are shown as mean ± s.d. *P < 0.05.
Next, we tested whether the recruitment of hSET1 could attribute to the transcriptional activity of the + 51 enhancer in K562 cells but not in Jurkat cells. Two differently sized DNA fragments containing the + 51 enhancer element were cloned into an SV40 minimal promoter-driven luciferase reporter and introduced into K562 and several T-ALL cell lines. Compared with the pGL3-SV40 vector that showed only minimal luciferase activity, the 2 Kb + 51 enhancer element specifically activated transcription of the luciferase reporter in K562 cells, but not in T-ALL cell lines, Jurkat, Rex, Molt4 and HPB-ALL (Figure 2d). Interestingly, the 4 Kb fragment containing the + 51 enhancer and an additional + 53 Kb CTCF site inserted between the + 51 enhancer and the SV40 minimal promoter blocks transactivation of the reporter in K562 cells, suggesting that the + 53 Kb CTCF site may block the + 51 enhancer from activating downstream neighboring genes (Figure 2d). Together, the data revealed that the + 51 enhancer is neither epigenetically nor transcriptionally active in T-ALL cells.
A long-range chromatin loop mediates enhancer/promoter interaction in the TAL1 locus in erythroid precursors but not in T-ALL cells
It has been reported from studies on transgenic mice that the + 51 enhancer is capable of driving reporter gene expression at physiological TAL1 expression sites during hematopoiesis.13,14 However, it is unclear how the + 51 enhancer activates the TAL1 gene from 51 Kb downstream in native chromatin location. An attractive model proposes that a chromosomal loop brings the + 51 enhancer and promoter 1 into close proximity. To test this possibility, we carried out chromosome conformation capture (3C) assays in CD36+ erythroid progenitor cells as shown in Figure 3a. A long-range chromosomal interaction between the + 51 enhancer and TAL1 promoter 1 was detected in primary CD36+ erythroid precursors (Figure 3b). Sequence analysis of the 197 bp 3C PCR product revealed a fusion molecule containing both the + 51 enhancer and the TAL1 promoter 1 sequences (Figure 3c). In contrast, no interactions were detected between TAL1 and Map17 promoters (Figure 3b). Thus, these data suggest that the + 51 enhancer activates TAL1 promoter 1 via a long-range enhancer/promoter chromatin loop.
Figure 3.
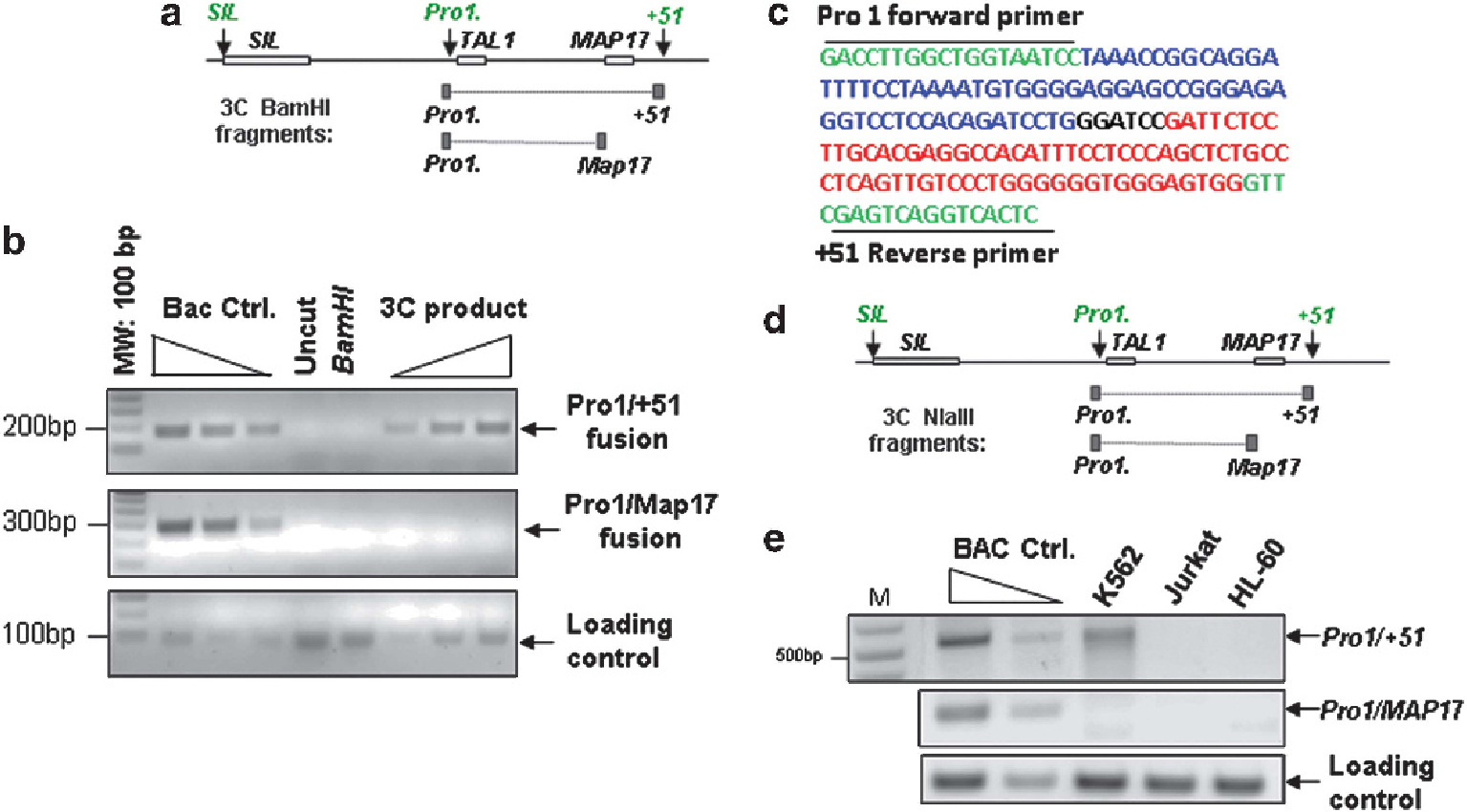
The TAL1 enhancer and promoter interact through a long-range chromatin loop in erythroid cells. (a) Schematic representation of the TAL1 locus. The STIL, TAL1 and MAP17 genes are indicated by gray boxes, and promoters and enhancers are indicated by arrows. The predicted 3C BamHI restriction fragments are shown in black boxes. (b) 3C analysis of the interaction between TAL1 promoter 1 and the + 51 enhancer in CD36+ erythroid precursors. (c) Sequencing analysis of the + 51/TAL1 promoter 1 3C fusion product. (d) Schematic representation of the TAL1 locus and the predicted 3C NlaIII restriction fragments. (e) 3C analysis of the interaction between TAL1 promoter 1 and the + 51 enhancer in K562, Jurkat and HL-60 cells.
Recent studies have highlighted that long-range chromatin interactions provide a topological basis for transcriptional regulation.39,40 To test whether the enhancer/promoter loop is specific for erythroid cells or is also present in TAL1-expressing T-ALL cells or in nonexpressing leukemic cells, 3C assays were carried out in K562, Jurkat, Rex, HPB-ALL and HL-60 cells. For these assays the restriction enzyme NlaIII was used (Figures 3d and e). Digestion with enzyme NlaIII generated on average 250–500 bp fragments across the genome, and the specific interaction between the + 51 enhancer and TAL1 promoter 1 produced a 527-bp PCR fragment (Figure 3d). Consistent with primary CD36+ cells, the + 51 enhancer physically interacts with promoter 1 only in K562 cells using either NlaIII (Figure 3e) or BamHI (Supplementary Figure S3A) digestion. The 527-bp PCR fragment was cloned and sequenced. It consists of a fusion of sequences from the + 51 enhancer and TAL1 promoter 1 (Supplementary Figure S3B). The long-range interaction is specific between the + 51 enhancer and promoter 1 in erythroid cells because it was detected neither between the Map17 promoter and TAL1 promoter 1 (Figure 3e) nor in TAL1-silenced HL-60 and HPB-ALL cell lines (Figure 3e and Supplementary Figure S3C). Interestingly, although both Jurkat and REX express the TAL1 gene, the chromatin interaction between the + 51 enhancer and TAL1 promoter 1 was not detected in these cells (Figure 3e and Supplementary Figure S3C), supporting the evidence that the + 51 enhancer is inactive in TAL1-expressing T-ALL cells (Figure 1).
Recruitment of the hSET1 complex is essential for long-range chromatin loop and transcription of TAL1 gene
Evidence suggests that H3K4me3 may be important for establishing chromatin loops.41 The hSET1 complex has been shown to regulate H3K4me3 methylation. Because the binding of the hSET1 complex correlates with TAL1 activation in erythroid cells (Figure 2), we further reasoned that recruitment of hSET1 may mediate the physical chromatin interaction between the + 51 enhancer and promoter 1 at the TAL1 locus. To test this hypothesis, we generated shRNA-mediated hSET1 KD in K562 cells (Supplementary Figure S4A). The KD of hSET1 led to a decrease in TAL1 expression in three individual hSET1 KD K562 clones (Figure 4a), but not in T-ALL Jurkat cells (Supplementary Figures S4B and C). Moreover, the levels of H3K4me2 and H3K4me3 enrichment at the + 51 enhancer and TAL1 promoter 1 also decreased in the hSET1 KD K562 clones (Figure 4b). Subsequently, in the hSET1 KD K562 clones the intrachromatin loop between the + 51 enhancer and TAL1 promoter 1 is inhibited (Figure 4c). In addition, the recruitment of RNA Pol II to both the enhancer and the promoter of the TAL1 gene is suppressed (Figure 4b). Consequently, the hSET1-mediated active histone modifications may be associated with specific chromatin loop formation. Finally, ablation of hSET1 in human CD34+ HSCs (Supplementary Figure S4D) resulted in a block of the ability of HSCs to differentiate into colony forming unit-erythroid (CFU-E) and burst-forming unit-erythroid colonies but not CFU-granulocyte, erythroid, macrophage, megakaryocyte and CFU-granulocyte and macrophage colonies (Figure 4d), implying that disruption of hSET1 function in HSCs specifically affects erythropoiesis by perturbing TAL1 transcription.
Figure 4.
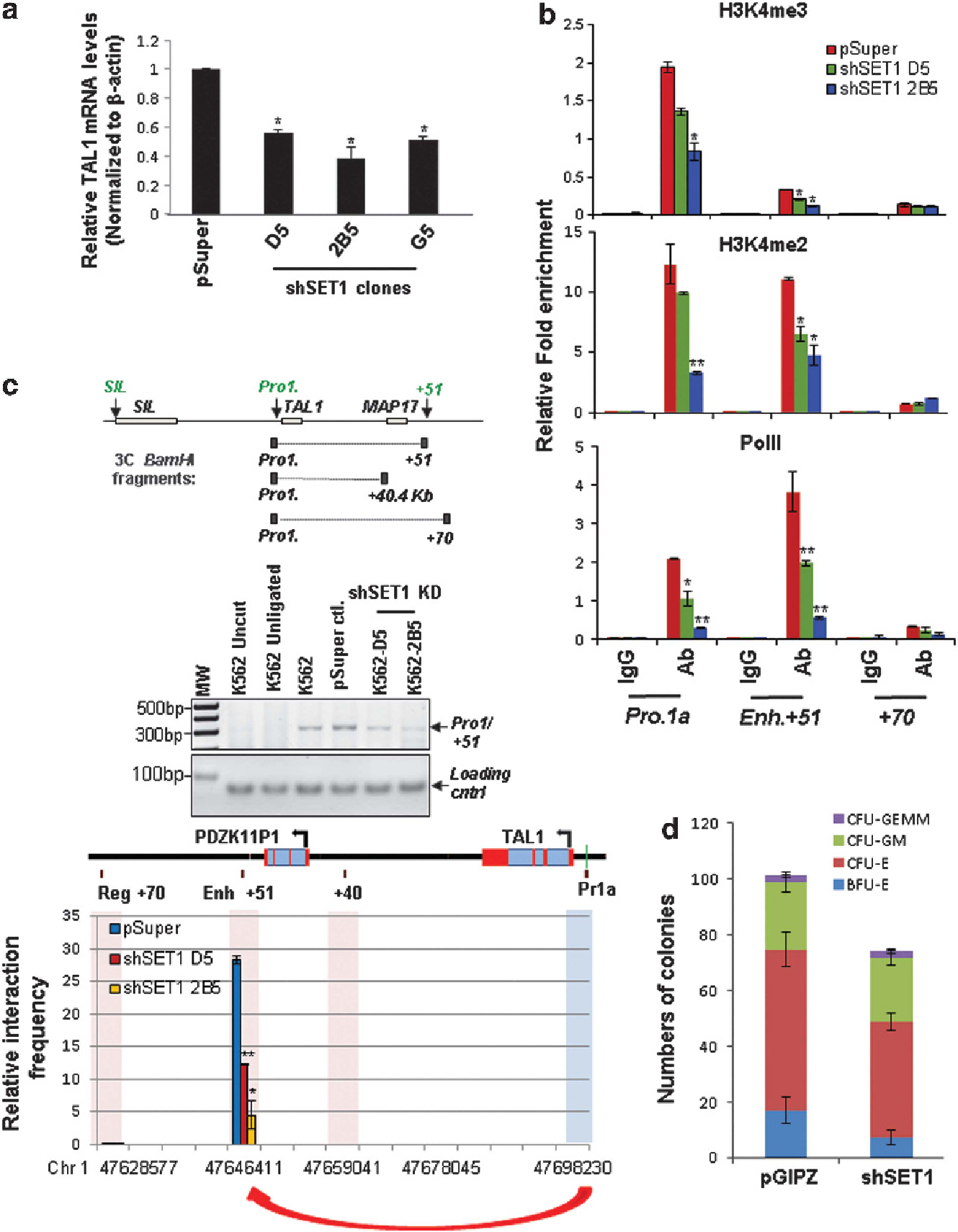
Loss of hSET1 recruitment disrupts the erythroid-specific enhancer/promoter long-range interaction in the TAL1 locus. (a) Real-time reverse transcription-quantitative PCR (RT-qPCR) analyses of TAL1 mRNA transcript levels in the vector control and three KD clones harboring shRNA specific for hSET1. Data are shown as mean ± s.d. *P < 0.05. (b) ChIP analyses of H3K4me2 and me3 levels as well as RNA PolII recruitment at the + 51 enhancer and TAL1 promoter 1 upon hSET1 KD. Data are shown as mean ± s.d. *P < 0.05; **P < 0.01. (c) The 3C analysis of the interaction between the + 51 enhancer and TAL1 promoter 1 in the TAL1 locus in the vector control and shSET1-transduced clones. (Top) Shown is gel electrophoresis of the 3C PCR products. (bottom) Shown is real-time qPCR quantitation of the 3C products. Data are shown as mean ± s.d. of three independent experiments. *P < 0.05; **P < 0.01. (d) CFC assay of CD34+ HSCs transduced with the vector control and shRNA-targeting hSET1.
CTCF-mediated cell-type specific chromatin loops in the TAL1 locus regulate expression of TAL1 gene in erythroid and leukemic cells
In the TAL1 locus there are four CTCF-binding elements that are occupied by CTCF in K562 cells.12,42 The −31 CTCF site is located between the STIL gene and the TAL1 gene; the + 40 CTCF site separates the TAL1 and MAP17 genes; and the + 53 and + 57 CTCF sites are located in the 3′ boundary of the TAL1 locus (downstream of the + 51 enhancer) (Figure 5a). Given the global role of CTCF in genome organization,43,44 CTCF may bind differently to CTCF elements in the TAL1 locus in erythroid and leukemic cells, thereby regulating the + 51 enhancer and TAL1 promoter 1 interaction. To examine this model, we carried out CTCF ChIP analysis in K562 and Jurkat cells. Interestingly, CTCF bound to all of the CTCF elements in both cell lines (Supplementary Figure S5A), suggesting that the binding of CTCF alone is not sufficient to modulate TAL1 gene activity. We next examined whether CTCF differentially regulates genome organization by controlling TAL1 promoter accessibility in K562 and Jurkat cells. To address this question, we performed 3C assays in K562 and Jurkat cells using the + 53 Kb CTCF element as bait. Figure 5b shows that in K562 cells, but not in T-ALL Jurkat cells, the − 31 Kb CTCF element interacts with the + 53 Kb CTCF site (Figure 5b). As a control, no CTCF-mediated loop is formed between − 10 and + 53 Kb CTCF elements (Figure 5b). The interaction between the − 31 Kb CTCF element and the + 53 Kb CTCF site was predominantly found in K562 cells (Figures 5b and c). This interaction brings the + 51 enhancer and promoter 1 of the TAL1 gene into close proximity, thereby facilitating enhancer/promoter regulation of TAL1 gene expression. Interestingly, the − 31/+ 53 CTCF loop is not dependent on the hSET1 complex, as hSET1 KD in K562 cells does not interfere with the loop formation (Supplementary Figures S5B and C). In contrast, when we carried out 3C assays to evaluate interactions between the + 40 Kb and + 53 Kb CTCF elements, the CTCF-mediated + 40/+ 53 chromatin loop was seen to predominantly exist in T-ALL Jurkat cells, but not in K562 cells (Figures 5b and c). Further, 3C analysis of normal bone marrow (BM) cells and a TAL1-overexpressing human T-ALL patient’s sample also showed different preferential loop interactions. The preferential − 31 Kb/+ 53 Kb CTCF loop found in normal BM cells switched to a predominantly smaller + 40 Kb/+ 53 Kb CTCF loop in T-ALL patient sample (Figures 5d and e), suggesting that CTCF may act to exclude the + 51 enhancer from TAL1 promoter 1 in T-cell leukemia.
Figure 5.
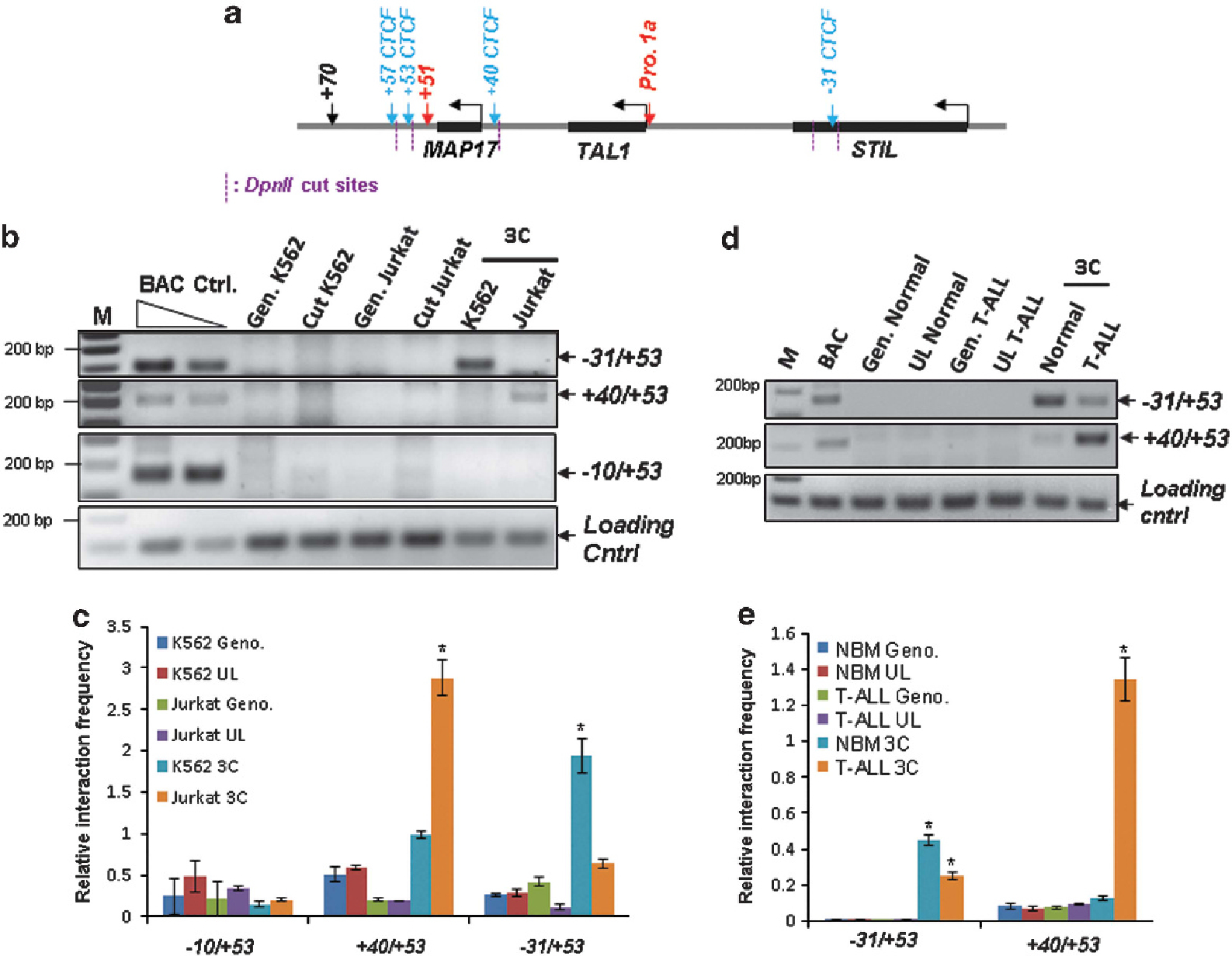
CTCF-mediated genome organization regulates enhancer and promoter interaction in the TAL1 locus. (a) Schematic representation of the TAL1 locus and CTCF sites is indicated by blue arrows. The predicted DpnII restriction fragments are indicated by magenta dashed lines. (b) The 3C analysis of long-range genome interaction among CTCF sites in the TAL1 locus comparing K562 and Jurkat cells. (c) A total of three independent 3C experiments were quantitated by densitometry. Data are shown as mean ± s.d. of three independent experiments. *P < 0.05. (d) The 3C analysis of long-range genome interaction among CTCF sites in the TAL1 locus comparing normal BM and T-ALL patient BM cells without chromosomal rearrangement detected in the TAL1 locus. (e) A total of three independent 3C experiments were quantitated by densitometry. Data are shown as mean ± s.d. of three independent experiments. *P < 0.05.
A novel cis-regulatory element in chromosome 16 interacts and drives TAL1 expression in leukemic T cells
Currently, it remains unknown how the TAL1 gene is aberrantly activated in the majority of T-ALL patients lacking chromosomal rearrangements in the TAL1 locus. To understand the molecular mechanisms underlying the regulation of the TAL1 oncogene in T-ALL, we used circular chromosome conformation capture (4C) technology to identify potential regulatory elements that associate with the TAL1 promoter in T-ALL (Figure 6a and Supplementary Figure S6A). Using TAL1 promoter 1a as bait, a 4C library was created and a total of 57 clones were sequenced. Supplementary Table 1 shows the potential TAL1 promoter 1-interacting elements found in the Jurkat genome. Most of the clones (54 out of 57 clones) are self-ligated products and one clone did not match with the human genome sequence (Supplementary Table S1). Of the two DNA elements in human T-ALL cells that interact with TAL1 promoter 1 (Supplementary Table S1), one is located ~2.1 Kb upstream of a long noncoding RNA LOC595101 and ~15 Kb downstream of the CD2BP2 gene, encoding a protein for T-cell signaling (Supplementary Figure S6B), and the other is located in the coding region of the RAP2A gene, encoding a member of the Ras superfamily of small GTPase (Supplementary Figure S6C). We confirmed by 3C analysis that the intergenic DNA element located downstream of the T-cell-specific CD2BP2 locus specifically interacts with TAL1 promoter 1 in the T-ALL cell line Jurkat, but not in K562 cells (Supplementary Figures S6D and E). The element was named TIL16 (TAL1-interacting locus located in chromosome 16) and contained p300, leukemia oncoproteins MEIS1/HoxA9 and T-cell specific transcription factor c-Maf binding sites (Supplementary Figure S7A). It is interesting to note that HoxA9, and its cofactor MEIS1 often co-occupy genomic sites in intergenic enhancers with high levels of H3K4me1 and CBP/p300 binding.45 The TIL16 element is located between the T-cell-specific CD2BP2 gene and a noncoding RNA gene LOC595101. Both transcripts are highly expressed in T lymphocytes (Supplementary Figures S7B and C). Interestingly, ChIP-seq analysis revealed that H3K4me1 and H3K4me2, which are epigenetic marks associated with regulatory enhancer elements,37 are enriched at the TIL16 element in CD4+ T lymphocytes (Supplementary Figure S8A). Further 3C analysis confirmed the presence of the interchromosomal interaction between the TIL16 region in chromosome 16 and TAL1 promoter 1 in chromosome 1 in three TAL1-overexpressing T-ALL cell lines, Jurkat, REX and Molt4 (Figure 6b). These assays did not detect the interaction in K562 cells (Figure 6b). Sequence analysis of the predicted 3C products revealed a fusion DNA containing both the TIL16 element and the TAL1 promoter 1 sequences (Figure 6c). In addition to T-ALL cell lines, 3C analysis was also performed in a BM sample from one T-ALL patient and in primary cells (COG-LL-317) from one pediatric T-ALL patient in whom TAL1 are aberrantly expressed (Supplementary Figures S9A–C). Genomic DNA analysis of these patient samples did not detect any chromosomal rearrangements in the TAL1 locus (Supplementary Figures S9D and E). The TIL16/TAL1 promoter 1 interchromosomal interaction was detected only in the primary T-ALL patient’s BM and in the patient-derived primary T-ALL cells, but was absent in normal BM cells (Figure 6d and Supplementary Figure S9F).
Figure 6.
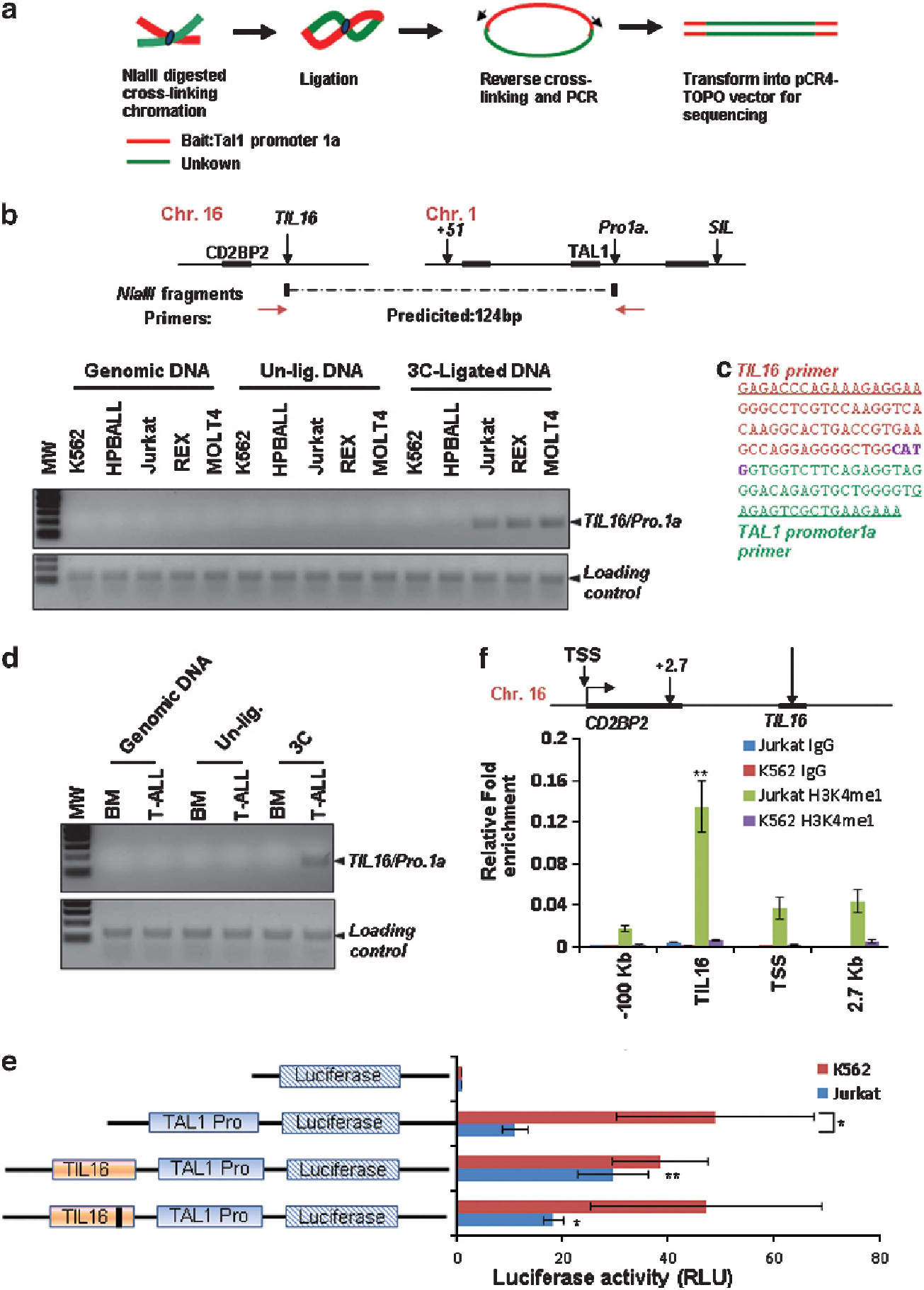
A T-cell-specific DNA element interacts with and activates the TAL1 promoter 1 via an interchromosomal interaction in leukemic T cells. (a) Schematic representation of the 4C procedure using TAL1 promoter 1 as bait. (b) The 3C analysis confirming the interchromosomal interaction identified by 4C experiments in K562 and a variety of T-ALL cell lines. (c) The predicted 3C product was confirmed by DNA sequencing (right). (d) 3C analysis of the interchromosomal interaction between TAL1 promoter 1 on chromosome 1 and the TIL16 element on chromosome 16 comparing normal BM and T-ALL patient BM cells. (e) K562 and Jurkat cells were transfected with the TAL1 promoter 1a-driven luciferase reporter, a TIL16 element linked to the TAL1 promoter 1-luciferase reporter, or a c-Maf site-deleted TIL16 element linked to the TAL1 promoter 1-driven luciferase reporter. A CMV-driven renilla luciferase plasmid was used as transfection control. Transfected cells were cultured for 48 h and lysed for the measurement of luciferase activity. Data are shown as mean ± s.d. *P < 0.05; **P < 0.01. (f) ChIP analysis of the enrichment of characteristic enhancer histone marker H3K4me1 in the CD2BP2 locus in Jurkat and K562 cells. Data are shown as mean ± s.d. **P < 0.01.
Next, to test whether TIL16 can act as a T-cell-specific enhancer to ectopically activate the TAL1 gene in T-ALL cells, a 995 bp of the TIL16 element containing the c-Maf, HoxA9/MEIS1, and p300/CBP binding sites was cloned into a TAL1 promoter 1-driven luciferase reporter and introduced into K562 and Jurkat cells. TAL1 promoter 1 is highly active in K562 cells, and the addition of the TIL16 element did not enhance reporter activity in K562 cells further (Figure 6e). However, in Jurkat cells in which TAL1 promoter 1 alone showed minimal activity when compared with K562 cells, the TIL16 element significantly stimulated the TAL1 promoter 1 activity, resulting in a nearly threefold increase in Jurkat cells (Figure 6e). Further, deletion of the c-Maf-binding site attenuates the TIL16-mediated transactivation in Jurkat cells (Figure 6e). In addition, H3K4me1 is highly enriched in the TIL16 element in Jurkat cells but not in K562 cells (Figure 6f). These results identify a novel regulatory element bound by T-cell specific transcription factor c-Maf that activates T-cell expression of TAL1 gene in trans in T-ALL.
c-Maf regulates TAL1 expression and interchromosomal interaction in T-cell leukemia
c-Maf is a member of the basic leucine zipper transcription factors belonging to the AP1 superfamily. c-Maf controls IL-4 expression in Th2 cells, and IL-4 and IL10 in macrophages.46,47 c-Maf is overexpressed in 60% of angioimmunoblastic T-cell lymphomas.48 Ectopic expression of c-Maf in the T-cell compartment contributes to T-cell lymphoma in mice,49 a phenotype similar to that of TAL1 overexpression.25,26 Interestingly, a consensus binding site for the c-Maf transcription factor was found in both the TIL16 element and TAL1 promoter 1 (Supplementary Figures S7A and S8B). c-Maf specifically occupies TIL16 and TAL1 promoter 1 elements in Jurkat cells (Figure 7a). To examine whether c-Maf regulates TAL1 expression in T-ALL and to determine the underlying mechanism of ectopic TAL1 activation in T-ALL, c-Maf was depleted in Jurkat cells using lentivirus-mediated shRNA specifically targeting the human c-Maf sequence (Figure 7b). c-Maf ablation led to a significant decrease in both TAL1 mRNA and protein levels (Figures 7b and c) and impaired Jurkat cell proliferation (Supplementary Figures S10 A and B). We further reasoned that inhibition of TAL1 expression by c-Maf KD may result from a disruption of the interchromosomal loop between the TIL16 element and TAL1 promoter 1. To test this possibility, 3C analysis was carried out in wild-type and c-Maf-depleted Jurkat cells. We found that c-Maf depletion destabilizes the TIL16 and TAL1 promoter 1 interchromosomal interaction (Figure 7d and Supplementary Figure S10C) and also inhibits H3K4me3 enrichment and TAF3 recruitment at TAL1 promoter 1 (Figures 7e and f). These data revealed that c-Maf plays an important role in maintenance of the topological TAL1 promoter 1 and TIL16 interaction as well as maintenance of aberrant TAL1 gene expression in human T-ALL.
Figure 7.
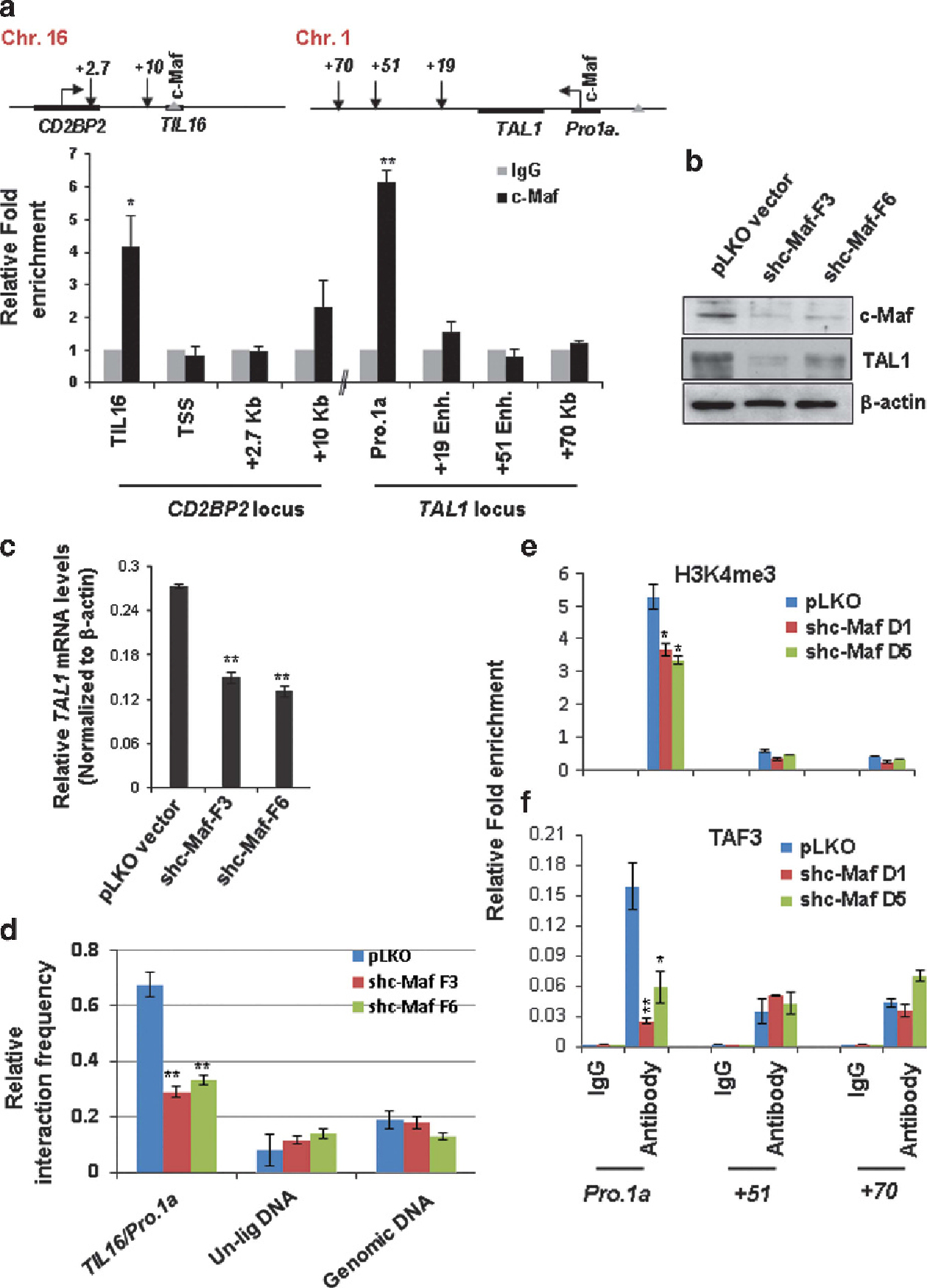
KD of c-Maf leads to disruption of the interchromosomal interaction and decrease in TAL1 transcription in T-ALL cells. (a) ChIP analysis of T-cell-specific transcription factor c-Maf binding in the CD2BP2 and TAL1 loci in Jurkat cells. Data are shown as mean ± s.d. of three independent experiments. *P < 0.05; **P < 0.01. (b, c) Western blot (b) and RT-qPCR (c) analyses of TAL1 expression in the vector control and two c-Maf KD clones. (d) 3C analysis of interchromosomal interaction between TAL1 promoter 1 and the TIL16 element comparing the vector control and c-Maf KD Jurkat clones. A total of three independent 3C experiments were quantitated by densitometry. (e, f) ChIP analyses of H3K4me3 levels (e) as well as TAF3 recruitment (f) at the + 51 enhancer and TAL1 promoter 1 upon c-Maf KD. Data are shown as mean ± s.d. of three independent experiments. *P < 0.05; **P < 0.01.
Finally, we performed a ChIP-3C experiment to test whether the T-cell specific transcription factor c-Maf is directly involved in interchromosomal interaction to aberrantly activate the TAL1 oncogene in T-ALL leukemia. The crosslinked chromatin was digested with the NlaIII restriction enzyme and immune-precipitated with antibody specific to c-Maf. The c-Maf-selected chromatin was subjected to 3C analysis for the loop interaction between the TIL16 element and TAL1 promoter 1 (Figure 8a). PCR reaction using the TIL16 and TAL1 promoter 1 primers yielded a unique band of the expected size and sequence in Jurkat cells (Figure 8a). Thus, we concluded that T-cell specific transcription factor c-Maf anchors an interchromatin loop that brings the TIL16 element and the TAL1 promoter into close proximity in T-ALL.
Figure 8.
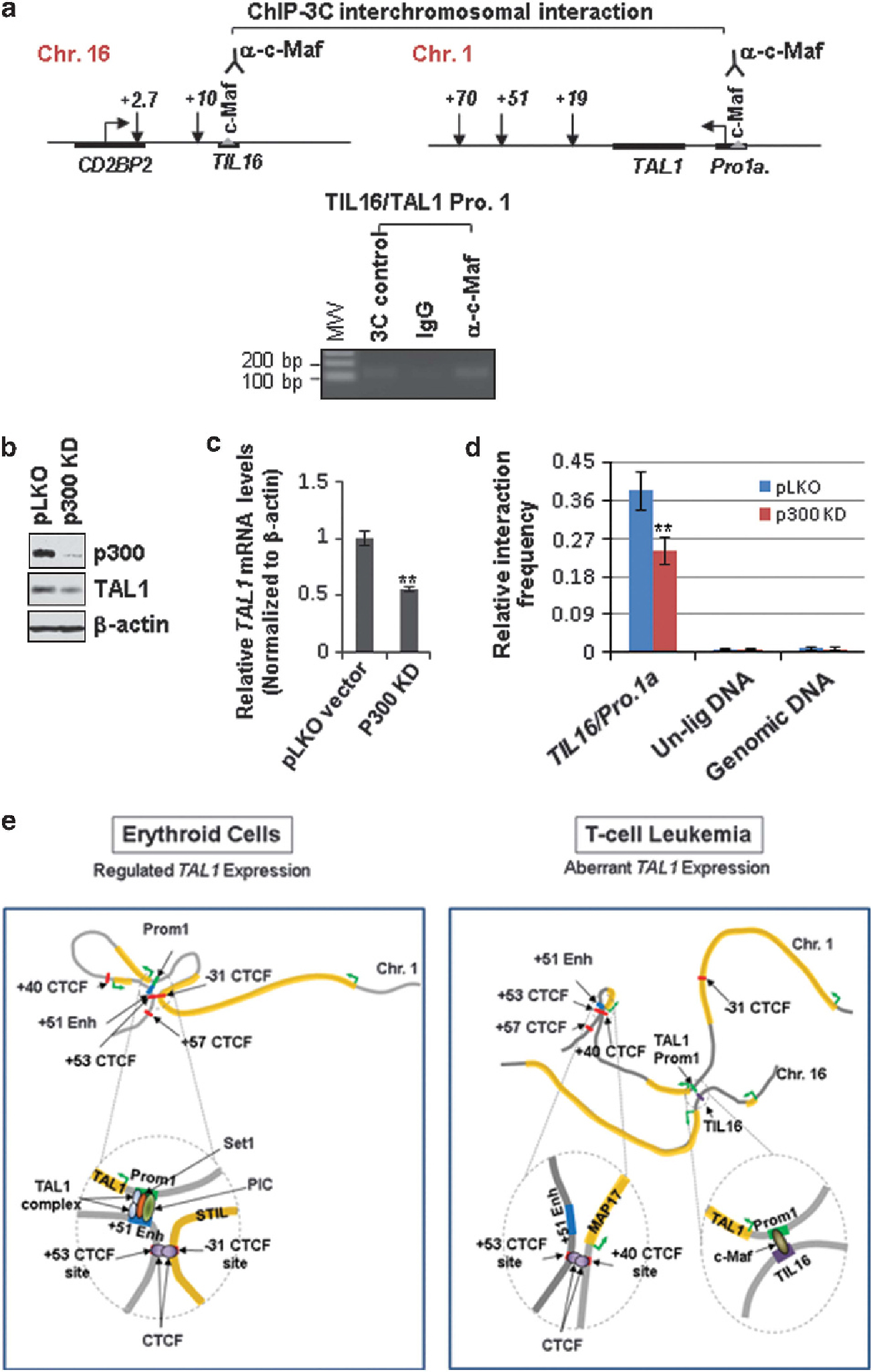
c-Maf anchors interchromosomal interaction between the TIL16 element and the TAL1 promoter in aberrant TAL1-expressed T-ALL cells. (a) The ChIP-3C assay was carried out in the NlaIII-digested Jurkat chromatin. The chromatin was selected with antibodies specific to c-Maf or control immunoglobulin G (IgG), followed by ligation and PCR amplification using the TIL16 and TAL1 promoter primers. (b, c) Western blot (b) and RT-qPCR (c) analyses of TAL1 expression in the vector control and p300 KD pool. (d) 3C analysis of interchromosomal interaction between TAL1 promoter 1 and the TIL16 element comparing the vector control and p300 KD Jurkat cells. A total of three independent 3C experiments were quantitated by densitometry. Data are shown as mean ± s.d. of three independent experiments. **P < 0.01. (e) A model depicting that cell-type-specific TAL1 transcription is regulated by CTCF, transcription factors and cofactor-mediated intra- or interchromosomal loops.
p300 is important for TAL1 expression and interchromosomal interaction in T-cell leukemia
There is a strong correlation between the presence of p300 and enhancer function in the human genome37,50, and enhancer-targeted p300/CBP is able to interact with the promoter-bound TATA-binding protein and other components of TFIID.51 Presence of the p300 binding site in the TIL16 and TAL1 promoter 1 elements suggests that p300 may mediate the interchromosomal interaction of these two elements. To test this possibility, p300 was depleted in Jurkat cells using lentivirus-mediated shRNA specifically targeting the human p300 sequence (Figure 8b). p300 ablation led to a significant decrease in both TAL1 mRNA and protein levels (Figures 8b and c). 3C analysis showed that p300 depletion interferes with the TIL16 and TAL1 promoter 1 interchromosomal interaction (Figure 8d), suggesting that c-Maf and p300 may cooperate to control the interchromosomal loop for aberrant TAL1 activation in T-ALL.
DISCUSSION
We identified a novel T-cell-specific cis-regulatory element in chromosome 16 that is involved in aberrant activation of the TAL1 gene through an interchromosomal loop in human T-ALL (Figure 8e). Interestingly, genome annotation revealed that the novel TIL16 element is flanked by a T-cell specific CD2BP2 gene and a long noncoding RNA LOC595101, which are highly expressed in T lymphocytes (Supplementary Figures S7B & C). The TIL16 element itself is bound by p300 and the T-cell specific transcription factor c-Maf. It is possible that the TIL16 element confers T-cell specificity on TAL1 genes and leads to malignant transformation. Except for TAL1 promoter 1, promoter IV of the TAL1 gene is also involved in T-ALL cases52 and is epigenetically active in Jurkat and REX T-ALL cells (Figure 1c and Supplementary Figure S1A). It still remains to be determined whether promoter IV is also activated by the interchromosomal interactions in human T-ALL.
TAL1 is a critical oncogenic transcriptional factor required for normal hematopoiesis.9 Aberrant activation of TAL1 in T lymphocytes leads to leukemic transformation in the majority of childhood T-ALL.23 It is essential to understand the molecular mechanisms that regulate TAL1 transcription activity in normal hematopoiesis and leukemic T cells. We found that the TAL1 gene is controlled by a long-range intrachromatin loop that brings the + 51 enhancer into close proximity with TAL1 promoter 1. This loop interaction is specific for erythroid cells and is absent in other hematopoietic cells in which TAL1 is silenced or even in T-ALL cells in which TAL1 is expressed (Figure 8e). Thus, an interesting question is what underlies differential selection of the + 51 enhancer usage in erythroid cells and how the tissue-specific chromatin loop is established and stabilized. Several lines of evidence support the notion that active histone modifications such as H3 acetylation have an important role in communication between genes and distal cis-regulatory elements by chromosomal loops.32,53 One example is the β-globin locus in which the LCR can serve as a primary site for recruiting transcription factors and chromatin-modifying and -remodeling factors and stably alter the topology of the β-globin locus during transcriptional activation in erythroid cells.32,54,55 Depletion of hSET1 led to reduced H3K4 methylation, disruption of the + 51 enhancer/promoter 1 chromatin loop and loss of TAL1 transcription (Figure 4), suggesting a plausible model that hSET1-mediated H3K4 methylation may be required for establishing or stabilizing the enhancer/promoter communication through a long-range chromatin loop. However, the loss of Pol II occupancy in hSET1-depleted cells (Figure 4b) makes it possible that hSET1-mediated H3K4 methylations also remodel local chromatin and facilitate the accessibility of the basal transcription factors to the TAL1 promoter (Figure 8e). Nevertheless, the data provide a potential mechanism that links H3K4 methylations and the long-range enhancer/promoter interaction. Further, the KD of hSET1 does not affect the CTCF-mediated looping in the TAL1 locus (Supplementary Figures S5B and C). This suggests that CTCF is not directly involved in the enhancer/promoter interaction. However, the CTCF-mediated chromatin loops probably facilitate interactions between enhancers and promoters by bringing them into close proximity.
One particularly interesting finding is that CTCF mediates different chromatin loops at the TAL1 locus in erythroid and T-ALL cells, thereby providing another layer of regulation to ensure proper TAL1 expression (Figures 5 and 8e). CTCF has been implicated in diverse regulatory functions, including transcriptional activation and repression, insulation, imprinting, and X chromosome inactivation.43,44 CTCF molecules are capable of interacting with each other to form a cluster, thereby creating closed looping domains.56 It has been proposed that CTCF may have a primary role in the global organization of chromatin architecture and lineage-specific gene expression.43,44 With regard to the TAL1 locus, our data revealed that CTCF differentially organized chromatin loop domains in erythroid and leukemic cells such that the + 51 enhancer was in close proximity to TAL1 promoter 1 in erythroid cells and the + 51 enhancer was separated from the TAL1 promoter in T-ALL cells (Figure 8e). It is likely that the closed chromatin loop between − 31 and + 53 CTCF sites also prevents the upstream STIL promoter from interfering with the TAL1 gene in erythroid cells. Therefore, the CTCF-mediated loop confines or facilitates interaction between the downstream + 51 enhancer and promoter 1 (Figure 8e).
In T-ALL patients, the TAL1 oncogene can be activated by chromosomal translocation and interstitial deletion.22,23,57 However, chromosomal rearrangements account for only less than 30% of cases with aberrant TAL1 expression.23,58,59 How the TAL1 oncogene is aberrantly activated in the majority of T-ALL patients who lack chromosomal rearrangements in the TAL1 locus, and whether dysregulation of enhancer/promoter interactions leads to a disease-causing regulatory variant, remains unknown. Although several enhancer elements have been identified in the TAL1 locus,16 epigenetic, chromatin looping and reporter analysis suggested that none of them is transcriptionally active in T-ALL cells (Figures 1–3). Many enhancers are specified by specific histone marks that may contribute to cellular memory to determine the timing and place of gene transcription.31,36,37 A typical enhancer has characteristics of p300/CBP, binding, H3K4me1 enrichment and transcription factor binding sites.60 The TIL16 element has all of these characteristics, including enrichment of H3K4me1, recruitment of p300/CBP, and T-cell specific c-Maf occupancy. It is conceivable that c-Maf confers a T-cell specific TAL1 promoter interaction that leads to an aberrant TAL1 activation in T-ALL (Figure 8e). There is a strong correlation between the presence of p300 and enhancer function in the human genome37,50, and enhancer-targeted p300/CBP is able to interact with TATA-binding protein and other components of TFIID,51 thereby bridging tissue-specific transcription factors with the Pol II holoenzyme to regulate assembly of a tissue- or cell-type specific transcription complex at promoters.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to members of the Huang laboratory for their suggestions and comments. We thank Drs Christopher Cogle and Zhixiong Xu for generously providing T-ALL samples and for their advice on the 4C assay. This work was supported by grants from the National Institute of Health (SH, R01HL090589, R01HL091929 and R01HL091929-01A1S1-the ARRA Administrative supplement; YQ, R01HL095674; BP, 5T32CA009126-35). KZ is supported by the Intramural Research programs, the National Heart Lung Blood Institute, and the National Institute of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
REFERENCES
- 1.Aplan PD, Begley CG, Bertness V, Nussmeier M, Ezquerra A, Coligan J et al. The SCL gene is formed from a transcriptionally complex locus. Mol Cell Biol 1990; 10: 6426–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begley CG, Aplan PD, Davey MP, Nakahara K, Tchorz K, Kurtzberg J et al. Chromosomal translocation in a human leukemic stem-cell line disrupts the T-cell antigen receptor delta-chain diversity region and results in a previously unreported fusion transcript. Proc Natl Acad Sci USA 1989; 86: 2031–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Q, Yang CY, Tsan JT, Xia Y, Ragab AH, Peiper SC et al. Coding sequences of the tal-1 gene are disrupted by chromosome translocation in human T cell leukemia. J Exp Med 1990; 172: 1403–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finger LR, Kagan J, Christopher G, Kurtzberg J, Hershfield MS, Nowell PC et al. Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma. Proc Natl Acad Sci USA 1989; 86: 5039–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robb L, Lyons I, Li R, Hartley L, Kontgen F, Harvey RP et al. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc Natl Acad Sci USA 1995; 92: 7075–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shivdasani RA, Mayer EL, Orkin SH. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature 1995; 373: 432–434. [DOI] [PubMed] [Google Scholar]
- 7.Robb L, Elwood NJ, Elefanty AG, Kontgen F, Li R, Barnett LD et al. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. Embo J 1996; 15: 4123–4129. [PMC free article] [PubMed] [Google Scholar]
- 8.Porcher C, Swat W, Rockwell K, Fujiwara Y, Alt FW, Orkin SH. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell 1996; 86: 47–57. [DOI] [PubMed] [Google Scholar]
- 9.Hu X, Ybarra R, Qiu Y, Bungert J, Huang S. Transcriptional regulation by TAL1: a link between epigenetic modifications and erythropoiesis. Epigenetics 2009; 4: 357–361. [DOI] [PubMed] [Google Scholar]
- 10.Cardoso BA, de Almeida SF, Laranjeira AB, Carmo-Fonseca M, Yunes JA, Coffer PJ et al. TAL1/SCL is downregulated upon histone deacetylase inhibition in T-cell acute lymphoblastic leukemia cells. Leukemia 2011; 25: 1578–1586. [DOI] [PubMed] [Google Scholar]
- 11.Hu G, Schones DE, Cui K, Ybarra R, Northrup D, Tang Q et al. Regulation of nucleosome landscape and transcription factor targeting at tissue-specific enhancers by BRG1. Genome Res 2011; 21: 1650–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhami P, Bruce AW, Jim JH, Dillon SC, Hall A, Cooper JL et al. Genomic approaches uncover increasing complexities in the regulatory landscape at the human SCL (TAL1) locus. PLoS ONE 2010; 5: e9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogilvy S, Ferreira R, Piltz SG, Bowen JM, Gottgens B, Green AR. The SCL + 40 enhancer targets the midbrain together with primitive and definitive hematopoiesis and is regulated by SCL and GATA proteins. Mol Cell Biol 2007; 27: 7206–7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delabesse E, Ogilvy S, Chapman MA, Piltz SG, Gottgens B, Green AR. Transcriptional regulation of the SCL locus: identification of an enhancer that targets the primitive erythroid lineage in vivo. Mol Cell Biol 2005; 25: 5215–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottgens B, Barton LM, Chapman MA, Sinclair AM, Knudsen B, Grafham D et al. Transcriptional regulation of the stem cell leukemia gene (SCL)--comparative analysis of five vertebrate SCL loci. Genome Res 2002; 12: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottgens B, Barton LM, Gilbert JG, Bench AJ, Sanchez MJ, Bahn S et al. Analysis of vertebrate SCL loci identifies conserved enhancers. Nat Biotechnol 2000; 18: 181–186. [DOI] [PubMed] [Google Scholar]
- 17.Follows GA, Dhami P, Gottgens B, Bruce AW, Campbell PJ, Dillon SC et al. Identifying gene regulatory elements by genomic microarray mapping of DNaseI hypersensitive sites. Genome Res 2006; 16: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silberstein L, Sanchez MJ, Socolovsky M, Liu Y, Hoffman G, Kinston S et al. Transgenic analysis of the stem cell leukemia + 19 stem cell enhancer in adult and embryonic hematopoietic and endothelial cells. Stem Cells 2005; 23: 1378–1388. [DOI] [PubMed] [Google Scholar]
- 19.Gottgens B, Gilbert JG, Barton LM, Grafham D, Rogers J, Bentley DR et al. Long-range comparison of human and mouse SCL loci: localized regions of sensitivity to restriction endonucleases correspond precisely with peaks of conserved noncoding sequences. Genome Res 2001; 11: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bockamp EO, McLaughlin F, Murrell AM, Gottgens B, Robb L, Begley CG et al. Lineage-restricted regulation of the murine SCL/TAL-1 promoter. Blood 1995; 86: 1502–1514. [PubMed] [Google Scholar]
- 21.Gottgens B, Broccardo C, Sanchez MJ, Deveaux S, Murphy G, Gothert JR et al. The scl + 18/19 stem cell enhancer is not required for hematopoiesis: identification of a 5’ bifunctional hematopoietic-endothelial enhancer bound by Fli-1 and Elf-1. Mol Cell Biol 2004; 24: 1870–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown L, Cheng JT, Chen Q, Siciliano MJ, Crist W, Buchanan G et al. Site-specific recombination of the tal-1 gene is a common occurrence in human T cell leukemia. Embo J 1990; 9: 3343–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bash RO, Hall S, Timmons CF, Crist WM, Amylon M, Smith RG et al. Does activation of the TAL1 gene occur in a majority of patients with T-cell acute lymphoblastic leukemia? A pediatric oncology group study. Blood 1995; 86: 666–676. [PubMed] [Google Scholar]
- 24.Carroll AJ, Crist WM, Link MP, Amylon MD, Pullen DJ, Ragab AH et al. The t(1;14)(p34;q11) is nonrandom and restricted to T-cell acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood 1990; 76: 1220–1224. [PubMed] [Google Scholar]
- 25.Condorelli GL, Facchiano F, Valtieri M, Proietti E, Vitelli L, Lulli V et al. T-cell-directed TAL-1 expression induces T-cell malignancies in transgenic mice. Cancer Res 1996; 56: 5113–5119. [PubMed] [Google Scholar]
- 26.Kelliher MA, Seldin DC, Leder P. Tal-1 induces T cell acute lymphoblastic leukemia accelerated by casein kinase IIalpha. Embo J 1996; 15: 5160–5166. [PMC free article] [PubMed] [Google Scholar]
- 27.Palii CG, Perez-Iratxeta C, Yao Z, Cao Y, Dai F, Davison J et al. Differential genomic targeting of the transcription factor TAL1 in alternate haematopoietic lineages. Embo J 2011; 30: 494–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu X, Li X, Valverde K, Fu X, Noguchi C, Qiu Y et al. LSD1-mediated epigenetic modification is required for TAL1 function and hematopoiesis. Proc Natl Acad Sci USA 2009; 106: 10141–10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Wang S, Li Y, Deng C, Steiner LA, Xiao H et al. Chromatin boundaries require functional collaboration between the hSET1 and NURF complexes. Blood 2011; 118: 1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S, Litt M, Felsenfeld G. Methylation of histone H4 by arginine methyltransferase PRMT1 is essential in vivo for many subsequent histone modifications. Genes Dev 2005; 19: 1885–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell 2009; 4: 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Hu X, Patel B, Zhou Z, Liang S, Ybarra R et al. H4R3 methylation facilitates beta-globin transcription by regulating histone acetyltransferase binding and H3 acetylation. Blood 2010; 115: 2028–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagege H, Klous P, Braem C, Splinter E, Dekker J, Cathala G et al. Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nature protocols 2007; 2: 1722–1733. [DOI] [PubMed] [Google Scholar]
- 34.Abou El Hassan M, Bremner R. A rapid simple approach to quantify chromosome conformation capture. Nucleic Acids Res 2009; 37: e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gondor A, Rougier C, Ohlsson R. High-resolution circular chromosome conformation capture assay. Nat Protoc 2008; 3: 303–313. [DOI] [PubMed] [Google Scholar]
- 36.Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature 2009; 459: 108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heintzman ND, Stuart RK, Hon G, Fu Y, Ching CW, Hawkins RD et al. Distinct and predictive chromatin signatures of transcriptional promoters and enhancers in the human genome. Nat Genet 2007; 39: 311–318. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Deng C, Hu X, Patel B, Fu X, Qiu Y et al. Dynamic interaction between TAL1 oncoprotein and LSD1 regulates TAL1 function in hematopoiesis and leukemogenesis. Oncogene 2012; 31: 5007–5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li G, Ruan X, Auerbach RK, Sandhu KS, Zheng M, Wang P et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 2012; 148: 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature 2012; 489: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z et al. High-resolution profiling of histone methylations in the human genome. Cell 2007; 129: 823–837. [DOI] [PubMed] [Google Scholar]
- 42.Follows GA, Ferreira R, Janes ME, Spensberger D, Cambuli F, Chaney AF et al. Mapping and functional characterisation of a CTCF-dependent insulator element at the 3’ border of the murine Scl transcriptional domain. PLoS ONE 2012; 7: e31484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr Opin Genet Dev 2007; 17: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips JE, Corces VG. CTCF: master weaver of the genome. Cell 2009; 137: 1194–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang Y, Sitwala K, Bronstein J, Sanders D, Dandekar M, Collins C et al. Identification and characterization of Hoxa9 binding sites in hematopoietic cells. Blood 2012; 119: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim JI, Ho IC, Grusby MJ, Glimcher LH. The transcription factor c-Maf controls the production of interleukin-4 but not other Th2 cytokines. Immunity 1999; 10: 745–751. [DOI] [PubMed] [Google Scholar]
- 47.Cao S, Liu J, Song L, Ma X. The protooncogene c-Maf is an essential transcription factor for IL-10 gene expression in macrophages. J Immunol 2005; 174: 3484–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murakami YI, Yatabe Y, Sakaguchi T, Sasaki E, Yamashita Y, Morito N et al. c-Maf expression in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol 2007; 31: 1695–1702. [DOI] [PubMed] [Google Scholar]
- 49.Morito N, Yoh K, Fujioka Y, Nakano T, Shimohata H, Hashimoto Y et al. Overexpression of c-Maf contributes to T-cell lymphoma in both mice and human. Cancer Res 2006; 66: 812–819. [DOI] [PubMed] [Google Scholar]
- 50.Birney E, Stamatoyannopoulos JA, Dutta A, Guigo R, Gingeras TR, Margulies EH et al. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 2007; 447: 799–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitsiou DJ, Stunnenberg HG. p300 is involved in formation of the TBP-TFIIA-containing basal transcription complex, TAC. Embo J 2003; 22: 4501–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard O, Azogui O, Lecointe N, Mugneret F, Berger R, Larsen CJ et al. A third tal-1 promoter is specifically used in human T cell leukemias. J Exp Med 1992; 176: 919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schubeler D, Francastel C, Cimbora DM, Reik A, Martin DI, Groudine M. Nuclear localization and histone acetylation: a pathway for chromatin opening and transcriptional activation of the human beta-globin locus. Genes Dev 2000; 14: 940–950. [PMC free article] [PubMed] [Google Scholar]
- 54.Song SH, Hou C, Dean A. A positive role for NLI/Ldb1 in long-range beta-globin locus control region function. Mol Cell 2007; 28: 810–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vakoc CR, Letting DL, Gheldof N, Sawado T, Bender MA, Groudine M et al. Proximity among distant regulatory elements at the beta-globin locus requires GATA-1 and FOG-1. Mol Cell 2005; 17: 453–462. [DOI] [PubMed] [Google Scholar]
- 56.Yusufzai TM, Tagami H, Nakatani Y, Felsenfeld G. CTCF tethers an insulator to subnuclear sites, suggesting shared insulator mechanisms across species. Mol Cell 2004; 13: 291–298. [DOI] [PubMed] [Google Scholar]
- 57.Aplan PD, Raimondi SC, Kirsch IR. Disruption of the SCL gene by a t(1;3) translocation in a patient with T cell acute lymphoblastic leukemia. J Exp Med 1992; 176: 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Breit TM, Mol EJ, Wolvers-Tettero IL, Ludwig WD, van Wering ER, van Dongen JJ. Site-specific deletions involving the tal-1 and sil genes are restricted to cells of the T cell receptor alpha/beta lineage: T cell receptor delta gene deletion mechanism affects multiple genes. J Exp Med 1993; 177: 965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aplan PD, Lombardi DP, Ginsberg AM, Cossman J, Bertness VL, Kirsch IR. Disruption of the human SCL locus by “illegitimate” V-(D)-J recombinase activity. Science 1990; 250: 1426–1429. [DOI] [PubMed] [Google Scholar]
- 60.Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet 2011; 12: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


