Abstract
Purpose:
To assess the effectiveness of 38% silver diamine fluoride (SDF) in arresting cavitated caries lesions in young U.S. children.
Methods:
Children 12–71 months of age with severe early childhood caries participated in this Phase III, multicenter, randomized, placebo-controlled trial. SDF was applied twice (baseline, 6-months), and children followed for 8 months. A planned interim analysis of only the 6-month primary outcome caries arrest data, for approximately half of the cohort (680 of 1,144 children), was conducted using a generalized estimating equation model, accounting for non-independence among caries lesions within a patient.
Results:
599 of the 680 participants, with 1,413 lesions, completed the 6-month exam. Lesions in the SDF group demonstrated 54% arrest vs. 21% in the placebo (P<0.001).
Conclusion:
SDF was effective at arresting active cavitated lesions in this population, leading to the early stop of the trial. Final analyses of all data and other outcomes is currently underway.
Keywords: cavitated lesion, children, primary teeth, SDF, Severe ECC
Introduction
Dental caries, one of the most prevalent chronic diseases among children in the U.S. and the world,1 has long-term dental, medical, societal, economic, and oral health-related quality of life consequences.2 The current standard of care for management of cavitated caries lesions focuses on restorations and extractions.3 Young children with severe early childhood caries (S-ECC)4 and who are uncooperative, have immature cognitive functioning, disabilities, or comorbid medical conditions often have dental treatment completed under general anesthesia (GA).5 Such treatment often results in associated health, economic, and relapse issues.6 In 2017, the U.S. Food and Drug Administration (FDA) approved a warning indicating that GA may negatively affect brain development in children younger than 3-years, and should be delayed if possible.7 Thus, safe, effective, acceptable, inexpensive, less-invasive, easy to implement strategies are needed to successfully manage cavitated lesions in young children. Systematic reviews of existing randomized clinical trials, conducted outside the U.S., have concluded that 38% Silver Diamine Fluoride (or Diammine Silver Fluoride; SDF) could meet these needs.8–10
SDF became available in the U.S. in 2014 for treatment of dental hypersensitivity11 and is being used “off label” for caries management. Because of the urgent need for effective strategies to safely manage S-ECC, the FDA granted Breakthrough Therapy Status to SDF in 2016. This status facilitated SDF efficacy trials to be carried out which are required to obtain a “drug claim” of caries arrest.
The present clinical trial (ClinicalTrials.gov=NCT03649659) was designed specifically to be acceptable to the FDA for a dental caries arrest “drug” claim. An efficient and ethical 8-month evaluation period was used, and sample size calculations included early stopping rules (or interim analyses) for efficacy and futility. Interim analyses allow for a trial to be stopped early if proven either effective or ineffective. The purpose of this study was to assess the effectiveness of 38 percent SDF in arresting cavitated caries lesions in young U.S. children. Findings of the interim analysis, which includes only an assessment of the primary FDA-labeling outcome after approximately half the sample completed the six-month visit, are presented.
Methods
The trial was approved by a central Institutional Review Board (WCG IRB). Written consent was obtained from the parent (parent, legal guardian) of each child.
Study Design:
This was a Phase III, multisite, randomized, quadruple-blinded, placebo-controlled trial, with two parallel groups: SDF (Advantage Arrest™, Elevate Oral Care, LLC., West Palm Beach, FL) and placebo (deionized water and Food Drug and Cosmetics Blue 1 dye, provided by Elevate Oral Care), applied twice (at baseline and 6-months), with participants followed closely for 8 months (Figure 1).
Figure 1-.

Trial Study Design including in Person and Remote Contacts.Blue lines above the horizontal lines indicate in-person visits, while yellow lines below the horizontal lines indicate remote contacts. Note that timeline is not to scale.
Objectives:
The primary FDA-labeling objective (Aim 1) was to assess the efficacy of one application of SDF to arrest active cavitated lesions at 6-month follow-up (also the focus of this manuscript’s interim analysis). The hypothesis was that SDF is superior to placebo. This was accomplished by assessing changes in dentin hardness (e.g., lesion going from active/soft to arrested/hard) using the International Caries Detection and Assessment System (ICDAS) criteria11 on frank cavitated lesions [ICDAS score 5 (less than half the surface of the tooth) or 6 (half or more)].
Secondary aims were to: assess the efficacy at 8-months follow-up when applied twice, 6 months apart (Aim 2), compare the effects at 3- and 6-months follow-up of one application (Sub-Aim 2a), assess the impact on children’s pain when applied twice, 6 months apart (Aim 3), and assess the impact on pain after a single application (assessed at 3- and 6-months follow-up; Sub-Aim 3a). Tertiary aims were to: assess the impact on family-level outcomes (Aim 4), including assessing the impact on oral health-related quality of life (Sub-Aim 4a), and on treatment satisfaction and acceptability (Sub-Aim 4b).
Recruitment:
Children were recruited between 2018–2022 from a variety of settings (e.g., Head Start Centers, preschools, dental and medical clinics, online) by study personnel at the University of Michigan, New York University and the University of Iowa. Children aged 12–71 months of age were eligible if their behavior allowed for examination of the oral cavity and application of treatment, had S-ECC, and had at least one SDF-target tooth with a soft cavitated caries lesion(s) that allowed for direct hardness assessment and application of treatment. In addition, the parent who provided written informed consent was at least 18 years old or an emancipated minor and participated in trial activities.
Exclusion criteria included: presence of pain due to caries, pulpal exposure, signs of pulpal infection (abscess, fistula, swelling), or mobility not associated with exfoliation; presence of any gingival or peri-oral ulceration, abscess, or stomatitis; known allergy/sensitivity to silver or other heavy metal ions; inability to comply with trial protocol requirements; chronic disease, conditions, or defects such as, but not limited to, osteopenia, osteoporosis, chronic kidney disease, Amelogenesis Imperfecta, Dentinogenesis Imperfecta, hypothyroidism, hyperparathyroidism, or hypocalcemia; and chronic glucocorticoid, anticonvulsants, chemotherapy, or bisphosphonate administration. Children were also ineligible if they were in foster care, or if parent demonstrated inability to comply with trial requirements, and/or inability to read and comprehend the consent document or trial questionnaires in English, Spanish, or Mandarin.
Treatment Procedures:
Allocation to SDF or placebo was 1:1. Products were concealed in identical unit-dose ampules, identified by random numbers, and presented as liquids of equal color (so they could not be identified). The biostatistician, parent, participant, examiners, and all trial team members remained masked regarding group allocation.
Randomization was at the participant-level; thus, all teeth within a participant received the same product. The personnel applying the concealed product (trained hygienists) were distinct from the examiners to minimize issues associated with bias and masking. In addition, the primary outcome of hardness was measured independently from the outcome of color, as lesions can be black yet still be active (Figure 2).13–14
Figure 2-.
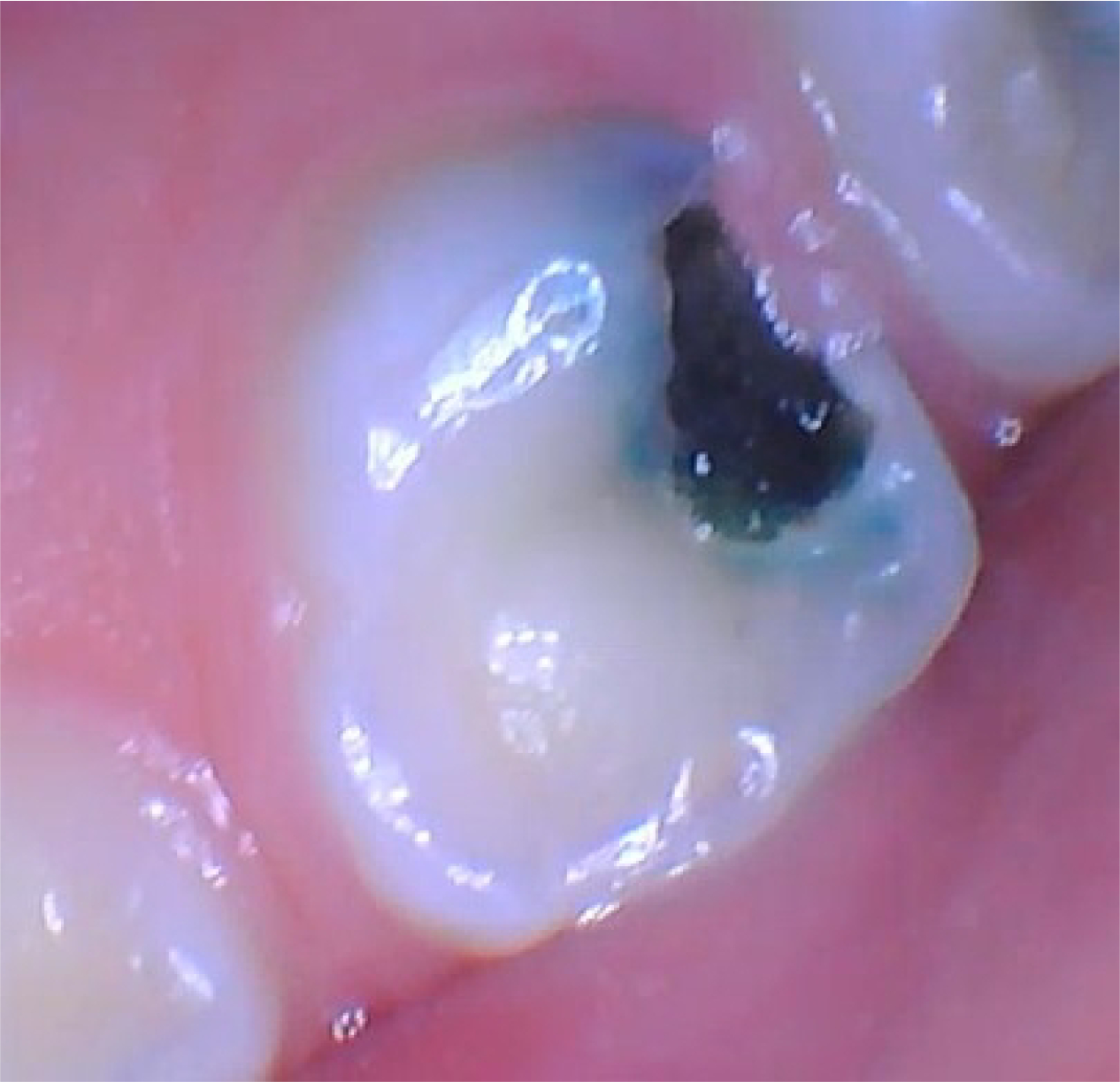
Color is a Poor Indicator of Lesion Activity (Example of Lesion at the 8 Month Clinical Visit). Images are of a study tooth showing an ICDAS 5 soft lesion that increased in size throughout the trial visits and thus was active, even though it was black.
Participants were examined and study lesions were treated at baseline and 6-months, in-person safety visits were conducted 24h after each application, and additional examinations occurred at 3- and 8-months (Figure 1). Status check-ins were made by phone by trained study personnel between all in-person visits.
Included caries lesions were dried, and treated by rubbing the solution onto the entire carious dentin for 10 seconds using a microbrush. Excess was blotted dry to prevent unnecessary swallowing. No other professional topical fluoride treatment was allowed the same day. There were 2–3 examiners per clinical site (dentists or pediatric dentists) who were trained and calibrated annually on ICDAS criteria for lesion severity and activity, and assessment of lesion color. Intra- and inter-examiner reliability were assessed with kappa statistics, with thresholds (0.75 for lesion severity and 0.70 for lesion hardness) met for all examiners annually.
Sample size calculation:
The target sample size was 1,144 enrolled children, based on a 2-sided test for a difference in proportions with cluster randomization (participant as the cluster) at significance level α=0.1%, with 80% power to detect a superiority difference of 10% for SDF compared to placebo. The calculations also accounted for the use of early stopping rules (interim analyses) for efficacy and futility after approximately half the sample completed the 6-month visit, using a Lan-Demets spending function with O’Brien-Fleming type boundary15 with p-values for efficacy and non-binding p-values for stopping due to futility at 50% and 100% completion.
Analyses:
Analyses included an intent-to-treat approach, where all randomized subjects were included (not only those who finished the study). The interim analysis focused on comparing the proportion of arrested caries lesions between the SDF and placebo-treated groups at the 6-month visit using a generalized estimating equation model for binary outcomes. An exchangeable correlation accounted for possible non-independence among multiple caries lesions within a patient (lesion is the unit of analysis). Trial site was included as a covariate since randomization was stratified by site. A separate unblinded statistician was responsible for the interim analyses.
Multiple imputation (based on race/ethnicity, gender, age, trial site, initial ICDAS score, tooth location, and most recent prior visit results) was used for handling missing data. Twenty-five imputed datasets were created, and results combined using standard methods.
Results
Interim analyses included 680 enrolled participants of which 599 (including 1,413 lesions) completed the 6-month examination. The trial’s 8-month follow-up timeline forced in-person visits into a tight 19-day window. The COVID-19 pandemic occurred halfway through this trial resulting in participant withdrawal due to clinic closure. Overall attrition rate was 12%. However, participants were retained if their 6-month visit occurred within a 60-day window given that in clinical practice there is often some latitude in biannual visits. Therefore, in the interim analyses, two definitions of valid 6-month exams were used: the original window (± 19 days) and an expanded window due to COVID-19 (± 60 days). For both windows, SDF was more effective than placebo at arresting caries lesions (54–55% SDF, 21% Placebo) (Table 1).
Table 1:
Interim Analysis for Caries Lesion Arrest at 6-Months after Treatment for Approximately half of the Planned Sample Size
| Treatment | Estimated Proportion of Arrested Lesions (95% confidence interval) | P-value* |
|---|---|---|
| Original Window 6-month visit | ||
| 38% Silver Diamine Fluoride | 0.54 (0.47–0.61) | <0.001 |
| Placebo | 0.21 (0.16–0.26) | |
| Expanded COVID-19 Window 6-month visit | ||
| 38% Silver Diamine Fluoride | 0.55 (0.49–0.62) | <0.001 |
| Placebo | 0.21 (0.15–0.26) | |
The probability of observing proportions of arrested caries lesions in the silver diamine fluoride and placebo-treated groups at the six-month visit is at least as different as the observed result if the null hypothesis (equal proportions) is true, using a generalized estimating equation model for binary outcomes with a logit link. An exchangeable correlation accounted for possible non-independence among multiple caries lesions within a patient. The trial site was included as a covariate since randomization was stratified by site.
Based on these findings, on September 12, 2022, it was determined that the trial should conclude early due to achieving its primary endpoint of effectiveness, with no safety concerns. At that time, 831 participants, representing a very diverse cohort (Table 2), had been enrolled in the trial and will be included in future final analyses.
Table 2-.
Socio-demographic Characteristics of Enrolled Participants included in the Interim analysis and all enrolled participants at the Time the Trial was Terminated Early Due to Efficacy
| At Interim analysis | Total Enrolled | |
|---|---|---|
| N | 599 | 831 |
| Sex | ||
| Female | 320 (53%) | 428 (52%) |
| Male | 279 (47%) | 403 (48%) |
| Ethnicity | ||
| Hispanic or Latino | 252 (42%) | 365 (44%) |
| Not Hispanic or Latino | 331 (55%) | 447 (54%) |
| Unknown | 4 (1%) | 6 (1%) |
| Not Reported | 12 (2%) | 13 (2%) |
| Race | ||
| White | 203 (34%) | 269 (32%) |
| Black or African American | 118 (20%) | 171 (21%) |
| Asian | 39 (7%) | 50 (6%) |
| American Indian or Alaskan Native | 5 (1%) | 6 (1%) |
| Multiracial | 41 (7%) | 61 (7%) |
| Unknown | 46 (8%) | 61 (7%) |
| Not Reported | 147 (25%) | 213 (26%) |
Discussion
Approval of SDF for caries arrest would be highly innovative, given how few products are approved by the FDA specifically to prevent or arrest dental caries (e.g., over-the-counter fluoride dentifrices and mouthrinses). Currently, SDF is cleared by the FDA as a medical “device” to manage hypersensitivity, while use to arrest caries lesions remains “off label”. Similar FDA clearance for other professionally-applied caries control products, such as fluoride varnishes (FV), has allowed great variability of products and characteristics (color, flavor, texture, etc.). Studies in vitro and in vivo of fluoride release and in vitro of remineralization from some commercially-available FV have shown wide variation in results, leading to concerns about the clinical efficacy of some products.16–18 However, without good clinical caries data or agreement on surrogate testing, it is not possible to ascertain the validity of these concerns or if changes in product formulation would impact efficacy. Labeling SDF with an indication for caries arrest would support dental professional clinical decision-making, enhance usage, facilitate insurance coverage, ensure public safety and efficacy for diverse U.S. populations, and transform the landscape for research and development of new SDF products.
Future final analyses will include the entire cohort and outcomes from Aims 2, 3 and 4 from a diverse sample. These outcomes are extremely relevant for the provider, patient and parent acceptance, and implementation. In addition, most SDF studies conducted outside the U.S. rely on participant and parents’ reports of safety rather than in-person assessments as it was done in this trial. The study protocol was carefully designed to minimize and control sources of bias to ensure high quality and confidence in the findings. For example, because color may not be an accurate indicator, the outcomes of hardness and color were separated in this study. Chu et al.13 found that while 100% of SDF-treated lesions turned black, 66% of FV-treated and 42% of not-treated lesions also turned black over time.
The cohort included in this trial had S-ECC. Little is known about natural caries arrest in such a high-caries risk group. Previous SDF studies on preschool children with ECC showed the mean number of arrested lesions in the SDF group was 1.5 to 2.6 times the mean in the control group.13–14 Therefore, a conservative mean ratio of 1.5 was used in sample size calculations. A 2020 trial in 1–3 year-old children showed a 21% caries arrest rate at 6 months in the SDF-treated group vs.12% in the placebo group after a single application.19 As this was significantly lower than the arrest rate for older children with ECC (~66–81%),9–10 the authors suggested that technique difficulties in this young cohort could be partly responsible for the findings. In the present trial, even when young participants had S-ECC, the arrest rate was higher than that reported previously for 1–3 year-old children,19 but still lower than averages for older children.9–10 Final analyses of the entire cohort will provide final estimates as well as results concerning secondary and tertiary aims.
Conclusions
Based on this study’s results, the following conclusions can be made:
Silver diamine fluoride can significantly improve the arrest of some active cavitated lesions in young children with severe early childhood caries.
Analyses of final trial data and other aims are currently underway.
Acknowledgements
This study was supported by grant UH3DE027372 from the U.S. National Institutes of Health. The authors wish to thank all the participating families, multiple recruitment partners (clinical sites and the many dentists, pediatric dentists, hygienists, pediatricians, schools, Head Start programs, and early childhood centers that participated), clinical trial staff members, examiners, co-investigators at the three clinical sites (At the University of Michigan: Livia M.A. Tenuta, DDS, MSc, PhD, Associate Professor, Marcia Campos, DDS, MS, PhD, Clinical Associate Professor, Elisabeta Karl, DDS, MS, PhD, Clinical Associate Professor, James Boynton, DDS, MS, Clinical Professor, Susan Flannagan, MA, Cariology Laboratory Manager, Maimoonah Riaz, RDA, RDH, BSDH, MS, Dental Hygiene Study Coordinator, Elizabeth I. Pitts, RDH, MS, Dental Hygiene Study Coordinator, Taylor Cezon, RDH, BSDH, Dental Hygiene Study Coordinator, Kristin Miller, RDH, BSDH, Dental Hygiene Study Coordinator, Meredith McEachern, RDH, BSDH, Dental Hygiene-Research Assistant, Andrew Chen, BS, Research Assistant, Chris Oshana, BS, Research Assistant and Shelby Yesney, BS, Research Assistant. At New York University: Courtney Chinn, DDS, MS, Clinical Associate Professor, Alex Sheen, DDS, MS, Clinical Assistant Professor, Xesubel Hernandez, Study Coordinator. At the University of Iowa: Justine Kolker, DDS, MS, PhD, Fuller-Denehy Professor of Operative Dentistry, John Warren, DDS, MS, Professor, Amy B. Lesch, DDS, MS, Assistant Professor, for making this study possible, Advantage Arrest and Peter Milgrom, DDS, MS, Professor, University of Washington for use of the Investigational New Drug Application to the FDA, and Elevate Oral Care for providing the drug used in the trial.
Footnotes
Declaration of Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Margherita Fontana, Department of Cariology, Restorative Sciences and Endodontics, School of Dentistry, University of Michigan, Ann Arbor, MI.
Divya Khera, Department of Pediatric Dentistry, College of Dentistry, New York University, New York, NY, USA.
Steven Levy, Department of Preventive and Community Dentistry, College of Dentistry, and Professor, Department of Epidemiology, College of Public Health, University of Iowa, Iowa City, Iowa, USA.
George Eckert, Department of Biostatistics and Health Data Science, School of Medicine and Richard M. Fairbanks School of Public Health, Indiana University, Indianapolis, Ind., USA.
Barry Katz, Department of Biostatistics and Health Data Science, School of Medicine and Richard M. Fairbanks School of Public Health, Indiana University, Indianapolis, Ind., USA.
Emily Yanca, Department of Cariology, Restorative Sciences and Endodontics, School of Dentistry, University of Michigan, Ann Arbor, MI.
Carlos González-Cabezas, Department of Cariology, Restorative Sciences and Endodontics, School of Dentistry, University of Michigan, Ann Arbor, MI.
Amr Moursi, Department of Pediatric Dentistry, College of Dentistry, New York University, New York, NY, USA.
References
- 1.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of untreated caries: A systematic review and meta regression. J Dent Res 2015;94(5):650–8. [DOI] [PubMed] [Google Scholar]
- 2.Tinanoff N, Baez RJ, Diaz Guillory C et al. Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: global perspective. Int J of Paediatr Dent 2019;29(3):238–48. [DOI] [PubMed] [Google Scholar]
- 3.Santamaría RM, Abudrya MH, Gül G, Mourad MS, Gomez GF, Zandona AGF. How to iIntervene in the caries process: Dentin caries in primary teeth. Caries Res 2020;54(4):306–23. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatric Dentistry. Policy on Early Childhood Caries (ECC): Consequences, and preventive strategies. The Reference Manual of Pediatric Dentistry. Chicago, Ill.: American Academy of Pediatric Dentistry; 2021: 90–93. [Google Scholar]
- 5.American Academy of Pediatric Dentistry. Policy on the use of deep sedation and general anesthesia in the pediatric dental office. The Reference Manual of Pediatric Dentistry. Chicago, Ill.: American Academy of Pediatric Dentistry; 2012: 94–95. [Google Scholar]
- 6.Foster T, Perinpanayagam H, Pfaffenbach A, Certo M. Recurrence of early childhood caries after comprehensive treatment with general anesthesia and follow-up. J Dent Child (Chic) 2006;73(1):25–30. [PubMed] [Google Scholar]
- 7.FDA Drug Safety Communication: FDA Drug Safety Communication: FDA approves label changes for use of general anesthetic and sedation drugs in young children; 2017. Accessed online June 15, 2023. “https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-approves-label-changes-use-general-anesthetic-and-sedation-drugs”.
- 8.Slayton RL, Urquhart O, Araujo MWB, et al. Evidence-based clinical practice guideline on non-restorative treatments for carious lesions: A report from the American Dental Association. J Am Dent Assoc 2018;149(10):837–49. [DOI] [PubMed] [Google Scholar]
- 9.Gao SS, Zhao IS, Hiraishi N et al. Clinical Trials of Silver Diamine Fluoride in Arresting Caries among Children: A Systematic Review. JDR Clin Trans Res 2016;1(3):201–10. [DOI] [PubMed] [Google Scholar]
- 10.Gao SS, Zhang S, Mei ML, Lo ECM, Chu CH. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment - a systematic review. BMC Oral Health 2016;16(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Food and Drug Administration. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K102973 Accessed online September 21, 2023.
- 12.International Caries Detection and Assessment System (ICDAS) Coordinating Committee. Criteria Manual: International Caries Detection and Assessment System (ICDAS II). Available at: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.iccms-web.com/uploads/asset/5ccb149905404942610729.pdf. Accessed online September 21, 2023.
- 13.Chu CH, Lo ECM, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Den Res 2002;81(11):767–70. [DOI] [PubMed] [Google Scholar]
- 14.Llodra JC, Rodriguez A, Ferrer B, Menardia V, Ramos T, Morato M. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res 2005;84(8):721–4. [DOI] [PubMed] [Google Scholar]
- 15.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659–663. [Google Scholar]
- 16.Lippert F, Hara AT, Martinez-Mier EA, Zero D. In vitro lesion rehardening and enamel fluoride update from fluoride varnishes as a function of application mode. Am J of Dent 2013;26(2):81–5. [PubMed] [Google Scholar]
- 17.Gonzalez-Cabezas C, Flannagan S. Impact of different fluoride varnish formulations on enamel demineralization prevention. J Dent Res 2014;93(SI B):76. [Google Scholar]
- 18.Gonzalez-Cabezas C, Flannagan S. Enamel Fluoride Uptake from Varnishes with Different Fluoride Release. J Dent Res 2015;94(SI A):0223. [Google Scholar]
- 19.Mabangkhru S, Duangthip D, Chu CH, Phonghanyudh A, Jirarattanasopha V. A randomized clinical trial to arrest dentin caries in young children using silver diamine fluoride. J Dent 2020;99:103375. [DOI] [PubMed] [Google Scholar]


