Abstract
Extracellular vesicles (EVs) are a new revelation in cross-kingdom communication, with increasing evidence showing the diverse roles of bacterial EVs (BEVs) in mammalian cells and host-microbe interactions. BEVs include outer membrane vesicles (OMVs) released by gram-negative bacteria and membrane vesicles (MVs) generated from gram-positive bacteria. Recently, BEVs have drawn attention for their potential as biomarkers and therapeutic tools because they are nano-sized and can deliver bacterial cargo into host cells. Importantly, exposure to BEVs significantly affects various physiological and pathological responses in mammalian cells. Herein, we provide a comprehensive overview of the various effects of BEVs on host cells (i.e., immune cells, endothelial cells, and epithelial cells) and inflammatory/infectious diseases. First, the biogenesis and purification methods of BEVs are summarized. Next, the mechanisms and pathways identified by BEVs that stimulate either pro-inflammatory or anti-inflammatory responses are highlighted. In addition, we discuss the mechanisms by which BEVs regulate host-microbe interactions and their effects on the immune system. Finally, this review focuses on the contribution of BEVs to the pathogenesis of sepsis/septic shock and their therapeutic potential for the treatment of sepsis.
Keywords: Bacterial extracellular vesicles (BEVs), Outer membrane vesicles (OMVs), Pro-inflammation, Anti-inflammation, Host-Microbe Interactions, Sepsis
1. Introduction
Extracellular vesicles (EVs) are non-replicative nano-sized particles with a diameter range of 20–300 nm, released by eukaryotic cells, bacteria, and archaea from their outer membranes into the surrounding environment1,2. EVs can also be referred to as membrane vesicles (MVs), outer membrane vesicles (OMVs), microvesicles, exosomes, or ectosomes, depending on their parent cells and characteristics3,4. In 1966, bacterial EVs (BEVs) were first observed under electron microscopy as lipid-like structures in Escherichia coli culture supernatants5. Both Gram-positive and Gram-negative bacteria produce EVs. However, EVs from Gram-positive bacteria were not reported until 20096. This is likely because Gram-positive bacteria lack an outer membrane and instead have a thick peptidoglycan layer, which hinders EV production.
Since their discovery, BEVs have been found to contain various types of molecular and surface cargo, including proteins, nucleic acids, lipids, and various other metabolites, such as virulence factors7. Importantly, these BEV cargoes can elicit diverse effects on bacterial surveillance, including nutrient uptake, biofilm formation, horizontal gene transfer, antimicrobial activity, virulence factor secretion, and toxin release7. In addition, BEVs can transport their contents to recipient cells either extracellularly through receptor-ligand interactions or can be absorbed via membrane fusion or receptor-mediated endocytosis into host cells8 9. BEVs also function as bridges for cross-kingdom communication. For example, BEVs have been demonstrated to interact with mammalian cells and thereby affect their behavior, including cell proliferation, angiogenesis, and immunoregulation, from triggering proinflammatory responses in immune cells to several other immunomodulatory effects in non-immune cells10–12. Interestingly, many studies have indicated that BEVs can play either pro-inflammatory or anti-inflammatory roles, depending on their cargoes13–16.
The immunomodulatory qualities and abilities of BEVs to deliver cargo to host cells have made them desirable tools for future therapies and disease prevention. Additionally, the non-replicative nature of BEVs renders them noninfectious and, therefore, relatively safe for various biomedical applications, such as targeted drug delivery and vaccine development12. In this review, we summarize the current knowledge about the role of BEVs in (i) pro-inflammatory responses, (ii) anti-inflammatory effects, (iii) alteration of host-microbe interactions, and (iv) sepsis development and prevention/treatment.
2. Biogenesis of bacterial extracellular vesicles (BEVs)
BEV synthesis varies between gram-negative and gram-positive bacteria because of differences in their outer cell structures. Gram-negative bacteria contain an outer membrane underlying a thin peptidoglycan layer, whereas gram-positive bacteria lack an outer membrane and contain a thick peptidoglycan layer. The detailed mechanisms of BEV synthesis in gram-negative bacteria have been summarized in our previous review7. Briefly, four models have been proposed: (i) reduced cross-links between the outer membrane and peptidoglycan layer lying underneath17,18; (ii) accumulation of some envelope proteins, peptidoglycan fragments, and membrane lipids that exert pressure on the membrane, resulting in membrane budding off in the form of BEVs19; (iii) accumulation of lipopolysaccharide (LPS) and phospholipids causing BEV biogenesis20 (iv) extracellular stimulation for local curvature formation in the bacterial outer membrane. For example, through extracellular stimulation, the Pseudomonas quinolone signal (PQS) can induce membrane blebbing in Pseudomonas aeruginosa (P. aeruginosa)21.
In addition, several factors (i.e. as growth conditions, environmental stress, and genetic factors) have been reported to affect BEV synthesis7. Notably, high growth temperatures22, oxygen stress23, chemical stress, and other forms of physical stress have been shown to increase EV production in various bacterial species24,25. Furthermore, pathogenic bacteria have been found to produce more EVs than their counterpart non-pathogenic strains26. This suggests that BEV production may have been adopted by pathogens to increase their virulence. Recent studies have demonstrated that BEV biogenesis does not occur randomly; rather, it is regulated by genetic factors. For example, PSMα3 can promote EV secretion, and deletion of PSMα3 results in significant reductions in EV secretion from Staphylococcus aureus27.
It is important to note that manipulation of BEV biogenesis by various means is species-specific in terms of their effects on bacterial growth and BEV production28–31. Interestingly, one effective approach is to alter culture media nutrients29, 30. For instance, depletion of sulfate from the culture system could increase EV production from N. meningitides, because the VacJ/Yrb ABC (ATP-binding cassette, a phospholipid transporter) is utilized to generate BEVs through removing excess phospholipid content caused by sulfate deletion29. A recent study by Hirayama et al.30 identified that glycine, a known disruptor of peptidoglycan, significantly promoted BEV yields from E. coli. In addition, nearly trifold amounts of Propionibacterium freudenreichii EVs are produced when cultured in milk ultrafiltrate (UF) medium compared with yeast extract lactate (YEL)32. Besides the media nutrient composition, pH also affects BEV production from Lactobacillus casei and Lactobacillus plantarum31.
Furthermore, genetic modifications of bacteria by altering the expression of PGase, a protein that interferes with peptidoglycan layer integrity, could favor BEV biogenesis33. With future in-depth mechanistic findings of BEV biogenesis, an increasing number of bioengineering tools will be developed to promote or block BEV generation.
3. Isolation and detection of BEVs in laboratory settings
Recently, BEVs have been used in advanced nanotherapeutics for the delivery of anti-microbials, chemotherapy, drugs, and vaccine developments34–36. However, the small- or large-scale production of BEVs is often challenging and requires an additional purification method to collect enough BEVs37. Therefore, an ideal approach for isolation is expected to yield a large number of BEVs with intact structure38. The most widely used techniques to isolate and concentrate BEVs are ultracentrifugation (UC) and ultrafiltration (UF)38. Notably, depending on the method used, the physical properties (morphology, size, and total yield) of BEVs differ. For instance, Reimer et al. (2021) reported that although UC and UF are both useful techniques for BEV extraction, UF is much faster and yields a higher concentration of small-sized vehicles than UC38. Similarly, upon isolation and purification, BEVs were further subjected to functional assays to test their ability to enter host cells and stimulate cytokine and chemokine production39. However, different methodologies used to extract BEVs can impact the following experimental analysis38. In this regard, we briefly summarize the methodologies below: An overall schematic of BEV isolation and quantification is outlined in Figure 1.
Figure1:
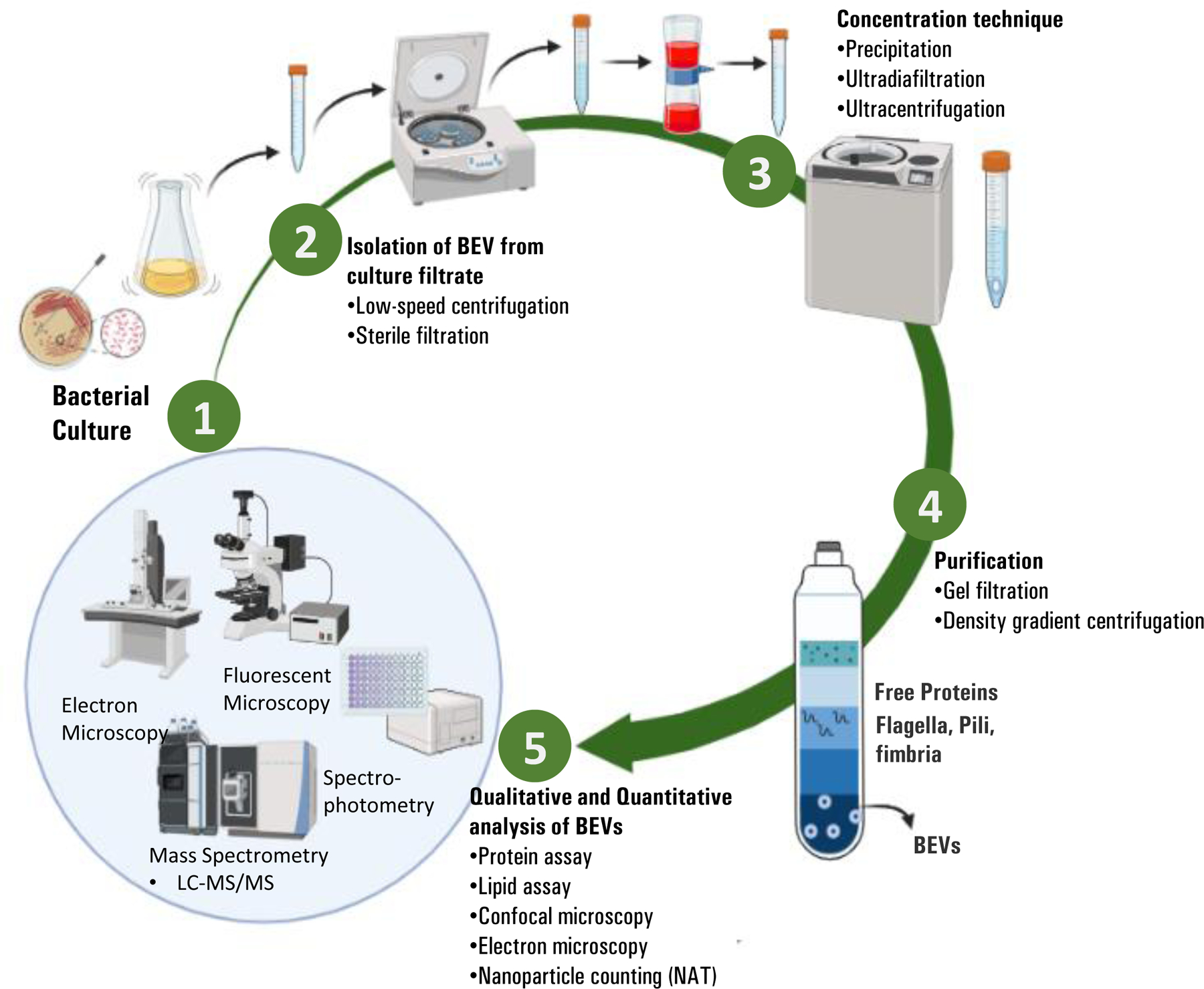
Overview of BEV preparations. (1) Bacterial Culture: properties of BEVs are affected by several factors including growth medium, temperature, oxidative stress, hyperosmotic shock, iron depletion etc. (2) Isolation of BEVs from culture filtrates: achieved by low-speed centrifugation. (3) Concentration: Additional steps to ensure the high yields and purity of BEVs are obtained by ultracentrifugation and ultra-diafiltration. (4) Purification: density gradient centrifugation or gel filtration allows the separation of BEVs from other interfering extracellular matrix (i.e., flagella, pili, and fimbria). (5) Qualitative and quantitative analysis of BEVs: different approaches either measure the BEV cargo content (i.e., protein, lipids, DNA, RNA, or other particles), or BEV morphology.
3.1. Isolation and purification of BEVs
Usually, BEVs can be harvested from the cell-free supernatants of bacterial culture37. Compared with gram-positive bacteria, more attention has been focused on the isolation and purification of gram-negative BEVs34, 40–44. Nevertheless, the isolation and purification steps for both gram-negative and gram-positive bacteria are similar. The isolation of BEVs begins by separating the supernatant containing vesicles from the bacterial cell debris, large proteins, and membrane aggregates, which is achieved by high-speed centrifugation or several differential centrifugation steps. The supernatant was further filtered to remove residual bacterial components. However, various BEVs may have differential size distributions and form large aggregates that can lead to an almost 50% loss of isolated EVs. Considering the low yield of BEVs, pre-concentration steps are crucial, which are usually achieved by ultracentrifugation or ultrafiltration40. Unfortunately, ultracentrifugation can also fail to separate BEVs from interfering with extracellular materials, such as flagella, fimbria, pills, and large protein complexes38. In this scenario, density gradient centrifugation or gel filtration played a significant role in the purification of BEVs (Fig. 1). For example, iodixanol is a commonly used density-gradient medium for the purification of BEVs40.
3.2. Qualitative and quantitative detection of BEVs.
Purified BEVs are further identified (qualitative) and quantified using various laboratory approaches37. The particulate structure of BEVs contains several proteins, lipids, DNA, RNA, and other biomolecules. The quantitative values of these biomolecules act as proxies for the quantification of isolated BEVs36. Several different protein tests, including Bradford, protein bicinchoninic acid (BCA), Lowry, and immunoblotting (tracking specific proteins) are usually performed for BEV quantification37,39. Likewise, quantification of BEV proteins using Qubit fluorometric analysis has been the method of choice in multiple studies34,37. Similarly, previous studies have shown that the quantification of lipids, particularly 3-deoxy-D-manno-octulosonic acid, a specific component of LPS, can reflect the number of BEVs isolated (Malachite Green)38,40. Quantification of lipids can be achieved by using a lipophilic dye (FM-64) or enzyme-linked immunosorbent assay (ELISA) targeting LPS40. Similarly, with the advancement of cutting-edge technologies (e.g., mass spectrometry), the absolute quantification of BEVs, including proteins and lipids (phospholipids and fatty acids) is possible40. In addition to absolute quantification of BEV properties, visualization of BEVs can be performed using transmission electron microscopy (TEM). This approach can provide critical information such as shape, size, and the presence of interfering non-EV components, mainly flagella and large protein aggregates40.
Growing research has shown that BEV components such as protein, DNA, RNA, and lipids vary between different species, and these components may not directly reflect BEV concentrations34. Thus, there is a dire need for a standardized approach to address the factors that may interfere with BEV isolation and quantification, such as bacterial growth conditions, bacterial stages at which BEVs are isolated, their size, and the content of BEVs. Considering these limitations, NanoSight nanoparticle tracking analysis (NAT) is gaining popularity because of its ability to quantify BEVs irrespective of BEV contents34.
Taken together, variation in the quantitative value of BEVs not only exists between different bacterial species and strains, but also between different types of quantitative assays used. Bitto et al.34, 39 reported that the BEV quantification method could alter the biological functions of BEVs, which was evident by the significantly different experimental outcomes. For example, they observed higher production of interleukin-8 (IL-8) in cells stimulated by an equivalent BEV concentration that was quantified using Bradford when compared to cells stimulated with BEVs quantified by BCA or Qubit protein assay39. This disparity in response is possible due to the lack of a standardized protocol for the isolation, purification, and quantification of BEVs. Thus, future studies will be paramount to establish a standardized method for isolating and quantifying BEVs by addressing interfering factors.
4. The effects of BEVs in pro-inflammatory responses
Over the past decade, numerous studies have indicated that BEVs elicit various effects on host cells, and many studies have further explored the functional role of BEVs in their parent bacteria45–47. Recently, multiple groups have demonstrated that BEVs stimulate diverse pro-inflammatory responses48,49, as different composition/contents encased in BEVs could affect distinct receptors of targeting cells and the intracellular signaling pathways50–52, as summarized below and depicted in Figure 2.
Figure 2:

Model of BEV-mediated pro-inflammation mechanisms. Gram-positive/gram-negative bacteria produce BEVs with MAMPs from cell membrane components LTA/lipoproteins and LPS respectively. LPS and LTA/lipoproteins activate membrane receptors TLR2 and TLR4 respectively, which results in intracellular signaling with MyD88. Further intracellular signaling activates NF-κB, a transcription factor that causes the production of pro-inflammatory cytokines and chemokines that contribute to various immune responses, as well as inactive cytokines. Intracellular receptors such as NOD1 can also contribute to NF-κB expression via activation from peptidoglycan of BEVs transported into the cell and aid from RIPK2 signaling. Endosomal TLR7, TLR8, TLR9, all activated by RNA, can also increase NF-κB expression, while other bacterial ligands such as gingipains can activate MAPK and NF-κB signaling pathways. Alternatively, intracellularly transported BEVs utilize other proteins such as GBPs, SNX-10, and Hemolysin for exposing LPS (or directly exposing bacterial toxins such as flagellum) to inflammasomes NLRP3/NLRC4, which activate caspase-1 and caspase-11 by transforming into inflammasome complexes. Then, caspase-1 can reactivate inactive cytokines, produce more pro-inflammatory cytokines, or can even induce pyroptosis via GSDMD. Caspase-11 is also capable of increasing pro-inflammatory cytokine production and inducing pyroptosis. Some BEVs can activate inflammasome pathways by K+ efflux or mitochondrial dysfunction, which can cause pyroptosis by reducing anti-apoptotic factor MCL-11.
4.1. Activation of pattern recognition receptors
Toll-like receptors (TLRs) are pattern recognition receptors (PRRs) present on mammalian cells that regulate the host immune system by responding to microbe-associated molecular patterns (MAMPs)53. TLRs belong to the type 1 transmembrane glycoprotein family, which is composed of a transmembrane, intracellular Toll-like/interleukin-1 receptor (TIR), and an extracellular domain54. The extracellular domain comprises 20 leucine-rich repeat (LRR) modules that determine how TLRs differentiate between different MAMPs54. On the other hand, the intracellular TIR domain is pivotal in controlling downstream signaling cascades and levels of signaling molecules and transcription factors, such as myeloid differentiation primary response 88 (MyD88) and nuclear factor kappa B (NF-κB), which subsequently promote the secretion of pro-inflammatory cytokines55. TLRs allow the immune system to identify unknown particles and protect against invading foreign particles53.
Ten different TLR types were identified based on location and ligand recognition. Of these, six TLRs were related to bacterial components and four were related to nucleic acids. Recent studies have demonstrated the ability of BEVs to interact with TLR2 and TLR4 and induce pro-inflammatory responses (Fig. 2)55–59. The BEV-mediated activation of TLRs can be attributed to the membrane composition of their parent bacteria. For example, BEVs from gram-positive bacteria contain lipoteichoic acid (LTA), and gram-negative BEVs contain LPS, which can activate TLR2 and TLR456. This concept of TLR activation by BEV-sourced ligands is supported by the fact that inhibition of TLR4-LPS binding suppresses the proinflammatory response elicited by LPS-containing EVs from gram-negative E. coli60. The role of LPS in BEV-activated TLR4 has been further documented, as Pep19–2.5, an inhibitor of several MAMPs, including LPS, can reduce pro-inflammatory cytokines stimulated by BEV-LPS and TLR4 activation58. Moreover, the importance of BEV-TLR interactions in immunomodulation is also demonstrated by how E. coli EVs struggle to invoke pro-inflammatory responses in immune cells (e.g., macrophages) when TLR4 is absent from the system59.
Despite the multitude of studies supporting the TLR4 and TLR2 pathways as the main mechanisms of BEV pathogenesis, recent studies have implicated that BEV pathogenesis is not fully dependent on LPS/LTA-activated receptors61–64. For example, several groups have highlighted that various MAMPs found in BEVs are involved in other TLR-associated pro-inflammatory responses61–64. In addition, S. aureus EVs containing RNA and DNA activate TLR7, TLR8, and TLR9, whereas S. aureus EV peptidoglycans activate TLR261. As a matter of fact, TLR2, TLR4, and TLR9, each has various protein- and lipid-based ligands, and with the aid of other molecules (e.g., superantigens and phenol-soluble modulins), they play a critical role in developing an inflammatory response. Thus, different BEVs may activate different inflammation pathways65. However, some studies suggest that S. aureus EVs produce a pro-inflammatory response preferably through the lipoprotein/TLR2 pathway, where lipoproteins act as the primary MAMPs compared to other MAMPs for TLR2, such as nucleic acids and peptidoglycan62. For example, lipoproteins have been identified as the main MAMP in Fillifactor alocis EVs for TLR2. Importantly, lipoprotein/TLR2-induced pro-inflammatory cytokines are responsible for the increase in osteoclasts and decrease in osteoblasts, leading to bone loss64. BEVs can interact with various TLRs, leading to the production of pro-inflammatory cytokines that are not typically induced by TLR2/4 pathways63. Furthermore, the function of a BEV acting as a MAMP may vary depending on its size, resulting in a variety of pro-inflammatory cytokines being produced from different signaling pathways63.
In addition to TLRs, NOD-like receptors (NLRs), such as NOD1 (nucleotide-binding oligomerization domain-containing protein 1), are intracellular receptors with a leucine-rich repeat (LRR) domain that is activated by BEV-peptidoglycan (Fig. 2)66. Once NLRs are activated, they recruit signaling molecules such as RIPK2 (receptor-interacting serine/threonine kinase 2) to induce inflammatory pathways such as NF-κB and MAPK (Fig. 2)66. Interestingly, E. coli EVs can activate NOD1 receptors to induce IL-8 and IL-6 in epithelial cells, which is EV source-dependent67. This may be ascribed to the varying amounts of peptidoglycan processing enzymes in various EVs released by different strains of E. coli67. Taken together, the ability of BEVs to induce a pro-inflammatory reaction is linked towards BEV-packaged ligands that activate PRRs, which stimulate distinct cell-signaling pathways responsible for host immune responses.
4.2. Regulation of cytokine release pathways
Various BEVs have been reported to invoke pro-inflammatory cytokines, such as interleukin (IL) and interferon (IFN) family cytokines, through different intracellular pathways that lead to a variety of inflammatory responses in host immune cells60,68–74. For example, Soult et al. (2015) showed that E. coli EVs could increase the secretion of IL-6 in endothelial cells60. This could potentially be explained by the increased LPS/TLR4-mediated activation of NF-κB60. In addition, P. aeruginosa EVs can induce pro-inflammatory chemokines, such as CXCL1 and CCL2, in neutrophils and macrophages through several receptors and MAMPs that combine to form a more potent inflammatory response75. Similarly, Helicobacter pylori (H. pylori) EVs stimulate the production of pro-inflammatory cytokines IL-1β, IL-6, IL-8, and TNF-α in epithelial and macrophage cells68. Notably, H. Pylori EVs also induced IL-17 and IFN-γ, which regulate immune responses by T helper cells TH17 and TH1 respectively68. Furthermore, H. Pylori EV-induced IL-6, which is integral towards host immune responses in gastric environments, may be attributed to the activation of either the LPS/TLR4 pathway or the PG (peptidoglycan)/NOD1 pathway76. Likewise, S. aureus EVs can cause skin inflammation via pro-inflammatory cytokines such as IFN-γ, but mainly IL-17, which stimulates complementary Th17 cells to produce IgE antibodies71. In keratinocytes, S. aureus EVs can induce CXCL8 to activate neutrophils towards skin inflammation. Additionally, lipoproteins and protein A found in S. aureus EVs also contribute to initiating an inflammatory pathway involving TLR2 and NF-κB72.
Given that E. coli EVs can induce more pro-inflammatory cytokine production than E. coli LPS alone73, other components of BEVs besides LPS may also contribute to the pro-inflammatory response. Indeed, Gingipains, found on the surface of Porphyromonas gingivalis (P. gingivalis) EVs, act as virulence factors to induce pro-inflammatory cytokines (i.e., IL-6, IL-1β, IL-8, and TNF-α) that are potentially regulated by the NF-κB, MAPK, and STING (stimulator of interferon genes) pathways69,70. Aggregatibacter actinomycetemcomitans EVs encase small RNAs (sRNAs) that control the production of TNF-α from immune cells and blood cells through the TLR8 and NF-κB pathways74.
4.3. Initiation of inflammasome pathways
Recent studies have also demonstrated that BEVs can initiate inflammasome pathways to recognize/eliminate pathogens and induce specific actions such as pyroptosis77–80. Inflammasomes are intracellular PRRs induced by various MAMPs found in BEV cargo, and most are NLRs such as NLRP3 and NLRC4 (Fig. 2)66,77,78,80. Inflammasomes are initiated by the production of pro-inflammatory cytokines, such as inactive IL-1β and IL-18, through the NF-κB pathway involving TLR4/TLR2. Activation of inflammasomes by MAMPs leads to caspase-1 cleavage, which activates inactive cytokines and results in the release of pro-inflammatory cytokines with the help of ASC (apoptosis-associated speck-like protein containing CARD)77–79,81. Responses involving caspase-1 are categorized as canonical, while non-canonical inflammasome responses involve either caspase-11 from mice or caspase 4/5 from humans79. In addition to the production of pro-inflammatory cytokines, inflammasome-activated caspase-1 can initiate pore formation in the cellular membrane via gasdermin-D (GSDMD), leading to inflammasome-induced pyroptosis, a type of immunoregulatory cell death that allows the removal of bacteria-infected host cells78,79.
Given that inflammasomes are cytosolic protein complexes, the mechanisms by which BEVs transfer their virulence factors from the extracellular space into the host cell cytosol have been of great interest, as there are several molecules involved in this process that perform entirely different tasks depending on their environment77–83. BEV-LPS has been linked to these inflammasome pathways, as enterohemorrhagic (EHEC) E. coli EVs can transport LPS into host cells, inducing a caspase-11 pathway that causes pyroptosis and IL-1 secretion80. Similarly, P. aeruginosa EVs contain LPS, which can induce a caspase-11 NLRP3 inflammasome pathway and a caspase-5 non-canonical inflammasome pathway, suggesting that LPS is a favored MAMP in the activation of most inflammasome-induced pathways77. However, some BEVs derived from H. pylori or periodontal pathogenic bacteria do not contain LPS, which can activate caspases. This suggests that several different MAMPs besides LPS may induce inflammasome responses77,78. For example, BEVs from the periodontal bacterium P. gingivalis seem to induce pro-inflammatory responses through inflammasomes, as evidenced by the appearance of IL-1β, NF-κB activation, and ASC requirement. However, they possess a different version of LPS that lacks hexa-acylated A, which plays a significant role in inflammasome activation and subsequent pro-inflammatory responses78. Since they lack LPS, B. pertussis is thought that Bordetella pertussis prioritize K+ efflux to activate the NLRP3 inflammasome complex through ASC assembly and IL-1β secretion (Fig. 2)79. In addition, S. aureus EVs combine the effects of lipoproteins and pore-forming toxins (PFT) to activate TLR2 and the NLRP3 inflammasome, respectively, resulting in pro-inflammatory cytokine production and pyroptosis in macrophages81. Interestingly, flagellin from pathogenic Salmonella typhimurium EVs induces the NLRC4 inflammasome pathway to produce IL-1β83 (Fig. 2). This is completely different from LPS-dependent pathways, as this pathway could trigger a stronger host response to pathogenic bacteria, as evidenced by quicker inflammasome assembly and prevention of excessive pyroptosis observed in LPS-containing BEVs83. In addition to receptor-ligand interactions, BEVs can induce apoptosis to control inflammasome responses, as they can increase IL-1β production through caspase-11 and NLRP3 by interfering with mitochondrial protein production, resulting in a lack of molecules such as MCL-11 that prevent cell death (Fig. 2)82.
Another mechanism by which MAMPs interact with inflammasome pathways is by guanylate binding proteins (GBPs), which play an essential role in host defense by destroying pathogen membranes and exposing their substituents to PRRs to induce an immune response84. GBPs are vital for inflammasome-mediated cytokine release and pyroptosis. Specific signals involved in inflammasome pathways, such as caspase-11, are activated by LPS carried by E. coli EVs and rely on GBPs, possibly due to lipid A found in LPS, to complete this process84,85. In addition, B. pertussis EVs can potentially interfere with interferon pathways through lipooligosaccharide (LOS) to induce the caspase-11 inflammasome pathway, resulting in changes in GBP regulation79. The mechanisms by which GBPs expose intracellular receptors to LPS are heavily disputed. Some studies suggest that GBPs use their ability to change membrane permeability to release LPS from outer membranes, while others suggest that BEVs transport LPS directly to these receptors84,85. However, it is important to note that GBP is limited in mouse models as it differs from human GBP. Other proteins, such as hemolysin, which are known for their ability to cause membrane dysfunction, may provide another mechanism for LPS exposure to inflammasomes.86. Edwardseilla tarda EVs associated with a significant concentration of hemolysin can induce significant amounts of the pro-inflammatory cytokines IL-1β and IL-18 through caspase-11 activation, suggesting that hemolysin plays a notable role in exposing LPS from BEVs to intracellular receptors86. SNX-10 is a protein that regulates endosomes, which are associated with the delivery of LPS to cytosolic receptors. SNX-10 may regulate PIKfyve, a lipid kinase that controls endosomes transporting LPS from BEVs into the cytosol to activate caspase-587.
Interestingly, inflammasomes and other immune interactions differ depending on exposure to bacteria alone versus EVs. P. gingivalis EVs, for example can cause a significantly stronger inflammatory response than P. gingivalis alone by inducing more pro-inflammatory cytokines, activating inflammasomes, and inducing pyroptosis, possibly due to a greater concentration of gingipains found on P. gingivalis EVs compared to bacteria88. This difference in responses could indicate how bacteria alter the host response for their benefit. For instance, the pro-inflammatory characteristics of P. gingivalis EVs can be used to extract nutrients from damaged host tissues. In contrast, a reduced inflammatory profile of whole P. gingivalis bacteria can be used to avoid host defenses88. In the future, the immunomodulatory effects of BEVs on inflammasome activation should be further investigated, as they may provide new avenues to develop vaccines and pharmaceuticals that protect against bacterial sepsis.
5. Role of BEVs in anti-inflammatory responses
Unlike the BEVs derived from pathogenic bacteria that stimulate pro-inflammatory response described above, recent studies have shown that BEVs released by probiotic/commensal bacteria may elicit anti-inflammatory responses, as highlighted below and depicted in Figure 3.
Figure 3:
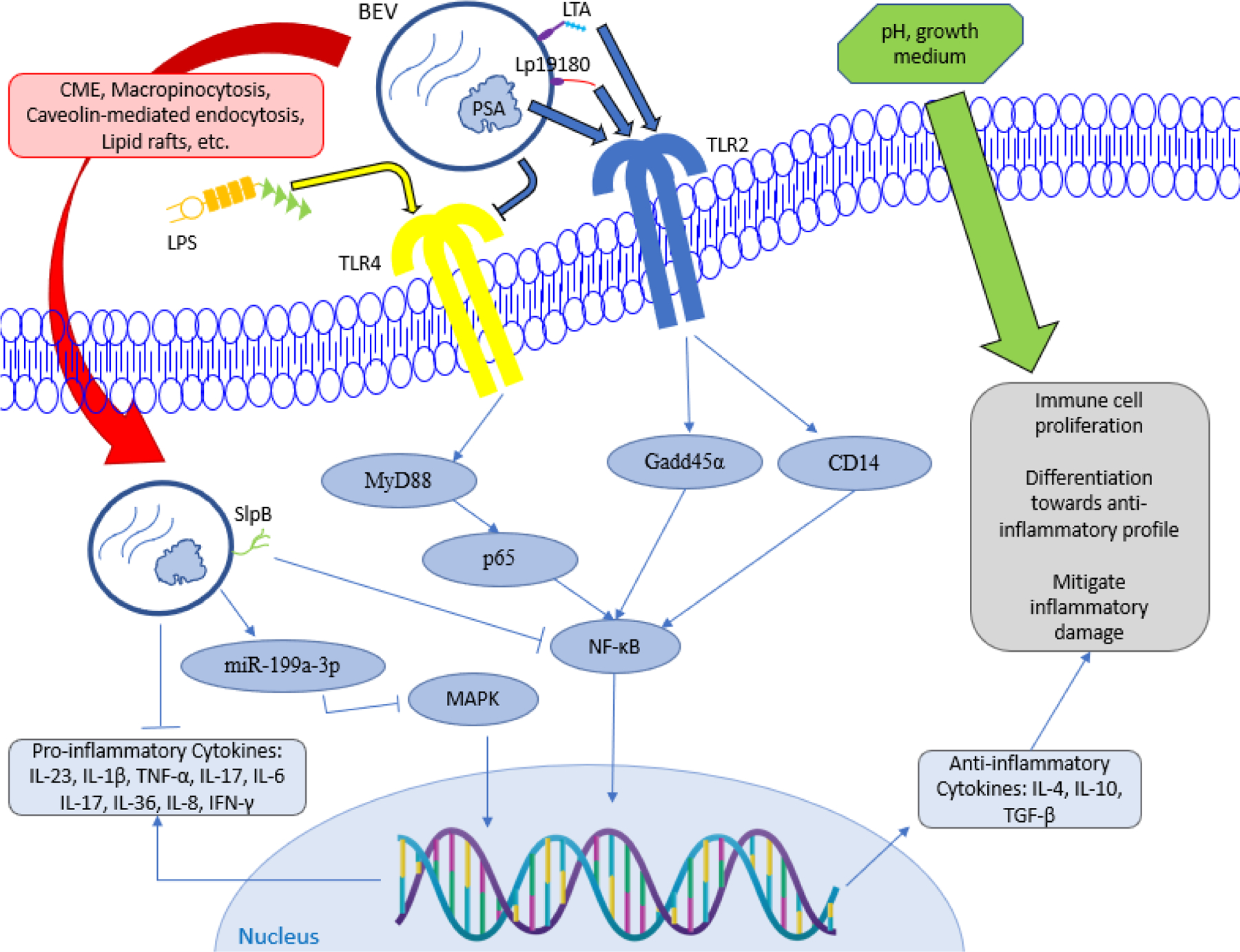
Model of BEV-mediated anti-inflammatory mechanisms. BEVs carry ligands LTA, Lp19180, and PSA to activate TLR2, which activates intracellular signals Gadd45α and CD14 that increase NF-κB expression. In contrast, BEVs can inhibit TLR4 activated from LPS stimuli, which subsequently lowers activity of other signaling molecules such as MyD88, p65, and NF-κB. Furthermore, BEVs transported into the host cell can inhibit certain pathways such as NF-κB by SlpB and MAPK by miR-199a-3p, and they can even inhibit pro-inflammatory cytokine production. Regulation of NF-κB expression results in greater concentration of anti-inflammatory cytokines, which provides beneficial action for the immune system. Other environmental factors such as pH and growth medium can enhance the anti-inflammatory effects of BEVs.
5.1. Manipulation of anti-inflammatory TLR pathways and cytokine production
Accumulating evidence has demonstrated that probiotic/commensal bacteria can affect inflammation through TLR pathways to invoke an anti-inflammatory response89–92. For example, one of the microorganisms prevalent in the human gastrointestinal system is the commensal bacterium, Bacteriodes fragilis (B. fragilis). The released BEVs contain polysaccharide A (PSA), which is known to trigger anti-inflammatory immune responses and aid in the prevention of colitis92. This PSA encased in B. fragilis-EVs acts as a MAMP for TLR2 on dendritic cells to induce the production of the anti-inflammatory cytokine, IL-10, through Gadd45α (growth arrest and DNA-damage-inducible protein) (Fig.3). Consequently, this leads to the appearance of regulatory T cells (TREGS), which further induce IL-1092. Similarly, B. vulgatus can release anti-inflammatory BEVs that contain polysaccharides to interact with TLR2/TLR4 on bone marrow-derived dendritic cells (BMDCs), resulting in tolerance towards pathogenic E. coli and preventing future inflammation91. In addition, EVs released by Lacticaseibacillus rhamnosus (L. rhamnosus) strain JB-1 contain LTA, which modulates anti-inflammatory mechanisms, such as intestinal epithelial cell (IEC) internalization and IL-10 production90. Moreover, in response to dextran sodium sulfate (DSS)-induced colitis, L. rhamnosus GG EVs suppressed the production of pro-inflammatory cytokines such as IL-1β and TNF-α through the inhibition of the TLR4/NF-κB pathway, as evidenced by the reduced expression of MyD88, p65, and p-p6593. It is important to note that TLRs seem to play a more important role in pro-inflammatory cytokine production89, however, anti-inflammatory BEVs rely on similar signaling mechanisms to enhance their immunomodulatory role in host responses, as TLRs are required to carry out anti-inflammatory responses such as anti-inflammatory cytokine production.
BEVs have been shown to play an important role in producing anti-inflammatory cytokines or blocking the production of pro-inflammatory cytokines15,94–98. When stimulated with LPS from E. coli, HT-29 epithelial cells can produce the pro-inflammatory cytokine IL-8. However, EVs from P. freudenreichii strain CIRM-BIA 129 can reduce the production of IL-8 by downregulating the NF-κB pathway, potentially owing to the presence of SlpB (surface-layer protein B) in EVs, as SlpB has anti-inflammatory properties98. Similarly, H. pylori EVs induced the production of anti-inflammatory cytokines IL-4 and IL-10 in RAW 264.7 macrophages94. Furthermore, Bifidobacterium longum EVs from strain AO44 induced IL-10 in murine splenocytes and DC/CD4+ cultures, which is potentially linked towards an increase in CD8+/CD4+ T cell activity97. Interestingly, BEVs can reverse the effects induced by pro-inflammatory stimuli, such as DSS-induced colitis15. For example, EcN (E. coli Nissle 1917) EVs can mitigate the effects of DSS-induced colitis, as they have been linked to a reduction in pro-inflammatory cytokines (i.e., IL-1β, TNF-α, and IL-17) and an increase in the anti-inflammatory cytokine IL-1015. Additionally, Bacteroides thetaiotaomicron EVs can induce the production of IL-10, which is typically absent in inflammatory bowel disease (IBD), in various types of cells upon DSS-induced colitis. Similarly, bone marrow-derived macrophages (BMDMs) treated with LPS produced more IL-10 and less TNF-α when exposed to B. thetaiotaomicron EVs because of the increased TLR2 activation by coreceptor CD14 or changes in the epigenetic marker H3K4me1 (histone 3 lysine 4 methylation), although this interpretation is highly disputed95.
In contrast to S. aureus EVs, Staphylococcus epidermidis ATCC12228 EVs can reverse the production of pro-inflammatory cytokines IL-6, IL-23, and IL-17F in murine skin caused by imiquimod-induced (IMQ) psoriasis, an inflammatory skin disease96. The underlying mechanism could potentially be associated with a decrease in IL-36, which is responsible for inflammatory responses from immune cells and other pro-inflammatory cytokines found in psoriasis96. Similarly, Clostridium butyricum (C. butyricum) EVs can reduce the production of pro-inflammatory cytokines IL-6, TNF-α, and IL-1β in LPS-treated RAW264.7 cells by blocking the NF-κB and MAPK pathways99. In addition, C. butyricum EVs can upregulate the expression of miR-199a-3p in RAW264.7 cells which consequently, suppresses map3k4 gene expression, leading to inactivation of the MAPK signaling pathway99.
Lactobacillus, a genus of probiotic bacteria, produces BEVs, which serve as one of the most widely studied anti-inflammatory BEVs. They have been implicated in a multitude of anti-inflammatory responses in the host100–102. For example, treatment of TNFα-stimulated Caco-2 cell model of IBD with Lactobacillus EVs greatly reduced the expression of phosphorylated p65, an important signal of the NF-κB pathway, and blocked pro-inflammatory IL-8 production102. Interestingly, while LPS stimulation alone caused an increase in pro-inflammatory cytokines (TNF-α, IL-1β, and IL-8) in broiler cells, Limosilactobacillus reuteri EVs from strain BBC3 combined with LPS resulted in decreased production of these pro-inflammatory cytokines and increased production of the anti-inflammatory cytokine IL-10 in broiler macrophages100. In addition, when splenic lymphocytes were co-cultured with broiler cells treated with L. reuteri BBC3 EVs, anti-inflammatory cytokines IL-10 and TGF-β were increased, whereas pro-inflammatory cytokines IL-17 and IFN-γ were blocked, indicating the large scope of anti-inflammatory responses induced by BEVs in the entire host system100.
Lactobacillus may also generate anti-inflammatory responses by negating the effects of pathogenic stimuli. This is exemplified by how L. plantarum EVs cause the decrease of pro-inflammatory cytokine IL-6 induced by S. aureus EVs in immune cells and skin cells103. Notably, despite being probiotic and providing benefits to the host, not all Lactobacillus bacteria have anti-inflammatory properties, as L. plantarum EVs induce not only the anti-inflammatory cytokine IL-10 but also the pro-inflammatory cytokines IL-1β and IL-6 to activate the host immune system101. Furthermore, L. plantarum EVs potentially utilize proteomic sections of Lp19180, a lipoprotein found in the cellular membrane, as a ligand of TLR2 to induce the NF-κB pathway101.
Recent studies have also indicated that some anti-inflammatory pathways induced by BEVs may depend on various environmental changes. BEVs from agitated L. casei and L. plantarum cultures grown at pH 5 resulted in the greatest IL-10 production in THP-1 cells compared to other conditions31. BEVs produced at these specific conditions may potentially be more apt to interfere with TLR4 and LPS binding interactions31. In addition, BEVs from L. casei and L. plantarum grown at pH 5 resulted in the reduction of TNF-α in THP-1 cells; however, it is noteworthy that the various proteomic compositions and particle concentrations caused by differing environmental conditions did not correlate with anti-inflammatory effects31. Thus, it is necessary to identify the anti-inflammatory mechanisms of BEVs caused by various BEV cargoes and culture environments, thereby proving beneficial in developing future BEV pharmaceuticals.
5.2. Pathogenic usage of anti-inflammatory mechanisms
Although we generally associate pathogenic bacterial EVs with pro-inflammatory, it is also important to consider some of their anti-inflammatory qualities. Such BEV-mediated anti-inflammatory cytokine pathways could provide possible explanations for how pathogenic bacteria utilize BEVs to cause persistent infection78,89,104–108. For example, although Streptococcus pyogenes EVs can induce pro-inflammatory cytokines (i.e., TNF-α, IL-1β and IL-8) through bacterial toxins such as SLO (streptolysin O) in monocytic cells, BEVs from invasive strain SSI-1 cannot induce pro-inflammatory cytokines that non-invasive strain JRS4 can. This could be interpreted by which BEVs aid in evading host defenses, because anti-inflammatory bacterial toxin C5a peptidase found in SSI-1 may contribute to the reduction of pro-inflammatory cytokines105. Interestingly, S. aureus EVs were found to induce only a small amount of TNF-α compared to the large amount of pro-inflammatory cytokines induced by S. aureus bacteria108. This suggests that pathogenic BEVs can deflect immune system alerts during the initial infection of host cells. P. gingivalis EVs also contain sphingolipids (SL) that regulate TLR2/TLR4 pathways, resulting in the reduced production of pro-inflammatory cytokines (i.e., IL-6, IL-8, IL-1β, and TNF-α) in THP-1 cells, allowing pathogenic bacteria to evade the development of host defenses against infection107. Furthermore, pathogenic BEVs can increase the chances of bacterial infection by producing a combination of pro-inflammatory and anti-inflammatory cytokines78. For example, P. gingivalis EVs induce not only the pro-inflammatory cytokines TNF-α and IL-8 through the NF-κB pathway, but also induce anti-inflammatory IL-10 secretion78. The presence of IL-10 could indicate inflammatory anergy and a reduction in pro-inflammatory responses, leading to the retention of bacterial populations. In addition, BEVs from P. gingivalis (and other periodontal pathogens) contain sRNAs that may contribute towards avoiding immune defense by blocking the production of inflammatory cytokines (i.e., IL-5 and IL-13) in T cells104. Indeed, when introduced into antibiotics such as metronidazole, pathogenic B. fragilis EVs cause an increase in both pro-inflammatory cytokines (i.e., IL-8, TNF-α, IL-1α) and anti-inflammatory cytokines (i.e., IL-10)106. This mixed production of cytokines can prevent excessive host cell damage and dampen the host immune response, thereby creating an opportunity for the pathogen to remain in the host environment. Pathogenic E. coli EVs can produce a significant amount of pro- and anti-inflammatory cytokines89, possibly because of the similarities between the two types of cytokine pathways that respond to LPS. The Anti-inflammatory cytokine IL-10 might be able to reduce the production of pro-inflammatory cytokines such as IL-17, allowing pathogenic bacteria to remain in the host by limiting the immune response to inflammation89. Taken together, these findings may explain chronic bacterial infections and ineffective antibiotic treatment. Thus, effective drugs targeting pathogenic bacteria should be considered to avoid BEV-mediated immune defense.
6. BEVs in the regulation of host-microbe interactions
Several studies have elucidated host-microbe relationships through the interactions of BEVs with the host system and have further identified how the host invokes diverse immune responses to BEVs21,109. BEVs play a critical role in the regulation of these cross-kingdom communications110–112 and gut microbiota homeostasis113,114, as discussed below.
6.1. Diverse approaches for BEVs to enter host cells/environment.
To better understand how BEVs communicate with host cells, we first introduced how BEVs are transported into host cells (Fig. 3, red arrow). There has been much evidence that BEVs enter intracellular environments, such as periodontal BEVs that can pass through the blood-brain barrier. More specifically, these EVs cross blood vessel barriers into brain monocytes by endothelial-interfering gingipains69,74,115. However, the manner in which BEVs enter host cells is dependent on the bacterial species and the type of host cells. In addition to phagocytic cells, non-phagocytic cells can absorb BEVs through macropinocytosis or endocytic approaches mediated by clathrin, caveolin, lipid rafts and membrane fusion9. Clathrin-mediated endocytosis (CME) utilizes clathrin, which surrounds vesicles that contain extracellular cargo with support from adaptor proteins (i.e., AP2), resulting in further processing of intracellular endosomes and lysosomes116. Caveolin-dependent endocytosis uses caveolin as a membrane coating, a cholesterol-dependent protein capable of manipulating the membrane shape and forming caveolae that carry out endocytosis117. Micropinocytosis is specialized towards intaking cargo of greater size compared to other endocytic methods, whereas lipid raft endocytosis can continue via caveolin or GTPases9.
BEV uptake via endocytosis is specific to a specific uptake method. For example, EHEC EVs require AP2 and dynamin to enter the host cells via CME118. Likewise, Cañas et al. (2016) demonstrated that E. coli EVs from the gut microbiome strains ECOR12 and EcN require CME for internalization by HT-29 cells119. In contrast, enterotoxigenic E. coli (ETEC) EVs contain heat-labile enterotoxin (LT), which initiates caveolin-dependent lipid raft endocytosis in HT-29 cells120. Moraxella catarrhalis EV uptake by A549 epithelial cells also requires lipid raft endocytosis, a mechanism that is common among other pathogenic gram-negative bacterial EVs121. Notably, BEV uptake can be blocked by the addition of chlorpromazine (an inhibitor of CME) and dynasore (an inhibitor of dynamin), both of which regulate clathrin-coated vesicle formation119. Similarly, the internalization of ETEC-EVs can be disrupted by the addition of filipin, an inhibitor of lipid raft formation120.
In addition, a combination of endocytic pathways can facilitate BEV uptake by host cells. For example, H. pylori EVs use CME as their main method of entry into host cells; however, they also utilize CME-independent endocytosis to a lesser degree122. Furthermore, BMDMs mainly utilize macropinocytosis to internalize F. tularensis EVs; however, internalization of F. tularensis EVs can also occur through other endocytic methods, as evidenced by the fact that dansylcadaverine and MβC, inhibitors of claritin-dependent and claritin-independent methods, respectively, caused a significant reduction in BEV entry123. Similarly, the gram-positive bacterium Bacillus cereus produces pro-inflammatory BEVs that enter Caco2 cells via dynamin through lipid raft cytosis or clathrin-mediated endocytosis (CME)124.
As described above, while the mechanism underlying the host internalization of BEVs may vary from cell to cell and species to species, it is a great advantage for bacteria to communicate widely between species. BEVs are particularly beneficial when bacteria are inaccessible to the host organism. This phenomenon creates new opportunities for bacteria to cause infections without direct contact with host cells125. For example, unlike whole bacteria, B. fragilis EVs not only activate more TLRs because of the increased concentrations of receptor ligands (i.e., LPS and RNA), but also travel inside host cells to induce intracellular receptors125. Interestingly, E. coli EVs can carry Cre proteins through the intestinal mucosa and spread among various organs126, demonstrating their ability to act as vehicles for protein transport into inaccessible areas.
6.2. BEV-mediated microbe-host interaction in the immune and endo/epithelium systems
As described above, BEVs can regulate both the pro- and anti-inflammatory responses. Hence, a significant portion of BEV-mediated microbe-host interactions are related to the immune system. In addition, the endothelium and epithelial structures act as the first lines to interact with infected bacteria. Accordingly, BEVs have been reported to greatly affect endothelial/epithelial cells. Therefore, the following discussion focuses on the effects of BEV-mediated microbe-host interactions on the host immune and endo/epithelium systems.
First, BEVs have been shown to affect macrophage polarization88, 127–130. For example, P. gingivalis EVs can alter murine macrophages towards an M1-like proinflammatory phenotype by reprogramming their metabolism from oxidative phosphorylation towards glycolysis by altering the expression of genes such as Irg-1, a regulator of TCA (the citric acid) cycle88. Similarly, E. coli EVs can increase the expression of reactive oxygen species (ROS) and CD86 in macrophages, which exhibit an M1-like phenotype128. In contrast, Pediococcus pentosaceus-derived EVs promote macrophages towards M2-like characteristics by increasing Arg-1, PD-L1, and IL-10 expression and stimulating the development of anti-inflammatory immune cells such as myeloid-derived suppressor cells (MDSCs)130. The anti-inflammatory effects of P. pentosaceus EVs were demonstrated by blocking immune cell recruitment and mitigating the harmful effects of DSS-induced colitis130. More interestingly, it has been reported that E. coli EVs may manipulate the communication between macrophages. For example, CirA, a BEV protein highly concentrated in lower iron inflammatory environments, has been shown to mediate the transport of inflammatory signals, such as iNOS and COX-2, in exosomes from infected macrophages toward uninfected macrophages131.
Second, other types of host immune cells are also susceptible to BEVs125,132–135. Lajqi et al. (2022) recently showed that neutrophils stimulated with lower concentrations of gut microbiota EVs activated the TLR2/MyD88 pathway to express more pro-inflammatory and antibacterial mediators, such as ROS, MCP-1, CD11a, and CD32134. In contrast, the anti-inflammatory cytokine IL-10 is expressed when neutrophils are stimulated with higher doses of gut microbiota EVs134. Regarding dendritic cells (DCs), it has been reported that gut microbiota EVs can augment DC anti-inflammatory reactions135. EVs released by different strains of E. coli can stimulate DCs to produce T cell-responsive cytokines, such as IL-12 for Th1 cells from EcN-EVs and TGF-β for Tregs from ECOR12-EVs135. These BEVs can also influence crosstalk between DCs and T-helper cells by increasing surface markers (i.e., CD86 for T-cell activation) and miR-155–5p in DC-derived exosomes (miR-155–5p for the regulation of T-cell differentiation)135.
Third, some BEVs can be used towards manipulate the immune response to favor bacterial infection/survival. For example, Legionella pneumophila EVs stimulate THP-1 cells to produce pro-inflammatory cytokines; however, THP-1 cells pretreated with L. pneumophila EVs exhibited an immune response beneficial towards L. pneumophila infection136. Pre-treatment of THP-1 cells with these BEVs causes the upregulation of miR-146a, an anti-inflammatory miRNA involved in the TLR2/NF-κB pathway, which allows L. pneumophila to avoid bacterial clearance from M1 macrophages while causing persistent infection of other immune cells through pro-inflammatory cytokines and anti-apoptotic factors136. Moreover, L. pneumophila EVs also contain the small RNA RsmY, which is similar in function and sequence to eukaryotic miRNAs that attenuate RIG-1 expression in THP-1 cells137. This interferes with the TLR pathways responsible for IFN-β production, resulting in enhanced bacterial survival137. Another pneumococcal bacterium, S. pneumoniae, also produces BEVs that help bacteria survive in macrophages to avoid host defenses138. However, S. pneumoniae EVs can be rapidly recognized by DCs, resulting in a significant increase in TNF-α139, demonstrating their inability to process antigens for subsequent immune responses. In addition, P. aeruginosa EVs can participate in immune invasion by controlling neutrophil activation and migration to avoid other immune cells from initiating bacterial killing140.
Finally, BEVs have been shown to affect endothelial and epithelial cell survival141–145. Bielaszewska et al. reported that BEVs from EHEC can initiate apoptosis by transporting enterohemorrhagic E. coli hemolysin (EHEC-Hly), a pore-forming toxin, into endothelial/epithelial cells141. In addition, Acinetobacter baumanii EVs play an important role in transporting outer membrane protein A (OmpA) into epithelial cells to induce mitochondrial dysfunction through the DRP1 pathway, resulting in cell death142. Lee et al. recently showed that E. coli EVs can induce cytokine IL-8 through the NF-κB pathway and cell adhesion molecules, such as ICAM-1, on human endothelial cells to encourage neutrophil recruitment143. Furthermore, E. coli EVs can increase leukocyte migration into mouse lungs via the upregulation of pro-inflammatory cytokines/chemokines in epithelial cells144. Likewise, pathogenic S. aureus and S. epidermis EVs can also induce the release of cytokines IL-8 and chemokine MCP-1 (monocyte chemoattractant protein 1) in epithelial cells, leading to the migration of leukocytes, neutrophils, and macrophages145.
7. BEVs in the pathogenesis and prevention/treatment of sepsis
Sepsis is defined as life-threatening multiorgan dysfunction caused by a dysregulated host response to pathogen infection. Bacterial sepsis occurs when the persistence of bacteria becomes uncontrolled and host proinflammatory immune responses are abnormally elevated. Given that BEVs play a crucial role in controlling either pro- or anti-inflammatory responses, it is well appreciated that BEVs can significantly affect the development of sepsis and septic shock. Additionally, BEVs can be used as vaccines or antibiotic delivery tools to prevent or treat sepsis.
7.1. BEVs contribute to the pathogenesis of sepsis
As mentioned above, BEVs can stimulate pro-inflammatory responses with a much higher potency than the parent bacteria themselves. This finding suggests that BEVs are critical factors in the development of severe sepsis and septic shock. More importantly, accumulating evidence has demonstrated that some bacteria (i.e., Acinetobacter baumannii) can use BEVs to help them survive and resist antibiotics, leading to severe sepsis7, 146–148. Indeed, BEVs can carry antibiotic-degrading enzymes that can act as distractors for antibiotics7. Kim et al. (2020) found that when sub-lethal doses of ampicillin were administered to methicillin-resistant S aureus (MRSA), the production of BEVs increased and encased higher levels of β-lactam-degrading enzymes149. In addition, other bacteria could use BEV cargo to help build up biofilm communities under stress conditions, and even partake in the horizontal transfer of antibiotic resistance genes7,147–150. For example, exposure of various bacterial species to MRSA-BEVs resulted in protection from death and lower growth rates when treated with ampicillin149. Interestingly, Ye et al. (2021) observed that the injection of imipenem in mice infected with multidrug-resistant Klebsiella pneumonia caused excretion of K. pneumonia BEVs and excessive inflammatory responses151. They further identified that these BEVs interacted with Lox-1 receptors and caspase-11, resulting in macrophage pyroptosis and a severe inflammatory response151. Taken together, these studies suggest that BEVs can promote and spread antibiotic resistance, thereby greatly contributing to the pathogenesis of sepsis and septic shock.
7.2. BEVs in the prevention and treatment of sepsis
Recently, BEVs isolated from probiotic bacterial strains have been shown to protect against sepsis7, 147, 152. Li et al. (2017) used probiotic L. plantarum EVs to enhance the host immune response against vancomycin-resistant Enterococcus (VRE) infection in C. elegans152. Likewise, Wang et al. (2020) observed that the antimicrobial compounds in B. thailandensis EVs were able to disrupt biofilm formation in antibiotic-resistant A. baumannii153. These studies indicate that natural BEVs can be used to combat antibiotic-resistant bacteria. Furthermore, components of BEV cargo and surface proteins can be further subjected to extraction, purification, and modification to develop vaccines against antibiotic-resistant bacteria147, 150. Additionally, BEVs can be modified to precisely deliver antibiotics to infected cells at lower doses, thereby mitigating the development of new resistant strains146, 153. For example, Gao et al. (2018) wrapped nanoparticles containing vancomycin and rifampicin in S. aureus-BEVs154. They observed that macrophages would take up these modified BEVs and deliver antibiotics to the internal S. aureus infection, which is more efficient than antibiotics administered alone155. Hence, these studies open possibilities for new specific and efficient targeting therapies to treat antibiotic-resistant sepsis.
In addition to antibiotic delivery, modification of BEV components is a promising strategy for vaccine development. Surface LPS and other surface markers allow BEVs to act as both antigens and adjuvants to trigger innate and adaptive immune responses155, 156. Kim et al. (2013) showed that, by injecting 0.5–1μg of E. coli EVs in advance, mice had 80–100% survival rates after a lethal infection of E. coli157. Other studies have used BEVs as adjuvants in vaccines. For instance, Prados-Rosales et al. (2014) found that mice had less viable Mycobacterium tuberculosis in their lungs when mycobacterial EV immunization was coupled with the BCG vaccine156. Most importantly, modified or unmodified BEVs can activate both humoral and cellular immunity, which are crucial for developing protective memory cells after vaccination157–159. Particularly, BEVs can increase levels of IFN-γ and thus encourage Th1-cell responses that strengthen macrophage and dendritic cell activity against intracellular infections157, 159. Recently, the European Medicines Agency approved the 4CMenB vaccine, which incorporates BEVs into the vaccine against meningitis B160. This vaccine was derived when BEVs were isolated and used to control the 2011 meningitis outbreak in New Zealand160. Therefore, BEVs may be a valid and promising tool for vaccine development to prevent sepsis and septic shock.
8. Conclusions and future perspectives
In this review, we summarize the current literature on: (i) the role of BEVs in pro-inflammatory responses, (ii) BEV-mediated anti-inflammatory effects, (iii) BEV-mediated host-microbe interactions, and (iv) the role of BEVs in sepsis. Specifically, the major characteristics of BEVs are their ability to affect pro- and anti-inflammatory responses, as well as their impact on the regulation of certain MAMPs and signaling molecules, resulting in the alteration of receptor activity, cytokine production, immune cell function, and host-bacterial communication109,161. This implies that BEVs play multiple roles in the host environment. Indeed, BEV-related immunology is of utmost importance, as it solves some of the current significant challenges in sepsis, including the rise in antibiotic resistance146,162, 163.
Despite overwhelming research addressing the effects of BEVs using animal models, future investigations need to transfer these results towards in vivo experiments for clinical relevance164. Although BEV cargo acts as a facilitator of cell signaling pathways, it remains unknown whether BEV cargo can perform other mechanisms to control the host response. For example, sRNAs encased in BEVs can regulate the immune response through different mechanisms such as regulation of transcription, epigenetics, cell metabolism, and miRNA activity in host cells165–170. Recently, Fan et al. (2023) identified sRNA45033 within P. gingivalis EVs that could induce pro-inflammatory and pro-apoptosis effects through CBX5 gene expression via epigenetic regulation171. This is exemplified by the changes in H3K9me3 in response to P. gingivalis EVs, suggesting the involvement of DNA methylation. However, the mechanism requires further investigation171. Additionally, bioinformatic technology may uncover a relationship between BEV-sRNAs and human mRNAs, which could affect gene expression if these interkingdom RNAs can link together172; however, the experimental methods proposed to test these theories have yet to be applied practically.
Given the diverse immunological effects of BEVs, it is expected that new discoveries from BEV research could lead to more advanced biomedical applications of BEVs, beyond the above-mentioned roles in antibiotic resistance and vaccine development. Antiviral defenses could become another perspective in the field of BEV research, as several BEVs have been shown to inhibit viral replication and infection through macrophages by inducing pro-inflammatory cytokine pathways associated with MX-1 (a GTPase known to inhibit viruses) or TLR4 to activate the immune system173. Moreover, these mechanisms could have therapeutic value, as S. typhimurium EVs were found to attach to the spike receptor-binding domain (RBD) and may act as a SARS-CoV-2 vaccine174. Nonetheless, further studies are required to utilize the antiviral properties of BEVs in a more flexible manner. Considering the broad spectrum of positive and negative effects on host systems and their potential implications for various therapeutics, BEVs will undoubtedly become a predominant focus of immunology and biomedical research for decades.
Acknowledgments:
The research in Dr. Guo-Chang Fan’s lab was supported by NIH grants R01 HL-160811 and R35 GM-149538.
Footnotes
Conflict of interest
All the authors include B. D. Liu and R. Akbar. A. Oliverio, K. Thapa, X. Wang, and G.-C. Fan confirms that this article has no conflicts of interest.
References:
- 1.McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. Journal of bacteriology. 2006;188(15):5385–5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jan A Outer membrane vesicles (OMVs) of Gram-negative bacteria: a perspective update. Front Microbiol 8: 1053. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gill S, Catchpole R, Forterre P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS microbiology reviews. 2019;43(3):273–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deatherage BL, Cookson BT. Membrane vesicle release in bacteria, eukaryotes, and archaea: a conserved yet underappreciated aspect of microbial life. Infection and immunity. 2012;80(6):1948–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Work E, Knox K, Vesk M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Annals of the New York Academy of Sciences. 1966;133(2):438–449. [DOI] [PubMed] [Google Scholar]
- 6.Lee EY, Choi DY, Kim DK, et al. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9(24):5425–5436. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y-j Wang X-h, Fan G-C. Versatile effects of bacterium-released membrane vesicles on mammalian cells and infectious/inflammatory diseases. Acta Pharmacologica Sinica. 2018;39(4):514–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caruana JC, Walper SA. Bacterial membrane vesicles as mediators of microbe–microbe and microbe–host community interactions. Frontiers in microbiology. 2020;11:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’donoghue EJ, Krachler AM. Mechanisms of outer membrane vesicle entry into host cells. Cellular microbiology. 2016;18(11):1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Martin R, Wang G, Brandão BB, et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601(7893):446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Geng Z, Su J. Engineered mammalian and bacterial extracellular vesicles as promising nanocarriers for targeted therapy. Extracell Vesicles Circ Nucleic Acids. 2022;3(2):63–86. [Google Scholar]
- 12.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nature Reviews Immunology. 2015;15(6):375–387. [DOI] [PubMed] [Google Scholar]
- 13.Patten DA, Hussein E, Davies SP, Humphreys PN, Collett A. Commensal-derived OMVs elicit a mild proinflammatory response in intestinal epithelial cells. Microbiology. 2017;163(5):702–711. [DOI] [PubMed] [Google Scholar]
- 14.Hiippala K, Kainulainen V, Suutarinen M, et al. Isolation of anti-inflammatory and epithelium reinforcing Bacteroides and Parabacteroides spp. from a healthy fecal donor. Nutrients. 2020;12(4):935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fábrega M-J, Rodríguez-Nogales A, Garrido-Mesa J, et al. Intestinal anti-inflammatory effects of outer membrane vesicles from Escherichia coli Nissle 1917 in DSS-experimental colitis in mice. Frontiers in microbiology. 2017;8:1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skerniškytė J, Karazijaitė E, Lučiūnaitė A, Sužiedėlienė E. OmpA protein-deficient Acinetobacter baumannii outer membrane vesicles trigger reduced inflammatory response. Pathogens. 2021;10(4):407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burdett I, Murray R. Electron microscope study of septum formation in Escherichia coli strains B and B/r during synchronous growth. Journal of bacteriology. 1974;119(3):1039–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonntag I, Schwarz H, Hirota Y, Henning U. Cell envelope and shape of Escherichia coli: multiple mutants missing the outer membrane lipoprotein and other major outer membrane proteins. Journal of bacteriology. 1978;136(1):280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwechheimer C, Kuehn MJ. Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nature reviews microbiology. 2015;13(10):605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chowdhury C, Jagannadham MV. Virulence factors are released in association with outer membrane vesicles of Pseudomonas syringae pv. tomato T1 during normal growth. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2013;1834(1):231–239. [DOI] [PubMed] [Google Scholar]
- 21.Tashiro Y, Ichikawa S, Nakajima-Kambe T, Uchiyama H, Nomura N. Pseudomonas quinolone signal affects membrane vesicle production in not only Gram-negative but also Gram-positive bacteria. Microbes and environments. 2010;25(2):120–125. [DOI] [PubMed] [Google Scholar]
- 22.McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram‐negative bacteria is a novel envelope stress response. Molecular microbiology. 2007;63(2):545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabra W, Lunsdorf H, Zeng A-P. Alterations in the formation of lipopolysaccharide and membrane vesicles on the surface of Pseudomonas aeruginosa PAO1 under oxygen stress conditions. Microbiology. 2003;149(10):2789–2795. [DOI] [PubMed] [Google Scholar]
- 24.Baumgarten T, Vazquez J, Bastisch C, et al. Alkanols and chlorophenols cause different physiological adaptive responses on the level of cell surface properties and membrane vesicle formation in Pseudomonas putida DOT-T1E. Applied microbiology and biotechnology. 2012;93:837–845. [DOI] [PubMed] [Google Scholar]
- 25.Baumgarten T, Sperling S, Seifert J, et al. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Applied and environmental microbiology. 2012;78(17):6217–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. Journal of Biological Chemistry. 2000;275(17):12489–12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Thompson CD, Weidenmaier C, Lee JC. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nature communications. 2018;9(1):1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerritzen MJ, Stangowez L, van de Waterbeemd B, Martens DE, Wijffels RH, Stork M. Continuous production of Neisseria meningitidis outer membrane vesicles. Applied microbiology and biotechnology. 2019;103:9401–9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerritzen MJ, Martens DE, Uittenbogaard JP, Wijffels RH, Stork M. Sulfate depletion triggers overproduction of phospholipids and the release of outer membrane vesicles by Neisseria meningitidis. Scientific Reports. 2019;9(1):4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirayama S, Nakao R. Glycine significantly enhances bacterial membrane vesicle production: a powerful approach for isolation of LPS‐reduced membrane vesicles of probiotic Escherichia coli. Microbial biotechnology. 2020;13(4):1162–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller L, Kuhn T, Koch M, Fuhrmann G. Stimulation of probiotic bacteria induces release of membrane vesicles with augmented anti-inflammatory activity. ACS Applied Bio Materials. 2021;4(5):3739–3748. [DOI] [PubMed] [Google Scholar]
- 32.Rodovalho VdR, da Luz BSR, Nicolas A, et al. Environmental conditions modulate the protein content and immunomodulatory activity of extracellular vesicles produced by the probiotic Propionibacterium freudenreichii. Applied and Environmental Microbiology. 2021;87(4):e02263–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Chen J, Raj K, et al. A Universal Strategy to Promote Secretion of G+/G–Bacterial Extracellular Vesicles and Its Application in Host Innate Immune Responses. ACS Synthetic Biology. 2023;12(1):319–328. [DOI] [PubMed] [Google Scholar]
- 34.Bitto NJ, Zavan L, Johnston EL, Stinear TP, Hill AF, Kaparakis-Liaskos M. Considerations for the analysis of bacterial membrane vesicles: methods of vesicle production and quantification can influence biological and experimental outcomes. Microbiology Spectrum. 2021;9(3):e01273–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cecil JD, Sirisaengtaksin N, O’Brien-Simpson NM, Krachler AM. Outer membrane vesicle-host cell interactions. Microbiology spectrum. 2019;7(1):7.1. 06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of extracellular vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohl P, Zingl FG, Eichmann TO, Schild S. Isolation of outer membrane vesicles including their quantitative and qualitative analyses. Vibrio Cholerae: Methods and Protocols. 2018:117–134. [DOI] [PubMed] [Google Scholar]
- 38.Reimer SL, Beniac DR, Hiebert SL, et al. Comparative analysis of outer membrane vesicle isolation methods with an Escherichia coli tolA mutant reveals a hypervesiculating phenotype with outer-inner membrane vesicle content. Frontiers in Microbiology. 2021;12:628801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bitto NJ, Kaparakis-Liaskos M. Methods of bacterial membrane vesicle production, purification, quantification, and examination of their immunogenic functions. Effector-Triggered Immunity: Methods and Protocols. Springer; 2022:43–61. [DOI] [PubMed] [Google Scholar]
- 40.Klimentová J, Stulík J. Methods of isolation and purification of outer membrane vesicles from gram-negative bacteria. Microbiological research. 2015;170:1–9. [DOI] [PubMed] [Google Scholar]
- 41.MacDonald IA, Kuehn MJ. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. Journal of bacteriology. 2013;195(13):2971–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van de Waterbeemd B, Zomer G, van den IJssel J, et al. Cysteine depletion causes oxidative stress and triggers outer membrane vesicle release by Neisseria meningitidis; implications for vaccine development. PloS one. 2013;8(1):e54314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van de Waterbeemd B, Streefland M, Van der Ley P, et al. Improved OMV vaccine against Neisseria meningitidis using genetically engineered strains and a detergent-free purification process. Vaccine. 2010;28(30):4810–4816. [DOI] [PubMed] [Google Scholar]
- 44.Grizot S, Buchanan SK. Structure of the OmpA‐like domain of RmpM from Neisseria meningitidis. Molecular microbiology. 2004;51(4):1027–1037. [DOI] [PubMed] [Google Scholar]
- 45.Ünal CM, Schaar V, Riesbeck K. Bacterial outer membrane vesicles in disease and preventive medicine. Springer; 2011:395–408. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Defourny KA, Smid EJ, Abee T. Gram-positive bacterial extracellular vesicles and their impact on health and disease. Frontiers in microbiology. 2018;9:1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Elsevier; 2015:97–104. [DOI] [PubMed] [Google Scholar]
- 48.Prados-Rosales R, Baena A, Martinez LR, et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. The Journal of clinical investigation. 2011;121(4):1471–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Bergenhenegouwen J, Kraneveld AD, Rutten L, Kettelarij N, Garssen J, Vos AP. Extracellular vesicles modulate host-microbe responses by altering TLR2 activity and phagocytosis. PloS one. 2014;9(2):e89121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giordano NP, Cian MB, Dalebroux ZD. Outer membrane lipid secretion and the innate immune response to gram-negative bacteria. Infection and Immunity. 2020;88(7): 10.1128/iai.00920-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bose S, Aggarwal S, Singh DV, Acharya N. Extracellular vesicles: An emerging platform in gram-positive bacteria. Microbial Cell. 2020;7(12):312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng Y, Yin S, Wang M. Extracellular vesicles of bacteria as potential targets for immune interventions. Human Vaccines & Immunotherapeutics. 2021;17(3):897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sheikh A, Taube J, Greathouse KL. Contribution of the microbiota and their secretory products to inflammation and colorectal cancer pathogenesis: the role of toll-like receptors. Carcinogenesis. 2021;42(9):1133–1142. [DOI] [PubMed] [Google Scholar]
- 54.Jin MS, Lee J-O. Structures of TLR–ligand complexes. Current opinion in immunology. 2008;20(4):414–419. [DOI] [PubMed] [Google Scholar]
- 55.Asami J, Shimizu T. Structural and functional understanding of the toll‐like receptors. Protein Science. 2021;30(4):761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park K-S, Lee J, Lee C, et al. Sepsis-like systemic inflammation induced by nano-sized extracellular vesicles from feces. Frontiers in microbiology. 2018;9:1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Soult M, Wahab K, Dobrydeneva Y, Britt L, Sullivan C. Bacterial Outer Membrane Vesicles Alter Mediators of Inflammation and Coagulation. Journal of Surgical Research. 2014;2(186):688. [DOI] [PubMed] [Google Scholar]
- 58.Pfalzgraff A, Correa W, Heinbockel L, et al. LPS-neutralizing peptides reduce outer membrane vesicle-induced inflammatory responses. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2019;1864(10):1503–1513. [DOI] [PubMed] [Google Scholar]
- 59.Kim Y-S, Lee W-H, Choi E-J, et al. Correction: Extracellular Vesicles Derived from Gram-Negative Bacteria, such as Escherichia coli, Induce Emphysema Mainly via IL-17A–Mediated Neutrophilic Inflammation. The Journal of Immunology. 2015;194(12):6193–6193. [DOI] [PubMed] [Google Scholar]
- 60.Soult MC, Lonergan NE, Shah B, Kim W-K, Britt L, Sullivan CJ. Outer membrane vesicles from pathogenic bacteria initiate an inflammatory response in human endothelial cells. journal of surgical research. 2013;184(1):458–466. [DOI] [PubMed] [Google Scholar]
- 61.Bitto NJ, Cheng L, Johnston EL, et al. Staphylococcus aureus membrane vesicles contain immunostimulatory DNA, RNA and peptidoglycan that activate innate immune receptors and induce autophagy. Journal of extracellular vesicles. 2021;10(6):e12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kopparapu PK, Deshmukh M, Hu Z, et al. Lipoproteins are responsible for the pro-inflammatory property of Staphylococcus aureus extracellular vesicles. International Journal of Molecular Sciences. 2021;22(13):7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ryu S, Ni K, Wang C, et al. Bacterial Outer Membrane Vesicles Promote Lung Inflammatory Responses and Macrophage Activation via Multi-Signaling Pathways. Biomedicines. 2023;11(2):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim HY, Song MK, Gho YS, Kim HH, Choi BK. Extracellular vesicles derived from the periodontal pathogen Filifactor alocis induce systemic bone loss through Toll‐like receptor 2. Journal of Extracellular Vesicles. 2021;10(12):e12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Asano K, Hirose S, Narita K, et al. Extracellular vesicles from methicillin resistant Staphylococcus aureus stimulate proinflammatory cytokine production and trigger IgE-mediated hypersensitivity. Emerging Microbes & Infections. 2021;10(1):2000–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Johnston EL, Heras B, Kufer TA, Kaparakis-Liaskos M. Detection of bacterial membrane vesicles by NOD-like receptors. International Journal of Molecular Sciences. 2021;22(3):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cañas M-A, Fábrega M-J, Giménez R, Badia J, Baldomà L. Outer membrane vesicles from probiotic and commensal Escherichia coli activate NOD1-mediated immune responses in intestinal epithelial cells. Frontiers in microbiology. 2018;9:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choi H-I, Choi J-P, Seo J, et al. Helicobacter pylori-derived extracellular vesicles increased in the gastric juices of gastric adenocarcinoma patients and induced inflammation mainly via specific targeting of gastric epithelial cells. Experimental & Molecular Medicine. 2017;49(5):e330–e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoshida K, Yoshida K, Seyama M, et al. Porphyromonas gingivalis outer membrane vesicles in cerebral ventricles activate microglia in mice. Oral Diseases. 2022; [DOI] [PubMed] [Google Scholar]
- 70.Uemura Y, Hiroshima Y, Tada A, et al. Porphyromonas gingivalis outer membrane vesicles stimulate gingival epithelial cells to induce pro-inflammatory cytokines via the MAPK and STING pathways. Biomedicines. 2022;10(10):2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong SW, Kim MR, Lee EY, et al. Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis‐like skin inflammation. Allergy. 2011;66(3):351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Staudenmaier L, Focken J, Schlatterer K, Kretschmer D, Schittek B. Bacterial membrane vesicles shape Staphylococcus aureus skin colonization and induction of innate immune responses. Experimental Dermatology. 2022;31(3):349–361. [DOI] [PubMed] [Google Scholar]
- 73.Svennerholm K, Park K-S, Wikström J, et al. Escherichia coli outer membrane vesicles can contribute to sepsis induced cardiac dysfunction. Scientific Reports. 2017;7(1):17434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han E-C, Choi S-Y, Lee Y, Park J-W, Hong S-H, Lee H-J. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood–brain barrier in mice. The FASEB Journal. 2019;33(12):13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park K-S, Lee J, Jang SC, et al. Pulmonary inflammation induced by bacteria-free outer membrane vesicles from Pseudomonas aeruginosa. American journal of respiratory cell and molecular biology. 2013;49(4):637–645. [DOI] [PubMed] [Google Scholar]
- 76.Choi MS, Ze EY, Park JY, Shin T-S, Kim JG. Helicobacter pylori-derived outer membrane vesicles stimulate interleukin 8 secretion through nuclear factor kappa B activation. The Korean Journal of Internal Medicine. 2021;36(4):857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bitto NJ, Baker PJ, Dowling JK, et al. Membrane vesicles from Pseudomonas aeruginosa activate the noncanonical inflammasome through caspase‐5 in human monocytes. Immunology and Cell Biology. 2018;96(10):1120–1130. [DOI] [PubMed] [Google Scholar]
- 78.Cecil JD, O’Brien-Simpson NM, Lenzo JC, et al. Outer membrane vesicles prime and activate macrophage inflammasomes and cytokine secretion in vitro and in vivo. Frontiers in immunology. 2017;8:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elizagaray ML, Gomes MTR, Guimaraes ES, et al. Canonical and non-canonical inflammasome activation by outer membrane vesicles derived from Bordetella pertussis. Frontiers in immunology. 2020;11:1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vanaja SK, Russo AJ, Behl B, et al. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell. 2016;165(5):1106–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, Eagen WJ, Lee JC. Orchestration of human macrophage NLRP3 inflammasome activation by Staphylococcus aureus extracellular vesicles. Proceedings of the National Academy of Sciences. 2020;117(6):3174–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deo P, Chow SH, Han M-L, et al. Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nature microbiology. 2020;5(11):1418–1427. [DOI] [PubMed] [Google Scholar]
- 83.Yang J, Hwang I, Lee E, et al. Bacterial outer membrane vesicle-mediated cytosolic delivery of flagellin triggers host NLRC4 canonical inflammasome signaling. Frontiers in immunology. 2020;11:581165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santos JC, Dick MS, Lagrange B, et al. LPS targets host guanylate‐binding proteins to the bacterial outer membrane for non‐canonical inflammasome activation. The EMBO journal. 2018;37(6):e98089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finethy R, Luoma S, Orench-Rivera N, et al. Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. MBio. 2017;8(5): 10.1128/mbio.01188-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S, Yang D, Wen Y, et al. Dysregulated hemolysin liberates bacterial outer membrane vesicles for cytosolic lipopolysaccharide sensing. PLoS Pathogens. 2018;14(8):e1007240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Ni J, You Y, et al. SNX10‐mediated LPS sensing causes intestinal barrier dysfunction via a caspase‐5‐dependent signaling cascade. The EMBO Journal. 2021;40(24):e108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fleetwood AJ, Lee MK, Singleton W, et al. Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by Porphyromonas gingivalis and its outer membrane vesicles. Frontiers in cellular and infection microbiology. 2017;7:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Behrouzi A, Vaziri F, Riazi Rad F, et al. Comparative study of pathogenic and non-pathogenic Escherichia coli outer membrane vesicles and prediction of host-interactions with TLR signaling pathways. BMC research notes. 2018;11(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Champagne-Jorgensen K, Mian MF, McVey Neufeld K-A, Stanisz AM, Bienenstock J. Membrane vesicles of Lacticaseibacillus rhamnosus JB-1 contain immunomodulatory lipoteichoic acid and are endocytosed by intestinal epithelial cells. Scientific Reports. 2021;11(1):13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maerz JK, Steimle A, Lange A, Bender A, Fehrenbacher B, Frick J-S. Outer membrane vesicles blebbing contributes to B. vulgatus mpk-mediated immune response silencing. Gut Microbes. 2018;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shen Y, Torchia MLG, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell host & microbe. 2012;12(4):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tong L, Zhang X, Hao H, et al. Lactobacillus rhamnosus GG derived extracellular vesicles modulate gut microbiota and attenuate inflammatory in DSS-induced colitis mice. Nutrients. 2021;13(10):3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmed AAQ, Qi F, Zheng R, et al. The impact of ExHp-CD (outer membrane vesicles) released from Helicobacter pylori SS1 on macrophage RAW 264.7 cells and their immunogenic potential. Life Sciences. 2021;279:119644. [DOI] [PubMed] [Google Scholar]
- 95.Fonseca S, Carvalho AL, Miquel-Clopés A, et al. Extracellular vesicles produced by the human gut commensal bacterium Bacteroides thetaiotaomicron elicit anti-inflammatory responses from innate immune cells. Frontiers in Microbiology. 2022;13:1050271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gómez-Chávez F, Cedillo-Peláez C, Zapi-Colín LA, et al. The extracellular vesicles from the commensal Staphylococcus epidermidis ATCC12228 strain regulate skin inflammation in the imiquimod-induced psoriasis murine model. International Journal of Molecular Sciences. 2021;22(23):13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mandelbaum N, Zhang L, Carasso S, et al. Extracellular vesicles of the Gram-positive gut symbiont Bifidobacterium longum induce immune-modulatory, anti-inflammatory effects. npj Biofilms and Microbiomes. 2023;9(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodovalho VdR Luz BSRd, Rabah H, et al. Extracellular vesicles produced by the probiotic Propionibacterium freudenreichii CIRM-BIA 129 mitigate inflammation by modulating the NF-κB pathway. Frontiers in microbiology. 2020;11:1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma L, Lyu W, Song Y, et al. Anti‐Inflammatory Effect of Clostridium Butyricum‐Derived Extracellular Vesicles in Ulcerative Colitis: Impact on Host microRNAs Expressions and Gut Microbiome Profiles. Molecular Nutrition & Food Research. 2023:2200884. [DOI] [PubMed] [Google Scholar]
- 100.Hu R, Lin H, Wang M, et al. Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. Journal of animal science and biotechnology. 2021;12:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kurata A, Kiyohara S, Imai T, et al. Characterization of extracellular vesicles from Lactiplantibacillus plantarum. Scientific Reports. 2022;12(1):13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Seo M, Park E, Ko S, Choi E, Kim S. Therapeutic effects of kefir grain Lactobacillus-derived extracellular vesicles in mice with 2, 4, 6-trinitrobenzene sulfonic acid-induced inflammatory bowel disease. Journal of Dairy Science. 2018;101(10):8662–8671. [DOI] [PubMed] [Google Scholar]
- 103.Kim M-H, Choi SJ, Choi H-I, et al. Lactobacillus plantarum-derived extracellular vesicles protect atopic dermatitis induced by Staphylococcus aureus-derived extracellular vesicles. Allergy, asthma & immunology research. 2018;10(5):516–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Choi J-W, Kim S-C, Hong S-H, Lee H-J. Secretable small RNAs via outer membrane vesicles in periodontal pathogens. Journal of dental research. 2017;96(4):458–466. [DOI] [PubMed] [Google Scholar]
- 105.Murase K, Aikawa C, Nozawa T, et al. Biological effect of Streptococcus pyogenes-released extracellular vesicles on human monocytic cells, induction of cytotoxicity, and inflammatory response. Frontiers in Cellular and Infection Microbiology. 2021:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de Freitas MCR, de Almeida PE, Vieira WV, et al. Inflammatory modulation and outer membrane vesicles (OMV) production associated to Bacteroides fragilis response to subinhibitory concentrations of metronidazole during experimental infection. Anaerobe. 2022;73:102504. [DOI] [PubMed] [Google Scholar]
- 107.Rocha FG, Ottenberg G, Eure ZG, Davey ME, Gibson III FC. Sphingolipid-containing outer membrane vesicles serve as a delivery vehicle to limit macrophage immune response to Porphyromonas gingivalis. Infection and Immunity. 2021;89(4): 10.1128/iai.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saenz-de-Juano MD, Silvestrelli G, Weber A, Röhrig C, Schmelcher M, Ulbrich SE. Inflammatory response of primary cultured bovine mammary epithelial cells to Staphylococcus aureus extracellular vesicles. Biology. 2022;11(3):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Doré E, Boilard E. Bacterial extracellular vesicles and their interplay with the immune system. Pharmacology & Therapeutics. 2023:108443. [DOI] [PubMed] [Google Scholar]
- 110.Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Host-and microbiota-derived extracellular vesicles, immune function, and disease development. International journal of molecular sciences. 2019;21(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Villard A, Boursier J, Andriantsitohaina R. Microbiota‐derived extracellular vesicles and metabolic syndrome. Acta Physiologica. 2021;231(4):e13600. [DOI] [PubMed] [Google Scholar]
- 112.Ñahui Palomino RA, Vanpouille C, Costantini PE, Margolis L. Microbiota–host communications: Bacterial extracellular vesicles as a common language. PLoS Pathogens. 2021;17(5):e1009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liang X, Dai N, Sheng K, et al. Gut bacterial extracellular vesicles: important players in regulating intestinal microenvironment. Gut Microbes. 2022;14(1):2134689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Taitz JJ, Tan JK, Potier‐Villette C, et al. Diet, commensal microbiota‐derived extracellular vesicles, and host immunity. European Journal of Immunology. 2023:2250163. [DOI] [PubMed] [Google Scholar]
- 115.Ha JY, Choi S-Y, Lee JH, Hong S-H, Lee H-J. Delivery of periodontopathogenic extracellular vesicles to brain monocytes and microglial IL-6 promotion by RNA cargo. Frontiers in molecular biosciences. 2020;7:596366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature reviews Molecular cell biology. 2011;12(8):517–533. [DOI] [PubMed] [Google Scholar]
- 117.Kumari S, Mg S, Mayor S. Endocytosis unplugged: multiple ways to enter the cell. Cell research. 2010;20(3):256–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rennick JJ, Johnston AP, Parton RG. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nature nanotechnology. 2021;16(3):266–276. [DOI] [PubMed] [Google Scholar]
- 119.Cañas M-A, Giménez R, Fábrega M-J, Toloza L, Baldomà L, Badia J. Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enter intestinal epithelial cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS One. 2016;11(8):e0160374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. The EMBO journal. 2004;23(23):4538–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schaar V, De Vries SP, Perez Vidakovics MLA, et al. Multicomponent Moraxella catarrhalis outer membrane vesicles induce an inflammatory response and are internalized by human epithelial cells. Cellular microbiology. 2011;13(3):432–449. [DOI] [PubMed] [Google Scholar]
- 122.Olofsson A, Nygård Skalman L, Obi I, Lundmark R, Arnqvist A. Uptake of Helicobacter pylori vesicles is facilitated by clathrin-dependent and clathrin-independent endocytic pathways. MBio. 2014;5(3): 10.1128/mbio.00979-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pavkova I, Klimentova J, Bavlovic J, et al. Francisella tularensis outer membrane vesicles participate in the early phase of interaction with macrophages. Frontiers in Microbiology. 2021;12:748706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Buchacher T, Digruber A, Kanzler M, Del Favero G, Ehling-Schulz M. Bacillus cereus extracellular vesicles act as shuttles for biologically active multicomponent enterotoxins. Cell Communication and Signaling. 2023;21(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gilmore WJ, Johnston EL, Zavan L, Bitto NJ, Kaparakis-Liaskos M. Immunomodulatory roles and novel applications of bacterial membrane vesicles. Molecular Immunology. 2021;134:72–85. [DOI] [PubMed] [Google Scholar]
- 126.Bittel M, Reichert P, Sarfati I, et al. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe‐host‐communication in vivo. Journal of Extracellular Vesicles. 2021;10(12):e12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qu M, Zhu H, Zhang X. Extracellular vesicle-mediated regulation of macrophage polarization in bacterial infections. Frontiers in Microbiology. 2022;13:1039040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guangzhang C, Fangfang F, Siqian D, et al. Outer membrane vesicles from Escherichia coli are efficiently internalized by macrophage cells and alter their inflammatory response. Microbial Pathogenesis. 2023;175:105965. [DOI] [PubMed] [Google Scholar]
- 129.Jiang Y, Kong Q, Roland KL, Curtiss III R. Membrane vesicles of Clostridium perfringens type A strains induce innate and adaptive immunity. International journal of medical microbiology. 2014;304(3–4):431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alpdundar Bulut E, Bayyurt Kocabas B, Aykut G, et al. Human gut commensal membrane vesicles modulate inflammation by generating M2-like macrophages and myeloid-derived suppressor cells. The Journal of Immunology. 2020;205(10):2707–2718. [DOI] [PubMed] [Google Scholar]
- 131.Imamiya R, Shinohara A, Yakura D, et al. Escherichia coli-derived outer membrane vesicles relay inflammatory responses to macrophage-derived exosomes. Mbio. 2023;14(1):e03051–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy, Asthma & Clinical Immunology. 2018;14(2):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu R, Lin H, Li J, et al. Probiotic Escherichia coli Nissle 1917-derived outer membrane vesicles enhance immunomodulation and antimicrobial activity in RAW264. 7 macrophages. BMC microbiology. 2020;20(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lajqi T, Köstlin-Gille N, Hillmer S, et al. Gut microbiota-derived small extracellular vesicles endorse memory-like inflammatory responses in murine neutrophils. Biomedicines. 2022;10(2):442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Diaz-Garrido N, Badia J, Baldomà L. Modulation of dendritic cells by microbiota extracellular vesicles influences the cytokine profile and exosome cargo. Nutrients. 2022;14(2):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jung AL, Stoiber C, Herkt CE, Schulz C, Bertrams W, Schmeck B. Legionella pneumophila-derived outer membrane vesicles promote bacterial replication in macrophages. PLoS pathogens. 2016;12(4):e1005592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sahr T, Escoll P, Rusniok C, et al. Translocated Legionella pneumophila small RNAs mimic eukaryotic microRNAs targeting the host immune response. Nature Communications. 2022;13(1):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Olaya-Abril A, Prados-Rosales R, González-Reyes JA, Casadevall A, Pirofski L-a, Rodríguez-Ortega MJ. Extracellular vesicles from different pneumococcal serotypes are internalized by macrophages and induce host immune responses. Pathogens. 2021;10(12):1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mehanny M, Koch M, Lehr C-M, Fuhrmann G. Streptococcal extracellular membrane vesicles are rapidly internalized by immune cells and alter their cytokine release. Frontiers in immunology. 2020;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ayilam Ramachandran R, Lemoff A, Robertson DM. Pseudomonas aeruginosa-Derived Extracellular Vesicles Modulate Corneal Inflammation: Role in Microbial Keratitis? Infection and Immunity. 2023;91(4):e00036–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bielaszewska M, Rüter C, Kunsmann L, et al. Enterohemorrhagic Escherichia coli hemolysin employs outer membrane vesicles to target mitochondria and cause endothelial and epithelial apoptosis. PLoS pathogens. 2013;9(12):e1003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tiku V, Kofoed EM, Yan D, et al. Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii. Scientific reports. 2021;11(1):618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee J, Yoon YJ, Kim JH, et al. Outer membrane vesicles derived from Escherichia coli regulate neutrophil migration by induction of endothelial IL-8. Frontiers in Microbiology. 2018;9:2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jang SC, Kim SR, Yoon YJ, et al. In vivo kinetic biodistribution of nano‐sized outer membrane vesicles derived from bacteria. Small. 2015;11(4):456–461. [DOI] [PubMed] [Google Scholar]
- 145.Zaborowska M, Vazirisani F, Shah FA, et al. Immunomodulatory effects exerted by extracellular vesicles from Staphylococcus epidermidis and Staphylococcus aureus isolated from bone-anchored prostheses. Biomaterials. 2021;278:121158. [DOI] [PubMed] [Google Scholar]
- 146.Collins SM, Brown AC. Bacterial outer membrane vesicles as antibiotic delivery vehicles. Frontiers in immunology. 2021;12:733064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Uddin MJ, Dawan J, Jeon G, Yu T, He X, Ahn J. The role of bacterial membrane vesicles in the dissemination of antibiotic resistance and as promising carriers for therapeutic agent delivery. Microorganisms. 2020;8(5):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huang W, Zhang Q, Li W, et al. Development of novel nanoantibiotics using an outer membrane vesicle-based drug efflux mechanism. Journal of controlled release. 2020;317:1–22. [DOI] [PubMed] [Google Scholar]
- 149.Kim SW, Seo J-S, Park SB, et al. Significant increase in the secretion of extracellular vesicles and antibiotics resistance from methicillin-resistant Staphylococcus aureus induced by ampicillin stress. Scientific Reports. 2020;10(1):21066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yang J, Jia F, Qiao Y, Hai Z, Zhou X. Correlation between bacterial extracellular vesicles and antibiotics: A potentially antibacterial strategy. Microbial Pathogenesis. 2023:106167. [DOI] [PubMed] [Google Scholar]
- 151.Ye C, Li W, Yang Y, et al. Inappropriate use of antibiotics exacerbates inflammation through OMV-induced pyroptosis in MDR Klebsiella pneumoniae infection. Cell Reports. 2021;36(12) [DOI] [PubMed] [Google Scholar]
- 152.Li M, Lee K, Hsu M, Nau G, Mylonakis E, Ramratnam B. Lactobacillus-derived extracellular vesicles enhance host immune responses against vancomycin-resistant enterococci. BMC microbiology. 2017;17(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, Hoffmann JP, Chou C-W, et al. Burkholderia thailandensis outer membrane vesicles exert antimicrobial activity against drug-resistant and competitor microbial species. Journal of Microbiology. 2020;58:550–562. [DOI] [PubMed] [Google Scholar]
- 154.Gao F, Xu L, Yang B, Fan F, Yang L. Kill the real with the fake: eliminate intracellular Staphylococcus aureus using nanoparticle coated with its extracellular vesicle membrane as active-targeting drug carrier. ACS infectious diseases. 2018;5(2):218–227. [DOI] [PubMed] [Google Scholar]
- 155.Qiao L, Rao Y, Zhu K, Rao X, Zhou R. Engineered remolding and application of bacterial membrane vesicles. Frontiers in Microbiology. 2021;12:729369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Prados-Rosales R, Carreño LJ, Batista-Gonzalez A, et al. Mycobacterial membrane vesicles administered systemically in mice induce a protective immune response to surface compartments of Mycobacterium tuberculosis. MBio. 2014;5(5):e01921–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kim OY, Hong BS, Park K-S, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. The Journal of Immunology. 2013;190(8):4092–4102. [DOI] [PubMed] [Google Scholar]
- 158.Zanella I, König E, Tomasi M, et al. Proteome‐minimized outer membrane vesicles from Escherichia coli as a generalized vaccine platform. Journal of Extracellular Vesicles. 2021;10(4):e12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee W-H, Choi H-I, Hong S-W, Kim K-s Gho YS, Jeon SG. Vaccination with Klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. Experimental & molecular medicine. 2015;47(9):e183–e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ladhani SN, Ramsay M, Borrow R, Riordan A, Watson JM, Pollard AJ. Enter B and W: two new meningococcal vaccine programmes launched. Archives of disease in childhood. 2016;101(1):91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tarashi S, Zamani MS, Omrani MD, et al. Commensal and pathogenic bacterial-derived extracellular vesicles in host-bacterial and interbacterial dialogues: Two sides of the same coin. Journal of Immunology Research. 2022;2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Suri K, D’Souza A, Huang D, Bhavsar A, Amiji M. Bacterial extracellular vesicle applications in cancer immunotherapy. Bioactive Materials. 2023;22:551–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zou C, Zhang Y, Liu H, Wu Y, Zhou X. Extracellular vesicles: Recent insights into the interaction between host and pathogenic bacteria. Frontiers in Immunology. 2022;13:840550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhao G, Jones MK. Role of Bacterial Extracellular Vesicles in Manipulating Infection. Infection and Immunity. 2023;91(5):e00439–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ahmadi Badi S, Bruno SP, Moshiri A, Tarashi S, Siadat SD, Masotti A. Small RNAs in outer membrane vesicles and their function in host-microbe interactions. Frontiers in Microbiology. 2020;11:1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Felden B, Augagneur Y. Diversity and versatility in small RNA-mediated regulation in bacterial pathogens. Frontiers in Microbiology. 2021;12:719977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Munhoz da Rocha IF, Martins ST, Amatuzzi RF, et al. Cellular and Extracellular Vesicle RNA Analysis in the Global Threat Fungus Candida auris. Microbiology Spectrum. 2021;9(3):e01538–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Stanton BA. Extracellular vesicles and host–pathogen interactions: a review of inter-kingdom signaling by small noncoding RNA. Genes. 2021;12(7):1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lécrivain A-L, Beckmann BM. Bacterial RNA in extracellular vesicles: A new regulator of host-pathogen interactions? Biochimica et Biophysica Acta (BBA)-Gene Regulatory Mechanisms. 2020;1863(7):194519. [DOI] [PubMed] [Google Scholar]
- 170.Lee H-J. Microbe-host communication by small RNAs in extracellular vesicles: vehicles for transkingdom RNA transportation. International journal of molecular sciences. 2019;20(6):1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fan R, Zhou Y, Chen X, et al. Porphyromonas gingivalis Outer Membrane Vesicles Promote Apoptosis via msRNA-Regulated DNA Methylation in Periodontitis. Microbiology Spectrum. 2023;11(1):e03288–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Krohmaly KI, Freishtat RJ, Hahn AL. Bioinformatic and experimental methods to identify and validate bacterial RNA-human RNA interactions. Journal of Investigative Medicine. 2023;71(1):23–31. [DOI] [PubMed] [Google Scholar]
- 173.Bierwagen J, Wiegand M, Laakmann K, et al. Bacterial vesicles block viral replication in macrophages via TLR4-TRIF-axis. Cell Communication and Signaling. 2023;21(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bhar S, Zhao G, Bartel JD, et al. Bacterial extracellular vesicles control murine norovirus infection through modulation of antiviral immune responses. Frontiers in Immunology. 2022;13:909949. [DOI] [PMC free article] [PubMed] [Google Scholar]


