Abstract
Background:
Post-acute Sequelae of SARS-CoV-2 (PASC) describes a syndrome of physical and cognitive decline that persists after acute symptoms of infection resolve. Few studies have explored PASC among nursing home (NH) residents.
Methods:
A retrospective cohort study was conducted at two NHs in Michigan. COVID-positive patients were identified from March 21, 2020 to October 26, 2021. The comparison group were patients who lived at the same NH but who were never infected during the study period. Minimum Data Set was used to examine trajectories of functional dependence (Activity of Daily Living [ADL] composite score) and cognitive function (Brief Interview for Mental Status [BIMS]). Linear mixed-effects models were constructed to estimate short term change in function and cognition immediately following diagnosis and over time for an additional 12 months, compared to pre-COVID and non-COVID trajectories and adjusting for sex, age, and dementia status.
Results:
We identified 171 residents (90 COVID-19 positive, 81 non-COVID) with 719 observations for our analyses. Cohort characteristics included: 108 (63%) ≥ 80 yrs.; 121 (71%) female; 160 (94%) non-Hispanic white; median of 3 comorbidities (IQR 2-4), with no significant differences in characteristics between groups. COVID-19 infection affected the trajectory of ADL recovery for the first nine months following infection, characterized by an immediate post-infection decrease in functional status post-infection (−0.60 points, p=0.002) followed by improvement toward the expected functional trajectory sans infection (0.04 points per month following infection, p=0.271).
Conclusions:
NH residents experienced a significant functional decline that persisted for 9 months following acute infection. Further research is needed to determine whether increased rehabilitation services after COVID-19 may help mitigate this decline.
Introduction
Survivors of COVID-19 often experience residual symptoms after infection, a phenomenon called Post-acute Sequelae of SARS-CoV-2 infection, or PASC.1 Symptoms can span multiple organ systems and include pulmonary2 and non-pulmonary manifestations, cognitive symptoms and psychological symptoms.3 Such symptoms should persist for more than 2 months and be severe enough to affect function.4 Risk factors for PASC include older age, female sex, severe symptoms at presentation, and decreased physical function.5,6
PASC can have a unique presentation in older adults with studies indicating worsening of geriatric syndromes after infection.7,8 Studies have found higher risk of cognitive decline, sarcopenia and worsened mobility in older survivors of COVID-19.7-10 Thus far, research has focused on community-dwelling adults9 even though NH residents were disproportionately affected by the pandemic.11,12
Interventions to treat PASC symptoms is an emerging area of research.13 To our knowledge, no study has followed NH residents to characterize the longitudinal effects of infection on physical and mental function. We hypothesized that NH residents would experience a decline in function and cognition that would persist for months following COVID-19 infection.
Methods
Study Population
Any resident who lived in the NH at any time from September 1, 2019 to February 1, 2022 was eligible for inclusion. A COVID-19 patient was a resident who tested positive on at least one SARS-CoV-2 PCR test during the study interval. Residents who lived in the NH but never tested positive on a PCR test were non-COVID controls. Long-term residents were defined as a NH length of stay surpassing 90 days.
Data was extracted from Minimum Data Set (MDS), a mandated assessment collected on NH residents at least quarterly. For COVID-positive patients, we collected up to two quarterly MDS assessments before the date of SARS-CoV-2 infection if available, and up to four quarterly MDS assessments post-infection. For non-COVID patients, we collected the six most recent MDS assessments from when data collection occurred in October 2021.
We screened 261 patients (123 COVID-19, 138 non-COVID) for inclusion (Figure 1). Patients were excluded if insufficient post-infection records were available or records contained an error. The final study cohort consisted of 171 patients (90 COVID-positive, 81 non-COVID) with a total of 719 MDS records.
Figure 1: Selection of nursing home residents and MDS observations.
Overview of the selection process used to include residents in the study sample.
In the follow-up period, 30% of COVID-19 cases (n=27) and 12.8% of non-COVID 19 cases (n=12) died, which is a statistically significant difference (p=0.02). Of the 70% of COVID patients that survived, 76.2% of residents had 2 or more ADL points (n=48).
Of the 171 residents, only 17 (9.9%) were hospitalized, 75 (43.9%) had no hospitalization and 79 (46.2) had an unknown hospitalization status. Among the 17 hospitalized patients, 7 (41.2%) were in the ICU.
Function and Cognition
Our primary outcome was change in function as reflected in composite ADL score. We modified composite ADL score14 to reflect higher scores as better function, calculated as the sum of a resident’s ability to transfer, dress, self-feed, toilet, and bathe. Each activity contributes up to 4 points on a scale of 0 (complete dependence) to 4 (independence).14 We assigned a score of zero if an activity had been assessed inconsistently. Functional status was measured in two ways: first, the short-term decline that occurred immediately post-infection (ie, difference between pre-infection MDS and first post-infection MDS), and second, the recovery post-infection as indicated by positive slope over time in MDS assessments.
Our secondary outcome was change in cognitive performance per Brief Interview for Mental Status (BIMS) assessment.15 BIMS evaluates cognition on a scale of 0-15 with higher scores indicating intact cognition. Cognitive trajectories were conceptualized as an initial decline post-infection followed by subsequent slope over time during subsequent MDS assessments.
Statistical Analysis
We compared characteristics between COVID-positive and non-COVID patients. We used Pearson’s chi-square test to compare categorical characteristics, and Fisher’s exact test for any cell with value under 10. We compared continuous characteristics between groups using the Wilcoxon rank-sum test.
We developed multivariable linear mixed-effects models to estimate change in composite ADL score following acute infection and longitudinally. Our predictive models included patient group (COVID-positive vs non-positive), sex, age, dementia status, and longitudinal covariates: time in study, COVID-19 status (0 for all pre-COVID and all non-COVID patients’ test observations; 1 for all observations post-infection) and time elapsed since COVID-19 diagnosis (null for all non-COVID observations). We used the same model omitting dementia to assess change in cognitive function acutely and longitudinally.
Using the coefficients from this model, we constructed graphs of the predicted functional and cognitive trajectories of two example patients, one of whom had a COVID-19 infection eight months after baseline observation, and the other who was never infected.16 We modeled functional trajectories with and without dementia.
We conducted a sensitivity analysis to examine the effect of dementia on the association between COVID-positive and functional status. We added the interaction term between dementia and acute change in function, and dementia and time to examine the effect in both the acute and follow-up change in function. The effect on trajectories over time were visually presented for COVID and non-COVID example patients. Finally, we limited the sample only to patients with all six possible MDS assessments during the study period. All analyses and graphing were conducted using Stata version 17.0 (StataCorp, College Station, TX).
Results
Population Characteristics
A median of 4(IQR 3-6) MDS assessments were available per patient, reflecting a median of 12 months’ follow-up (Table 1). More COVID-positive patients (86/90 [95.6%]) were long-term care patients compared to non-COVID patients (69/81 [85.2%], p=0.020). Almost two-thirds of patients (108/171 [63.2%]) were over 80 years. Age distribution did not differ by COVID status (p=0.418). Most patients were female (121/171 [70.8%]) and non-Hispanic white (160/171 [93.6%], which did not differ significantly between groups (p=0.366 and 0.542).
Table 1.
Clinical and Demographic Characteristics of the Study Population
| Characteristic | All (N=171) |
COVID-19 Patients (N=90) |
Non-COVID Patients (N=81) |
p-value |
|---|---|---|---|---|
| Patient Observations, median (IQR) | ||||
| No. of observations | 4 (3-6) | 5 (2-6) | 4 (3-6) | 0.669a |
| Follow-up time in months | 12.0 (4.1-15) | 12.1 (3.1-15) | 12.0 (6-15) | 0.568a |
| Age, N(%) | ||||
| 35-69 | 14 (8.2%) | 5 (5.6%) | 9 (11.1%) | 0.418b |
| 70-79 | 49 (28.7%) | 26 (28.9%) | 23 (28.4%) | |
| 80-89 | 61 (35.7%) | 36 (40.0%) | 25 (30.1%) | |
| Age >89 | 47 (27.5%) | 23 (25.6%) | 24 (29.6%) | |
| Sex, N(%) | ||||
| Female | 121 (70.8%) | 61 (67.8%) | 60 (74.1%) | 0.366c |
| Race, N(%) | ||||
| Non-Hispanic white | 160 (93.6%) | 83 (92.2%) | 77 (95.1%) | 0.542b |
| Length of Stay, N(%) | ||||
| Long-term care | 155 (90.6%) | 86 (95.6%) | 69 (85.2%) | 0.020 b |
| Comorbidities, N(%) | ||||
| Dementia | 87 (50.9%) | 52 (57.8%) | 35 (43.2%) | 0.057c |
| Congestive heart failure | 58 (33.9%) | 31 (34.4%) | 27 (33.3%) | 0.878c |
| Diabetes | 45 (26.3%) | 27 (30.0%) | 18 (22.2%) | 0.249c |
| COPD | 38 (22.2%) | 22 (24.4%) | 16 (19.8%) | 0.461c |
| No. of comorbidities, median (IQR) | 3 (2-4) | 3 (2-4) | 3 (2-4) | 0.285a |
| Activities of Daily Living, median (IQR) | ||||
| Transferring | 3 (2-3) | 3 (2-3) | 3 (2-3) | 0.337a |
| Dressing | 3 (2-3) | 3 (2-3) | 3 (2-3) | 0.461a |
| Eating | 1 (0-1) | 1 (0-2) | 1 (0-1) | 0.126a |
| Toilet | 3 (2-3) | 3 (2-3) | 3 (2-3) | 0.695a |
| Bathing | 3 (3-4) | 4 (3-4) | 3 (3-4) | 0.072a |
| ADL Sum Score | 13 (10-14) | 13 (11-15) | 13 (10-14) | 0.245a |
| BIMS Score, median (IQR) | 12 (6-15) | 13 (7-15) | 12 (4.5-14) | 0.662a |
Significance evaluated using Wilcoxon rank-sum test
Significance evaluated using Fisher’s exact test
Significance evaluated using Pearson’s chi-square
A median of 3 comorbidities were present and did not differ significantly between groups (Table 1). 57.8% (52/90) of COVID-positive and 43.2% (35/81) of non-COVID patients had dementia (p=0.057). Congestive heart failure (33.9% of all patients), diabetes (26.3%), and COPD (22.2%) were also common.
Baseline ADLs were similar between groups, with median initial composite ADL score of 13 (COVID-positive: median 13, IQR 11-15; non-COVID: median 13, IQR: 10-14) and distributions did not differ significantly (p=0.245) (Table 1). COVID-positive patients had median BIMS score of 13 (IQR 7-15) and non-COVID patients had median score of 12 (IQR 4.5-14) (p=0.662).
ADL Composite
Our multivariable linear mixed-effects modeling indicates that COVID-19 infection affected the trajectory of composite ADL score for nine months post-infection (Figure 2a). Compared with pre-COVID slopes, a significant decline in function occurred acutely post-infection (adjusted decrease in composite ADL score, 0.60 points [95% CI, −0.97 to −0.19], p=0.003). This acute decline was followed by improvement toward baseline function (adjusted increase in composite ADL score per month, 0.05 [95% CI, −0.02 to 0.11], p=0.187) which was not statistically significant.
Figure 2A. Modeled Trajectory of ADL Impairment over Time, by COVID-19 Status and Dementia Status.
This figure illustrates the progression of ADL impairment over time according to COVID-19 and cognitive status. Participant-specific predicted values of ADL impairment were calculated for an 80-year-old female resident. Multivariable linear mixed- effects model was used to estimate change in composite ADL score acutely following COVID-10 diagnosis as well as over time. Linear-mixed models included patient-level variables of patient group (COVID-positive vs non-positive), sex, age (greater than or equal to 80) and dementia status, as well as the following longitudinal covariates: time in study, COVID-19 status and time elapsed since COVID-19 diagnosis (null for all non-COVID patients’ observations). Green dashed line indicates the trajectory for non-COVID residents without dementia; blue dashed line, the trajectory for non-COVID residents with dementia; gold dashed line, the trajectory for COVID patient without dementia; red dashed line, the trajectory for COVID patient with dementia.
BIMS
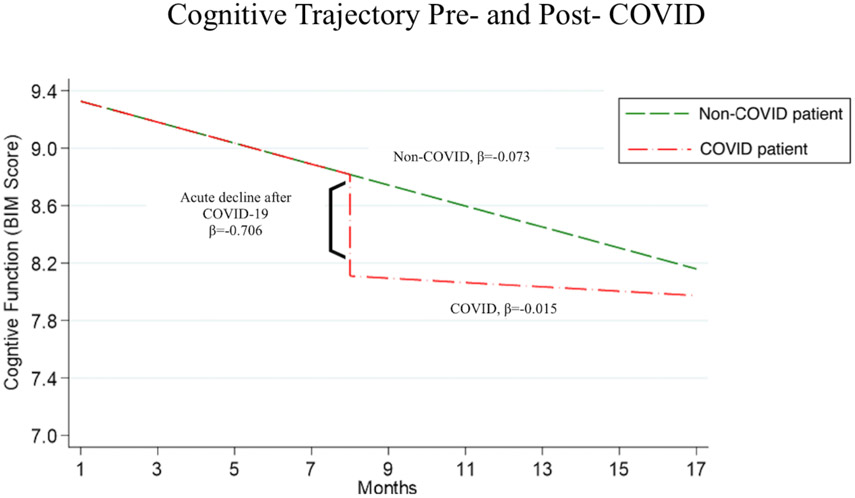
We modeled change in BIMS score over time using 605 BIMS measures of 159 patients (Figure 2b). This model predicted a decline of 0.07 in score per month for all patients regardless of COVID-19 status (95% CI, −0.13 to −0.02; p=0.006). The model predicted an additional acute decline following COVID-19 of 0.71 points (95% CI, −1.35 to −0.06; p=0.032). The model predicted a significant decline of 2.76 points per month among patients over 80 years, adjusting for all other factors (95% CI, −4.33 to −1.20; p=0.001).
Figure 2B. Modeled Trajectory of Cognitive Function over Time, by COVID-19 Status:
This figure illustrates the progression of BIM score over time according to COVID-19 infection status. Participant-specific predicted values of BIM score were calculated for an 80-year-old female resident. Multivariable linear mixed- effects model was used to estimate change in BIM score score acutely following COVID-10 diagnosis as well as over time.
Sensitivity Analyses
Regardless of COVID-19 status, the decline in ADL function was significantly faster among dementia patients. COVID-19 infection did not statistically affect the acute decline in ADL score (−0.25 ADL, 95% CI −1.03 to 0.52; p=0.522) nor the slope of decline post-COVID (0.11 ADL/month, 95% CI −0.02 to 0.25; p=0.091). Limiting the analysis to only patients with all six MDS observations (60 patients for ADL outcome; 47 for BIMS outcome), our results did not differ substantially nor statistically from the full models.
Discussion
Our study indicates that PASC in NH residents includes an immediate short-term decline in ADL function. The median acute decrement associated with infection was 1 point in the composite ADL score, corresponding to a resident requiring substantially more assistance with completing an essential self-care activity post-infection. Importantly, residents in general showed a slow but steady improvement over one year. Most residents show functional recovery by one year follow-up. In addition, there was a detectable but more modest decrease in cognitive score in the short term that also showed recovery by one year.
Functional decline after COVID-19 is common and research is unclear as to the mechanism. One potential mechanism may be weight loss associated with COVID-19. One US study explored weight changes among NH residents between March 2020 and May 2020 and found that all residents lost weight over this time period but residents who had COVID-19 experienced 4.6% greater weight loss compared to non-infected residents.17 Potential mechanisms of this weight decrease include loss of taste and smell as a symptom of COVID-19, as well as decreased mealtime assistance in quarantine.18,19 NH residents are vulnerable to weight loss that could potentially lead to sarcopenia and nutritional deficiencies, fatigue, and subsequently, functional decline.10 This study is limited by lack of precise weight trajectories that precludes a direct link with our findings, as weight loss is recorded in MDS data only if greater than 5% of body weight in the past 1 month or 10% in the past 180 days. Future studies should be designed to explore this association.
Quarantine may play a role in the acute decline in function and cognition after COVID-19 infection. Throughout the pandemic, NHs suffered severe staffing shortages and the degree to which the usual care services could be provided is not known, although recent research indicates that SNFs provided a level of rehabilitation services similar to pre-pandemic levels.20-22 During quarantine, however, NH patients experienced additional disruptions to their routine where they were confined to a smaller area within the facility and had less contact with residents and staff members for 14 days.23 Social isolation during the pandemic has been associated with negative health outcomes among NH residents including significant weight loss,24 increase in non-COVID mortality,25 increase in depressive symptoms20,24 worsening cognition, 25 and increase in incontinence.25
Our study has limitations that should be addressed. First, we missed asymptomatic COVID-19 infections early in the study period since universal screening testing and infection control policies improved throughout the pandemic. Second, our study took place at two NHs in southeast Michigan limiting its generalizability. However, it is hypothesis generating and should be explored using larger data sets. Finally, all infections in this study were diagnosed during the early phases of the pandemic between March 2020 and October 2021, and most residents were unvaccinated at the time of their infection (84/90, 93% of cases were not vaccinated). Recent data suggests that complete primary vaccination protects against PASC6 and that the pre-Omicron variants of COVID-19 are associated with the highest rates of PASC.26 Therefore, our study likely captured the highest rates of PASC early in the pandemic prior to widespread vaccination.
In conclusion, our study begins to describe how PASC manifests in the NH population as a short-term decline in function and cognition with a slow recovery. Further research with a larger sample size is needed to understand how interventions can mitigate the effects of COVID-19 on this vulnerable population.
Key Points:
PASC in NH residents includes a short-term decline in activities of daily living with residents on average requiring assistance with one additional self-care activity after infection
NH residents also experienced a modest decline in cognitive scores following infection
Both cognitive and functional status scores showed recovery by one year post-infection.
Why does this matter?
PASC is poorly understood among NH resident survivors of COVID-19 infection, despite disproportionate impact on NHs by the pandemic. Characterizing PASC is the first step in designing interventions that can aid this population.
Acknowledgements
We thank all nursing home patients and healthcare workers who participated in this research study.
Funding sources:
This work was supported by the Agency for Healthcare Research & Quality [grant RO1HS25451]. Dr. Mody is also supported by the National Institute on Aging Mid-Career Mentorship Grant [K24 AG050685]; Michigan Institute for Clinical and Health Research [UL1TR002240]; the Claude D. Pepper Older Americans Independence Center [P30 AG024824, 3P30AG024824-16S1]. Dr. Mody is also supported by the Geriatrics Research, Education and Clinical Centers, Veterans Affairs Ann Arbor Healthcare System.
Footnotes
Conflict of interest. All authors report no conflicts of interest relevant to this article.
Sponsor’s Role: The authors’ funding sources did not participate in the planning, collection, analysis, or interpretation of data or in the decision to submit for publication. The investigators had full access to the data and were responsible for the study protocol, progress of the study, analysis, reporting of the study, and the decision to publish.
Presentation: poster presentation at American Geriatric Society, Long Beach CA, May 2023
References
- 1.Levine RL. Addressing the long-term effects of COVID-19. JAMA. 2022;328(9):823. doi: 10.1001/jama.2022.14089 [DOI] [PubMed] [Google Scholar]
- 2.Willigen H, Wynberg E, Verveen A, et al. One-fourth of COVID-19 patients have an impaired pulmonary function after 12 months of illness onset. 2023. doi: 10.1164/ajrccmconference.2023.207.1_meetingabstracts.a6792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid—mechanisms, risk factors, and management. BMJ. 2021. doi: 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 4.Diaz JV, Soriano JB. A Delphi consensus to advance on a clinical case definition for post covid-19 condition: A who protocol. 2021. doi: 10.21203/rs.3.pex-1480/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlis RH, Santillana M, Ognyanova K, et al. Prevalence and correlates of long covid symptoms among US adults. JAMA Network Open. 2022;5(10). doi: 10.1001/jamanetworkopen.2022.38804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selvakumar J, Havdal LB, Drevvatne M, et al. Prevalence and characteristics associated with post–COVID-19 condition among nonhospitalized adolescents and young adults. JAMA Network Open. 2023;6(3). doi: 10.1001/jamanetworkopen.2023.5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Havervall S, Rosell A, Phillipson M, et al. Symptoms and functional impairment assessed 8 months after mild covid-19 among health care workers. JAMA. 2021;325(19):2015. doi: 10.1001/jama.2021.5612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y-H, Wang Y-R, Wang Q-H, et al. Post-infection cognitive impairments in a cohort of elderly patients with covid-19. Molecular Neurodegeneration. 2021;16(1). doi: 10.1186/s13024-021-00469-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen AB, McAvay GJ, Geda M, et al. Rationale, design, and baseline characteristics of the valiant (covid-19 in older adults: A longitudinal assessment) cohort. 2022. doi: 10.1101/2022.09.14.22279932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piotrowicz K, Gąsowski J, Michel J-P, Veronese N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clinical and Experimental Research. 2021;33(10):2887–2898. doi: 10.1007/s40520-021-01942-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorensen JM, Crooks VA, Freeman S, et al. A call to action to enhance understanding of long covid in long-term care home residents. Journal of the American Geriatrics Society. 2022;70(7):1943–1945. doi: 10.1111/jgs.17889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conlen M, Ivory D, Yourish K, et al. 43% of U.S. Coronavirus Deaths Are Linked to Nursing Homes. The New York Times. https://www.nytimes.com/interactive/2020/us/coronavirus-nursing-homes.html. Accessed July 7, 2020. [Google Scholar]
- 13.Fairbank R. Long Covid Exercise Trials proposed by NIH Raise Alarm. Nature. 2023;616(7956):228–229. doi: 10.1038/d41586-023-00900-w [DOI] [PubMed] [Google Scholar]
- 14.Morris JN, Fries BE, Morris SA. Scaling adls within the MDS. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 1999;54(11). doi: 10.1093/gerona/54.11.m546 [DOI] [PubMed] [Google Scholar]
- 15.Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care 2017; 55:e68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aubert CE, Kabeto M, Kumar N, Wei MY. Multimorbidity and long-term disability and physical functioning decline in middle-aged and older Americans: An observational study. BMC Geriatrics. 2022;22(1). doi: 10.1186/s12877-022-03548-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinchek M, Beiting KJ, Walker J, et al. Weight loss in Covid-19–Positive Nursing Home Residents. Journal of the American Medical Directors Association. 2021;22(2):257–258. doi: 10.1016/j.jamda.2020.11.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grund S, Bauer JM. Malnutrition and sarcopenia in covid-19 survivors. Clinics in Geriatric Medicine. 2022;38(3):559–564. doi: 10.1016/j.cger.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courtois-Amiot P, Allart H, de Cathelineau C, et al. Covid-19 as an independent risk factor for weight loss in older adults living in nursing homes. Gerontology. 2023. doi: 10.1159/000529357 [DOI] [PubMed] [Google Scholar]
- 20.Coe AB, Montoya A, Chang CH, et al. Behavioral symptoms, depression symptoms, and medication use in Michigan nursing home residents with dementia during covid -19. Journal of the American Geriatrics Society. 2022;71(2):414–422. doi: 10.1111/jgs.18116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang XS, Charland K, Quach C, Nguyen QD, Zinszer K. Institutional, therapeutic, and individual factors associated with 30-day mortality after covid -19 diagnosis in Canadian long-term care facilities. Journal of the American Geriatrics Society. 2022;70(11):3210–3220. doi: 10.1111/jgs.17975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi SM, Kosar CM, Gouskova N, Berry SD. Post–Acute Care Rehabilitation Services and outcomes in skilled nursing facilities before and during the COVID-19 pandemic. JAMA Health Forum. 2023;4(3). doi: 10.1001/jamahealthforum.2023.0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montoya A, Jenq G, Mills JP, et al. Partnering with local hospitals and public health to manage covid -19 outbreaks in nursing homes. Journal of the American Geriatrics Society. 2020;69(1):30–36. doi: 10.1111/jgs.16869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danilovich MK, Norrick CR, Hill KC, Conroy DE. Nursing home resident weight loss during coronavirus disease 2019 restrictions. Journal of the American Medical Directors Association. 2020;21(11):1568–1569. doi: 10.1016/j.jamda.2020.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levere M, Rowan P, Wysocki A. The adverse effects of the COVID-19 pandemic on Nursing Home resident well-being. Journal of the American Medical Directors Association. 2021;22(5). doi: 10.1016/j.jamda.2021.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kahlert CR, Strahm C, Güsewell S et al. Post-acute sequelae after severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection by viral variant and vaccination status: A multicenter cross-sectional study. Clinical Infectious Diseases 2023. doi: / 10.1093/cid [DOI] [PMC free article] [PubMed] [Google Scholar]