Abstract
Background:
Although immunotherapy has emerged as a therapeutic strategy for many cancers, there are limited studies establishing the safety and efficacy in people living with human immunodeficiency virus (PLWH) and cancer.
Methods:
PLWH and solid tumors or Kaposi Sarcoma (KS) receiving antiretroviral therapy (ART) and a suppressed HIV viral load received nivolumab at 3 mg/kg every 2 weeks, in two dose deescalation cohorts stratified by CD4 count (Stratum 1: CD4 count ≥ 200/uL and Stratum 2: CD4 count 100–199/uL). An expansion cohort of 24 participants with a CD4 count ≥ 200/uL was then enrolled.
Results:
36 PLWH received nivolumab, including 15 with KS and 21 with a variety of other solid tumors. None of the first 12 participants had dose-limiting toxicity in both CD4 strata, and 5 patients (14%) overall had grade 3 or higher immune related AEs. Objective partial response occurred in 9 PLWH and cancer (25%), including in 6 of 15 with KS (40%; 95% confidence intervals (CI): 16.3% - 64.7%), The median duration of response was 9.0 months overall, and 12.5 months in KS. Responses were observed regardless of PDL1 expression. There were no significant changes in CD4 count or HIV viral load.
Conclusions:
Nivolumab has a safety profile in PLWH similar to HIV-negative subjects with cancer, and also efficacy in KS. Plasma HIV remained suppressed and CD4 counts remained stable during treatment and ART, indicating no adverse impact on immune function.
Trial Registration:
ClinicalTrials.gov Identifier: NCT02408861
Keywords: HIV cancer, Immunotherapy, Nivolumab, CD4 count >100/uL
Precis:
The anti-PD1 inhibitor nivolumab may be used safely for treatment of cancer, and has activity in Kaposi Sarcoma, in people living with HIV receiving antiretroviral therapy, a suppressed HIV viral load, and CD4 lymphocyte count of at least 100/uL
Introduction
Since the advent of effective antiretroviral therapy (ART) in 1996, opportunistic diseases have become less common, and mortality for people living with HIV (PLWH) has declined such that most of them now have near-normal life expectancy [1, 2]. In the United States, more than 50% of the 1.3 million PLWH are over 50 years old; and by 2030, it is estimated that 70% will be over the age of 50 years [3]. People aging with HIV share many of the same health concerns as the general population, including cancer. There is an increasing incidence of non-AIDS-defining cancers in PLWH, including cancers that may benefit from immunotherapy [4, 5].
Programmed death 1 (PD-1) protein, a T-cell co-inhibitory receptor, and one of its ligands, PD-L1, play a pivotal role in the ability of tumor cells to evade the host’s immune system [6]. PD-L1 is the primary PD-1 ligand that is up-regulated in solid tumors, where it can inhibit cytokine production and the cytolytic activity of PD-1+, tumor-infiltrating CD4+ and CD8+ T cells [7]. Nivolumab is a high-affinity, fully humanized, PD-1–specific, IgG4 (S228P) monoclonal antibody that inhibits the binding of PD-L1 to both PD-1 and CD80 [8]. Immune checkpoint inhibitors (ICIs) such as nivolumab and other inhibitors of PD-1 and PD-L1 have become an important mainstay of immunotherapy for several cancers [9–16]. Nivolumab is FDA-approved for a broad range of cancers. However, PLWH who develop cancers were often excluded from clinical trials evaluating ICI because of concerns that HIV-associated perturbations in T-cell repertoires may compromise anti-tumor efficacy of immunotherapy, promote HIV reactivation, and increase the potential for immune-related toxicity or infection. As a result, clinical trial data on immune checkpoint blockade in PLWH were sparse at the time this trial was initiated, and remain limited. We therefore conducted a prospective study of the humanized anti–programmed cell death-1 (anti–PD-1) antibody nivolumab in PLWH with advanced cancers, including Kaposi Sarcoma, to determine the safety in patients with a CD4 count as low as 100/uL and an undetectable or very low HIV viral load while receiving ART, effect of HIV viral load and CD4/CD8 cell populations.
Methods
Study Oversight
The AIDS Malignancy Consortium (AMC) coordinated this multi-center phase 1 study (AMC-095) which was reviewed and approved by the National Cancer Institute Cancer Therapy Evaluation Program and the institutional review boards at each participating institution (clinicaltrials.gov NCT02408861). All participants provided written informed consent.
Eligibility
Participants were required to have HIV infection and a histologically confirmed solid tumor malignancy that was metastatic or unresectable and for which standard curative or palliative measures did not exist or were no longer effective. For participants in the solid tumor expansion cohort, patients with KS were eligible, but cancers known to be not responsive to single agent nivolumab were ineligible. KS was required to be progressing despite ART and HIV suppression for at least 2 months or stable despite ART for at least 3 months. HIV-associated eligibility criteria required a CD4 count greater than or equal to 100 cells/μL and effective ART for at least 4 weeks with an HIV viral load of less than 75 copies/mL. Participants had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and measurable or assessable disease by RECIST1.1 criteria for solid tumors [17], or assessable by ACTG response criteria for KS [18]. Laboratory criteria included an absolute neutrophil count of greater than 1,000/μL, a platelet count greater than 75,000/μL, hemoglobin level greater than 9 g/dL, total bilirubin less than 1.5 × upper limit of normal (ULN) (< 3 mg/dL allowed for patients on atazanavir), aspartate and alanine aminotransferase levels less than 3 times ULN, creatine kinase level less than 5 times ULN, serum creatinine level less than 1.5 times ULN (or creatinine clearance greater than 50 mL/min, and albumin greater than 2.8 g/dL. Key exclusion criteria included prior treatment with an anti-PD-1/PDL1 anti-CTLA-4 antibody, allogeneic transplant, active immunosuppressive therapy, and uncontrolled hepatitis B or C infection or history of autoimmune disease requiring systemic therapy.
Sample size and design
The study was designed as phase 1 dose de-escalation (DDE) component to be administered in two strata based on CD4 count, followed by an expansion component in solid tumors. The first component (DDE) was planned for two dose levels of nivolumab: 3 mg/kg q 2 week × 46 doses and 1 mg/kg q 2 week × 46 doses. The DDE followed a rolling six design aiming at establishing the maximum tolerable dose (MTD) for single agent nivolumab. The study would start with stratum 1 (CD4 counts ≥ 200/uL). Six participants would need to be enrolled unless two or more of the first 3 participants in that cohort experience a dose limiting toxicity (DLT). DLT is defined in the following section. If 2 DLTs are experienced at the first dose level, one dose de-escalation would be permitted. The Phase I MTD was defined as the dose level at which ≤1/6 participants experience DLT, with the next higher dose having ≥2 participants encountering DLT (unless the MTD is the highest pre-specified dose level). Stratum 2 dosing was planned to begin at the single-agent therapy MTD for Stratum 1. Each subject was followed up for the safety evaluation period of 6 weeks. The dose expansion component was planned for 24 subjects with the goal of obtaining more safety data and to estimate preliminary efficacy.
Treatment and Procedures
Patients described in this report received nivolumab 3mg/kg administered intravenously every 2 weeks with continued ART. Dose limiting toxicity (DLT) was defined as permanently stopping protocol treatment during the DLT period (defined as 6 weeks from the first dose) for either stratum. Any drug-related death was a DLT, or any grade 3 or 4 events requiring permanent discontinuation of nivolumab, including those that required long term, high dose corticosteroids. Management of immune-related adverse events (irAEs) included withholding nivolumab and administration of corticosteroids based on AE severity following standard protocol guidelines. Zero or one unacceptable AE in the de-escalating cohort were required to expand the solid tumor cohort. Safety monitoring occurred on all cycles. AEs were graded and attributions were assigned using the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 until January 29, 2018, and then v5.0 was used. The HIV viral load and CD4 counts were measured at baseline and once over the first 8 weeks, and then repeated every 12 weeks.
Objective responses, stable disease and disease progression as defined by RECIST1.1 [17] were monitored by appropriate imaging at 12-week intervals during the study for those with solid tumors; for those with KS, the ACTG KS criteria [18] were followed to assess disease and define response at baseline and every 4 weeks. Clinical benefit rate (CBR) was defined as percentage of subjects who achieved either a complete response, partial response, or at least six months of stable disease because of the study treatment. Treatment beyond progression was allowed with repeat imaging 4 to 6 weeks later to confirm progressive disease (PD) in select patients. Treatment for patients who achieved stable disease (SD) or better could continue for up to 2 years. Drug administration was discontinued for confirmed PD, unacceptable AEs, intercurrent serious illness, investigator’s decision, consent withdrawal, or completion of 2 years of therapy.
Statistical Analysis
The safety population included all participants who received at least one dose of nivolumab. After completion of accrual to strata 1, 2, and the solid tumor expansion cohorts, the database was locked on August 1, 2021. Baseline characteristics were tabulated and summarized. Treatment emergent adverse events (TEAEs) are the undesirable events that were attributed as possibly, probably, or definitely to the therapy due to being either absent pre-treatment, or a present and worsening in either intensity or frequency post-treatment. Changes in CD4+ T-cell counts and CD4/CD8 ratio from baseline to each time points (C5D1, C11D1, post-treatment follow-ups at 6 and 16 weeks) were evaluated by Wilcoxon signed rank test for paired data. Statistical analyses were performed using R computing environment [27].
Tumor tissue and PDL1 testing
Baseline tumor formalin fixed paraffin-embedded (FFPE) tissue was acquired prior to enrollment if tissue biopsy was available. PD-L1 immunostaining was performed on tissue biopsies using the commercially available PD-L1 immunohistochemistry 22C3 pharm Dx assay (Dako North America). A PD-L1 expression (combined positive score [CPS]) level of ≥1% was defined as positive staining.
Results
Participant Characteristics
38 patients were accrued between October 21, 2015 and January 6, 2020 at 15 sites, 36 patients received at least a single dose of study treatment; one patient withdrew consent prior to treatment and one patient died before initiation of treatment (see Figure 1 Consort Diagram). The characteristics of the study population including 36 patients who received at least 1 dose of nivolumab are outlined in Table 1 and summarized herein. The median age was 54.6 [IQR: 47.5–59.3] years, 33 (91.7%) were male, and all had had an ECOG PS of 0 (n=15) or 1 (n=21). Regarding race and ethnicity, 21 (58.3%) were non-Hispanic White, 10 (27.8%) were non-Hispanic Black or African American, 3 (8.3%) were Hispanic White, 1 (2.8%) was American Indian or Alaskan Native, and 1 (2.8%) did not report race. All patients were taking stable ART at the time of registration as required by the protocol. The median CD4 count was 315 cells/μL (inter-quartile range, 228–441 cells/μL) and all had an HIV viral load less than 75 copies/mL at study enrollment. Regarding tumor histology, 15 had KS, 9 had cancers for which PD-1 directed agents had known single-agent efficacy (including 4 with anal cancer, 2 with non-small cell lung cancer, 2 with head and neck cancer, 1 with hepatocellular cancer), and 12 had a variety of other solid tumors. Most participants were pretreated; 35 (97%) had progressed after 1 or more lines of previous cancer treatment. Among 19 patients who had tumor available for PD-L1 immunostaining, only 2 (5.6%) of tumors had a PD-L1 of at least 1%, including 1 patient each with KS and follicular dendritic sarcoma.
Figure 1.

CONSORT diagram.
Table 1:
Baseline Patient Demographic and Clinical Characteristics
| Nivolumab dose de-escalation cohort (N=13) |
Nivolumab Solid Tumor Expansion (N=23) |
Overall (N=36) |
|
|---|---|---|---|
| Sex | |||
| Male | 12 (92.3%) | 21 (91.3%) | 33 (91.7%) |
| Female | 1 (7.7%) | 2 (8.7%) | 3 (8.3%) |
| Age | |||
| Median [Q1-Q3] | 56.8 [53.7–59.4] | 51.6 [40.6–58.9] | 54.6 [47.5–59.3] |
| Race and Ethnicity | |||
| American Indian or Alaska Native | 1 (7.7%) | 0 (0%) | 1 (2.8%) |
| Hispanic White | 0 (0%) | 3 (13.0%) | 3 (8.3%) |
| Non-Hispanic Black | 4 (30.8%) | 6 (26.1%) | 10 (27.8%) |
| Non-Hispanic White | 8 (61.5%) | 13 (56.5%) | 21 (58.3%) |
| Other/Unknown | 0 (0%) | 1 (4.3%) | 1 (2.8%) |
| Baseline CD4 count | |||
| >=200 | 5 (38.5%) | 23 (100%) | 28 (77.8%) |
| 100–199 | 8 (61.5%) | 0 (0%) | 8 (22.2%) |
| Prior treatments received | |||
| 0 | 0 (0%) | 1 (4.3%) | 1 (2.8%) |
| 1 | 13 (100%) | 20 (87.0%) | 33 (91.7%) |
| 2 | 0 (0%) | 2 (8.7%) | 2 (5.6%) |
| ECOG performance status | |||
| 0 | 4 (30.8%) | 11 (47.8%) | 15 (41.7%) |
| 1 | 9 (69.2%) | 12 (52.2%) | 21 (58.3%) |
| Tumor histology | |||
| Anal cancer | 4 (30.8%) | 0 (0%) | 4 (11.1%) |
| Head & neck squamous cell carcinoma | 2 (15.4%) | 0 (0%) | 2 (5.6%) |
| Kaposi’s Sarcoma | 0 (0%) | 15 (65.2%) | 15 (41.7%) |
| Liver cancer | 0 (0%) | 1 (4.3%) | 1 (2.8%) |
| Non-small cell lung cancer | 1 (7.7%) | 1 (4.3%) | 2 (5.6%) |
| Others* | 6 (46.2%) | 6 (26.1%) | 12 (33.3%) |
Pancreatic cancer, skin squamous cell cancer, breast cancer, gallbladder cancer, follicular dendritic sarcoma, small cell lung cancer, colon cancer, squamous cell cancer of unknown primary, liposarcoma, and ovarian cancer
Treatment Administered
Thirty-six participants received 396 doses of therapy. In the dose-de-escalation cohort, 5 and 8 subjects having a baseline CD4 count ≥200/uL (stratum 2) and 100–200/uL (stratum 1), respectively, were treated with single agent nivolumab; 23 subjects with a CD4 count ≥200/UL (stratum 2) received nivolumab therapy in the solid tumor expansion cohort (Table 1). The median number of doses was 5.5 (interquartile range, 4–13). 7 subjects experienced delayed administration of nivolumab, and nivolumab dose reduction was performed in one subject. 3 participants completed 2 years of therapy. Reasons for discontinuation of therapy in 30 participants included disease progression (n =17), adverse events (n=9), deaths on study (n=2 due to diabetes mellitus or disease progression), and patient withdrawal of consent or refusal to study intervention (n=2). Of the 9 discontinuations due to adverse effects, 7 were attributed to treatment, including hyperglycemia (grade 4), lipase elevation (grade 3), colitis (grade 3), adrenal insufficiency (grade 2), pain in extremity (grade 2), and optic nerve disorder (grade 3) later diagnosed as secondary malignancy (Burkitt’s lymphoma) and considered a separate event.
Adverse Events and Dose De-escalation
None of the first 12 participants in both CD4 strata experienced a protocol-defined DLT, and thus dose de-escalation was not invoked. In the overall population, 29 out of 36 (80.5%) subjects experienced at least one treatment-related adverse event (AE) (111 AEs overall), including 9 subjects (25%) with grade 3 or greater severity. The majority of the treatment-related AEs were grade 1 or 2 (88%). Among 28 subjects with baseline CD4 count ≥ 200/uL in stratum 1, at least one treatment-related AE (82 overall) occurred in 22 subjects (79%), including 6 subjects experienced at least one grade 3–4 treatment-related AEs (21.4%; 95% CI: 10.2–39.5%) Among 8 patients with a baseline CD4 count of 100–200/uL in stratum 2, 7 (88%) had a least 1 treatment related AE (29 overall), including 3 subjects who experienced at least one grade 3–4 treatment-related AE (37.5%; 95% CI:13.7–69.4%). No statistically significant difference was observed in incidence of treatment related AEs of grade 3 or greater severity between the two CD4 count-based strata (p>0.5).
Treatment related adverse events with at least possible attribution to nivolumab are shown in Table 2. The table excludes grade 1 or 2 AEs that occurred in less than 5% of subjects. In case of multiple occurrences of the same AE in any subject, the occurrence with the highest severity grade of that adverse event was selected for the patient. Fatigue, nausea, diarrhea, rash and lymphopenia were the most common adverse events occurring in more than 10% of subjects, but were all grade 1 or 2. There were 71 adverse events overall, including 58 grade 1–2 (82%), 12 grade 3–4 (17%), and 1 grade 5 (1%), further described in the next paragraph. A full list of treatment related adverse events with at least possible attribution to nivolumab stratified by CD4 count stratum is provided in supplemental tables 1 and 2.
Table 2.
Worst grade adverse events that were possibly, probably, or definitely attributed to nivolumab
| Adverse Events | Severity N (%) | Total | ||||
|---|---|---|---|---|---|---|
| Grade 1 * | Grade 2 * | Grade 3 | Grade 4 | Grade 5 | N (%) | |
| Immune-related | ||||||
| Alanine aminotransferase increased | 2(5.6) | 0(0) | 1(2.8) | 0(0) | 0(0) | 3(8.3) |
| Alkaline phosphatase increased | 2(5.6) | 0(0) | 0(0) | 0(0) | 0(0) | 2(5.6) |
| Aspartate aminotransferase increased | 0(0) | 1(2.8) | 1(2.8) | 0(0) | 0(0) | 2(5.6) |
| Serum amylase increased | 2(5.6) | 1(2.8) | 0(0) | 0(0) | 0(0) | 3(8.3) |
| Colitis | 0(0) | 0(0) | 1(2.8) | 0(0) | 0(0) | 1(2.8) |
| Hyperglycemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 1 (2.8) |
| Hypothyroidism | 0(0) | 2(5.6) | 0(0) | 0(0) | 0(0) | 2(5.6) |
| Lipase increased | 1(2.8) | 0(0) | 0(0) | 1(2.8) | 0(0) | 2(5.6) |
| Pruritus | 2(5.6) | 0(0) | 0(0) | 0(0) | 0(0) | 2(5.6) |
| Maculo-papular rash | 4(11.1) | 2(5.6) | 0(0) | 0(0) | 0(0) | 6(16.7) |
| Hematologic | ||||||
| Lymphocyte count decreased | 4(11.1) | 1(2.8) | 0(0) | 0(0) | 0(0) | 5(13.9) |
| Neutrophil count decreased | 2(5.6) | 0(0) | 0(0) | 0(0) | 0(0) | 2(5.6) |
| Platelet count decreased | 2(5.6) | 0(0) | 0(0) | 0(0) | 0(0) | 2(5.6) |
| White blood cell decreased | 2(5.6) | 0(0) | 1(2.8) | 0(0) | 0(0) | 3(8.3) |
| Cardiopulmonary and systemic | ||||||
| Pericardial tamponade | 0(0) | 0(0) | 0(0) | 1(2.8) | 0(0) | 1(2.8) |
| Lung infection | 0(0) | 0(0) | 0(0) | 1(2.8) | 0(0) | 1(2.8) |
| Atelectasis | 0(0) | 0(0) | 0(0) | 1(2.8) | 0(0) | 1(2.8) |
| Dyspnea | 2(5.6) | 0(0) | 0(0) | 0 (0) | 0(0) | 2(5.6) |
| Sepsis | 0(0) | 0(0) | 0(0) | 1(2.8) | 0(0) | 1(2.8) |
| Gastrointestinal | ||||||
| Nausea/Anorexia | 4(11.1) | 0 (0) | 0(0) | 0(0) | 0(0) | 4(11.1) |
| Diarrhea | 3(8.3) | 1(2.8) | 0(0) | 0(0) | 0(0) | 4(11.1) |
| Other | ||||||
| Headache | 2(5.6) | 0(0) | 0(0) | 0(0) | 0(0) | 2(5.6) |
| Arthralgia | 2(5.6) | 1(2.8) | 0(0) | 0(0) | 0(0) | 3(8.3) |
| Fatigue | 7(19.4) | 5(13.9) | 0(0) | 0(0) | 0(0) | 12(33.3) |
| Hyperglycemia | 0(0) | 0(0) | 0(0) | 0(0) | 1(2.8) | 1(2.8) |
| Glaucoma | 0(0) | 0(0) | 1(2.8) | 0(0) | 0(0) | 1(2.8) |
| Edema face | 2(5.6) | 0(0) | 0(0) | 0(0) | 0(0) | 2(5.6) |
includes only grade 1–2 adverse events occurring in more than 10%
Regarding immune related AEs listed in Table 2, there were 3 grade 3 (2 with alanine or aspartate aminotransferase elevation, 1 with colitis), 1 grade 4 (elevated lipase), and 1 grade 5 event (hyperglycemia). The grade 5 event occurred in a patient who developed diabetic ketoacidosis after 10 doses of nivolumab, and died 31 months after discontinuing nivolumab due to recurrence diabetic ketoacidosis. The remaining immune related AEs shown in Table 2 were grade 1–2 in severity.
Responses
Objective response and clinical benefit data are depicted in Figure 2, which is a swimmer plot demonstrating the duration of therapy in months, best response and time of documented disease progression. Objective response occurred in 9 (25%; 95% CI: 12.1–42.2%) subjects, all of which were in patients with a baseline CD4 count ≥200 with stratum-specific response rate of 32.1% [95% CI: 15.9–52.3%]. No subject in a stratum with CD4 100–199 experienced objective response. There were no complete responses. Partial response occurred in 6 of 15 PLWH and KS (40%; 95% binomial exact CI: 16.3% - 64.7%), and in 3 other PLWH with squamous cell carcinoma of skin (n=1), colon cancer (n=1), and adenocarcinoma of the gall bladder (n=1). Clinical benefit, defined as objective response or stable disease for at least 6 months, occurred in 11 subjects (30.6%), including 5 out of 36 (13.9%) subjects had responses lasting longer than 6 months. The median duration of response in the responders was 9 months (IQR: 5.5– 20.0 months), and median time to response was 2.5 months (IQR: 2.0 −4.8 months); for those with KS, the median response duration was 12.5 months.
Figure 2.
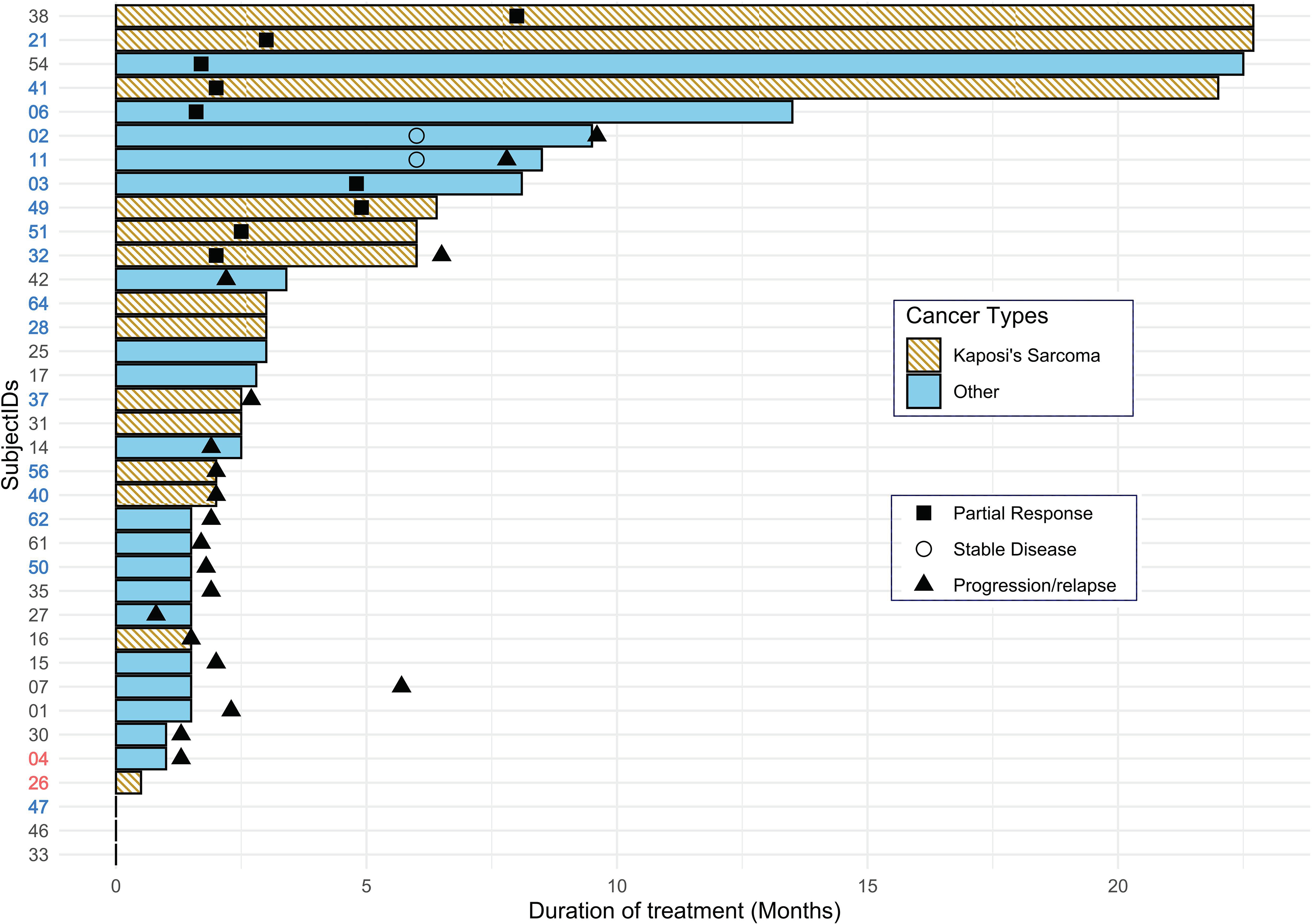
Swimmer plot demonstrating the duration of therapy in months, best response and time of documented disease progression (months). Unique cases(cases (N=36) are represented as SubjectIDs in y-axis. Axis text in red and blue indicates PD-L1 positive and negative cases, respectively. Cancer types other than KS shown as blue bars, Others included anal cancer (11, 27, 15, and 07), lung cancer (42, 25, 17, 50, and 47), cancers of head & neck (02, 35), colon (54), liver (62), gall bladder (03) and pancreas (61), breast (01), ovary (30), follicular dendritic sarcoma (04), liposarcoma (14), squamous cell carcinoma of skin (06) and inguinal squamous cell cancer (33). Some patients who discontinued therapy before disease. Patients with disease progression were switched to other therapy or not further followed (64, 28, 25, 17, 31, and 26),
Only 2 out of 19 patients tested for PD-L1 immunostaining had PD-L1-positive tumors (10.5%), including 1 with KS (5% PD-L1 expression score), and 1 with follicular dendritic sarcoma (10% PD-L1 expression score). Neither of the two patients experienced either objective response or stable disease. The patient with follicular dendritic sarcoma enrolled in the nivolumab dose de-escalation cohort and experienced progression within 5 weeks of initiation of treatment. The KS patient with PD-L1 positive tumor was enrolled in the solid tumor expansion cohort. Treatment was discontinued for this subject at third treatment cycle due to adverse effects.
CD4 and HIV monitoring
CD4 and HIV RNA monitoring were performed during the study and are shown in Figure 3. Compared with the CD4 count at baseline, there were no statistically significant changes in the CD4 count after 4 cycles (Figure 3a), 10 cycles (Figure 3b) of nivolumab, and after completion therapy at 6 weeks (Figure 3c) or 16 weeks (Figure 3d); p>0.05. Compared with the CD4/CD8 ratio at baseline, there were no significant changes in the CD4/8 ratio after 4 cycles (Figure 3a) or 10 cycles (Figure 3f) of nivolumab; although the CD4/8 ratio showed statistically significant increase at 6 weeks post-completion (Figure 3g) of nivolumab therapy (p=0.03), there was not statistically insignificant increase at 16 weeks (Figure 3h); p=0.05. Detectable HIV viremia greater than 75 copies/mL was noted in 2 (5.5%) participants during at least 1 treatment visit. Both subjects had detectable viremia with <200 copies/mL. One of those two subjects had two consecutive results between 75 and 100 copies/mL. No subject experienced consistent upward trend in the HIV viremia during the treatment. All participants were taking ART, and there was no significant change of CD4 count in relationship with nivolumab.
Figure 3.
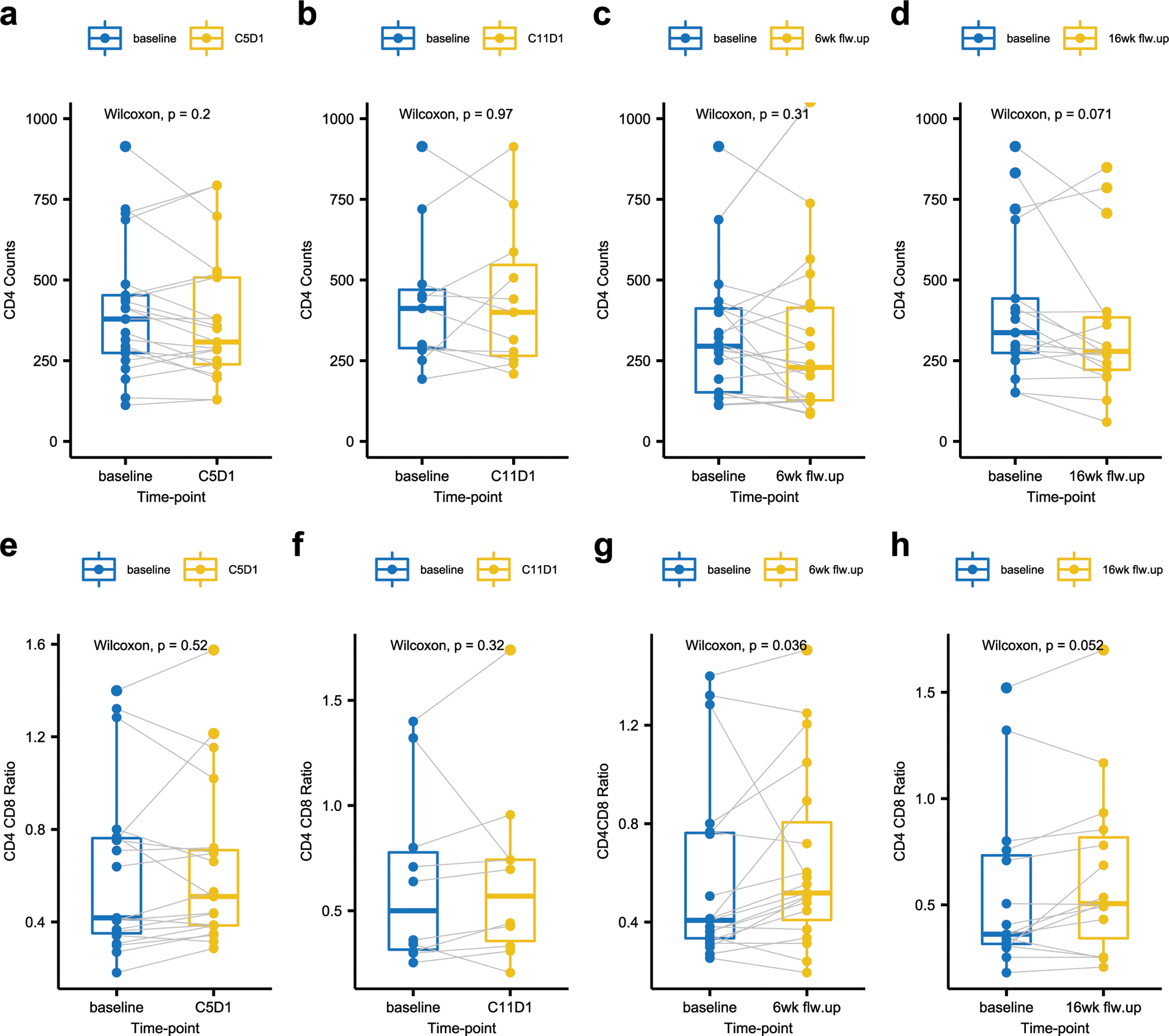
Box and whisker plots of CD4 absolute counts and CD4 CD8 ratio. CD4 absolute counts[a-d] and CD4 CD8 ratio [e-h]: Comparison of the baseline assessment [in blue] to the assessment at four different time points [in yellow; C5D1, C11D1, post-treatment 6 and 16 weeks follow-ups] in same subject. The P-values based on Wilcoxon rank-sum test is shown. Red and blue lines in plots a to d indicates CD4 count levels of 200 and 100 cells/mm3. P-values are based on Wilcoxon test for comparing paired
Discussion
Anti−PD-1/PD-L1 therapy is approved for a variety of cancers, including several that occur with increased incidence in people living with HIV. Given that nivolumab is an immunotherapeutic agent whose efficacy relies on enhancing T-cell immunity, we focused on evaluating the efficacy and safety of nivolumab in the solid tumor expansion cohort in patients who were less immunocompromised, as reflected by a CD4 count of at least 200/uL. Having demonstrated safety in the cohort with a CD4 count ≥ 200/uL, we then explored the safety of nivolumab in patients with a CD4 count. 100–200/uL, although few patients had a CD4 count in this range given the high level of effectiveness of antiretroviral therapy. The study demonstrated the safety of nivolumab in PLWH and a CD4 count as low as 100/uL and an undetectable or low HIV viral load receiving ART. The study also demonstrated efficacy in PLWH who have KS and other cancers known to benefit from immunotherapy. The proportion of grade 3 and 4 irAEs was generally similar to that previously described in patients without HIV infection receiving anti−PD-1 therapy for FDA-approved indications. The most common irAE was grade 1 rash and liver function test elevations in 6 (16%) of participants. Hypothyroidism was noted in 2 (5%) participants and was successfully managed through thyroid hormone supplementation. The results of our study are consistent with studies evaluating immune checkpoint blockade targeting PD-1 and PD-L1 in PLWH and advanced cancers [19–21], establish the safety of such therapy in PLWH receiving ART and suppressed HIV viral load with a CD4 count as low as 100/uL, and demonstrates the efficacy of such therapy in PLWH and KS. This report includes the largest cohort of PLWH and KS (n=15) treated prospectively with a PD1 inhibitor in a clinical trial, demonstrating a response rate of 40% and median duration of response of 12.5 months. Only 2 of 19 (10.5%) had a PD-L1 positive tumor, and response did not correlate with tumor PD-L1 expression. ART exposure has been associated with decreased PD-L1 expression in cervical cancer [22, 23]. However, other groups have found that colorectal cancer and lung cancer in PLWH have similar PD-L1 expression and PD-1 expression of tumor-infiltrating lymphocytes [24, 25].
Another objective of this study was to evaluate the effect of nivolumab on CD4/C8 populations and HIV viral load. We found that nivolumab monotherapy does not have a clinically relevant detrimental effect on CD4+ T-cell counts. CD4+ T-cell counts did not significantly change from baseline after 2 doses (at 6 weeks), 5 doses (at 12 weeks) of nivolumab and post-treatment at 6 or 16 weeks. Additionally, HIV viral load remained suppressed below 75 copies/ml in 94% of subjects. No participant required a change of ART, thus nivolumab is safe regarding sustained control of HIV-1 infection. A previously reported analysis including this cohort showed that nivolumab had no effect on HIV latency or the latent HIV reservoir [26]. A limitation of the trial was that most of the patients enrolled were men.
In conclusion, nivolumab has an acceptable safety profile in PLWH and cancer who have a suppressed HIV viral load receiving ART and a CD4+ T-cell count of at least 100 cells/μL Plasma HIV viral load remained suppressed, the CD4 lymphocyte count remained stable during nivolumab therapy. when used concurrently with ART. Clinically meaningful activity was observed in PLWH and KS, suggesting that nivolumab and other PD-1/PD-L1 directed agents merit further evaluation in KS.
Supplementary Material
Acknowledgements:
Coordinated by the AIDS Malignancy Consortium (Joseph A. Sparano, MD, Chair) and supported in part by Public Health Service Grant No. UM1CA121947 and the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the or NIH or NCI.
Footnotes
Disclosures: The authors declare no conflict of interest.
Data availability statement:
Data can be made available by submitting a written request by email to the AIDS Malignancy Consortium (AMC) Coordinating Center (amcpm@emmes.com) and/or via the AMC Coordinating Center website (https://amcoperations.com/about).
References:
- 1.Teeraananchai S, et al. , Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV Med, 2017. 18(4): p. 256–266. [DOI] [PubMed] [Google Scholar]
- 2.Samji H, et al. , Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One, 2013. 8(12): p. e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wing EJ, HIV and aging. Int J Infect Dis, 2016. 53: p. 61–68. [DOI] [PubMed] [Google Scholar]
- 4.Coghill AE, et al. , Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer, 2019. 125(16): p. 2868–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shiels MS, et al. , Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Ann Intern Med, 2018. 168(12): p. 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han Y, Liu D, and Li L, PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res, 2020. 10(3): p. 727–742. [PMC free article] [PubMed] [Google Scholar]
- 7.Taube JM, et al. , Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med, 2012. 4(127): p. 127ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brahmer JR, et al. , Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med, 2012. 366(26): p. 2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albiges L, et al. , Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open, 2020. 5(6): p. e001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrios DM, et al. , Immune checkpoint inhibitors to treat cutaneous malignancies. J Am Acad Dermatol, 2020. 83(5): p. 1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green SE, McCusker MG, and Mehra R, Emerging immune checkpoint inhibitors for the treatment of head and neck cancers. Expert Opin Emerg Drugs, 2020. 25(4): p. 501–514. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, et al. , Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol, 2019. 20(11): p. 1506–1517. [DOI] [PubMed] [Google Scholar]
- 13.Pirker R, Immunotherapy combinations in advanced nonsmall cell lung cancer. Curr Opin Oncol, 2021. 33(1): p. 73–79. [DOI] [PubMed] [Google Scholar]
- 14.Ready N, et al. , First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol, 2019. 37(12): p. 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tambaro R, et al. , From clinical trials to clinical use of checkpoint inhibitors for patients with metastatic urothelial cancer. Immunotherapy, 2021. 13(1): p. 67–77. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S and Bi M, The efficiency and safety of immune checkpoint inhibitors in the treatment of small cell lung cancer: a meta-analysis. Ann Palliat Med, 2020. 9(6): p. 4081–4088. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 18.Krown SE, Metroka C, and Wernz JC, Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol, 1989. 7(9): p. 1201–7. [DOI] [PubMed] [Google Scholar]
- 19.Cook MR and Kim C, Safety and Efficacy of Immune Checkpoint Inhibitor Therapy in Patients With HIV Infection and Advanced-Stage Cancer: A Systematic Review. JAMA Oncol, 2019. 5(7): p. 1049–1054. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Cao M, et al. , Assessment of the Feasibility and Safety of Durvalumab for Treatment of Solid Tumors in Patients With HIV-1 Infection: The Phase 2 DURVAST Study. JAMA Oncol, 2020. 6(7): p. 1063–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai S, Suzuki H, and Okuma Y, Durvalumab Consolidation Treatment after Chemoradiotherapy for an HIV-Positive Patient with Locally Advanced Non-Small Cell Lung Cancer. Case Rep Oncol, 2020. 13(2): p. 747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loharamtaweethong K, et al. , PD-L1 protein expression and copy number gains in HIV-positive locally advanced cervical cancer. Ther Adv Med Oncol, 2020. 12: p. 1758835920963001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loharamtaweethong K, et al. , Impact of antiretroviral drugs on PD-L1 expression and copy number gains with clinical outcomes in HIV-positive and -negative locally advanced cervical cancers. Oncol Lett, 2019. 18(6): p. 5747–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao Y, et al. , Lymphocytes Infiltration and Expression of PD-1 and PD-L1 in Colorectal Cancer Between HIV-Infected and Non-HIV-Infected Patients: A Propensity Score Matched Cohort Study. Front Oncol, 2022. 12: p. 827596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scilla KA, et al. , Case-control study of PD-1, PD-L1 and B7-H3 expression in lung cancer patients with and without human immunodeficiency virus (HIV) infection. Lung Cancer, 2018. 123: p. 87–90. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen TA, et al. , Impact of Anti-PD-1 and Anti-CTLA-4 on the Human Immunodeficiency Virus (HIV) Reservoir in People Living With HIV With Cancer on Antiretroviral Therapy: The AIDS Malignancy Consortium 095 Study. Clin Infect Dis, 2021. 73(7): p. e1973–e1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.project.org/]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be made available by submitting a written request by email to the AIDS Malignancy Consortium (AMC) Coordinating Center (amcpm@emmes.com) and/or via the AMC Coordinating Center website (https://amcoperations.com/about).


