Abstract
Purpose:
We sought to evaluate critically ill patients with delirium to evaluate inflammatory cytokine production and delirium progression and the role of antipsychotics.
Materials and Methods:
Adult critically ill patients with confirmed delirium according to a positive CAM-ICU score were included and IL-6 and IL-8 levels were trended for 24 h in this single-center, prospective, observational cohort study.
Results:
A total of 23 patients were consented and had blood samples drawn for inclusion. There was no difference in IL-6 and IL-8 levels at baseline, 4 to 8 h, and 22 to 28 h after enrollment when comparing patients based on antipsychotic exposure. We identified 2 patient clusters based on age, APACHE III, need for mechanical ventilation, and concomitant infection. In cluster 1, 5 (33.3%) patients received antipsychotics versus 5 (62.5%) patients in cluster 2 (P = .18). Patients in cluster 1 had more co-inflammatory conditions (P < .0001), yet numerically lower baseline IL-6 (P = .18) and IL-8 levels (P = .80) compared to cluster 2. Patients in cluster 1 had a greater median number of delirium-free days compared to cluster 2 (17.0 vs 6.0 days; P = .05).
Conclusions:
In critically ill patients with delirium, IL-6 and IL-8 levels were variable and antipsychotics were not associated with improvements in delirium or inflammatory markers.
Keywords: delirium, critically ill, antipsychotic, inflammatory marker, interleukin
Introduction
The reported incidence of delirium in critically ill patients varies based on the patient population studied and assessment strategies, but reported rates have ranged from 12% to 80%.1 Delirium has been associated with increased morbidity and mortality, due to prolonged duration of mechanical ventilation, inability to provide early mobility resulting in prolonged hospital stays and intensive care unit (ICU)-acquired weakness, and long-term cognitive consequences and psychiatric effects.1,2 Risk factors for delirium include the severity of illness, need for mechanical ventilation, older age, medications like opioids, benzodiazepines, and corticosteroids, female gender, baseline mental health disorder, and hypertension.1,2
The pathogenesis of delirium in critically ill patients is likely multifactorial, but mechanisms thought to play a role include neurotransmitter imbalance, reduced cerebral blood flow, endocrine imbalance, dysregulated sleep-wake cycle and stress response, and inflammation.3,4 In patients with delirium, plasma pro-inflammatory cytokines/chemokines, C-reactive protein (CRP), and interleukin (IL) levels have been shown to be elevated.4–6 Levels of IL-6 were also found to be elevated in patients with schizophrenia, which shares some clinical features, including hallucinations and delusions, with delirium.5,7
Due to these similarities between delirium and psychological conditions and the potential correlation with inflammatory markers/cytokines, antipsychotics have been studied for delirium prevention and treatment. Data evaluating the effects of antipsychotic medications on inflammatory markers/cytokines has been inconsistent, but some studies have demonstrated decreases in systemic interleukin (IL-1β and IL-6) and TNF-α levels.5,8–10 Although limited, this data has been of interest to critical care clinicians as there is currently no proven treatment for delirium, and biomarker identification would be valuable. It is unclear, however, if decreased levels of inflammatory markers correlate with delirium improvement, but has been demonstrated in patients with schizophrenia although other markers likely also play a role.5,7,8
Due to limited data evaluating IL-6 and IL-8 in critically ill patients with delirium and the effect of antipsychotics on these levels, we sought to perform an exploratory analysis evaluating systemic cytokine expression over time in delirious patients and the effect of antipsychotics on these levels.
Materials and Methods
This was a single-center prospective observational cohort study of medical ICU patients with delirium enrolled between February 1, 2019, and January 31, 2020. Patients were included if they were between 18 and 85 years old, admitted to a medical ICU at the Cleveland Clinic, had confirmed delirium according to a positive Confusion Assessment Method-ICU (CAM-ICU) score (assessed every 8 h), and had an arterial/central venous/peripheral line prior to enrollment. Patients were screened for study inclusion the first time they had a positive CAM-ICU score after being admitted to the medical ICU. Patients were excluded if they had a baseline psychiatric diagnosis (including schizophrenia, bipolar disorder, schizoaffective disorder, or borderline personality disorder), active alcohol withdrawal or admission for drug overdose, history of dementia, immunodeficiency (including hematologic malignancies, active solid tumor, AIDS, receiving >20 mg/day of prednisolone equivalents within last 3 months, or receiving > 3 months of immunosuppressants within last 6 months), ICU length of stay > 7 days at assessment for enrollment, hospital length of stay > 14 days at assessment of enrollment, chronic antipsychotic use, received quetiapine or haloperidol < 3 days from enrollment, or severe liver dysfunction (Child-Pugh > 7 or INR > 1.6 due to liver disease). Exclusion criteria were selected to limit the number of co-inflammatory and confounding conditions accounting for delirium.4 The decision to administer quetiapine or haloperidol, as well as the prescribed dose and frequency, was at the discretion of the primary medical ICU team. These antipsychotics were selected based on our institution’s delirium practice patterns. All patients received non-pharmacologic delirium interventions according to our institution’s nursing protocol which includes reorientation, noise/stimulation minimization, mobilization/range-of-motion exercises, circadian rhythm preservation, use of hearing aids and eyeglasses if appropriate, and encouragement of family at bedside.
This study “Inflammatory markers and antipsychotics in delirium” was approved by the Cleveland Clinic institutional review board (IRB#18–020) on December 3, 2018, and consent was obtained from all patients’ legal representatives at the time of enrollment. Research procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) and with the Helsinki Declaration of 1975.
IL-6 and IL-8 levels were measured at baseline prior to the first dose of quetiapine/haloperidol administration, 4 to 8 h after enrollment or quetiapine/haloperidol administration, and 22 to 28 h after enrollment or quetiapine/haloperidol administration. Blood samples were drawn into chilled K2EDTA tubes, inverted 8 to 10 times to mix, and then centrifuged at 2000g for 15 min at 4 °C to isolate plasma. Plasma samples were transferred into microtubes and stored at −70 °C until analyzing using the Human IL-6 Quantikine ELISA kit and Human IL-8/CXCL8 Quantikine ELISA kit. Baseline demographics including co-inflammatory conditions, SOFA and APACHE III scores, pertinent laboratory values, concomitant sedative and analgesic use, quetiapine/haloperidol exposure (experimental group), and delirium duration were collected.
Patients’ information collected was described using counts, means, standard deviations, medians, quartiles and ranges for all continuous variables and counts and percentages for categorical variables. Continuous covariates were compared using the Kruskal–Wallis test or t-test and categorical covariates were compared using the chi-square test or Fisher’s exact test. All analyses were two-tailed and were performed at a significance level of 0.05. An exploratory post-hoc analysis of delirium subphenotypes based on clinical factors for delirium4,11,12 was performed to detect a correlation with inflammatory biomarkers, if present. K-medoid clustering analysis (also known as partition around medoids clustering) was used to assess the heterogeneity effect of antipsychotics in this population. The variables age, APACHE III score, need for mechanical ventilation, and concomitant infection, were used to cluster included patients. The optimal number of clusters was determined based on the silhouette method. Logistic regression was performed to further analyze the association of quetiapine use and biomarkers accounting for confounding variables. As this was an exploratory pilot study, a power calculation was not performed. All analyses were performed by using the R software program (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 327 patients were evaluated for study enrollment and 23 patients were consented and had blood samples drawn with isolation of plasma for inclusion in this study. Reasons for patient exclusion are included in Supplemental Table S1 and baseline characteristics for included patients are listed in Table 1. Patients were similar at baseline with the exception of the non-neurologic SOFA score which was higher in patients with no antipsychotic exposure. The incidence of concomitant infection was numerically higher in patients with no antipsychotic exposure, but was not statistically significant (5 [50%] vs 10 [76.9%] patients; P = .22). Medication exposure and patient outcomes are listed in Table 2. The median (range) dose of quetiapine received by patients prior to the 4 to 8 h IL-6 and IL-8 samples was 25 (12.5–50) mg. The median (range) total dose of quetiapine within 24 h after enrollment was 31.25 (25–275) mg. Of the 10 patients who received quetiapine, 3 patients received a rescue dose of haloperidol following the initial quetiapine dose administered (haloperidol dose range 5–15 mg). There was no difference in exposure to opioids or sedatives between the 2 groups. There were numerically fewer delirium-free days at day 28 in the quetiapine group (14 vs 17 days; P = .20).
Table 1.
Baseline Characteristics.
| Variable | Antipsychotic Exposure (N = 10) | No Antipsychotic Exposure (N = 13) | P value |
|---|---|---|---|
| Age, yearsa | 71.5 (49–77) | 64 (63–72) | .59 |
| Male, n (%) | 6 (60) | 7 (53.8) | .99 |
| Weight, kga | 97.7 (75.7–121.7) | 88.5 (77.5–95.2) | .35 |
| BMI, kg/m2a | 32.7 (24.9–35.6) | 30.9 (26.8–32.5) | .51 |
| Race, n (%) | |||
| White or Caucasian | 3 (30) | 6 (46.1) | .66 |
| Black or African American | 6 (60) | 4 (30.7) | .22 |
| Not reported | 1 (10) | 3 (23) | .60 |
| Not Hispanic or Latino | 8 (80) | 13 (100) | .17 |
| Mechanical ventilation, n (%) | 9 (90) | 9 (69.2) | .25 |
| Admission diagnosis | |||
| Pneumonia | 4 (40) | 3 (23) | .65 |
| Heart failure | 3 (30) | 2 (15.3) | .61 |
| Renal failure | 0 (0) | 2 (15.3) | .48 |
| COPD exacerbation | 1 (10) | 0 (0) | .43 |
| Other | 5 (50) | 9 (69.2) | .41 |
| Other inflammatory conditions, n (%) | |||
| Concomitant infection | 5 (50) | 10 (76.9) | .22 |
| Drug or alcohol abuse history | 1 (10) | 0 (0) | .43 |
| Prior cognitive deficit | 1 (10) | 1 (7.6) | .99 |
| None | 4 (40) | 2 (15.3) | .34 |
| Comorbidities, n (%) | |||
| Hypertension | 7 (70) | 7 (54) | .45 |
| Diabetes | 5 (50) | 5 (38) | .60 |
| Chronic kidney disease | 3 (30) | 3 (23) | .72 |
| End-stage liver disease | 1 (10) | 1 (8) | .85 |
| Cancer | 1 (10) | 0 (0) | .39 |
| Stroke | 1 (10) | 2 (15) | .41 |
| Depression | 1 (10) | 2 (15) | .41 |
| APACHE IIIa | 74 (59–91) | 71 (59–92) | .99 |
| SOFA scorea | 6 (4–11) | 9 (7–13) | .12 |
| SOFA score without neuroa | 5 (4–8) | 8 (6–13) | .03 |
Abbreviations: COPD, chronic obstructive pulmonary disease; SOFA, Sequential Organ Failure Assessment; APACHE III, Acute Physiology and Chronic Health Evaluation III; IQR, interquartile range.
Median (IQR).
Table 2.
Medication Exposure and Outcomes.
| Outcome | Antipsychotic Exposure (N = 10) | No Antipsychotic Exposure (N = 13) | P value |
|---|---|---|---|
| Benzodiazepine infusion, n (%) | |||
| Any time point | 0 (0) | 0 (0) | N/A |
| Propofol infusion, n (%) | |||
| Day 0 | 4 (40) | 4 (30.7) | .68 |
| Day 1 | 4 (40) | 3 (23) | .65 |
| Day 2 or later | 1 (10) | 5 (38.4) | .17 |
| Any time point | 5 (50) | 6 (46.1) | .99 |
| Opioid infusion, n (%) | |||
| Day 0 | 6 (60) | 8 (61.5) | .99 |
| Day 1 | 6 (60) | 6 (46.1) | .68 |
| Day 2 or later | 4 (40) | 4 (30.7) | .68 |
| Any time point | 7 (70) | 8 (61.5) | .99 |
| Dexmedetomidine infusion, n (%) | |||
| Day 0 | 1 (10) | 0 (0) | .43 |
| Day 1 | 1 (10) | 0 (0) | .43 |
| Day 2 or later | 3 (30) | 1 (7.6) | .28 |
| Any time point | 3 (30) | 1 (7.6) | .28 |
| Benzodiazepine bolus, n (%) | |||
| Day 0 | 1 (10) | 2 (15.3) | .99 |
| Day 1 | 0 (0) | 0 (0) | N/A |
| Day 2 or later | 1 (10) | 5 (38.4) | .17 |
| Any time point | 1 (10) | 6 (46.1) | .08 |
| Propofol bolus, n (%) | |||
| Day 0 | 2 (20) | 0 (0) | .17 |
| Day 1 | 0 (0) | 0 (0) | N/A |
| Day 2 or later | 0 (0) | 1 (7.6) | .99 |
| Any time point | 2 (20) | 1 (7.6) | .56 |
| Opioid bolus, n (%) | |||
| Day 0 | 4 (40) | 1 (7.6) | .12 |
| Day 1 | 3 (30) | 3 (23) | .99 |
| Day 2 or later | 7 (70) | 8 (61.5) | .99 |
| Any time point | 9 (90) | 8 (61.5) | .17 |
| Melatonin, n (%) | 1 (10) | 1 (8) | .85 |
| Delirium-free days day-14, daysa | 2.5 (0–8) | 3 (0–12) | .50 |
| Delirium-free days day-28, daysa | 14 (0–20) | 17 (12–26) | .20 |
| Mechanical ventilation duration, daysa | 5.8 (2.9–7.5) | 8.3 (3.6–10) | .17 |
| 28-day mortality | 3 (30) | 2 (15) | .62 |
Abbreviation: IQR, interquartile range.
Median (IQR).
The baseline, 4 to 8 h sample, and 22 to 28 h sample for IL-6 and IL-8 are presented in Figure 1. There were no differences between groups in regards to IL-6 and IL-8 at baseline, 4 to 8 h after enrollment, and 22 to 28 h after enrollment. There was no difference between the change in IL-6 from baseline to sample 3 when comparing patients who were exposed to antipsychotics and patients who were not exposed to antipsychotics (−0.12 [−0.35–0.47] vs −0.21 [−0.54–0.65] pg/mL; P = .74). There was also no difference between the change in IL-8 from baseline to sample 3 when comparing patients who were exposed to antipsychotics and patients who did not receive antipsychotics (0.02 [−0.07–0.13] vs −0.19 [−0.62–0.31] pg/mL; P = 0.26). Logistic regression also did not reveal an association between antipsychotic exposure and inflammatory biomarkers (Supplemental Table S1).
Figure 1.
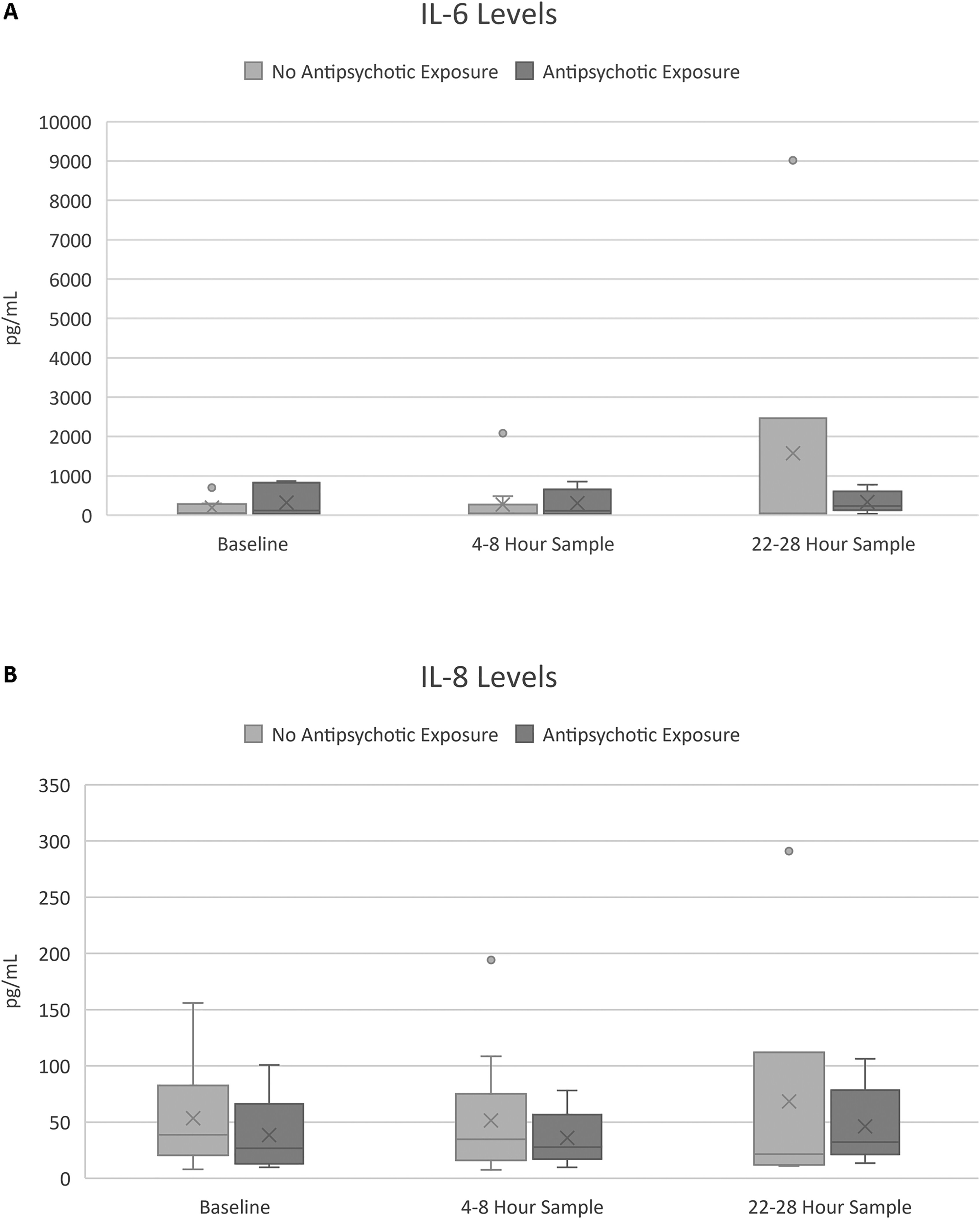
Inflammatory markers by patient. (A) IL-6 levels for antipsychotic and no antipsychotic exposure groups at baseline, 4 to 8 h, and 22 to 28 h; (B) IL-8 levels for antipsychotic and no antipsychotic exposure groups at baseline, 4 to 8 h, and 22 to 28 h.
In an exploratory post-hoc analysis of potential delirium subphenotypes, we identified 2 patient clusters based on age, APACHE III score, need for mechanical ventilation, and concomitant infection. Patient clusters are reported in Figure 2. All patients in cluster 1 had concomitant infection compared to 0 patients in cluster 2 (P < .0001) and 12 (80.0%) patients in cluster 1 required mechanical ventilation compared to 6 (75%) patients in cluster 2 (P = .78). Patients in cluster 2 had numerically higher bilirubin, AST, INR, and white blood cell count compared to patients in cluster 1. Cluster baseline characteristics are presented in Table 3. In terms of antipsychotic exposure, 5 (33.3%) patients in cluster 1 received antipsychotics and 5 (62.5%) patients in cluster 2 received antipsychotics (P = .18). There were no differences in benzodiazepine, opioid, propofol, and dexmedetomidine exposure between clusters. Patients in cluster 1 had numerically lower median IL-6 and IL-8 levels compared to cluster 2, but this was not statistically significant. Ultimately, patients in cluster 1 had a numerically greater number of delirium-free days at day 28 compared to patients in cluster 2 (17 [0–28] vs 6 [−2–24], respectively; P = .05). Medication exposure and patient outcomes for the exploratory delirium subphenotype clusters are listed in Table 4.
Figure 2.
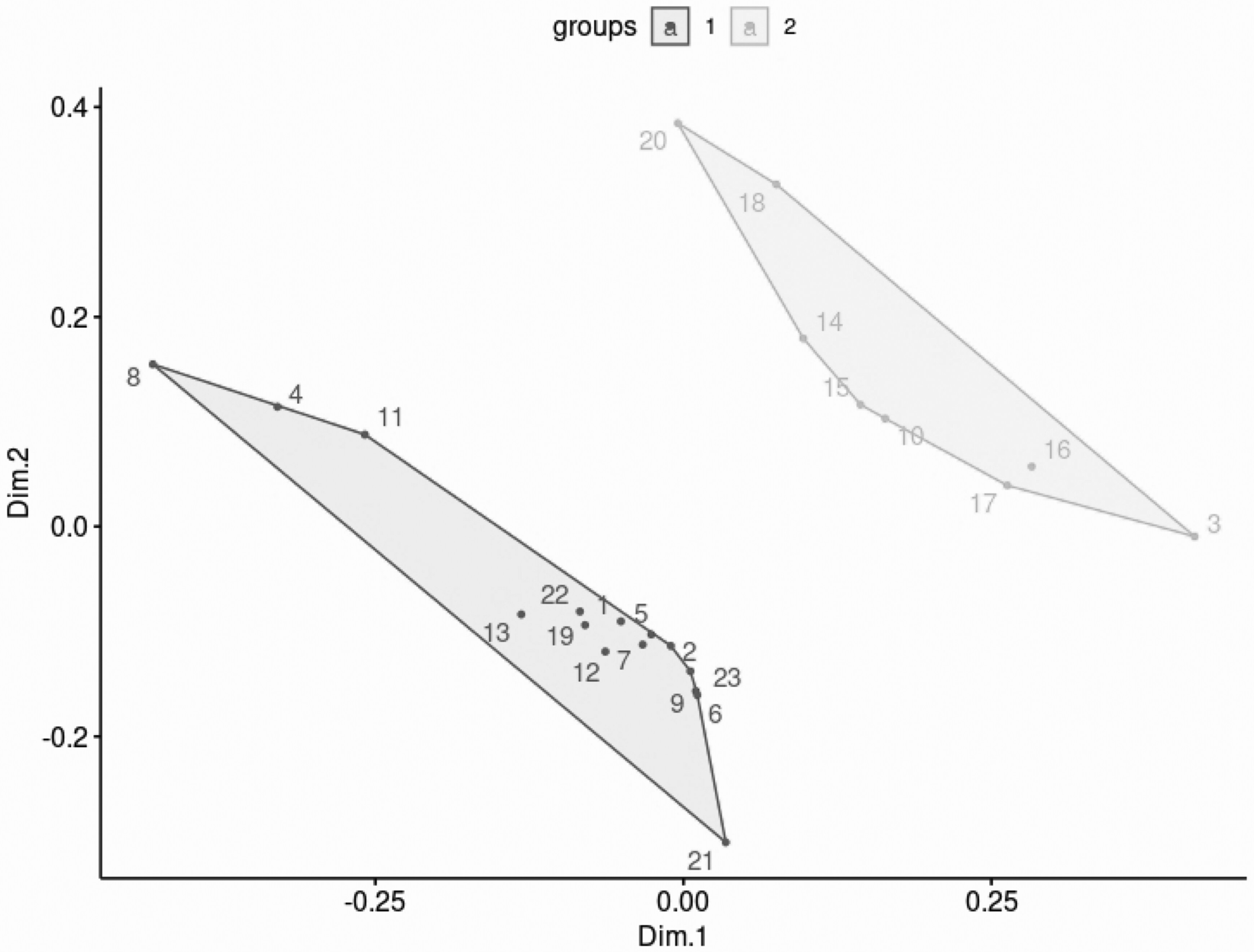
The principal component score plot. Through principal component analysis, we transformed high-dimensional data into lower-dimension while retaining as much information as possible. The score plot indicates the projection of the data onto the span of the principal components. The x-axis is the first principal component, while the y-axis denotes the second principal component. The graph shows that the patients are separated into 2 clusters by the 2 principal component scores.
Table 3.
Cluster Baseline Characteristics.
| Variable | Cluster 1 (N = 15) | Cluster 2 (N = 8) | Total (N = 23) | P value |
|---|---|---|---|---|
| Age, yearsc | 71 (35–82) | 65.5 (47–83) | 67 (35–83) | .75a |
| Male, n (%) | 8 (53.3%) | 5 (62.5%) | 13 (56.5%) | .67b |
| Weight, kgc | 88.5 (68.9–135.0) | 94.9 (43.2–133.5) | 88.5 (43.2–135.0) | .42a |
| BMI, kg/m2c | 31.0 (21.3–42.7) | 31.5 (16.9–52.1) | 31.0 (16.9–52.1) | .85a |
| Co-inflammatory condition, n (%) | <.0001b | |||
| Concomitant infection | 15 (100.0%) | 0 (0.0%) | 15 (65.2%) | |
| Drug/alcohol abuse | 0 (0.0%) | 1 (12.5%) | 1 (4.3%) | |
| Prior cognitive deficit | 0 (0.0%) | 1 (12.5%) | 1 (4.3%) | |
| None | 0 (0.0%) | 6 (75.0%) | 6 (26.1%) | |
| APACHE III scorec | 71 (35–105) | 80 (27–119) | 71 (27–119) | .67a |
| SOFA scorec | 8 (1–15) | 6.5 (1–14) | 7 (1–15) | .85a |
| Mechanical ventilation, n (%) | 12 (80.0%) | 6 (75.0%) | 18 (78.3%) | .78b |
| White blood cell count, k/μLc | 12.2 (3.8–36.0) | 17.2 (5.6–30.6) | 14.2 (3.8–36.0) | .70a |
| Platelets, k/μLc | 141 (47–544) | 147.5 (42–290) | 141 (42–544) | .90a |
| Bilirubin, μmol/Lc | 0.4 (0.2–2.5) | 1.3 (0.4–7.6) | 0.6 (0.2–7.6) | .06a |
| Albumin, g/dLc | 2.5 (2.0–4.8) | 2.7 (1.9–3.3) | 2.6 (1.9–4.8) | .62a |
| AST, units/Lc | 25 (9–507) | 64 (23–22 134) | 53.5 (9–22 134) | .19a |
| ALT, units/Lc | 20 (4–256) | 21 (12–12 495) | 20.5 (4–12 495) | .84a |
| INRc | 1.2 (1.0–1.4) | 4.4 (4.4–4.4) | 1.2 (1.0–4.4) | .11a |
Abbreviations: SOFA, Sequential Organ Failure Assessment; APACHE III, Acute Physiology and Chronic Health Evaluation III; BMI, body mass index; ALT, alanine transaminase; AST, aspartate transaminase; INR, international normalized ratio.
Kruskal-Wallis.
Chi-square.
Median (range).
Table 4.
Cluster Medication Exposure and Outcomes.
| Outcome | Cluster 1 (N = 15) | Cluster 2 (N = 8) | Total (N = 23) | P value |
|---|---|---|---|---|
| Antipsychotic exposure, n (%) | 5 (33.3) | 5 (62.5) | 10 (43.5) | .18b |
| Propofol exposure, n (%) | 6 (40.0) | 5 (62.5) | 11 (47.8) | .30b |
| Opioid exposure, n (%) | 9 (60.0) | 6 (75.0) | 15 (65.2) | .47b |
| Dexmedetomidine exposure, n (%) | 3 (20.0) | 1 (12.5) | 4 (17.4) | .65b |
| Benzodiazepine exposure, n (%) | 4 (26.7) | 3 (37.5) | 7 (30.4) | .59b |
| Melatonin exposure, n (%) | 2 (13) | 0 (0) | 2 (9) | .30b |
| Baseline IL-6, pg/mLc | 54.5 (24.3–821.8) | 285.7 (4.5–866.4) | 94.8 (4.5–866.4) | .18a |
| 4 to 8 h IL-6, pg/mLc | 49.4 (20.4–535.8) | 448.4 (11.5–2085.1) | 86.9 (11.5–2085.1) | .06a |
| 22 to 28 h IL-6, pg/mLc | 38.0 (13.3–774.1) | 197.6 (3.8–9015.6) | 83.7 (3.8–9015.6) | .15a |
| Baseline IL-8, pg/mLc | 38.6 (8.0–117.2) | 30.9 (9.7–155.9) | 37.9 (8.0–155.9) | .80a |
| 4 to 8 h IL-8, pg/mLc | 34.6 (7.4–108.5) | 30.4 (16.2–194.1) | 30.8 (7.4–194.1) | .52a |
| 22 to 28 h IL-8, pg/mLc | 23.9 (10.2–115.4) | 24.0 (7.7–290.9) | 23.9 (7.7–290.9) | .42a |
| Delirium-free days at day-14, daysc | 3 (−1–14) | 1.5 (−2–10) | 3 (−2–14) | .20a |
| Delirium-free days at day-28, daysc | 17.0 (0–28) | 6 (−2–24) | 16 (−2–28) | .05a |
| Duration of mechanical ventilation, daysc | 4.2 (2.6–34.1) | 8.8 (0.8–14.1) | 5.9 (0.8–34.1) | .26a |
Kruskal-Wallis.
Chi-square.
Median (range).
Discussion
In our patient population of critically ill patients with delirium, we found no difference overall in IL-6 and IL-8 levels at baseline or over a 22 to 28 h period regardless of antipsychotic exposure. However, we did identify 2 distinct delirium subphenotype clusters in our cohort of critically ill patients with delirium. Factors resulting in similar clusters included age, APACHE III score, need for mechanical ventilation, and concomitant infection. There was also no statistically significant difference in IL-6 and IL-8 levels between delirium subphenotype clusters, but patients in cluster 1 had numerically lower inflammatory markers and greater delirium-free days.
Our findings add to a growing body of literature evaluating inflammatory markers in critically ill patients with delirium.4,6,13–15 In the largest study to date, Khan et al15 evaluated 321 critically ill patients with delirium and found that elevated IL-6, IL-8, and IL-10 levels were associated with delirium severity and IL-8 was associated with increased in-hospital mortality. This study did not, however, evaluate the impact of antipsychotics on these levels, and baseline IL-6 and IL-8 levels were significantly lower at baseline compared to our patient population. Alexander et al14 sought to evaluate the association between inflammatory markers and genotype on the development of ICU delirium and outcomes. The included 77 patients had similar baseline APACHE III scores and IL-6 levels compared to our patient population, but they found that subjects with higher IL-6 levels were more likely to have delirium and worse outcomes, which we did not observe.
Outcomes from existing data attempting to correlate inflammatory markers with delirium progression in critically ill patients have been inconsistent.6,13–15 The patient clusters we identified in our analysis support the need to also incorporate delirium phenotypes and subphenotypes beyond the level of motoric activity12 into further analysis of this clinical question, as patients in cluster 1 had greater concomitant infection, yet lower IL-6 and IL-8 levels and greater delirium-free days. The use of antipsychotics is not without risk and studies have demonstrated that patients are often unintentionally continued on these medications beyond ICU and hospital discharge.16,17 The pathophysiology of delirium is complex and studies evaluating interventions for delirium often include heterogeneous patient populations.2 Delirium subphenotypes have identified risk factors, symptoms, precipitants, and mechanisms that may impact the efficacy of treatment strategies and outcomes.11,12,18 Delirium in the setting of sedation, hypoxia, and sepsis has been associated with worse outcomes18 and future studies should consider these subphenotypes before initiating delirium prophylaxis and treatment.
Our study has a number of limitations and should be considered an exploratory analysis given the small sample size and P values should be cautiously interpreted. We were not able to control for which patients received antipsychotics and what regimen was prescribed, which may have resulted in selection bias and impacted findings. We began enrolling patients on February 1, 2019. On February 16, 2019, the 2018 PADIS Guideline update was published which recommended against the routine use of antipsychotics for treatment of delirium.2 This guideline update resulted in a change in practice at our institution and it was likely that patients prescribed an antipsychotic were those patients with more hyperactive and recalcitrant delirium. Additionally, given that we were not able to control for the dose and frequency of quetiapine prescribed, it is unclear if higher doses would have demonstrated a greater impact on IL-6 and IL-8 levels as has been studied in schizophrenia.8 We attempted to collect data that may have confounded results related to inflammation and delirium including additional medications that could have impacted IL-6 and IL-8 levels, but given that this was a noninterventional study we could not eliminate these confounders. We also excluded patients with baseline psychiatric disorders, dementia, substance abuse disorders, or those who were immunosup-pressed or had liver dysfunction in an attempt to limit confounding, but this may also limit the applicability of our findings to real-world critical care delirium management. Finally, we only evaluated IL-6 and IL-8 levels during a 24-h time period within 7 days of ICU admission and 14 days within hospital admission, so it is unclear if timing or trending interleukin levels beyond this time period would have resulted in different outcomes or if measuring other inflammatory cytokines would have resulted in a different outcome.
The results of our analysis highlight the heterogeneity of ICU delirium and emphasize the need for studies evaluating delirium subphenotypes to help better understand patient populations that may benefit from pharmacologic interventions and the use of precision medicine to reduce adverse effects.
Conclusion
In an exploratory analysis of inflammatory markers in critically ill patients with delirium, IL-6 and IL-8 levels were variable and antipsychotics were not associated with improvements in delirium or inflammatory markers. Studies evaluating inflammatory markers and antipsychotics in delirium subphenotypes are needed to better understand the complex pathogenesis of delirium and identify clinical factors that may better respond to pharmacologic intervention.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Joanne Smith, PharmD.
Funding
This project received US$12 500 from the Cleveland Clinic Research Programs Committees and US$12 500 from the Cleveland Clinic Department of Pharmacy.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Stollings JL, Kotfis K, Chanques G, Pun BT, Pandharipande PP, Ely EW. Delirium in critical illness: clinical manifestations, outcomes, and management. Intensive Care Med. 2021;47(10):1089–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. [DOI] [PubMed] [Google Scholar]
- 3.Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018;33(11):1428–1457. [DOI] [PubMed] [Google Scholar]
- 4.Ormseth CH, LaHue SC, Oldham MA, Josephson SA, Whitaker E, Douglas VC. Predisposing and precipitating factors associated with delirium: a systematic review. JAMA Netw Open. 2023;6(1):e2249950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simone MJ, Tan ZS. The role of inflammation in the pathogenesis of delirium and dementia in older adults: a review. CNS Neurosci Ther. 2011;17(5):506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith RJ, Rabinstein AA, Cartin-Ceba R, et al. Chemokines in ICU delirium: an exploratory study. Crit Care Explor. 2022;4(7):e0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fragus D, Diaz-Caneja CM, Ayora M, et al. Oxidative stress and inflammation in first-episode psychosis: a systematic review and meta-analysis. Schizophr Bull. 2019;45(4):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumeister D, Ciufolini S, Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology (Berl). 2016;233(9):1575–1589. Epub 2015/08/14. doi: 10.1007/s00213-015-4044-5. [DOI] [PubMed] [Google Scholar]
- 9.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tourjman V, Kouassi É, Koué M, et al. Antipsychotics’ effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophr Res. 2013;151(1–3):43–47. [DOI] [PubMed] [Google Scholar]
- 11.Bowman EML, Cunningham EL, Page VJ, McAuley DF. Phenotypes and subphenotypes of delirium: a review of current categorisations and suggestions for progression. Crit Care. 2021;25(1):334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oldham MA, Slooter AJC, Ely EW, Crone C, Maldonado JR, Rosenthal LJ. An interdisciplinary reappraisal of delirium and proposed subtypes. J Acad Consult Liaison Psychiatry. 2023;64(3):248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritter C, Tomasi CD, Dal-Pizzol F, et al. Inflammation biomarkers and delirium in critically ill patients. Crit Care. 2014;18(3):R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander SA, Ren D, Gunn SR, et al. Interleukin 6 and apolipo-protein E as predictors of acute brain dysfunction and survival in critical care patients. Am J Crit Care. 2014;23(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan BA, Perkins AJ, Prasad NK, et al. Biomarkers of delirium duration and delirium severity in the ICU. Crit Care Med. 2020;48(3):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilchrist NA, Asoh I, Greenberg B. Atypical antipsychotics for the treatment of ICU delirium. J Intensive Care Med. 2012;27(6): 354–361. [DOI] [PubMed] [Google Scholar]
- 17.Farrokh S, Castle AC, Heavner M, Pisani MA. Continuation rate of atypical antipsychotics after discharge when initiated in the intensive care unit. J Pharm Pract. 2017;30(3):342–346. [DOI] [PubMed] [Google Scholar]
- 18.Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study. Lancet Respir Med. 2018;6(3):213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


