Abstract
Animals in nature are constantly managing multiple demands, and decisions about how to adjust behavior in response to ecologically relevant demands is critical for fitness. Evidence for behavioral correlations across functional contexts (behavioral syndromes) and growing appreciation for shared proximate substrates of behavior prompts novel questions about the existence of distinct neural, molecular and genetic mechanisms involved in decision-making. Those proximate mechanisms are likely to be an important target of selection, but little is known about how they evolve, their evolutionary history or where they harbor genetic variation. I provide a conceptual framework for understanding the evolution of mechanisms for decision-making, highlighting insights on decision-making in humans and model organisms, and sketch an emerging synthesis.
Keywords: animal behavior, behavioral syndromes, neurogenomics, social decision-making network, RNA sequencing
How do animals manage multiple demands?
If you spend much time watching animals in nature, you’re bound to notice that animals are rarely doing just one thing at a time. Territorial males are not only defending their territory from intruders, but they’re also trying to attract mates, for example. How animals manage multiple demands is probably just as, if not more, critical for fitness, than behavior in any given context on its own. There’s little benefit to being great at finding food resources if the forager can’t switch into hiding mode in the face of danger, for instance.
Behavioral ecology provides abundant evidence that animals are very capable of adaptively modulating their behavior in the face of multiple demands, with at least four potential behavioral outcomes (Box 1). Therefore it seems reasonable to suppose that there is some mechanism for making those decisions, and for integrating information about multiple demands. Behavioral reaction norms [1,2] and optimality theory [3] provide a strong framework for understanding the outcome of behavioral decision-making when there are multiple demands by assuming that decision-making favors the decision that will ultimately increase fitness the most, depending on the animal’s state and the immediate environment. But if the actual target of selection is the way that animals make those decisions, then a full understanding of the evolution of decision-making when there are multiple demands requires study of the underlying mechanisms, not just their outcomes.
Box 1. Experimental design and behavioral responses to multiple demands.
Imagine we study animals in different contexts or situations representing different ecologically relevant demands, e.g. male courtship (A) and territorial aggression (B), or parental care (A) and foraging behavior (B), and those demands are simulated by providing different cues, e.g. a receptive female, an intruding male conspecific, a food item, and/or by measuring animals in specific behavioral states, e.g. while providing parental care, or when hungry.
Animals’ response to different demands can be studied using a simple experimental design where demands occur either separately or together (A, B, A+B), with appropriate controls (e.g. no stimulus, stimulus that lacks a key feature, etc., Figure I). The same (e.g., activity level) or different (e.g., courtship displays, aggressive behavior) behaviors could then be compared across conditions, or levels of an experimental treatment (Figure I). If individuals are measured repeatedly, personality could be quantified as the repeatability of behavior within a condition, behavioral syndromes could be quantified as among-individual correlations in different behaviors across conditions and plasticity could be quantified as the slope of behavioral reaction norms [2]. Changes in behavior in the A+B condition relative to the A and B condition could be interpreted as multidimensional plasticity across environmental gradients [57,58], and if multiple behaviors change, that could reflect multivariate plasticity [59].
At the behavioral level, a common outcome of this type of experiment is that animals behave differently in the A+B condition compared to the A or B condition, with at least four potential behavioral responses in the A+B condition: compromise, amplification, switching and multi-tasking. Males might compromise courtship by adjusting the conspicuousness of their courtship displays, for example, when predators are near [60–63] because attracting mates and predator avoidance can be in direct conflict with one another and result in fitness tradeoffs. When different demands are not in conflict with each other, e.g. parenting behavior is attractive to potential mates [19], or the presence of a competitor increases rates of courtship (the audience effect, [20]), that could result in amplification. Alternatively, an animal might switch between different demands over relatively short time scales, such as alternating between courtship and aggressive behavior when both mates and competitors are present. Finally, an animal might multi-task in the presence of multiple demands, e.g. vigilance and foraging. A related important concept are time budget constraints, such that time performing one behavior (e.g., courtship) takes away from time available to perform another behavior (e.g. territorial aggression), so that by definition, levels of a behavior in the A+B condition must be lower than the behavior in the A or B condition. For simplicity, this hypothetical example just considers two demands, but in reality animals are often managing more than two demands, and more complicated experimental designs could be insightful.
Knowing more about the proximate mechanistic basis by which animals juggle multiple demands and measuring those mechanisms directly has the potential to reveal where the mechanisms harbor genetic variation and therefore how they are likely to evolve [4,5]. However, until recently, we have not had the tools to empirically understand how those mechanisms evolve, their evolutionary history or where they harbor genetic variation. The value of integrating information on mechanisms into studies on the utility and evolution of behavior has been appreciated for decades [6]. Fortunately, new tools for studying proximate mechanisms in diverse species is allowing this highly-valued integration to become realized, especially for understanding how animals balance multiple demands, which is the reality of behavior.
Shared mechanisms of behavior
Behavioral syndromes (see Glossary) draw attention to the idea that behaviors in different contexts, or in response to different ecologically-relevant demands, are often linked together, sometimes via a shared proximate mechanism [34]. When the behavioral syndromes framework was formulated, little was known about their mechanistic bases. But over the last 20 years there have been numerous findings in neuroscience and genomics which bear directly on this question. This work is showing widespread evidence of shared mechanisms – brain regions, hormones, genes – across behaviors that are typically treated separately. The idea that proximate mechanisms can act as constraints is central to the idea of limited plasticity (Box 2) but much of the relevant mechanistic work is not typically on the radar of behavioral ecologists and is not carried out in an ecological or evolutionary context.
Box 2. Have we been studying behavioral syndromes wrong?
When individuals have a behavioral type that is maintained through contexts or across situations, then it could reflect limited plasticity [64]. In the context of multiple demands, e.g., between courtship and antipredator behavior, the tendency of a behavioral type to prioritize mate attraction might spillover, causing them to be more vulnerable to predators, while behavioral types which prioritize safety could miss out on mating opportunities. Behavioral syndromes and personality have important implications for ecological and evolutionary processes [65,66] and the suggestion they reflect tradeoffs and result in behavioral spillovers was part of the reason why they attracted attention [67–69].
To study behavioral syndromes, typically the same set of individuals is measured multiple times in different behavioral assays designed to capture behavior in different contexts (but see [70]). This is done to accurately characterize behavioral types and repeatability; separate assays are used to elicit behavior in different contexts, while carefully controlling for order and carryover effects, because we know they can be important [71,72]. Negative within-individual correlations across contexts could reflect tradeoffs that result from a proximate constraint that links behaviors across contexts. One approach to understanding those mechanistic links is to compare the proximate (genetic, neural, hormonal, molecular) correlates of behavior in each of these contexts separately, and infer that shared mechanisms are constraints [14,69].
However, if there are specific mechanisms for managing multiple demands that are only engaged when they co-occur, this approach will be misleading because the outcome of the conflict between multiple demands will be impossible to predict based on studies of behavior in separate contexts, one at a time. Instead, it would be necessary to study animals while they are making decisions in the face of multiple demands. From this perspective, in our urgency to accurately characterize repeatable behavioral types, we may have inadvertently missed the opportunity to understand the mechanisms of limited plasticity, which is ironic considering that limited plasticity was one of the mysteries originally motivating work in this area.
This tension – about whether to view plasticity as correlations between separate traits in different environments or as a trait unto itself with its own mechanistic basis – has a long history [73], but has primarily been considered in the context of morphology. Highly labile traits such as behavior change the parameters of the problem and push the limits of our understanding. In particular, behavioral plasticity while animals are simultaneously balancing multiple demands in real time over short timescales is likely to involve higher-order processes involved in decision-making and integration, presumably in the nervous system. However, we know essentially nothing about natural phenotypic and genetic variation in those mechanisms, and how they evolve.
An influential idea from neuroethology is that different forms of ecologically relevant social behavior in vertebrates – aggression, mating, parental care – are associated with distinct patterns of activation across nodes in a deeply conserved network of interacting subcortical brain regions, the social decision-making network [7–9]. Male aggression in rodents, for example, is associated with brain activation in all of the nodes in the network except for the preoptic area, while mating behavior is associated with brain activation in all of the nodes except the anterior hypothalamus [7]. This begs the (rhetorical) question: if the same part of the brain is activated during both territorial defense (aggression) and courtship, then what happens when the animal has to defend and court at the same time, or rapidly switch between them?
There is also evidence for shared mechanisms underlying behavior in different functional contexts at the molecular and genetic level [10,11]. Work in behavioral genomics is showing that pleiotropic genes related to behavior operate within networks of interacting genes in the brain [12]. The expression of the same genes and gene regulatory networks can be triggered in what at first glance appears to be very different behavioral contexts or situations, e.g. territorial defense and parental care, well-studied in the context of the challenge hypothesis [13]. For example, many of the same genes were differentially expressed in the brain following either a territorial challenge by an intruder or during parental care in male sticklebacks (Gasterosteus aculeatus) [14]. 8 out of the 10 transcription factors that were identified by bioinformatic analysis were regulated in opposite directions in response to either a territorial challenge or during parental care. In other words, different salient experiences – providing parental care and territorial aggression – affected the same genes but in opposite directions.
Evidence for shared substrates prompts novel questions about the mechanisms by which animals manage multiple demands (Figure 1). For example, if the same underlying mechanisms are involved in both courtship and aggression, perhaps in opposing ways, then how do those mechanisms work while animals are simultaneously defending resources and attracting mates? Detailed study of the specific neuronal basis of behaviors that appear to be in conflict may reveal that conflicts are resolved via fine partitioning within a brain area, with heterogeneous subpopulations of neurons within a brain area playing distinct roles [15]. For example, specific sub-populations of galanin neurons in the preoptic area (POA) of the hypothalamus have specific functions in parenting behavior in mice: galanin cells projecting from the POA to one brain area promote pup grooming, cells projecting to another brain area promote approaching pups, and cells projecting to yet another area suppress interactions with adults [16]. Another (nonexclusive) possibility is that different molecules acting within the same brain area function competitively to influence opposing behaviors, e.g. isotocin and arginine vasotocin signaling have opposite effects in the POA to mediate the balance between offspring care and offspring defense in clownfish [17].
Figure 1: Proximate mechanisms involved in decision-making when there are multiple demands.
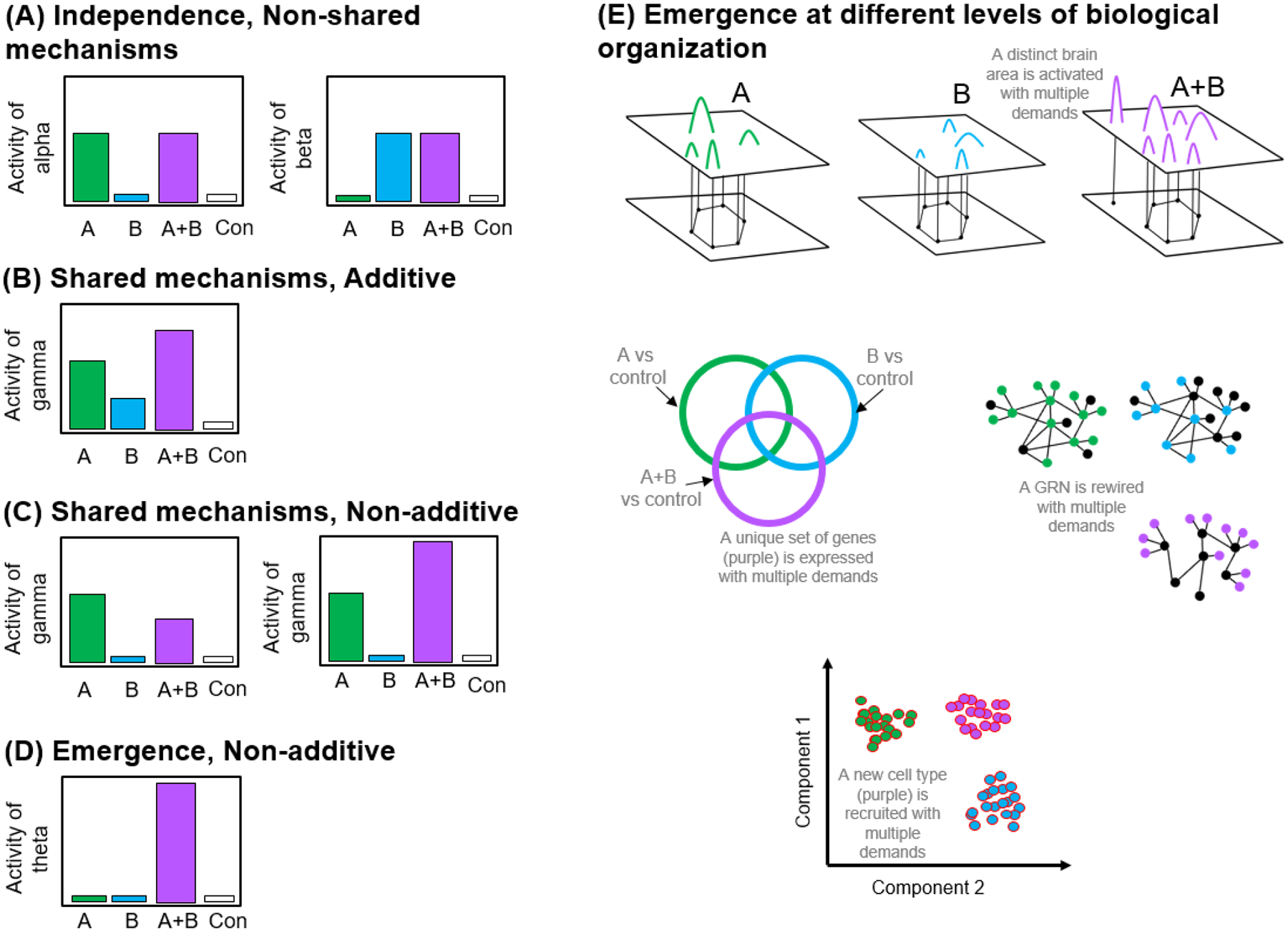
Greek letters refer to a mechanism, e.g. neural activity, brain gene expression, cell type activity, hormones etc. The activity of the mechanism in the simultaneous condition (A+B) will depend on the extent to which the response to demand A and demand B is influenced by distinct versus shared mechanisms. (A) Independence. The response to demand A and demand B utilize distinct, nonoverlapping proximate mechanisms; demand A triggers mechanism alpha (e.g. neural activity in one brain area), B triggers beta (e.g. neural activity in another brain area), and A+B triggers mechanisms alpha and beta (i.e. neural activity in both brain areas). (B) Additivity. The response to demand A and demand B depend on the activity of the same mechanism (gamma), e.g. neural activity in the same brain area, and the response to A+B is additive, such that demand A triggers gamma by x amount, while demand B triggers gamma by y amount so that when A and B are presented together, the activity of gamma is x+y. (C) Nonadditivity. The activity of gamma in the A+B condition is greater or less than x+y. (D) Emergence. An entirely new mechanism (“theta”) is recruited in the A+B condition. Note that in both (C) and (D), the response of the shared mechanism to A+B can not be predicted from the response to demand A versus demand B alone. (E) Theta might operate at the brain, transcriptomic, gene regulatory and/or cell type level.
Nonadditive behavioral responses to multiple demands are the rule rather than the exception, both in theory (e.g. nonadditive effects of state on behavior [18]) and in practice (e.g. amplification ([19,20], Box 1), which implies that behavioral plasticity while animals are simultaneously balancing multiple demands in real time involves higher-order processes involved in decision-making and integration, presumably in the nervous system. Support for this possibility comes from work in humans and model organisms showing that distinct mechanisms are often recruited in the face of multiple demands. For example, fMRI studies of multi-tasking in humans have implicated a set of distinct brain regions (e.g. the superior parietal cortex) that are consistently activated when people have to alternate between different tasks [21,22], as compared to when they repeatedly perform the same task.
The fMRI work in humans tends to focus on human-specific brain areas, but detailed work on the neural basis of decision-making in nonhuman animals suggests that a prefrontal cortex is not required [23]. Moreover, all sorts of animals – from flies to worms to monkeys – have figured out how to manage competing demands. For example, ants, predacious beetles, dragonflies, damselflies, caddisfly larvae, copepods and shrimps adjust their foraging behavior in response to predation risk (reviewed in [24]). Therefore there may be fundamental features of nervous systems such as inhibitory neuronal mechanisms, hierarchically organized integration of information from many sources and top-down suppression of distracting stimuli [25] that are deeply conserved. However, essentially nothing is known about natural phenotypic and genetic variation within species that influences the operation of these mechanisms, or how divergence in these mechanisms over microevolutionary timescales is related to the emergence of behavioral diversity.
If distinct mechanisms are invoked during decision-making about competing demands, then simply knowing if mechanisms are shared will not tell us everything we need to know to understand constraints on plasticity. Specifically, it means that we need to study the operation of proximate mechanisms while animals are faced with multiple demands rather than relying on comparisons between the mechanisms involved in managing one demand at a time (Box 1). However, empirical studies of the mechanistic basis of behavioral syndromes and behavioral plasticity in natural populations are not yet routinely designed to incorporate this complexity, e.g., by comparing (at a mechanistic level) animals engaged in courtship to animals engaged in aggression to animals confronted by a tradeoff between courtship and aggression (with appropriate controls, Box 2). The importance of this sort of integration has been explored conceptually from a modeling perspective [4,26] and new tools for more species have the potential to facilitate this integration empirically. It is also important to note that although I focus on decision making when there are multiple demands, the conceptual framework and experimental strategy could be readily applied to numerous other timely topics in behavioral ecology that involve integrating multiple cues, e.g. multi-modal communication [27], integrating different sources of information [28], how foragers gather and weigh information [29].
What is theta and how does it evolve?
Growing evidence from work in humans and model organisms suggests that a distinct mechanism is often invoked during decision-making about multiple demands. Those distinct mechanisms might occur at various levels of biological organization and serve a number of different functions, and are hereafter generally referred to as “theta” (Figure 1). For example, a higher order brain area, circuit or cell type whose role is to integrate and/or evaluate conflicting information might become activated when animals are making decisions about multiple demands (Figure 1E). There is not necessarily a one-to-one correspondence between the activity of the mechanism and levels of a particular behavior, or exclusive mapping between mechanisms and behaviors because the mechanism could serve multiple functions [30], e.g. buffering, maintaining homeostasis, decision-making or integrating information, so that its activity is not directly proportional to behavioral output.
I propose three ways to find and study theta following the experimental design in Box 1: in the brain, in the genome and in different cell types. Importantly, these mechanisms are neither comprehensive nor mutually exclusive, and they operate over different timescales and at different levels of biological organization. Equally important, new tools allow them to be investigated in organisms from natural populations where the ecological constraints are known. Which demands, exactly, individuals need to manage is likely to be highly species-specific, depending on the species’ umwelt and ecology. If decisions over how to negotiate between foraging and predation risk is the primary determinant of fitness for that species, then selection should favor thetas to solve that problem. Alternatively, if decisions over how to maximize fitness in the face of competing demands between mating opportunities versus territorial defense are most critical to the animal, then we might expect selection to favor thetas that are useful in that regard.
Critical to understanding how theta-like mechanisms diverge over microevolutionary timescales is knowing where they might vary, so comparing theta’s operation in behaviorally divergent but closely related groups could be insightful (Box 3). Those subjects could comprise individuals differing in salient experiences, age classes, coping styles [31] or different genotypes, populations or closely related species, for example. There is often important variation in how animals balance conflicting demands (risks versus rewards) across each of these comparisons, and this is a critically important issue for major questions in ecology and evolution [3]. These scenarios form testable hypotheses to help explain longstanding questions about behavior, including why some behaviors are more plastic than others, which behaviors tend to cluster together in syndromes, and why animals are not as plastic as optimality theory predicts.
Box 3: Four interpretations of individual differences in behavior in the A+B condition.
There is often extensive behavioral variation among individuals in how they manage competing demands, some of which might be consistent over time and reflect “personality” differences, and there are at least four interpretations of that variation. Challenges for future work are to integrate these hypotheses into an adaptive framework and to test among them.
1. Variation is a byproduct of the underlying cognitive architecture.
The brain uses simple and imperfect but resilient heuristics to solve problems because the natural environment is often heterogeneous, unpredictable and dynamic, and the relevant sensory input is often partial, inaccurate and ambiguous. Coming up with accurate assessments of the true values of everything may take time that the individual can ill afford to waste [4,74]. Application of simple heuristics (“Darwinian algorithms”) can occasionally lead to error in the short term but are robust in the long term [4,25].
2. Variation reflects differences in state.
When animals manage multiple demands they often need to both balance aspects of the external environment and aspects of their internal environment – their state, e.g. energy reserves, condition, resource holding potential, reproductive value. There might be state-dependent ways the brain is organized that influence responses to multiple demands. Signals of state might be detected by theta-like mechanisms and influence their operation via steroid action which sets up the neuroendocrine system [75], via emotion circuits [76] or via signals from the periphery, from sensory systems or from the microbiome. Signals of state might influence how different alternatives (A vs B) are prioritized via inhibitory and/or stimulatory switching mechanisms [77].
3. Variation reflects non-state-dependent differences in priority setting.
Individuals might differ in how they prioritize different demands, regardless of state. In mice, neurons within the VMHv1 that were activated during attack were inhibited during mating, and the majority of male-excited cells were actively suppressed during encounters with females [52]. However, there was less evidence that cells that were excited by females were inhibited in response to males [52]. The asymmetry in inhibitory responses could explain why the conflict between fighting and mating in mice is usually resolved in favor of fighting. Individual differences in the number of cells that are excited by males versus females are related to stable individual differences in the priority setting of mating versus aggression, but little is known about this.
4. Variation reflects “behavioral skill” [78] and/or “social competence” [79].
Some individuals might be better than others at adaptively modulating their behavior in the face of competing demands, and this could be generated by several different mechanisms, e.g. more cells in theta, more connections between theta and what it integrates/what feeds into it, more modular structure of the network of interacting circuits or genes, but little is known about this. Lack of understanding has contributed to a troubling and problematic history of related ideas in the past, e.g. correlating brains and IQ in humans [80].
Theta in the brain
The human fMRI literature suggests that a distinct brain area is often activated when there are multiple demands [21,22], but little is known about variation in theta-like mechanisms in the brain, or how they evolve, despite their likely importance for fitness and evolution. That being said, studies documenting phenotypic variation in cognitive performance in fMRI tasks offer some clues. For example, older adults typically struggle more with multitasking, and reduced performance has been linked to crosstalk conflict, bottleneck processing limitations [32] and modularity, which in the fMRI context refers to the relative extent of integration and segregation of distributed functional brain networks [33]. Individuals with greater modularity perform better during dual tasking [34], perhaps by reducing behavioral interference [35].
The little that is known about genetic variation in theta-like mechanisms in the brain comes from fMRI work correlating polymorphisms in candidate genes with brain activity and, more recently, genome-wide association studies (GWAS) for imaging phenotypes (i.e. from fMRI data), e.g. [36,37], but those studies have suffered from many of the same problems plaguing GWAS of other complex traits, including noisy phenotypic data and small effect sizes [38]. Moreover, for the most part this work is carried out within a biomedical context, and is largely motivated by interest in understanding how the decision-making process itself works, rather than how it evolves, and in the context of multiple demands, often focuses on human-specific brain areas (e.g. frontal cortex, but see [39,40]).
While the previous paragraphs focus on human fMRI studies, and fMRI is less amenable for use in nonhuman animals (but see [41]), there are ample opportunities for studies in diverse organisms to make significant contributions to our understanding of the evolution of neural mechanisms involved in managing multiple demands. For example, comparing brain-wide neural activity across the A, B and A+B conditions using immediate early gene expression could identify brain areas that are exclusively activated in the A+B condition. The next step would then be to compare neural activity across behaviorally divergent populations or species, a relatively unexplored area rife with potential evolutionary insights [8].
Theta as a gene or set of genes
Decision-making about multiple demands might trigger the expression of a distinct set of genes in the brain. Brain gene expression profiling via RNA sequencing can identify genes that are expressed in response to changes in the environment and can therefore give a readout of the relevant biological processes [42]. By comparing brain gene expression profiles across the conditions in Figure I, genes that are unique to the simultaneous (A+B) condition, and which are specifically involved in the conflict between multiple demands, can be identified.
Figure I: Experimental design for studying competing demands.

Animals are compared across ecologically relevant conditions where they are asked to manage one demand (A), a different demand (B), or both demands simultaneously (A+B). (A) Example experimental design to investigate the tradeoff between courtship and territorial defense, where a male is presented with a courtship opportunity (A), a territorial intruder (B), or both a courtship opportunity and a territorial intruder at the same time (A+B, control: no stimulus). (B) Example comparing animals in different behavioral states (satiated versus hungry) in the presence versus absence of predation risk as a 2×2 factorial design.
There are at least three attractions of measuring gene expression on a genome-wide scale. First, it can nominate candidate genes that might harbor genetic variation necessary for decision-making to evolve. If we are looking for genes that are specifically important for plasticity, the genes that are uniquely expressed in the simultaneous (A+B) condition are especially good candidates, with the caveat that the relevant genetic variation might not be in coding regions and DNA sequencing might be required to find genetic variation in regulatory regions. Second, gene expression data can give insights into the role of genetic constraints by revealing which genes are shared between conditions A and B, with the caveat that if the same gene is expressed in different populations of neurons, then functions can be uncoupled. Finally, transcriptomics provides a common currency for comparing across evolutionarily divergent groups, which opens up the possibility of looking for signatures of selection and evolution at the genomic level.
Similar to the way network theory has been used in community ecology to understand community stability and invasibility, e.g. [43], gene regulatory network (GRN) analysis of gene expression data can offer insights into flexibility and constraints in the context of competing demands. One possibility is that a GRN might be rewired in response to multiple demands and/or depend on state (Figure 1E). For example, the way that transcription factors and their target genes are co-expressed in honey bee brains depends on the bees’ behavioral state, i.e. whether the measurements were made from a bee engaged in nursing or foraging [44]. A highly connected GRN is expected to result in relatively stable gene expression profiles that are harder to change [12]. Another possibility is that the structure of the GRN could bias the outcome of a conflict between competing demands, by guiding the information coming in or out of the network more towards A or B. Finally, theta-like genes might have particular attributes, e.g. by occupying key positions within a GRN, perhaps by integrating many inputs and regulating many outputs [45].
While the evolution of gene regulatory networks is an exciting and active area in evo-devo [46], applying similar concepts to brain and behavior introduces a new set of challenges because gene regulatory networks in the brain operate within another level of complexity, i.e. within neural networks [12,47]. Gene expression networks can influence the architecture and activity of neural networks by modulating neural excitability and connectivity [48], but the interplay between them is not well understood. The specific ways that network function change as behavior evolves is an even greater mystery; do neural networks evolve by adding or deleting nodes, i.e. by incorporating novel cell populations, or by recruiting a different set of genes?
Theta as a distinct cell type
Another potential outcome is that a distinct cell type in the brain is activated in the A+B condition. New single cell RNA sequencing technologies (scRNAseq) offer an opportunity to test this hypothesis in a wide range of organisms. While traditional (“bulk”) RNAseq profiles gene expression within a tissue often comprising multiple cell types, scRNAseq allows gene expression to be profiled within single cells of a tissue. The output is essentially a list – for thousands of cells in the sample – of all of the transcripts expressed within each cell in the sample [49]. scRNAseq technology can be especially insightful in the context of brain and behavior because brain tissue is highly heterogenous, comprising multiple cells types [49], and it is possible to index neural activity through immediate early gene expression (IEG) in single cell datasets [50].
Another reason why this technology is likely to be a game-changer is because it offers the possibility to determine whether differences in gene expression reflect differential gene regulation (typically the assumption of most RNAseq studies) or differences in the distribution, abundance or activity of cell types across conditions. Until now, for example, we have not been able to determine whether the genes that are shared across conditions (e.g. A and B) are expressed in the same or different cells. Similarly, it might appear that the GRN is rewired in the A+B condition based on bulk RNAseq data, but a closer look at IEGs in single cell data might reveal activation of different subcircuits in the A+B condition. Single cell data could also offer insights into other fundamental questions about the relative importance of activation versus inhibition for behavior. Comparing the number and activity of excitatory versus inhibitory neurons in single cell data across the conditions in Figure I could offer some clues.
To examine the evolution of mechanisms for resolving multiple demands at the cell type level, behaviorally divergent groups could be sampled for scRNAseq profiling (with the caveat that identifying homologous cell types can be non-trivial [51]) following the experimental design in Figure I, with several outcomes of interest. For example, the identity, number, or activity of cell types that are unique to the A+B condition might differ among groups.
That being said, the proximate processes involved in balancing multiple demands are probably highly dynamic, and a single snapshot in time taken after lethal sampling could be misleading. For example, in mice, there is considerable overlap between the cells that are activated in the presence of either a male or female during the initial stages of a social encounter [52]. However, the subpopulations of cells are increasingly distinct as the social encounter progresses, an insight that was only available because neural activity was recorded in real time [52], see [34] for something similar with fMRI. The move to conceptualize neural populations as dynamical systems is gaining traction in model organisms where imaging and optogenetics tools are available [53]; neural assemblies coactivated or distinctively activated across two time points could potentially be investigated in non-model vertebrates with IEGs using CATfish [52].
Concluding Remarks
There is a long and rich tradition of integrating proximate and ultimate approaches to behavior (e.g. [54]). This article asks a specific question: how do the proximate mechanisms by which animals manage multiple demands evolve? Which parts of the system – structures, cell types, circuits, molecules, GRNs – are most tweakable during evolution and how does that generate the extensive variation in the specific behavioral means by which animals manage multiple demands? Obviously the first step toward answering these questions is detailed study of those mechanisms in a diversity of subjects, then we can start asking questions about how they vary, and where they harbor genetic variation.
A central message of this article is that the mechanisms involved in managing multiple demands occur at different levels of biological organization. Processes at different levels likely serve different functions (integration, decision-making, priority setting, etc.), therefore studies at different levels may provide complementary and mutually informative insights. Neural mechanisms are likely to be important over immediate timescales, but others (molecular, genetic, endocrine) could be important over longer timescales, by setting the stage for neural mechanisms to operate [48].
A new wave of studies on decision-making with tools that toggle at the interface between the neural, genetic and molecular are poised to make major headway in our understanding of the evolution of behavioral plasticity by identifying its proximate substrates [12,47] (see Outstanding Questions). The challenge, then, will be to place this integrated view within an evolutionary context. The genetic variation that is targeted by selection when differences in decision-making evolve is likely to be expressed in the brain area, circuit or cell type that is activated in the presence of multiple stimuli, i.e. in theta. Spatial transcriptomic technologies – which can measure gene expression at fine levels of resolution in a spatial context [55,56] – hold a lot of promise for bridging across the molecular and neural levels, which could be key to resolving these issues. By comparing spatial transcriptomic data across groups that are actively diverging such as different selected lines or populations in the early stages of divergence, the level of analysis (gene, cell type, brain network) that is most divergent could be identified, which would suggest which elements of the system are most readily modified by selection.
Outstanding questions.
How to reconcile widespread sharing of mechanisms across functional contexts with distinct behavioral outputs? Is it because we failed to look at the right level (if we had better resolution we’d realize there’s less sharing than we think), or because there are emergent properties of networks that generate specificity? Understanding if the answer lies in reductionism (eventually we will get it right if we drill down enough) or about emergent properties of networks or interactions among layers of networks is an outstanding question with implications for the evolution of complex traits.
How often are behavioral trade-offs characterized at the proximate level by fine spatial neuronal substructure, distinctive transcriptional signatures, competing brain substrates or neuromolecules, emergent properties of networks, distinct mechanisms that are only recruited in the face of competing demands (theta) or combinations all of the above?
How does incorporating more realistic assumptions about the underlying genetic architecture (e.g. many interacting, pleiotropic genes of small effect) into theoretical models alter predictions about the evolution of mechanisms for resolving tradeoffs? What is the right common currency for comparing across demands? How can modeling offer insights into questions such as biases toward certain outcomes, variation in the A+B condition and its adaptive significance?
How does considering proximate mechanisms as evolvable traits (rather than as constraints) change the way we think about the evolution of decision-making?
Are the proximate mechanisms that are recruited in the face of multiple demands the same across different tradeoffs (foraging versus vigilance, courtship versus aggression, etc.), or is there a theta that is unique to each of them? Are features that are at the intersection of many multi-tasking assays the strongest evidence for theta?
Are there common principles of decision-making about competing demands across taxa? To what extent are general principles versus specific architectures conserved?
Another central message is that if there are distinct mechanisms that are only invoked in the presence of multiple demands, experimental designs need to incorporate this complexity, and failing to consider those mechanisms could result in misunderstanding selection and phenotypic evolution. Fortunately, thanks to a growing toolbox, there are many opportunities for studies of theta in diverse organisms exhibiting divergent strategies to transform our understanding of the evolution of behavioral plasticity and decision-making.
Highlights.
Animals in natural populations are constantly managing multiple demands on their time and energy, and decisions about ecologically relevant tradeoffs have important fitness consequences.
Growing evidence for behavioral syndromes and shared mechanisms underlying behavior in different functional contexts prompts novel hypotheses about how animals balance competing demands.
Mechanistic work in humans and model organisms suggests that distinct molecular, neural and genetic mechanisms are often involved in decision-making, but little is known about variation in those mechanisms or how they evolve.
Applying neurogenomic tools to non-traditional model organisms where the ecological constraints are understood has the potential to offer exciting insights into the evolution and adaptive significance of mechanisms for decision-making.
Acknowledgements
Thanks to Tina Barbasch, Ross De Angelis, Eva Fischer, Meghan Maciejewski, Marc Mangel, Jonathan Perelmuter, Andy Sih, Judy Stamps and two anonymous reviewers for helpful comments on previous versions of the manuscript.
Glossary
- Behavioral syndrome
a suite of correlated behaviors quantified by examining among-individual correlations of behaviors in different contexts
- Compromise
a behavioral response to multiple demands that occurs when levels of a behavior that serves multiple functions is intermediate between the behavior which would be optimal in response to one cue and the behavior that would be optimal in response to the other cue
- Amplification
a behavioral response to multiple demands that occurs when levels of a behavior that serves multiple functions are higher in the presence of multiple demands compared to when there is only one demand
- Gene regulatory network (GRN)
a collection of regulatory relationships among genes within cells. GRNs can be estimated by analyzing patterns of co-expression within an RNA-seq dataset
- Immediate early genes (IEGs)
genes which are activated transiently in the brain as the first wave of transcription in response to a stimulus and can be used as a proxy of neural activity, including in scRNAseq data
- Limited behavioral plasticity
less than optimal behavioral plasticity based on what is expected to be the optimum in each context. Limited behavioral plasticity can result from behavioral syndromes and can reflect tradeoffs
- Multidimensional plasticity
more than one environmental cue influences the same trait. In the context of multiple demands, each demand is an environmental cue which influences a behavior
- Multi-tasking
a behavioral response to multiple demands that occurs when the animal simultaneously performs two behaviors that serve different functions
- Multivariate plasticity
phenotypic plasticity in multiple traits. Multiple demands can trigger multivariate plasticity if they cause changes in more than one behavior
- Neural activity
electrical activity in the brain which can be measured at the whole-brain level via fMRI, calcium imaging, or by recording the activity of single cells or groups of cells with electrophysiology, for example. Neural activity can also be approximated by the expression of immediate early genes, which can be gleaned from scRNAseq data
- RNA sequencing
a technique which uses next generation sequencing to quantify the presence and quantity of different RNA transcripts in a tissue sample
- Single cell RNA sequencing (scRNASeq)
uses next generation sequencing to quantify different RNAs within single cells of a sample. By analyzing patterns of gene expression within and across cells, statistical clustering methods can be used to group cells into different clusters with similar expression profiles, which may correspond to different cell types
- Social decision-making network in the brain
a hypothesized system of reciprocally connected brain structures and neurochemicals important for social behavior with dynamic patterns of activity that are conserved across vertebrate species
- Switching
a behavioral response to multiple demands that occurs when the animal alternates between different behaviors that serve different functions
- Theta
a proximate mechanism that is only engaged when there are multiple demands. Theta could serve diverse functions (sensory integration, decision-making) and can occur at different levels of biological organization (neural circuit, brain area, genes, cell type)
- Time budget constraint
time spent engaging in a behavior that serves one function takes away from time available to perform a behavior that serves a different function
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Dingemanse NJ and Dochtermann NA (2013) Quantifying individual variation in behaviour: Mixed-effect modelling approaches. J Anim Ecol 82, 39–54. [DOI] [PubMed] [Google Scholar]
- 2.Dingemanse NJ et al. (2010) Behavioural reaction norms: Animal personality meets individual plasticity. Trends Ecol Evol 25, 81–89. [DOI] [PubMed] [Google Scholar]
- 3.McNamara JM and Houston AI (1986) The common currency for behavioral decisions. The Am Nat 127, 358–378. [Google Scholar]
- 4.Budaev S et al. (2019) Decision-making from the animal perspective: Bridging ecology and subjective cognition. Front in Ecol and Evol 7. DOI 10.3389/fevo.2019.00164 [DOI] [Google Scholar]
- 5.McNamara JM and Houston AI (2009) Integrating function and mechanism. Trends Ecol Evol 24, 670–675. [DOI] [PubMed] [Google Scholar]
- 6.Tinbergen N (1963) On aims and methods of ethology. Zeitschrift fur Tierpsychologie 20, 410–433. [Google Scholar]
- 7.Newman SW (1999) The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci 877, 242–257. [DOI] [PubMed] [Google Scholar]
- 8.Goodson JL (2005) The vertebrate social behavior network: Evolutionary themes and variations. Horm Behav 48, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell LA and Hofmann HA (2011) The vertebrate mesolimbic reward system and social behavior network: A comparative synthesis. J Comp Neurol 519, 3599–3639. [DOI] [PubMed] [Google Scholar]
- 10.Flint J and Mackay TFC (2009) Genetic architecture of quantitative traits in mice, flies, and humans. Genome Research 19, 723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anholt RRH and Mackay TFC (2004) Quantitative genetic analyses of complex behaviours in Drosophila. Nat Rev Genetics 5, 838–849. [DOI] [PubMed] [Google Scholar]
- 12.Sinha S et al. (2020) Behavior-related gene regulatory networks: A new level of organization in the brain. Proc Nat Acad Sci 117, 23270–23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wingfield JC et al. (1990) The challenge hypothesis: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. The Am Nat 136, 829–846. [Google Scholar]
- 14.Bukhari SA et al. (2019) Neurogenomic insights into paternal care and its relation to territorial aggression. Nat Comm 10, 4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson DJ (2016) Circuit modules linking internal states and social behaviour in flies and mice. Nat Rev Neurosci 17, 692–704. [DOI] [PubMed] [Google Scholar]
- 16.Kohl J et al. (2018) Functional circuit architecture underlying parental behaviour. Nature 556, 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeAngelis R et al. (2020) Nonapeptides mediate trade-offs in parental care strategy. Horm and Behav 121, 104717. [DOI] [PubMed] [Google Scholar]
- 18.Sih A et al. (2015) Animal personality and state-behaviour feedbacks: A review and guide for empiricists. Trends Ecol Evol 30, 50–60. [DOI] [PubMed] [Google Scholar]
- 19.Lindström K et al. (2006) Sexual selection for male parental care in the sand goby, Pomatoschistus minutus. Behav Ecol and Sociobiol 60, 46–51. [Google Scholar]
- 20.Zajonc RB (1965) Social facilitation. Science 149, 269–274. [DOI] [PubMed] [Google Scholar]
- 21.Wager TD et al. (2004) Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage 22, 1679–1693. [DOI] [PubMed] [Google Scholar]
- 22.Alavash M et al. (2016) Dynamic coupling of complex brain networks and dual-task behavior. Neuroimage 129, 233–246. [DOI] [PubMed] [Google Scholar]
- 23.Rook N et al. (2021) A hierarchical processing unit for multi-component behavior in the avian brain. iScience 24, 103195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima SL and Dill LM (1990) Behavioral decisions made under the risk of predation - A review and prospectus. Can J Zool 68, 619–640. [Google Scholar]
- 25.Adams GK et al. (2012) Neuroethology of decision-making. Curr Opin Neurobiol 22, 982–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eliassen S et al. (2016) From sensing to emergent adaptations: Modelling the proximate architecture for decision-making. Ecol Modelling 326, 90–100. [Google Scholar]
- 27.Higham JP and Hebets EA (2013) An introduction to multimodal communication. Behav Ecol Sociobiol 67, 1381–1388. [Google Scholar]
- 28.Dall SR et al. (2015) Genes as cues: Phenotypic integration of genetic and epigenetic information from a Darwinian perspective. Trends Ecol Evol 30, 327–333. [DOI] [PubMed] [Google Scholar]
- 29.Stephens DW et al. (2007) Foraging: Behavior and Ecology University of Chicago Press [Google Scholar]
- 30.Martin LB et al. (2011) Integrator networks: Illuminating the black box linking genotype and phenotype. Int Comp Biol 51, 514–527. [DOI] [PubMed] [Google Scholar]
- 31.Coppens CM et al. (2010) Coping styles and behavioural flexibility: towards underlying mechanisms. Philos Trans R Soc Lond B Biol Sci 365, 4021–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clapp WC et al. (2011) Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proc Nat Acad Sci 108, 7212–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meunier D et al. (2010) Modular and hierarchically modular organization of brain networks. Front Neurosci 4. DOI 10.3389/fnins.2010.00200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alavash M et al. (2015) Persistency and flexibility of complex brain networks underlie dual-task interference. Human Brain Mapping 36, 3542–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sporns O (2013) Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol 23, 162–171. [DOI] [PubMed] [Google Scholar]
- 36.Elliott LT et al. (2018) Genome-wide association studies of brain imaging phenotypes in UK Biobank. Nature 562, 210–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mufford MS et al. (2017) Neuroimaging genomics in psychiatry-a translational approach. Genome Medicine 9, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marek S et al. (2022) Reproducible brain-wide association studies require thousands of individuals. Nature 603, 654–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collette F et al. (2006) Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience 139, 209–221. [DOI] [PubMed] [Google Scholar]
- 40.Andres P (2003) Frontal cortex as the central executive of working memory: Time to revise our view. Cortex 39, 871–895. [DOI] [PubMed] [Google Scholar]
- 41.Van der Linden A et al. (2007) Current status of functional MRI on small animals: application to physiology, pathophysiology, and cognition. NMR in Biomedicine 20, 522–545. [DOI] [PubMed] [Google Scholar]
- 42.Bell AM and Robinson GE (2011) Genomics. Behavior and the dynamic genome. Science 332, 1161–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alcántara JM and Rey PJ (2012) Linking topological structure and dynamics in ecological networks. The Am Nat 180, 186–199. [DOI] [PubMed] [Google Scholar]
- 44.Traniello IM and Robinson GE (2021) Neural and molecular mechanisms of biological embedding of social interactions. Ann Rev Neurosci 44, 109–128. [DOI] [PubMed] [Google Scholar]
- 45.Stern DL and Orgogozo V (2009) Is genetic evolution predictable? Science 323, 746–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDonald JMC and Reed RD (2022) Patterns of selection across gene regulatory networks. Seminars in Cell & Developmental Biology 145, 60–67. [DOI] [PubMed] [Google Scholar]
- 47.Baran NM et al. (2017) Applying gene regulatory network logic to the evolution of social behavior. Proc Nat Acad Sci 114, 5886–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clayton DF (2000) The genomic action potential. Neurobiol Learn Mem 74, 185–216. [DOI] [PubMed] [Google Scholar]
- 49.Poulin J-F et al. (2016) Disentangling neural cell diversity using single-cell transcriptomics. Nat Neurosci 19, 1131–1141. [DOI] [PubMed] [Google Scholar]
- 50.Tyssowski KM et al. (2018) Different neuronal activity patterns induce different gene expression programs. Neuron 98, 530–546.e511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tosches MA (2021) From cell types to an integrated understanding of brain evolution: The case of the cerebral cortex. In Annual Review of Cell and Developmental Biology, Vol 37 (Lehmann R, ed), pp. 495–517, [DOI] [PubMed] [Google Scholar]
- 52.Lin DY et al. (2011) Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nair A et al. (2023) An approximate line attractor in the hypothalamus encodes an aggressive state. Cell 186, 178–193.e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Holekamp KE and Sherman PW (1989) Why male ground squirrels disperse: A multilevel analysis explains why only males leave home. Am Sci 77, 232–239. [Google Scholar]
- 55.Moffitt JR et al. (2018) Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaughn E et al. (2022) Three-dimensional interrogation of cell types and instinctive behavior in the periaqueductal gray. bioRxiv, 2022.2006.2027.497769. [Google Scholar]
- 57.Westneat DF et al. (2019) Causes and consequences of phenotypic plasticity in complex environments. Trends Ecol Evol 34, 555–568. [DOI] [PubMed] [Google Scholar]
- 58.Chevin LM and Lande R (2015) Evolution of environmental cues for phenotypic plasticity. Evolution 69, 2767–2775. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen ME and Papaj DR (2022) Why study plasticity in multiple traits? New hypotheses for how phenotypically plastic traits interact during development and selection. Evolution 76, 858–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Endler JA (1987) Sexual selection and predation risk in guppies. Nature 332, 593–594. [Google Scholar]
- 61.Hedrick AV (2000) Crickets with extravagant mating songs compensate for predation risk with extra caution. Proc Roy Soc B 267, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dill LM et al. (1999) Male mating strategies under predation risk: do females call the shots? Behav Ec 10, 452–461. [Google Scholar]
- 63.Ryan MJ (1985) The Tungara Frog: A Study in Sexual Selection and Communication University of Chicago Press [Google Scholar]
- 64.Sih A et al. (2004) Behavioral syndromes: An integrative overview. Quart Rev Biol 79, 241–277. [DOI] [PubMed] [Google Scholar]
- 65.Wolf M and Weissing FJ (2012) Animal personalities: consequences for ecology and evolution. Trends Ecol Evol 27, 452–461. [DOI] [PubMed] [Google Scholar]
- 66.Sih A et al. (2012) Ecological implications of behavioural syndromes. Ecology Letters 15, 278–289. [DOI] [PubMed] [Google Scholar]
- 67.Neff BD and Sherman PW (2004) Behavioral syndromes versus Darwinian algorithms. Trends Ecol Evol 19, 621–622. [Google Scholar]
- 68.Sih A et al. (2004) Reply to Neff and Sherman. Behavioral syndromes versus Darwinian algorithms. Trends Ecol Evol 19, 622–623. [Google Scholar]
- 69.Duckworth RA and Sockman KW (2012) Proximate mechanisms of behavioural inflexibility: Implications for the evolution of personality traits. Func Ecol 26, 559–566. [Google Scholar]
- 70.Bell AM and Sih A (2007) Exposure to predation generates personality in threespined sticklebacks. Ecology Letters 10, 828–834. [DOI] [PubMed] [Google Scholar]
- 71.Dochtermann NA (2010) Behavioral syndromes: Carryover effects, false discovery rates, and a priori hypotheses. Behav Ecol 21, 437–439. [Google Scholar]
- 72.Bell A (2013) Randomized or fixed order for studies of behavioral syndromes? Behav Ecol 24, 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Via S et al. (1995) Adaptive phenotypic plasticity - Consensus and controversy. Trends Ecol Evol 10, 212–217. [DOI] [PubMed] [Google Scholar]
- 74.Trimmer PC et al. (2011) Decision-making under uncertainty: biases and Bayesians. Animal Cognition 14, 465–476. [DOI] [PubMed] [Google Scholar]
- 75.Atkins-Regan E (2005) Hormones and Animal Social Behavior Princeton University Press [Google Scholar]
- 76.LeDoux J (2012) Rethinking the emotional brain. Neuron 73, 653–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu M et al. (2022) Make war not love: The neural substrate underlying a state-dependent switch in female social behavior. Neuron 110, 841–856.e846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sih A et al. (2019) On the importance of individual differences in behavioural skill. Anim Behav 155, 307–317. [Google Scholar]
- 79.Taborsky B and Oliveira RF (2012) Social competence: an evolutionary approach. Trends Ecol Evol 27, 679–688. [DOI] [PubMed] [Google Scholar]
- 80.Fischer CS et al. (1996) Inequality by Design: Cracking the Bell Curve Myth Princeton University Press [Google Scholar]


