Abstract
Individuals with autism spectrum disorder (ASD) frequently exhibit difficulties in retrieving autobiographical memories of specific events from their life. Such memory deficits are frequently attributed to underlying disruptions in self-referential or social cognition processes. This makes intuitive sense as these are hallmarks of ASD. However, an emerging literature suggests that parallel deficits also exist in ASD individuals’ ability to reconstruct the rich spatial contexts in which events occur. This is a capacity known as scene construction, and in typically developing individuals is considered a core process in retrieving autobiographical memories. In this review, we discuss evidence of difficulties with scene construction in ASD, drawing upon experiments that involve autobiographical memory retrieval, other forms of mental time travel, and spatial navigation. We also highlight aspects of extant data that cannot be accounted for using purely social explanations of memory deficits in ASD. We conclude by identifying key questions raised by our framework and suggest how they might be addressed in future research.
Keywords: Autism spectrum disorder, autobiographical memory, navigation, scene construction, self-projection
LAY SUMMARY
Difficulties in retrieving memories of specific events are well documented in autism spectrum disorder (ASD). Although it is commonly thought that these difficulties arise from a deficit in ASD individuals’ social cognitive abilities, a growing literature suggests that a separate, parallel deficit also exists in the ability to mentally reconstruct aspects of the space, or scene, in which a remembered event occurred. In this review, we discuss what is known about the scene construction deficit in ASD and how its consideration might impact future research.
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder that is characterized by behavioral challenges1 in social interaction, verbal and non-verbal communication, and cognitive flexibility. Although social deficits are prominently associated with ASD, a long-standing literature has also highlighted deficits in memory-related processes, particularly for episodic, autobiographical memories (AMs) (Kanner, 1943). This is apparent even in ASD individuals without intellectual disability, who will be the focus of this review. Such memory deficits are frequently attributed to underlying deficits in self-referential or social cognition (e.g. Henderson et al., 2009; Kristen et al., 2014; Robinson et al., 2017; Wantzen et al., 2021), and this makes intuitive sense given the central role the self is thought to play in AM retrieval (e.g., Tulving, 1985; Conway, 2005). However, an emerging body of research has found that not all aspects of AM deficits are directly attributable to social deficits—in particular, information related to “where” an event occurred also appears to be disrupted in the memories of ASD individuals. It may therefore be the case that ASD additionally involves deficits related to one’s ability to mentally reconstruct scenes from memory. We consider this possibility throughout this review, first by evaluating the shortcomings of a purely “social” account in explaining empirical data, then by offering a complimentary account by which parallel scene construction deficits also contribute to memory deficits in ASD, and finally by identifying specific questions to motivate future research that might better identify the underlying cause(s) of AM deficits in ASD.
Current ideas on ASD social deficits explaining AM deficits
Autobiographical memory, as a construct, consists of both personally experienced events (personal episodic memories; deficits related to which are the primary focus of this review) and self-related information (personal semantic memory or knowledge; Tulving, 1983; Conway, 2005). AM is not, however, thought to function as a passive store for these different forms of memories. Rather, it is thought to support diverse functions that relate to oneself and to interactions with others. For example, a review by Bluck (2003) highlighted crucial roles of AM in supporting the continuity and development of the self (Pillemer, 1992; Conway, 1996); in anticipating and solving future problems (including social problems) by recalling aspects of past experiences (Neisser, 1988; Goddard et al., 1996; Gerlach et al., 2011); and in communication to form and maintain social relationships (Pillemer, 1992; Davidson et al., 2012; Brien & Hutchins, 2022). Irrespective of other factors, the prominent roles of AM in social and self-referential domains render it theoretically relevant to the study of ASD. That said, the broader social and communicative difficulties, restrictive and repetitive behavioral patterns, and deficits associated with Theory of Mind (ToM; one’s ability to make inferences about others’ mental states) in ASD have been described at length in prior work and are not the intended focus of this review (Kanner, 1943; Bishop, 1989; Happé & Frith, 1995; Baron-Cohen et al., 1985; Turner, 1999; Baron-Cohen, 2000; Frith, 2001; Stone & Gerrans, 2006). Rather, it is important to consider social behavioral difficulties as they provide the foundation for dominant ideas regarding the AM deficits observed in ASD.
Episodic AMs engage self-referential processing as they necessarily involve an awareness that we have a past, will have a future, and can engage in “mental time travel” to a place and time outside the present (Tulving, 1983, 1985). Episodic AMs also involve social cognitive elements or ToM insofar as they frequently involve interactions with other humans that must be represented in the memory (Klein et al., 2009; Gilmore et al., 2021b). However, the linkage of the self to AM is perhaps most pronounced in the Self-Memory System framework (Conway & Pleydell-Pearce, 2000; Conway et al., 2004), which describes a system with three main components: the long-term self, the working self, and the episodic memory system. The long-term self reflects (semantic) self-knowledge and one’s life story. The working self is dynamic, reflecting our current goals, motivations, and self-identity, and interacts with the other two system components. The episodic memory system contains specific sensory, emotional, and cognitive details about specific experiences that allow for a given event, or episode, to be subjectively re-experienced (as in Tulving, 1985). According to this model, cues to retrieve episodic memories are generated by one’s working self, which also serves as a lens through which we can reflect upon our broader life story. At the same time, the episodes and events we experience serve to modify and add to one’s self-knowledge and sense of self, which will then impact how goal-relevant memories are cued and retrieved in the future.
It is therefore notable that ASD research has demonstrated specific, theoretically predicted impairments in memory, in the self, and between the two constructs (e.g. Crane & Goddard, 2008; Millward et al., 2000; Lind, 2010; Tanweer et al., 2010; Crane et al., 2012; Coutelle et al., 2020). From a neuroscience perspective, the observations of overlap in large-scale meta-analyses (e.g., Spreng et al., 2009) between brain regions associated with social cognition and those associated with AM retrieval lends further credence to accounts whereby social disruptions in ASD might be expected to impact AM. However, recent behavioral findings have highlighted several aspects of the AM deficit in ASD, particularly those regarding episodic AMs, that do not appear to be consistent with an account hypothesizing purely social deficits. Furthermore, recent imaging evidence suggests that some of the neural overlap observed at the group level may not be recapitulated at the level of the individual. These findings, discussed in the remainder of this review, are cause for a reconsideration of how we previously thought of the AM impairment—and memory impairments in general—in ASD.
Memory impairments in ASD
The memory impairments observed in ASD are selective rather than global. In particular, evidence suggests a deficit in memories for specific events or episodes (autobiographical episodic memories), including the specific details associated with an event and the broader spatiotemporal contexts in which they occur (Bowler et al., 2011; Boucher et al., 2012; Cooper & Simon, 2019). In contrast, semantic memory—that is, general world knowledge— appears intact (Shalom, 2003; Lind, 2010; Gaigg et al., 2014). This general pattern has been observed across studies over the past several decades, with semantic knowledge commonly serving as a control condition when assessing AM ability in ASD (Bowler et al., 2000; Bowler et al., 2007; Bruck et al., 2007; Crane et al., 2009; Crane & Goddard, 2008; see also McDonnell et al., 2017). For example, studies investigating the retrieval of personally experienced events have observed episodic AM difficulties in ASD participants, who nonetheless possess similar levels of explicit semantic (acontextual) self-knowledge, both self and non-self-related, as typically-developing controls (TDs; Crane & Goddard, 2008; Lind & Bowler, 2010; Cooper & Simon, 2019). Crane et al. (2012) observed that ASD individuals generate fewer AMs and take significantly longer to do so when compared with TD individuals. Tanweer et al. (2010) found that retrieved memories of ASD individuals, (identified by the authors as having Asperger syndrome), were less often purely episodic than in TDs, while more were described to be “known” (in a semantic or “general knowledge” sense) rather than subjectively re-experienced and “remembered.” In convergent work, Crane and Goddard (2008) used an episodic and semantic interview task to independently examine episodic and semantic AMs across different time periods in ASD participants. The questions designed to assess episodic AM asked about events such as what happened on one’s first day of secondary school, while the questions designed to assess semantic AM asked about personal life facts such as the name of one’s primary school teacher. In addition, participants generated episodic and semantic memories from different time periods at speed in a fluency task. Consistent with previous research, episodic AM was impaired in ASD despite preserved personal semantic memory (in both tasks) suggesting a deficit and dissociation between personal episodic and personal semantic memory in ASD. Although the above evidence supports a clear deficit in episodic AMs rather than in all forms of memory, the intact nature of both non-personal semantic and personal semantic memories makes it difficult to assume that a simple self/social cognitive impairment was driving AM reductions in ASD. It therefore appears that a separate mechanism likely contributes to the pattern of episodic memory deficits observed in the existing literature.
Using a multilevel Bayesian meta-analysis to quantify episodic memory differences between ASD individuals and TD controls, Griffin et al. (2022) found that the effect size of episodic recollection deficits was high when probing memory at the event or “story” level, but modest when examining memory for simple stimulus items such as words or pictures (including objects, figures, and shapes). For example, no difference was found between ASD and TD groups on immediate and delayed word recognition, whereas a disadvantage was observed for the ASD group on tests of story recognition. A similar conclusion was reached by Cooper and Simon (2019), who argued in a recent review that existing social accounts of AM impairments in ASD could explain some, but not all, memory results and that AM impairments were likely also attributable to difficulties in the ability to reconstruct (and monitor) past experiences.
Looking beyond the social domain: A scene construction impairment in ASD?
Work in cognitive psychology and cognitive neuroscience has stressed the importance of scene construction in the retrieval of AMs. Scene construction refers to the ability to mentally generate and maintain a complex and coherent representation of a spatial context, or “scene” (Hassabis et al., 2007; Hassabis & Maguire, 2007, 2009). Scenes are constructed by consciously reactivating and/or maintaining both sensory and semantic information (Hassabis & Maguire, 2009; Ladyka-Wojcik et al., 2022), and are thought to serve as a “scaffold” (Robin, 2018) that supports the recollection of past events, the imagination of hypothetical future events (a capacity termed episodic future thought, or “EFT”), and the memory-based navigation of an environment (Hassabis et al., 2007; Hassabis & Maguire, 2009; Lind et al., 2014a; see also Lind et al., 2013; Rubin & Umanath, 2015). Some have argued that scene construction is explicitly necessary for retrieving episodic AMs, as it provides a “where” component that subsequently allows the “what” component to be accessed (Hassabis & Maguire, 2007). Others have taken the similar but less extreme position that without an accompanying scene component, AMs lack a foundational context and are accompanied by a diminished sense of reliving and subjective vividness (Rubin et al., 2019).
The central role played by scene construction in AM retrieval was elegantly demonstrated in work by Robin et al. (2015) with TD individuals. Cues involving locations were more effective at eliciting specific and vivid memories than cues with information about a person. Moreover, participants frequently reported adding a spatial context (i.e., scene) to events cued with information about a person but rarely added person-related details to events cued with location information (for further discussion, see Robin, 2018). It therefore appears that retrieving social information about a prior event engages constructive processes relating to the spatial location in which it occurred, suggesting a privileged role for scenes in AM. This is further demonstrated in work by Rubin et al. (2019), who investigated the role of scene construction in TDs using self-report measures of memory qualities in a sample of 200 participants. Using structural equation modeling, the authors found that clearer spatial layouts in scenes—more so than any other collected measure, including the amount of detail retrieved or the subjective emotionality of a memory—were associated with greater subjective senses of reliving an event, more overall vividness, and belief that a retrieved memory had occurred. In sum, a growing body of evidence suggests that scene construction is a core component of AM retrieval.
Roughly coinciding with the proposal of scene construction was a conceptually similar idea of self-projection (Buckner & Carroll, 2007): the ability to shift perspective from one’s immediate (first-person perspective) present to an alternate time (past or future), location (present whereabouts or elsewhere), or visual perspective (including adopting the thoughts and/or visual perspective of another individual through one’s ToM). Self-projection is posited to be driven by an internal mode of mental simulation that is referenced to oneself. Under this view, AMs are accessed by self-projecting to another time and place. Underlying deficits in social cognition or self-referential processing would therefore be expected to cause disruptions in AM if self-projection is a critical explanatory factor of AM deficits in ASD.
Almost a decade ago, Lind and colleagues (2013; see also Lind et al., 2014b) argued that scene construction and self-projection should be theoretically separated. We adopt this perspective ourselves in this review. The two constructs certainly share conceptual similarities—both enable leaving the “here and now” to mentally construct a complex scenario and both appear necessary to recall AMs or engage in episodic future thinking. However, as highlighted by Lind et al. (2013), scene construction’s primary emphasis is spatial in nature and involves the representation of complex environments, whereas self-projection instead emphasizes a self-referential component that is social-cognitive in nature (Figure 1; see also Lind et al., 2014b).
Figure 1.
Schematic separation of self-projection and scene construction, as well as cognitive abilities they are thought to support, as proposed by Lind et al. (2014b). Self-projection and scene construction are distinct processes that emphasize social and spatial cognition, respectively (outlined in blue and red), yet are thought to commonly support episodic memory retrieval (including AM retrieval) and the related process of episodic future thinking (outlined in purple). Deficits in either self-projection or scene construction would be expected to cause disruptions in AM, but for different reasons. Figure adapted and modified from Lind et al. (2014b) and used under a Creative Commons Attribution License (CC BY 3.0).
Evidence of scene construction deficits in ASD
Deficits in scene construction, with their emphasis on generating spatial contexts, make unique predictions that differ from an account based on social deficits, and few studies have directly investigated a scene construction impairment as a possible underlying cause of AM deficits in ASD. This stands in contrast to the frequently investigated and well-supported link between scene construction and AM in TDs (e.g., Hassabis et al., 2007; Hassabis & Maguire, 2009; Madore et al., 2019; Rubin et al., 2019). However, the research that does exist suggests that scene construction warrants further investigation in ASD. We consider these studies below.
Lind et al. (2014b) asked ASD individuals to imagine or remember vivid scenes in their mind based on 3 types of cue cards with short descriptions: personally experienced past events (last week, last birthday) to assess AM, plausible self-relevant future events (next Christmas, next camping trip) to assess EFT, and atemporal, non-self-relevant fictitious scenes (beach, pub, museum) to assess scene construction. A control task without any constructive memory demands, in which participants used a picture book to tell the experimenter a story, assessed general narrative ability. Vividness and specificity were measured by external raters looking at the subcomponents of the narrative content and using participant experiential ratings. Consistent with previous accounts of AM deficits in ASD, the descriptions of events were significantly less vivid and less specific in ASD individuals compared to TD individuals. However, the critical finding was that the descriptions of non-self-relevant fictitious scenes, which lacked a self-projection component, were also impaired. This impairment was independent of general narrative ability, and the authors argued that a scene construction deficit was a parsimonious explanation of their results. Similarly, a meta-analysis of AM and EFT deficits in ASD by Ye et al. (2023) found that these processes, which the authors note rely heavily on the (re)construction of a coherent scene, cannot be explained by variables such as language ability, the direction of time travel involved (i.e., the past or future), the specific dependent measure employed, or demographic factors such as age, sex ratios2, or IQ.
In other work, Ciaramelli et al. (2018) examined the contents of descriptions that differed in self-relevance (personal past events, personal future events, and events involving others) and found that ASD individuals provided fewer internal (episodic) event details but a similar number of external (semantic) details to controls in all conditions. This deficit was particularly evident in conditions of high retrieval support (i.e., where specific verbal probes were provided to encourage highly detailed retrieval), wherein the TD group’s performance was significantly improved, but the performance of the ASD group was not. This general pattern held for personal past events, personal future events, and events involving others. Although one could, in principle, attribute their results to either self-projection or scene construction deficits, Ciaramelli and colleagues concluded that scene construction was a more likely candidate because the deficits persisted in non-self-oriented conditions.
Anger et al. (2019) also compared the amount of information retrieved between ASD and TD groups under different levels of retrieval support but utilized visual (cartoon) cues instead of verbal prompts. Free recall performance was higher in TDs than in ASD participants, consistent with the general pattern of results discussed in this review. However, in contrast to the results of Ciaramelli et al. (2018), event recall performance in the cued (high support) condition provided a benefit to ASD participants as well as TDs, such that event recall scores no longer differed between groups (although some specific, subjective sensory detail deficits were still observed in the ASD group in follow-up analyses). Recall scores were near ceiling for both the ASD and TD groups, so the discrepancy between the results of Ciaramelli et al. to those of Anger et al. should not be over-interpreted, but the data nevertheless provide empirical evidence of visual cues improving ASD performance. Furthermore, as Anger et al. note, if (re)constructing scene contexts is impaired in ASD individuals, then providing visual cues could be expected to reduce construction demands and therefore facilitate performance.
Further investigating the link between scene construction and EFT ability, Marini et al. (2016) found that ASD children were impaired in tasks requiring them to imagine themselves in plausible future scenarios. Although a social or self-projection account would predict this pattern, ASD children also had difficulties in tasks requiring them to mentally imagine (nonsocial) objects interacting mechanically. The authors suggested that both self-projection and scene construction might be impaired in ASD, while also positing that the former was “more severely compromised” (p. 3359). Other research by Chua (2020) sought to establish the extent to which scene construction and/or self-projection might underpin EFT deficits in ASD children. Participants were instructed to imagine and provide descriptions of atemporal events, plausible self-relevant future events, and engage in a narrative control task, similar to the one employed by Lind et al. (2014b). These conditions were meant to vary demands on scene construction, self-projection, and narrative processing (for related discussion, see also Mercuri et al., 2016). Consistent with previous findings, ASD children generated a similar number of internal details in the atemporal and future conditions, both of which contained significantly fewer details than the control condition. The ASD group, however, also exhibited a reduced capacity to describe themselves experiencing an atemporal scene in which self-projection demands were reduced in comparison to the future condition.
It therefore appears likely that deficits in scene construction may contribute to AM difficulties in ASD, and that such difficulties may not be attributable to self-projection alone (also discussed by Westby, 2022). However, convergent evidence from studies involving spatial navigation also supports a scene construction deficit, as will be considered below.
Spatial navigation abilities in ASD also suggest a scene construction deficit
Spatial navigation involves finding one’s way around an environment while maintaining a sense of direction and location. It is a task context in which scene construction plays an active role in organizing spatial information to build an accurate and flexible mental representation of one’s environment, but which lacks a clear “social” component (and thereby sets itself aside from social or self-projection deficits). Broadly speaking, navigation can be based on either, or both, of two distinct frames of reference, which are referred to as “egocentric” and “allocentric” (e.g., Burgess, 2006; Wolbers & Hegarty, 2010). Egocentric navigation is a self-oriented approach relying on one’s own perspective and their body’s position in space (i.e., egocentric navigation utilizes self-referential cues), in which landmarks and objects are perceived in relation to oneself. Allocentric navigation, in contrast, relies on understanding the relationships between objects and landmarks in an environment independent of one’s own position (O’Keefe & Nadel, 1978). Allocentric navigation allows for creating a flexible mental map of one’s environment from an external, objective viewpoint, although it is thought to emerge after egocentric approaches (Dilks et al., 2022).
In principle, egocentric navigation can be impaired because of deficits in either self-projection or scene construction, as it involves representation of an environment in an inherently self-referential manner. However, allocentric navigation involves no direct projection of oneself; rather, it involves the construction of rich spatial interrelations that are, by definition, independent of one’s current location or perspective. Thus, if ASD individuals have a deficit in scene construction, one would expect difficulties with allocentric navigation in particular (Lind et al., 2013). At least some evidence supports this prediction. Across two studies, Lind et al. (2013; 2014a) assessed allocentric and egocentric spatial navigation ability using a memory island navigation paradigm in ASD and TD participants. During the initial phase, participants completed four “visible” (egocentric) trials. Participants moved through a realistic 3D virtual environment to several target objects in different flagged locations. The next phase consisted of four “hidden” (allocentric) trials in which the markers were removed, and participants had to rely on their learned internal map built during visible trials, without additional aids, to locate these same target objects. Across both studies, the ASD group spent significantly less time in the target quadrant, took longer to find the targets, successfully completed fewer trials, took significantly longer routes, and covered a broader search area compared to the TD group during the hidden condition. Importantly, navigation performance did not differ between the groups during the visible condition. Instead, the ASD group’s diminished performance was specific to the hidden condition, which required memory-based scene construction for successful navigation.
Ring et al. (2018) extended the study of navigation in ASD using a human virtual reality adaptation of the Morris Water Maze task that assessed both egocentric and allocentric navigation abilities. During the task, participants had to find a hidden platform in a virtual swimming pool in which either object cues (egocentric condition) or the participants’ starting location (allocentric condition) changed in relation to the original object, participant, and platform location of the initial learning phase. Performance did not differ between groups in the egocentric condition. Although both ASD and TD groups found the allocentric condition more difficult, ASD individuals were significantly more impaired than TDs. Additional control tasks involving visual short-term memory and mental rotation tasks, intended to identify participants’ ability to process and manipulate spatial information, revealed no between group differences, and no significant correlations were found between control tasks and allocentric navigation performance. It therefore seems unlikely that these abilities had any influence on the significant between-group difference in allocentric spatial navigation performance. These findings support a diminished ability in allocentric spatial navigation, as would be predicted by a scene construction deficit.
More recently, Yang et al. (2021) explored spatial navigation in an ASD cohort with intellectual impairment. Performance was compared to separate control groups that included age-matched TDs in one case and IQ-matched controls in another. Experimental tasks involved learning specific routes in a complex virtual environment; learning and identifying the direction of certain objects from a cued location in a separate environment; and following a 2-dimensional path and indicating whether each turn would be to one’s subjective left or right as they imagined walking through it. Although the ASD group sometimes matched the performance of the IQ-matched controls, both groups scored consistently lower than the age-matched TDs. However, in both the “reverse navigation” of a learned route (i.e., starting at the end and ending at the standard start point) and in the 2-dimensional path task, the ASD group performed significantly lower than either control group. Yang et al. (2021) concluded that the ASD group had particular difficulties in adopting allocentric reference frames or using them to update egocentric positions within an environment. Such a conclusion appears consistent with scene construction difficulties in ASD and does so using a more impaired population than is typically examined in navigation studies.
Functional network interdigitation may partially explain why scene construction has not been examined in more detail
The past decade has seen growing behavioral evidence for the presence of a scene construction deficit in ASD. One may therefore wonder why this possibility has not been raised more broadly, particularly given the wealth of imaging data collected from ASD participants over the past several decades. This may reasonably have been expected to identify disruptions in regions associated with scene construction. One possible reason this has not occurred concerns the framing of questions regarding deficits in ASD. These have, understandably, focused on social or self-referential questions. Although entirely logical, such a focus limits the task conditions being compared in any given experiment, and consequently the conclusions that might be drawn from it. Independent of that possibility, we suggest that the large-scale organization of the human brain itself has hindered wider observations of potential differences in scene construction.
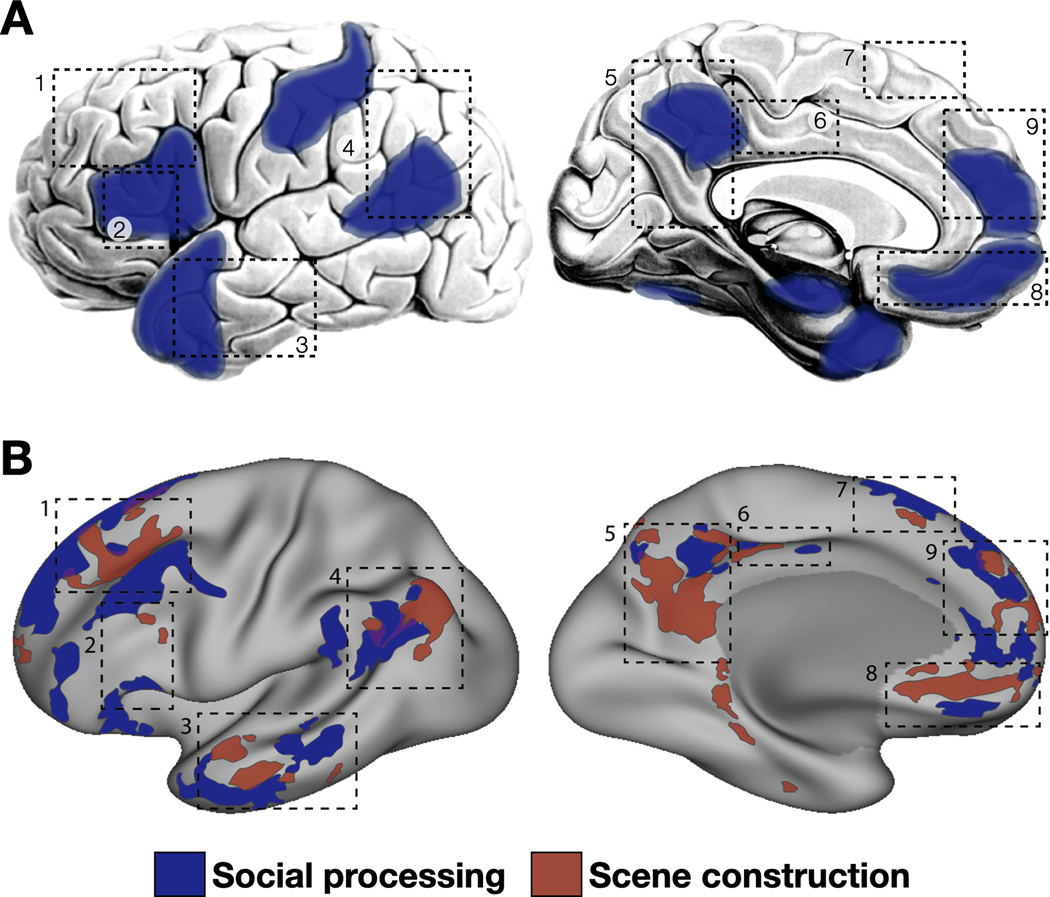
Brain regions associated with ASD are reliable and include aspects of the temporal lobe (especially the temporal pole and posterior lateral ventral cortex), medial prefrontal cortex, medial and lateral parietal cortex, and the anterior hippocampus and amygdala (Figure 2A). These regions are associated with social or self-related cognition with a sufficient frequency that they have been described as the “social brain” (e.g., Adolphs, 2009; Gotts et al., 2012). However, regions associated with social processing are often adjacent to, or even interdigitated with, those associated with scene construction (Figure 2B), which canonically include ventral medial parietal cortex (sometimes referred to as the retrosplenial complex or retrosplenial cortex), parahippocampal cortex, and portions of the angular gyrus (e.g., Hassabis et al., 2007). The extent of the interdigitation was first appreciated within highly sampled individual participants (Braga & Buckner, 2017; see also DiNicola et al., 2020; DiNicola et al., 2023), but in recent years has been observed in group studies as well (Silson et al., 2019; Woolnough et al., 2020; Gilmore et al., 2021b; for further discussion, see Gilmore et al., 2021a). However, in older studies and meta-analyses, group averaging could have blurred the networks together (e.g., Gordon et al., 2017), such that they appeared to be largely overlapping rather than distinct (e.g., Buckner & Carroll, 2007; Spreng et al., 2009). Some additional studies found suggestions evidence of social/scene related differences, but even assumed this was interpreted to indicate that a common functional network served as a hub, with the more specialized functional networks serving as spokes (Andrews-Hanna et al., 2010; Andrews-Hanna et al., 2014a;or subnetworks (Andrews-Hanna et al., 2010; Andrews-Hanna, Saxe, & Yarkoni, 2014; see also Andrews-Hanna et al., 2014b). Andrews-Hanna, Smallwood, & Spreng, 2014). Given the close juxtaposition/interdigitation of networks supporting distinct functions, and the between-groups averaging associated with typical comparisons of ASD and TD groups, significant effects in regions associated with scene construction may have simply been eclipsed (either on a statistical parametric map or in terms of researchers’ attention) by those in regions associated with social cognition.
Figure 2.
Brain regions supporting social cognition and scene construction in the brain are juxtaposed and/or interdigitated. (a) Canonical social processing regions, defined from large-group studies, are frequent targets of investigation in autism spectrum disorder (ASD) research. (b) However, precision fMRI investigations using highly sampled individuals demonstrate that social regions (blue) are adjacent to, and often interdigitated with, regions involved in scene processing (red), with little apparent overlap (purple). Functionally distinct, the two networks can nevertheless be difficult to disentangle at the group level (see e.g., Gilmore et al., 2021). Numbered boxes reflect corresponding locations on the different brain surfaces. Panel A images adapted from Gotts et al. (2012); panel B images adapted from Braga & Buckner (2017) under a Creative Commons CC-BY license.
Future directions
In this review, we have considered evidence that a scene construction deficit contributes to the diminished AM recollections in ASD, as well as in the related capacities of EFT and spatial navigation. Many questions still exist regarding the role and differentiation of scene construction and self-projection in the overall pattern of AM deficits and behavioral challenges observed in ASD. We identify several such questions below.
Does neuroimaging provide evidence of abnormal task-evoked activity or functional connectivity in regions associated with scene construction?
In contrast to the large neuroimaging literature examining social and self-referential cognition in ASD, there are very few neuroimaging studies investigating AM in the disorder or the underlying neural components contributing to the AM deficit. fMRI has been employed to study memory for laboratory-type materials (e.g., object arrays superimposed on a background image; Cooper et al., 2017) or knowledge about one’s past tendencies (Cygan et al., 2018), but functional imaging studies have not been conducted to complement behavioral studies of autobiographical recall or EFT in ASD. Nevertheless, the framework described in this review allows for several testable predictions to be articulated.
First, differences in task-evoked activity and/or connectivity should be observed between ASD and TD participants in regions during AM retrieval. If one were to use independent functional localizer or resting-state data to separate regions associated with scene construction and social cognition at the level of individual subjects (rather than at the group level), then deficits should be expected in both sets of regions. Furthermore, our hypothesis predicts similar disruptions in regions associated with scene construction during allocentric navigation. Particularly beneficial might be studies that employ cross-task contexts involving high and low scene construction and ToM demands in ASD and TD individuals, as these allow for the observation of dissociations that might support or falsify the hypothesis we forward here. Alternatively, one might use overt AM recall in fMRI so that activity during the retrieval of both social and scene-related information can be captured in a naturalistic manner (Gilmore et al., 2021b), enabling researchers to measure how activity might differ between ASD patients and TDs during these time periods. Such an approach may also prove useful in improving our understanding of how the hippocampus—a structure associated with both social and spatial cognition, as well as AM more broadly—may differentially contribute to retrieval for ASD individuals and TDs (see Banker et al., 2021; Gilmore et al., 2022).
When do scene construction deficits emerge?
The divergence of ASD individuals from typical development suggests that ASD can be characterized as a progressive disorder, in which the expected developmental trajectory is disrupted, and atypical behaviors gradually emerge (Landa et al., 2013). Increasing divergence from typical development can be detected as soon as 14 months in social-communication and motor related tasks (Estes et al., 2015). A key question stemming from the framework we provide here is how scene construction develops in ASD, and in what ways it may differ from its development in TDs. Early developmental data will be critical in understanding how scene construction and AM may relate to one another in ASD, yet data from this period are lacking when compared to data from adolescent or adult cohorts. Scene construction might be studied using several different approaches, depending on the target age of the children in question, but among the most useful would be approaches that allow the dissociation of scene construction from self-projection.
In preverbal children, the development of motor skills is closely connected with the development of spatial cognition (Vasileyva & Lourenco, 2012; Newcome, 2019; Cortes et al., 2022; Dilks et al., 2022), and appears promising as an early life candidate of study. Of relevance here, impaired motor learning has been proposed as a core symptom of ASD (see Bo et al., 2016), and the question therefore becomes how well motor learning impairments may predict scene construction in ASD. For slightly older children, adaptations of standard spatial navigation tasks in animals, such as radial arm or Morris water maze tasks, have been used to assess performance in children as young as 20 months (Overman et al., 1996) but can be used in school-aged children as well (e.g., Ring et al., 2018). This offers an opportunity to study scene construction with a common approach across a range of ages. Verbal descriptions of memories, imagined hypothetical occurrences, or spatial layouts remain useful tools for future research in this area as well (as in Lind et al., 2014a; Ciaramelli et al., 2018; Marini et al., 2016). Along with behavioral measures, a multidisciplinary approach that combines neuroimaging, clinical diagnosis, neuropsychological tests, and parent/family/school reports may be particularly fruitful in addressing this gap in the field’s knowledge.
Is there a link between scene construction deficits and repetitive behaviors?
Restricted and repetitive patterns of behaviors and interests, characterized by sameness, rigidity, and repetitiveness, are among the earliest infantile predictors of later ASD diagnosis (Kim & Lord, 2010; Ozonoff et al., 2008) and are characteristic of ASD individuals (Kanner, 1943; Bishop, 1989; Honey et al., 2012). At present, there is a continued need to integrate ASD research on memory, behavior, and development. Terrett et al. (2013) discussed how difficulties with EFT might relate to the core feature of behavioral inflexibility, given that a person’s current behavior is strongly influenced by how they anticipate the future. A reduced capacity to project forward in time may be reflected by more limited behavioral repertoires and a greater insistence on sameness. Supporting this hypothesis, Lind et al. (2014a) found that ASD children who showed more repetitive behaviors showed poorer spatial navigation (which, according to our framework, is likely the result of scene construction difficulties). As a corollary, one may hypothesize that scene construction is a contributor to one particular form of repetitive behavior: an insistence on following specific routes to specific destinations (Turner, 1999). An empirical investigation of the relationship between memory deficits associated with scene construction and restrictive and repetitive behaviors would be a valuable avenue to pursue in the future to facilitate efforts in understanding the diversity in ASD etiology and inform the design of future interventions.
Concluding remarks
Behavioral deficits in the social domain lie at the heart of ASD, yet these alone seem insufficient to account for deficits in episodic AMs or other areas such as spatial navigation. Rather, emerging data suggest that a parallel deficit in scene construction is also present in ASD. The need for further insights into the extent of scene construction deficits in ASD—and the separation of these from more traditionally-appreciated social deficits and their behavioral sequelae—represents a challenge and an opportunity for future research that can inform our understanding of ASD and human memory more broadly.
ACKNOWLEDGEMENTS:
We thank Andrew Persichetti for thoughtful comments and discussions relating to this work.
FUNDING:
This work was supported by the Intramural Research Program at the National Institute of Mental Health (ZIAMH002920).
Footnotes
In this manuscript, we use terminology such as “impairment” and “deficit” to describe specific cognitive differences thought to produce difficulties or challenges in ASD individuals. We acknowledge that not all forms of cognitive diversity lead to functional impairments and support the use of language that combats the stigma surrounding ASD.
Sex ratios in most ASD studies skew heavily toward males, as do rates of ASD diagnosis in general (Looms et al., 2017). Sex imbalances in empirical studies therefore reflect the general population but may hinder our understanding of sex differences in ASD that relate to AM (among other domains). Studies that explicitly balance sex ratios (as done by, e.g., Crane et al., 2009; Goddard et al., 2014) and that recruit large sample sizes will be important in future research
CONFLICTS OF INTEREST:
The authors declare no conflicts of interest.
DATA AVAILABILITY:
No new data were collected for this review.
REFERENCES
- Adolphs R. (2009). The social brain: Neural basis of social knowledge. Annual Review of Psychology, 60, 693–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, & Buckner RL (2010). Functional-anatomic fractionation of the brain’s default network. Neuron, 65, 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Saxe R, & Yarkoni T. (2014a). Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage, 91, 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, & Spreng RN (2014b). The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences, 1316, 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anger M, Wantzen P, Le Vaillant J, Malvy J, Bon L, Guénolé F, Moussaoui E, Barthelemy C, Bonnet-Brilhault F, Eustache F, Baleyte J-M, & Guillery-Girard B. (2019). Positive effect of visual cuing in episodic memory and episodic future thinking in adolescents with autism spectrum disorder. Frontiers in Psychology, 10, 1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker AM, Gu X, Schiller D, & Foss-Feig JH (2021). Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends in Neurosciences, 44, 793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. (2000). Theory of mind and autism: A review. International review of research in mental retardation, 23, 169–184. [Google Scholar]
- Baron-Cohen S, Leslie AM, & Frith U. (1985). Does the autistic child have a “theory of mind”? Cognition, 21, 37–46. [DOI] [PubMed] [Google Scholar]
- Bishop DV (1989). Autism, Asperger’s syndrome and semantic-pragmatic disorder: where are the boundaries? British Journal of Disorders of Communication, 24, 107–121. [DOI] [PubMed] [Google Scholar]
- Bluck S. (2003). Autobiographical memory: Exploring its functions in everyday life. Memory, 11(2), 113–123. [DOI] [PubMed] [Google Scholar]
- Bo J, Lee C-M, Colbert A, & Shen B. (2016). Do children with autism spectrum disorders have motor learning difficulties. Research in Autism Spectrum Disorders, 23, 50–62. [Google Scholar]
- Boucher J, Mayes A, & Bingham A. (2012). Memory in autistic spectrum disorder. Psychological Bulletin, 138, 458–496. [DOI] [PubMed] [Google Scholar]
- Bowler D, Gaigg SB, & Lind S. (2011). Memory in autism: Binding, self, and brain. In Roth I. & Rezaie P. (Eds.), Researching the Autism Spectrum: Contemporary perspectives (pp. 316–346). Cambridge: Cambridge University Press. [Google Scholar]
- Bowler DM, Gardiner JM, & Gaigg SB (2007). Factors affecting conscious awareness in the recollective experience of adults with Asperger’s syndrome. Consciousness and Cognition, 16, 124–143. [DOI] [PubMed] [Google Scholar]
- Bowler DM, Gardiner JM, & Grice SJ (2000). Episodic memory and remembering in adults with Asperger syndrome. Journal of Autism and Developmental Disorders, 30, 295–304. [DOI] [PubMed] [Google Scholar]
- Braga RM, & Buckner RL (2017). Parallel interdigitated distributed networks within the individual estimated by intrinsic functional connectivity. Neuron, 95, 457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien A, & Hutchins T. (2022). Development of a manualized intervention to support episodic memory in autistic children: Elaborative reminiscing is key. Seminars in Speech and Language, 43, 299–315. [DOI] [PubMed] [Google Scholar]
- Bruck M, London K, Landa R, & Goodman J. (2007). Autobiographical memory and suggestibility in children with autism spectrum disorder. Development and Psychopathology, 19, 49–57. [DOI] [PubMed] [Google Scholar]
- Buckner RL, & Carroll DC (2007). Self-projection and the brain. Trends in Cognitive Sciences, 11(2), 49–57. [DOI] [PubMed] [Google Scholar]
- Burgess N. (2006). Spatial memory: How egocentric and allocentric combine. Trends in Cognitive Sciences, 10, 551–557. [DOI] [PubMed] [Google Scholar]
- Chua SJS (2020). An investigation of episodic future thinking, episodic foresight and prospective memory in children with Autism Spectrum Disorder Australian Catholic University, [Google Scholar]
- Ciaramelli E, Spoglianti S, Bertossi E, Generali N, Telarucci F, Tancredi R, Muratori F, & Igliozzi R. (2018). Construction of Past and Future Events in Children and Adolescents with ASD: Role of Self-relatedness and Relevance to Decision-Making. Journal of Autism and Developmental Disorders, 48, 2995–3009. [DOI] [PubMed] [Google Scholar]
- Conway MA (1996). Autobiographical memory. In Memory (pp. 165–194): Academic Press. [Google Scholar]
- Conway MA (2005). Memory and the self. Journal of Memory and Language, 53, 594–628. [Google Scholar]
- Conway MA, & Pleydell-Pearce CW (2000). The construction of autobiographical memories in the self-memory system. Psychological Review, 107, 261–288. [DOI] [PubMed] [Google Scholar]
- Conway MA, Singer JA, & Tagini A. (2004). The self and autobiographical memory: Correspondence and coherence. Social Cognition, 22, 491–529. [Google Scholar]
- Cooper RA, Richter FR, Bays PM, Plaisted-Grant KC, Baron-Cohen S, & Simons JS (2017). Reduced hippocampal functional connectivity during episodic memory retrieval in autism. Cerebral Cortex, 27, 888–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RA, & Simon JS (2019). Exploring the neurocognitive basis of episodic recollection in autism. Psychonomic Bulletin & Review, 26, 163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes RA, Green AE, Barr RF, & Ryan RM (2022). Fine motor skills during early childhood predict visuospatial deductive reasoning in adolescence. Developmental psychology, 58, 1264–1276. [DOI] [PubMed] [Google Scholar]
- Coutelle R, Goltzene MA, Bizet E, Schoenberger M, Berna F, & Danion JM (2020). Self-concept clarity and autobiographical memory functions in adults with autism spectrum disorder without intellectual deficiency. Journal of Autism and Developmental Disorders, 50, 3874–3882. [DOI] [PubMed] [Google Scholar]
- Crane L, & Goddard L. (2008). Episodic and semantic autobiographical memory in adults with autism spectrum disorders. Journal of Autism and Developmental Disorders, 28, 498–506. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, & Pring L. (2009). Specific and general autobiographical knowledge in adults with autism spectrum disorders: The role of personal goals. Memory, 17, 557–576. [DOI] [PubMed] [Google Scholar]
- Crane L, Pring L, Jukes K, & Goddard L. (2012). Patterns of autobiographical memory in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 42, 2100–2112. [DOI] [PubMed] [Google Scholar]
- Cygan HB, Marchewka A, Kotlewska I, & Nowicka A. (2018). Neural correlates of reflection on present and past selves in autism spectrum disorder. Journal of Autism and Developmental Disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PSR, Drouin H, Kwan D, Moscovitch M, & Rosenbaum RS (2012). Memory as social glue: Close interpersonal relationships in amnesic patients. Frontiers in Psychology, 3, 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks DD, Kramps FS, & Persichetti AS (2022). Three cortical scene systems and their development. Trends in Cognitive Sciences, 26, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicola LM, Ariyo OI, & Buckner RL (2023). Functional specialization of parallel distributed networks revealed by analysis of trial-to-trial variation in processing demands. Journal of Neurophysiology, 129, 17–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNicola LM, Braga RM, & Buckner RL (2020). Parallel distributed networks dissociate episodic and social functions within the individual. Journal of Neurophysiology, 123, 1144–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes A, Zwaigenbaum L, Gu H, St. John T, Paterson S, Elison JT, Hazlett H, Botteron K, Dager SR, Schultz RT, Kostopoulos P, Evans A, Dawson G, Eliason J, Alvarez S, Piven J, & network, t. I. (2015). Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. Neurodevelopmental disorders, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U. (2001). Mind blindness and the brain in autism. Neuron, 32, 969–979. [DOI] [PubMed] [Google Scholar]
- Gaigg SB, Bowler DM, & Gardiner JM (2014). Episodic but not semantic order memory difficulties in autism spectrum disorder: Evidence from the Historical Figures Task. Memory, 22, 669–678. [DOI] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Gilmore AW, & Schacter DL (2011). Solving future problems: Default network and executive activity associated with goal-directed mental simulations. NeuroImage, 55(4), 1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AW, Audrain S, & Martin A. (2022). Specifying ‘where’ and ‘what’ is critical for testing hippocampal contributions to memory retrieval. Cognitive Neuroscience, 13, 144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AW, Nelson SM, & McDermott KB (2021a). Precision functional mapping of human memory systems. Current Opinion in Behavioral Sciences, 40, 52–57. [Google Scholar]
- Gilmore AW, Quach A, Kalinowski SE, Gotts SJ, Schacter DL, & Martin A. (2021b). Dynamic content reactivation supports naturalistic autobiographical recall in humans. Journal of Neuroscience, 41, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard L, Dritschel B, & Burton A. (1996). Role of autobiographical memory in social problem solving and depression. Journal of Abnormal Psychology, 105, 609–616. [DOI] [PubMed] [Google Scholar]
- Goddard L, Dritschel B, & Howlin P. (2014). A preliminary study of gender differences in autobiographical memory in children with an autism spectrum disorder. Journal of Autism and Developmental Disorders, 44, 2087–2095. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, Hampton JM, Coalson RS, Nguyen AL, McDermott KB, Shimony JS, Snyder AZ, Schlaggar BL, Petersen SE, Nelson SM, & Dosenbach NUF (2017). Precision functional mapping of individual human brains. Neuron, 95, 791–807.e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotts SJ, Simmons WK, Milbury LA, Wallace GL, Cox RW, & Martin A. (2012). Fractionation of social brain circuits in autism spectrum disorders. Brain, 125, 2711–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin JW, Bauer R, & Gavett BE (2022). The episodic memory profile in autism spectrum disorder: A bayesian meta-analysis. Neuropsychology Review, 32, 316–351. [DOI] [PubMed] [Google Scholar]
- Happé F, & Frith U. (1995). Theory of mind in autism. In Schopler E. & Mesibov GB (Eds.), Learning and Cognition in Autism (pp. 177–197). New York: Springer. [Google Scholar]
- Hassabis D, Kumaran D, & Maguire EA (2007). Using imagination to understand the neural basis of episodic memory. Journal of Neuroscience, 27(52), 14365–14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, & Maguire EA (2007). Deconstructing episodic memory with construction. Trends in Cognitive Sciences, 11(7), 299–306. [DOI] [PubMed] [Google Scholar]
- Hassabis D, & Maguire EA (2009). The construction system of the brain. Philosophical Transactions of the Royal Society B: Biological Sciences, 364(1521), 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Zahka NE, Kojkowski NM, Inge AP, Schwartz CB, Hileman CM, Coman DC, & Mundy PC (2009). Self-referenced memory, social cognition, and symptom presentation in autism. Journal of Child Psychology and Psychiatry, 50, 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey E, McConachie H, Turner M, & Rodgers J. (2012). Validation of the repetitive behaviour questionnaire for use with children with autism spectrum disorder. Research in Autism Spectrum Disorders, 6, 355–364. [Google Scholar]
- Kanner L. (1943). Autistic disturbances of affective contact. Nervous child, 2, 217–250. [PubMed] [Google Scholar]
- Kim SH, & Lord C. (2010). Restricted and repetitive behaviors in toddlers and preschoolers with autism spectrum disorders based on the Autism Diagnostic Observation Schedule (ADOS). Autism Research, 3, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, Robertson TE, & Delton AW (2009). Facing the future: Memory as an evolved system for planning future acts. Memory & Cognition, 38(1), 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristen S, Rossman F, & Sodian B. (2014). Theory of own mind and autobiographical memory in adults with ASD. Research in Autism Spectrum Disorders, 8, 827–837. [Google Scholar]
- Ladyka-Wojcik N, Zhong-Xu L, & Ryan JD (2022). Unrestricted eye movements strengthen effective connectivity from hippocampal to oculomotor regions during scene construction. NeuroImage, 260, 119497. [DOI] [PubMed] [Google Scholar]
- Landa R, Gross AL, Stuart EA, & Faherty A. (2013). Developmental trajectories in children with and without autism spectrum disorders: The first 3 years. Child development, 84, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind SE (2010). Memory and the self in autism: A review and theoretical framework. Autism, 14, 430–456. [DOI] [PubMed] [Google Scholar]
- Lind SE, & Bowler DM (2010). Episodic memory and episodic future thinking in adults with autism. Journal of Abnormal Psychology, 119, 896–905. [DOI] [PubMed] [Google Scholar]
- Lind SE, Bowler DM, & Raber J. (2014a). Spatial navigation, episodic memory, episodic future thinking, and theory of mind in children with autism spectrum disorder: evidence for impairments in mental simulation. Frontiers in Psychology, 5, 1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind SE, Williams DM, Bowler DM, & Peel A. (2014b). Episodic memory and episodic future thinking impairments in high-functioning autism spectrum disorder: An underlying difficulty with scene construction or self-projection? Neuropsychology, 28, 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind SE, Williams DM, Raber J, Peel A, & Bowler DM (2013). Spatial navigation impairments among intellectually high-functioning adults with autism spectrum disorder: exploring relations with theory of mind, episodic memory, and episodic future thinking. Journal of Abnormal Psychology, 122, 1189–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looms R, Hull L, & Mandy WPL (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 56, 466–474. [DOI] [PubMed] [Google Scholar]
- Madore KP, Jing HG, & Schacter DL (2019). Episodic specificity induction ands cene construction: Evidence for an event construction account. Consciousness and Cognition, 68, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini A, Ferretti F, Chiera A, Magni R, Adornetti I, Nichiarelli A, Vicari A, & Valeri G. (2016). Brief report: Self-based and mechanical-based future thinking in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 46, 3353–3360. [DOI] [PubMed] [Google Scholar]
- McDonnell CG, Valentino K, & Diehl JJ (2017). A developmental psychopathology perspective on autobiographical memory in autism spectrum disorder. Developmental Review, 44, 59–81. [Google Scholar]
- Mercuri K, Terrett G, Bailey PE, Henry JD, Curran HV, & Rendell PG (2016). Deconstructing the nature of episodic foresight deficits assocaited with chronic opiate use. British Journal of Clinical Psychology, 55, 401–413. [DOI] [PubMed] [Google Scholar]
- Millward C, Powell S, Messer D, & Jordan R. (2000). Recall for self and other in autism: Children’s memory for events experienced by themselves and their peers. Journal of Autism and Developmental Disorders, 30, 15–28. [DOI] [PubMed] [Google Scholar]
- Neisser U. (1988). Five kinds of self-knowledge. Philosophical psychology, 1, 35–59. [Google Scholar]
- Newcome NS (2019). Navigation and the developing brain. Journal of experimental biology, 222, jeb186460. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, & Nadel L. (1978). The hippocampus as a cognitive map: Oxford University Press. [Google Scholar]
- Overman WH, Pate BJ, Moore K, & Peuster A. (1996). Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behavioral Neuroscience, 110, 1205–1228. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Heung K, Byrd R, Hansen R, & Hertz-Picciotto I. (2008). The onset of autism: patterns of symptom emergence in the first years of life. Autism Research, 1, 320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillemer DB (1992). Remembering personal circumstances: A functional analysis. In Winograd E. & Neisser U. (Eds.), Affect and accuracy in recall: Studies of “flashbulb” memories (pp. 236–264). Cambridge: Cambridge University Press. [Google Scholar]
- Ring M, Gaigg SB, Altgassen M, Barr P, & Bowler DM (2018). Allocentric versus egocentric spatial memory in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48, 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin J. (2018). Spatial scaffold effects in event memory and imagination. WIREs Cognitive Science, 9, e1462. [DOI] [PubMed] [Google Scholar]
- Robin J, Wynn J, & Moscovitch M. (2015). The spatial scaffold: The effects of spatial context on memory for events. Journal of Experimental Psychology: Learning, Memory, & Cognition, 42, 308–315. [DOI] [PubMed] [Google Scholar]
- Robinson S, Howlin P, & Russell A. (2017). Personality traits, autobiographical memory and knowledge of self and others: A comparative study in young people with autism spectrum disorder. Autism, 21, 357–367. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Deffler SA, & Umanath S. (2019). Scenes enable a sense of reliving: Implications for autobiographical memory. Cognition, 183, 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DC, & Umanath S. (2015). Event memory: A theory of memory for laboratory, autobiographical, and fictional events. Psychological Review, 122, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalom DB (2003). Memory in autism: Review and synthesis. CORTEX, 39, 1129–1138. [DOI] [PubMed] [Google Scholar]
- Silson EH, Steel AD, Kidder A, Gilmore AW, & Baker CI (2019). Distinct subdivisions of human medial parietal cortex support recollection of people and places. eLife, 8, e47391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, & Kim ASN (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. [DOI] [PubMed] [Google Scholar]
- Stone VE, & Gerrans P. (2006). What’s domain-specific about theory of mind? Social Neuroscience, 1, 309–319. [DOI] [PubMed] [Google Scholar]
- Tanweer T, Rathbone CJ, & Souchay C. (2010). Autobiographical memory, autonoetic consciousness, and identity in Asperger syndrome. Neuropsychologia, 48, 900–908. [DOI] [PubMed] [Google Scholar]
- Terrett G, Rendell PG, Raponi-Saunders S, Henry JD, Bailey PE, & Altgassen M. (2013). Episodic future thinking in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 43, 2558–2568. [DOI] [PubMed] [Google Scholar]
- Tulving E. (1983). Elements of Episodic Memory. New York: Oxford University Press. [Google Scholar]
- Tulving E. (1985). Memory and consciousness. Canadian Psychology, 26(1), 1–12. [Google Scholar]
- Turner M. (1999). Repetitive behaviour in autism: A review of psychological research. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 40, 839–849. [PubMed] [Google Scholar]
- Vasileyva M, & Lourenco SF (2012). Development of spatial cognition. WIREs Cognitive Science, 3, 349–362. [DOI] [PubMed] [Google Scholar]
- Wantzen P, Boursette A, Zante E, Mioche J, Eustache F, Guénolé F, Baleyte J-M, & Guillery-Girard B. (2021). Autobiographical memory and social identity in autism: Preliminary results of social positioning and cognitive intervention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westby C. (2022). Nature and effects of autobiographical memory issues in persons with autism spectrum disorders. Neuropsychiatric Disease and Treatment, 2279–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, & Hegarty M. (2010). What determines our navigational abilities. Trends in Cognitive Sciences, 14, 138–146. [DOI] [PubMed] [Google Scholar]
- Woolnough O, Rollo PS, Forseth KJ, Kadipasaoglu CM, Ekstrom AD, & Tandon N. (2020). Category selectivity for face and scene recognition in human medial parietal cortex. Current Biology, 30, 2707–2715.e2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Li W, Huang D, He W, Zhang Y, & Merrill E. (2021). An evaluation of wayfinding abilities in adolescent and young adult males with autism spectrum disorder. Research in Autism Spectrum Disorders, 80, 101697. [Google Scholar]
- Ye JY, Qin XJ, Cui JF, Ren Q, Jia LX, Wang Y, Pantelis C, & Chan RC (2023). A meta-analysis of mental time travel in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 53, 1509–1528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were collected for this review.