Abstract
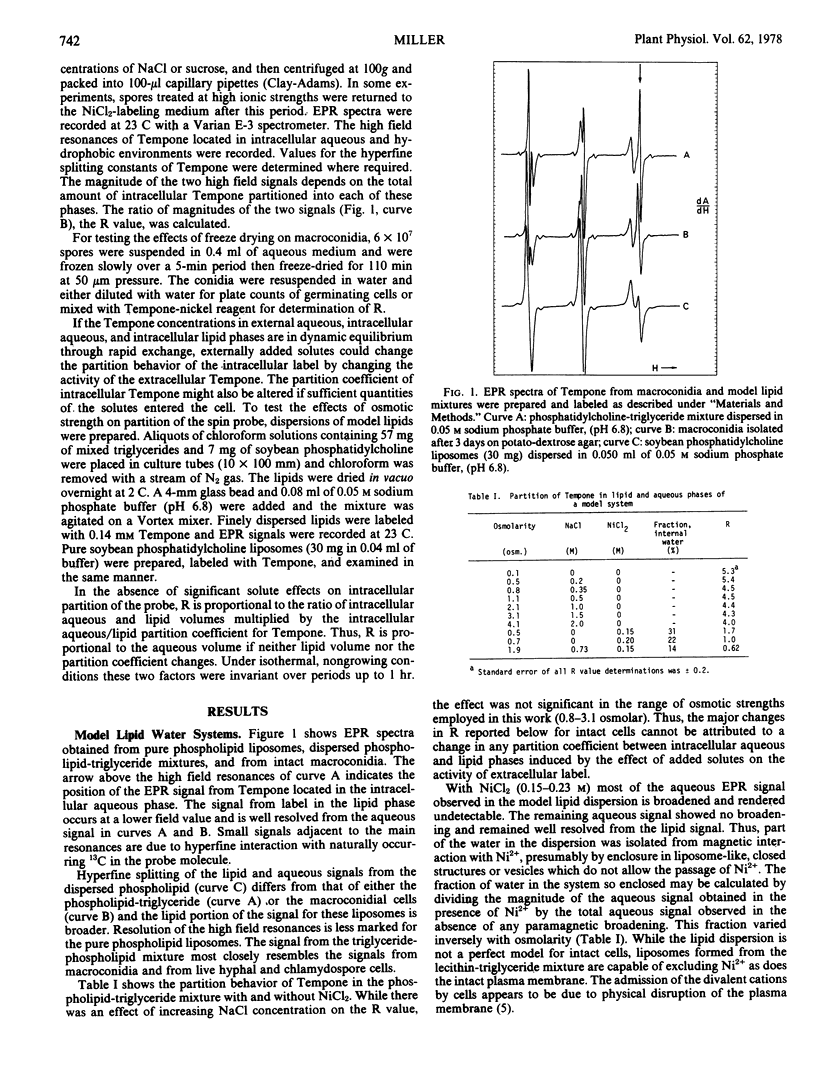
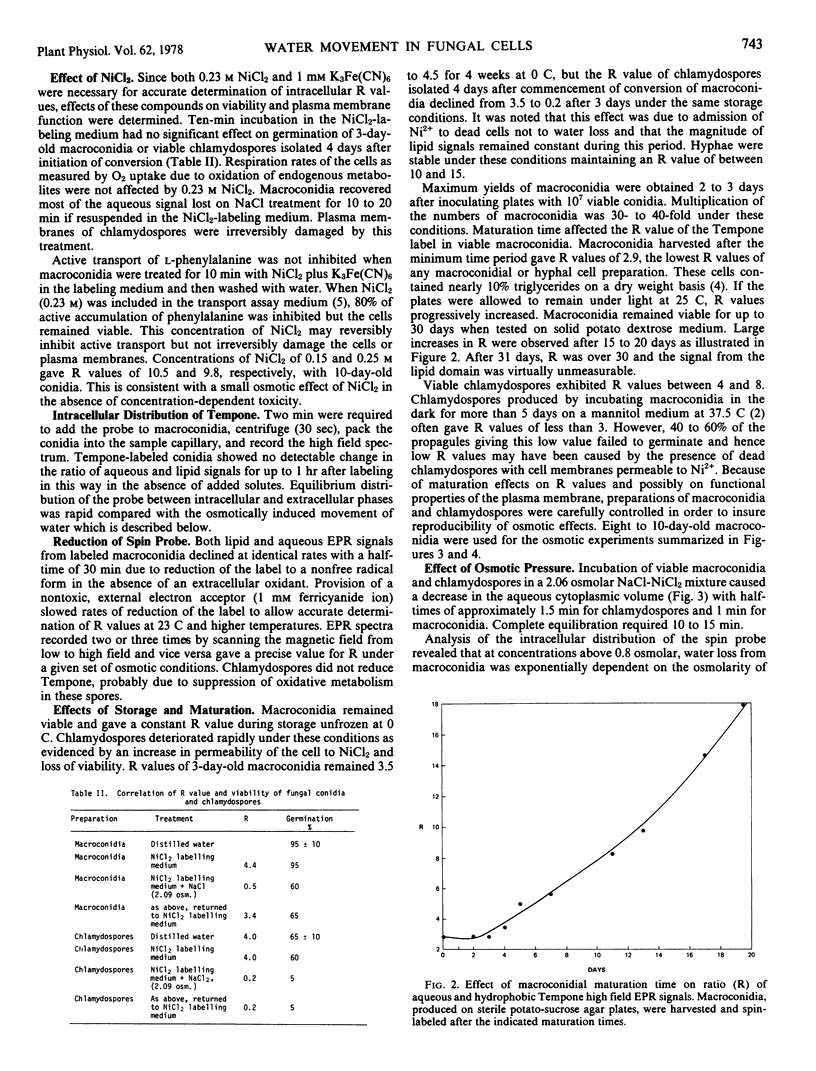
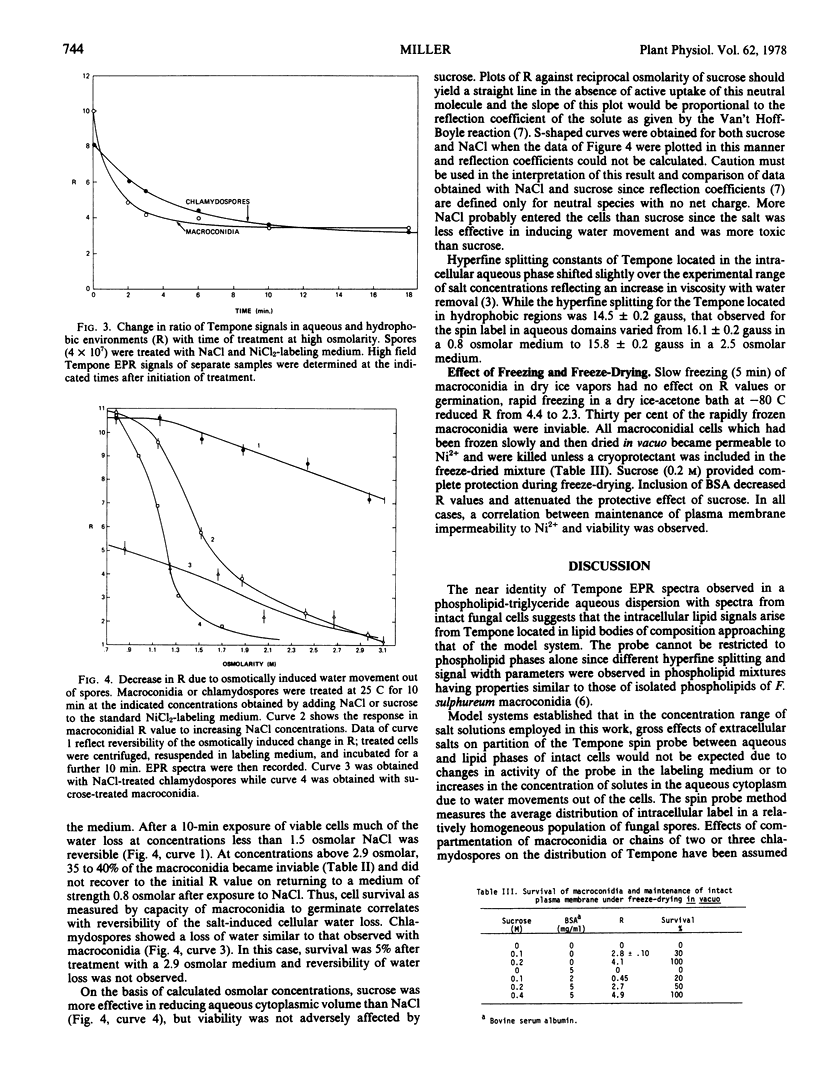
Effects of physical environment on plasma membrane semipermeability and osmotic induction of changes in aqueous cytoplasmic volume were studied in vegetative and spore cells of a plant pathogenic fungus, Fusarium sulphureum. A direct method, employing a spin probe molecule that partitioned between intracellular aqueous and hydrophobic phases, allowed measurement of reversible water movement out of macroconidial cells and chlamydospores exposed to solutions of high osmolarity. Equilibrium distribution of the spin probe between intracellular aqueous and lipid phases was more rapid than movement of water in and out of the cells. The extent of water removal was exponentially dependent on osmotic strength. Some cells became irreversibly permeable to divalent cations on treatment with sodium chloride above 1.5 osmolar but addition of sucrose to the suspension medium at equivalent osmolar concentrations caused water removal without adversely affecting the viability. Sucrose also protected the plasma membrane against damage during freeze-drying. Induction of plasma membrane damage by osmotic shock or freeze-drying permitted rapid permeation of nickel ions. Neither slow equilibration of intracellular components with divalent paramagnetic cations nor partial permeability of damaged plasma membranes to these ions was observed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barran L. R., Schneider E. F., Seaman W. L. Requirements for the rapid conversion of macroconidia of Fusarium sulphureum to chlamydospores. Can J Microbiol. 1977 Feb;23(2):148–151. doi: 10.1139/m77-021. [DOI] [PubMed] [Google Scholar]
- Barran L. R. Transport of phenylalanine by conidia of Fusarium sulphureum. Can J Microbiol. 1976 Sep;22(9):1390–1396. doi: 10.1139/m76-203. [DOI] [PubMed] [Google Scholar]
- Keith A. D., Snipes W. Viscosity of cellular protoplasm. Science. 1974 Feb 15;183(4125):666–668. doi: 10.1126/science.183.4125.666. [DOI] [PubMed] [Google Scholar]
- Miller R. W., Barran L. R. The effect of ionic surface-active agents on macroconidial plasma membrane of Fusarium sulphureum. Can J Microbiol. 1977 Oct;23(10):1373–1383. doi: 10.1139/m77-206. [DOI] [PubMed] [Google Scholar]
- Miller R. W., De La Roche I. A. Properties of spin labelled membranes of Fusarium oxysporum f. sp. lycopersici. Biochim Biophys Acta. 1976 Aug 4;443(1):64–80. doi: 10.1016/0005-2736(76)90491-0. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L., Becker S. A. The fine structure and development of chlamydospores of Fusarium oxysporum. Can J Microbiol. 1972 Jul;18(7):997–1002. doi: 10.1139/m72-155. [DOI] [PubMed] [Google Scholar]


