To the Editor,
Asthma is a common respiratory illness, with approximately 8% lifetime prevalence in the United States (1). In most patients, asthma is characterized by a chronic type 2 inflammatory airway response, accompanied by an increase in pro-phlogistic mediators, eosinophil tissue accumulation, bronchial epithelial mucous cell metaplasia and airway hyperresponsiveness (AHR) (2). Current anti-inflammatory therapies, including corticosteroids and type 2 targeted biologics, can decrease airway inflammation, asthmatic symptoms, and exacerbation frequency (3); however, asthma pathobiology does not fully resolve. Thus, resolution of T2 inflammation and disease modification remain significant unmet therapeutic goals.
Resolution is an active process mediated, in part, by specialized pro-resolving mediators (SPMs) (4, 5). SPMs comprise a family of enzymatically derived mediators from dietary essential polyunsaturated fatty acids that promote resolution via interactions with high affinity cognate receptors with low nanomolar KDs (6, 7). SPMs can regulate type 2 inflammation in human and mouse experimental model systems (6, 8, 9). In some patients, disruption of SPM synthesis is linked to asthma pathogenesis and severity (4). SPMs engage receptors to transduce cell-type specific response. SPM resolvin D2 (RvD2) interacts with the DRV2 receptor (GPR18, ID: 2841) (10), which was recently identified as a potential asthma susceptibility locus (11). RvD2 is a product of DHA metabolism, catalyzed by the sequential actions of 15-lipoxygenase and 5-lipoxygenase (4, 7), with potent counter-regulatory actions for tissue and systemic inflammation (12–14). RvD2 decreases TNFα-induced AHR (15), so together, these findings suggest pro-resolving potential for RvD2-DRV2 signaling in T2 inflammation.
To assess RvD2-DRV2 actions in lung T2 inflammation, BALB/c mice were sensitized and challenged by intratracheal administration of house dust mite (HDM) extract (100 μg) on protocol days 0, 7, and 14. RvD2 (100 ng) was authenticated (Fig-S1), administered intranasally on days 15 and 16, and mice were immunophenotyped on days 17, 19 and 21 (Fig-1A). Compared to vehicle, RvD2 significantly decreased bronchoalveolar lavage (BAL) total cell counts, eosinophils, total macrophages (especially infiltrating macrophages (iMacs) and exudative macrophages (exMacs)), and lymphocytes (including CD4 and CD8 T cells, B cells, and regulatory T cells) (Fig-1B,C and S2A–I). The resolution interval (Ri) for BAL eosinophils, defined as the time to decrease from maximal (Tmax) to half maximal (T50) counts, was >3 days for vehicle, and 1.4 days for RvD2 (Fig-1D), a marked acceleration of resolution by RvD2.
Figure 1. RvD2 promotes resolution of HDM-induced lung inflammation and AHR.
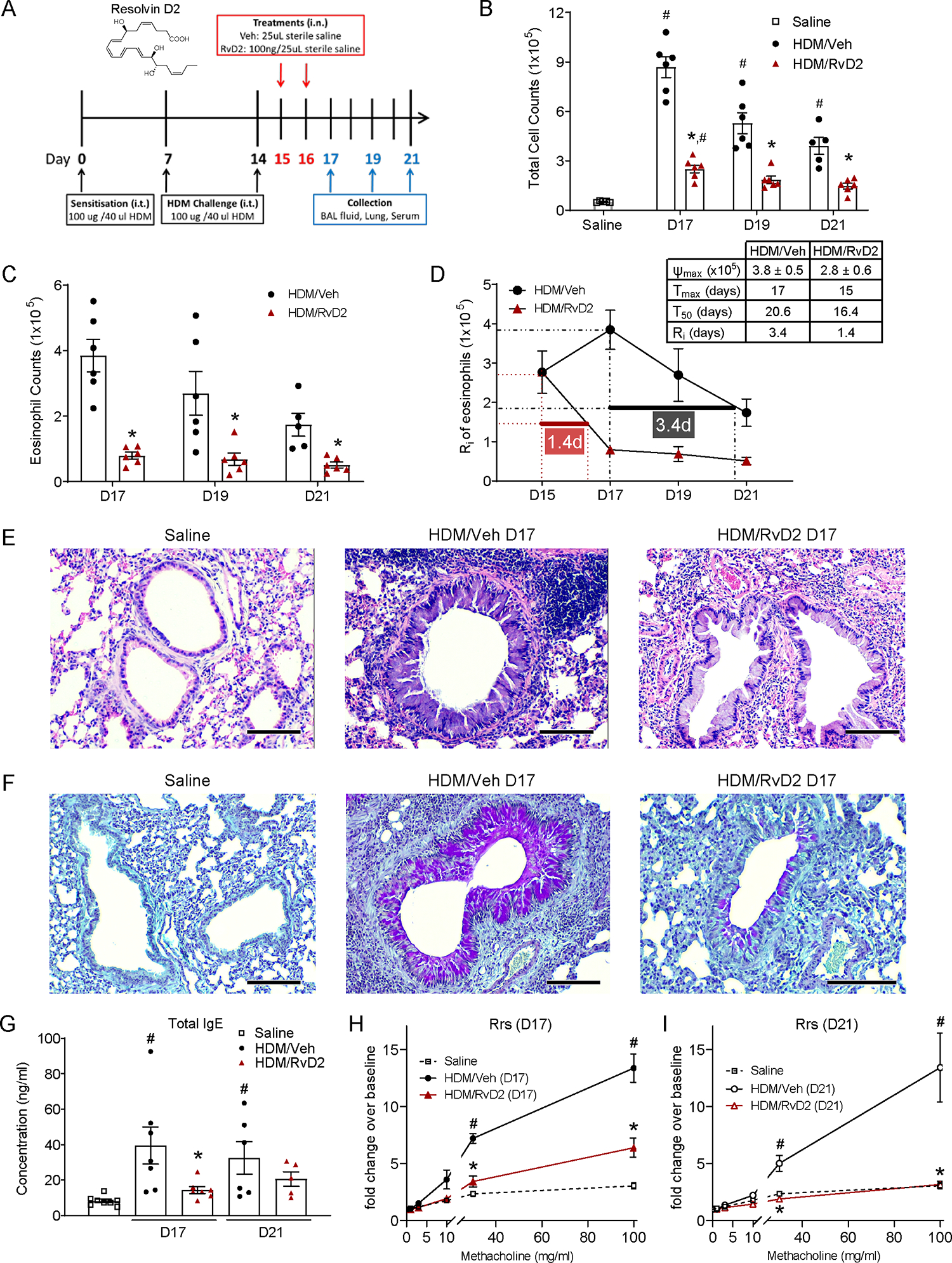
(A) Mice were sensitized, and challenged with HDM, and then given vehicle or RvD2 (100 ng, intranasally). BAL (B) total cells, and (C) eosinophils were enumerated. (D) The resolution interval (Ri) (Tmax to T50) for BAL eosinophils was calculated. (E) Representative H&E- and (F) PAS-stained lung sections are shown (original magnification x100, d17). Scale bar on photomicrographs = 50 μm. (G) Total serum IgE was measured by ELISA. Respiratory resistance (Rrs) was determined after methacholine and expressed as fold change from baseline on (H) d17 and (I) d21. Values are mean ± SEM. (B-D) n=5–6, (G) n=5–8, and (H-I) n=4–5. #p<0.05 compared with saline, *p<0.05 compared with vehicle, by one-way ANOVA (C,G) or two-way ANOVA (B,H-I) followed by Bonferroni’s test for multiple comparisons.
Lung histology showed that RvD2 markedly decreased inflammatory changes to bronchial epithelium, peri-bronchial and perivascular leukocyte infiltration, and airway epithelial mucous cell metaplasia (Fig-1E,F and S2J,K). RvD2 also significantly decreased lung Muc5ac expression (Fig-S2L), and serum total IgE levels (Fig-1G).
Given the changes in lung histology, RvD2’s impact on methacholine evoked AHR was determined. Resistance of the respiratory system (Rrs) with methacholine was significantly attenuated by RvD2 (Fig-1H,I), which returned the methacholine dose response to basal levels of saline control animals by day 21 (Fig-1I). The calculated ED200 for methacholine initiated Rrs is defined as the effective dose of methacholine to increase the Rrs by 200%. On days 17 and 21, HDM decreased the methacholine Rrs ED200, which was significantly increased by RvD2 to near baseline (Fig-S2M). RvD2 decreased Ri for the methacholine Rrs ED200 (time from maximal AHR to the half-maximal response) from 6.9 days with vehicle to 2.4 days (Fig-S2N).
HDM upregulated BAL levels of several pro-inflammatory mediators, including T2 cytokines (IL-4, IL-5 and IL-13), all of which were decreased by RvD2 (Fig-S3A–D), and the increased lung expression of Sele (E-selectin), Icam1 (ICAM-1), Il33 (IL-33), Ccl5 (RANTES), Il13 (IL-13) and Postn (periostin) were significantly attenuated by RvD2 (Fig-S3E).
HDM challenge increased DRV2 expression on BAL leukocytes (CD45+) with significantly increased DRV2 mean fluorescent intensity (MFI) on BAL macrophages (Fig-2A,B). Among the macrophage populations, aMacs have the highest MFI, followed by exMacs and iMacs, respectively (Fig-2C). All BAL leukocytes expressed DRV2 receptor after HDM, with the highest MFI in macrophages and eosinophils (Fig-2B–D). RvD2 did not significantly increase DRV2 expression on BAL leukocytes (Fig-2B–D). To translate to human asthma, DRV2 expression on peripheral blood eosinophils was determined from several asthma patients (n=5) and healthy non-asthmatic participants (n=3). Asthma patients had significantly higher numbers of peripheral blood eosinophils, with markedly higher DRV2 expression (MFI) (Fig-2E). In addition to eosinophils, DRV2 was also expressed on circulating monocytes, neutrophils, CD4 T cells, CD8 T cells, and B cells in humans (Fig-S4, Fig-S5). Given the widespread expression of DRV2 on many cell types, multiple direct and indirect mechanisms are likely engaged given the potent actions of RvD2.
Figure 2. DRV2 expression on HDM-challenged mice and asthmatic patients’ leukocytes, and DRV2-dependence for RvD2 pro-resolving actions in allergic lung inflammation.
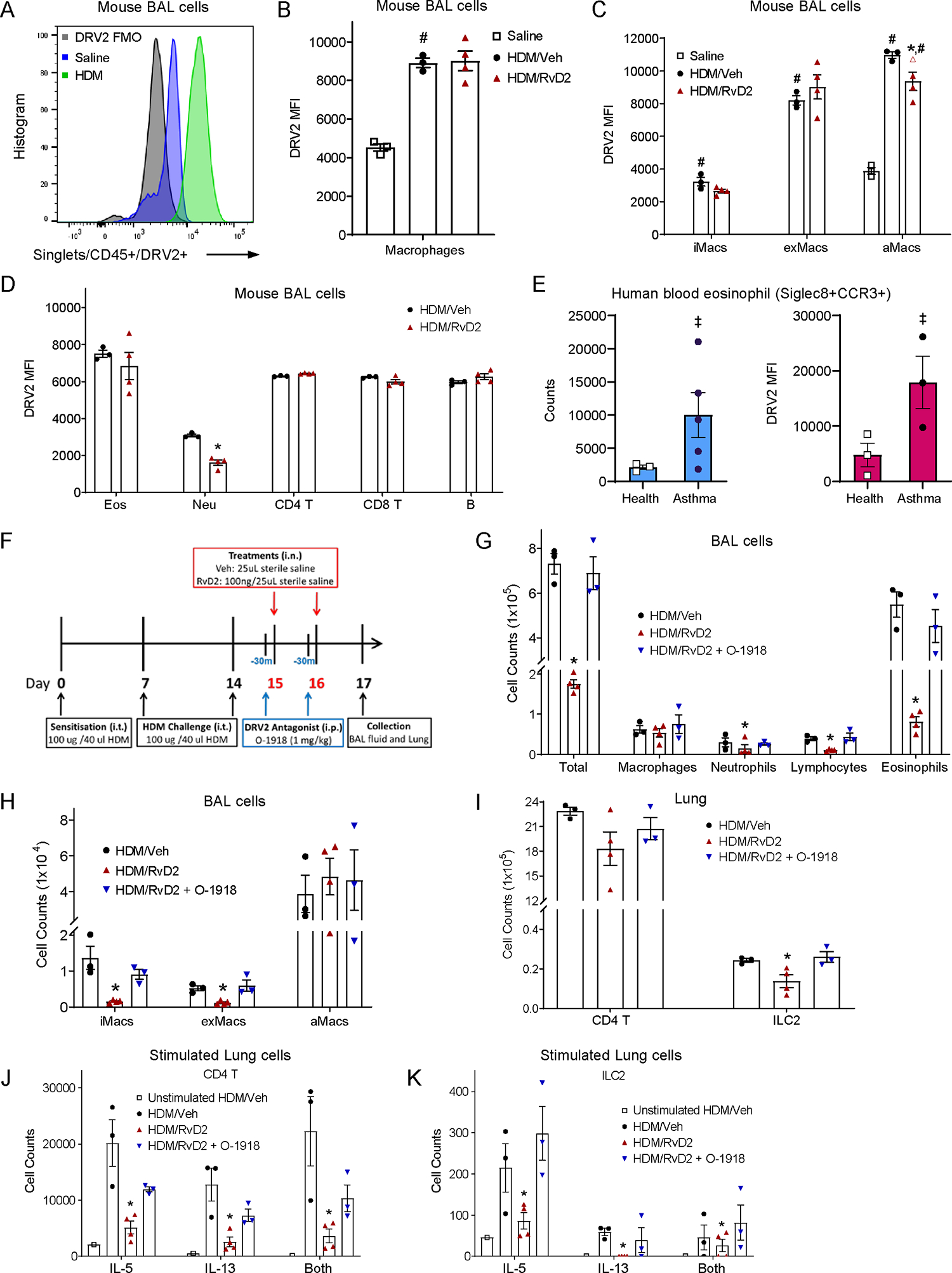
(A) Representative histogram of DRV2 MFI on BAL CD45+ cells after HDM, saline, or with FMO control. (B) DRV2 MFI on BAL macrophages, (C) infiltrating, exudative and alveolar macrophages, (D) eosinophils, neutrophils, CD4 T cells, CD8 T cells, and B cells on d17. (E) Left, eosinophils per 100uL blood in healthy (n=3) and asthma (n=5) participants. Right, DRV2 MFI for eosinophils from healthy (n=3) and asthma (n=3) participants. (F) Schema for intervention with a DRV2 antagonist O-1918 (1 mg/kg, intraperitoneal). (G) BAL total cells, macrophages, neutrophils, lymphocytes, and eosinophils; and (H) infiltrating, exudative and alveolar macrophages were enumerated. (I) Lung CD4 T cells and ILC2 by FACS. (J) Lung IL-5, IL-13, and dual IL-5/13 expressing CD4 T cells and (K) ILC2 upon stimulation. Values are mean ± SEM. (A-D) n=3–4, (E) n=3–5, and (G-K) n=3. #p<0.05 versus saline, *p<0.05 versus vehicle, ‡p<0.05 versus healthy, by two-tailed unpaired Student’s t test (D-E) or one-way ANOVA followed by Bonferroni’s test for multiple comparisons (B-C,G-K).
To determine if RvD2’s actions were DRV2-dependent, O-1918 (1 mg/kg), a competitive antagonist for DRV2, was given 30 minutes before RvD2 on days 15 and 16 (Fig-2F). O-1918 blocked RvD2-induced reductions in BAL total cell numbers (including BAL eosinophils, lymphocytes, neutrophils, iMacs and exMacs), and lung T2 innate lymphoid cells (ILC2) (Fig-2G–I). Upon stimulation of isolated CD4 T cells and ILC2s ex vivo there was increased expression of T2 cytokines (Fig-2J,K). RvD2 in vivo exposure reduced CD4 T cells and ILC2s numbers expressing IL-5, IL-13 or both, and these RvD2 actions were blocked by O-1918 (Fig-2J,K, Fig-S5).
In conclusion, DRV2 receptors are expressed on immune cells linked to asthma pathobiology, and RvD2 accelerated the resolution of mouse T2 inflammation evoked by HDM sensitization and challenge. RvD2 potently regulated T2 cytokine production and action in a DRV2 receptor-dependent manner. The findings here suggest RvD2-DRV2 interactions have the potential to mediate the pro-resolving actions for T2 inflammation required for disease modification of asthma and other “T2-high” allergic diseases.
Supplementary Material
Acknowledgements:
The authors thank Dr. Guangli Zhu for technical support, and Meghan Le for her help with the human samples.
Sources of Funding:
The National Heart, Lung, and Blood Institute R01 HL122531 (B.D.L.) and the National Institute of General Medical Sciences P01 GM095467 (C.N.S., B.D.L.) and R35 GM139430 (C.N.S., R.N.); a postdoctoral fellowship and early career development award from the National University of Singapore (HY.P.).
Footnotes
Competing Interests: B.D.L. is an inventor on patents (SPMs) assigned to Brigham and Women’s Hospital which are managed by the BWH according to conflict of interest policies. The other authors have no conflicts of interest to declare.
REFERENCES
- 1.National Center for Health Statistics. Percentage of current asthma for adults aged 18 and over in United States: National Health Interview Survey; 2021. [Aug 12 2023]. Available from: https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm.
- 2.Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015;1(1):15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brusselle GG, Koppelman GH. Biologic Therapies for Severe Asthma. New England Journal of Medicine. 2022;386(2):157–71. [DOI] [PubMed] [Google Scholar]
- 4.Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. The Journal of Clinical Investigation. 2018;128(7):2657–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins : A Family of Bioactive Products of Omega-3 Fatty Acid Transformation Circuits Initiated by Aspirin Treatment that Counter Proinflammation Signals. Journal of Experimental Medicine. 2002;196(8):1025–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnamoorthy N, Abdulnour R-EE, Walker KH, Engstrom BD, Levy BD. Specialized Proresolving Mediators in Innate and Adaptive Immune Responses in Airway Diseases. Physiological Reviews. 2018;98(3):1335–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy BD, Serhan CN. Resolution of Acute Inflammation in the Lung. Annual Review of Physiology. 2014;76(1):467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 Is Generated in Asthma and Dampens Airway Inflammation and Hyperresponsiveness1. The Journal of Immunology. 2007;178(1):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nature Reviews Immunology. 2016;16(1):51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. Journal of Experimental Medicine. 2015;212(8):1203–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valette K, Li Z, Bon-Baret V, Chignon A, Bérubé J-C, Eslami A, et al. Prioritization of candidate causal genes for asthma in susceptibility loci derived from UK Biobank. Communications Biology. 2021;4(1):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dort J, Orfi Z, Fabre P, Molina T, Conte TC, Greffard K, et al. Resolvin-D2 targets myogenic cells and improves muscle regeneration in Duchenne muscular dystrophy. Nature Communications. 2021;12(1):6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brüggemann TR, Peh HY, Tavares LP, Nijmeh J, Shay AE, Rezende RM, et al. Eosinophil Phenotypes are Functionally Regulated by Resolvin D2 During Allergic Lung Inflammation. American Journal of Respiratory Cell and Molecular Biology. 2023. DOI: 10.1165/rcmb.2023-0121OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaddaj-Mallat R, Sirois C, Sirois M, Rizcallah E, Marouan S, Morin C, et al. Pro-Resolving Effects of Resolvin D2 in LTD4 and TNF-α Pre-Treated Human Bronchi. PLOS ONE. 2016;11(12):e0167058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


