Abstract
Saccharomyces cerevisiae Los1p, which is genetically linked to the nuclear pore protein Nsp1p and several tRNA biogenesis factors, was recently grouped into the family of importin/karyopherin-β-like proteins on the basis of its sequence similarity. In a two-hybrid screen, we identified Nup2p as a nucleoporin interacting with Los1p. Subsequent purification of Los1p from yeast demonstrates its physical association not only with Nup2p but also with Nsp1p. By the use of the Gsp1p-G21V mutant, Los1p was shown to preferentially bind to the GTP-bound form of yeast Ran. Furthermore, overexpression of full-length or N-terminally truncated Los1p was shown to have dominant-negative effects on cell growth and different nuclear export pathways. Finally, Los1p could interact with Gsp1p-GTP, but only in the presence of tRNA, as revealed in an indirect in vitro binding assay. These data confirm the homology between Los1p and the recently identified human exportin for tRNA and reinforce the possibility of a role for Los1p in nuclear export of tRNA in yeast.
In eukaryotic cells, all transport between the nuclear interior and the cytoplasm occurs through the nuclear pore complexes (NPCs) (reviewed in reference 16). According to the data that have accumulated during the last few years, proteins destined to enter the nucleus associate in the cytoplasm with receptors that recognize and bind specific sequences, termed nuclear localization signals (NLSs). These complexes are targeted to the NPC and are translocated into the nucleoplasm, where the import cargo is released and the receptor is recycled to the cytoplasm (reviewed in references 13, 31, 33, 65, and 68). In the case of the basic-type (classical) NLS, the receptor consists of importin α (karyopherin α), the NLS-binding component, and importin β (karyopherin β), which can interact with repeat-containing nucleoporins and is responsible for docking to the NPC. Importin β belongs to a large protein family whose members are characterized by the presence of an amino-terminally located Ran-GTP binding domain (23, 32). Other members of this family include transportin and Kap123p (Yrb4p), which respectively directly bind to some hnRNP proteins and ribosomal proteins, and mediate their nuclear import (24, 72, 79, 83, 96). Similar functions have also been proposed for their homologues Kap104p (1) and karyopherin β3 (105). Recently two more importin β homologues, Mtr10p and Sxm1p, have been shown to function as import receptors for Npl3p (a yeast hnRNP protein) and Lhp1p (the yeast La homologue), respectively (71, 78, 86).
The principles of active nuclear protein import may also apply to active nuclear export of proteins and RNA. Indeed, two members of the importin β family have been shown to be involved in nuclear export processes and were therefore termed exportins (reviewed in reference 102). Export of importin α from the nucleus is mediated by CAS (57), while CRM1 functions as an export receptor for the leucine-rich nuclear export signal (NES) (22, 26, 56, 67, 69, 98). This type of NES can mediate nuclear export of proteins or, as is the case for the human immunodeficiency virus protein Rev, of RNA-protein complexes (for a review, see reference 27). Export of U snRNAs, which requires the cap binding protein complex (50), has been suggested to follow the same route as export of Rev (19). Moreover, a NES-containing receptor has been implicated in the nuclear export of mRNA (70). The M9 domain of hnRNP A1 represents an additional type of NES (48, 62). hnRNP proteins shuttle between the nucleus and the cytoplasm and are required for mRNA export from the nucleus (65). Genetic screens in the yeast Saccharomyces cerevisiae have led to the identification of additional factors that are involved in mRNA nuclear export (2, 16, 54); among them, Nup159p (35), Mtr2p (53), Gle1p (64), Npl3p (60), Mex67p (85), and Dbp5p/Rat8p (97, 101) are candidates for proteins having a direct role in the mRNA export process.
A central role in the nucleocytoplasmic transport machinery is fulfilled by the small GTPase Ran and its effectors (30, 55, 63). Hydrolysis of GTP by Ran may provide the energy required for the translocation of transport complexes through the NPCs. However, recent data suggest that nuclear export of several substrates requires the presence of Ran-GTP in the nucleus (49, 77). Ran-GTP triggers the dissociation of the importin (karyopherin)-import substrate complex (34, 49, 76) while, on the other hand, promoting the association of an exportin with the corresponding export cargo (22, 57). According to these models, the abundance of the Ran-GTP form in the nucleoplasm may be due to the nuclear localization of the Ran nucleotide exchange factor RCC1 (Prp20p in yeast) and the nuclear exclusion of the GTPase-activating protein RanGAP1 (Rna1p in yeast). RanGAP1 and RanBP1 hydrolysis of the Ran-bound GTP may occur then only in the cytoplasm or close to the NPC and may represent the last step of an export reaction, the release of the export substrate. Although there is no evidence for the nucleotide-bound state of Ran in vivo, the in vitro results suggest that the directionality of the nuclear transport processes may be ensured by the distinct subcellular distribution of the components of the Ran system.
tRNA molecules are synthesized by RNA polymerase III as precursors which undergo a complex maturation pathway before they are exported from the nucleus. These sequencial events include trimming of the 5′ and 3′ ends, addition of three terminal CCA residues, modification of a number of nucleosides, and splicing (43). Export of tRNA from Xenopus oocyte nuclei was shown to be carrier mediated (106). Furthermore, structural integrity and maturation of the tRNA molecules are also required for efficient nuclear export (41, 100). For intron-containing pre-tRNAs, it has been suggested that the tRNA-splicing reaction may be coupled to the translocation step, since the key enzymes of splicing, the tRNA endonuclease and tRNA ligase, were found to be localized at the inner side of the nuclear membrane, in close proximity to the nuclear pores (103). It has been also shown that in yeast, several nucleoporin mutants are defective in pre-tRNA splicing as well as in biogenesis of active suppressor tRNA (87, 94), suggesting that pre-tRNA splicing and nuclear export of tRNA require functional nuclear pores. Furthermore, modification of tRNA appears to be also coupled to the nuclear export process (94).
Some aspects of nuclear export of tRNA differ from export of other RNA species. Microinjected tRNA in Xenopus oocyte nuclei is transported into the cytoplasm much faster than other RNA classes, and its nuclear export is not inhibited by homopolymeric RNAs, unlike the export of 5S RNA, mRNA, and U snRNAs (51). Export of tRNA is not affected by antibodies against the nucleoporin Nup98 or by the matrix protein of vesicular stomatitis virus, both of which impair export of other cellular RNAs (42, 73). Concerning the role of Ran in the export of tRNA, the available data are not consistent. While tRNA export was apparently not affected in a mammalian RCC1 mutant in which nuclear export of mRNA and snRNA was inhibited (12), it was recently reported that nuclear export of tRNA from oocyte nuclei requires the presence of Ran-GTP in the nucleus (49). Furthermore, the nuclear export of tRNA from oocyte nuclei can be significantly impaired by injection of dominant-negative importin β mutants (58). Therefore, the tRNA export mechanism may be similar, at least in part, to the mechanisms regulating nuclear export of proteins or other RNAs.
The LOS1 gene was originally identified as a mutant exhibiting a conditional loss of tRNATyr suppressor activity (44). los1 mutants produce reduced levels of functional suppressor tRNAs and accumulate end-trimmed, but unspliced, pre-tRNA, although they apparently contain normal levels of tRNA splicing endonuclease and ligase activities (45, 88, 89). We have recently shown that a significant pool of Los1p is localized at the nuclear pores and that Los1p genetically interacts with the nuclear pore protein Nsp1p as well as with components required for tRNA biogenesis, such as Pus1p, a nuclear tRNA pseudouridine synthase; Tfc4p, a subunit of the tRNA transcription factor TFIIIC; and Arc1p, a cytoplasmic protein that delivers at least two tRNA species to the corresponding aminoacyl-tRNA synthetases (92–94). These data suggested a close relationship between tRNA processing and tRNA transport and indicated that Los1p might be required for the nuclear export step of tRNA biogenesis. Sequence homology searches have shown that Los1p contains an amino-terminal Ran-GTP binding motif that characterizes the importin β protein family (32). In addition, two groups have recently reported the cloning and characterization of a mammalian member of the importin β protein family that can function as an exportin for tRNA (3, 59). This protein also displays significant sequence homology to yeast Los1p.
To gain further insight into the function of Los1p in yeast, we used the two-hybrid system to search for proteins that physically interact with it. The results of this analysis, as well as biochemical data, show that Los1p binds to nucleoporins and to the GTP-bound form of Gsp1p, the yeast Ran. Furthermore, the formation of the Los1p–Gsp1-GTP complex is stimulated in the presence of tRNA, as revealed in an indirect in vitro binding assay. Thus, Los1p appears to be a nuclear export factor for tRNA.
MATERIALS AND METHODS
Yeast strains, media, and plasmids.
The yeast strains used in this work are listed in Table 1. Cells were grown in rich yeast-peptone-dextrose medium or in synthetic dextrose complete medium containing the necessary amino acids and nutrients. For counterselection of URA3- or CYH2-containing plasmids, 5-fluoroorotic acid (5-FOA; Toronto Research Chemicals) or cycloheximide (Sigma) was used, respectively. Test plates for the two-hybrid system contained SDC-Trp-Leu-His plus 100 mM NaPi (pH 7.0), 15 to 25 mM 3-aminotriazole (3AT; Sigma), and 65 mg of 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (X-Gal; Biomol) per liter. Yeast cells were transformed by the lithium acetate method (80). Genetic manipulations, including mating, sporulation, and tetrad dissection of yeasts, were performed as described elsewhere (90). The following yeast plasmids were used: pUN100 (17), pRS313 (91), pRS316 (91), YCplac111 (29), pAS2(pAS1-CYH2) (40), and pACTII (40). YEp13-GAL10-CYC1 was constructed by inserting the GAL10-CYC1 hybrid yeast promoter, cut as an EcoRV-BamHI fragment from vector pLGSD5 (39), between the unique PvuII and BamHI sites of YEp13. Plasmid pEMBLyex4-ATG was constructed as follows: the sequence between the XhoI and BamHI sites of plasmid pEMBLyex4 (11), containing the CYC1 promoter, was replaced by the corresponding sequence of plasmid YEp13-GAL10-CYC1, which contains the CYC1 promoter followed by an ATG start codon.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| RS453 | MATa/MATα ade2/ade2 his3/his3 leu2/leu2 trp1/trp1 ura3/ura3 | R. Serrano |
| Y190 | MATa gal4 gal80 ade2 his3 leu2 trp1 ura3 URA3::GAL-lacZ LYS2::GAL(UAS)-HIS3 cyh2 | 40 |
| Y547 | MATα ade2 his3 leu2 trp1 ura3 los1::HIS3 | 94 |
| Y680 | MATα ade2 his3 leu2 trp1 ura3 los1::HIS3 pus1::HIS3 | 94 |
| Y748 | MATα ade2 his3 leu2 trp1 ura3 los1::HIS3 arc1::HIS3 + pHT4467-LOS1 (CEN ARS ADE3 URA3) | 94 |
| YKH44 | MATa/MATα ade2/ade2 his3/his3 leu2/leu2 trp1/trp1 ura3/ura3 gcd11::HIS3/GCD11 | This study |
| YKH45 | MATa ade2 his3 leu2 trp1 ura3 gcd11::HIS3; pRS316-GCD11 (CEN ARS URA3) | This study |
| YKH53 | MATa ade2 his3 leu2 trp1 ura3 los1::HIS3 gcd11::HIS3; pRS316-GCD11 (CEN ARS URA3) | This study |
| YKH64 | MATα ade2 his3 leu2 trp1 ura3 gsp1::HIS3; pRS316-GSP1 (CEN ARS URA3) | This study |
| YMO106 | MATa/α his3/his3 leu− trp− ura3 gsp1::HIS3/GSP1 | 52 |
Two-hybrid screen.
Cells of Y190(pAS2-LOS1) (100 ml; see below) grown in SDC-Trp to an optical density at 600 nm (OD600) of 1.0 were transformed with 50 μg of library DNA. With the help of glass beads, transformants were spread on four plates (25 cm by 25 cm) of SDC-Trp-Leu-His plus 25 mM 3AT that had been covered with nitrocellulose filters; the plates were then incubated for 7 days at 30°C, and the resultant colonies were then transferred to SDC-Trp-Leu-His plus 25 mM 3AT and X-Gal and incubated for 3 days at 30°C. The theoretical number of transformants was calculated by plating an aliquot of the cell suspension on SDC-Trp-Leu. In the first screen, an S. cerevisiae cDNA library in pACT (40) yielded 3.0 × 106 transformants, of which 18 colonies grew and turned blue on the test plates. In a second screen, an S. cerevisiae genomic library in pACTIIStopBis (25) yielded 6.4 × 106 transformants, of which 25 were positive. Blue colonies were restreaked on SDC-Leu (additionally containing 2.5 mg of cyclohexamide per ml to select against the CYH2 marker on the pAS2 plasmid) and on SDC-Trp (the loss of the pACTII plasmid was verified by restreaking on SDC-Trp-Leu). Plasmids from those colonies which no longer showed β-galactosidase activity on X-Gal-containing plates were recovered by glass bead lysis and transformation of Escherichia coli MC1061. Y190(pAS2-LOS1) was retransformed with the recovered plasmid DNA and again tested for β-galactosidase activity. Two and 15 clones from the first and second screens, respectively, passed all false-positivity tests.
Two-hybrid interaction assay.
The DNA fragments of interest were PCR amplified, using genomic copies of LOS1 and NUP2 in plasmids pUN100-LOS1 (94) and pJON37 (61) as the template, respectively. The GSP1 open reading frame (ORF) was amplified from genomic DNA, while the GSP2 ORF was amplified from pUN100-GSP2 (55a). The PCR products were cut with NcoI and BamHI, ligated into the GAL4 DNA binding domain vector pAS2, and then subcloned into the GAL4 activation domain vector pACTII. Yeast strain Y190 was transformed with each of these plasmids, and expression of the resulting Gal4p-hemagglutinin fusion proteins was checked by Western blotting with the monoclonal mouse antibody 12CA5, produced against the hemagglutinin epitope tag. Colonies that contained both bait and prey plasmids were tested on SDC-Trp-Leu-His plus 15 mM 3AT and X-Gal. Development of a blue color was scored after 3 to 4 days. To quantify the β-galactosidase activity, an o-nitrophenyl-β-d-galactopyranoside (ONPG) liquid assay was used. Briefly, the yeast cells in 0.5 to 1 ml of a liquid culture in SDC-Trp-Leu at an OD600 of 0.5 to 1 were harvested by centrifugation, incubated in 500 μl of Z buffer (100 mM NaPi [pH 7.2], 10 mM KCl, 1 mM MgSO4) with 0.36% β-mercaptoethanol, permeabilized with 200 μl of water-saturated diethyl ether, and incubated with 100 μl of a 4-mg/ml solution of ONPG (Sigma). The reaction was stopped by addition of 250 μl of 1 M Na2CO3. The activity (per milliliter per minute) was calculated with the equation 1,000 × (OD420/OD600 × V × t), where V is culture volume and t is reaction time.
Mutation of genes and construction of shuffle strains.
The GCD11 gene was cloned into pRS316 as a HindIII-EcoRI fragment from pUN100 GCD11, which contains a 2.1-kb PCR-amplified HindIII-SnaBI fragment, using genomic DNA as a template. For disruption of the whole GCD11 ORF, a direct gene deletion method was used (4). A heterozygous diploid (RS453 derivative) harboring the correct gcd11::HIS3 disruption at the GCD11 gene locus was verified by PCR. The positive strain (YKH44) was sporulated. A 2:2 segregation pattern for cell growth was observed in a tetrad analysis. To construct a GCD11 shuffle strain, YKH44 was transformed with pRS316-GCD11 and sporulated. A His+ Ura+ FOA− spore (YKH45) of a 4:0 tetrad was chosen as the shuffle strain. This strain was mated to Y547 (los1::HIS3) in order to obtain YKH53 (los1::HIS3 gcd11::HIS3) containing pRS316-GCD11, which was then transformed with the plasmids Ep552 (pSB32-GCD11), Ep653 (pSB32 gcd11-G397A), Ep620 (pSB32 gcd11-R510H), and Ep654 (pSB32 Ty525), which were kindly provided by E. Hannig (15). The GSP1 gene was excised from plasmid YEp352-GSP1 (5) as a 3.2-kb SalI-SacI fragment and ligated into pRS316. To construct a GSP1 shuffle strain, the heterozygous diploid strain YMO106 harboring a gsp1::HIS3 disruption (52) was transformed with pRS316-GSP1 and sporulated. A His+ Ura+ FOA− spore (YKH64) of a 4:0 tetrad was chosen as the shuffle strain. The gsp1::HIS3 disruption in YKH64 was verified by PCR. A GAL1-driven version of GSP1(G21V) was constructed based on plasmid pGPCNR1 (52), which contains a wild-type GSP1 BamHI-HindIII fragment. A version of this fragment coding for Gsp1(G21V) was generated by a two-step PCR procedure (28) (mutagenizing primer, 5′-CTTACCAGTACCAACATCACCGACAAG-3′) and inserted into the corresponding sites in YEp352GAL (6). The mutation was verified by nucleotide sequence analysis.
Construction and affinity purification of fusion proteins.
A DNA fragment coding for two immunoglobulin G (IgG) binding domains of protein A (ProtA), under the control of the NOP1 promoter, followed by a TEV proteolytic cleavage site (ENLYEQG) was fused to the 5′ end of the LOS1 gene, yielding plasmid pUN100-NOP1::ProtA-TEV-LOS1, as will be described elsewhere (41a). The ORF of GSP1 or the GSP1-G21V mutant version was PCR amplified from YEpCNR1 (52) or YEp352GAL-GSP1-G21V, respectively, and was used to generate pUN100-NOP1::ProtA-TEV-GSP1::ADH1(terminator) and pUN100-NOP1::ProtA-TEV-GSP1-G21V::ADH1(terminator). The GSP1 shuffle strain was transformed with one of these plasmids and tested for growth on SDC-FOA. Affinity purification by IgG-Sepharose chromatography was done as described earlier (95), with modifications, and elution from the IgG-Sepharose column was performed with recombinant TEV protease. Typically, 10 g of spheroplasts was lysed in 50 ml of a lysis buffer containing 150 potassium acetate, 20 mH HEPES-KOH (pH 7.0), 2 mM magnesium acetate 0.1% Tween 20, and a cocktail of protease inhibitors (Boehringer Mannheim). The crude cell extract was then centrifuged at 25,000 × g for 10 min at 4°C. The supernatant was applied onto a column packed with 250 μl of IgG-Sepharose beads (Pharmacia). The beads were extensively washed with lysis buffer and equilibrated with 1 ml of a cleavage buffer containing 150 mM potassium acetate, 20 mH HEPES-KOH (pH 7.0), 0.5 mM EDTA, and 1 mM dithiothreitol and transferred onto a spin column (MoBiTec). One volume of beads was mixed with one volume of cleavage buffer, and 100 U of TEV protease was added. The mixture was incubated for 2 h at 16°C on a rotating wheel prior to removal of the eluate by a short centrifugation. The elution was completed with an additional bed volume of cleavage buffer.
Overexpression of LOS1.
To construct an amino-terminal truncation of Los1p, a fragment of LOS1 (codons 195 to 425) was PCR amplified, cut with PstI and XbaI, and ligated to a BamHI-PstI NOP1::ProtA cassette and an XbaI-BamHI fragment of pUN100-LOS1, which contained the rest of the LOS1 gene. The coding sequences for ProtA-Los1p and ProtA-Los1ΔNp were removed as SphI fragments from the vectors pUN100-NOP1::ProtA-LOS1 and pUN100-NOP1::ProtA-LOS1ΔN, respectively, blunt ended, and inserted downstream of the strong and inducible GAL10-CYC1 hybrid promoter of BamHI-cut and blunt-ended YEp13-GAL10-CYC1, creating plasmids YEp13-GAL-ProtA-LOS1 and YEp13-GAL-ProtA-LOS1ΔN, respectively. Wild-type yeast cells carrying these plasmids were allowed to grow in raffinose-containing medium before induction by dilution in medium containing 2% galactose. To obtain higher levels of LOS1 expression, the coding sequences for ProtA-Los1p and ProtA-Los1ΔNp were also individually subcloned as BamHI fragments into vector pEMBLyex4-ATG. This vector contains as a selectable markers URA3 and the poorly expressed leu2-d allele of LEU2, which increases the copy number of the plasmid under leucine selection conditions (14).
Green fluorescent protein (GFP) tagging and localization of proteins.
pRS314 carrying an in-frame YRB1-GFP(S65T) fusion was constructed by introducing a unique BamHI site before the translation termination codon of a chromosomal EcoRI-XbaI YRB1 fragment on pRS314 by a single-step PCR. Insertion of a BamHI-GFP(S65T) cassette (85) into this BamHI site yielded plasmid pRS314-YRB1-GFP(S65T). Tagging of MEX67 and NPL3 with GFP was described previously (85, 86). The plasmid expressing NLS-GFP-lacZ was kindly provided by G. Schlenstedt, and the plasmid expressing GFP-NOP1 was provided by B. Senger. Amino-terminal tagging of Pus1p with GFP was done by subcloning the PUS1 ORF as a PstI fragment, cut from pUN100-ProtA-PUS1 (94), into pRS315 containing the NOP1::GFP cassette (41a). All GFP-tagged proteins were functional because they could complement the corresponding mutant yeast strains (data not shown).
The localization of GFP-tagged proteins in living cells was examined in the fluorescein channel of a Zeiss Axioskop fluorescence microscope. Pictures were obtained with a Xillix Microimager charge-coupled device camera and processed with Improvision Openlab 1.7 software.
Purification of E. coli recombinant proteins.
The cloning of the LOS1 and MTR10 ORFs into the E. coli expression vector pET8c has been described previously (86, 94). To produce only the amino-terminal part of Los1p (amino acids 1 to 563), the ORF downstream of the internal SpeI site was deleted by cutting pET-LOS1 with SpeI-MluI and inserting at the blunt-ended sites a palindromic 29-mer oligonucleotide that contains stop codons in all six frames and a BamHI site in the middle. The vectors containing the fusion genes were transformed into E. coli BL21 cells. Cultures (500 ml each) were grown in minimal medium at 37°C to an OD600 of 0.7, shifted to 25°C, and induced by addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). The bacterial cell pellet was lysed by sonication in 10 ml of a solution containing 50 mM Tris-Cl, 100 mM NaCl, 10 mM MgCl2, 5% glycerol, and 5 mM β-mercaptoethanol (pH 7.5). The lysate was cleared by ultracentrifugation (Beckman SW40 rotor, 35,000 rpm, 45 min) and applied onto a nickel-nitrilotriacetic acid (Ni-NTA) resin (QIAGEN, Hilden, Germany) column. Bound proteins were eluted with 200 mM imidazole in lysis buffer and, when necessary, dialyzed against a solution consisting of 20 mM Tris-Cl, 100 mM NaCl, and 1 mM dithiothreitol (pH 7.4). Los1p and Mtr10p were further purified by chromatography on a Mono Q HR 5/5 column (Pharmacia) equilibrated in the same buffer and eluted with an NaCl gradient. The expression and purification of Rna1p and Kap95p were done as described previously (9, 34). The concentrations of the recombinant proteins in partially purified preparations were determined by comparing the intensities of the Coomassie-stained bands and using bovine serum albumin as a marker.
Enzymatic Gsp1p-GTP binding assays.
GTPase assays were carried out as described elsewhere (8, 10, 83). Briefly, 50 pM Gsp1p-[γ-32P]GTP was incubated in a 50-μl volume with the corresponding Gsp1p-binding protein in incubation buffer. The GTPase reaction was started by addition of 20 nM Rna1p. After a 2-min incubation at 25°C, released [32P]phosphate was determined by the charcoal assay (8). Total yeast tRNA and tRNAPhe (Sigma) were used after purification by phenol extraction and ethanol precipitation. 5S rRNA was from Boehringer Mannheim.
Antibodies.
The following antisera were used in this study: anti-Nsp1p (EC9-1), which cross-reacts with FXFG repeat-containing nucleoporins like Nup1p and Nup2p and was originally raised against an insoluble nuclear fraction (46); anti-Los1p, which was raised against the His6-tagged protein produced in E. coli (94); and anti-Gsp1p, which was a gift of J. Becker (MPI für Molekulare Physiologie, Dortmund, Germany). The preparation of a rabbit polyclonal anti-Yrb1p antiserum will be described elsewhere (56a).
RESULTS
Nup2p and Gcd11p were found in a two-hybrid screen with Los1p as the bait.
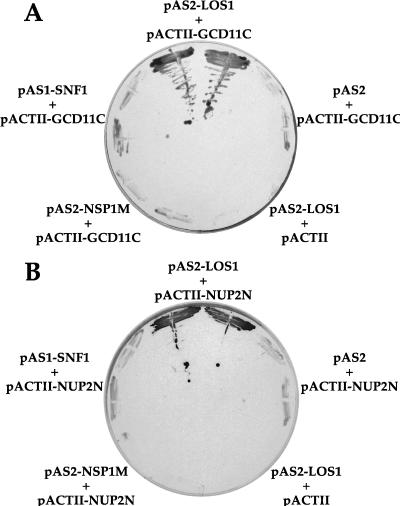
To identify possible binding partners of Los1p, we utilized the two-hybrid system, with full-length LOS1 (attached to the GAL4 DNA binding domain) as the bait. After transformation with a yeast cDNA library fused to the GAL4 activation domain, two clones that showed activation of both reporter genes, HIS3 and lacZ, and passed all false-positivity tests were isolated (see Materials and Methods). Sequence analysis of the inserts revealed that both corresponded to GCD11. In a second screen, using another yeast genomic library, positive clones which contained genomic inserts encoding GCD11 or NUP2 were isolated. The inserts isolated in the screen correspond to the amino-terminal region of Nup2p (amino acids 42 to 177) or three fragments of the carboxy-terminal part of eIF-2γ (amino acids 320 to 527, 329 to 527, and 368 to 527), which is encoded by GCD11. eIF-2γ is the γ subunit of the eukaryotic protein translation initiation factor 2, which is responsible for binding to the charged initiator tRNAMet and delivering it to the small ribosomal subunit. Nup2p, like Nsp1p and Nup1p, is a nuclear pore protein containing FXFG-type repeat sequences. The two-hybrid interactions were specific since only the combination of the LOS1 bait plasmid with the GCD11 or the NUP2 prey plasmid allowed growth and development of a blue color on the test plates (Fig. 1 and Table 2). Neither LOS1, GCD11, nor NUP2 alone activated transcription of the reporter genes; additionally, GCD11 or NUP2 in combination with an irrelevant bait plasmid did not activate transcription of these genes.
FIG. 1.
Two-hybrid interactions of Los1p. Los1p interacts with Gcd11p (A) and Nup2p (B). The yeast screening strain Y190 was transformed with the indicated bait (pAS2) and prey (pACTII) plasmids in various combinations, and in each case two independent transformants were tested for growth (His+) and β-galactosidase activity (LacZ+) on plates of SDC-Trp-Leu-His plus 3AT and X-Gal. Only in the presence of both pAS2-LOS1 as bait and pACTII-GCD11C (codons 368 to 527) or pACTII-NUP2N (codons 42 to 177) as prey were blue-colored yeast cells found growing on selective plates. The bait plasmids pAS1-SNF1 (40) and pAS2-NSP1M (codons 273 to 564 of NSP1) (3a) served as negative controls.
TABLE 2.
Quantification of the two-hybrid interaction between Los1p and Nup2p, Gcd11p, Gsp1p, or Gsp2p with LOS1 baita
| Activity with LOS1 bait and prey plasmid:
| |||||
|---|---|---|---|---|---|
| NUP2N (42–177) | GCD11C (368–527) | GSP1 | GSP2 | SNF4 | No insert |
| 23 | 1.9 | 0.70 | 0.73 | 0.01 | 0.03 |
β-Galactosidase activities of yeast strain Y190(pAS2-LOS1), which contained the indicated prey plasmids (pACTII derived), were measured by the ONPG liquid assay (see Materials and Methods). For each combination, the average of data for at least three independent transformants was calculated. Prey plasmids pACT-SNF4 and pACTII without inserts served as negative controls. Activity, calculated by the equation 1,000 × (OD420/OD600 × V × t), is expressed in milliliters per minute.
Because of the sequence homology between the amino-terminal region of Los1p and the Ran-GTP binding domain of importin β, we included Gsp1p and Gsp2p, the two yeast Ran homologues, in the two-hybrid analysis. Los1p exhibited a weaker but specific two-hybrid interaction with both Gsp1p and Gsp2p, which is seen in a quantitative β-galactosidase assay (Table 2).
GCD11 genetically interacts with LOS1.
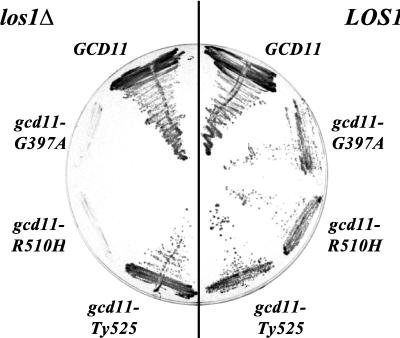
The interaction between Los1p and the carboxy-terminal part of eIF-2γ in the two-hybrid system was unexpected. To find out whether these two proteins also functionally interact, we checked for a synthetic-lethal relationship. Various mutations in GCD11, the gene coding for the yeast translation initiation factor subunit eIF-2γ, have been described previously (15). While four of these mutants show severe growth defects and were therefore not suitable for the plasmid shuffling assay, three of the mutants map in the carboxy-terminal region of Gcd11p and grow well under permissive conditions. These alleles, gcd11-G397A and gcd11-R510H, which contain point mutations in codons 397 and 510, respectively, and gcd11-508, which contains a Ty insertion after the second-to-last codon, 525 (and therefore is referred to here as gcd11-Ty525), were combined with a los1::HIS3 disruption. While the wild-type control and the Ty insertion mutant still grew at normal levels in the absence of Los1p, both point mutants failed to grow in a los1Δ background (Fig. 2, left). This synthetic-lethal phenotype is specific, since the nongrowing strains could be rescued by cotransformation with a single-copy plasmid that contains the LOS1 wild-type gene (Fig. 2, right). This is indicative of a genetic interaction between Los1p and the carboxy-terminal region of Gcd11p, which is reported to be part of the tRNA binding domain and which also interacts with Los1p in the two-hybrid system (see also discussion).
FIG. 2.
Genetic interaction between LOS1 and GCD11. Shuffle strain YKH53 (los1::HIS3 gcd11::HIS3) containing pRS316-GCD11 was transformed with various GCD11 alleles on a LEU2 plasmid and then streaked on 5-FOA-containing medium, on which cells with the URA3 plasmid, which contains the GCD11 wild-type gene, were selected against. The GCD11 point mutations G397A and R510H, combined with a los1::HIS3 disruption, led to synthetic lethality (left), which was overcome by cotransformation with a LOS1 wild-type gene on a TRP1 plasmid (right).
Nsp1p, Nup2p, and Gsp1p-GTP associate with Los1p.
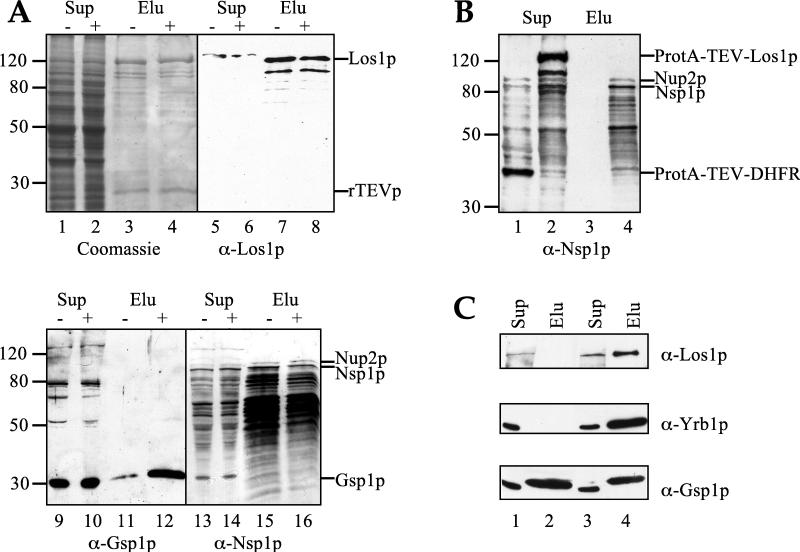
To confirm the interaction between Los1p and nucleoporins such as Nup2p in an independent way, Los1p was purified from yeast by affinity chromatography and analyzed for copurifying components. For this purpose, full-length Los1p was amino-terminally tagged with two IgG-binding domains of Staphylococcus aureus protein A followed by a proteolytic cleavage site for the TEV protease. The ProtA-TEV-LOS1 construct was able to complement the thermosensitive Los1− Pus1− double mutant and the synthetic-lethal Los1− Arc1− double mutant and thus was functional (data not shown). Furthermore, to test whether the GTP-bound form of Gsp1p would affect association of Los1p with other cellular proteins, the ProtA-TEV-Los1p-producing strain was cotransformed with a plasmid that expresses the mutant form Gsp1p-G21V. This mutation (a substitution of glycine for valine at position 21) stabilizes the GTP-bound form of Gsp1p and inhibits both nuclear protein import and mRNA export (82). For these experiments, the GSP1-G21V gene was controlled by the galactose-inducible GAL promoter. We prepared extracts from cells that do not produce the mutant form of Gsp1p, by growing them in glucose, and from cells in which the expression of the dominant-negative mutant GSP1-G21V was induced, by shifting the cells from glucose to galactose medium for 7 h. Each extract was passed through an IgG-Sepharose column, and the bound proteins were eluted by incubation with the TEV protease. Analysis of the eluates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and protein staining showed some weaker bands, in addition to the 125-kDa band that corresponds to Los1p, but did not reveal major differences between the two eluates (Fig. 3A, lanes 3 and 4). Furthermore, none of the major visible bands were specific for the Los1p purification, since they could also be observed when a control protein (ProtA-TEV-DHFR, with the last component being mouse dihydrofolate reductase) was purified under the same conditions (data not shown).
FIG. 3.
Affinity purification of Los1p and Gsp1p. (A) Cells expressing ProtA-TEV-LOS1 and carrying the plasmid YEp352GAL-GSP1-G21V were grown in the absence (−) or presence (+) of galactose. After lysis, the soluble proteins (supernatants [Sup]) were passed over an IgG-Sepharose column, to which ProtA-TEV-Los1p and associated proteins bound. Los1p was then eluted from the column by incubation with recombinant TEV protease, which cleaves and removes the protein A tag. Supernatants and eluates (Elu) were analyzed by SDS-PAGE and Coomassie staining (lanes 1 to 4) or by Western blotting with the indicated antibodies (lanes 5 to 16). The eluates were shown to contain Los1p, Gsp1p, Nsp1p, and Nup2p. The amount of Gsp1p in the eluate was increased when the cells were grown in the presence of galactose and, therefore, expressed the GSP1-G21V mutant form. (B) Soluble proteins (supernatants) derived from cells expressing ProtA-TEV-DHFR (lanes 1 and 3) or ProtA-TEV-LOS1 (lanes 2 and 4) were applied onto an IgG-Sepharose column, and bound proteins were eluted by treatment with TEV protease. The material loaded on the column (Sup) and the eluates (Elu) were analyzed by Western blotting with anti-Nsp1p antibodies. In the two soluble-protein preparations (lanes 1 and 2), the antibody recognized, in addition to Nup2p and Nsp1p, the protein A tag of Los1p and DHFR. Note that Nup2p and Nsp1p specifically copurified with Los1p and were present in the eluate derived from the ProtA-TEV-Los1p-expressing cells (lane 4) but absent from the eluate from Prot-TEV-DHFR-expressing cells (lane 3). (C) Soluble extracts derived from cells expressing ProtA-TEV-GSP1 (lanes 1 and 2) or ProtA-TEV-GSP1-G21V (lanes 3 and 4) were passed through an IgG-Sepharose column, and bound proteins were eluted by TEV-mediated proteolytic cleavage. The SDS-PAGE-separated extracts (Sup) and the eluates (Elu) were probed with anti-Los1p, anti-Yrb1p, and anti-Gsp1p antibodies. Only the relevant parts of the blots are shown. In the extracts (lanes 1 and 3), equal amounts of Los1p, Yrb1p, and chromosomally encoded Gsp1p were evident. The eluate derived from ProtA-TEV-GSP1-expressing cells contained only Gsp1p (lane 2), while the eluate derived from ProtA-TEV-GSP1-G21V-expressing cells contained, besides Gsp1p-G21V (which migrates slower than native Gsp1p, due to the small amino-terminal addition present after TEV cleavage), also Los1p and Yrb1p (lane 4).
To look for specifically copurifying polypeptides that might not be readily detectable by protein staining, we probed the eluates with antibodies produced against the proteins that were suggested by the two-hybrid data to interact with Los1p. Using an antibody against Gsp1p, we detected very little Gsp1p in the eluate derived from cells grown in glucose (Fig. 3A, lane 11). This weak interaction could be due to Los1p preferentially interacting with Gsp1p-GTP and Gsp1p existing mainly in the GDP-bound form after cell lysis. Indeed, the amount of Gsp1p that copurified with Los1p was significantly increased in the eluate derived from cells that were grown in galactose and, therefore, overproduced the GTP-bound form of Gsp1p (Fig. 3A, compare lanes 11 and 12). We also probed the eluates with an antibody which had been raised against Nsp1p but also cross-reacted with Nup2p. By Western blotting, we could detect Nup2p, Nsp1p, and many Nsp1p degradation bands in the eluates (Fig. 3A, lane 15). Nsp1p is very susceptible to degradation during purification, resulting in many degradation products (37). The identity of the Nup2p band was confirmed by probing with the same antibody extracts, derived from a strain in which the NUP2 gene had been disrupted (data not shown). Similar amounts of Nsp1p and Nup2p copurified with Los1p also when the dominant-negative Gsp1p-G21V was expressed in the yeast cells (Fig. 3A, lane 16). To show that the presence of Nsp1p and Nup2p in the eluates reflects a specific association of these two nucleoporins with Los1p, we prepared extracts from cells expressing ProtA-TEV-LOS1 or ProtA-TEV-DHFR, passed them through an IgG-Sepharose column, and eluted them with TEV protease under identical conditions. Both Nsp1p and Nup2p copurified with Los1p, but they were completely absent from the eluate derived from cells expressing the DHFR control protein (Fig. 3B, compare lanes 3 and 4).
To corroborate the interaction of Los1p with Ran-GTP, Gsp1p was amino-terminally tagged with ProtA-TEV and expressed under the control of the constitutive NOP1 promoter. The ProtA-TEV-GSP1 construct could complement the lethal gsp1 disruption mutant (data not shown). Another construct, ProtA-TEV-GSP1-G21V, was not able to complement the Gsp1− strain, but it could be expressed in a GSP1+ strain without causing a dominant-negative effect because, as revealed by Western blotting with anti-Gsp1p antibodies (data not shown), its level of expression, driven from the constitutive NOP1 promoter and a centromeric plasmid, was relatively low. Therefore, ProtA-TEV-GSP1 and ProtA-TEV-GSP1-G21V were both expressed in GSP1+ strains and affinity purified by IgG-Sepharose chromatography. Gsp1p and Gsp1p-G21V were eluted by TEV cleavage and analyzed for associated proteins. Western blot analysis revealed that binding of Yrb1p and Los1p to Gsp1p-G21V, which is blocked in the GTP-bound form, is strongly stimulated compared to that of wild-type Gsp1p (Fig. 3C, lanes 2 and 4). Increased binding of Yrb1p to Gsp1-G21V was also described earlier (84). Thus, binding of Los1p to the GTP-bound form of Gsp1p occurs preferentially.
Overexpression of LOS1 impairs nuclear export.
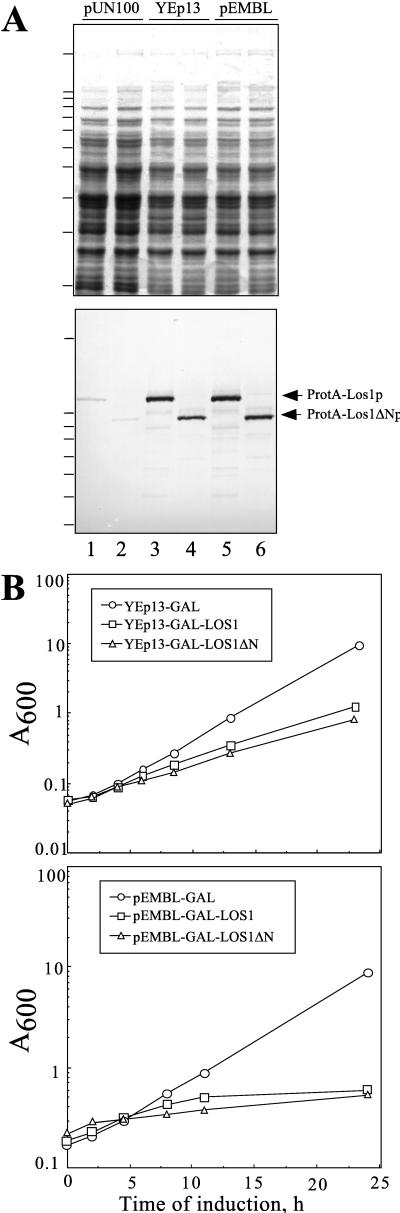
The binding of Los1p to Gsp1-GTP and its association with the nuclear pore complexes indicate that Los1p participates in nuclear transport processes. However, a disruption mutant of LOS1 was viable and was not impaired in nuclear import of NLS reporter proteins or in nuclear mRNA export (94). Therefore, we tested whether overproduction of Los1p or a truncated form, Los1ΔNp, that lacks the amino-terminal 194 amino acids comprising the putative Ran-GTP binding domain can cause a dominant-negative phenotype with regard to nuclear transport mechanisms. Expression of both ProtA-LOS1 and ProtA-LOS1ΔN, driven from a YEp13 high-copy-number plasmid and the GAL10 promoter in the presence of galactose, was approximately fivefold higher than expression from a centromeric plasmid and a constitutive promoter (Fig. 4A, compare lanes 1 and 2 with 3 and 4). This overexpression led to a reduction of cell growth (Fig. 4B, upper panel). On the other hand, the colony sizes of these strains on plates were very heterogeneous, suggesting differing levels of expression of Los1p due to different plasmid copy numbers in individual cells. To overcome this problem, ProtA-LOS1 and ProtA-LOS1ΔN were subcloned in a modified pEMBLyex4 plasmid, which ensures very high and controlled copy numbers in all cells under selective conditions (see Materials and Methods). These constructs were transformed in wild-type cells, and overexpression was induced by adding galactose to cultures pregrown in raffinose medium. Under these conditions, ProtA-Los1p or ProtA-Los1ΔNp production, which was further increased by a factor of 2 to 3 (Fig. 4A, lane 5 and 6), now caused a complete inhibition of growth in liquid cultures (Fig. 4B, lower panel) and on plates (data not shown). Thus, overproduction of full-length or truncated Los1p causes a dominant-negative growth defect.
FIG. 4.
Inhibition of cell growth by overexpression of LOS1. (A) Total lysates of cells grown for 4 h in the presence of galactose were analyzed by SDS-PAGE and Coomassie staining (upper panel) or Western blotting (lower panel) to determine the level of the protein A-tagged proteins. The cells were carrying the following plasmids: lane 1, pUN100-ProtA-LOS1; lane 2, pUN100-ProtA-LOS1ΔN; lane 3, YEp13-GAL-ProtA-LOS1; lane 4, YEp13-GAL-ProtA-LOS1ΔN; lane 5, pEMBLyex4-ProtA-LOS1; and lane 6, pEMBLyex4-ProtA-LOS1ΔN. The positions of molecular mass markers (10-kDa ladder) are indicated by the bars on the left (highest bar, 200 kDa; next bars, 120, 110, and 100 kDa; etc.) (B) Wild-type cells transformed with YEp13 (upper panel) or pEMBLyex4 (lower panel) containing no inserts (○) or carrying ProtA-LOS1 (□) or the truncation mutant ProtA-LOS1ΔN (▵) under the control of the GAL10-CYC1 inducible promoter were grown in raffinose-containing medium. At time zero, expression was induced by diluting the cultures in medium containing 2% galactose. Growth rates were determined by monitoring the OD600.
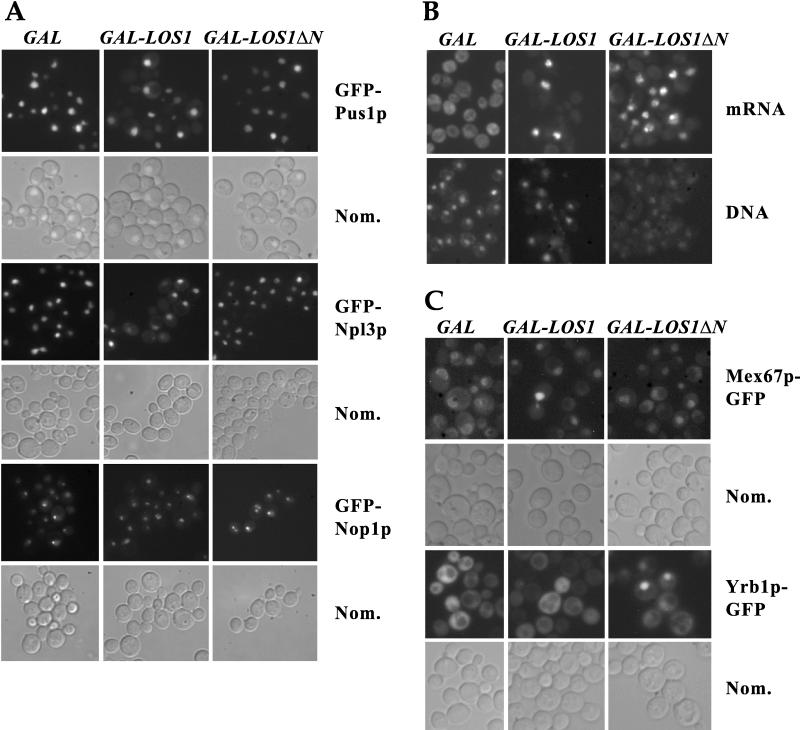
To find out whether the growth phenotype is related to defects in nuclear transport, we localized in the LOS1-overexpressing cells several GFP-tagged reporter proteins known to be imported into the nucleus or to shuttle between the nucleus and the cytoplasm. Specifically, we looked at the localization of Pus1p, an intranuclear tRNA-pseudouridine synthase that genetically interacts with Los1p (94); Npl3p, an RNA binding protein that shuttles between the nucleus and the cytoplasm (20); and Nop1p, a nucleolar protein (81). As shown in Fig. 5A, all three proteins exhibited their expected localization in galactose-grown cells containing an empty pEMBLyex4 plasmid. GFP-Pus1p and GFP-Npl3p were localized exclusively in the nucleus, while GFP-Nop1p localized predominantly in the nucleolus. Induction of the overexpression of ProtA-LOS1 or ProtA-LOS1ΔN by growing the cells for 7 h in galactose medium did not inhibit the nuclear accumulation of either GFP-Pus1p or GFP-Npl3p. However, in the case of ProtA-Los1p-overproducing cells only, we observed the appearance of weak cytoplasmic staining for both proteins, indicating a possible defect in nuclear import of proteins. GFP-Nop1p, on the other hand, remained inside the nucleus in all cases. Furthermore, in some of the cells that overexpressed ProtA-LOS1, GFP-Nop1p was no longer concentrated in a single spot but rather was evident in more than two intranuclear spots, suggesting that the nucleolus had been fragmented (Fig. 5A). This phenotype was even more pronounced when ProtA-Los1ΔNp was overproduced. Fragmentation of the nucleolus has often been observed in yeast mutant strains deficient in mRNA nuclear export (see also below). Finally, examination of the localization of the artificial nuclear reporter GFP-NLS-LacZ in cells containing the YEp13-GAL-ProtA-LOS1 or the YEp13-GAL-ProtA-LOS1ΔN plasmid and grown in galactose medium gave results similar to those of GFP-Pus1p and GFP-Npl3p (data not shown). These results suggested that the dominant-negative phenotype of LOS1 or LOS1ΔN overexpression was not due to a defect in the nuclear import of proteins.
FIG. 5.
Inhibition of nuclear transport processes by overexpression of LOS1. (A) Cells containing pEMBlyex4 (GAL), pEMBLyex4-ProtA-LOS1 (GAL-LOS1), or pEMBLyex4-ProtA-LOS1ΔN (GAL-LOS1ΔN) and expressing GFP-PUS1, GFP-NPL3, or GFP-NOP1, as indicated, were grown in the presence of galactose for 7 h. The intracellular location of the GFP-tagged proteins was revealed by fluorescence microscopy. (B) Cells carrying pEMBlyex4 (GAL), pEMBLyex4-ProtA-LOS1 (GAL-LOS1), or pEMBLyex4-ProtA-LOS1ΔN (GAL-LOS1ΔN) were grown in galactose medium for 3 h and then processed for in situ localization of mRNA and stained for DNA with 4′,6-diamidino-2-phenylindole (DAPI). (C) Mex67− cells expressing MEX67-GFP were transformed with pEMBLyex4 (GAL), pEMBLyex4-ProtA-LOS1 (GAL-LOS1), or pEMBLyex4-ProtA-LOS1ΔN (GAL-LOS1ΔN) (upper two rows), and wild-type cells expressing YRB1-GFP were transformed with YEp13-GAL (GAL), YEp13-GAL-ProtA-LOS1 (GAL-LOS1), or YEp13-GAL-ProtA-LOS1ΔN (GAL-LOS1ΔN) (lower two rows). After growth in galactose medium for 7 h, the localization of the GFP-tagged proteins was observed. Nom., Nomarski image.
To test nuclear export, we localized poly(A)+ RNA by fluorescent in situ hybridization. We observed that LOS1 overexpression caused nuclear accumulation of poly(A)+ RNA in a significant number of cells; this was already evident after a 3-h induction period with galactose (Fig. 5B), and it became stronger with time (data not shown). Overexpressed ProtA-LOS1ΔN was even a stronger inducer of mRNA export defects in terms of the number of cells displaying the defect (Fig. 5B). Finally, we analyzed whether overexpression of LOS1 or LOS1ΔN affects the intracellular distribution of two nucleocytoplasmic transport factors, Mex67p and Yrb1p. In the case of Mex67p-GFP, an essential mRNA export factor that normally associates with the nuclear pores and concentrates around the nuclear envelope, overexpression of LOS1 or LOS1ΔN mediated by the pEMBLyex4 plasmid caused a predominant accumulation of the tagged protein inside the nucleus in almost all of the cells (Fig. 5C). Yrb1p, the yeast homologue of RanBP1, is essential for nucleocytoplasmic transport and normally localizes in the cytoplasm. On overexpression of full-length LOS1, Yrb1p-GFP remains cytoplasmic (Fig. 5C). In contrast, overexpression of LOS1ΔN caused nuclear accumulation of Yrb1p-GFP, but only in a small number of cells (Fig. 5C). However, in this case, the number of accumulating cells did not increase when the pEMBLyex4 plasmid was used for the overexpression of LOS1ΔN (data not shown). Yrb1p also was previously shown to accumulate in the nucleus when a truncated form of YRB4, a member of the importin-β-like protein family required for nuclear import of ribosomal proteins, was overexpressed (83).
In summary, several nucleocytoplasmic transport routes become impaired in cells that overexpress LOS1. It appears, however, that nuclear export is predominantly inhibited by overproduction of this importin-β-like protein (see also Discussion).
Los1p interacts with Gsp1p-GTP in vitro only in the presence of tRNA.
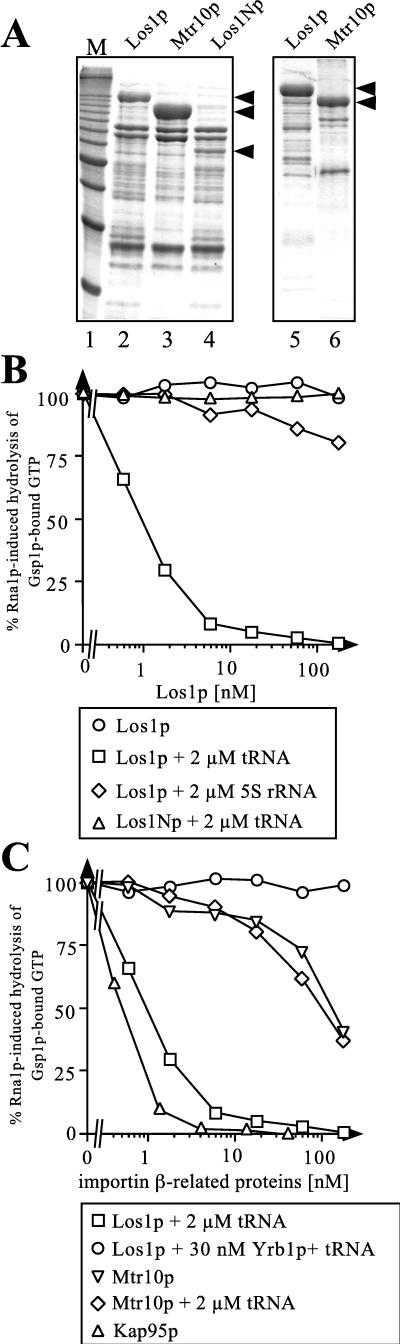
Since Los1p was found to bind preferentially to the GTP-bound form of Gsp1p (see above), we wished to characterize this interaction in more detail. Since binding of an importin-β-related protein to Ran-GTP is reflected by its ability to inhibit the exchange and hydrolysis of bound GTP (7, 21, 34), this interaction can be used to estimate the dissociation constants of the resulting complexes. For this purpose, we expressed and isolated the following proteins from E. coli: recombinant Los1p; its amino-terminal domain (Los1Np, amino acids 1 to 563), which is expected to contain the Ran-GTP binding site; and, as controls, Mtr10p and Kap95p (yeast importin β). The results of the protein composition analysis of the Los1p, Mtr10p, and Los1Np preparations are shown in Fig. 6A. Both highly and partially purified samples were used, without any difference in the results since contaminating E. coli proteins, present also in the control samples, did not affect the assay. When Los1p- or Los1Np-containing preparations were incubated with Gsp1p loaded with GTP, no inhibition of GTP exchange (data not shown) or Rna1p-induced hydrolysis of Gsp1p-bound GTP (Fig. 6B) could be observed, even at micromolar concentrations of the respective Los1p construct. However, under the same conditions, recombinant yeast importin β (Kap95p) and importin-β-related Mtr10p, which were used as controls, inhibited GTP hydrolysis at concentrations corresponding to the previously reported affinities (Fig. 6C). A similarly weak binding to Ran-GTP has previously been shown for CAS, the nuclear export receptor for importin α (57). The affinity of CAS for Ran-GTP is very low but is substantially increased in the presence of the export cargo. We therefore assumed that Los1p could be a nuclear export factor and hence included in the Ran-binding assay, in addition to recombinant Los1p, potential candidates for export cargos, such as tRNA or Arc1p and Pus1p, two tRNA binding proteins that genetically interact with Los1p.
FIG. 6.
Interaction of recombinant Los1p with Gsp1p-GTP and tRNA. (A) His6-tagged Los1p-, Mtr10p-, and Los1Np-containing E. coli lysates were passed through a Ni-NTA–agarose column, and bound proteins were eluted with 200 mM imidazole, desalted, concentrated, and analyzed by SDS-PAGE and Coomassie staining (lanes 2 to 4). In the case of Los1p and Mtr10p, Ni-NTA column eluates were subsequently passed through an ion-exchange column (Mono Q) and bound proteins were eluted with an NaCl gradient. The fractions containing Los1p or Mtr10p were pooled, desalted, concentrated, and analyzed by SDS-PAGE and Coomassie staining (lanes 5 and 6). Minor bands in these preparations represented mostly degradation products. From top to bottom, arrowheads indicate the positions of Los1p, Mtr10p, and Los1Np, respectively. Lane 1 contains molecular mass standards (M; 10-kDa ladder; the most intense band corresponds to 50 kDa). (B and C) Cooperative binding of Gsp1p-GTP and tRNA to Los1p. Gsp1p-[γ-32P]GTP (50 pM) was preincubated for 15 min either with the importin-β-related proteins alone, with these proteins as mixtures with tRNA or 5S rRNA in the indicated concentrations, or with incubation buffer. Then 20 nM Rna1p was added, and the reaction was allowed to proceed for 2 min. Where indicated, Yrb1p was added immediately before the addition of Rna1p. Hydrolysis of Gsp1p-bound GTP was determined as released [32P]phosphate.
Surprisingly, the affinity of Los1p for Gsp1p-GTP increased dramatically even when tRNA alone was added (Fig. 6B), while addition of Arc1p or Pus1p had no effect (data not shown). In the presence of tRNA, the concentration of Los1p required for half-maximal inhibition of the Rna1p-induced GTPase activity (an estimate for the dissociation constant of the Los1p–Gsp1p-GTP complex) was found to be close to 1 nM (Fig. 6B). The half-life of the complex (off rate) was determined to be approximately 7 min (data not shown). The induction of the Los1p–Ran-GTP interaction by tRNA is specific since (i) tRNA does not increase the weak affinity of Mtr10p for Gsp1p-GTP (Fig. 6C) and (ii) a different RNA species (5S rRNA) exhibits no stimulatory effect on Los1p–Gsp1p-GTP binding (Fig. 6B). Furthermore, the cooperative binding of Los1p to Gsp1p-GTP can also be seen when a single tRNA species (e.g., tRNAPhe) is used instead of total yeast tRNA (data not shown). Binding of Los1Np to Gsp1p-GTP was not increased in the presence of tRNA, suggesting that the C-terminal domain of Los1p is essential for the interaction with tRNA (Fig. 6B). Finally, the tRNA-induced binding of Los1p to Gsp1p-GTP can be abolished by the addition of Yrb1p, which mediates the release of Gsp1p-GTP from other importin-β-related nuclear import or export receptors (Fig. 6C) (7).
We wanted to confirm this observed heterotrimeric-complex formation between tRNA, Gsp1p-GTP, and Los1p in an independent in vitro binding assay. However, to date we have not been able to biochemically isolate a Los1p–tRNA–Ran-GTP complex by incubating immobilized His6- or protein A-tagged Los1p with tRNA in the presence of Gsp1p-GTP. This negative result may indicate that such a trimeric Los1p–Gsp1p-GTP–tRNA complex is kinetically unstable and thus may fall apart during biochemical purification (see also Discussion).
DISCUSSION
Los1p is required for suppressor tRNA activity in yeast; it is genetically linked to different tRNA binding and modification enzymes, and it is associated with the nuclear pore complexes and physically interacts in vivo with Nsp1p, Nup2p, and Ran-GTP. Furthermore, Los1p can form a complex with Ran-GTP and tRNA, as detected in vitro by an established indirect binding assay (86). Taken together, these results provide experimental evidence that yeast Los1p is indeed a bona fide member of the importin-β-like family, with a possible role in nuclear export of tRNA. Human LOS1, which shows a distinct sequence homology to yeast Los1p, has already been shown to act as a tRNA exportin in higher-eukaryotic cells (3, 59).
Formation of a complex between Los1p, Ran-GTP, and tRNA has so far been observed only in an indirect in vitro assay. By biochemical purification, we were not able to obtain sufficient amounts of a Los1p-tRNA complex in the presence of Ran-GTP (41a). The reason for this difference is not clear, but it could be due to the fact that a trimeric Los1p–Gsp1p-GTP–tRNA complex is kinetically unstable and therefore dissociates when the excess free components are removed in the necessary washing steps preceding biochemical isolation. Indeed, we have experimental evidence that the Los1p–Gsp1p-GTP–tRNA complex is very short-lived (half-life, 7 min) in the indirect binding assay compared to the corresponding complexes of importin β or transportin and Ran-GTP (half-lives, 2 to 4 hours [7]).
It appears relevant, in this respect, that Los1p is genetically linked to the intranuclear tRNA modification enzyme Pus1p (94). Pus1p catalyzes the formation of pseudouridine in several tRNAs, at positions which are specific for the eukaryotic cell. Therefore, it is possible that these modifications act as positive determinants for association of tRNA with the nuclear export machinery and in particular with Los1p. In support of this, the human homologue of Los1p was shown to bind stronger to native tRNA than to tRNA that was produced in vitro and therefore lacked all modifications (59).
After assembly in the nucleus, a putative Los1p–Ran-GTP–tRNA complex must be targeted to the nuclear pores and subsequently translocated into the cytoplasm. Targeting to the pores and translocation could be achieved through the interaction of Los1p with nucleoporins (see below), but in general these steps are the least understood in the nuclear transport process. Finally, once in the cytoplasmic environment, the export complex may be dissociated by RanBP1 followed by RanGAP-induced hydrolysis of Ran-bound GTP. Indeed, we have shown that Yrb1p (the yeast RanBP1) inhibits the tRNA-induced association of Los1p with Ran-GTP, and a similar effect has also been shown for importin α and CAS (57) and for the human homologue of Los1p (59). The dissociation of Los1p from Ran would trigger the release of tRNA. However, it is very likely that tRNA is not set free to diffuse in the cytosol but is instead directly delivered to cytoplasmic tRNA-binding proteins, such as Arc1p and Gcd11p, that would guide it through the subsequent steps, tRNA aminoacylation and delivery to the ribosome.
Surprisingly, we have also found in the two-hybrid screen a potential interaction between Los1p and the translation initiation factor eIF-2γ (Gcd11p), specifically with its carboxy-terminal domain, which is thought to mediate the binding of eIF-2 to tRNA (15). Since we were not able to copurify eIF-2 with Los1p, the two-hybrid interaction could be indirect, through a tRNA molecule that bridges eIF-2γ with the tRNA-binding domain in Los1p. On the other hand, we have found that certain point mutations in the carboxy-terminal domain of eIF-2γ cause synthetic lethality when combined with the los1 disruption mutant; i.e., Los1p and Gcd11p also functionally interact. Independent of a direct or indirect interaction of eIF-2γ with Los1p, high-affinity binding sites for tRNA in the cytoplasm may ensure its efficient and direct transfer from Los1p to the protein translation machinery in a channeling mode, without the involvement of free diffusion (66, 99). It has recently been shown that tRNA aminoacylation can also occur inside the nucleus and may be required for nuclear export of tRNA in Xenopus oocytes (61a). If this is also the case in yeast, the interaction of Los1p with eIF-2γ may occur inside the nucleus as part of the delivery of the aminoacylated tRNA to the nuclear export machinery.
Binding to and translocation through the nuclear pore complexes are necessary intermediate steps for all reactions involving transport between the nucleus and cytoplasm. We have demonstrated an interaction of Los1p with Nup2p both in the two-hybrid system and biochemically. We have also shown biochemically that Los1p interacts with Nsp1p, to which it is also genetically linked. The fact that we did not find Nsp1p in the two-hybrid screen probably indicates that the interaction domains involved are sensitive to the orientational or conformational changes that result from the fusion of the proteins to GAL activation or DNA binding sequences. Direct binding to the FXFG or GLFG nucleoporin repeat sequences has been previously reported for members of the importin/karyopherin-β-like protein family (1, 47, 74–76, 79). Although Nsp1p is genetically and physically linked to many other nucleoporins (36–38, 104), it has never been found in genetic screens to be linked to soluble transport factors, with the exception of Los1p. Therefore, the physical and genetic interactions between Nsp1p and Los1p (94), as well as the genetic interaction between Nsp1p and Arc1p (92), suggest that Nsp1p (most likely in one of its different subcomplexes) is involved in tRNA export through the nuclear pores. Since Nsp1p has recently been localized on both sides of the central gated channel of the NPC (18), it is possible that Nsp1p (or one of its different subcomplexes) is involved in tRNA export. The role of Nup2p in nucleocytoplasmic transport is less-well studied. Nup2p is not essential in yeast, but it is required for viability of strains carrying mutations in nucleoporin Nsp1p or Nup1p, with which Nup2p is homologous, in the central FXFG repeat-containing domain (61). Interestingly, Nup2p shares a redundant function with the other two FXFG repeat sequence-containing nucleoporins, Nup1p and Nsp1p (61). Therefore, all of these nucleoporins could provide docking sites for Los1p at the NPC or facilitate translocation through the NPC.
The converging of several transport pathways at the NPC, where soluble receptors encounter the same group of nucleoporins, could explain the dominant-negative phenotype of LOS1 overexpression. Dominant-negative phenotypes have been reported previously in the case of importin β and yeast Yrb4p mutants which caused inhibition of both nuclear import and export pathways (58, 83). Overexpression of LOS1 or LOS1ΔN, however, predominantly affects nuclear export processes, as shown for mRNA export, and causes intranuclear accumulation of Yrb1p and Mex67p. Therefore, an excess of Los1p may saturate sites at the NPC that are preferentially involved in nuclear export. Alternatively, excess Los1p may bind to and inactivate another transport factor, such as Gsp1p, which is required for both nuclear import and export. This could explain why overproduction of Los1p also affects slightly the nuclear import of Npl3p and Pus1p. On the other hand, overproduction of Los1ΔNp, which lacks the putative Gsp1p-binding domain but can still interact with nucleoporins, has no effect on nuclear import and causes a stronger RNA export defect, suggesting that in this case the defect is due to unproductive binding of Los1ΔNp to NPC sites also used by other nuclear export factors.
What remains puzzling is that LOS1 is not required for cell growth. On the other hand, Los1p’s function becomes essential if upstream or downstream steps in tRNA biogenesis are affected; e.g., the synthesis rate of tRNA is reduced by mutating the tRNA transcription factor Tfc4p, intranuclear tRNA modification is inhibited by mutating Pus1p, aminoacylation is slowed down by mutating Arc1p (92–94), or delivery of initiator tRNAMet to the ribosomes is affected by mutating a subunit of eIF-2. It is therefore possible that all of these impairments render Los1p essential for viability because, when combined with a deficiency in tRNA nuclear export, they cause the pool of functional tRNA molecules in the cytoplasm to drop below a threshold level required to sustain cell growth. Also, the initial finding that LOS1 is a gene involved in suppressor tRNA activity (44) can be most easily explained by a requirement of Los1p for efficient export of tRNA. However, it is clear that there must be a redundant pathway(s) in the cell to compensate for a putative tRNA export defect in los1 mutants. Since there are still uncharacterized importin-β-like proteins in yeast, it is possible that some of them act as mediators of tRNA export as well and overlap with the Los1p function. Alternatively, there may be additional tRNA export pathways which do not require the action of importin-β-like transport factors.
ACKNOWLEDGMENTS
We especially thank Helge Grosshans for performing the poly(A)+ RNA in situ hybridization experiment, Anke Sauer and Karina Deinert for excellent technical assistance, Kerstin Kloke for the ProtA-TEV-DHFR construct, and S. Bailer for the pAS2-NSP1M plasmid. We are also grateful to S. Elledge (Baylor College of Medicine, Houston, Tex.), C. Gamberi (EMBL, Heidelberg, Germany), E. Hannig (University of Texas at Dallas, Dallas, Tex.), M. Fromont-Racine and P. Legrain (Institut Pasteur, Paris, France), J. Becker (MPI für Molekulare Physiologie, Dortmund, Germany), and D. Wong (Dana-Farber Cancer Institute, Boston, Mass.) for strains, plasmids, and antisera and to all members of the lab for useful comments.
E.H. and G.S. are recipients of grants from the Deutsche Forschungsgemeinschaft (SFB352). M.K. was supported by a long-term fellowship from the Human Frontier Science Program organization.
REFERENCES
- 1.Aitchison J D, Blobel G, Rout M P. Kap104p: a karyopherin involved in the nuclear transport of messenger RNA binding proteins. Science. 1996;274:624–627. doi: 10.1126/science.274.5287.624. [DOI] [PubMed] [Google Scholar]
- 2.Amberg D C, Goldstein A L, Cole C N. Isolation and characterization of RAT1: an essential gene of Saccharomyces cerevisiae required for the efficient nucleocytoplasmic trafficking of mRNA. Genes Dev. 1992;6:1173–1189. doi: 10.1101/gad.6.7.1173. [DOI] [PubMed] [Google Scholar]
- 3.Arts G-J, Fornerod M, Mattaj I W. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. doi: 10.1016/s0960-9822(98)70130-7. [DOI] [PubMed] [Google Scholar]
- 3a.Bailer, S. Unpublished data.
- 4.Baudin A, Ozier-Kaloeropoulos O, Denoul A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993;21:3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belhumeur P, Lee A, Tam R, DiPaolo T, Fortin N, Clark M W. GSP1 and GSP2, genetic suppressors of the prp20-1 mutant in Saccharomyces cerevisiae: GTP-binding proteins involved in the maintenance of nuclear organization. Mol Cell Biol. 1993;13:2152–2161. doi: 10.1128/mcb.13.4.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benton B M, Eng W-K, Dunn J J, Studier F W, Sternglanz R, Fisher P A. Signal-mediated import of bacteriophage T7 RNA polymerase into the Saccharomyces cerevisiae nucleus and specific transcription of target genes. Mol Cell Biol. 1990;10:353–360. doi: 10.1128/mcb.10.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischoff F R, Görlich D. RanBP1 is crucial for the release of RanGTP from importin β-related nuclear transport factors. FEBS Lett. 1997;419:249–254. doi: 10.1016/s0014-5793(97)01467-1. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff F R, Klebe C, Kretschmer J, Wittinghofer A, Ponstingl H. RanGAP1 induces GTPase activity of nuclear Ras-related Ran. Proc Natl Acad Sci USA. 1994;91:2587–2591. doi: 10.1073/pnas.91.7.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bischoff F R, Krebber H, Kempf T, Hermes I, Ponstingl H. Human RanGTPase-activating protein RanGAP1 is a homologue of yeast Rna1p involved in mRNA processing and transport. Proc Natl Acad Sci USA. 1995;92:1749–1753. doi: 10.1073/pnas.92.5.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bischoff F R, Krebber H, Smirnova E, Dong W, Ponstingl H. Co-activation of RanGTPase and inhibition of GTP dissociation by Ran-GTP binding protein RanBP1. EMBO J. 1995;14:705–715. doi: 10.1002/j.1460-2075.1995.tb07049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cesareni G, Murray J A H. Plasmid vectors carrying the replication origin of filamentous single-stranded phages in genetic engineering. Genet Eng. 1987;9:135–154. [Google Scholar]
- 12.Cheng Y, Dahlberg J E, Lund E. Diverse effects of the guanine nucleotide exchange factor RCC1 on RNA transport. Science. 1995;267:1807–1810. doi: 10.1126/science.7534442. [DOI] [PubMed] [Google Scholar]
- 13.Corbett A H, Silver P A. Nucleocytoplasmic transport of macromolecules. Microbiol Rev. 1997;61:193–211. doi: 10.1128/mmbr.61.2.193-211.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dente L, Cesareni G, Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983;11:1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorris D R, Erickson F L, Hannig E M. Mutations in GCD11, the structural gene for eIF-2γ in yeast, alter translational regulation of GCN4 and the selection of the start site for protein synthesis. EMBO J. 1995;14:2239–2249. doi: 10.1002/j.1460-2075.1995.tb07218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doye V, Hurt E C. From nucleoporins to nuclear pore complexes. Curr Opin Cell Biol. 1997;9:401–411. doi: 10.1016/s0955-0674(97)80014-2. [DOI] [PubMed] [Google Scholar]
- 17.Elledge S J, Davis R W. A family of versatile centromeric vectors designed for use in the sectoring-shuffle mutagenesis assay in Saccharomyces cerevisiae. Gene. 1988;70:303–312. doi: 10.1016/0378-1119(88)90202-8. [DOI] [PubMed] [Google Scholar]
- 18.Fahrenkrog, B., E. C. Hurt, U. Aebi, and N. Panté. Molecular architecture of the yeast nuclear pore complex: localization of Nsp1p subcomplexes. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 19.Fischer U, Huber J, Boelens W C, Mattaj I W, Lührmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 20.Flach J, Bossie M, Vogel J, Corbett A, Jinks T, Aker Willins D, Silver P A. A yeast RNA-binding protein shuttles between the nucleus and the cytoplasm. Mol Cell Biol. 1994;14:8399–8407. doi: 10.1128/mcb.14.12.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Floer M, Blobel G. The nuclear transport factor karyopherin β binds stoichiometrically to Ran-GTP and inhibits the Ran GTPase activating protein. J Biol Chem. 1996;271:5313–5316. doi: 10.1074/jbc.271.10.5313. [DOI] [PubMed] [Google Scholar]
- 22.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 23.Fornerod M, Vandeursen J, Vanbaal S, Reynolds A, Davis D, Gopal Murti K, Fransen J, Grosveld G. The human homologue of yeast Crm1 is in a dynamic subcomplex with Can/Nup214 and a novel nuclear pore component, Nup88. EMBO J. 1997;16:807–816. doi: 10.1093/emboj/16.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fridell R A, Truant R, Thorne L, Benson R E, Cullen B R. Nuclear import of hnRNP A1 is mediated by a novel cellular cofactor related to karyopherin-β. J Cell Sci. 1997;110:1325–1331. doi: 10.1242/jcs.110.11.1325. [DOI] [PubMed] [Google Scholar]
- 25.Fromont-Racine M, Rain J C, Legrain P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat Genet. 1997;16:277–282. doi: 10.1038/ng0797-277. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 27.Gerace L. Nuclear export signals and the fast track to the cytoplasm. Cell. 1995;82:341–344. doi: 10.1016/0092-8674(95)90420-4. [DOI] [PubMed] [Google Scholar]
- 28.Giebel L B, Spritz R A. Site-directed mutagenesis using a double-stranded DNA fragment as a PCR primer. Nucleic Acids Res. 1990;18:4947. doi: 10.1093/nar/18.16.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base-pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 30.Goldfarb D S. Nuclear transport—whose finger is on the switch? Science. 1997;276:1814–1816. doi: 10.1126/science.276.5320.1814. [DOI] [PubMed] [Google Scholar]
- 31.Goldfarb D S. Nuclear transport: proliferating pathways. Curr Biol. 1997;7:R13–R16. doi: 10.1016/s0960-9822(06)00009-1. [DOI] [PubMed] [Google Scholar]
- 32.Görlich D, Dabrowski M, Bischoff F R, Kutay U, Bork P, Hartmann E, Prehn S, Izaurralde E. A novel class of RanGTP binding proteins. J Cell Biol. 1997;138:65–80. doi: 10.1083/jcb.138.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Görlich D, Mattaj I W. Protein kinesis—nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 34.Görlich D, Panté N, Kutay U, Aebi U, Bischoff F R. Identification of different roles for RanGDP and RanGTP in nuclear protein import. EMBO J. 1996;15:5584–5594. [PMC free article] [PubMed] [Google Scholar]
- 35.Gorsch L C, Dockendorff T C, Cole C N. A conditional allele of the novel repeat-containing yeast nucleoporin RAT7/NUP159 causes both rapid cessation of mRNA export and reversible clustering of nuclear pore complexes. J Cell Biol. 1995;129:939–955. doi: 10.1083/jcb.129.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grandi P, Doye V, Hurt E C. Purification of NSP1 reveals complex formation with ‘GLFG’ nucleoporins and a novel nuclear pore protein, NIC96. EMBO J. 1993;12:3061–3071. doi: 10.1002/j.1460-2075.1993.tb05975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandi P, Emig S, Weise C, Hucho F, Pohl T, Hurt E C. A novel nuclear pore protein, Nup82p, which specifically binds to a fraction of Nsp1p. J Cell Biol. 1995;130:1263–1273. doi: 10.1083/jcb.130.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grandi P, Schlaich N, Tekotte H, Hurt E C. Functional interaction of Nic96p with a core nucleoporin complex consisting of Nsp1p, Nup49p and a novel protein, Nup57p. EMBO J. 1995;14:76–87. doi: 10.1002/j.1460-2075.1995.tb06977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guarente L, Yocum R R, Gifford P. A GAL10-CYC1 hybrid yeast promoter identifies the GAL4 regulatory region as an upstream site. Proc Natl Acad Sci USA. 1982;79:7410–7414. doi: 10.1073/pnas.79.23.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. The p21 CdK-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 41.Haselbeck R C, Greer C L. Minimum intron requirements for tRNA splicing and nuclear transport in Xenopus oocytes. Biochemistry. 1993;32:8575–8581. doi: 10.1021/bi00084a026. [DOI] [PubMed] [Google Scholar]
- 41a.Hellmuth, K., et al. Unpublished data.
- 42.Her L S, Lund E, Dahlberg J E. Inhibition of Ran guanosine triphosphatase-dependent nuclear transport by the matrix protein of vesicular stomatitis virus. Science. 1997;276:1845–1848. doi: 10.1126/science.276.5320.1845. [DOI] [PubMed] [Google Scholar]
- 43.Hopper A K, Martin N C. Processing of yeast cytoplasmic and mitochondrial precursor tRNAs. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces. II. Gene expression. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. pp. 99–141. [Google Scholar]
- 44.Hopper A K, Schultz L D. Processing of intervening sequences: a new yeast mutant which fails to excise intervening sequences from precursor tRNAs. Cell. 1980;19:741–751. doi: 10.1016/s0092-8674(80)80050-x. [DOI] [PubMed] [Google Scholar]
- 45.Hurt D J, Wang S S, Lin Y-H, Hopper A K. Cloning and characterization of LOS1, a Saccharomyces cerevisiae gene that affects tRNA splicing. Mol Cell Biol. 1987;7:1208–1216. doi: 10.1128/mcb.7.3.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hurt E C. A novel nucleoskeletal-like protein located at the nuclear periphery is required for the life cycle of Saccharomyces cerevisiae. EMBO J. 1988;7:4323–4334. doi: 10.1002/j.1460-2075.1988.tb03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iovine M K, Watkins J L, Wente S R. The GLFG repetitive region of the nucleoporin Nup116p interacts with Kap95p, an essential yeast nuclear import factor. J Cell Biol. 1995;131:1699–1713. doi: 10.1083/jcb.131.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Izaurralde E, Jarmolowski A, Beisel C, Mattaj I W, Dreyfuss G, Fischer U. A role for the M9 transport signal of hnRNP A1 in mRNA nuclear export. J Cell Biol. 1997;137:27–35. doi: 10.1083/jcb.137.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izaurralde E, Kutay U, von Kobbe C, Mattaj I W, Görlich D. The asymmetric distribution of the constituents of the Ran system is essential for transport into and out of the nucleus. EMBO J. 1997;16:6535–6547. doi: 10.1093/emboj/16.21.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Izaurralde E, Lewis J, Gamberi C, Jarmolowski A, McGuigan C, Mattaj I W. A cap-binding protein complex mediating U snRNA export. Nature. 1995;376:709–712. doi: 10.1038/376709a0. [DOI] [PubMed] [Google Scholar]
- 51.Jarmolowski A, Boelens W C, Izaurralde E, Mattaj I W. Nuclear export of different classes of RNA is mediated by specific factors. J Cell Biol. 1994;124:627–635. doi: 10.1083/jcb.124.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadowaki T, Goldfarb D, Spitz L M, Tartakoff A M, Ohno M. Regulation of RNA processing and transport by a nuclear guanine nucleotide release protein and members of the Ras superfamily. EMBO J. 1993;12:2929–2937. doi: 10.1002/j.1460-2075.1993.tb05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadowaki T, Hitomi M, Chen S, Tartakoff A M. Nuclear mRNA accumulation causes nucleolar fragmentation in yeast mtr2 mutant. Mol Biol Cell. 1994;5:1253–1263. doi: 10.1091/mbc.5.11.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadowaki T, Zhao Y, Tartakoff A M. A conditional yeast mutant deficient in mRNA transport from nucleus to cytoplasm. Proc Natl Acad Sci USA. 1992;89:2312–2316. doi: 10.1073/pnas.89.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koepp D M, Silver P A. A GTPase controlling nuclear trafficking: running the right way or walking RANdomly? Cell. 1996;87:1–4. doi: 10.1016/s0092-8674(00)81315-x. [DOI] [PubMed] [Google Scholar]
- 55a.Kosova, B. Unpublished data.
- 56.Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, Horinouchi S. Molecular cloning and cell cycle-dependent expression of mammalian CRM1, a protein involved in nuclear export of proteins. J Biol Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- 56a.Künzler, M., et al. Unpublished data.
- 57.Kutay U, Bischoff F R, Kostka S, Kraft R, Görlich D. Export of importin α from the nucleus is mediated by a specific nuclear transport factor. Cell. 1997;90:1061–1071. doi: 10.1016/s0092-8674(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 58.Kutay U, Izaurralde E, Bischoff F R, Mattaj I W, Gorlich D. Dominant-negative mutants of importin-β block multiple pathways of import and export through the nuclear pore complex. EMBO J. 1997;16:1153–1163. doi: 10.1093/emboj/16.6.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kutay U, Lipowsky G, Izaurralde E, Bischoff F R, Schwarzmaier P, Hartmann E, Görlich D. Identification of a tRNA-specific nuclear export receptor. Mol Cell. 1998;1:359–369. doi: 10.1016/s1097-2765(00)80036-2. [DOI] [PubMed] [Google Scholar]
- 60.Lee M S, Henry M, Silver P A. A protein that shuttles between the nucleus and the cytoplasm is an important mediator of RNA export. Genes Dev. 1996;10:1233–1246. doi: 10.1101/gad.10.10.1233. [DOI] [PubMed] [Google Scholar]
- 61.Loeb J D J, Davis L I, Fink G R. NUP2, a novel yeast nucleoporin, has functional overlap with other proteins of the nuclear pore complex. Mol Biol Cell. 1993;4:209–222. doi: 10.1091/mbc.4.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61a.Lund, E., and J. E. Dahlberg. Proofreading and aminoacylation of tRNAs prior to export from the nucleus. Submitted for publication. [DOI] [PubMed]
- 62.Michael W M, Choi M Y, Dreyfuss G. A nuclear export signal in hnRNP A1: a signal-mediated, temperature-dependent nuclear protein export pathway. Cell. 1995;83:415–422. doi: 10.1016/0092-8674(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 63.Moore M S, Blobel G. A G protein involved in nucleocytoplasmic transport: the role of Ran. Trends Biochem Sci. 1994;19:211–216. doi: 10.1016/0968-0004(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 64.Murphy R, Wente S R. An RNA-export mediator with an essential nuclear export signal. Nature. 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- 65.Nakielny S, Fischer U, Michael W M, Dreyfuss G. RNA transport. Annu Rev Neurosci. 1997;20:269–301. doi: 10.1146/annurev.neuro.20.1.269. [DOI] [PubMed] [Google Scholar]
- 66.Negrutskii B S, Stapulionis R, Deutscher M P. Supramolecular organization of the mammalian translation system. Proc Natl Acad Sci USA. 1994;91:964–968. doi: 10.1073/pnas.91.3.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Neville M, Stutz F, Lee L, Davis L I, Rosbash M. The importin-beta family member Crm1p bridges the interaction between Rev and the nuclear pore complex during nuclear export. Curr Biol. 1997;7:767–775. doi: 10.1016/s0960-9822(06)00335-6. [DOI] [PubMed] [Google Scholar]
- 68.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 69.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 70.Pasquinelli A E, Powers M A, Lund E, Forbes D J, Dahlberg J E. Inhibition of mRNA export in vertebrate cells by nuclear export signal conjugates. Proc Natl Acad Sci USA. 1997;94:14394–14399. doi: 10.1073/pnas.94.26.14394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pemberton L F, Rosenblum J S, Blobel G. A distinct and parallel pathway for nuclear import of an mRNA-binding protein. J Cell Biol. 1997;139:1645–1653. doi: 10.1083/jcb.139.7.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pollard V W, Michael W M, Nakielny S, Siomi M C, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 73.Powers M A, Forbes D J, Dahlberg J E, Lund E. The vertebrate GLFG nucleoporin, Nup98, is an essential component of multiple RNA export pathways. J Cell Biol. 1997;136:241–250. doi: 10.1083/jcb.136.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radu A, Blobel G, Moore M S. Identification of a protein complex that is required for nuclear protein import and mediates docking of import substrate to distinct nucleoporins. Proc Natl Acad Sci USA. 1995;92:1769–1773. doi: 10.1073/pnas.92.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Radu A, Moore M S, Blobel G. The peptide repeat domain of nucleoporin Nup98 functions as a docking site in transport across the nuclear pore complex. Cell. 1995;81:215–222. doi: 10.1016/0092-8674(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 76.Rexach M, Blobel G. Protein import into nuclei: association and dissociation reactions involving transport substrate, transport factors, and nucleoporins. Cell. 1995;83:683–692. doi: 10.1016/0092-8674(95)90181-7. [DOI] [PubMed] [Google Scholar]
- 77.Richards S A, Carey K L, Macara I G. Requirement of guanosine triphosphate-bound Ran for signal-mediated nuclear protein export. Science. 1997;276:1842–1844. doi: 10.1126/science.276.5320.1842. [DOI] [PubMed] [Google Scholar]
- 78.Rosenblum J S, Pemberton L F, Blobel G. A nuclear import pathway for a protein involved in tRNA maturation. J Cell Biol. 1997;139:1655–1661. doi: 10.1083/jcb.139.7.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rout M P, Blobel G, Aitchison J D. A distinct nuclear import pathway used by ribosomal proteins. Cell. 1997;89:715–725. doi: 10.1016/s0092-8674(00)80254-8. [DOI] [PubMed] [Google Scholar]
- 80.Schiestl R H, Gietz R D. High efficiency transformation of intact yeast cells using single stranded nucleic acid as carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 81.Schimmang T, Tollervey D, Kern H, Frank R, Hurt E C. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989;8:4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlenstedt G, Saavedra C, Loeb J D, Cole C N, Silver P A. The GTP-bound form of the yeast Ran/TC4 homologue blocks nuclear protein import and appearance of poly(A)+ RNA in the cytoplasm. Proc Natl Acad Sci USA. 1995;92:225–229. doi: 10.1073/pnas.92.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schlenstedt G, Smirnova E, Deane R, Solsbacher J, Kutay U, Görlich D, Ponstingl H, Bischoff F R. Yrb4p, a yeast Ran-GTP-binding protein involved in import of ribosomal protein L25 into the nucleus. EMBO J. 1997;16:6237–6249. doi: 10.1093/emboj/16.20.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schlenstedt G, Wong D H, Koepp D M, Silver P A. Mutants in a yeast Ran binding protein are defective in nuclear transport. EMBO J. 1995;14:5367–5378. doi: 10.1002/j.1460-2075.1995.tb00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Segref A, Sharma K, Doye V, Hellwig A, Huber J, Hurt E C. Mex67p, which is an essential factor for nuclear mRNA export, binds to both Poly(A)+ RNA and nuclear pores. EMBO J. 1997;16:3256–3271. doi: 10.1093/emboj/16.11.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Senger B, Simos G, Bischoff F R, Podtelejnikov A V, Mann M, Hurt E C. Mtr10p functions as a nuclear import receptor for the mRNA binding protein Npl3p. EMBO J. 1998;17:2196–2207. doi: 10.1093/emboj/17.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharma K, Fabre E, Tekotte H, Hurt E C, Tollervey D. Yeast nucleoporin mutants are defective in pre-tRNA splicing. Mol Cell Biol. 1996;16:294–301. doi: 10.1128/mcb.16.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen W-C, Selvakumar D, Stanford D R, Hopper A K. The Saccharomyces cerevisiae LOS1 gene involved in pre-tRNA splicing encodes a nuclear protein that behaves as a component of the nuclear matrix. J Biol Chem. 1993;268:19436–19444. [PubMed] [Google Scholar]
- 89.Shen W-C, Stanford D R, Hopper A K. Los1p, involved in yeast pre-tRNA splicing, positively regulates members of the SOL gene family. Genetics. 1996;143:699–712. doi: 10.1093/genetics/143.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sherman F. Guide to yeast genetics and molecular biology. Getting started with yeast. Methods Enzymol. 1990;194:3–20. [Google Scholar]
- 91.Sikorski R S, Hieter R. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Simos G, Sauer A, Fasiolo F, Hurt E C. A conserved domain within Arc1p delivers tRNA to aminoacyl-tRNA synthetases. Mol Cell. 1998;1:235–242. doi: 10.1016/s1097-2765(00)80024-6. [DOI] [PubMed] [Google Scholar]
- 93.Simos G, Segref A, Fasiolo F, Hellmuth K, Shevchenko A, Mann M, Hurt E C. The yeast protein Arc1p binds to tRNA and functions as a cofactor for methionyl- and glutamyl-tRNA synthetases. EMBO J. 1996;15:5437–5448. [PMC free article] [PubMed] [Google Scholar]
- 94.Simos G, Tekotte H, Grosjean H, Segref A, Sharma K, Tollervey D, Hurt E C. Nuclear pore proteins are involved in the biogenesis of functional tRNA. EMBO J. 1996;15:2270–2284. [PMC free article] [PubMed] [Google Scholar]
- 95.Siniossoglou S, Wimmer C, Rieger M, Doye V, Tekotte H, Weise C, Emig S, Segref A, Hurt E C. A novel complex of nucleoporins, which includes Sec13p and a Sec13p homolog, is essential for normal nuclear pores. Cell. 1996;84:265–275. doi: 10.1016/s0092-8674(00)80981-2. [DOI] [PubMed] [Google Scholar]
- 96.Siomi M C, Eder P S, Kataoka N, Wan L L, Liu Q, Dreyfuss G. Transportin-mediated nuclear import of heterogeneous nuclear RNP proteins. J Cell Biol. 1997;138:1181–1192. doi: 10.1083/jcb.138.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Snay-Hodge C A, Colot H V, Goldstein A L, Cole C N. Dbp5p/Rat8p is a yeast nuclear pore-associated DEAD-box protein essential for RNA export. EMBO J. 1998;17:2663–2676. doi: 10.1093/emboj/17.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stade K, Ford C S, Guthrie C, Weis K. Exportin 1 (Crm1p) is an essential nuclear export factor. Cell. 1997;90:1041–1050. doi: 10.1016/s0092-8674(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 99.Stapulionis R, Deutscher M P. A channeled tRNA cycle during mammalian protein synthesis. Proc Natl Acad Sci USA. 1995;92:7158–7161. doi: 10.1073/pnas.92.16.7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tobian J A, Drinkard L, Zasloff M. tRNA nuclear transport: defining the critical region of human tRNAmet by point mutagenesis. Cell. 1985;43:415–422. doi: 10.1016/0092-8674(85)90171-0. [DOI] [PubMed] [Google Scholar]
- 101.Tseng S S L, Weaver P L, Liu Y, Hitomi M, Tartakoff A M, Chang T H. Dbp5p, a cytosolic RNA helicase, is required for poly(A)+ RNA export. EMBO J. 1998;17:2651–2662. doi: 10.1093/emboj/17.9.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ullman K S, Powers M A, Forbes D J. Nuclear export receptors: from importin to exportin. Cell. 1997;90:967–970. doi: 10.1016/s0092-8674(00)80361-x. [DOI] [PubMed] [Google Scholar]
- 103.Westaway S K, Abelson J. Splicing of tRNA precursors. In: Söll D, RajBhandary U L, editors. tRNA: structure, biosynthesis, and function. Washington, D.C: ASM Press; 1995. pp. 79–92. [Google Scholar]
- 104.Wimmer C, Doye V, Grandi P, Nehrbass U, Hurt E. A new subclass of nucleoporins that functionally interact with nuclear pore protein NSP1. EMBO J. 1992;11:5051–5061. doi: 10.1002/j.1460-2075.1992.tb05612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yaseen N R, Blobel G. Cloning and characterization of human karyopherin β3. Proc Natl Acad Sci USA. 1997;94:4451–4456. doi: 10.1073/pnas.94.9.4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zasloff M. tRNA transport from the nucleus in a eukaryotic cell: carrier-mediated translocation process. Proc Natl Acad Sci USA. 1983;80:6436–6440. doi: 10.1073/pnas.80.21.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]