Abstract
OBJECTIVE:
Postoperative pancreatic fistula is a potentially devastating complication after pancreatoduodenectomy (PD). We aim to identify features on preoperative computed tomography (CT) imaging that correlate with an increased risk of postoperative pancreatic fistula (POPF).
METHOD:
Patients who underwent PD at our high-volume pancreatic surgery center from 2019-2021 were included if CT imaging was available within eight weeks of surgical intervention. Pancreatic neck thickness (PNT), abdominal wall thickness (AWT), and intraabdominal distance from pancreas to peritoneum (PTP) were measured by two board-certified radiologists blinded to the clinical outcomes. Radiographic measurements, as well as preoperative patient characteristics and intraoperative data were assessed with univariate and multivariable analysis (MVA) to determine risk for clinically-relevant POPF (CR-POPF, grade B and C).
RESULTS:
204 patients met inclusion criteria. Median PTP was 5.8cm, AWT 1.9cm, and PNT 1.3cm. CR-POPF occurred in 33/204(16.2%) patients. MVA revealed PTP>5.8cm (OR:2.86, p=0.023), PNT>1.3cm (OR:2.43, p=0.047), soft pancreas consistency (OR:3.47, p=0.012) and pancreatic duct size ≤3.0mm (OR:4.55, p=0.01) as independent risk factors for CR-POPF after PD. AWT and obesity were not associated with increased risk of CR-POPF. Patients with PTP>5.8cm or PNT>1.3cm were significantly more likely to suffer a major complication after PD (39.6% vs. 22.3% and 40% vs. 22.1%, p<0.008).
CONCLUSION:
Patients with a thick pancreatic neck and increased intraabdominal girth have a heightened risk of CR-POPF after pancreatoduodenectomy and they experience more serious postoperative complications. We defined a simple CT scan based measurement tool to identify patients at increased risk of CR-POPF.
1. Introduction
Postoperative pancreatic fistula (POPF) is a well-described complication following pancreatic resection, with reported incidence of up to 30% even in high-volume centers 1. Clinically relevant POPF (CR-POPF), grade B and C, are associated with an increased risk of infectious complications, post-pancreatectomy hemorrhage, and mortality 1–4. The reported mortality of a grade C POPF is 44% 5. The development of a CR-POPF also carries a significant economic burden for patients, nearly doubling overall costs after surgery 6.
Several risk factors are described to estimate the risk of POPF, including soft pancreas, small pancreatic duct diameter, high intraoperative blood loss, and “high-risk pathology,” such as ampullary, duodenal, or cystic lesions 3,7,8. Some of these risk factors can only be assessed intraoperatively, limiting the ability of clinicians to appropriately stratify patients in the preoperative setting who may be at increased risk for development of CR-POPF. There exists a need to identify preoperative patient characteristics that carry an increased risk for CR-POPF development.
Cross-sectional imaging such as contrast-enhanced computed tomography (CT) or magnetic resonance (MR) is the standard modality for the diagnosis of pancreatic pathologies and commonly used for pancreatectomy planning 9,10. Our study aims to explore and assess the value of preoperative CT-derived risk factors in predicting an increased risk of CR-POPF development, aiming to facilitate preoperative patient risk-stratification and optimize perioperative management.
2. Methods
2.1. Study Population
Approval for this study was obtained from the Emory University Institutional Review Board. All patients who underwent pancreatoduodenectomy (PD) within the Emory Healthcare System between January 1, 2019 and December 31, 2021 were identified from a previously consolidated database of all pancreatic resections. Patients were excluded from the study if no preoperative CT scan was available or if the preoperative CT scan was not within eight weeks of surgical resection.
2.2. Radiographic Measurements
CT scans were reviewed by two board-certified abdominal radiologists (SS, HS) using the picture archiving system PACS (Sectra IDS7, Sectra). These radiologists were blinded to clinical outcomes. Pancreatic neck diameter was measured at the level of the portal-superior mesenteric vein (SMV) confluence. At this level, we obtained two additional measurements – the distance between the peritoneum and the anterior surface of the pancreas (PTP) and the abdominal wall thickness assessed as the distance between the skin and peritoneum.
2.3. Study variables
Patient characteristics, histopathology, operative, and postoperative data were obtained from patient electronic medical records. Data evaluated included patient demographics (age, gender, race), body mass index (BMI), preoperative diagnosis, intraoperative information (pancreatic duct size and pancreatic gland texture), postoperative complications, and 30-day as well as in-hospital mortality. Preoperative malignant diagnosis includes adenocarcinomas of the pancreas, bile duct, ampulla, and duodenum and neuroendocrine tumors of the pancreas. The Clavien-Dindo (CD) classification of surgical complications was applied to standardize postoperative complication severity 11,12. For the purpose of analyses, CD grades I-II were classified as “minor” complications, and CD grades III-V were classified as “major” complications. The POPF grading is based on the 2016 update of the International Study Group in Pancreatic Surgery (ISGPS). CR-POPF is defined as grade B or C POPF based on ISGPS 4. Small pancreatic duct is defined as duct diameter ≤ 3.0mm estimated intraoperatively. Obesity is defined as BMI ≥30.
2.4. Statistical Analysis
Interrater reliability between the two board-certified radiologists was assessed using a subset of fifteen patients and determining the intraclass correlation coefficient. Frequency distributions and summary statistics were calculated for all variables. Continuous variables were expressed as median and range, and categorical values were expressed as frequency and percentage. Univariate associations were evaluated using Pearson’s chi square and Fisher’s exact t tests (as appropriate) for dichotomous variables and independent t tests for continuous variables. Multivariable analysis was conducted utilizing a binary logistic regression model using the radiographically measured variables in addition to clinical variables approaching statistical significance (p value ≤ 0.10) on univariate analysis. Backward selection with an alpha level of removal of 0.2 was used for the binary logistic regression model. A p value of ≤0.05 was accepted to indicate a statistically significant association. All analyses were performed using SPSS software version 28.0.1 (SPSS, IBM Corp., Armonk, NY, USA) and Graphpad Prism 9.4.1 (Graphpad Inc., San Diego, CA, USA).
3. Results
3.1. Comparison of patients with and without CR-POPF
Of 461 patients who underwent pancreas resections from 2019-2021, 305 underwent PD. Patients were further screened based on available preoperative CT scan and 204 patients had preoperative CT scan within 8 weeks of surgical resection (Figure 1). The median age of patients was 65 years (range 20-88), and 50% (n=102) were female. Seventy-three percent (n=150) of patients in the study were white, 16.7% (n=34) were black, and 9.8% (n=20) were of Asian or Hispanic descent. The median BMI was 24.9 (range 14.8-53.1). PD was performed for known malignancy in 79.4% (n=162) of cases.
Figure 1:
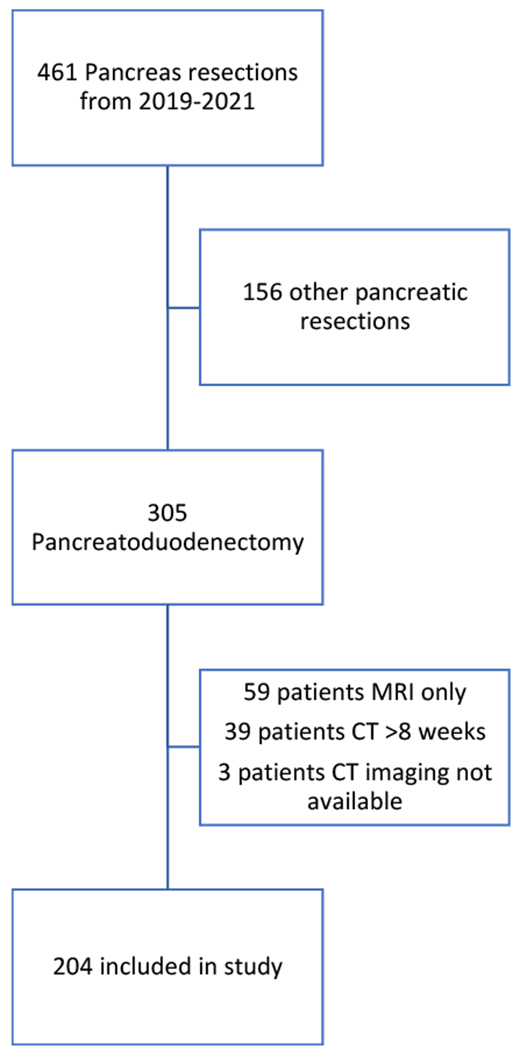
Schematic depicting inclusion and exclusion criteria of patients selected for study.
CR-POPF occurred in 33 (16.2%) patients. Univariate analysis (UVA) demonstrated that patients with a pancreatic duct 3mm or smaller compared to >3mm (23.8% vs. 4.9%, p<0.001) as well as patients with a soft pancreatic gland consistency compared to those with intermediate or firm glands (31.8% vs. 9.9% and 7.5%, p<0.001) were significantly more likely to develop CR-POPF. Age, sex, race, obesity, neoadjuvant chemotherapy or radiotherapy, and preoperative diagnosis of malignancy were not associated with CR-POPF formation (Table 1).
Table 1:
Univariate analysis of patient and lesion factors and preoperative CT imaging measurements as risk factors for CR-POPF.
| Univariate Analysis | ||
|---|---|---|
| CR-POPF (%) | p value | |
| Sex | ||
| Female | 14/102 (13.7%) | 0.342 |
| Male | 19/102 (18.6%) | |
|
| ||
| Race | ||
| White | 21/149 (14.1%) | 0.367 |
| Black | 7/35 (20.0%) | |
| Other | 5/20 (25.0%) | |
|
| ||
| Age (years) | ||
| ≥ 70 | 11/75 (14.7%) | 0.655 |
| < 70 | 22/129 (17.1%) | |
|
| ||
| BMI (kg/m2) | ||
| ≥ 30 | 11/46 (23.9%) | 0.105 |
| < 30 | 22/158 (13.9%) | |
|
| ||
| Pre-operative Diagnosis | ||
| Malignant | 23/162 (14.2%) | 0.132 |
| Non-malignant | 10/42 (23.8%) | |
|
| ||
| Neoadjuvant Chemotherapy | ||
| No | 21/107 (19.6%) | 0.160 |
| Yes | 12/97 (12.4%) | |
|
| ||
| Neoadjuvant Radiotherapy | ||
| No | 29/189 (15.4%) | 0.272 |
| Yes | 4/15 (26.7%) | |
|
| ||
| Gland Texture | ||
| Soft | 21/66 (31.8%) | <0.001 |
| Intermediate | 7/71 (9.9%) | |
| Firm | 5/67 (7.5%) | |
|
| ||
| Duct Size (mm) | ||
| > 3.0 | 4/82 (4.9%) | <0.001 |
| ≤ 3.0 | 29/122 (23.8%) | |
|
| ||
| Abdominal wall | ||
| > 1.95cm | 20/102 (19.6%) | 0.183 |
| ≤ 1.95cm | 13/102 (12.7%) | |
|
| ||
| PTP | ||
| > 5.8cm | 24/101 (23.8%) | 0.004 |
| ≤ 5.8cm | 9/103 (8.7%) | |
|
| ||
| Pancreatic Neck | ||
| > 1.3cm | 22/100 (22.0%) | 0.027 |
| ≤ 1.3cm | 11/104 (10.6%) | |
BMI = Body mass index, PTP = pancreas to peritoneum.
3.2. Radiographic measurements
Preoperative CT scan was evaluated to determine if pancreatic neck diameter, intra-abdominal distance to pancreas, or abdominal wall thickness were associated with an increased risk of development of CR-POPF. Two board-certified radiologists measured these distances in the preoperative CT scan (Figure 2). Interrater reliability of the two radiologists was conducted using a subset of 15 patients. The intraclass coefficient for the radiographic measurements was >0.97 (Table 2).
Figure 2:
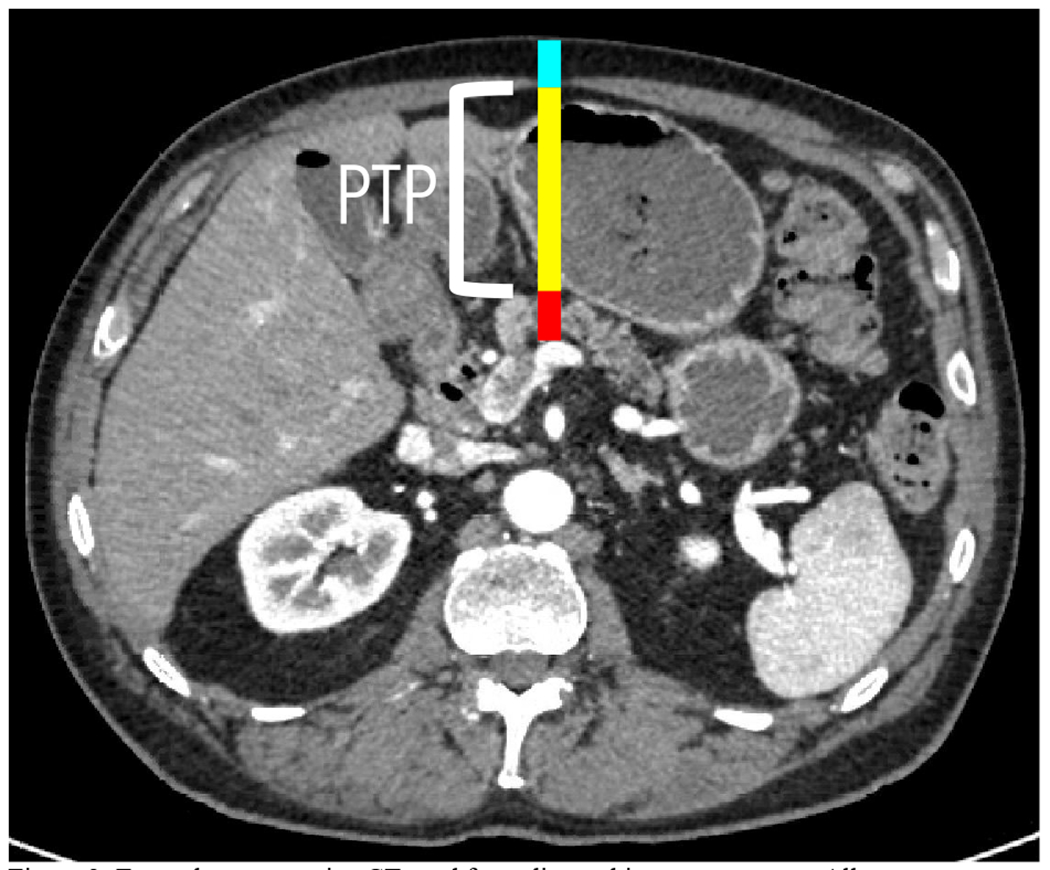
Example preoperative CT used for radiographic measurements. All measurements were taken at the level of the portal-SMV confluence. Red line = pancreatic neck diameter. Yellow line = PTP. Cyan line = abdominal wall thickness.
Table 2:
Radiographic measurement interrater reliability as assessed by intraclass correlation coefficient (ICC).
| Interrater Reliability | |
|---|---|
| CT Measurement | ICC |
| Abdominal Wall | 0.996 |
| PTP | 0.996 |
| Pancreatic Neck | 0.979 |
Patients undergoing PD had a median pancreatic neck thickness of 1.3cm (range 0.4-3.2cm), PTP of 5.8cm (range 0.8-11.3cm) and abdominal wall thickness of 1.95cm (range 0.4-5.9cm). A PTP >5.8cm compared to ≤5.8cm was significantly associated with CR-POPF on univariate analysis (23.8% vs. 8.7%, p=0.004). Pancreatic neck thickness >1.3cm compared to ≤1.3cm was also significantly associated with CR-POPF (22.0% vs. 10.6%, p=0.027). Abdominal wall thickness >1.95cm was not associated with CR-POPF (p=0.18). These data are shown in Table 1.
3.3. Multivariable analysis to identify independent factors associated with CR-POPF
Binomial logistic regression was carried out using the radiographically measured variables pancreatic neck thickness, PTP, and abdominal wall thickness as well as pancreatic duct size, pancreas gland quality, and BMI. CT imaging factors that were found to be independently associated with CR-POPF development were increased PTP (OR 2.86, 95%CI: 1.16-7.14), increased pancreas neck thickness (OR 2.43, 95%CI: 1.01-5.88). Radiographically measured abdominal wall thickness was not associated with CR-POPF development (OR 1.92, 95%CI 0.8-4.55). Additional clinical factors independently associated with CR-POPF development were small duct size (OR 4.55, 95%CI: 1.44-14.38) and soft pancreatic gland consistency (OR 3.47, 95%CI: 1.13-10.64) (Table 3). BMI was removed from the model during backward selection.
Table 3:
Multivariable analysis of independent risk factors for CR-POPF. Dash indicates reference variable.
| Clinically Significant Pancreatic Fistula=Yes |
|||||
|---|---|---|---|---|---|
| Covariate | Level | N | Odds Ratio (95% CI) |
OR P-value | Overall P-value |
| Gland Textme | Soft | 66 | 3.47 (1.13-10.64) | 0.029 | 0.012 |
| Intermediate | 71 | 0.93 (0.26-3.32) | 0.913 | ||
| Firm | 67 | - | - | ||
| Pancreatic duct size | ≤3.0 mm | 122 | 4.55 (1.44-14.38) | 0.010 | 0.010 |
| >3.0 mm | 82 | - | - | ||
| Abdominal wall thickness | ≤1.95 cm | 102 | - | - | 0.146 |
| >1.95 cm | 102 | 1.92 (0.8-4.55) | 0.146 | ||
| PTP | ≤5.8 cm | 103 | - | - | 0.023 |
| ≤ 5.8 cm | 101 | 2.86 (1.16-7.14) | 0.023 | ||
| Thickness of pancreatic neck | ≤1.3 cm | 104 | - | - | 0.047 |
| >1.3 cm | 100 | 2.44 (1.01-5.88) | 0.047 | ||
OR = Odds ratio, CI = Confidence interval.
Number of observations in the original data set = 204. Number of observations used = 204.
Backward selection with an alpha level of removal of .2 was used. The following variables were removed from the model: BMI (kg/m2).
3.4. Overall complication rate associated with increased PTP and pancreatic neck thickness
Because it was determined that PTP >5.8cm and pancreatic neck thickness >1.3cm were independently associated with CR-POPF development, we then compared postoperative outcomes of patients with increased PTP and pancreatic neck thickness against patients with PTP ≤5.8cm and pancreatic neck thickness ≤1.3cm. Sixty-seven percent (n=68) of patients with increased PTP experienced a postoperative complication, whereas 56% (n=58) of patients with PTP at or below the median had a complication (p=0.105). Increased PTP was associated with a significantly increased risk of a major (CD grade ≤3) postoperative complication (39.6% vs. 22.3%, p=0.008). Seventy percent (n=70) of patients with increased pancreatic neck thickness experienced a postoperative complication, whereas 53.8% (n=56) of patients with pancreatic neck thickness at or below the median had a complication (p=0.018). Increased pancreatic neck thickness was associated with a significantly increased risk of a major postoperative complication (40% vs. 22.1%, p=0.006). Length of stay, 30-day readmission rates, and death were similar across groups (Table 4).
Table 4:
Comparison of post-operative outcomes of patients based on PTP and pancreatic neck thickness measurement on preoperative CT imaging.
| Post-Operative Outcomes | ||||||
|---|---|---|---|---|---|---|
| PTP ≤ median |
PTP > median |
p value | PN ≤ median |
PN > median |
p value | |
| Any Complication | 58/103 (56.3%) |
68/101 (67.3%) |
0.105 | 56/104 (53.8%) |
70/100 (70.0%) |
0.018 |
| Serious Complication | 23/103 (22.3%) |
40/101 (39.6%) |
0.008 | 23/104 (22.1%) |
40/100 (40.0%) |
0.006 |
| Death | 2/103 (1.9%) |
4/101 (4.0%) |
0.443 | 3/104 (2.9%) |
3/100 (3.0%) |
1.00 |
| LOS (days) | 9.4 ± 1.0 | 11.8 ± 1.4 | 0.156 | 9.4 ± 1.1 | 11.9 ± 1.3 | 0.077 |
| 30-Day Readmission | 22/103 (21.4%) |
27/101 (27.7%) |
0.291 | 21/104 (20.2%) |
29/100 (29.0%) |
0.144 |
PN = pancreatic neck; LOS = length of stay; Serious complication = Clavien-Dindo classification 3-5. LOS reported as mean ± standard error of the mean.
4. Discussion
This study assessed the value of preoperative CT-derived risk factors in predicting an increased risk of CR-POPF development, with a goal to facilitate preoperative patient risk-stratification and optimize perioperative management. Patients with CR-POPF requiring adjuvant therapy after PD may experience a delay in the initiation of adjuvant therapy, or may be unable to receive adjuvant therapy, which may affect long-term survival 13–15. It is critical to identify novel factors that may predict the likelihood of CR-POPF, and develop strategies to manage these risk factors to improve patient outcomes. The results of the present study suggest that two relatively simple measurements obtained from a preoperative CT scan, the diameter of the pancreatic neck and the distance from the peritoneum to the anterior surface of the pancreas, have an association with an increase in odds of CR-POPF by nearly 2.5- and 3-fold, respectively. Our study also demonstrates that the occurrence of CR-POPF was significantly associated with well-established risk factors such as small pancreatic duct size and soft pancreas based on intraoperative assessment. Based on these results, PTP and PNT can be used as additional independent variables to identify patients who are at increased risk of CR-POPF, even when the patient does not have a small pancreatic duct or a soft pancreas.
Pancreatic thickness and its relationship to CR-POPF has been well-established for patients undergoing distal pancreatectomy (DP) 16–21. The role that parenchymal thickness plays in the development of CR-POPF after PD requires more exploration. Multiple studies have examined the association of preoperatively measured pancreatic neck thickness at the portomesenteric confluence with CR-POPF and have reported mixed results. Two retrospective studies have found no association between pancreatic thickness measured on preoperative CT and CR-POPF 22,23. Work by Roberts et al. and Sugimoto et al. reported results similar to the results of our current study, demonstrating an increased risk of CR-POPF in patients with increasing pancreatic neck thickness 24,25. These studies were limited by sample size, as all examined fewer than 200 patients, in contrast to the present study. The mixed results of these studies indicate that larger sample sizes are required to truly determine the effect that pancreatic neck thickness has on the development of CR-POPF.
Although it has been previously suggested that obesity might be a useful predictor of complications after PD, we found that BMI did not predict CR-POPF on UVA or MVA. PTP, the intraabdominal distance from the peritoneum to the pancreas was independently associated with increased risk of CR-POPF26,27. PTP suggests a measurement of intraabdominal visceral fat rather than true obesity. Our findings suggest that the distribution of fat is more important than obesity, and visceral fat plays a central role in the process of CR-POPF development. The relationship between fatty tissue and the development of pancreatic fistula is complex and may be partially explained by the emerging view that considers visceral fat as an endocrine organ, able to modulate inflammatory pathways 28,29. It has been observed that adipose tissue can produce hormone-like adipokines that are involved in the regulation of metabolism and the immune system and can secrete proinflammatory cytokines 30–33. This proinflammatory microenvironment can be exacerbated and potentiated by an anastomotic dehiscence like a CR-POPF. Tumor development at inflammatory sites has been observed in multiple tissues, including pancreas, suggesting that the microenvironment of a chronic wound may stimulate cancer cell growth and recurrence 34–37. Neoplastic cells may acquire metastatic potential and preferential growth in wound sites with persisting inflammation 38,39. Furthermore, the effects of locally activated pancreatic enzymes, with their intense lytic activity in a fatty tissue, may be devastating. Indeed, we observed a significant increase in serious complications in patients with increased PTP. The systemic spillover of mediators from visceral fat persistently activated by a pancreatic fistula may also account for the well-known generalized consequences of a severe POPF such as systemic inflammatory response syndrome and subsequent organ dysfunction. Increased visceral fat as indicated by increased PTP may also make the technical aspects of the operation more challenging for the operating surgeon, including dissection and ease of anastomosis.
Multiple previous studies have shown that visceral fat found on preoperative CT scan is a risk factor for CR-POPF 40–43. These studies have utilized various methods for assessing visceral fat, including measurements of visceral fat area, total fat area, and retro-renal fat thickness. Measurements of this nature require specialized software and expertise that may not be widely available to clinicians 44–46. In our study we describe a surrogate measurement of visceral fat that can easily be utilized in the clinic setting with similar predictive power as more technologically intricate methods. This information may help patients make more informed decisions about the benefits and risks of surgery. Preoperative identification of patients at higher risk of CR-POPF may help physicians optimize and individualize perioperative management by filtering patients into high-risk perioperative management pathways or through the utilization of drains or the use of somatostatin analogues such as pasireotide, based on surgeon or institutional preference47,48.
The present study has several limitations. First, we performed a retrospective analysis of the data at a single institution. Therefore, the biases and limitations of a retrospective data apply to our study. Also, a prospective validation of the results is lacking and the risk of POPF was stratified by estimates derived from a logistic analysis. Pancreatic texture was defined as soft, intermediate, or firm based on the surgeon’s intraoperative assessment, rendering it challenging to utilize the commonly used Fistula Risk Score and alternative Fistula Risk Score, which define pancreatic texture as firm or soft, to predict patients at risk for CR-POPF. Patients were analyzed based on the median value for measurements of PTP, pancreatic neck thickness, and abdominal wall thickness, which limits its applicability to other populations. Preoperative CT acquisition settings such as slice thickness were not standardized and were acquired at multiple locations on different machines. Additionally, patients with only MRI or remote CT scan were excluded from the study. This was done in an effort to ensure that measured parameters on imaging were similar to how the patient presented during surgery. In doing so, patients with benign pathology not requiring multiple restaging scans before surgery may have been filtered due to timing of their imaging. The data include patients undergoing surgery with multiple surgeons of different experience curves and methodology. Internal review of surgeon outcomes revealed no differences in CR-POPF rates across surgeons included in the study. Despite these limitations, our study determines a novel factor that is associated with development of CR-POPF in a large number of patients at a single institution.
5. Conclusion
We demonstrated that a preoperative computed tomography scan evaluating the thickness of the pancreatic neck as well as measurement of the intraabdominal distance from the peritoneum to the pancreas may help identify patients at increased risk of clinically significant postoperative pancreatic fistula after pancreatoduodenectomy. This may help physicians risk-stratify, optimize and individualize perioperative patient management to identify strategies to improve clinical outcomes.
Synopsis.
Postoperative pancreatic fistula is a potentially devastating complication after pancreatoduodenectomy. This article identified two simple measurements on preoperative computed tomography that can indicate that a patient is at increased risk of developing this serious complication.
Acknowledgements:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number K12 CA237806 from the Emory K12 Clinical Oncology Training Program. The Content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support in part by the Contardi Research Fellowship and the Georgia CTSA UL1 Program (grant number UL1 TR002378). The acknowledged parties had no role in study design, data collection, analysis and interpretation of data, manuscript writing and decision to submit the manuscript for publication.
References
- 1.McMillan MT, Soi S, Asbun HJ, et al. Risk-adjusted Outcomes of Clinically Relevant Pancreatic Fistula Following Pancreatoduodenectomy: A Model for Performance Evaluation. Ann Surg. Aug 2016;264(2):344–52. doi: 10.1097/SLA.0000000000001537 [DOI] [PubMed] [Google Scholar]
- 2.McMillan MT, Vollmer CM Jr., Asbun HJ, et al. The Characterization and Prediction of ISGPF Grade C Fistulas Following Pancreatoduodenectomy. J Gastrointest Surg. Feb 2016;20(2):262–76. doi: 10.1007/s11605-015-2884-2 [DOI] [PubMed] [Google Scholar]
- 3.Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. Jan 2013;216(1):1–14. doi: 10.1016/j.jamcollsurg.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. Mar 2017;161(3):584–591. doi: 10.1016/j.surg.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 5.Pulvirenti A, Ramera M, Bassi C. Modifications in the International Study Group for Pancreatic Surgery (ISGPS) definition of postoperative pancreatic fistula. Transl Gastroenterol Hepatol. 2017;2:107. doi: 10.21037/tgh.2017.11.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma LW, Dominguez-Rosado I, Gennarelli RL, et al. The Cost of Postoperative Pancreatic Fistula Versus the Cost of Pasireotide: Results from a Prospective Randomized Trial. Ann Surg. Jan 2017;265(1):11–16. doi: 10.1097/SLA.0000000000001892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mungroop TH, Klompmaker S, Groot Koerkamp B, Besselink MG, Dutch Pancreatic Cancer G. Added Value of Body Fat Distribution in Predicting Clinically Significant Pancreatic Fistula in the a-FRS Following Pancreatoduodenectomy Currently Unclear. Ann Surg. Jan 2019;269(1):e2–e3. doi: 10.1097/SLA.0000000000002831 [DOI] [PubMed] [Google Scholar]
- 8.Mungroop TH, van Rijssen LB, van Klaveren D, et al. Alternative Fistula Risk Score for Pancreatoduodenectomy (a-FRS): Design and International External Validation. Ann Surg. May 2019;269(5):937–943. doi: 10.1097/SLA.0000000000002620 [DOI] [PubMed] [Google Scholar]
- 9.Joo I, Lee JM, Lee ES, et al. Preoperative CT Classification of the Resectability of Pancreatic Cancer: Interobserver Agreement. Radiology. Nov 2019;293(2):343–349. doi: 10.1148/radiol.2019190422 [DOI] [PubMed] [Google Scholar]
- 10.Zins M, Matos C, Cassinotto C. Pancreatic Adenocarcinoma Staging in the Era of Preoperative Chemotherapy and Radiation Therapy. Radiology. May 2018;287(2):374–390. doi: 10.1148/radiol.2018171670 [DOI] [PubMed] [Google Scholar]
- 11.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. Aug 2009;250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. Aug 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol. Sep 2014;21(9):2873–81. doi: 10.1245/s10434-014-3722-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hank T, Sandini M, Ferrone CR, et al. Association Between Pancreatic Fistula and Long-term Survival in the Era of Neoadjuvant Chemotherapy. JAMA Surg. Oct 1 2019;154(10):943–951. doi: 10.1001/jamasurg.2019.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neoptolemos JP, Kleeff J, Michl P, Costello E, Greenhalf W, Palmer DH. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol. Jun 2018;15(6):333–348. doi: 10.1038/s41575-018-0005-x [DOI] [PubMed] [Google Scholar]
- 16.De Pastena M, van Bodegraven EA, Mungroop TH, et al. Distal Pancreatectomy Fistula Risk Score (D-FRS): Development and International Validation. Ann Surg. Jul 7 2022;doi: 10.1097/SLA.0000000000005497 [DOI] [PubMed] [Google Scholar]
- 17.He C, Zhang Y, Li L, Zhao M, Wang C, Tang Y. Risk factor analysis and prediction of postoperative clinically relevant pancreatic fistula after distal pancreatectomy. BMC Surg. Jan 11 2023;23(1):5. doi: 10.1186/s12893-023-01907-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okano K, Oshima M, Kakinoki K, et al. Pancreatic thickness as a predictive factor for postoperative pancreatic fistula after distal pancreatectomy using an endopath stapler. Surg Today. Feb 2013;43(2):141–7. doi: 10.1007/s00595-012-0235-4 [DOI] [PubMed] [Google Scholar]
- 19.Sugimoto M, Kendrick ML, Farnell MB, et al. Relationship between pancreatic thickness and staple height is relevant to the occurrence of pancreatic fistula after distal pancreatectomy. HPB (Oxford). Mar 2020;22(3):398–404. doi: 10.1016/j.hpb.2019.07.010 [DOI] [PubMed] [Google Scholar]
- 20.Maeda K, Kuriyama N, Yuge T, et al. Risk factor analysis of postoperative pancreatic fistula after distal pancreatectomy, with a focus on pancreas-visceral fat CT value ratio and serrated pancreatic contour. BMC Surg. Jun 22 2022;22(1):240. doi: 10.1186/s12893-022-01650-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mungroop TH, van der Heijde N, Busch OR, et al. Randomized clinical trial and meta-analysis of the impact of a fibrin sealant patch on pancreatic fistula after distal pancreatectomy: CPR trial. BJS Open. May 7 2021;5(3)doi: 10.1093/bjsopen/zrab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akamatsu N, Sugawara Y, Komagome M, et al. Risk factors for postoperative pancreatic fistula after pancreaticoduodenectomy: the significance of the ratio of the main pancreatic duct to the pancreas body as a predictor of leakage. J Hepatobiliary Pancreat Sci. May 2010;17(3):322–8. doi: 10.1007/s00534-009-0248-6 [DOI] [PubMed] [Google Scholar]
- 23.Barbier L, Mege D, Reyre A, Moutardier VM, Ewald JA, Delpero JR. Predict pancreatic fistula after pancreaticoduodenectomy: ratio body thickness/main duct. ANZ J Surg. May 2018;88(5):E451–E455. doi: 10.1111/ans.14048 [DOI] [PubMed] [Google Scholar]
- 24.Roberts KJ, Storey R, Hodson J, Smith AM, Morris-Stiff G. Pre-operative prediction of pancreatic fistula: is it possible? Pancreatology. Jul-Aug 2013;13(4):423–8. doi: 10.1016/j.pan.2013.04.322 [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto M, Takahashi S, Kojima M, Kobayashi T, Gotohda N, Konishi M. In Patients with a Soft Pancreas, a Thick Parenchyma, a Small Duct, and Fatty Infiltration Are Significant Risks for Pancreatic Fistula After Pancreaticoduodenectomy. J Gastrointest Surg. May 2017;21(5):846–854. doi: 10.1007/s11605-017-3356-7 [DOI] [PubMed] [Google Scholar]
- 26.Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. Mar 2008;32(3):419–28. doi: 10.1007/s00268-007-9388-5 [DOI] [PubMed] [Google Scholar]
- 27.Hashimoto Y, Sclabas GM, Takahashi N, et al. Dual-phase computed tomography for assessment of pancreatic fibrosis and anastomotic failure risk following pancreatoduodenectomy. J Gastrointest Surg. Dec 2011;15(12):2193–204. doi: 10.1007/s11605-011-1687-3 [DOI] [PubMed] [Google Scholar]
- 28.Kawai T, Autieri MV, Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am J Physiol Cell Physiol. Mar 1 2021;320(3):C375–C391. doi: 10.1152/ajpcell.00379.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coelho M, Oliveira T, Fernandes R. Biochemistry of adipose tissue: an endocrine organ. Arch Med Sci. Apr 20 2013;9(2):191–200. doi: 10.5114/aoms.2013.33181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia SH, Li Y, Parodo J, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. May 2004;113(9):1318–27. doi: 10.1172/JCI19930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Yang Q, Cai D, et al. Resistin, a Novel Host Defense Peptide of Innate Immunity. Front Immunol. 2021;12:699807. doi: 10.3389/fimmu.2021.699807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stoffel NU, El-Mallah C, Herter-Aeberli I, et al. The effect of central obesity on inflammation, hepcidin, and iron metabolism in young women. Int J Obes (Lond). Jun 2020;44(6):1291–1300. doi: 10.1038/s41366-020-0522-x [DOI] [PubMed] [Google Scholar]
- 33.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. Oct 2006;6(10):772–83. doi: 10.1038/nri1937 [DOI] [PubMed] [Google Scholar]
- 34.Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. Jul 12 2021;6(1):263. doi: 10.1038/s41392-021-00658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greten FR, Grivennikov SI. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. Jul 16 2019;51(1):27–41. doi: 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh N, Baby D, Rajguru JP, Patil PB, Thakkannavar SS, Pujari VB. Inflammation and cancer. Ann Afr Med. Jul-Sep 2019;18(3):121–126. doi: 10.4103/aam.aam_56_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagai N, Kudo Y, Aki D, Nakagawa H, Taniguchi K. Immunomodulation by Inflammation during Liver and Gastrointestinal Tumorigenesis and Aging. Int J Mol Sci. Feb 24 2021;22(5)doi: 10.3390/ijms22052238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Lin PC, Zhou BP. Inflammation fuels tumor progress and metastasis. Curr Pharm Des. 2015;21(21):3032–40. doi: 10.2174/1381612821666150514105741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathur A, Hernandez J, Shaheen F, et al. Preoperative computed tomography measurements of pancreatic steatosis and visceral fat: prognostic markers for dissemination and lethality of pancreatic adenocarcinoma. HPB (Oxford). Jun 2011;13(6):404–10. doi: 10.1111/j.1477-2574.2011.00304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandini M, Bernasconi DP, Ippolito D, et al. Preoperative Computed Tomography to Predict and Stratify the Risk of Severe Pancreatic Fistula After Pancreatoduodenectomy. Medicine (Baltimore). Aug 2015;94(31):e1152. doi: 10.1097/MD.0000000000001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tajima Y, Kawabata Y, Hirahara N. Preoperative imaging evaluation of pancreatic pathologies for the objective prediction of pancreatic fistula after pancreaticoduodenectomy. Surg Today. Feb 2018;48(2):140–150. doi: 10.1007/s00595-017-1529-3 [DOI] [PubMed] [Google Scholar]
- 42.Tranchart H, Gaujoux S, Rebours V, et al. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg. Jul 2012;256(1):139–45. doi: 10.1097/SLA.0b013e318256c32c [DOI] [PubMed] [Google Scholar]
- 43.Park CM, Park JS, Cho ES, Kim JK, Yu JS, Yoon DS. The effect of visceral fat mass on pancreatic fistula after pancreaticoduodenectomy. J Invest Surg. Jun 2012;25(3):169–73. doi: 10.3109/08941939.2011.616255 [DOI] [PubMed] [Google Scholar]
- 44.Savin ML, Mihai F, Gheorghe L, et al. Proposal of a Preoperative CT-Based Score to Predict the Risk of Clinically Relevant Pancreatic Fistula after Cephalic Pancreatoduodenectomy. Medicina (Kaunas). Jun 24 2021;57(7)doi: 10.3390/medicina57070650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolbinger FR, Lambrecht J, Leger S, et al. The image-based preoperative fistula risk score (preFRS) predicts postoperative pancreatic fistula in patients undergoing pancreatic head resection. Sci Rep. Mar 8 2022;12(1):4064. doi: 10.1038/s41598-022-07970-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frozanpor F, Loizou L, Ansorge C, Lundell L, Albiin N, Segersvard R. Correlation between preoperative imaging and intraoperative risk assessment in the prediction of postoperative pancreatic fistula following pancreatoduodenectomy. World J Surg. Sep 2014;38(9):2422–9. doi: 10.1007/s00268-014-2556-5 [DOI] [PubMed] [Google Scholar]
- 47.Menahem B, Guittet L, Mulliri A, Alves A, Lubrano J. Pancreaticogastrostomy is superior to pancreaticojejunostomy for prevention of pancreatic fistula after pancreaticoduodenectomy: an updated meta-analysis of randomized controlled trials. Ann Surg. May 2015;261(5):882–7. doi: 10.1097/SLA.0000000000000806 [DOI] [PubMed] [Google Scholar]
- 48.Garg PK, Sharma J, Jakhetiya A, Chishi N. The Role of Prophylactic Octreotide Following Pancreaticoduodenectomy to Prevent Postoperative Pancreatic Fistula: A Meta-Analysis of the Randomized Controlled Trials. Surg J (N Y). Oct 2018;4(4):e182–e187. doi: 10.1055/s-0038-1675359 [DOI] [PMC free article] [PubMed] [Google Scholar]


