Abstract
Complex chromosome rearrangements, known as chromoanagenesis, are widespread in cancer. The most frequent type based on large-scale DNA sequencing of human tumors is chromothripsis, a massive, localized and clustered rearrangement of one (or a few) chromosomes seemingly acquired in a single event. Chromothripsis can be initiated by mitotic errors that produce a micronucleus encapsulating a single chromosome or chromosomal fragment. Rupture of the unstable micronuclear envelope exposes its chromatin to cytosolic nucleases and induces chromothriptic shattering. Found in up to half of tumors included in pan-cancer genomic analyses, chromothriptic rearrangements can contribute to tumorigenesis through inactivation of tumor suppressor genes, activation of proto-oncogenes, or gene amplification through production of self-propagating extrachromosomal circular DNAs (ecDNAs) encoding oncogenes or genes conferring anti-cancer drug resistance. Here, we discuss what has been learned about the mechanisms which enable these complex genomic rearrangements and their consequences in cancer.
Introduction
Most cancer genomes are highly rearranged. Karyotyping first identified abnormal chromosome morphology as a common feature of cancers1–5 (reviewed in ref. 6,7), and advances in DNA sequencing and analysis tools8,9 subsequently led to the identification of complex chromosome rearrangements in cancer. Along with a myriad of mutations10–13, 80–90% of cancer genomes14,15 (reviewed in ref. 16) are now known to possess large-scale genomic rearrangements, which include numerical and structural alterations that can inactivate tumor suppressor genes (by loss or disruption) or activate proto-oncogenes (by amplification, translocation or generation of oncogenic fusions) (reviewed in ref. 17,18). An umbrella, mechanism-independent term has been coined to describe the full spectrum of these large-scale rearrangements19: ‘chromoanagenesis’ (or ‘rebirth of the chromosome’ from the Greek chromo for chromosomes and anagenesis for to be reborn). The most frequent, and by far the best studied, is chromothripsis20, which is the focus of this review. In addition to chromothripsis, two less well understood processes have been reported: chromoanasynthesis21 and chromoplexy22 (Box 1).
Box 1. Types of chromoanagenesis
In addition to chromothripsis, two less well understood processes have been reported: chromoanasynthesis and chromoplexy (see the table below for similarities and differences between these three phenomena).
Chromoanasynthesis was identified in constitutional genomic disorders21 as a phenomenon occurring in the germline, driving localized gains and losses of chromosomal regions via DNA replication-based mechanisms, including microhomology-mediated template switching21. It was later detected in ~5% of cancers33 and expanded to other contexts of aberrant DNA replication, such as those caused by the actions of DNA crosslinking agents175. Chromoplexy is characterized by rearrangements between multiple chromosomes (in some cases involving five chromosomes ‘woven’ together22,175), which represent largely balanced translocations (in contrast to profound losses of chromatin material observed in chromothripsis). Initially described in prostate cancer22, chromoplexy was later reported in sarcomas142 and carcinomas176,177 and observed in ~10% of tumors included in a pan-cancer analysis33. Experimental models are needed to shed light on the mechanisms of chromoplexy, and in particular, to test whether chromoplexy is an ‘all-at-once’ event or is an outcome of multiple aberrant cell cycles driving tumorigenesis.
| Chromoanagenesis | |||
|---|---|---|---|
| Chromothripsis | Chromoplexy | Chromoanasynthesis | |
| Origin | ‘chromo’ for chromosomes and ‘thripsis’ for shattering into pieces | ‘chromo’ for chromosomes and ‘pleko’ for braid or weave together | ‘chromo’ for chromosomes and ‘anasynthesis’ for reconstitution |
| Definition | A single event of local chromosome shattering that produces tens to hundreds of fragments, followed by random restitching driven by NHEJ | Co-occurrence of multiple inter- and intra-chromosomal translocations and (to a lesser extent) deletions, frequently involving five or more chromosomes | A replication-based process with local rearrangements and altered gene copy numbers due to serial fork stalling or template switching, or microhomology-mediated break-induced replication |
| Single event | Yes | ? | Yes |
| Chromosomes | 1–2 | ≥5 | 1–2 |
| Deletions | Yes | Yes | Yes |
| Duplications | Rare | Rare | Frequent |
| Translocations | Yes | Yes | Yes |
| Frequency in cancer a | 40–60% | 10% | 5% |
Inferred from Ref 33.
Chromothripsis was first described in 201120 and defined as the shattering of one or a few chromosomes into tens to hundreds of 0.1–10 Mb fragments, followed by their re-ligation in a random order. In this process, some fragments (especially ones lacking a functional centromere) might fail to be incorporated into a derivative chromosome. As a result, the genome of a cancer cell contains a massively rearranged ‘chromothriptic’ chromosome composed of randomly localized and oriented pieces, with some regions amplified and others missing, compared with sequenced reference chromosomes20,23. Starting with a striking example of a tumor isolated from a patient with chronic lymphocytic leukemia (CLL), Stephens et al.20 expanded their analysis to 746 cancer cell lines and 2,792 cancers, and observed a common, distinctive pattern of genomic rearrangements. These findings were consistent with cytogenetic analysis, which confirmed multiple segments from different chromosomes assembled into derivative ‘chromothriptic’ chromosomes20. Subsequently, a set of criteria were proposed to distinguish chromothripsis from other complex genomic rearrangements, in addition to the slow acquisition of somatic cell mutations: (1) breakpoint clustering, followed by regions with intact chromosome sequence; (2) copy-number oscillation; (3) regular oscillating pattern of segments with retained heterozygosity interspersed with loss-of-heterozygosity; (4) rearrangements detected on a single parental copy (haplotype); (5) random joining of chromosome fragments; and (6) random position of joined fragments and the ability to ‘reconstruct’ the derivative chromosome by joining breakpoints23.
Chromothripsis can be initiated by mitotic errors that produce micronuclei encapsulating a single chromosome or chromosomal fragment as a result of chromosome missegregation or breakage of a chromosomal bridge24–28. Rupture of the unstable micronuclear envelope exposes its chromatin to cytosolic nucleases and induces chromothriptic shattering. Thus, chromothripsis is posited to result from a single rearrangement event rather than a stepwise series of somatic cell mutations. Conceptually, the identification of complex genomic rearrangements occurring in a single event challenged the then-prevailing view of cancer evolution in which mutations and genomic alterations were gradually acquired29 (reviewed in ref. 17,18).
The initial recognition of chromothripsis in a broad range of cancers20,30–32 was confirmed and expanded by subsequent large-scale efforts including the international collaboration within the framework of the Pan-Cancer Analysis of Whole Genome (PCAWG) Consortium (Table 1). Estimates by the PCAWG Consortium, which analysed 2,658 tumors from 38 cancer types33,34, and similar efforts35–38 showed a very high frequency of chromothripsis in the majority of human cancers reaching 100% for liposarcomas33. Chromothripsis has been associated with poor clinical outcomes in multiple cancer types30–32,39–42, as also observed by pan-cancer efforts33,35. In addition, chromothripsis was implicated in the formation of oncogene-bearing extrachromosomal circular DNAs (ecDNAs)20,26,43,44, another increasingly recognized hallmark of the rearranged cancer genome45,46.
Table 1.
Pan-cancer analyses of chromothripsis
| Study | Methods of detection | Algorithms of detection | Sources | Samples n | Cancer types n | Estimated frequency | Associated oncogene amplifications | Associated loss of suppressors |
|---|---|---|---|---|---|---|---|---|
| Cortes-Ciriano et al., Nat Genet 2020-Ref.33 | WGS | ShatterSeq | PCAWG | 2,658 | 38 | 40–60% | 20% | 2% |
| Voronina et al., Nat Commun 2020-Ref.35 | WGS, WES | ShatterSeq | NCT/DKTK MASTER | 634 | 28 | 49% | Examples identified, % not reported | |
| Rasnic and Linial, Cancers 2021-Ref.36 | CNA | ML based on ShatterSeq | TCGA | 10,728 | 20 | 39% | Examples identified, % not reported | |
| Steele et al., Nature 2022-Ref.37 | WGS, WES, SNP6 profiling | NA | TCGA PCAWG | 9,873 | 33 | NA, 5 signatures of chromothripsis | Examples identified, % not reported | |
| Bao et al., Nat Cancer 2022-Ref.38 | WGS | Starfish | PCAWG | 2,428 | 37 | 53%, 6 CGR signatures | Examples identified, % not reported | |
WGS: whole genome sequencing; WES: whole exome sequencing; CNA: copy number alteration; SNP6: single nucleotide polymorphism (SNP) 6.0 microarrays; ML: machine learning; PCAWG: Pan-Cancer Analysis of Whole Genomes; NCT/DKTK MASTER program: Molecularly Aided Stratification for Tumour Eradication; TCGA: The Cancer Genome Atlas; CGR: Complex genomic rearrangements, NA: not available.
Here, we review the methods to identify chromothripsis in cancer genomes, discuss the mechanisms of formation of these complex genomic rearrangements as a result of micronuclei or chromosomal bridge ruptures, and showcase their consequences in cancer. In addition, we highlight the link between chromothripsis and the accumulation of ecDNA.
Methods to detect chromothripsis in cancer genomes
Given its strikingly high prevalence and association with poor prognosis, the efficient detection of chromothripsis in cancer is important in both clinical and research contexts. Whole-genome sequencing (WGS) relying on paired-end20, mate-pair47 or long-read sequencing48, followed by subsequent in silico analyses, and combined with a variety of imaging-based techniques enables the identification of chromothripsis in cancer genomes (Fig. 1). Ideally, detection of chromothripsis relies on using complementary techniques. A ‘gold-standard’ approach combined paired-end WGS with single-nucleotide polymorphism (SNP) microarrays, spectral karyotyping and multicolor fluorescence in situ hybridization (FISH)20.
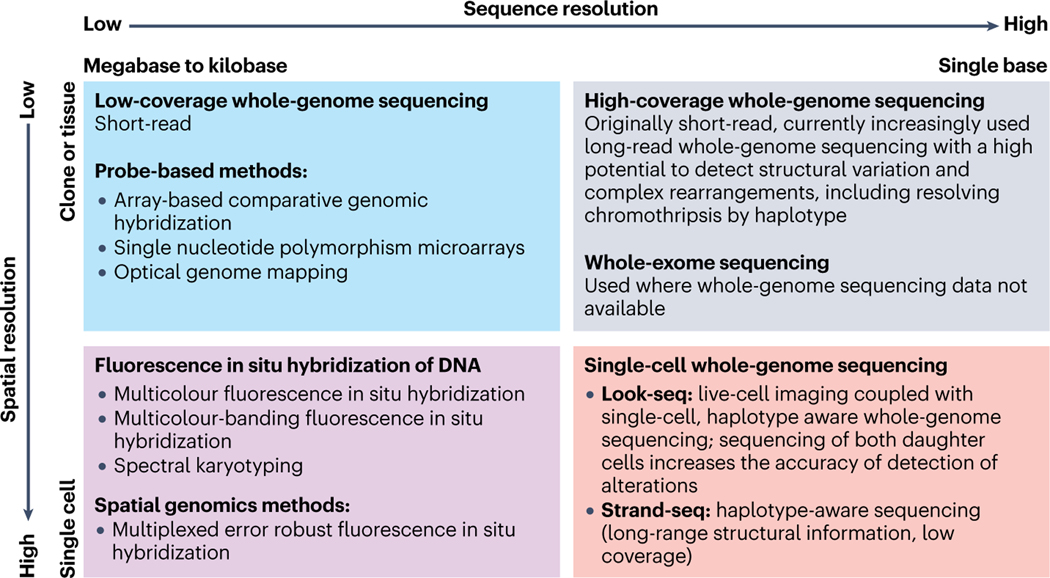
Fig. 1. Methods to detect chromothripsis with different spatial and sequence resolution.
Complementary techniques applied for the detection of chromothripsis vary in the precision of sequence resolution (ranging from the megabases to single nucleotides, horizontal axis) and spatial resolution (from clones or tissue samples to single cells, vertical axis). Top left square: examples of high-throughput techniques with relatively low spatial/sequence resolution: low coverage whole genome sequencing (WGS) and in situ hybridization probe-based methods; top right square: the highest sequence resolution with low spatial resolution is achieved by high coverage WGS (might be combined with whole exome sequencing); bottom left square: advanced fluorescent-in-situ-hybridization techniques provide the highest (single-cell) spatial/relatively low (within kilobases to megabases) sequence resolution; bottom right square: single-cell WGS aims at achieving the highest spatial/sequence resolution, but the scarcity of genomic material remains a significant challenge.
Sequence analysis algorithms that align multiple reads generated by high-coverage WGS of control and cancer samples, followed by comparison with a reference human genome (the latest version being produced by the Telomere-to-Telomere (‘T2T’) Consortium49), and perhaps manual curation of putative cases, represent the most thorough approach available today to identify complex genome rearrangements within tumor-derived DNA isolated from millions of cells20,33,35–38 (Fig. 1, top right square). limitation of the sequence analysis algorithms include arbitrary cut-offs for the number/density of rearrangements or copy number variations in the sequence analysis, as well as lack of haplotype information, which impact the frequency of detection of true events. The recent advent of long-read WGS inarguably facilitates the detection of structural variations and complex rearrangements, including resolving chromothripsis events by haplotype (see, for example, ref. 50–52. Besides WGS, whole-exome sequencing (WES) can be used to infer chromothripsis-related copy number changes35,42. Of note, low-coverage WGS, sufficient to detect copy number variations, has been reported to be highly predictive of chromothripsis, albeit at the cost of lower sensitivity42 (Fig. 1, top left square). Included here are array-based comparative genomic hybridization (aCGH), which identifies small-size chromosome aberrations and copy number alterations21,39,53, SNP microarrays22,47,54,55, and optical genome mapping (OGM) (which directly introduces fluorescent labels into genomic DNA and detects genome-wide structural variations ranging from ~500 bp to megabases)44,56–58. These analyses are, unavoidably, confounded by continuing genomic instability and the presence of a variable proportion of normal cells within each tumor sample.
A single-cell level of resolution would be a powerful addition, partially achievable in experimental instances by single-cell WGS approaches, such as in ‘Look-Seq’26, an elegant method that combines long-term live imaging of cultured dividing cells with single-cell WGS of their progeny (Fig. 1, bottom right square). While this method is limited by low throughput, amplification noise, and the incomplete sequence coverage, sequence assessment of both daughter cells markedly increases accuracy of detection of small copy number alterations. Successful attempts at identifying chromothripsis using DNA sequencing of single cells isolated from tumors have been reported for glioblastoma59, T cell acute lymphoblastic leukemia60–61 (using the Strand-Seq approach)61, and medulloblastoma62. The development of long-read sequencing of single cells (such as in ref. 63) is expected to further facilitate the detection of chromothripsis at the single-cell level.
It should be recognized that methods to detect chromothripsis at the single-cell level within an intact tumor sample would be of great advantage, including extension of the recently developed in situ genome sequencing64 or optical methods using multi-probe in situ hybridization with subchromosomal resolution, such as multicolor DNA FISH20, multicolor-banding FISH (M-BAND)65, and spectral karyotyping (SKY)20 (Fig. 1, bottom left square). The genomic resolution of FISH-based detection of chromothripsis is likely to be massively improved with the help of high-resolution techniques such as Multiplexed Error Robust Fluorescence In Situ Hybridization (MERFISH) that is capable of simultaneously measuring spatial distribution and copy number of thousands of DNA loci66,67 (Fig. 1, bottom left square).
Finally, resources such as ChromothripsisDB (http://cailab.labshare.cn/ChromothripsisDB)68 and Chromothripsis Explorer (http://compbio.med.harvard.edu/chromothripsis/)33 allow defining in detail case-specific chromothriptic events and chromothripsis frequencies in different cancer types. Identification of links between tumor genetic landscapes, phenotypes and chromothripsis in cancer is likely to be significantly enhanced as these or similar tools are integrated into the massive cancer genome databases (such as cBioPortal69 https://www.cbioportal.org/, ICGC70 https://dcc.icgc.org/, COSMIC71 https://cancer.sanger.ac.uk/cosmic and GDC72 https://portal.gdc.cancer.gov/).
How to scramble the genome: Mechanisms of chromothripsis
A salient feature of chromothripsis – its confinement to one or a few chromosomes – immediately raised the mechanistic question of how the affected fraction of the genome is singled out to undergo shattering and subsequent re-ligation. This ‘localized’ action might occur if a chromosome or chromosomal region to be shattered is initially separated from the rest of the chromosomes (Fig. 2). Two errors in cell division do just that: 1) if a chromosome (or acentric fragment of a broken chromosome) is not attached to the mitotic spindle and correspondingly lags behind when other chromosomes segregate to the spindle poles in anaphase and thereby generating a micronucleus in the next interphase or 2) if a chromosome containing two active centromeres (a ‘dicentric chromosome’) is simultaneously pulled to opposite spindle poles, resulting in a chromosomal bridge, whose subsequent breakage produces broken pieces that become micronucleated (Fig. 2).
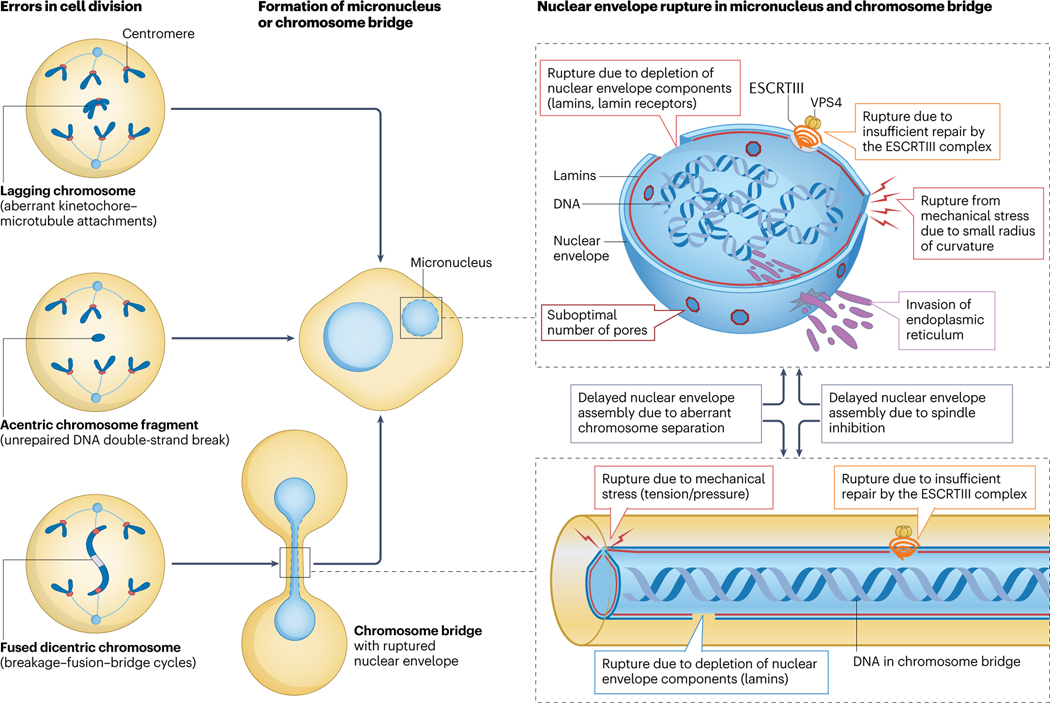
Fig. 2. Mechanisms of micronuclei and chromosome bridges formation and rupture.
Errors in cell division, which involve physical separation of a chromosome or chromosome region from the rest of the chromosomes (such as a lagging chromosome or acentric chromosome fragments), lead to the formation of a micronucleus in the subsequent interphase. Dicentric chromosomes result in the formation of a chromosome bridge when two centromeres are pulled apart to the opposite poles in mitosis; subsequently, chromosome fragments generated following the breakage of the chromosome bridge become micronucleated. Nuclear envelope assembly in micronuclei and chromosome bridges is delayed due to aberrant chromosome separation in anaphase and spindle inhibition. Furthermore, nuclear envelopes of both micronuclei and chromosome bridges are prone to rupture due to reduced levels of some nuclear envelope proteins (including lamin B1), suboptimal numbers of nuclear pores, mechanical tension, and stress due to small radius of curvature, endoplasmic reticulum (ER) invasion and insufficient repair of the rupture sites by the ESCRTIII complex.
Chromothripsis due to chromosome shattering in a micronucleus
Micronuclei are small, nuclei-like compartments, spatially separated from the primary nucleus and whose chromosomal DNA is encapsulated by a frequently abnormal nuclear envelope (reviewed in ref. 73,74). A common feature of cancer cells, micronuclei are formed by lagging chromosomes or chromosome fragments that persist into interphase after failing to be included into a primary nucleus after cell division (Fig. 2). As early as in 1968, it was recognized that chromosomes within micronuclei were delayed in DNA replication, continuing to replicate well after chromosomes in the main nucleus had finished replication75.
The nuclear envelope encapsulating micronuclei is frequently deficient in incorporation of sufficient nuclear pores24,76 into topologically constrained envelopes with high curvature77, leading to delayed completion of micronuclear DNA replication and DNA repair, and inhibited transcription (reviewed in ref. 74) (Fig. 2 and Fig. 3). It also undergoes spontaneous, irreversible rupture76,78, exposing the previously encapsulated chromatin to cytosolic factors (Fig. 2). Multiple contributing factors have been implicated in the rupture of a micronuclear envelope, including the loss or mislocalization of the nuclear lamina component Lamin B178,79, depletion of proteins of nuclear envelope76, invasion of endoplasmic reticulum78,80, and aberrant accumulation of proteins normally involved in the repair of nuclear envelopes (including endosomal sorting complexes required for transport-III (ESCRT-III))80,81. Compelling experimental evidence from cellular models suggests that chromosomes from ruptured micronuclei undergo chromothriptic shattering24,26 (Fig. 3).
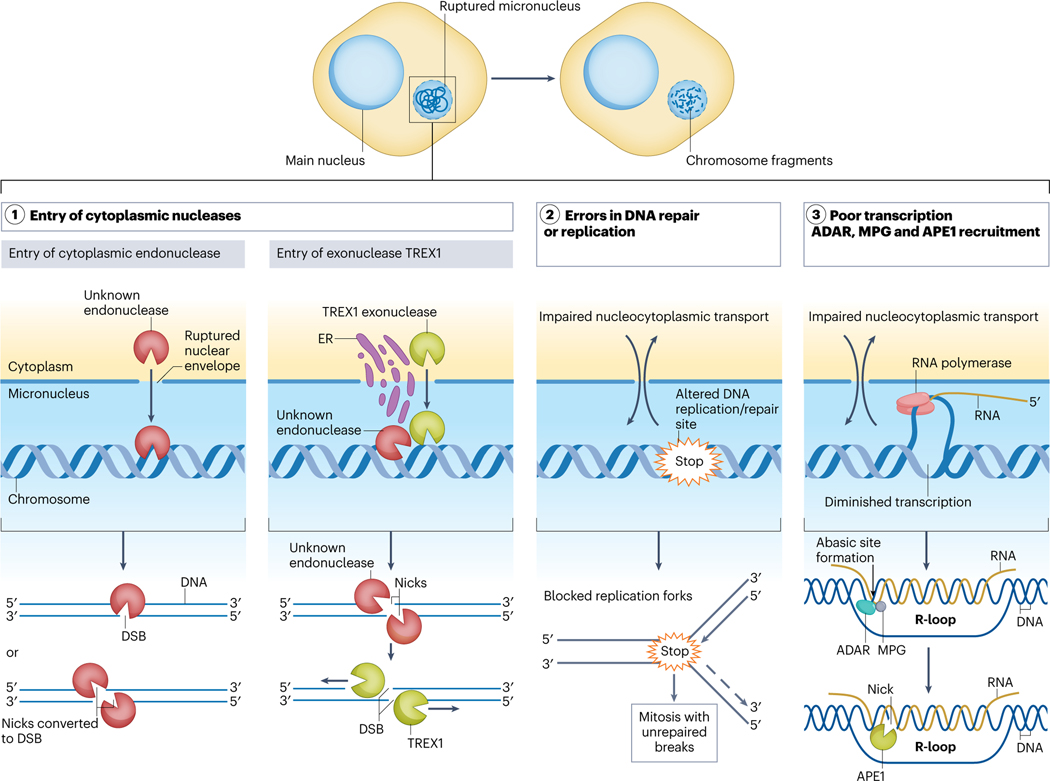
Fig. 3. Molecular mechanisms initiating chromosome fragmentation.
Chromosomes in ruptured micronuclei are prone to the accumulation of DNA damage and subsequent chromosome fragmentation. (1) Entry of cytoplasmic nuclease(s) upon nuclear envelope rupture including (a) endonucleases that cause generation of multiple nicks and double strand breaks and can lead to chromosome shattering; (b) the entry of endoplasmic reticulum- and nuclear envelope-associated exonuclease TREX1 (the Three-prime Repair EXonuclease 1) induces DNA damage, however, additional factors are likely to be involved, including endonucleases/nickases which generate the initial nicks serving as the substrates for TREX1; (2) Loss of nuclear components and entry of cytosolic factors in ruptured micronuclei leads to altered DNA replication and repair, enzyme inhibition, accumulation of unrepaired DNA crosslinks and nucleotide misincorporation. As a result, a micronucleated chromosome enters mitosis with unrepaired DNA breaks, which may contribute to its fragmentation; or (3) Ruptured micronuclei-associated attenuation of transcription and accumulation of RNA-DNA hybrids triggers DNA base excision repair. RNA-DNA hybrids are edited by adenine deaminases acting on RNA (ADAR) transforming deoxyadenosine in these hybrids into deoxyinosine; next, N-methyl-purine DNA glycosylase (MPG) removes deoxyinosine to produce an abasic site. The abasic sites are next cleaved by apurinic/apyrimidinic endonuclease (APE1), which generates single-stranded DNA nicks.
Experimentally induced missegregation of a specific chromosome into a micronucleus (by selectively inactivating the centromere of the Y chromosome)28, followed by selection for retention of a selectable marker gene inserted into that chromosome, generated complex genomic rearrangements mimicking those found in human cancer, producing the first experimentally generated chromothriptically rearranged chromosomes in which pieces of the shattered Y chromosome were incorporated into an autosome82. Micronuclei formation is now recognized as a major triggering event for chromosome shattering, followed by aberrant reassembly of the resulting fragments24,26,28,82. The size and position of a chromosome of a chromosome has been argued to be a predictive factor for its missegregation into micronuclei, with the larger and most peripheral chromosomes frequently forming micronuclei83,84 to initiate chromothripsis33,84 and with nuclear envelope rupture occurring more often in micronuclei with larger chromosomes and lower gene density85. Future genomic analyses of cancer samples will likely determine whether chromothriptic breakpoints preferentially happen in gene poor regions of larger chromosomes.
Finally, certain viruses have been implicated in the generation of micronuclei, which contribute to chromothripsis in infected cells, including cancer-related human papilloma virus86,87 and Epstein–Barr virus (EBV)88, a virus to which almost the entire human population has been exposed and which has been linked to multiple human tumors9. In the latter case, the breakage of a fragile DNA site on chromosome 11 induced by the sequence-specific DNA binding protein EBNA188 encoded by EBV, occurs between the tumor suppressor ATM and the mixed lineage leukaemia (MLL) proto-oncogene, producing an acentric MLL-containing fragment that will be micronucleated and subjected to chromothripsis88.
Chromothripsis due to chromosome shattering in a bridge
Besides micronuclei, spatial separation of a chromosome or chromosomal region occurs when chromosomes form bridges between nascent daughter cells in anaphase, which then persist into interphase (reviewed in ref. 89) (Fig. 2). The major underlying mechanism behind bridge formation is the ‘breakage-fusion-bridge’ (BFB) process discovered by Barbara McClintock in the late 1930’s90. BFB can be triggered by the loss of telomere protection, upon which the DNA repair machinery recognizes unprotected chromosome ends as the sides of a DNA double-strand break and acts to ligate them together to produce fusions of duplicated chromosomes (reviewed in ref. 91). The resulting fused chromosomes contain two active centromeres (‘dicentric chromosomes’) and thus can be simultaneously pulled to opposite poles during anaphase, forming a bridge. BFB may well be recurrent, repeating in the next cell divisions, as the subsequent resolving (‘breakage’) of this bridge would again produce chromosomes without telomeres (reviewed in ref. 89,91). Similar to micronuclei, the nuclear envelope assembled around DNA in bridges is frequently abnormal38,25 (Fig. 2), with the ‘breakage’ itself producing small micronuclei on both sides of the bridge43,92,93. Not surprisingly, multiple lines of evidence indicate that BFB leading to dicentric bridges could serve to initiate chromothriptic rearrangements20,25,43,44,94–96.
Factors inducing chromosome shattering in micronuclei and bridges
Chromothripsis has been proposed as an ‘all-at-once’ event based on the analysis of mathematical simulations favoring a single cellular catastrophe over progressive accumulation of rearrangements19. This has been validated in experimentally induced chromothripsis following micronucleation26,82. Mechanistically, chromosome shattering (i.e., the generation of numerous DNA double-strand breaks) is likely an early step of such genome rearrangements, producing chromosome fragments in the cells with micronuclei or bridges, as has been observed in multiple models24,26,75,97 and directly linked to the micronucleated chromosome28,82.
How is localized DNA damage achieved? For chromatin within a micronucleus, likely drivers of DNA damage are nuclear envelope rupture-provoked abnormalities, including entry of one or more cytoplasmic nucleases or disrupted DNA repair or DNA replication24,26 (Fig. 3). The endoplasmic reticulum- and nuclear envelope-associated three-prime repair exonuclease 1 (TREX1), a major exonuclease established to degrade single-stranded DNA in mammalian cells, especially in protecting against autoimmune induction of DNA-sensing pathways98, has been reported to accumulate in micronuclei99 and chromosome bridges25. TREX1 has been reported to contribute to bridge resolution and chromothripsis in a model of telomere crisis-induced bridges25,100, albeit another study found no role for TREX1 in chromosome bridge breakage in a similar example of telomere crisis-induced bridges96. TREX1-mediated DNA damage upon nuclear envelope rupture has been reported in some101 but not other102 cancer models. This controversy aside, it seems likely that additional nucleases are involved, especially yet to be identified cytosolic endonucleases that may enter ruptured micronuclei or bridge encapsulated chromatin (reviewed in ref. 74) (Fig. 3). Indeed, in instances where TREX1 is involved, its known exonuclease activity implies that other nuclease(s) or factor(s) would be needed to co-operate with TREX1 to produce chromosome fragments25,100, such as Ankyrin repeat and LEM domain-containing protein 1 (ANKLE-1/LEM-3) nuclease, which has most recently been implicated in the bridge resolution along with TREX1103–105.
Beyond nuclease-induced shattering, chromosome breakage has been proposed to be initiated by micronuclei-associated inhibition of transcription followed by aberrant DNA repair106 (Fig. 3), a process requiring a chain of at least four events: 1) an accumulation of RNA–DNA hybrids due to disrupted transcription, with 2) adenine deaminases acting on RNA (ADAR) transforming deoxyadenosine in these hybrids into deoxyinosine, which is 3) then removed by a DNA base excision repair (BER) glycosylase such as MPG (N-methyl-purine DNA glycosylase) to produce an abasic site, which is in turn 4) cleaved by the BER endonuclease, apurinic/apyrimidinic endonuclease APE1 to create single-strand DNA nicks that are somehow converted into DNA double-strand breaks106. For DNA within chromosome bridges, action of mechanical forces may also physically tear chromatin into pieces, an idea proposed for the actin cytoskeleton-mediated resolution of bridges in interphase96 and for chromosomes within micronuclei in which mechanical stress was imposed by premature chromatin condensation107. In addition, exerting tension on DNA within chromosome bridges could increase accessibility to nucleases that may provide another contributor to induction of chromosome shattering.
Additional possibilities for initiating chromosome fragmentation could be 1) abortive apoptosis, referred to as anastasis108,109; 2) escape from digestion of chromatin by the autophagy–lysosome system, which has been reported to participate in what seems to be an evolutionary conserved removal of micronuclei110–114; 3) double-strand break-generating mechanisms through micronucleus formation, including EBNA1-induced breakage of a fragile site on chromosome 1188, and CRISPR-Cas9-mediated DNA double strand breaks, which both can produce acentric chromosome fragments that will be micronucleated.
Chromothripsis inadvertently induced by therapy
Recognizing that a broken, acentromeric chromosome fragment is expected to undergo micronucleation, two types of therapeutic avenues are potential triggers of this initiating step of chromothripsis. First, ionizing radiation, well known to induce DNA double-strand breaks, was proposed20 and then reported to induce chromothripsis-like chromosomal rearrangements in oral squamous cell carcinoma cell lines, possibly via direct localized action in nuclei and/or by inducing micronuclei115. Of note, ionizing radiation-mediated generation of DNA double-strand breaks is likely not restricted to one chromosome, and thus might also potentially trigger chromoplexy, which involves translocations between multiple chromosomes (Box 1). Second, CRISPR-Cas9-mediated genome editing, which generates targeted DNA double-strand breaks followed by repair (reviewed in ref. 116,117) can produce an acentric fragment that will missegregate into micronuclei following cell division, while the centromere-bearing fragments might fuse, leading to the formation of bridges. Indeed, this has been documented experimentally during gene editing in retinal pigment epithelial cells (with chromothripsis detected using Look-Seq analysis), in CD34+ hematopoietic stem and progenitor cells (HSPCs)118, and in editing of hyperploid hepatocellular carcinoma (HepG2)119.
Furthermore, chemotherapy-induced chromothripsis was shown in hyperploid cell lines treated with DNA double strand breaks-inducing doxorubicin95 and in cancer cell lines treated with a dihydrofolate reductase inhibitor methotrexate27. Most relevant, metastases of human tumors caused by an activating mutation in the B-Raf kinase were found to have undergone chromothripsis including incorporation and amplification of the mutant BRAF gene into ecDNA following treatment of the initial tumor with vemurafenib, a selective inhibitor of the mutant kinase27. These findings are particularly important in light of the fact that multiple anti-cancer therapies are known to induce DNA damage that generates micronuclei; thus, chromothripsis needs to be considered among potential adverse outcomes (reviewed in ref. 74).
Constructing a chromothriptic chromosome: putting fragments back together
The images of chromosome fragments occurring in metaphase spreads (see, for example28,75) raise the question of how the many, scattered pieces could be re-assembled to produce a heritable, rearranged chromosome. Live imaging of intact cells has demonstrated that without the mechanical and/or osmolarity-mediated forces produced during chromosome spreading, micronuclei-derived chromosome fragments remain largely clustered in mitosis typically with one daughter cell inheriting most or all of the pieces24,120,121 (Fig. 4). The bundle of fragments either becomes a new micronucleus or is incorporated into the main nucleus of one daughter cell. This tethering has been proposed to be mediated by a three-protein complex consisting of Mediator of DNA damage checkpoint 1 (MDC1) (which directly binds to γH2AX, a histone H2AX phosphorylated on Ser139122, that marks double stranded DNA breaks (DSBs)), Cellular Inhibitor of Protein phosphatase 2A (CIP2A), and DNA Topoisomerase II Binding Protein 1 (TOPBP1)120 or a two-protein complex of just CIP2A and TOPBP1120,121 (Fig. 4). Transient degron-induced reduction in CIP2A during the mitosis following chromosome micronucleation-dependent chromosome shattering was shown to drive acquisition of segmental deletions and inversions120, and a FISH-based analysis of Y chromosome rearrangements identified less complex rearrangements in CIP2A knockout cells121.
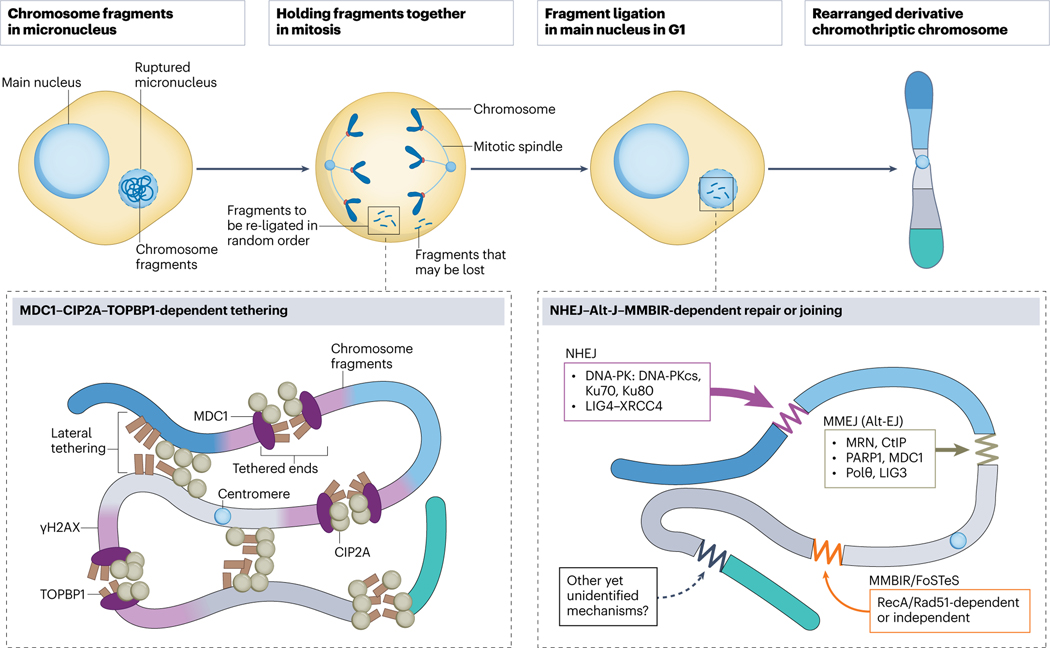
Fig. 4. Molecular mechanisms for chromosome reassembly.
Chromosome fragments from a ruptured micronucleus are held together in mitosis, which prevents their loss and is crucial for the subsequent reassembly of a derivative chromothriptic chromosome. This tethering is mediated by a three-protein complex which includes Mediator of DNA damage checkpoint 1 (MDC1) directly binding to γH2AX at double strand DNA breaks, Cellular Inhibitor of Protein phosphatase 2A (CIP2A) and DNA Topoisomerase II Binding Protein 1 (TOPBP1) or a two-protein complex of just CIP2A and TOPBP1. Reassembly of the random fragments into a chromothriptic chromosome occurs in a main nucleus in the next G1 phase. Non-homologous end-joining (NHEJ) is considered a major pathway involved in fragment re-ligation, followed by Microhomology-mediated end-joining, MMEJ (a type of alternative end-joining, Alt-EJ) which repairs clusters of DNA double strand breaks. Microhomology-mediated break-induced replication (MMBIR) and fork stalling and template switching (FoSTeS) are other replication-associated pathways which rely on microhomology.
Once deposited into a daughter cell nucleus, a plethora of intertwined DNA repair mechanisms seem to contribute to the reassembly of the pieces into a derivative chromothriptic chromosome (Fig. 4). Two complementary types of analyses have directly implicated key players in DNA repair20,28,43,82 or the potentially involved DNA repair pathways by inspection of breakpoints (i.e., junctions of fragments in reassembled chromothriptic chromosomes)26,33. These efforts have firmly supported that canonical non-homologous end-joining (NHEJ) is a major pathway involved in fragment re-ligation following selective chromosome missegregation into micronuclei, as reduction in key NHEJ components DNA Ligase IV (LIG4) or DNA-dependent protein kinase, catalytic subunit (DNA-PKcs) resulted in a twofold increase in Y chromosome fragmentation frequency28. In addition, other pathways have been implicated including the DNA replication-related repair pathway, termed fork stalling and template switching (FoSTeS)123 (later renamed microhomology-mediated break-induced replication- MMBIR124) has been implicated in micronuclei-associated chromothripsis26,125, as well chromoanasynthesis21. Another mechanism has been reported126 to rely on alternative end-joining, alt-EJ, (or more specifically microhomology-mediated end joining, MMEJ) via Poly (ADP-ribose) Polymerase 1 (PARP1) and MDC1 (ref. 127). Both canonical NHEJ43 and MMBIR128 were also reported to be necessary for bridge- and BFB-associated chromothripsis. Finally, a pan-cancer analysis of chromothripsis confirmed a principal role of NHEJ, with the involvement of alt-EJ, MMBIR and other replication-associated mechanisms33, overall suggesting that the primary repair pathway is likely to depend on cell type, cell cycle stage and origin of chromosomal breaks.
Consequences of chromothripsis in cancer
Chromothripsis is frequently detected as a clonal event, indicating that it occurs early and thus likely contributes to tumorigenesis20,23,30. This is puzzling, as at first glance chromothripsis may seem to be unlikely to provide any selective advantage to the cancer cell: multiple gene disruptions are expected to be neutral at best, or even fatal if essential genes are targeted. Furthermore, in normal cells, chromothripsis-causing cell division errors activate multiple protective mechanisms that can arrest the cell cycle and ultimately trigger cell death if the errors remain unresolved129. Therefore, it seems quite likely that the majority of ‘chromothripsogenic’ events are quickly eliminated.
However, chromothriptic rearrangements can result in positive effects on cell survival or proliferation (including activation of proto-oncogenes or inactivation of tumor suppressors), which lead to the transformation into a cancer cell (Fig. 5). A strong association of chromothripsis with disruption of the major tumor suppressor, ‘the guardian of the genome’, p53 (encoded by TP53), has been shown for medulloblastoma30,130 and acute myeloid leukemia (AML)40,114. Subsequent pan-cancer analyses confirmed the correlation between chromothripsis and mutated or deleted TP53, reporting ~50% higher prevalence of chromothripsis in the patients with inactivating TP53 mutations compared to patients with the wild-type TP53 (ref. 33). While a contributing factor, inactivation of the p53 pathway does not seem to be necessary, as ~60% of identified tumor samples with chromothripsis have been reported to bear neither TP53 mutations nor amplification33 of MDM2 (a negative regulator of p53), in addition, humans with congenital cases of chromothripsis lack TP53 mutations131–134.
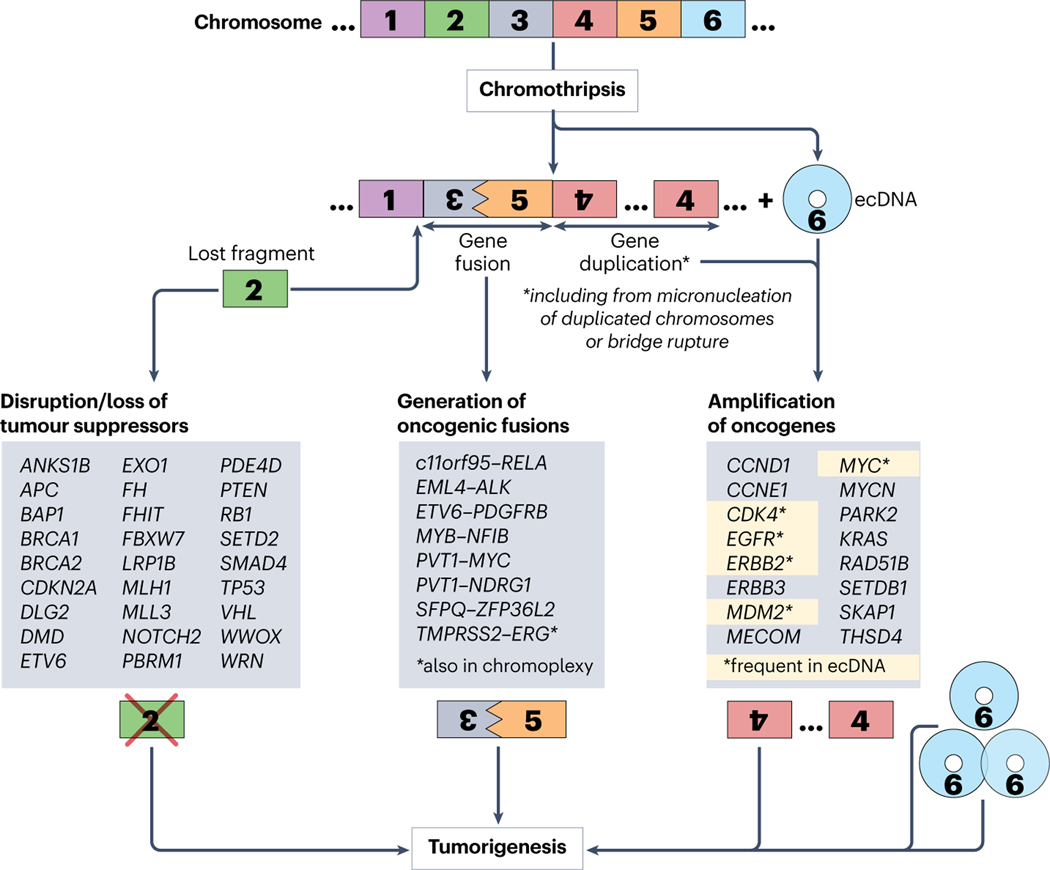
Fig. 5. Mechanisms through which chromothripsis drives tumorigenesis.
Three chromothripsis-associated mechanisms are thought to be capable of driving tumorigenesis: 1) disruption or loss of tumor suppressors, 2) generation of oncogenic fusions, and 3) amplification of oncogenes, with the most prevalent pathway being through the generation of circular, self-propagating and oncogene-bearing extrachromosomal DNA (ecDNA). Chromothripsis-affected genes, identified in patient-derived tumors, are listed for each mechanism.
How can chromothripsis promote cancer cell survival or proliferation? The outcomes of random reshuffling of DNA within coding sequences might lead to the gene disruption, amplification or fusion. Below, we discuss in detail the gene-altering consequences of chromothripsis, potentially contributing to oncogenesis.
Genes affected by chromothripsis: lost suppressors, amplified oncogenes and gene fusions
In their pioneering study, Campbell and colleagues20 identified chromothripsis-mediated losses of the tumor suppressors CDKN2A (in CLL, a thyroid cancer cell line, and a bone sarcoma type, chordoma), FBXW7 and WRN (in chordoma), as well as amplifications of MYC (in a small cell lung cancer cell line), and seventeen potential in-frame fusions. Subsequent efforts identified chromothripsis-mediated disruption or deletion of multiple tumor suppressors (Fig. 5), including ETV6, EXO1, FH, FHIT, MLL3 and NOTCH2 in colorectal cancer47, RB1 in retinoblastoma135, VHL, PBRM1, BAP1, and SETD2 in clear cell renal cell carcinoma136, SMAD4 in esophageal adenocarcinoma137 and pancreatic cancer (along with the chromothripsis-mediated loss of CDKN2A and TP53, and amplification of MYC and KRAS138). Reported examples of chromothripsis-mediated gene fusions that may enhance/alter oncogene expression or activity include PVT1-MYC and PVT1-NDRG1 in medulloblastoma130, c11orf95-RELA in ependymoma139, EML4-ALK in a non–small-cell lung cancer cell line140, ETV6-PDGFRB in myelodysplastic syndrome/myeloproliferative neoplasm141, EWSR1-ETS in Ewing sarcoma142, and SFPQ-ZFP36L2 in T-cell acute lymphoblastic leukemia143.
A pan-cancer analysis of whole genome sequences of 2,658 tumors from 38 cancer types (by the PCAWG Consortium) identified chromothriptic rearrangements that included both focal amplifications of oncogenes (at a frequency of ~20%, including amplifications of CCND1, CDK4, ERBB2, ERBB3, MDM2, MYC, MYCN and SETDB1) and losses of tumor suppressors or DNA repair genes (with respective frequencies of 2.1% and 1.9%) including the loss of APC, BRCA1, BRCA2, MLH1, PTEN, SMAD4 and TP53) (ref. 33 and Table1). Analysis of complex chromoanagenic rearrangements in an initial 799 tumors overlapping between PCAWG and The Cancer Genome Project (TCGA), and then expanded to an additional ~10,000 tumors from TCGA, identified deletions in tumor suppressors ANKS1B, DLG2, DMD, LRP1B, PDE4D and WWOX and potentially oncogenic amplifications of MECOM, PARK2, RAD51B, SKAP1, and THSD4 (ref. 36). A subsequent analysis of PCAWG/TCGA datasets also reported positive association between chromothriptic signatures and amplification of oncogenes, including CCNE1, EGFR, ERBB2, MDM2 and MYC 37. Further analyses of the PCAWG sequences identified that almost half of the identified 2,493 gene fusions (1116, or 44.8%) had at least one fusion partner derived from a chromothriptic region33 and estimated fivefold increase in fusion genes in tumors with chromothripsis35, including highly oncogenic MYB-NFIB (in head and neck adenoid cystic carcinoma35) and TMPRSS2-ERG (in prostate cancer38, this fusion also associated with chromoplexy22). Interference with gene regulatory elements is another potential outcome of chromothripsis, with chromothripsis-mediated enhancer hijacking reported for multiple myeloma41.
Chromothripsis as a major driver of ecDNA
Originally described as small, abundant intranuclear chromatin bodies distinct from chromosomes, and initially designated as double minutes due to a characteristic ‘double-dot’ appearance on chromosomal spreads144, extrachromosomal DNAs (ecDNAs) are circular, typically 1–3 Mb-long chromosome-derived DNA species that Paul Michel and colleagues45 identified to be present in nearly half of human cancers (reviewed in ref 145–147). Earlier efforts established that double minutes/ecDNAs contained drug resistance genes (in particular, dihydrofolate reductase encoding DHFR, whose amplification results in the resistance to a long used anticancer drug methotrexate148, a dihydrofolate reductase inhibitor, and oncogenes, such as MDM2 (ref. 149). A recent pan-cancer analysis found ecDNAs in a large majority (25 out of 29) of analysed cancer types46. Examples of oncogenes frequently found in ecDNA include AKT1, CDK4, E2F3, EGFR, ERBB2, MDM2, MYC, MYCL, NEDD9, PDGFRA, SOX2 and TERT46,146 (Fig. 5). Having no centromeres and telomeres, ecDNAs behave as self-propagating entities that may undergo multiple rounds of replication and are asymmetrically inherited; their amplification and accumulation provides ecDNA-containing cancer cells with selective growth advantages and increased resistance to chemotherapy, and promotes tumor heterogeneity (reviewed in 145–147).
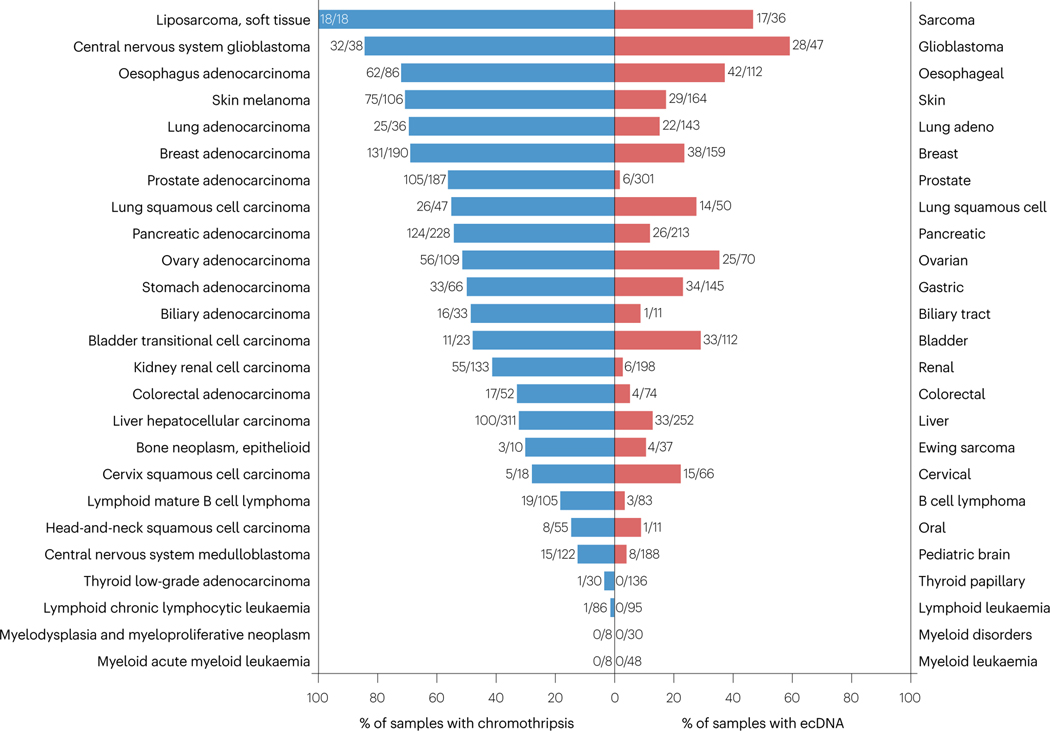
As ecDNAs are circularized fragments of chromosomes, chromothripsis seems a ‘primary suspect’ in promoting ecDNA biogenesis. Indeed, chromosomal shattering and re-ligation via chromothripsis has been shown to contribute to double minute/ecDNA formation20. Both major initiating mechanisms of chromothripsis drive ecDNA formation: shattering of missegregated chromosomes within micronuclei26 and breakage of dicentric chromosomes formed during a BFB cycle43,44. In the latter case, the resulting ecDNAs are sometimes encapsulated into small-size micronuclei43. To highlight the link between chromothripsis and ecDNA on a pan-cancer level, we compared prior PCAWG- and TCGA-based analyses of chromothripsis33 and ecDNA46 and plotted the frequencies of chromothripsis and ecDNA for 25 cancer types matching between these two studies (Fig. 6). Clearly, there is a high degree of co-occurrence between chromothripsis and ecDNA in analysed cancers: tumors (soft tissue sarcoma, glioblastoma and esophageal cancers) with the highest frequencies of chromothripsis (according to ref. 33) were also the ones with the highest frequency of ecDNA46, while thyroid, lymphoid leukemia and myeloid cancers (characterized by the absence/very low levels of chromothripsis) were ecDNA-negative (Fig. 6). It is therefore very likely that chromothripsis plays a major role in generating ecDNA, thus driving oncogene amplification or drug resistance. Correspondingly, chromothripsis and ecDNA need to be taken into consideration together when developing potential therapies and/or studying mechanisms of cancer.
Fig. 6. Pan-cancer genome analysis reveals frequent co-occurrence of chromo-thripsis and ecDNA in 25 cancer types.
Left, blue: percentages of chromothripsis-positive samples per cancer type are from Cortes-Ciriano et al., Nat Genet 2020; descending from top to bottom. Right, red: percentages of ecDNA-positive samples per cancer type are from Kim et al., Nat Genet 2020. Cancer types, similar or matching between the two studies are shown. Fractions show the number of chromothripsis-positive (left, blue) or ecDNA-positive (right, red) samples per total number of samples analyzed per given cancer type in the corresponding study. Left (blue): the cancer types abbreviations (top to bottom) are: SoftTissue-Liposarc, liposarcoma, soft tissue; CNS-GBM, central nervous system glioblastoma; Eso-AdenoCA, esophagus adenocarcinoma; Skin-Melanoma, skin melanoma; Lung-AdenoCA, lung adenocarcinoma; Breast-AdenoCA, breast adenocarcinoma; Prost-AdenoCA, prostate adenocarcinoma; Lung-SCC, lung squamous cell carcinoma; Panc-AdenoCA, pancreatic adenocarcinoma; Ovary-AdenoCA, ovary adenocarcinoma; Stomach-AdenoCA, stomach adenocarcinoma; Biliary-AdenoCA, biliary adenocarcinoma; Bladder-TCC, bladder transitional cell carcinoma; Kidney-RCC, kidney renal cell carcinoma; ColoRect-AdenoCA, colorectal adenocarcinoma; Liver-HCC, liver hepatocellular carcinoma; Bone-Epith, bone neoplasm, epithelioid; Cervix-SCC, cervix squamous cell carcinoma; Lymph-BNHL, lymphoid mature B-cell lymphoma; Head-SCC, head-and-neck squamous cell carcinoma; CNS-Medullo, CNS medulloblastoma; Thy-AdenoCA, thyroid low-grade adenocarcinoma; Lymph-CLL, lymphoid chronic lymphocytic leukemia; Myeloid-MDS, myeloid myelodysplastic syndrome; Myeloid-MPN, myeloid myeloproliferative neoplasm; Myeloid-AML, myeloid acute myeloid leukemia.
Chromothripsis and immunity
Besides their immediate impact on cancer cell survival and proliferation, chromothripsis-produced gene fusions may result in the generation of neoantigens, which can be presented by the major histocompatibility complex (MHC) proteins and recognized by immune T cells, thus contributing to anti-cancer immunity. This ‘neoantigenic potential’ of chromothripsis has been recently demonstrated for malignant pleural mesothelioma, associated with exposure to asbestos150; further studies will likely expand these observations to other cancer types.
Micronuclei and bridges, the DNA-containing structures associated with the induction of chromothripsis, also play an important role in cellular immunity. Rupture of micronuclei or formation of DNA bridges exposes DNA/chromatin to the cytosol and thus might trigger anti-DNA immunity151–159 via recruitment and activation of the DNA-sensing cyclic GMP-AMP synthase (cGAS)160–162 that produces the second messenger cyclic GMP-AMP (cGAMP), which in turn activates innate immune responses, including the induction of interferons via the Stimulator of interferon genes (STING)160,162. That a DNA-sensing immune pathway is seemingly activated concomitantly with the occurrence of chromothripsis suggests the link between genome rearrangements, cellular immunity, and tumorigenesis. The complexity of this potential link is further enhanced by the observations of cGAS exacerbating DNA damage163,164 and cGAMP inducing a DNA damage response165.
Chromothripsis beyond malignant tumors
Besides malignant tumors, chromothripsis is also implicated in- the development and progression of benign tumors131–133. As such, WGS analysis of uterine leiomyomas, benign smooth-muscle tumors, prevalent among women of reproductive age166, identified chromothripsis in 19% of MED12-mutated leiomyomas131,133 and chromothripsis was associated with the tissue-specific changes observed in these tumors, such as translocations of the HMGA2 and RAD51B loci and aberrations at the COL4A5–COL4A6 locus131,133. Interestingly, leiomyomas with chromothriptic rearrangements do not show any signs of malignancy and are characterized by the presence of normal TP53131–134. A substantial proportion of such rearrangements led to the activation of apoptosis and senescence due to functional cell cycle checkpoints, but some were associated with the growth and proliferation131–133.
Indeed, chromothripsis is not restricted to tumors. An unprecedented cure of an inherited disease as a result of chromothriptic rearrangements was described in 2015 (ref. 167). A patient with WHIM syndrome (Warts, Hypogammaglobulinemia, recurrent Infections and Myelokathexis), an immunodeficiency caused by dominant mutations in the chemokine receptor CXCR4, provided an example of inarguably beneficial chromothripsis, which occurred after the disease had been diagnosed and led to the deletion of the disease-producing allele CXCR4R334X, along with 163 other genes from one copy of chromosome 2. These rearrangements presumably took place in a single hematopoietic stem cell, which later repopulated the whole myeloid lineage.
Complex constitutional (i.e., occurring in germ cells and preimplantation embryos) chromosome rearrangements that resemble chromothripsis and chromoanasynthesis can be found in various congenital diseases. A first example was reported in 2011, when 12 apparently simultaneous rearrangements involving 3 chromosomes were identified in a child with severe mental retardation and multiple developmental abnormalities168. Subsequent efforts characterized ‘constitutional chromothripsis’ in individuals with various developmental phenotypes (such as growth retardation, facial dysmorphism, hypotonia and mental disabilities)125,169–174. Compared to what has been observed in cancers, constitutional chromothripsis is much more often characterized by balanced rearrangements and fewer number of breakpoints and can involve multiple chromosomes125,169–174, thus somewhat resembling chromoplexy and chromoanasynthesis (due to the presence of microhomologies) (Box 1).
Conclusions and perspectives
Research over the past decade has established that the most frequent form of chromoanagenesis, chromothripsis, is a hallmark of the majority of human cancers. Elegant cellular models have provided important mechanistic clues on how chromothripsis can be initiated as a consequence of chromosome segregation errors. Widespread occurrence of chromothripsis is one of the major lessons that has been learned from pan-cancer sequencing.
Understanding what factors — and why, how, where and when — induce chromothripsis and allow for reassembly and functioning of heavily rearranged chromosomes in different cancer types is still far from complete. Even less is known about the two other types of chromoanagenesis, chromoanasynthesis and chromoplexy (Box 1). More is understood as to how chromothripsis can drive tumorigenesis; identified mechanisms include: 1) oncogene amplification, with the most common (and efficient) way being through the generation of ecDNA, 2) the disruption of tumor suppressors, and 3) the formation of oncogenic fusions. Furthermore, the mere presence of chromothripsis might be indicative of spindle assembly checkpoint malfunction129 that is permissive of an initiating chromosome missegregation.
In clinical practice, we expect further improvement of the analysis tools to detect chromothripsis and ecDNAs in tumor samples, and accurately assess its potential consequences, including increased resistance to therapies. The key feature of chromothripsis, its apparently random reshuffling of chromosomal DNA, leads to the generation of genomically unique tumors, including neoantigens. This might be exploited on multiple levels as a potential ‘Achilles heel’, by developing tailored and personalized therapies, which 1) target unique genomic regions in a given tumor; 2) accelerate anti-tumor immune response via detection of chromothripsis-produced cancer neoantigens, and 3) target metabolic vulnerabilities arising from simultaneously acquired multiple and random gene deletions, amplifications, and/or fusions.
Finally, besides tumors, chromothripsis has been found in non-cancer cells and in both animals and plants (Box 2). With the development of single-cell methods of detection, it may be possible to determine whether chromothripsis occurs in postmitotic cells (such as neurons). We believe that rapid development of genome sequencing and analysis will allow addressing the role of chromothripsis in this broader context, including its potential role(s) in the establishment of cellular specificity and the evolution of eukaryotes.
Box 2. Genome rearrangements in plants
Extensive genome rearrangements have also been reported in plants. One of the most striking examples was in Arabidopsis thaliana upon haploid induction mediated by mutation in the centromeric histone H3 (CENH3), which resulted in both chromothripsis and chromoplexy, along with the formation of micronuclei during early zygotic divisions178. Additional instances of chromoanagenesis have been found in Poplar trees (Populus sp.) produced from gamma-irradiated pollen, which are characterized by clustered rearrangements and high copy number variations179,180. In this example, multiple DNA breaks and reassembly were described, with the breakpoints clustered on a single chromosome. In addition, multiple copy number states (two to five) and high occurrence of fragment duplications and multiplications were consistent with chromoanasynthesis179,180. Other examples of extensive chromoanagenesis-like rearrangements were observed in transformed maize (Zea mays)181, rice (Oryza sativa)181, potato (Solanum tuberosum)182 and grapewine (Vitis vinifera)183. Largely protected from negative consequences of chromosome rearrangements due to polyploidy, plants provide another powerful model to study the mechanisms and consequences of chromoanagenesis.
Acknowledgements
The authors apologize to all colleagues whose work has not been included due to space and format constraints. The authors received salary support from NIH grant R35 GM122476.
REFERENCES
- 1.Nowell PC & Hungerford DA Minute Chromosome in Human Chronic Granulocytic Leukemia. Science 132, 1497–1497 (1960). [Google Scholar]
- 2.Kaung DT & Swartzendruber AA Effect of chemotherapeutic agents on chromosomes of patients with lung cancer. Dis Chest 55, 98–100, doi: 10.1378/chest.55.2.98 (1969). [DOI] [PubMed] [Google Scholar]
- 3.Van Steenis H. Chromosomes and cancer. Nature 209, 819–821, doi: 10.1038/209819a0 (1966). [DOI] [PubMed] [Google Scholar]
- 4.Ishihara T, Kikuchi Y. & Sandberg AA Chromosomes of twenty cancer effusions: correlation of karyotypic, clinical, and pathologic aspects. J Natl Cancer Inst 30, 1303–1361 (1963). [PubMed] [Google Scholar]
- 5.Rowley JD Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243, 290–293, doi: 10.1038/243290a0 (1973). [DOI] [PubMed] [Google Scholar]
- 6.Thompson SL & Compton DA Chromosomes and cancer cells. Chromosome Res 19, 433–444, doi: 10.1007/s10577-010-9179-y (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frohling S. & Dohner H. Chromosomal abnormalities in cancer. N Engl J Med 359, 722–734, doi: 10.1056/NEJMra0803109 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Lander ES et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921, doi: 10.1038/35057062 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Venter JC et al. The sequence of the human genome. Science 291, 1304–1351, doi: 10.1126/science.1058040 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Cibulskis K. et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31, 213–219, doi: 10.1038/nbt.2514 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tate JG et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res 47, D941–D947, doi: 10.1093/nar/gky1015 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexandrov LB et al. Signatures of mutational processes in human cancer. Nature 500, 415–421, doi: 10.1038/nature12477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandrov LB et al. The repertoire of mutational signatures in human cancer. Nature 578, 94–101, doi: 10.1038/s41586-020-1943-3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor AM et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 33, 676–689 e673, doi: 10.1016/j.ccell.2018.03.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drews RM et al. A pan-cancer compendium of chromosomal instability. Nature 606, 976–983, doi: 10.1038/s41586-022-04789-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-David U. & Amon A. Context is everything: aneuploidy in cancer. Nat Rev Genet 21, 44–62, doi: 10.1038/s41576-019-0171-x (2020). [DOI] [PubMed] [Google Scholar]
- 17.Stratton MR, Campbell PJ & Futreal PA The cancer genome. Nature 458, 719–724, doi: 10.1038/nature07943 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yates LR & Campbell PJ Evolution of the cancer genome. Nat Rev Genet 13, 795–806, doi: 10.1038/nrg3317 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland AJ & Cleveland DW Chromoanagenesis and cancer: mechanisms and consequences of localized, complex chromosomal rearrangements. Nat Med 18, 1630–1638, doi: 10.1038/nm.2988 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stephens PJ et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 144, 27–40, doi: 10.1016/j.cell.2010.11.055 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P. et al. Chromosome catastrophes involve replication mechanisms generating complex genomic rearrangements. Cell 146, 889–903, doi: 10.1016/j.cell.2011.07.042 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baca SC et al. Punctuated evolution of prostate cancer genomes. Cell 153, 666–677, doi: 10.1016/j.cell.2013.03.021 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korbel JO & Campbell PJ Criteria for inference of chromothripsis in cancer genomes. Cell 152, 1226–1236, doi: 10.1016/j.cell.2013.02.023 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Crasta K. et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature 482, 53–58, doi: 10.1038/nature10802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maciejowski J, Li Y, Bosco N, Campbell PJ & de Lange T. Chromothripsis and Kataegis Induced by Telomere Crisis. Cell 163, 1641–1654, doi: 10.1016/j.cell.2015.11.054 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang CZ et al. Chromothripsis from DNA damage in micronuclei. Nature 522, 179–184, doi: 10.1038/nature14493 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoshani O. et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature 591, 137–141, doi: 10.1038/s41586-020-03064-z (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ly P. et al. Selective Y centromere inactivation triggers chromosome shattering in micronuclei and repair by non-homologous end joining. Nat Cell Biol 19, 68–75, doi: 10.1038/ncb3450 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A 68, 820–823, doi: 10.1073/pnas.68.4.820 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rausch T. et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell 148, 59–71, doi: 10.1016/j.cell.2011.12.013 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molenaar JJ et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483, 589–593, doi: 10.1038/nature10910 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Magrangeas F, Avet-Loiseau H, Munshi NC & Minvielle S. Chromothripsis identifies a rare and aggressive entity among newly diagnosed multiple myeloma patients. Blood 118, 675–678, doi: 10.1182/blood-2011-03-344069 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortes-Ciriano I. et al. Comprehensive analysis of chromothripsis in 2,658 human cancers using whole-genome sequencing. Nat Genet 52, 331–341, doi: 10.1038/s41588-019-0576-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Consortium ITP-CA o. W. G. Pan-cancer analysis of whole genomes. Nature 578, 82–93, doi: 10.1038/s41586-020-1969-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voronina N. et al. The landscape of chromothripsis across adult cancer types. Nat Commun 11, 2320, doi: 10.1038/s41467-020-16134-7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasnic R. & Linial M. Chromoanagenesis Landscape in 10,000 TCGA Patients. Cancers (Basel) 13, doi: 10.3390/cancers13164197 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steele CD et al. Signatures of copy number alterations in human cancer. Nature 606, 984–991, doi: 10.1038/s41586-022-04738-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao L, Zhong X, Yang Y. & Yang L. Starfish infers signatures of complex genomic rearrangements across human cancers. Nat Cancer 3, 1247–1259, doi: 10.1038/s43018-022-00404-y (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirsch D. et al. Chromothripsis and focal copy number alterations determine poor outcome in malignant melanoma. Cancer Res 73, 1454–1460, doi: 10.1158/0008-5472.CAN-12-0928 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fontana MC et al. Chromothripsis in acute myeloid leukemia: biological features and impact on survival. Leukemia 32, 1609–1620, doi: 10.1038/s41375-018-0035-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rustad EH et al. Revealing the impact of structural variants in multiple myeloma. Blood Cancer Discov 1, 258–273, doi: 10.1158/2643-3230.BCD-20-0132 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maclachlan KH et al. Copy number signatures predict chromothripsis and clinical outcomes in newly diagnosed multiple myeloma. Nat Commun 12, 5172, doi: 10.1038/s41467-021-25469-8 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoshani O. et al. Chromothripsis drives the evolution of gene amplification in cancer. Nature, doi: 10.1038/s41586-020-03064-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosswog C. et al. Chromothripsis followed by circular recombination drives oncogene amplification in human cancer. Nat Genet 53, 1673–1685, doi: 10.1038/s41588-021-00951-7 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Turner KM et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature 543, 122–125, doi: 10.1038/nature21356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim H. et al. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers. Nat Genet 52, 891–897, doi: 10.1038/s41588-020-0678-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kloosterman WP et al. Chromothripsis is a common mechanism driving genomic rearrangements in primary and metastatic colorectal cancer. Genome Biol 12, R103, doi: 10.1186/gb-2011-12-10-r103 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakamoto Y. et al. Phasing analysis of lung cancer genomes using a long read sequencer. Nat Commun 13, 3464, doi: 10.1038/s41467-022-31133-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nurk S. et al. The complete sequence of a human genome. Science 376, 44–53, doi: 10.1126/science.abj6987 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lei M. et al. Long-read DNA sequencing fully characterized chromothripsis in a patient with Langer-Giedion syndrome and Cornelia de Lange syndrome-4. J Hum Genet 65, 667–674, doi: 10.1038/s10038-020-0754-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schopflin R. et al. Integration of Hi-C with short and long-read genome sequencing reveals the structure of germline rearranged genomes. Nat Commun 13, 6470, doi: 10.1038/s41467-022-34053-7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rausch T. et al. Long-read sequencing of diagnosis and post-therapy medulloblastoma reveals complex rearrangement patterns and epigenetic signatures. Cell Genom 3, 100281, doi: 10.1016/j.xgen.2023.100281 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Przybytkowski E. et al. Chromosome-breakage genomic instability and chromothripsis in breast cancer. BMC Genomics 15, 579, doi: 10.1186/1471-2164-15-579 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berry NK, Dixon-McIver A, Scott RJ, Rowlings P. & Enjeti AK Detection of complex genomic signatures associated with risk in plasma cell disorders. Cancer Genet 218–219, 1–9, doi: 10.1016/j.cancergen.2017.08.004 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Ortega V, Mendiola C. & Velagaleti GV N. Identification of Chromothripsis in Biopsy Using SNP-Based Microarray. Methods Mol Biol 1769, 85–117, doi: 10.1007/978-1-4939-7780-2_7 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Chan EKF et al. Optical mapping reveals a higher level of genomic architecture of chained fusions in cancer. Genome Res 28, 726–738, doi: 10.1101/gr.227975.117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang H. et al. High-resolution structural variant profiling of myelodysplastic syndromes by optical genome mapping uncovers cryptic aberrations of prognostic and therapeutic significance. Leukemia 36, 2306–2316, doi: 10.1038/s41375-022-01652-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramos-Campoy S. et al. TP53 Abnormalities Are Underlying the Poor Outcome Associated with Chromothripsis in Chronic Lymphocytic Leukemia Patients with Complex Karyotype. Cancers (Basel) 14, doi: 10.3390/cancers14153715 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francis JM et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov 4, 956–971, doi: 10.1158/2159-8290.CD-13-0879 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jeong H. et al. Functional analysis of structural variants in single cells using Strand-seq. Nat Biotechnol, doi: 10.1038/s41587-022-01551-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanders AD et al. Single-cell analysis of structural variations and complex rearrangements with tri-channel processing. Nat Biotechnol 38, 343–354, doi: 10.1038/s41587-019-0366-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalo Parra RP,MJ; Simovic M; Susak H; Ratnaparkhe M; Wong JKL; Körber V; Mallm P; Sill M; Kolb T; Kumar R; Casiraghi N; Norali Ghasemi DR; Maaβ KK; Pajtler KW; Jauch A; Korshunov A; Höfer T; Zapatka M; Pfister SM; Stegle O; Ernst A. Single cell multi-omics analysis of chromothriptic medulloblastoma highlights genomic and transcriptomic consequences of genome instability. bioRxiv, doi: 10.1101/2021.06.25.449944 (2021). [DOI] [Google Scholar]
- 63.Shiau CK et al. High throughput single cell long-read sequencing analyses of same-cell genotypes and phenotypes in human tumors. Nat Commun 14, 4124, doi: 10.1038/s41467-023-39813-7 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Payne AC et al. In situ genome sequencing resolves DNA sequence and structure in intact biological samples. Science 371, doi: 10.1126/science.aay3446 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mackinnon RN & Campbell LJ Chromothripsis under the microscope: a cytogenetic perspective of two cases of AML with catastrophic chromosome rearrangement. Cancer Genet 206, 238–251, doi: 10.1016/j.cancergen.2013.05.021 (2013). [DOI] [PubMed] [Google Scholar]
- 66.Chen KH, Boettiger AN, Moffitt JR, Wang S. & Zhuang X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090, doi: 10.1126/science.aaa6090 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Su JH, Zheng P, Kinrot SS, Bintu B. & Zhuang X. Genome-Scale Imaging of the 3D Organization and Transcriptional Activity of Chromatin. Cell 182, 1641–1659 e1626, doi: 10.1016/j.cell.2020.07.032 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, Deng G. & Cai H. ChromothripsisDB: a curated database of chromothripsis. Bioinformatics 32, 1433–1435, doi: 10.1093/bioinformatics/btv757 (2016). [DOI] [PubMed] [Google Scholar]
- 69.Gao J. et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 6, pl1, doi: 10.1126/scisignal.2004088 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.International Cancer Genome C. et al. International network of cancer genome projects. Nature 464, 993–998, doi: 10.1038/nature08987 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bamford S. et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer 91, 355–358, doi: 10.1038/sj.bjc.6601894 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heath AP et al. The NCI Genomic Data Commons. Nat Genet 53, 257–262, doi: 10.1038/s41588-021-00791-5 (2021). [DOI] [PubMed] [Google Scholar]
- 73.Fenech M. et al. Micronuclei as biomarkers of DNA damage, aneuploidy, inducers of chromosomal hypermutation and as sources of pro-inflammatory DNA in humans. Mutat Res Rev Mutat Res 786, 108342, doi: 10.1016/j.mrrev.2020.108342 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Krupina K, Goginashvili A. & Cleveland DW Causes and consequences of micronuclei. Curr Opin Cell Biol 70, 91–99, doi: 10.1016/j.ceb.2021.01.004 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kato H. & Sandberg AA Chromosome pulverization in human cells with micronuclei. J Natl Cancer Inst 40, 165–179 (1968). [PubMed] [Google Scholar]
- 76.Liu S. et al. Nuclear envelope assembly defects link mitotic errors to chromothripsis. Nature 561, 551–555, doi: 10.1038/s41586-018-0534-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xia Y. et al. Nuclear rupture at sites of high curvature compromises retention of DNA repair factors. J Cell Biol 217, 3796–3808, doi: 10.1083/jcb.201711161 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hatch EM, Fischer AH, Deerinck TJ & Hetzer MW Catastrophic nuclear envelope collapse in cancer cell micronuclei. Cell 154, 47–60, doi: 10.1016/j.cell.2013.06.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vargas JD, Hatch EM, Anderson DJ & Hetzer MW Transient nuclear envelope rupturing during interphase in human cancer cells. Nucleus 3, 88–100, doi: 10.4161/nucl.18954 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Willan J. et al. ESCRT-III is necessary for the integrity of the nuclear envelope in micronuclei but is aberrant at ruptured micronuclear envelopes generating damage. Oncogenesis 8, 29, doi: 10.1038/s41389-019-0136-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vietri M. et al. Unrestrained ESCRT-III drives micronuclear catastrophe and chromosome fragmentation. Nat Cell Biol 22, 856–867, doi: 10.1038/s41556-020-0537-5 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Ly P. et al. Chromosome segregation errors generate a diverse spectrum of simple and complex genomic rearrangements. Nat Genet 51, 705–715, doi: 10.1038/s41588-019-0360-8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bochtler T. et al. Micronucleus formation in human cancer cells is biased by chromosome size. Genes Chromosomes Cancer 58, 392–395, doi: 10.1002/gcc.22707 (2019). [DOI] [PubMed] [Google Scholar]
- 84.Klaasen SJ et al. Nuclear chromosome locations dictate segregation error frequencies. Nature 607, 604–609, doi: 10.1038/s41586-022-04938-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mammel AE, Huang HZ, Gunn AL, Choo E. & Hatch EM Chromosome length and gene density contribute to micronuclear membrane stability. Life Sci Alliance 5, doi: 10.26508/lsa.202101210 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schutze DM et al. Immortalization capacity of HPV types is inversely related to chromosomal instability. Oncotarget 7, 37608–37621, doi: 10.18632/oncotarget.8058 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dacus D. et al. Beta Human Papillomavirus 8 E6 Induces Micronucleus Formation and Promotes Chromothripsis. J Virol 96, e0101522, doi: 10.1128/jvi.01015-22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li JSZ et al. Chromosomal fragile site breakage by EBV-encoded EBNA1 at clustered repeats. Nature 616, 504–509, doi: 10.1038/s41586-023-05923-x (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barra V. & Fachinetti D. The dark side of centromeres: types, causes and consequences of structural abnormalities implicating centromeric DNA. Nat Commun 9, 4340, doi: 10.1038/s41467-018-06545-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics 26, 234–282, doi: 10.1093/genetics/26.2.234 (1941). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maciejowski J. & de Lange T. Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol 18, 175–186, doi: 10.1038/nrm.2016.171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffelder DR et al. Resolution of anaphase bridges in cancer cells. Chromosoma 112, 389–397, doi: 10.1007/s00412-004-0284-6 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Pampalona J, Soler D, Genesca A. & Tusell L. Telomere dysfunction and chromosome structure modulate the contribution of individual chromosomes in abnormal nuclear morphologies. Mutat Res 683, 16–22, doi: 10.1016/j.mrfmmm.2009.10.001 (2010). [DOI] [PubMed] [Google Scholar]
- 94.Li Y. et al. Constitutional and somatic rearrangement of chromosome 21 in acute lymphoblastic leukaemia. Nature 508, 98–102, doi: 10.1038/nature13115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mardin BR et al. A cell-based model system links chromothripsis with hyperploidy. Mol Syst Biol 11, 828, doi: 10.15252/msb.20156505 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Umbreit NT et al. Mechanisms generating cancer genome complexity from a single cell division error. Science 368, doi: 10.1126/science.aba0712 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gisselsson D. et al. Telomere dysfunction triggers extensive DNA fragmentation and evolution of complex chromosome abnormalities in human malignant tumors. Proc Natl Acad Sci U S A 98, 12683–12688, doi: 10.1073/pnas.211357798 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang YG, Lindahl T. & Barnes DE Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell 131, 873–886, doi: 10.1016/j.cell.2007.10.017 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Christmann M, Tomicic MT, Aasland D, Berdelle N. & Kaina B. Three prime exonuclease I (TREX1) is Fos/AP-1 regulated by genotoxic stress and protects against ultraviolet light and benzo(a)pyrene-induced DNA damage. Nucleic Acids Res 38, 6418–6432, doi: 10.1093/nar/gkq455 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maciejowski J. et al. APOBEC3-dependent kataegis and TREX1-driven chromothripsis during telomere crisis. Nat Genet 52, 884–890, doi: 10.1038/s41588-020-0667-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nader GPF et al. Compromised nuclear envelope integrity drives TREX1-dependent DNA damage and tumor cell invasion. Cell 184, 5230–5246 e5222, doi: 10.1016/j.cell.2021.08.035 (2021). [DOI] [PubMed] [Google Scholar]
- 102.Xia Y. et al. Rescue of DNA damage after constricted migration reveals a mechano-regulated threshold for cell cycle. J Cell Biol 218, 2545–2563, doi: 10.1083/jcb.201811100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hong Y. et al. LEM-3 is a midbody-tethered DNA nuclease that resolves chromatin bridges during late mitosis. Nat Commun 9, 728, doi: 10.1038/s41467-018-03135-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Song J, Freeman ADJ, Knebel A, Gartner A. & Lilley DMJ Human ANKLE1 Is a Nuclease Specific for Branched DNA. J Mol Biol 432, 5825–5834, doi: 10.1016/j.jmb.2020.08.022 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jiang H, Kong N, Liu Z, West SC & Chan YW Human Endonuclease ANKLE1 Localizes at the Midbody and Processes Chromatin Bridges to Prevent DNA Damage and cGAS-STING Activation. Adv Sci (Weinh), e2204388, doi: 10.1002/advs.202204388 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang S, Stokasimov E, Cui Y. & Pellman D. Breakage of cytoplasmic chromosomes by pathological DNA base excision repair. Nature 606, 930–936, doi: 10.1038/s41586-022-04767-1 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terzoudi GI et al. Stress induced by premature chromatin condensation triggers chromosome shattering and chromothripsis at DNA sites still replicating in micronuclei or multinucleate cells when primary nuclei enter mitosis. Mutat Res Genet Toxicol Environ Mutagen 793, 185–198, doi: 10.1016/j.mrgentox.2015.07.014 (2015). [DOI] [PubMed] [Google Scholar]
- 108.Tang HL et al. Cell survival, DNA damage, and oncogenic transformation after a transient and reversible apoptotic response. Mol Biol Cell 23, 2240–2252, doi: 10.1091/mbc.E11-11-0926 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tubio JM & Estivill X. Cancer: When catastrophe strikes a cell. Nature 470, 476–477, doi: 10.1038/470476a (2011). [DOI] [PubMed] [Google Scholar]
- 110.Rello-Varona S. et al. Autophagic removal of micronuclei. Cell Cycle 11, 170–176, doi: 10.4161/cc.11.1.18564 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Nassour J. et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature 565, 659–663, doi: 10.1038/s41586-019-0885-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao M. et al. CGAS is a micronucleophagy receptor for the clearance of micronuclei. Autophagy 17, 3976–3991, doi: 10.1080/15548627.2021.1899440 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J. et al. Comprehensive Chromosome End Remodeling during Programmed DNA Elimination. Curr Biol 30, 3397–3413 e3394, doi: 10.1016/j.cub.2020.06.058 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rucker FG et al. Chromothripsis is linked to TP53 alteration, cell cycle impairment, and dismal outcome in acute myeloid leukemia with complex karyotype. Haematologica 103, e17–e20, doi: 10.3324/haematol.2017.180497 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morishita M. et al. Chromothripsis-like chromosomal rearrangements induced by ionizing radiation using proton microbeam irradiation system. Oncotarget 7, 10182–10192, doi: 10.18632/oncotarget.7186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Doudna JA & Charpentier E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096, doi: 10.1126/science.1258096 (2014). [DOI] [PubMed] [Google Scholar]
- 117.Hsu PD, Lander ES & Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278, doi: 10.1016/j.cell.2014.05.010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leibowitz ML et al. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat Genet 53, 895–905, doi: 10.1038/s41588-021-00838-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Geng K. et al. Target-enriched nanopore sequencing and de novo assembly reveals co-occurrences of complex on-target genomic rearrangements induced by CRISPR-Cas9 in human cells. Genome Res 32, 1876–1891, doi: 10.1101/gr.276901.122 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trivedi P, Steele CD, Au FKC, Alexandrov LB & Cleveland DW Mitotic tethering enables inheritance of shattered micronuclear chromosomes. Nature, doi: 10.1038/s41586-023-06216-z (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin YF et al. Mitotic clustering of pulverized chromosomes from micronuclei. Nature, doi: 10.1038/s41586-023-05974-0 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Paull TT et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 10, 886–895, doi: 10.1016/s0960-9822(00)00610-2 (2000). [DOI] [PubMed] [Google Scholar]
- 123.Lupski JR Genomic disorders: structural features of the genome can lead to DNA rearrangements and human disease traits. Trends Genet 14, 417–422, doi: 10.1016/s0168-9525(98)01555-8 (1998). [DOI] [PubMed] [Google Scholar]
- 124.Hastings PJ, Lupski JR, Rosenberg SM & Ira G. Mechanisms of change in gene copy number. Nat Rev Genet 10, 551–564, doi: 10.1038/nrg2593 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kloosterman WP et al. Constitutional chromothripsis rearrangements involve clustered double-stranded DNA breaks and nonhomologous repair mechanisms. Cell Rep 1, 648–655, doi: 10.1016/j.celrep.2012.05.009 (2012). [DOI] [PubMed] [Google Scholar]
- 126.Ratnaparkhe M. et al. Defective DNA damage repair leads to frequent catastrophic genomic events in murine and human tumors. Nat Commun 9, 4760, doi: 10.1038/s41467-018-06925-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schipler A. et al. Chromosome thripsis by DNA double strand break clusters causes enhanced cell lethality, chromosomal translocations and 53BP1-recruitment. Nucleic Acids Res 44, 7673–7690, doi: 10.1093/nar/gkw487 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cleal K, Jones RE, Grimstead JW, Hendrickson EA & Baird DM Chromothripsis during telomere crisis is independent of NHEJ, and consistent with a replicative origin. Genome Res 29, 737–749, doi: 10.1101/gr.240705.118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nigg EA Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2, 21–32, doi: 10.1038/35048096 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Northcott PA et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature 488, 49–56, doi: 10.1038/nature11327 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mehine M, Kaasinen E. & Aaltonen LA Chromothripsis in uterine leiomyomas. N Engl J Med 369, 2160–2161, doi: 10.1056/NEJMc1310230 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Holzmann C. et al. Cytogenetically normal uterine leiomyomas without MED12-mutations - a source to identify unknown mechanisms of the development of uterine smooth muscle tumors. Mol Cytogenet 7, 88, doi: 10.1186/s13039-014-0088-1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mehine M, Makinen N, Heinonen HR, Aaltonen LA & Vahteristo P. Genomics of uterine leiomyomas: insights from high-throughput sequencing. Fertil Steril 102, 621–629, doi: 10.1016/j.fertnstert.2014.06.050 (2014). [DOI] [PubMed] [Google Scholar]
- 134.Pendina AA et al. Case of chromothripsis in a large solitary non-recurrent uterine leiomyoma. Eur J Obstet Gynecol Reprod Biol 219, 134–136, doi: 10.1016/j.ejogrb.2017.10.028 (2017). [DOI] [PubMed] [Google Scholar]
- 135.McEvoy J. et al. RB1 gene inactivation by chromothripsis in human retinoblastoma. Oncotarget 5, 438–450, doi: 10.18632/oncotarget.1686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mitchell TJ et al. Timing the Landmark Events in the Evolution of Clear Cell Renal Cell Cancer: TRACERx Renal. Cell 173, 611–623 e617, doi: 10.1016/j.cell.2018.02.020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nones K. et al. Genomic catastrophes frequently arise in esophageal adenocarcinoma and drive tumorigenesis. Nat Commun 5, 5224, doi: 10.1038/ncomms6224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Notta F. et al. A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature 538, 378–382, doi: 10.1038/nature19823 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Parker M. et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature 506, 451–455, doi: 10.1038/nature13109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kodama T. et al. A novel mechanism of EML4-ALK rearrangement mediated by chromothripsis in a patient-derived cell line. J Thorac Oncol 9, 1638–1646, doi: 10.1097/JTO.0000000000000311 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Singh ZN et al. Cryptic ETV6-PDGFRB fusion in a highly complex rearrangement of chromosomes 1, 5, and 12 due to a chromothripsis-like event in a myelodysplastic syndrome/myeloproliferative neoplasm. Leuk Lymphoma 60, 1304–1307, doi: 10.1080/10428194.2018.1480774 (2019). [DOI] [PubMed] [Google Scholar]
- 142.Anderson ND et al. Rearrangement bursts generate canonical gene fusions in bone and soft tissue tumors. Science 361, doi: 10.1126/science.aam8419 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Arniani S. et al. Chromothripsis is a frequent event and underlies typical genetic changes in early T-cell precursor lymphoblastic leukemia in adults. Leukemia 36, 2577–2585, doi: 10.1038/s41375-022-01671-5 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cox D, Yuncken C. & Spriggs AI Minute Chromatin Bodies in Malignant Tumours of Childhood. Lancet 1, 55–58, doi: 10.1016/s0140-6736(65)90131-5 (1965). [DOI] [PubMed] [Google Scholar]
- 145.Ilic M, Zaalberg IC, Raaijmakers JA & Medema RH Life of double minutes: generation, maintenance, and elimination. Chromosoma 131, 107–125, doi: 10.1007/s00412-022-00773-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pecorino LT, Verhaak RGW, Henssen A. & Mischel PS Extrachromosomal DNA (ecDNA): an origin of tumor heterogeneity, genomic remodeling, and drug resistance. Biochem Soc Trans 50, 1911–1920, doi: 10.1042/BST20221045 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.van Leen E, Bruckner L. & Henssen AG The genomic and spatial mobility of extrachromosomal DNA and its implications for cancer therapy. Nat Genet 54, 107–114, doi: 10.1038/s41588-021-01000-z (2022). [DOI] [PubMed] [Google Scholar]
- 148.Kaufman RJ, Brown PC & Schimke RT Amplified dihydrofolate reductase genes in unstably methotrexate-resistant cells are associated with double minute chromosomes. Proc Natl Acad Sci U S A 76, 5669–5673, doi: 10.1073/pnas.76.11.5669 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fakharzadeh SS, Trusko SP & George DL Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J 10, 1565–1569, doi: 10.1002/j.1460-2075.1991.tb07676.x (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mansfield AS et al. Neoantigenic Potential of Complex Chromosomal Rearrangements in Mesothelioma. J Thorac Oncol 14, 276–287, doi: 10.1016/j.jtho.2018.10.001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mackenzie KJ et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465, doi: 10.1038/nature23449 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Harding SM et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470, doi: 10.1038/nature23470 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bartsch K. et al. Absence of RNase H2 triggers generation of immunogenic micronuclei removed by autophagy. Hum Mol Genet 26, 3960–3972, doi: 10.1093/hmg/ddx283 (2017). [DOI] [PubMed] [Google Scholar]
- 154.Bakhoum SF et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 553, 467–472, doi: 10.1038/nature25432 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gratia M. et al. Bloom syndrome protein restrains innate immune sensing of micronuclei by cGAS. J Exp Med 216, 1199–1213, doi: 10.1084/jem.20181329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ku JWK et al. Bacterial-induced cell fusion is a danger signal triggering cGAS-STING pathway via micronuclei formation. Proc Natl Acad Sci U S A 117, 15923–15934, doi: 10.1073/pnas.2006908117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lohard S. et al. STING-dependent paracriny shapes apoptotic priming of breast tumors in response to anti-mitotic treatment. Nat Commun 11, 259, doi: 10.1038/s41467-019-13689-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Flynn PJ, Koch PD & Mitchison TJ Chromatin bridges, not micronuclei, activate cGAS after drug-induced mitotic errors in human cells. Proc Natl Acad Sci U S A 118, doi: 10.1073/pnas.2103585118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heijink AM et al. BRCA2 deficiency instigates cGAS-mediated inflammatory signaling and confers sensitivity to tumor necrosis factor-alpha-mediated cytotoxicity. Nat Commun 10, 100, doi: 10.1038/s41467-018-07927-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun L, Wu J, Du F, Chen X. & Chen ZJ Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791, doi: 10.1126/science.1232458 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu J. et al. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 339, 826–830, doi: 10.1126/science.1229963 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ablasser A. et al. cGAS produces a 2'-5'-linked cyclic dinucleotide second messenger that activates STING. Nature 498, 380–384, doi: 10.1038/nature12306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu H. et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature 563, 131–136, doi: 10.1038/s41586-018-0629-6 (2018). [DOI] [PubMed] [Google Scholar]
- 164.Jiang H. et al. Chromatin-bound cGAS is an inhibitor of DNA repair and hence accelerates genome destabilization and cell death. EMBO J 38, e102718, doi: 10.15252/embj.2019102718 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Banerjee D. et al. A non-canonical, interferon-independent signaling activity of cGAMP triggers DNA damage response signaling. Nat Commun 12, 6207, doi: 10.1038/s41467-021-26240-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cramer SF & Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol 94, 435–438, doi: 10.1093/ajcp/94.4.435 (1990). [DOI] [PubMed] [Google Scholar]
- 167.McDermott DH et al. Chromothriptic cure of WHIM syndrome. Cell 160, 686–699, doi: 10.1016/j.cell.2015.01.014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kloosterman WP et al. Chromothripsis as a mechanism driving complex de novo structural rearrangements in the germline. Hum Mol Genet 20, 1916–1924, doi: 10.1093/hmg/ddr073 (2011). [DOI] [PubMed] [Google Scholar]
- 169.de Pagter MS et al. Chromothripsis in healthy individuals affects multiple protein-coding genes and can result in severe congenital abnormalities in offspring. Am J Hum Genet 96, 651–656, doi: 10.1016/j.ajhg.2015.02.005 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chiang C. et al. Complex reorganization and predominant non-homologous repair following chromosomal breakage in karyotypically balanced germline rearrangements and transgenic integration. Nat Genet 44, 390–397, S391, doi: 10.1038/ng.2202 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nazaryan L. et al. The strength of combined cytogenetic and mate-pair sequencing techniques illustrated by a germline chromothripsis rearrangement involving FOXP2. Eur J Hum Genet 22, 338–343, doi: 10.1038/ejhg.2013.147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Redin C. et al. The genomic landscape of balanced cytogenetic abnormalities associated with human congenital anomalies. Nat Genet 49, 36–45, doi: 10.1038/ng.3720 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weckselblatt B, Hermetz KE & Rudd MK Unbalanced translocations arise from diverse mutational mechanisms including chromothripsis. Genome Res 25, 937–947, doi: 10.1101/gr.191247.115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gamba BF, Richieri-Costa A, Costa S, Rosenberg C. & Ribeiro-Bicudo LA Chromothripsis with at least 12 breaks at 1p36.33-p35.3 in a boy with multiple congenital anomalies. Mol Genet Genomics 290, 2213–2216, doi: 10.1007/s00438-015-1072-0 (2015). [DOI] [PubMed] [Google Scholar]
- 175.Meier B. et al. C. elegans whole-genome sequencing reveals mutational signatures related to carcinogens and DNA repair deficiency. Genome Res 24, 1624–1636, doi: 10.1101/gr.175547.114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lee JK et al. Complex chromosomal rearrangements by single catastrophic pathogenesis in NUT midline carcinoma. Ann Oncol 28, 890–897, doi: 10.1093/annonc/mdw686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee JJ et al. Tracing Oncogene Rearrangements in the Mutational History of Lung Adenocarcinoma. Cell 177, 1842–1857 e1821, doi: 10.1016/j.cell.2019.05.013 (2019). [DOI] [PubMed] [Google Scholar]
- 178.Tan EH et al. Catastrophic chromosomal restructuring during genome elimination in plants. Elife 4, doi: 10.7554/eLife.06516 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Guo W, Comai L. & Henry IM Chromoanagenesis from radiation-induced genome damage in Populus. PLoS Genet 17, e1009735, doi: 10.1371/journal.pgen.1009735 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guo W, Comai L. & Henry IM Chromoanagenesis in plants: triggers, mechanisms, and potential impact. Trends Genet 39, 34–45, doi: 10.1016/j.tig.2022.08.003 (2023). [DOI] [PubMed] [Google Scholar]
- 181.Liu J. et al. Genome-Scale Sequence Disruption Following Biolistic Transformation in Rice and Maize. Plant Cell 31, 368–383, doi: 10.1105/tpc.18.00613 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fossi M, Amundson K, Kuppu S, Britt A. & Comai L. Regeneration of Solanum tuberosum Plants from Protoplasts Induces Widespread Genome Instability. Plant Physiol 180, 78–86, doi: 10.1104/pp.18.00906 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Carbonell-Bejerano P. et al. Catastrophic Unbalanced Genome Rearrangements Cause Somatic Loss of Berry Color in Grapevine. Plant Physiol 175, 786–801, doi: 10.1104/pp.17.00715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]