Abstract
Introduction:
Smoking is the leading preventable cause of death and disease in the US. This study evaluates the cost-effectiveness from a healthcare system perspective of a comprehensive primary care intervention to reduce smoking rates.
Methods:
This pragmatic trial implemented electronic health record prompts during primary care visits and employed certified tobacco cessation specialists to offer proactive outreach and smoking cessation treatment to patients who smoke. The data, analyzed in 2022, included 10,683 patients in the smoking registry from 2017 to 2020. Pre-post analyses compared intervention costs to treatment engagement, successful self-reported smoking cessation, and acute health care utilization (urgent care, emergency department visits, and inpatient hospitalization). Cost per quality adjusted life year was determined by applying conversion factors obtained from the tobacco research literature to the cost per patient who quit smoking.
Results:
Tobacco cessation outreach, medication, and counseling costs increased from $2.64 to $6.44 per patient per month, for a total post-implementation intervention cost of $500,216. Smoking cessation rates increased from 1.3% pre-implementation to 8.7% post-implementation, for an incremental effectiveness of 7.4%. The incremental cost-effectiveness ratio was $628 (95% CI: $568, $695) per person who quit smoking, and $905 (95% CI: $822, $1,001) per quality adjusted life year gained. Acute health care costs decreased by an average $42 (95% CI: - $59, $145) per patient per month for patients in the smoking registry.
Conclusions:
Implementation of a comprehensive and proactive smoking cessation outreach and treatment program for adult primary care patients who smoke meets typical cost-effectiveness thresholds for healthcare.
Keywords: cost-effectiveness, primary care, smoking cessation, ICER, system change, EHR
INTRODUCTION
Smoking is the leading preventable cause of death and disease in the US, causing about 30% of cancer and cardiovascular deaths and nearly 80% of mortality from chronic obstructive pulmonary disease.1-4 To reduce the smoking-attributable disease burden, it is imperative to reduce smoking prevalence rates by helping more patients who smoke successfully quit and maintain abstinence.5-10 Evidence-based treatments, comprising counseling and medication, have been associated with two-to-three-fold increased smoking cessation rates, but they are underused.11-15
Primary care offers a propitious opportunity to deliver proactive chronic-illness care management to increase the reach of tobacco cessation treatment and the rates of smoking abstinence.16 However, primary care providers offer smoking cessation counseling and evidence-based medications to half or fewer of their patients.17 Even with electronic health record (EHR) prompts and tools to refer patients who smoke to free, low-barrier treatments (e.g., tobacco quitlines), only 5% of patients receive treatment, which is the practice population rate of assisted quitting.18-20
To address gaps in tobacco treatment access, this study implemented the Comprehensive Tobacco Intervention Program (CTIP),21 a pragmatic trial of EHR prompts, use of opt-out treatment offer, and dedicated staff and tools to facilitate proactive, repeated, outreach and smoking cessation treatment to all adult patients who smoked cigarettes.19,22-24 Our prior analyses of CTIP implementation indicated that roughly twice as many patients (24%) received smoking treatment during CTIP than before its adoption (12%).21 The present study examines the costs, cost-effectiveness, and potential savings, or cost-offsets, of CTIP from a healthcare system perspective.25-27 All costs are reported in 2020 US$. To the authors’ knowledge, this is the first health economic analysis of an integrated set of recently developed and validated healthcare system enhancements to smoking treatment. These enhancements have been shown to increase smoking abstinence in a healthcare population, but their cost-effectiveness is unknown.
METHODS
The CTIP intervention comprised EHR-based alerts and order sets to facilitate clinician-delivered smoking-cessation care during primary care visits. In addition, two full-time Certified Tobacco Treatment Specialists (CTTS) were hired to provide telephone-based outreach and quarterly bulk outreach to patients by mail or EHR patient portal on smoking cessation resources. This included mailing one 2-week nicotine patch starter kit to patients in the smoking registry. More details on the study design, reach, and effectiveness are provided in McCarthy et al. (2022).21
This study was approved by the Health Sciences Institutional Review Board of the University of Wisconsin (protocol number 2017-0529). EHR data collected during routine clinical care were de-identified for analysis, obviating informed consent. This pragmatic multiple baseline design was considered a quality improvement project and was not registered in clinicaltrials.gov.
Study Sample
CTIP was conducted in six clinics, launched in 3 waves of 2 clinics each, from a non-profit, member-owned healthcare system located in and around Madison, Wisconsin. The sample comprised all patients in the smoking registry at the study clinics from January 2017 to February 2020. Individuals were included in the clinics’ smoking registry if they had tobacco use status recorded as currently smoking within the past 3 years, if they had a diagnostic code for current tobacco dependence in the past year, or if they had a prescription or billing code for stop-smoking medication or tobacco intervention in the past year.
Data on cessation treatment, smoking quit rates, and health care use were extracted from the EHR for January 1, 2017, to February 29, 2020 and analyzed in 2022. The initiation of the EHR intervention protocol was staggered with one clinic beginning in January 2018, one in March 2018, two in July 2018, and two in November 2018. The study analysis employed a pre-post design at all sites, with assessment ranging from 12 to 21 months pre-CTIP and 16 to 25 months post-CTIP launch, depending on the site. The launch month was excluded from analysis as data were aggregated at the patient-month level and not delineated specifically as pre- or post-intervention.
Measures
Effectiveness was measured by the rate at which patients in the smoking registry self-reported that they converted to ‘former smoking’ status, as recorded in the EHR. Multiple members of a patient’s care team could update patient self-reported smoking status, including the CTTS, who updated status to ‘former smoking’ if a patient reported at least one week of current abstinence at the 4- to 6-week post-quit date follow-up call. During outreach calls to patients in the smoking registry, the CTTS could update status to ‘former smoking’ if patients reported they did not smoke and had not for one month or longer.
Intervention costs included CTTS salary, benefits, and supervisor costs, resources for quarterly bulk mailings and outreach, physician tobacco cessation counseling, and tobacco cessation medications associated with stop smoking intervention. Resource cost details are provided in the Appendix.
Claims data drawn from the EHR included visit counts for acute care such as urgent care, emergency department visits, and inpatient hospitalization for all system patients pre- and post-CTIP. The staff model health maintenance organization operating the study clinics provided claims data for all hospital and urgent care visits covered through the health insurance plan. Acute care costs were derived by applying average healthcare costs to utilization counts of urgent care visits, emergency department visits, and hospitalizations.28,29 All health care costs were converted to 2020 dollars using the Consumer Price Index for Medical Care.30
Statistical Analysis
Cost-effectiveness is reported in terms of the incremental cost-effectiveness ratio31 (ICER), calculated as:
Costs for tobacco cessation counseling and medication were calculated monthly and totaled over the entire pre- and post-intervention periods. Due to the rolling enrollment of clinics into the intervention, months of post-intervention follow-up varied across clinics. Total incremental costs of counseling and medication were derived by multiplying the per member per month differential cost between pre-implementation and post-implementation by the number of months of assessment for each clinic.
Program reach and effectiveness rates included the number of individuals per month who received counseling and/or medication to stop smoking and the number of individuals who converted to ‘former smoking’ status in the EHR (based on patient self-report). The primary CTIP effectiveness outcome was the differential rate at which patients in the smoking registry switched from active smoking status to ‘former smoking’ status pre- vs post-implementation. The total number of patients converting from current to former smoker as a result of the intervention was computed by multiplying the monthly differential stop smoking rate by the number of months of follow-up at each clinic.
The unadjusted incremental cost-effectiveness ratio (ICER) assessing the cost per additional individual who quit smoking was calculated by dividing the incremental cost of the intervention by the incremental effectiveness rate of smoking cessation. Next, an adjusted ICER was computed accounting for the possibility that detecting ‘former smoking’ status was more likely post-implementation due to increased rates of inquiry about smoking status.
The ICER assessing the cost per additional individual who quit smoking was then translated into a lifetime cost per additional quality adjusted life year (QALY) from a healthcare system perspective using conversion factors from Stapleton and West (2012).32 Stapleton and West account for life years gained from stopping smoking at various ages, probabilities of relapse back to smoking, and discounting of future benefits (i.e., life years gained are accrued in the future, at the end of life) to current value in converting quit rate differentials pre- and post-intervention into life years. Stapleton and West note (on p.466) that smoking cessation will likely improve health, and thereby add QALYs over the life course. They note that QALYs may be reported as 1 life-year = 1 QALY but that life years have previously been valued at levels both above and below a full one QALY per life year gained.32 A valuation of one additional QALY per one additional life year gained was used as a reasonable estimate based on previously used values. A 3% per year discount rate was applied for the Stapleton and West conversion.
A distribution for QALYs gained was created based on the estimated confidence interval for the cessation rate, which was extrapolated to a distribution of life-years saved. The ICER assessing the cost per additional individual who quit smoking was calculated by drawing a random sample with replacement from the distribution for QALYs (μQALYi) and health-care costs (μCOSTi). This process was repeated (N=1000) to produce bootstrap estimates of the 95% confidence intervals for the ICER per additional individual who quit smoking and the ICER per QALY gained.
A combination of probabilistic sensitivity analysis and non-parametric bootstrap analysis was employed to account for uncertainty in parameters within the implementation stage and to determine confidence intervals for the ICER assessing the cost per additional individual who quit smoking.33,34 The sensitivity analysis accounts for the possibility that ascertainment of smoking status might have changed during the intervention with more focus on identifying adults who had quit smoking.
In the sensitivity analysis, a defined proportion of individuals from the smoking registry who reported not smoking prior to contact with the CTTS were excluded from ICER calculations based on a bulk outreach quit rate parameter drawn from a uniform distribution. The parameter value ranged from 0 (no patients quit smoking because of bulk outreach) to 1 (all former smokers at initial CTTS contact quit because of bulk outreach). The sensitivity analysis produced ICERs and confidence intervals that accounted for quit rate inflation uncertainty related to more frequent CTTS updates of smoking status prior to intervention. Details on the sensitivity analysis are provided in the Appendix.
RESULTS
A total of 10,683 adults were in the system smoking registry during the study time frame of January 1, 2017-February 29, 2020 (Table 1). Due to patient turnover, the number of adults in the smoking registry in a given month varied from a low of 9,068 to a high of 10,447. Overall, most patients identified as White, non-Hispanic, and commercially insured. Mean age was 42.5 years (sd 14.1). Across all clinics, 6,672 (62.5%) patients in the smoking registry had at least one clinic visit, and patients averaged 1.7 clinic visits per year of enrollment (Table 1).
Table 1:
Demographic Variables for Individuals in the Smoking Registry (n=10,683)
| Variable | % (n) |
|---|---|
| Age, mean (sd) | 42.5 (14.1) |
| Sex: | |
| Men | 54.3 (5801) |
| Women | 45.7 (4882) |
| Race: | |
| White | 72.3 (7724) |
| Black | 10.7 (1143) |
| Other minority | 4.3 (459) |
| Unknown | 12.7 (1357) |
| Ethnicity: | |
| Hispanic | 3.8 (406) |
| Insurance: | |
| Commercial | 53.7 (5737) |
| Medicaid | 14.5 (1549) |
| Medicare | 6.2 (662) |
| None or Unknown | 25.6 (2735) |
| Annual Clinic Visits, mean (sd) | 1.7 (2.2) |
Pre-intervention, an average of 68 patients per month (out of all patients in the smoking registry) received tobacco cessation counseling and/or medications (Table 3). Post-implementation counseling and/or medication rates, reflecting both front-line clinician treatment as well as CTTS intervention, increased to 104 patients per month. Based on EHR documentation, a total of 2,166 patients out of the 10,683 patients in the smoking registry (20.3% of patients) received tobacco cessation counseling and/or medication in the post-implementation period. To determine the incremental treatment effect of the intervention, Monte Carlo simulations (1,000 runs) were run under the condition that the pre-implementation treatment rate remained constant for the post-implementation months. This simulated treatment rate indicated that 1,421 patients would have received treatment post-implementation if the treatment rate did not change from pre- to post-implementation; however, records showed that 2,166 patients actually received treatment post-implementation. This represents an incremental treatment effectiveness of 745 (95% CI: 559, 1,047) additional patients who received tobacco cessation treatment as a result of the intervention.
Table 3:
Incremental Cost-Effectiveness Ratios (ICER)
| Outcome | Pre- Implementation Effectiveness (95% CI) |
Post- Implementation Effectiveness (95% CI) |
Incremental Effectiveness (95% CI) |
Incremental Cost- Effectiveness Ratio (95% CI) |
|---|---|---|---|---|
| Received tobacco cessation treatment (counseling and/or medication) | 68 patients per month (61, 75) | 104 patients per month (94, 114) | 36 patients per month (24, 48) | $671 per patient ($447, $895) |
| Quit smoking (converted from current smoker to former smoking status) | 7 patients per month (6, 9) | 48 patients per month (44, 53) | 41 patients per month (37, 45) | $628 per quit ($568, $695) |
| QALYs | . | . | . | $905 per QALY ($822, $1,001) |
Smoking cessation rates based on patient self-reported smoking status.
Total number of patients receiving tobacco cessation treatment and quitting smoking determined by applying the incremental effectiveness to the total number of follow-up months for each study clinic. Due to rolling enrollment of clinics into the study, the number of follow-up months varied by clinic.
QALY: Quality-Adjusted Life Year
For the primary outcome of tobacco cessation (i.e., changed from ‘active smoking’ to ‘former smoking status, as recorded in the EHR), pre-intervention rates averaged 7 patients per month who quit smoking. Post-implementation, an average of 48 patients per month quit smoking (Table 3). A total of 930 patients out of the 10,683 patients on smoking registry (8.7%) quit smoking post-implementation. To determine the incremental quit effect of the intervention, Monte Carlo simulations (1,000 runs) were run under the condition that the pre-implementation quit rate remained constant for the post-implementation months. This simulated rate indicated that only 133 patients would have quit in the post-implementation period had the pre-to-post quit rates stayed the same. In fact, 930 patients were recorded as having quit post-implementation, representing an incremental effectiveness of 797 (95% CI: 720, 880) additional patients who quit smoking as a result of the intervention.
Resource costs used for the intervention are detailed in Table 2. Total CTTS salary, benefits, and supervisor costs for the 2 FTEs were $237,315, for an average of $1.85 per smoking patient per month post-implementation for patients in the smoking registry (Table 2).
Table 2:
Intervention Costs
| Resource | Pre- Implementation (per patient per month) |
Post- Implementation (per patient per month) |
Incremental cost (per patient per month) |
Total Costs (95% CI) |
|---|---|---|---|---|
| Certified Tobacco Treatment Specialist | $0 | $1.85 | $1.85 | $237,315 |
| Physician Cessation Counseling | $0.07 | $0.43 | $0.36 | $46,303 ($39,017, $53,589) |
| Tobacco Cessation Medications | $2.57 | $3.49 | $0.92 | $118,330 ($99,710, $136,950) |
| Bulk Mail Outreach | $0 | $0.76 | $0.76 | $98,268 |
| Total costs | $2.64 | $6.44 | $3.80 | $500,216 ($474,310, $527,537) |
Total intervention costs are based on monthly incremental costs per patient in the smoking registry per month applied to the total number of follow-up months for each study clinic. Due to staggered enrollment of clinics into the study, the number of follow-up months varied by clinic. Pre-implementation and post-implementation counseling and medication rates were estimated by repeated random draws from the monthly pre- and post-implementation distributions.
Physician-based tobacco cessation counseling increased from $0.07 per smoking patient per month pre-intervention to $0.43 per smoking patient per month post-intervention, for an incremental cost of $0.36 per smoking patient per month for patients in the smoking registry. The total post-implementation cost of increased physician tobacco cessation counseling was $46,303, or approximately 492 hours of physician time.
Tobacco cessation medication costs increased from $2.57 per smoking patient per month pre-intervention to $3.49 per smoking patient per month post-intervention, for an incremental cost of $0.92 per smoking patient per month. The total post-implementation cost of increased tobacco cessation medications was $118,330 (Table 2).
Bulk outreach and intervention mailing costs totaled $98,268 post-implementation. This included $71,964 for the trial samples of 2-week supplies of nicotine patches that were mailed to patients in the smoking registry. At the first two clinics, patches were sent to all patients in the smoking registry. At subsequent study clinics, patients who were already prescribed smoking cessation medication were excluded from the patch mailings. A total of 3,577 patients were sent nicotine patch samples. The outreach costs averaged $0.76 per smoking patient per month (Table 2).
The total incremental cost of the intervention was $500,216 post-implementation, for an average of $3.80 per smoking registry patient per month over the average 21-month follow-up (Table 2). Of the total cost, 49% was attributable to adding the two CTTS positions and 26% was a result of increased tobacco cessation medication prescribing. Approximately 10% of the total cost was a result of increased clinician time for tobacco cessation counseling.
The incremental cost effectiveness ratio (ICER) per additional patient receiving treatment was calculated by dividing the total cost of the intervention ($500,216) by the incremental treatment effectiveness of 745 (95% CI: 559, 1,047) additional patients receiving treatment, resulting in an ICER of $671 (95%CI: $447, $895) per additional patient receiving treatment (Table 3). For the primary outcome of interest, the ICER per additional patient who quit smoking was calculated by dividing the total cost of the intervention ($500,216) by the incremental quit effectiveness of 797 (95% CI: 720, 880) additional patients who quit smoking, resulting in an ICER of $628 (95% CI: $568, $695) per additional patient who quit. After converting smoking quit rates to quality adjusted life years, the incremental cost was $905 (95% CI: $822, $1,001) per QALY.
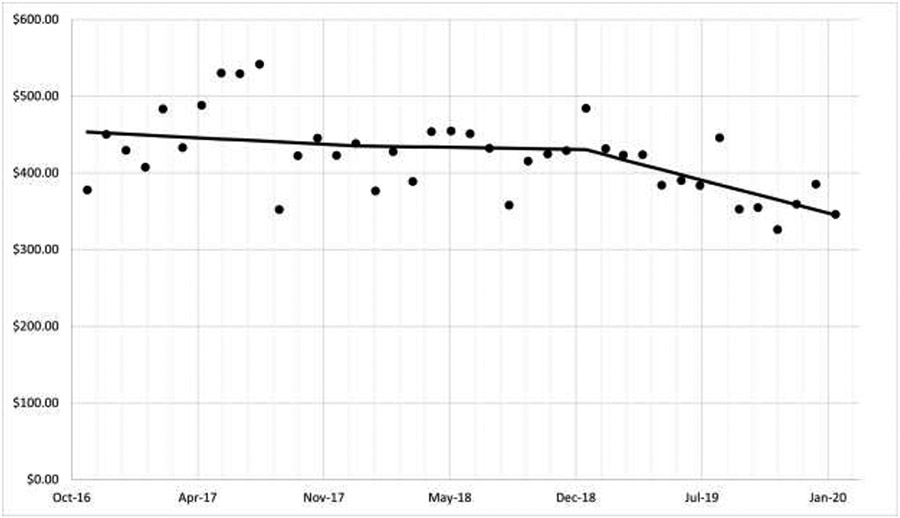
Figure 1 shows average monthly acute health care utilization costs including urgent care, emergency department, and hospital inpatient stays for patients in the smoking registry. Implementation of CTIP began in two clinics in January 2018, with all 6 clinics active by November 2018. Pre-implementation acute care costs averaged $432 (95% CI: $329, $535) per patient per month, while post-implementation acute care costs averaged $390 (95% CI: $308, $471) per patient per month. The incremental decrease in acute care costs pre- to post-implementation averaged $42 (95% CI: -$59, $145) per smoking registry patient per month.
Figure 1:
Monthly Acute Care Costs for Persons in the Primary Care Clinic Smoking Registry
Monthly urgent care, emergency department, and hospitalization costs, adjusted to US 2020 Dollars ($)
Sensitivity analysis that excluded a proportion of patients reporting they had quit smoking when first contacted by the CTTS, with a parameter ranging from 0 (no patients quit smoking because of bulk outreach) to 1 (all former smokers at initial CTTS contact quit because of bulk outreach), yielded incremental quit rates ranging from 2.9% to 7.4% of the individuals in the smoking registry. The ICER in the sensitivity analysis was $931 (95% CI: $772, $1,172) per additional patient who quit smoking and $1,518 (95% CI: $1,259, $1,911) per QALY added.
DISCUSSION
A comprehensive approach to treating smoking in primary care was found to be cost-effective in increasing rates of evidence-based treatment use and rates of quitting smoking among adult patients who smoke, relative to typical healthcare interventions. The cost per additional smoker who quit was $628, which translates into $905 per QALY added. The proactive intervention invested in: (1) integrating centralized certified tobacco treatment specialists into primary care teams to provide smoking cessation assistance by phonInd EHR patient portal; (2) outreach efforts through mailings and sample nicotine replacement therapy; and (3) incremental costs of counseling time and medications provided by CTTSs and existing clinic health professionals. Approximately one-half of the intervention costs resulted from hiring certified tobacco treatment specialists to provide outreach to patients before and after scheduled primary care visits and support to patients indicating a desire to quit smoking. Increased prescribing of tobacco cessation medications constituted another one-quarter of the overall intervention costs. Additional clinician-delivered counseling contributed a modest amount (9.8%) to incremental costs.
Previous analyses demonstrated that CTIP increases the reach of smoking cessation treatment.21 The current analyses suggest that it achieves this effect in an efficient manner. The cost of providing an additional patient with evidence-based-smoking treatment was $671, including the cost of medications. This is in line with data from a systematic review of smoking cessation intervention, where costs ranged from $101 to $340 per individual.35
More importantly, the results suggest that CTIP is highly cost-effective in helping more adult patients quit smoking. The cost per QALY gained for the added patients who quit smoking was $905/QALY, significantly below the cost-effectiveness threshold of $50,000 per QALY generally accepted as demonstrating cost-effectiveness and sufficient to justify investment of health care resources.36 Our sensitivity analysis shows that even accounting for possible inflation in the quit rate as a result of increased reporting of former smoker status, the ICER was only $1,518 (95% CI: $1,253, $1,911) per additional QALY. This confirms that CTIP offers a significant cost-effective health benefit for the resources allocated to the intervention.
These results arise from a comparison with the healthcare system’s earlier approach to smoking cessation prior to implementation. Smoking cessation intervention by primary care clinicians alone faces known barriers to high quality tobacco cessation treatment delivery (i.e., both counseling and pharmacotherapy) in primary care, such as clinician time burden and lack of knowledge about cessation resources.16,37-40
In addition, acute healthcare costs for individuals in the smoking registry decreased an average of $42 per member per month, with greater decreases noted in the period of full implementation than during the period of phased implementation in the multiple baseline period. While average health care costs in this sample decreased by $42 per member per month, there is insufficient evidence to determine whether the decrease was due to other factors including chance.
Limitations
The study has potential limitations. First, the multiple baseline design may not have wholly isolated intervention effects from temporal trends or external factors that may have influenced treatment utilization or smoking cessation. Notably, there was a general decrease in smoking rates during the 2017 to 2020 period when the study was conducted. Approximately 19% of US adults used at least one tobacco product in 2020 which was down from 19.3% of US adults who reported smoking in 2017.41,42 While the secular trend in decreasing smoking rates is encouraging, it alone could not fully explain our study findings. Second, a 16- to 25-month post-intervention follow-up may not be long enough to determine the full effects of smoking cessation on long-term health outcomes for patients who smoked.43,44 Third, the study took place in a single health system in one region of Wisconsin, so results may not be fully generalizable nationwide. Fourth, the smoking cessation outcome was assessed by patient self-report and not biochemically verified. The gold standard in smoking cessation research is bioverification,45 either through exhaled CO or saliva, blood, or urine cotinine. However, the meta-analysis by Patrick et al.46 demonstrates the validity of self-reported smoking. It is also notable that estimated increments in quitting smoking were greater than increments in use of treatment in this study, for reasons unknown. This may reflect several factors, such as: self-quitting (without formal treatment) spurred by program outreach, undocumented clinician advice, or other influences (e.g., secular trends); increased reporting of quitting among patients who wished to cease receiving outreach about smoking treatment; and/or more frequent updating of smoking status post-implementation than at baseline. We conducted sensitivity analyses to address some of these possibilities, and still found evidence of cost-effectiveness, but future studies are needed to explore this further.
CONCLUSIONS
A comprehensive EHR-enabled smoking treatment program with the engagement of CTTSs in primary care clinics is a cost-effective option to encourage smoking cessation among patients who smoke.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Epic Systems Corp., Verona, Wisconsin, USA, for their assistance and support in developing EHR enhancements that enabled the conduct of this research. The authors also thank Group Health Cooperative of South Central Wisconsin for partnering in CTIP and implementing it throughout primary care in their system. We particularly thank Katherine Coates, Hannah Wallenkamp, Margaret Steiner, Margaret Nolan, Christian Kastman, Oliver Eng, and Paul Rake for their critical contributions to this study.
This study was funded by a grant (R35CA197573 to Michael C. Fiore) from the National Cancer Institute (www.cancer.gov). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
No financial disclosures have been reported by the authors of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
Marlon Mundt: Conceptualization, Investigation, Methodology, Validation, Formal analysis, Writing-Original Draft, Visualization. Danielle McCarthy: Conceptualization, Investigation, Writing-Review & Editing, Supervision. Timothy Baker: Conceptualization, Investigation, Writing-Review & Editing, Supervision, Funding Acquisition. Mark Zehner: Conceptualization, Investigation, Writing-Review & Editing, Project administration. Deejay Zwaga: Software, Validation, Data Curation, Writing-Review & Editing. Michael Fiore: Conceptualization, Investigation, Writing-Review & Editing, Supervision, Funding Acquisition.
REFERENCES
- 1.Lortet-Tieulent J, Goding Sauer A, Siegel RL, et al. State-level cancer mortality attributable to cigarette smoking in the United States. JAMA Intern Med. 2016;176(12):1792–1798. doi: 10.1001/jamainternmed.2016.6530 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Smoking and tobacco use. 2019. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm. Accessed 12 April 2020.
- 3.U.S. Department of Health and Human Services. Smoking cessation: a report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. [Google Scholar]
- 4.Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin. 2018;68(1):31–54. doi: 10.3322/caac.21440 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Health effects of cigarette smoking. 2017. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm. Accessed 6 Sep 2017.
- 6.Chelland Campbell S, Moffatt RJ, Stamford BA. Smoking and smoking cessation -- the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis. 2008;201(2):225–235. 10.1016/j.atherosclerosis.2008.04.046 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Tobacco Key Facts. https://www.who.int/news-room/fact-sheets/detail/tobacco. Accessed 10 Oct 2023.
- 8.U.S. Department of Health and Human Services. Smoking Cessation. A Report of the Surgeon General. Atlanta, GA: 2020. [Google Scholar]
- 9.Banks E, Joshy G, Weber MF, et al. Tobacco smoking and all-cause mortality in a large Australian cohort study: findings from a mature epidemic with current low smoking prevalence. BMC Med. 2015;13:38. doi: 10.1186/s12916-015-0281-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Quitting smoking among adults - United States 2001-2010. MMWR. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 11.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008. [Google Scholar]
- 12.Stead LF, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2012;10:CD008286. doi: 10.1002/14651858.CD008286.pub2 [DOI] [PubMed] [Google Scholar]
- 13.Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. doi: 10.1002/14651858.CD008286.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahill K, Stevens S, Lancaster T. Pharmacological treatments for smoking cessation. JAMA. 2014;311(2):193–194. doi: 10.1001/jama.2013.283787 [DOI] [PubMed] [Google Scholar]
- 15.Richard P, West K, Ku L. The return on investment of a Medicaid tobacco cessation program in Massachusetts. PLoS One. 2012;7(1):e29665. doi: 10.1371/journal.pone.0029665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papadakis S, McDonald P, Mullen KA, Reid R, Skulsky K, Pipe A. Strategies to increase the delivery of smoking cessation treatments in primary care settings: a systematic review and meta-analysis. Prev Med. 2010;51(3-4):199–213. doi: 10.1016/j.ypmed.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Manolios E, Sibeoni J, Teixeira M, Revah-Levy A, Verneuil L, Jovic L. When primary care providers and smokers meet: a systematic review and metasynthesis. NPJ Prim Care Respir Med. 2021;31(1):31. doi: 10.1038/s41533-021-00245-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker TB, Berg KM, Adsit RT, et al. Closed-Loop Electronic Referral From Primary Care Clinics to a State Tobacco Cessation Quitline: Effects Using Real-World Implementation Training. Am J Prev Med. 2021;60(3 Suppl 2):S113–S122. doi: 10.1016/j.amepre.2019.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiore M, Adsit R, Zehner M, et al. An electronic health record-based interoperable eReferral system to enhance smoking Quitline treatment in primary care. J Am Med Inform Assoc. 2019;26(8-9):778–786. doi: 10.1093/jamia/ocz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults - United States, 2000-2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. doi: 10.15585/mmwr.mm6552a1 [DOI] [PubMed] [Google Scholar]
- 21.McCarthy DE, Baker TB, Zehner ME, et al. A comprehensive electronic health record-enabled smoking treatment program: Evaluating reach and effectiveness in primary care in a multiple baseline design. Prev Med. 2022:107101. doi: 10.1016/j.ypmed.2022.107101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adsit RT, Fox BM, Tsiolis T, et al. Using the electronic health record to connect primary care patients to evidence-based telephonic tobacco quitline services: a closed-loop demonstration project. Transl Behav Med. 2014;4(3):324–332. doi: 10.1007/s13142-014-0259-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linder JA, Rigotti NA, Schneider LI, Kelley JH, Brawarsky P, Haas JS. An electronic health record-based intervention to improve tobacco treatment in primary care: a cluster-randomized controlled trial. Arch Intern Med. 2009;169(8):781–787. doi: 10.1001/archinternmed.2009.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flocke SA, Seeholzer E, Lewis SA, et al. 12-Month Evaluation of an EHR-Supported Staff Role Change for Provision of Tobacco Cessation Care in 8 Primary Care Safety-Net Clinics. J Gen Intern Med. 2020;35(11):3234–3242. doi: 10.1007/s11606-020-06030-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javitz HS, Zbikowski SM, Deprey M, et al. Cost-effectiveness of varenicline and three different behavioral treatment formats for smoking cessation. Transl Behav Med. 2011;1(1):182–190. doi: 10.1007/s13142-010-0009-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekpu VU, Brown AK. The Economic Impact of Smoking and of Reducing Smoking Prevalence: Review of Evidence. Tob Use Insights. 2015;8:1–35. doi: 10.4137/TUI.S15628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy DE, Klinger EV, Linder JA, et al. Cost-Effectiveness of a Health System-Based Smoking Cessation Program. Nicotine Tob Res. 2017;19(12):1508–1515. doi: 10.1093/ntr/ntw243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore BJ, Liang L. Costs of Emergency Department Visits in the United States, 2017: Statistical Brief #268. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD)2020. [Google Scholar]
- 29.Liang L, Moore B, Soni A. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2017. HCUP Statistical Brief #261 Rockville, MD: 2020. [PubMed] [Google Scholar]
- 30.U.S. Bureau of Labor Statistics. Consumer Price Index for All Urban Consumers: Medical Care in U.S. City Average [CPIMEDSL]. Federal Reserve Bank of St. Louis. https://fred.stlouisfed.org/series/CPIMEDSL. Published 2022. Accessed June 1, 2022. [Google Scholar]
- 31.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 32.Stapleton JA, West R. A direct method and ICER tables for the estimation of the cost-effectiveness of smoking cessation interventions in general populations: application to a new cytisine trial and other examples. Nicotine Tob Res. 2012;14(4):463–471. doi: 10.1093/ntr/ntr236 [DOI] [PubMed] [Google Scholar]
- 33.Davidson R, MacKinnon JG. Estimation and inference in econometrics. New York, NY: Oxford University Press; 1993. [Google Scholar]
- 34.Efron B, Tibshirani RJ. An introduction to the bootstrap. London: Chapman and Hall; 1993. [Google Scholar]
- 35.Hoogendoorn M, Feenstra TL, Hoogenveen RT, Rutten-van Molken MP. Long-term effectiveness and cost-effectiveness of smoking cessation interventions in patients with COPD. Thorax. 2010;65(8):711–718. doi: 10.1136/thx.2009.131631 [DOI] [PubMed] [Google Scholar]
- 36.Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med. 1998;13(10):716–717. doi: 10.1046/j.1525-1497.1998.00211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longo DR, Stone TT, Phillips RL, et al. Characteristics of smoking cessation guideline use by primary care physicians. Mo Med. 2006;103(2):180–184. [PubMed] [Google Scholar]
- 38.DePue JD, Goldstein MG, Schilling A, et al. Dissemination of the AHCPR clinical practice guideline in community health centres. Tob Control. 2002;11(4):329–335. doi: 10.1136/tc.11.4.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piper ME, Fiore MC, Smith SS, et al. Use of the vital sign stamp as a systematic screening tool to promote smoking cessation. Mayo Clin Proc. 2003;78(6):716–722. doi: 10.4065/78.6.716 [DOI] [PubMed] [Google Scholar]
- 40.Holtrop JS, Malouin R, Weismantel D, Wadland WC. Clinician perceptions of factors influencing referrals to a smoking cessation program. BMC Fam Pract. 2008;9:18. doi: 10.1186/1471-2296-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cornelius ME, Loretan CG, Wang TW, Jamal A, Homa DM. Tobacco Product Use Among Adults - United States, 2020. Mmwr-Morbid Mortal W. 2022;71(11):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang TW, Gentzke A, Sharapova S, Cullen KA, Ambrose BK, Jamal A. Tobacco Product Use Among Middle and High School Students - United States, 2011-2017. Mmwr-Morbid Mortal W. 2018;67(22):629–633. doi: 10.15585/mmwr.mm6722a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368(4):341–350. doi: 10.1056/NEJMsa1211128 [DOI] [PubMed] [Google Scholar]
- 44.Duncan MS, Freiberg MS, Greevy RA Jr., Kundu S, Vasan RS, Tindle HA. Association of Smoking Cessation With Subsequent Risk of Cardiovascular Disease. JAMA. 2019;322(7):642–650. doi: 10.1001/jama.2019.10298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benowitz NL, Bernert JT, Foulds J, et al. Biochemical Verification of Tobacco Use and Abstinence: 2019 Update. Nicotine Tob Res. 2020;22(7):1086–1097. doi: 10.1093/ntr/ntz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994;84(7):1086–1093. doi: 10.2105/ajph.84.7.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.