Abstract
Patients with transfemoral amputation (TFA) are at an increased risk of secondary musculoskeleteal comorbidities, primarily due to asymmetric joint loading. Amputated limb muscle weakness is also prevalent in the TFA population, yet all factors that contribute to muscle strength and thus joint loading are not well understood. Our objective was to bilaterally compare gluteus medius (GMED) muscle factors (volume, fatty infiltration, moment arm) that all contribute to joint loading in patients with TFA. Quantitative magnetic resonance (MR) images of the hip were collected from eight participants with unilateral TFA (2M/6F; age: 47.3 ± 14.7 y/o; BMI: 25.4 ± 5.3 kg/m2; time since amputation: 20.6 ± 15.0 years) and used to calculate normalized GMED muscle volume and fatty infiltration. Six participants participated in an instrumented gait analysis session that collected whole-body kinematics during overground walking. Subject-specific musculoskeletal models were used to calculate bilateral GMED (anterior, middle, posterior) moment arms and frontal plane hip joint angles across three gait cycles. Differences in volume, fatty infiltration, hip adduction–abduction angle, and peak moment arms were compared between limbs using paired Cohen’s d effect sizes. Volume was smaller by 36.3 ± 18.8% (d = 1.7) and fatty infiltration was greater by 6.4 ± 7.8% (d = 0.8) in the amputated limb GMED compared to the intact limb. The amputated limb GMED abduction moment arms were smaller compared to the intact limb for both overground walking (anterior: d = 0.9; middle: d = 0.1.2) and during normal range of motion (anterior: d = 0.8; middle: d = 0.8) while bilateral hip adduction–abduction angles were similar during overground walking (d = 0.5). These results indicate that in patients with TFA, the amputated limb GMED is biomechanically disadvantaged compared to the intact limb, which may contribute to the etiology of secondary comorbidities. This population might benefit from movement retraining to lengthen the amputated limb GMED abduction moment arm during gait.
Keywords: Transfemoral amputation, Above knee amputation, Muscle volume, Fatty infiltration, Gluteus medius, Moment arm, Hip biomechanics, Socket prosthesis
Introduction
Patients with transfemoral amputation (TFA) are more likely to develop secondary musculoskeletal comorbidities (e.g., osteoarthritis (OA), osteopenia/osteoporosis, and low back pain (LBP)) that are largely attributed asymmetric joint loading caused by compensatory movement patterns required for the loss of the ankle and knee joints [16]. Notably, patients with TFA are three- to six-fold more likely to develop hip OA in either or both hips compared to able-bodied individuals [34, 49], and nearly 52% of this population will experience LBP as compared to only 15–25% in the general population [13]. Patients with TFA also demonstrate muscle weakness in the amputated limb, which is primarily attributed to residual limb disuse within the socket [24]. Proper joint loading is crucial to healthy cartilage maintenance and muscles are the primary contributors to joint loading [11, 20]. Therefore, adequate force generation from surrounding musculature is necessary for healthy joint loading required to sustain joint health.
Muscle weakness influences the muscle’s ability to stabilize and load the joint and thus is a known primary mechanical etiological factor in OA and LBP [2]. Muscle volume is a commonly used metric to describe the muscle strength because volume is associated with physiological cross-sectional area [1]. Furthermore, muscles tend to hypertrophy with increased chronic force demand (e.g., resistance training) and atrophy with decreased chronic force demand (e.g., disuse) [7, 45]. It is well documented that patients with TFA have smaller volumes of the amputated limb musculature compared to the intact limb, demonstrating greater muscular atrophy in the amputated limb [25, 26, 46]. However, volume alone is not a holistic representation of muscle strength.
Muscle composition, or the amount of contractile and non-contractile tissue within the muscle, is also fundamental to the muscle’s ability to generate force [18]. Prior work has demonstrated that high levels of fatty infiltration (i.e., non-contractile tissue) within musculature impair muscle strength and functional performance [18, 19, 28, 39, 51]. Quantification of muscular fatty infiltration within the TFA population is lacking, though prior work within the transtibal population has demonstrated increased fatty infiltration of the amputated limb musculature [8]. In patients with TFA, a previous study by Sherk et al. showed increased fatty infiltration in distal residual limb musculature [46]; however, distal residual limb musculature contributes minimally to hip joint loading. Correa et al. have previously shown that the gluteus medius (GMED) muscle force is the greatest contributor to hip joint loading [11]. Furthermore, GMED muscle strength is emphasized in rehabilitation for patients with TFA as it is critical to maintain stability and control the trunk; prior work by Jaegers et al. in this population has demonstrated smaller GMED muscle volume in the amputated limb [26]. However, to our knowledge, there is still a lack of quantitative data on GMED muscle composition in this population, which will influence the muscle’s strength gain potential in rehabilitation.
Muscle-induced joint loading is also dependent on the muscle’s anatomical path (i.e., attachment sites), which can be partially described by the muscle’s moment arm [6, 9]. The moment arm of a muscle is defined as the orthogonal distance between the joint center of rotation and the muscle line of action [37, 47, 52]. Specifically, a decrease in moment arm will require more force generation from the muscle to create the same moment about the joint [57]. Because the muscle line of action is directly affected by its origin and insertion sites, changes in bony morphology will directly influence the muscle moment arm [44]. For example, prior work has demonstrated that changes in moment arms and attachment sites directly impact the forces and moments applied to a joint in patients with hip dysplasia [48]. We have previously shown that patients with TFA demonstrated morphologic variation in greater trochanteric shape on the amputated limb compared to an age- and sex-matched control group [42]. Since the greater trochanter is the insertion site for the primary hip abductors (e.g., GMED), trochanteric shape is of clinical relevance as it will likely alter the GMED moment arm and thus overall joint loading, yet this has not yet been directly quantified in patients with TFA. This mechanical disadvantage could potentially be mitigated by compensatory altered frontal plane kinematics [22, 24, 43], yet this has also not yet been explored.
The objectives of this investigation were to compare, in patients with unilateral TFA, (1) bilateral GMED muscle volume and composition (i.e., fatty infiltration) measured using magnetic resonance (MR) imaging; (2) bilateral GMED abduction moment arm during overground walking and range of motion estimated using marker-based motion capture and musculoskeletal modeling; and (3) bilateral hip adduction–abduction angle during overground walking estimated using marker-based motion capture and musculoskeletal modeling. We hypothesized that volume would be decreased while fatty infiltration would be increased for all muscles of the amputated limb compared to the intact limb. Additionally, due to the altered bony geometry of the amputated limb proximal femur, we hypothesized that moment arms would be smaller for the amputated limb GMED compared to the intact limb.
Materials and Methods
Participants and Experimental Collection
With Institutional Review Board approval, eight participants with unilateral TFA were enrolled as part of a larger study (Table 1).
Table 1.
Patient demographics with mean ± 1 standard deviation (SD)
| Participant | Sex | Age (years) | BMI (kg/m2) | Time since amputation (years) | Amputation side |
|---|---|---|---|---|---|
|
| |||||
| 1 | M | 48 | 27.7 | 39.6 | L |
| 2 | F | 61 | 31.7 | 4.7 | L |
| 3 | F | 26 | 23.2 | 19.9 | R |
| 4a | F | 32 | 17.7 | 2.2 | L |
| 5 | M | 60 | 30.0 | 31.1 | R |
| 6 | F | 60 | 19.0 | 32.1 | L |
| 7 | F | 33 | 23.7 | 31.3 | R |
| 8a | F | 58 | 29.9 | 4.3 | R |
| Mean ± SD | 2 M/6F | 47.3 ± 14.7 | 25.4 ± 5.3 | 20.6 ± 15.0 | 4L/4R |
Non-ambulatory participants who did not participate in motion capture analysis
Inclusion criteria for the current study included having a unilateral TFA with a non-vascular amputation etiology. Participants were excluded if they had a vascular amputation etiology, had contraindication for MR imaging, had a diagnosed orthopedic pathology to the hip (hip dysplasia, femoroacetabular impingement, Perthes disease, or slipped capital femoral epiphysis), or undergone prior orthopedic hip surgery on either hip. Each participant provided a signed, written informed consent prior to the onset of experimental collection.
Quantitative MR images were collected using two sequences from the iliac crest to distal of the lesser trochanter from eight participants with unilateral TFA in a supine position on a 3T GE Signa PET/MR Scanner (GE Healthcare, Chicago, IL). T1-weighted LAVA (GE) fast-spin echo images with fat suppression (1 × 1 × 1 mm voxels, TR/TE = 6.6/3.1 ms, FOV = 44 cm, flip angle = 3°, axial), followed by 3D IDEAL [40] IQ images with a 6-point Dixon sequence (TR = 6.6, FOV = 44 cm, flip angle = 3°, matrix 256×256, slice thickness = 4 mm, axial) were collected.
Six participants then participated in an instrumented gait analysis collection. Two participants were non-ambulatory and thus were excluded from the motion analysis testing (Table 1). Whole-body kinematics were collected using 72 reflective markers recorded from 10 infrared cameras (Fs = 120 Hz) (Vicon, Centennial, CO) during overground walking at self-selected speeds. Reflective markers were placed on palpable bilateral bony anatomical landmarks on the pelvis (anterior superior iliac spine, posterior superior iliac spine, iliac crest, and sacrum), thighs (medial/lateral femoral condyles and greater trochanter), shanks (medial/lateral malleolus and tibial tuberosity), feet (heel, toe, tip, first metatarsal, and fifth metatarsal), torso (acromion, clavicular notch, sternal notch, T10 vertebrae), arms, and head. Tracking markers were placed on the lower extremities using rigid clusters (Supplemental Fig. 1).
Image Processing
The bilateral GMED was segmented from the T1 images via semi-automatic thresholding within Amira (v2020.2; Thermo Fisher Scientific, Waltham, MA). The bilateral gluteus maximus and tensor fascia latae were also segmented (see Supplementary Material). Three-dimensional surface reconstructions were triangulated from segmentations and used to calculate volumes (normalized by body mass (kg) and height (mm)) (Fig. 1) [21]. Repeatability of muscle segmentation and volume measurements of the amputated limb GMED was tested on two occasions (separated by two weeks) by one rater (GR) using five subjects.
Fig. 1.
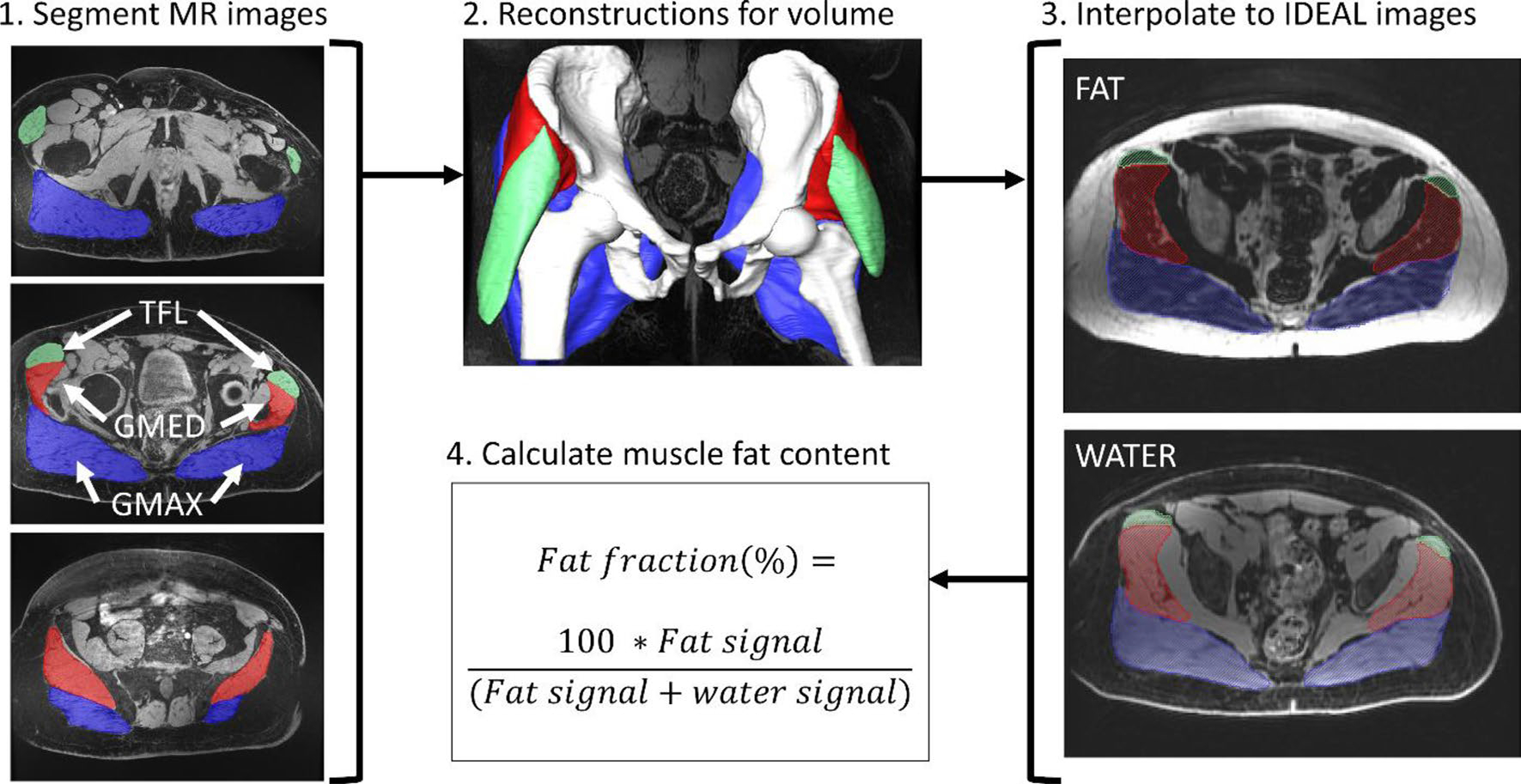
Quantitative imaging workflow where the tensor fascia latae (TFL) is in green, the gluteus medius (GMED) is in red, and the gluteus maximus (GMAX) is in blue
Muscle composition was quantified by separating water and fat signals based on the IDEAL algorithm [40]. Fatty infiltration, or percent fat fraction, was calculated as the fat signal divided by the sum of the fat and water signal multiplied by 100 (Fig. 1). Muscle volume and fatty infiltration were also calculated for the gluteus maximus and tensor fascia latae (Supplementary Fig. 2).
Musculoskeletal Modeling
Subject-specific musculoskeletal models were created by modifying a pre-existing model with 23° of freedom and 92 muscles [31]. The existing model was applied to individual subjects by using patient-specific bony reconstructions of the pelvis and bilateral proximal femurs (Fig. 2A) to attain subject-specific bony geometry, muscle paths, and hip joint center locations, with methods previously described in greater detail [15, 48, 53]. The remaining segments within the musculoskeletal model were non-uniformly scaled to match the patient’s anthropometry via individual body-segment scale factors determined by marker locations from the static motion capture trial. Due to the large attachments of the GMED, this muscle was broken sections (anterior, middle, and posterior) represented by three linear actuators (Fig. 2B) [3, 31, 32]. GMED attachments were updated using 3D reconstructions of the femur, pelvis, and GMED; MR images; and a human anatomy atlas (Fig. 2B) [35]. Repeatability of bilateral GMED origin and insertion sites were tested on two occasions (separated by at least two weeks) by one rater (GR) using five subjects.
Fig. 2.
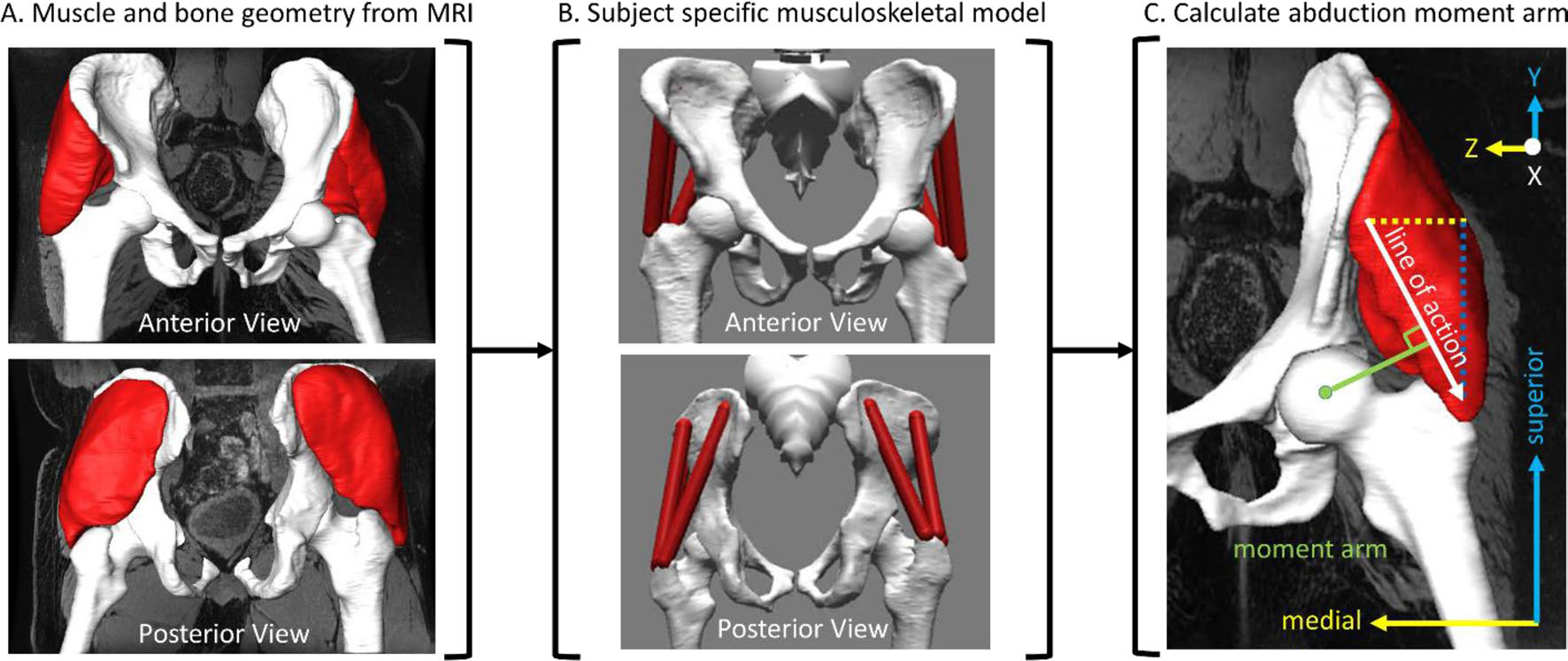
Moment arm workflow where A geometry from MRI is used in creating B the subjectspecific musculoskeletal model which is used to calculate C the moment arm (distance relative to the line of action), given in the pelvis frame. X, Y, and Z describe the anterior-posterior, superior-inferior, and medial-lateral directions, respectively.
Joint angles for all degrees of freedom were calculated using inverse kinematics via weighted least squares minimization between experimental and model markers within OpenSim. Only hip adduction–abduction angle (i.e., frontal plane hip joint angle) was considered for further analyses. The bilateral abduction moment arms (the orthogonal distance between the hip joint center of rotation and the linear actuator’s effective line of action) of the GMED sections were calculated using the generalized force approach within OpenSim (Fig. 2C), as previously described [47].
Statistical Analyses
Repeatability was assessed for segmentation (muscle volume), GMED attachment coordinates, and peak GMED abduction moment arm over the entire gait cycle with the absolute agreement intra-class correlation coefficient (ICC) and coefficient of variation (COV). ICC thresholds are defined as poor (ICC ≤ 0.5), moderate (0.5 < ICC ≤ 0.75), good (0.75 < ICC ≤ 0.9), and excellent (ICC ≥ 0.9) reliability [29]. The magnitude of COV thresholds are defined as low (≤ 10%), intermediate (11–20%), high (21–30%), and very high (> 30%) [27]. As the COV is the ratio of the standard deviation and the mean, the lower the COV, the more repeatable the measurements. Repeatability for all metrics was assessed on two occasions (separated by at least two weeks) by one rater (GR) using the same set of subjects for each metric. Bilateral differences in volume, composition, hip adduction–abduction angle extrema, and peak GMED abduction moment arm were compared using paired Cohen’s d effect sizes (medium: 0.5 ≤ d < 0.8; large: d ≥ 0.8) [10]. Descriptive results are presented as mean ± 1 standard deviation of the relative percent difference between the intact and amputated limbs.
Results
Repeatability/Reliability Results
Repeatability measures of the GMED segmentation demonstrated an ICC of 0.99 and a COV of 3.8%, indicating excellent repeatability for segmentation methods [12, 27, 29].
Repeatability measures of the bilateral anterior, middle, and posterior GMED origin points (on pelvis) demonstrated an ICC of 0.84, 0.83, and 0.93, with a COV of 5.5%, 12.0%, and 2.0%, respectively. Repeatability measures of the bilateral anterior, middle, and posterior GMED insertion points (on the greater trochanter) demonstrated an ICC of 0.94, 0.92, and 0.97, with a COV of 8.7%, 14.6%, and 2.0%, respectively. Collectively, this indicates repeatable placement of GMED origin and insertion attachment sites, and thus repeatable moment arm results.
Muscle Volume and Composition
The amputated limb GMED had smaller volumes and higher fatty infiltration compared to the intact limb GMED. Compared to the intact limb, amputated limb normalized GMED muscle volume was smaller by 36.3 ± 18.8% (d = 1.7) (Fig. 3A). Fatty infiltration was larger in the amputated limb GMED compared to the intact limb GMED by 35.5 ± 16.3% (d = 0.8) (Fig. 3B).
Fig. 3.

Mean ± 1 SD of A normalized muscle volume and B muscle fat content for the amputated limb (blue) and intact limb (red) gluteus medius with paired Cohen’s d values given above each subplot
Musculoskeletal Modeling
Hip Adduction–Abduction Angle
The peak hip abduction angle was larger on the intact limb than the amputated limb during early swing (d = 0.5), while there were no differences in the peak hip adduction angle during the stance period (Fig. 4).
Fig. 4.
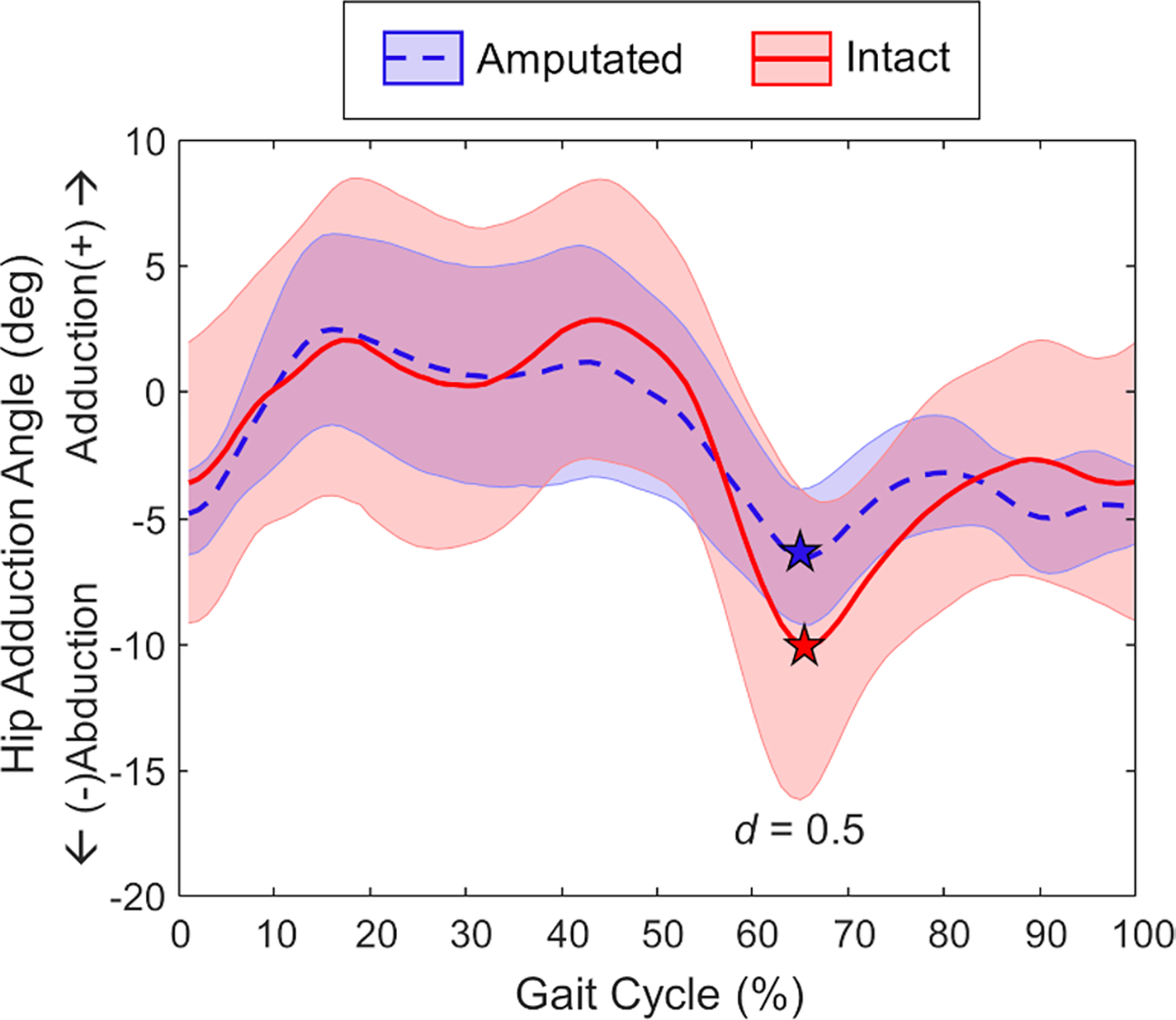
Ensemble average for hip adduction angle (mean ± 1 SD) across the amputated limb (blue dashed) and intact limb (red solid) gait cycle. The stars correspond to peak hip abduction angle during the gait cycle, which was smaller on the amputated limb (d = 0.5)
Gluteus Medius Moment Arm
During overground walking, the peak abduction moment arm was smaller in the amputated limb compared to the intact limb for anterior (12.0 ± 6.2%, d = 0.9), middle (11.0 ± 7.5%, d = 1.2), and posterior (11.1 ± 11.1%, d = 0.6) sections of the GMED (Fig. 5).
Fig. 5.

Ensemble average abduction moment arm (mean ± 1 SD) for the three components of the gluteus medius (A anterior, B middle, C posterior) across the amputated limb (blue dashed) and intact limb (red solid) gait cycle. The stars correspond to moment arm peaks for amputated and intact GMED components with Cohen’s d values between limbs given in the bottom right of each subfigure
Across the entire range of hip adduction–abduction, the GMED abduction moment arm was smaller in the amputated limb compared to the intact limb for all three components (Fig. 6). Specficially, peak abduction moment arm was smaller for the anterior (d = 0.8) and middle (d = 0.8) portions of the amputated limb GMED compared to the intact limb with (Fig. 6A, B).
Fig. 6.

Ensemble average abduction moment arm length (mean ± 1 SD) during adduction–abduction model range of motion for the three components of the gluteus medius (A anterior, B middle, C posterior) on the amputated limb (blue dashed) and intact limb (red solid). The stars correspond to moment arm peaks for amputated and intact GMED components with Cohen’s d values between limbs given in the bottom right of each subfigure
Discussion
The objective of this investigation was to quantify primary factors of the GMED muscle that contribute to joint loading in patients with unilateral TFA. Our results showed that muscle volume was smaller and fatty infiltration was larger in the amputated limb compared to the intact limb, indicating atrophy and increased fat content of the amputated limb GMED. We also found that the amputated limb GMED moment arm in the frontal plane was smaller than the intact limb during both range of motion and overground walking, indicating a decreased ability of the amputated limb GMED to generate stabilizing moments about the hip. These results indicate that the amputated limb GMED is biomechanically disadvantaged compared to the intact limb, which will influence its ability to properly stabilize and load the amputated limb hip joint.
We found the amputated limb frontal plane GMED moment arm was smaller than the intact limb during both overground walking and anatomic range of motion. A muscle’s moment arm is dependent upon both the movement of the joint itself and the muscle path; thus, it is important to consider both factors when elucidating the mechanism of these findings. As we found minimal differences in the hip abduction angle across limbs (excluding early swing), we interpret that the smaller amputated limb GMED moment arm is caused by a change in its path and not the joint motion. It has previously been established that skeletal deformities directly influence a muscle moment arm, as changes in bone shape will alter the muscle path due to changes in origin and insertion sites [48]. We have previously shown that the proximal femur of the amputated limb is different than that of able-bodied controls, most prominently in the shape of the greater trochanter, which we hypothesize occurs due to the habitually altered mechanical stimulus applied to the bone [42]. As such, because the GMED inserts on the greater trochanter, we interpret our current findings of a smaller GMED moment arm to be caused by bony morphological abnormalities of the amputated limb proximal femur. This represents a biomechanical disadvantage that necessitates greater muscle force generation to create the same moment about the joint [57]. For example, prior work by Harris et al. in hips with developmental dysplasia has demonstrated that smaller moment arms caused by bony morphological changes corresponded to higher hip abductor forces and resultant hip joint reaction forces [23]. Collectively, our current results indicating changes in muscle biomechanics likely plays a role in altered joint loading and could possibly contribute to the development of secondary comorbidities.
The results are clinically relevant as gait retraining (i.e., targeted foot placement to change hip adduction–abduction angle) could be used to create symmetrical abductor moment arms because the movement pattern is more modifiable via rehabilitation than muscle path changes. Thus, our results could potentially inform future interventions aimed at normalizing joint biomechanics through movement retraining (e.g., targeted foot placement) to support the mechanically disadvantaged GMED by maximizing its abduction moment arm. However, future work is needed to directly assess the impact of gait retraining on GMED abduction moment arm and joint loading in patients with unilateral TFA.
Additionally, our results suggest that TFA causes changes to intrinsic musculature factors that affect force generation capabilities of the amputated limb GMED (i.e., smaller volume and larger fatty infiltration). Jaegers et al. have shown smaller GMED muscle volumes on the amputated limb compared to the intact limb, which is indicative of muscular atrophy that is most likely caused by residual limb disuse [24]. As the abductors are critical for limb stability within the socket [24, 48], healthy joint loading [11], and balance during gait [36], abductor strengthening remains a primary focus during rehabilitation after limb amputation [54]. However, muscle volume alone is not the only factor that contributes to a muscle’s ability to generate force. We found that fatty infiltration was higher in the GMED of the amputated limb compared to the intact limb. Although bilateral fatty infiltration and volume differences both possessed large paired Cohen’s d effect sizes, the effect of side on GMED volume was more than double the effect of side on fatty infiltration, indicating amputation may have a larger effect on GMED volume compared to fatty infiltration. Prior evidence from Wentink et al. has shown increased duration of activation of amputated limb abductors during walking in patients with TFA compared to able-bodied individuals [55]. We hypothesize that this increase in duration of activation likely relates to our current findings of more pronounced differences in muscle volume, compared to fatty infiltration. Nonetheless, fatty infiltration is associated with lower muscle torques [41] and is potentially irreversible even with exercise or surgery [30, 56]. Overall, these results suggest that patients with TFA would continue to benefit from targeted abductor strengthening to increase muscle volume and mitigate fatty infiltration.
There are several limitations of the current investigation. First, marker-based motion capture is prone to skin artifact and does not account for residual limb motion within the socket prosthesis, which will inherently influence the moment arm. Prior evidence utilizing biplane fluoroscopy has shown that the residual limb is not static within the socket during walking [17]. Conversely, it has also previously been shown that skin artifact does not significantly affect hip abduction angles during gait compared to direct imaging of bone motion with dual fluoroscopy [14]. As the magnitude of the motion of the residual limb within the socket is substantially smaller than the hip angle during walking, we do not believe that this will have a significant effect on our results, yet future work is needed to confirm this. Second, the sample size is small and is considered a case-series. A Shapiro–Wilk goodness of fit normality test of our results indicated inconsistent results of both normal (e.g., fatty infiltration) and non-normal (e.g., volume) distributions, which is likely spurious due to such a small sample size. Furthermore, a post hoc power analysis (α = 0.05) of our variables presented ranged from 0.52 to 1.00. While some variables showed strong effects that yielded appropriate power for inferential statistics (e.g., muscle volume, anterior abductor moment arm), others were not. Thus, we advocate caution in interpreting all of our results as future work is needed for a fully powered sample size to perform inferential statistics. Third, this study is limited by the inherent heterogeneity of the participant demographics, including variation in residual limb length, physical activity, and time since amputation. However, we expect that this would only help to diminish the effect of transfemoral prosthesis use on bilateral muscle differences. Since bilateral differences in volume, fatty infiltration, and moment arm possessed large effect sizes (except for the posterior section of the GMED during normal hip adduction–abduction range), we expect this heterogeneity did not significantly influence our results. Lastly, this study is limited by the lack of a control group to compare bilateral differences in a healthy population. However, previous studies have shown no bilateral differences between limbs in muscle volume or fatty infiltration for the hip abductors [4, 5, 33].
In conclusion, our results imply the amputated limb GMED is biomechanically disadvantaged compared to the intact limb GMED, with differences observed in volume, composition, and moment arm. As all of these factors are known to contribute to joint loading, the biomechanical disadvantage of the amputated limb GMED could potentially exacerbate hip OA development or progression. Furthermore, due to regional interdependence, these results also have potential implications pertaining to both LBP and knee OA as changes to hip biomechanics will change the biomechanics in the surrounding joints. For example, in addition to hip OA, hip abductor weakness is also a known etiological factor to both LBP and knee OA [38, 50]. Understanding all components that influence muscle force generation and joint loads may advance future interventions aimed at improving function and normalizing joint loading in patients with TFA, possibly mitigating the risk of secondary musculoskeletal conditions. Future investigations should explore additional parameters which contributing to muscle weakness to further elucidate the etiology of secondary comorbidities (e.g., hip and knee OA, LBP) in able-bodied patients and patients with unilateral TFA.
Supplementary Material
Acknowledgements
This project was supported by the University of Colorado Osseointegration Research Consortium and the National Institutes of Health (K01AR080776 and UL1TR002535). We would like to acknowledge Taylor Wittwer for her role in segmentation and Cassie Wong for her role in motion capture data processing.
Funding
Funding was provided by National Institute of Arthritis and Musculoskeletal and Skin Diseases (K01AR080776) and National Institutes of Health (UL1TR002535).
Abbreviations
- OA
Osteoarthritis
- TFA
Transfemoral amputation
- GMED
Gluteus medius
Footnotes
Declarations
Conflict of interest There are no competing interests to disclose.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s10439-023-03400-0.
References
- 1.Albracht K, Arampatzis A, and Baltzopoulos V. Assessment of muscle volume and physiological cross-sectional area of the human triceps surae muscle in vivo. J. Biomech. 41:2211–2218, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Amaro A, Amado F, Duarte JA, and Appell HJ. Gluteus medius muscle atrophy is related to contralateral and ipsilateral hip joint osteoarthritis. Int. J. Sports Med. 28:1035–1039, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Arnold EM, Ward SR, Lieber RL, and Delp SL. A model of the lower limb for analysis of human movement. Ann. Biomed. Eng. 38:269–279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belzunce MA, Henckel J, Di Laura A, and Hart A. Intramuscular fat in gluteus maximus for different levels of physical activity. Sci. Rep. 11:1–10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belzunce MA, Henckel J, Di Laura A, and Hart AJ. Reference values for volume, fat content and shape of the hip abductor muscles in healthy individuals from Dixon MRI. NMR Biomed. 35:1–13, 2022. [DOI] [PubMed] [Google Scholar]
- 6.Blemker SS, and Delp SL. Three-dimensional representation of complex muscle architectures and geometries. Ann. Biomed. Eng. 33:661–673, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bodine SC Disuse-induced muscle wasting. Int. J. Biochem. Cell Biol. 45:2200–2208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bramley JL, Worsley PR, Bader DL, Everitt C, Darekar A, King L, and Dickinson AS. Changes in tissue composition and load response after transtibial amputation indicate biomechanical adaptation. Ann. Biomed. Eng. 49:3176–3188, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chopp-Hurley JN, Langenderfer JE, and Dickerson CR. Probabilistic evaluation of predicted force sensitivity to muscle attachment and glenohumeral stability uncertainty. Ann. Biomed. Eng. 42:1867–1879, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Cohen J Statistical power analysis for the behavioral sciences. Stat. Power Anal. Behav. Sci. 2013. 10.4324/9780203771587. [DOI] [Google Scholar]
- 11.Correa TA, Crossley KM, Kim HJ, and Pandy MG. Contributions of individual muscles to hip joint contact force in normal walking. J. Biomech. 43:1618–1622, 2010. [DOI] [PubMed] [Google Scholar]
- 12.Davico G, Bottin F, Di Martino A, Castafaro V, Baruffaldi F, Faldini C, and Viceconti M. Intra-operator repeatability of manual segmentations of the hip muscles on clinical magnetic resonance images. J. Digit. Imaging. 36:143–152, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehde DM, Smith DG, Czerniecki JM, Campbell KM, Malchow DM, and Robinson LR. Back pain as a secondary disability in persons with lower limb amputations. Arch. Phys. Med. Rehabil. 82:731–734, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino NM, Atkins PR, Kutschke MJ, Foreman KB, and Anderson AE. Soft tissue artifact causes underestimation of hip joint kinematics and kinetics in a rigid-body musculoskeletal model. J. Biomech. 108:109890, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaffney BMM, Vandenberg NW, Davis-Wilson HC, Christiansen CL, Roda GF, Schneider G, Johnson T, and Stoneback JW. Biomechanical compensations during a stand-to-sit maneuver using transfemoral osseointegrated prostheses: a case series. Clin. Biomech.98:105715, 2022. [DOI] [PubMed] [Google Scholar]
- 16.Gailey R, Allen K, Castles J, Kucharik J, and Roeder M. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J. Rehabil. Res. Dev. 45:15–30, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Gale T, Yang S, McGough R, Fiedler G, and Anderst W. Motion of the residual femur within the socket during gait is associated with patient-reported problems in transfemoral amputees. J. Biomech.112:110050, 2020. [DOI] [PubMed] [Google Scholar]
- 18.Gerber C, Schneeberger AG, Hoppeler H, and Meyer DC. Correlation of atrophy and fatty infiltration on strength and integrity of rotator cuff repairs: a study in thirteen patients. J. Shoulder Elb. Surg. 16:691–696, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, and Newman AB. Attenuation of skeletal muscle and strength in the elderly: the health ABC study. J. Appl. Physiol. 90:2157–2165, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Griffin TM, and Guilak F. The role of mechanical loading in the onset and progression of oestoarthritis: exercise and sport sciences reviews. Exerc. Sport Sci. Rev. 33:195–200, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Handsfield GG, Meyer CH, Hart JM, Abel MF, and Blemker SS. Relationships of 35 lower limb muscles to height and body mass quantified using MRI. J. Biomech. 47:631–638, 2014. [DOI] [PubMed] [Google Scholar]
- 22.Harandi VJ, Ackland DC, Haddara R, Lizama LEC, Graf M, Galea MP, and Lee PVS. Gait compensatory mechanisms in unilateral transfemoral amputees. Med. Eng. Phys. 77:95–106, 2020. [DOI] [PubMed] [Google Scholar]
- 23.Harris MD, Shepherd MC, Song K, Gaffney BMM, Hillen TJ, Harris-Hayes M, and Clohisy JC. The biomechanical disadvantage of dysplastic hips. J. Orthop. Res. 40:1387–1396, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heitzmann DWW, Leboucher J, Block J, Günther M, Putz C, Götze M, Wolf SI, and Alimusaj M. The influence of hip muscle strength on gait in individuals with a unilateral transfemoral amputation. PLoS ONE.15:e0238093, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henson DP, Edgar C, Ding Z, Sivapuratharasu B, Le Feuvre P, Finnegan ME, Quest R, McGregor AH, and Bull AMJ. Understanding lower limb muscle volume adaptations to amputation. J. Biomech.125:110599, 2021. [DOI] [PubMed] [Google Scholar]
- 26.Jaegers SMHJ, Arendzen JH, and De Jongh HJ. Changes in hip muscles after above-knee amputation. Clin. Orthop. Relat. Res. 319:276–284, 1995. [PubMed] [Google Scholar]
- 27.Juffermans JF, Westenberg JJM, van den Boogaard PJ, Roest AAW, van Assen HC, van der Palen RLF, and Lamb HJ. Reproducibility of aorta segmentation on 4D flow MRI in healthy volunteers. J. Magn. Reson. Imaging. 53:1268–1279, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoja SS, Moore CG, Goodpaster BH, Delitto A, and Piva SR. Skeletal muscle fat and its association with physical function in rheumatoid arthritis. Arthritis Care Res. 70:333–342, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo TK, and Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15:155–163, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzel BR, Grindel S, Papandrea R, and Ziegler D. Fatty infiltration and rotator cuff atrophy. J. Am. Acad. Orthop. Surg. 21:613–623, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Lai AKM, Arnold AS, and Wakeling JM. Why are antagonist muscles co-activated in my simulation? a musculoskeletal model for analysing human locomotor tasks. Ann. Biomed. Eng. 45:2762–2774, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Marra MA, Verdonschot N, and Lu Y. A three-dimensional finite-element model of gluteus medius muscle incorporating inverse-dynamics-based optimization for simulation of nonuniform muscle contraction. Med. Eng. Phys. 87:38–44, 2021. [DOI] [PubMed] [Google Scholar]
- 33.Marcon M, Berger N, Manoliu A, Fischer MA, Nanz D, Andreisek G, and Ulbrich EJ. Normative values for volume and fat content of the hip abductor muscles and their dependence on side, age and gender in a healthy population. Skelet. Radiol. 45:465–474, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Morgenroth DC, Gellhorn AC, and Suri P. Osteoarthritis in the disabled population: a mechanical perspective. PM R. 4:S20–S27, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Netter. Netter Atlas of Human Anatomy English. 1951
- 36.Pandy MG, Lin YC, and Kim HJ. Muscle coordination of mediolateral balance in normal walking. J. Biomech. 43:2055–2064, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Phillips ATM, Villette CC, and Modenese L. Femoral bone mesoscale structural architecture prediction using musculoskeletal and finite element modelling. Int. Biomech. 2:43–61, 2015. [Google Scholar]
- 38.Prather H, Cheng A, Steger-May K, Maheshwari V, and VanDillen L. Association of hip radiograph findings with pain and function in patients presenting with low back pain. PM&R. 10:11–18, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahemi H, Nigam N, and Wakeling JM. The effect of intramuscular fat on skeletal muscle mechanics: implications for the elderly and obese. J. R. Soc. Interface. 12:20150365, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, Gold GE, Beaulieu CH, and Pelc NT. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn. Reson. Med. 54:636–644, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Robles PG, Sussman MS, Naraghi A, Brooks D, Goldstein RS, White LM, and Mathur S. Intramuscular fat infiltration contributes to impaired muscle function in COPD. Med. Sci. Sports Exerc. 47:1334–1341, 2015. [DOI] [PubMed] [Google Scholar]
- 42.Roda GF, Stoneback JW, Gimarc D, and Gaffney BMM. Above knee socket prosthesis use changes proximal femur morphology. Bone. 172:116752, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rueda FM, Diego IMA, Sánchez AM, Tejada MC, Montero FMR, and Page JCM. Knee and hip internal moments and upper-body kinematics in the frontal plane in unilateral transtibial amputees. Gait Posture. 37:436–439, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Sangeux M Biomechanics of the hip during gait. Pediatr. Adolesc. Hip. 2019. 10.1007/978-3-030-12003-0_3. [DOI] [Google Scholar]
- 45.Schoenfeld BJ, Grgic J, Ogborn D, and Krieger JW. Strength and hypertrophy adaptations between low- vs. high-load resistance training: a systematic review and meta-analysis. J. Strength Cond. Res. 31:3508–3523, 2017. [DOI] [PubMed] [Google Scholar]
- 46.Sherk VD, Bemben MG, and Bemben DA. Interlimb muscle and fat comparisons in persons with lower-limb amputation. Arch. Phys. Med. Rehabil. 91:1077–1081, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Sherman MA, Seth A, and Delp SL. What is a moment arm? Calculating muscle effectiveness in biomechanical models using generalized coordinates. Proc. ASME Des. Eng. Tech. Conf. 2014. 10.1115/DETC2013-13633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song K, Gaffney BMM, Shelburne KB, Pascual-Garrido C, Clohisy JC, and Harris MD. Dysplastic hip anatomy alters muscle moment arm lengths, lines of action, and contributions to joint reaction forces during gait. J. Biomech.110:109968, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Struyf PA, van Heugten CM, Hitters MW, and Smeets RJ. The prevalence of osteoarthritis of the intact hip and knee among traumatic leg amputees. Arch. Phys. Med. Rehabil. 90:440–446, 2009. [DOI] [PubMed] [Google Scholar]
- 50.Sueki DG, Cleland JA, and Wainner RS. A regional interdependence model of musculoskeletal dysfunction: research, mechanisms, and clinical implications. J. Man. Manip. Ther. 21:90–102, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuttle LJ, Sinacore DR, and Mueller MJ. Intermuscular adipose tissue is muscle specific and associated with poor functional performance. J. Aging Res. 2012. 10.1155/2012/172957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Arkel RJ, Modenese L, Phillips ATM, and Jeffers JRT. Hip abduction can prevent posterior edge loading of hip replacements. J. Orthop. Res. 31:1172–1179, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vandenberg NW, Stoneback JW, Davis-Wilson H, Christiansen CL, Awad ME, Melton DH, and Gaffney BMM. Unilateral transfemoral osseointegrated prostheses improve joint loading during walking. J. Biomech.155:111658, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vieira RI, da Luz SCT, dos Santos KPB, Gonçalvez Junior E, and Campos PVC. Physiotherapy intervention during pre and post-prosthetic fitting of lower limb amputees: a systematic review. Acta Fisiatr. 24:98–104, 2017. [Google Scholar]
- 55.Wentink EC, Prinsen EC, Rietman JS, and Veltink PH. Comparison of muscle activity patterns of transfemoral amputees and control subjects during walking. J. Neuroeng. Rehabil. 10:1–11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wesselink EO, Pool JJM, Mollema J, Weber KA, Elliott JM, Coppieters MW, and Pool-Goudzwaard AL. Is fatty infiltration in paraspinal muscles reversible with exercise in people with low back pain? A systematic review. Eur. Spine J. 32:787–796, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanagawa T, Goodwin CJ, Shelburne KB, Giphart JE, Torry MR, and Pandy MG. Contributions of the individual muscles of the shoulder to glenohumeral joint stability during abduction. J. Biomech. Eng. 130:021024, 2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


