Abstract
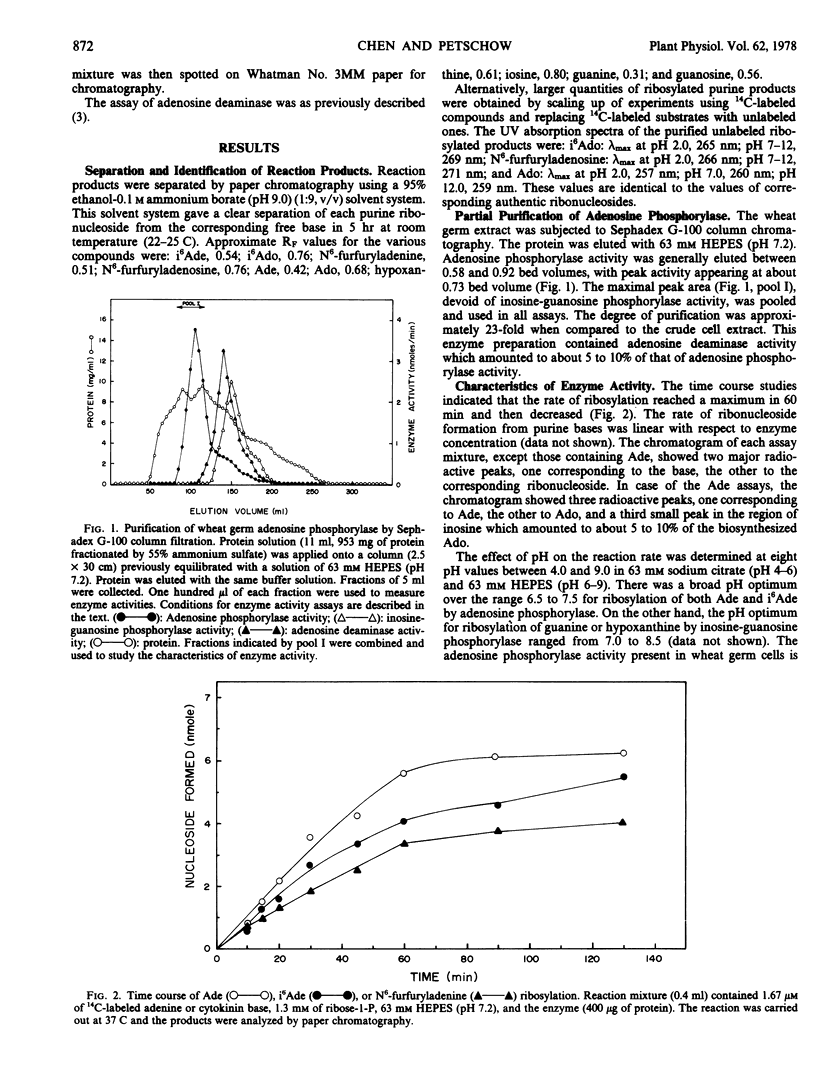
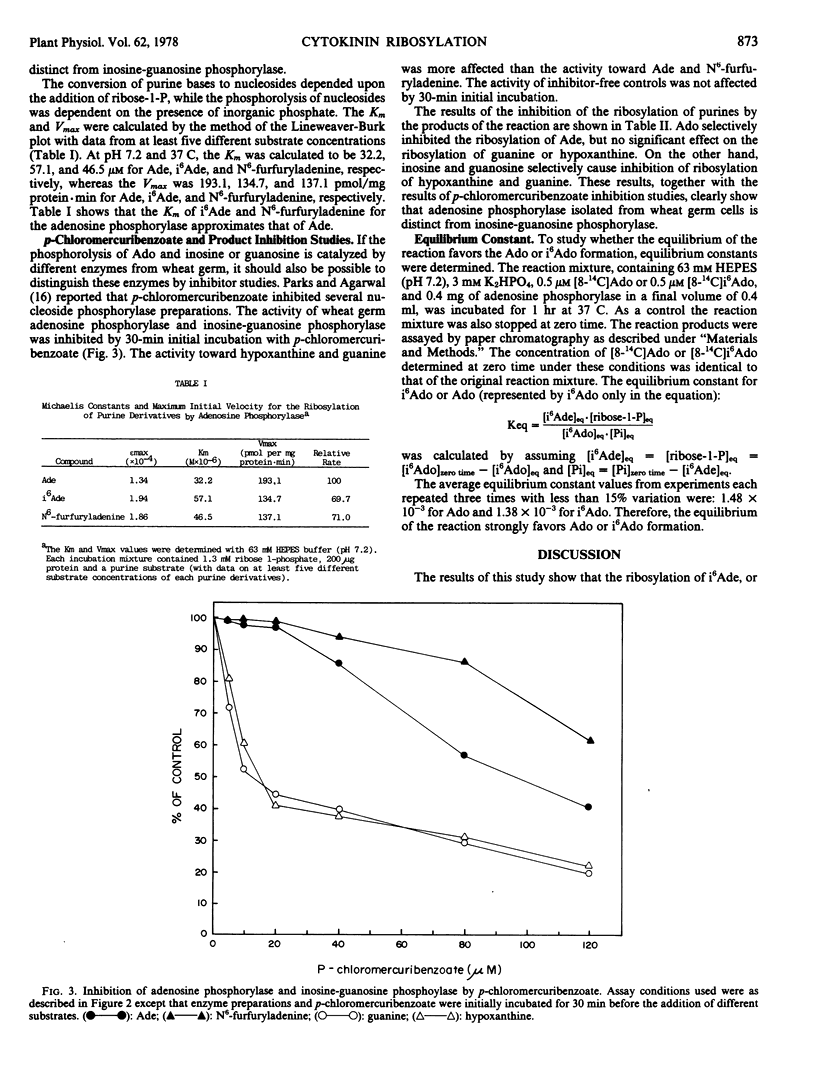
As part of the study of cytokinin metabolic pathways, an enzyme, adenosine phosphorylase (EC 2.4.2.-), which catalyzed the ribosylation of N6-(Δ2-isopentenyl)adenine, N6-furfuryladenine, and adenine to form the corresponding nucleosides, was partially purified from wheat (Triticum aestivum) germ. The pH optimum for the ribosylation of the cytokinins and adenine was from 6.5 to 7.8; for guanine and hypoxanthine it was from 7.0 to 8.5 At pH 7.2 (63 millimolar N-2-hydroxyethyl piperazine-N′-ethanesulfonic acid) and 37 C the Km for N6-(Δ2-isopentenyl)adenine was 57.1 micromolar; N6-furfuryladenine, 46.5 micromolar; adenine, 32.2 micromolar; and the Vmax for N6-(Δ2-isopentenyl)adenine, N6-furfuryladenine, and adenine were 134.7, 137.1, and 193.1 nanomoles per milligram protein per minute, respectively. The equilibrium constants of the phosphorolysis of N6-(Δ2-isopentenyl)adenosine and adenosine by this enzyme indicated that the reaction strongly favored nucleoside formation. This enzyme was shown to be distinct from inosine-guanosine phosphorylase based on the differences in the Sephadex G-100 gel filtration behaviors, pH optima, and the product and p-hydroxymercuribenzoate inhibitor studies. These results suggest that adenosine phosphorylase may play a significant role in the regulation of cytokinin metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C. M., Eckert R. L. Phosphorylation of cytokinin by adenosine kinase from wheat germ. Plant Physiol. 1977 Mar;59(3):443–447. doi: 10.1104/pp.59.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. M., Smith O. C., Hartnell G. F. Biological activity of ribose-modified N 6 (delta 2-isopentenyl) adenosine derivative. Can J Biochem. 1974 Dec;52(12):1154–1161. doi: 10.1139/o74-160. [DOI] [PubMed] [Google Scholar]
- Chen C., Smith O. C., McChesney J. Biosynthesis and cytokinin activity of 8-hydroxy and 2,8-dihydroxy derivatives of zeatin and N-6(increment-2-isopentenyl)adenine. Biochemistry. 1975 Jul 15;14(14):3088–3093. doi: 10.1021/bi00685a008. [DOI] [PubMed] [Google Scholar]
- Divekar A. Y. Adenosine phosphyorylase activity as distinct from inosine-guanosine phosphorylase activity in Sarcoma 180 cells and rat liver. Biochim Biophys Acta. 1976 Jan 23;422(1):15–28. doi: 10.1016/0005-2744(76)90004-8. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Cornette J., Deleuze G., Dyson W., Giersak C., Niu P., Zapata J., McChesney J. The formation, isolation, and biological activity of a cytokinin 7-glucoside. Plant Physiol. 1973 Dec;52(6):627–632. doi: 10.1104/pp.52.6.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. H. N6-(delta 2-isopentenyl)adenosine: chemical reactions, biosynthesis, metabolism, and significance to the structure and function of tRNA. Prog Nucleic Acid Res Mol Biol. 1970;10:57–86. doi: 10.1016/s0079-6603(08)60561-9. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Del Giudice R., Long C. Adenine formation from adenosine by mycoplasmas: adenosine phosphorylase activity. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1401–1405. doi: 10.1073/pnas.72.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H. The cytokinins. Int Rev Cytol. 1971;31:301–338. doi: 10.1016/s0074-7696(08)60061-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murai N., Armstrong D. J., Taller B. J., Skoog F. Distribution of incorporated, synthetic cytokinins in ribosomal RNA preparations from tobacco callus. Plant Physiol. 1978 Mar;61(3):318–322. doi: 10.1104/pp.61.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senesi S., Falcone G., Mura U., Sgarrella F., Ipata P. L. A specific adenosine phosphorylase, distinct from purine nucleoside phosphorylase. FEBS Lett. 1976 May 1;64(2):353–357. doi: 10.1016/0014-5793(76)80327-4. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. P., Gersten N. B., Ross A. F., Miech R. P. Adenine as substrate for purine nucleoside phosphorylase. Can J Biochem. 1971 Sep;49(9):1050–1054. doi: 10.1139/o71-153. [DOI] [PubMed] [Google Scholar]


