Abstract
Introduction:
Age-related blood-brain barrier (BBB) disruption, cerebromicrovascular senescence, and microvascular rarefaction substantially contribute to the pathogenesis of vascular cognitive impairment (VCI) and Alzheimer's disease (AD). Previous studies established a causal link between age-related decline in circulating levels of insulin-like growth factor-1 (IGF-1), cerebromicrovascular dysfunction, and cognitive decline. The aim of our study was to determine the effect of IGF-1 signaling on senescence, BBB permeability, and vascular density in middle-age and old brains.
Methods:
Accelerated endothelial senescence was assessed in senescence reporter mice (VE-Cadherin-CreERT2/Igf1rfl/fl × p16-3MR) using flow cytometry. To determine the functional consequences of impaired IGF-1 input to cerebromicrovascular endothelial cells, BBB integrity and capillary density were studied in mice with endothelium-specific knockout of IGF1R (VE-Cadherin-CreERT2/Igf1rfl/fl) using intravital two-photon microscopy.
Results:
In VE-Cadherin-CreERT2/Igf1rfl/fl mice 1) there was an increased presence of senescent endothelial cells; 2) cumulative permeability of the microvessels to fluorescent tracers of different molecular weights (0.3 kDa to 40 kDa) is significantly increased, as compared to that of control mice, whereas decline in cortical capillary density does not reach statistical significance.
Conclusions:
These findings support the notion that IGF-1 signaling plays a crucial role in preserving a youthful cerebromicrovascular endothelial phenotype and maintaining the integrity of the BBB.
Keywords: IGF-1, cerebral microcirculation, endothelial senescence, blood-brain barrier, accelerated aging
Introduction
The exponential increase in the prevalence of vascular cognitive impairment and dementia (VCID) with advancing age poses a significant healthcare challenge in the aging societies of the Western world1. Aging-induced structural and functional alterations in the cerebral microcirculation play a crucial role in the development of VCID2. Blood-brain barrier (BBB) disruption3–6, microvascular endothelial dysfunction, and a decline in brain capillarization, referred to as "cerebromicrovascular rarefaction," are prominent age-related microvascular pathologies, which are causally linked to the pathogenesis of VCID3–10.
The mechanisms responsible for age-related BBB disruption and cerebromicrovascular rarefaction are multifaceted and involve a combination of cell-autonomous pathways and non-autonomous neuroendocrine mechanisms. Extensive research has provided compelling evidence that non-autonomous neuroendocrine mechanisms of vascular aging encompass the age-related decline of insulin-like growth factor-1 (IGF-1) signaling11. IGF-1, an anabolic hormone synthesized by the liver, exerts diverse vasoprotective and anti-aging effects12–41. As individuals age, circulating levels of IGF-1 significantly decrease due to the decline in growth hormone (GH) release20,38,42–45. Notably, studies utilizing transgenic mouse models with circulating IGF-1 deficiency have demonstrated cerebromicrovascular rarefaction, impaired microvascular endothelial function, and neurovascular coupling responses in young animals, effectively mimicking the aging phenotype17,46. Importantly, IGF1R, the receptor for IGF-1, is abundantly expressed in all cell types of the neurovascular unit, including neurons, astrocytes, and endothelial cells. There is initial evidence that disruption of endothelial IGF-1 signaling by genetic depletion of IGF1R specifically in endothelial cells promotes cerebromicrovascular endothelial dysfunction47. Recent studies demonstrate that with advanced aging there is an increased presence of senescent endothelial cells in the cerebral microcirculation and that endothelial senescence contributes to the genesis of cerebromicrovascular aging phenotypes, including vasomotor dysfunction48–50. Moreover, IGF-1 was shown to regulate induction of cellular senescence in vascular diseases such as atherosclerosis22. However, the specific roles of IGF-1 signaling in endothelial cells in regulation of BBB integrity, cortical capillarization, and endothelial senescence remain to be determined.
The present study was designed to test the hypotheses that IGF-1 signaling modulates BBB integrity and capillary density in the brain and that disruption of IGF-1 specifically in endothelial cells leads to BBB disruption and capillary rarefaction, mimicking aspects of the aging phenotype. To test our hypotheses, intravital two-photon microscopy-based imaging was used to assess BBB permeability and cerebromicrovascular density in chronic cranial window equipped mice with adult-onset, endothelial cells-specific disruption of IGF-1 signaling (VE-Cadherin-CreERT2/Igf1rfl/fl). To determine whether disruption of IGF-1 signaling in endothelial cells leads to accelerated cellular senescence, we used senescence reporter mice with endothelial cells-specific disruption of IGF1R (VE-Cadherin-CreERT2/Igf1rfl/fl x × p16-3MR), which allows the detection of senescent cells51.
Materials and Methods
Animal model
To establish a mouse model with endothelium-specific depletion of IGFR1, Igf1rfl/fl (B6;129-Igf1rtm2Arge/J; loxP sites flanking exon 3) and VE-Cadherin-CreERT2 (B6.FVB-Tg(Cdh5-cre)7Mlia/J; Stock No: 006137) animals were acquired from Jackson Laboratories. The mice were housed in Allentown XJ cages with Anderson's Enrich-o-cob bedding (Maumee, OH), accommodating 3–4 mice per cage. Igf1rfl/fl and VE-Cadherin-CreERT2 mice were bred to generate experimental cohorts. The animals were kept under specific pathogen-free conditions, in the Rodent Barrier Facility at the University of Oklahoma Health Sciences Center. The mice were maintained on a 14-hour light/10-hour dark cycle during breeding, and the weaned mice were transitioned to a 12-hour light/12-hour dark cycle at a temperature of 21 °C. Mice had ad libitum access to standard irradiated bacteria-free rodent chow (5053 Pico Lab, Purina Mills, Richmond, IN) and reverse osmosis filtered water. Male VE-Cadherin-CreERT2 mice were crossed with female Igf1rfl/fl mice to generate VE-Cadherin-CreERT2/Igf1r+/− males. These males were then bred with Igf1rfl/fl female mice to establish the founder colony of VE-Cadherin-CreERT2/Igf1rfl/fl mice, according to published protocols52. The founder colony was subsequently bred with Igf1rfl/fl mice to generate experimental cohorts consisting of VE-Cadherin-CreERT2/Igf1rfl/fl mice with endothelium-specific knockdown of IGF1R as well as Cre-/Igf1rfl/fl control mice. When the mice reached three months of age, they were injected intraperitoneally with tamoxifen (75 mg/kg body weight, dissolved in corn oil) or vehicle (corn oil) for five consecutive days (Fig. 1).
Figure 1: Animal Model for Endothelial-Specific IGF1R Deficiency.
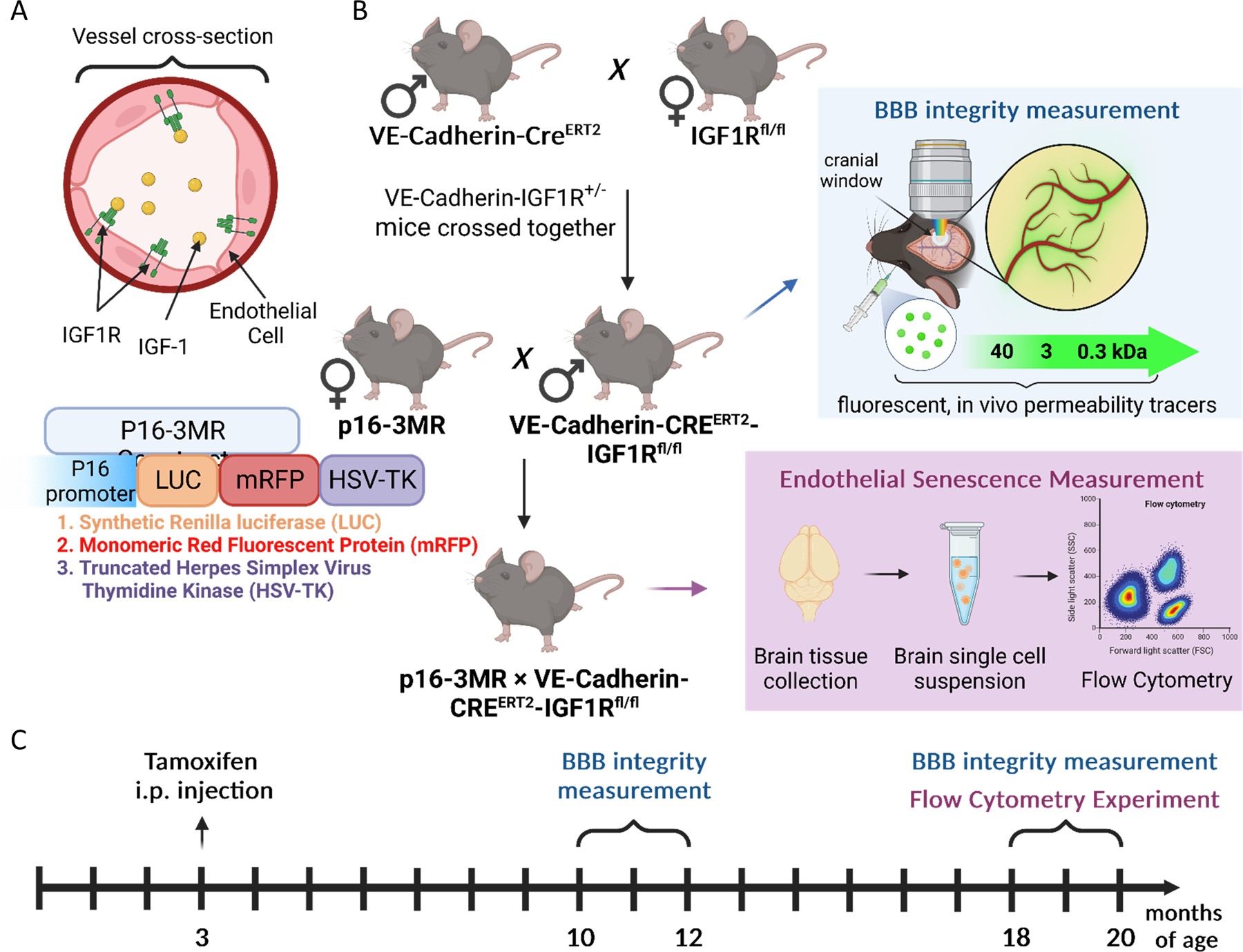
A) Illustration of the IGF1R receptor expressed on brain endothelial cells, highlighting its role in mediating IGF-1 signaling. B) Generation of the animal model: Male VE-Cadherin-CreERT2 mice were crossbred with female Igf1rfl/fl mice. This mating strategy produced VE-Cadherin-CreERT2/Igf1r+/− males, which were then mated with Igf1rfl/fl female mice, following our previously described protocol 106. This breeding approach resulted in the establishment of a founder colony of VE-Cadherin-CreERT2/Igf1rfl/fl mice. Experimental cohorts were generated by breeding these mice with Igf1rfl/fl mice, yielding VE-Cadherin-CreERT2/Igf1rfl/fl mice as the experimental group, and Cre-/Igf1rfl/fl mice as the control group. For senescence reporter triple-transgene colony, VE-Cadherin-CreERT2/Igf1rfl/fl males were bred with senescence reporter p16-3MR females resulting in p16-3MR × VE-Cadherin-CreERT2/Igf1rfl/fl animals, which were then used for flow cytometry studies. C) Experimental timeline: Three-month-old mice received intraperitoneal injections of tamoxifen. Experiments were conducted in mice at 10–12 months (adult stage) and 18–20 months (aging stage). A subset of the experimental cohort underwent chronic cranial window implantation, while the triple-transgene senescence reporter animals were euthanized for single-cell brain sample collection for analysis using flow cytometry. This animal model and experimental timeline allowed us to investigate the effects of endothelial-specific IGF1R deficiency in both adult and aging mice, providing valuable insights into various aspects of cerebromicrovascular and endothelial health.
To evaluate the effect of disruption of IGF-1 signaling on induction of senescence, VE-Cadherin-CreERT2/Igf1rfl/fl females were bred with male senescence reporter p16-3MR mice51,53 (Fig. 1). This novel transgenic mouse model of accelerated endothelial aging carries a fusion protein (3MR) under control of the p16Ink4a promoter. 3MR contains monomeric red fluorescent protein (mRFP), which enables us to FACS-sort senescent cells from tissues. Experiments were conducted in 10-12-month-old (middle-aged) and 18-20-month old (aged) mice. All procedures were approved by the Institutional Animal Use and Care Committee of the University of Oklahoma Health Sciences Center.
Determination of senescent cell burden by flow cytometry analysis
Mice were euthanized and perfused using ice-cold PBS. After 12 mins of perfusion, brains were collected, then under sterile conditions in a cell culture hood, were transferred onto Petri dish. As a next step the olfactory bulb, cerebellum and brainstem were removed using sterile scalpel, and meningeal vessels were removed by rolling brain on Whatman paper. Subsequently, the brain tissue was finely chopped, transferred to 50 ml conical tube, and centrifuged (50×g, 5 mins, 4 °C). Samples was washed in 5 mL of sterile PBS, then digestive enzymes were added (50 µL collagenase, 2 µL elastase, 2 µL dispase, and 3 µL hyaluronidase) and incubated in rotating incubator for 45 mins at 37 °C. Samples were then gently dissociated by resuspension with first with 10 ml, followed by 5 ml tips with pipettor. The tissue suspension was then filtered through 100 µm and 30 µm filters. Cells were then centrifuged (300×g, 10 mins, 4 °C) and resuspended in 3.1 mL of PBS. Subsequently, 0.9 mL of Debris Removal Solution (Miltenyi Biotec) was added, and tubes were mixed by inverting five times. Following, 4 mL of PBS were slowly overlaid, and samples were centrifuged at 3000×g for 10 mins with full brake and full acceleration. This results in the formation of a top clear buffer layer, white interphase containing myelin debris, and a bottom clear layer containing pelleted cells. The top and middle layers were completely removed, and cells were washed in sterile PBS. Samples were centrifuged (1000×g, 10 mins, 4 °C), supernatant was removed, and cells were fixed in 1% paraformaldehyde for 15 mins at room temperature. Finally, cells were resuspended in MACS Buffers (Miltenyi Biotech) for further analysis.
We used sorted cells obtained from the single-cell suspensions from the brain samples to analyze senescent cell burden using our published protocols51,53. In brief, single-cell suspensions were prepared. Cell concentration was assessed for each sample (Scepter™ 2.0, Handheld Automated Cell Counter, EMD Millipore) to provide the same cell number for each measured sample. Samples were then blocked with 0.5% BSA in PBS. To assess senescent cell burden, fixed cells were stained with the RFP-Booster (AlexaFluor-488, 1:1000; Chromotek; US-QUO201590, 0.5gm/L) for 30 minutes, centrifuged (300×g, 10 min), and resuspended in MACS buffer (Miltenyi Biotech). The RFP-Booster allows for the detection of senescent cells that express the RFP-containing 3MR construct under the control of the p16Ink4a promoter. RFP-Booster-AlexaFluor488-stained (1:300; Chromotek, Proteintech Group, US) samples were also labeled with an antibody directed against the endothelium-specific surface marker CD31 (anti-CD31 PE-Vio770 antibody (1:50; 130-102-375, Miltenyi Biotec, US). Antibody staining was performed at room temperature, protected from light, for 45 minutes on a horizontal shaker (150 rpm). Cells were washed (300 g, 10 mins, at room temperature) and resuspended in MACS buffer. First, the fraction of RFP+ senescent cells was determined as a percentage of total cells in the single cell suspensions from whole brain lysates using a Guava® EasyCyte™ BGR HT Flow Cytometer (Luminex). Then, the ratio of RFP+/CD31+ senescent endothelial cells as a percentage of all CD31+ endothelial cells were determined. Single-stained samples and non-stained controls were used to determine adequate compensation settings and gates for the data analysis, respectively (data not shown). For flow cytometry data analysis and to generate representative plots FCS Express™ 7 (De Novo Software) analysis software was used.
Chronic cranial window surgery
Animals in each experimental group underwent chronic cranial window surgery to measure BBB permeability and cerebromicrovascular density. For the surgery, the animals were anesthetized using 2–3% isoflurane (ISOTHESIA, Henry Schein Animal Health, OH, USA) via inhalation. An eye ointment was applied to prevent ocular dehydration, and the animals were placed on a heating pad to maintain body temperature. The animal's head was fixed in a stereotaxic frame, and the skin on the top of the skull was removed after disinfection. Lidocaine (Sigma-Aldrich, MO, USA) solution was dripped onto the periosteum, and a small area over the sensorimotor cortex was gently thinned using a dental drill (Foredom, Blackstone Industries, Bethel, CT, USA). Room temperature saline was used to cool the surface and remove debris. Once the bone was sufficiently thin, a craniotomy was performed under sterile saline. The dura mater's surface was carefully cleaned, and any bleeding was stopped using a gelatin sponge, Hemosponge (Goodwill Lifesciences, India) soaked in sterile saline. A glass coverslip (diameter: 5 mm, Thomas Scientific, Swedesboro, NJ, USA), previously soaked in ethanol and rinsed in saline, was applied to cover the cranial window and fixed to the skull with adhesive and dental acrylic resin. After the surgery, the animals received Buprenorphine as pain medication (1 ml/kg body weight, i.p.; Zoopharm, WY, USA) and Enrofloxacin (5 mg/kg body weight, s.c.; Baytril, Bayer, Germany) as preventive antibiotic. They were closely monitored until they regained consciousness. Intravital imaging studies were conducted at least ten days after the surgery.
Intravital two-photon microscopy
We utilized our established protocol to conduct two-photon imaging and evaluate microvascular density and BBB integrity54. The intravital imaging was performed using a FluoView 1000 MPE two-photon microscope (Olympus, Tokyo, Japan) equipped with a MaiTai HP DeepSee-OL laser (Spectra-Physics, San Jose, CA, USA) emitting at wavelengths ranging from 690 nm to 1040 nm. A water immersion objective (XLPLN25XWMP, 1.05 numerical aperture; Olympus, Tokyo, Japan) with a 25× magnification was used to focus and collect emitted light. We used an 800 nm laser line for excitation and the emitted light was captured by PMT detectors. Three different filter sets were employed for the three channels: 420–460 nm, 495–540 nm, and 575–630 nm.
To initiate the experiment, mice were anesthetized with isoflurane, and their heads were secured in a stereotaxic frame. For visualizing the vascular glycocalyx and brain vasculature, Wheat Germ Agglutinin, Alexa Fluor™ 594 Conjugate (4 ml/kg body weight of 1 mg/ml WGA-AF594, Thermo Fisher Scientific, MA, USA) was retro-orbitally injected. The reference 0 µm depth point was determined using superior pial vessels. Cerebral microvessels were examined at a depth of approximately 0–150 µm. Consistent laser intensity and detector sensitivity were maintained regardless of tissue depth. Images captured immediately after WGA-AF594 injection were used as a control for background intensity without any other tracer.
To evaluate BBB integrity, fluorescent tracer dyes with different molecular weights (4 ml/kg body weight of 2 mg/ml FITC-dextran 40 kDa, 3 kDa, Thermo Fisher Scientific, MA, USA; and sodium fluorescein, Sigma-Aldrich, MO, USA) were injected retro-orbitally as a bolus in descending order of molecular weight after each other separately. Imaging of the predetermined area was performed for 15 minutes after each tracer injection separately, immediately after injection. Volumes of interest (VOIs) were selected as 508 µm × 508 µm × 50–150 µm (x, y, z) z-stacks. The corresponding pixel numbers were 512 × 512 × 21, resulting in a spatial resolution of approximately 1 µm × 1 µm × 5 µm in the x, y, and z directions (objective point spread function (PSF) < 1.5 μm).
Image subtraction analysis for BBB permeability determination
The captured images were subjected to analysis using the 1.52p version of FIJI ImageJ software (Wayne Rasband, National Institutes of Health, USA). Analysis of the z-stack images was performed using a custom-made macro in FIJI, following modified methods described in reference54. To provide a summary, the imported TIFF images were first compiled and aligned, and then after background fluorescent subtraction, images underwent processing using maximal intensity projection and machine learning-based adaptive thresholding techniques55 to generate binary vascular masks. These binary images were further skeletonized to enable further quantification. For quantifying permeability, the vascular image masks were subtracted from the maximum intensity projections of background-corrected tracer z-stacks. This subtraction removed areas corresponding to intravascular volumes, allowing selective measurement of tracer intensities that had extravasated at each time point. The intensity of the post-subtraction images was then measured. The measured integrated density values, representing extravascular tracer fluorescent intensity in the z-stack maximum projections, were normalized to the baseline intensity. The intensities were plotted as a function of the elapsed time between the initial and subsequent recordings. The resulting function was further analyzed by calculating the "area under the curve" (AUC), which provided a relative difference in permeability between the groups.
Microvascular density measurement
To assess capillarization in the cerebral cortex, we used our previously described method supplemented with machine learning-based vascular segmentation53. To summarize, the cortical recordings obtained from two-photon microscopy were processed by employing maximum intensity projection of z-stacks for dimension reduction. Following that, different filters were utilized to improve contrast, eliminate noise, and remove outliers from the images. The processed images underwent supervised machine learning55 for classification and were converted into binary format, enabling measurement of pixel values. Ultimately, the vascular network was visualized using various analysis formats (Fig. 3A).
Figure 3: Analysis of the effects of endothelial-specific IGF1R deficiency on cortical capillary density by intravital two-photon microscopy.

Panel A) Segmentation of blood vessels on two-photon microscopy images. Original z-stack images obtained from the brains of middle-aged (10–12 months old) control, middle-aged endothelial-specific IGF1R deficient, aged (18–20 months old) control, and aged endothelial-specific IGF1R deficient mice were processed using a modified image analysis macro54 to generate binary vascular images. Skeleton and false-color local thickness images were used to calculate additional indices corresponding to microvascular density. Scale bar: 100 μm. Panels B-F: Assessment of microvascular density reveals a trend of decrease in endothelial-specific IGF1R deficiency. B) Vascular coverage, represented as the percentage of the area of the maximal intensity projection of the volume of interest (VOI). C) Vascular length density, measured as the length of the vascular skeleton in micrometers per µm2 of the maximal intensity projection of the VOI. D) Normalized number of branches, calculated by normalizing the number of branches to the area (μm2) of the same images. E) Normalized number of junctions, quantified by counting the number of junction points in the vascular network. F) Histogram of local thickness of the vascular images, averaged by group. Notably, a significant difference is observed between middle-aged control and middle-aged VE-Cadherin-CreERT2/Igf1rfl/fl mice, indicating a loss of microvascular density. Data are mean ± SEM. *p<0.05, **p<0.01 (n=4 for middle-aged control, n=8 for middle-aged endothelial-specific IGF1R deficient, n=8 for aged control, and n=13 for aged endothelial-specific IGF1R deficient) with one-way ANOVA followed by Fisher LSD post hoc test or an equivalent non-parametric Kruskal-Wallis test. Local thickness distribution of the vasculature was analyzed with repeated-measures ANOVA.
Statistical analyses
All data are presented as means ± standard error of the mean (SEM). GraphPad Prism 9 Software (La Jolla, CA, USA) was employed for the statistical analyses. Comparisons between groups of experimental results were conducted using a Student’s t-test, one-way ANOVA with Fisher LSD post hoc test or an equivalent non-parametric Kruskal-Wallis test. For the analysis of local thickness distribution of the vasculature, a repeated-measures ANOVA was utilized, and data visualization was achieved by applying non-linear curve fitting with a lognormal function. Statistical significance was defined as a p-value less than 0.05. A minimum of three independent measurements were performed for all data (n ≥ 3), and the exact "n" animal numbers are specified in the figure legends. Levels of significance were denoted as follows: * p < 0.05, ** p < 0.01, *** p < 0.001.
Results
Disruption of IGF-1 signaling promotes endothelial senescence in mouse brain
To investigate the impact of endothelial-specific IGF-1 deficiency on cellular senescence, flow cytometry was employed to identify p16-RFP+/CD31+ senescent endothelial cells in 18-20-month-old control mice and VE-Cadherin-CreERT2/Igf1rfl/fl × p16-3MR animals (Fig. 2). After gating to exclude cellular debris and doublet, senescent cells, endothelial cells, and senescent endothelial cells were identified based on Alexa488 (RFP-booster) and PE-Vio770 (CD31) signal intensity (Fig. 2A–B). We found a significant increase in the prevalence of p16-RFP+ senescent cells (Fig. 2C) in general and p16-RFP+/CD31+ cells in particular (Fig. 2D) in brains of VE-Cadherin-CreERT2/Igf1rfl/fl × p16-3MR mice.
Figure 2: Flow cytometric assessment of cerebromicrovascular endothelial senescence in endothelial-specific IGF1R deficient p16-3MR mice.

We performed flow cytometric analysis to assess the senescence cell burden in the brain microvasculature of senescence reporter 18-20-month-old control mice and p16-3MR × VE-Cadherin-CreERT2/Igf1rfl/fl animals, which exhibit endothelial-specific IGF1R deficiency. Transcardial perfusion was conducted to euthanize the mice, and whole brain samples were collected and digested to obtain single-cell suspension. Senescent cells were quantified by co-labeling CD31 with p16-RFP, with the RFP signal enhanced using a custom-ordered RFP-Booster Alexa Fluor 488. Panel A displays a representative flow cytometry gating procedure. Cellular debris was excluded, followed by selection of singlet cells, and finally, quantification of CD31 (PE-Vio770) positive and RFP-Booster AF488 positive senescent endothelial cells. Panel B presents representative flow cytometry dot plots from control (n=4) and endothelial-specific IGF1R deficient (n=5) mouse brains. Note that the endothelial-specific IGF1R deficient samples exhibit an elevated presence of senescent cells. Panels C and D provide the quantification of the flow cytometry results. Endothelial-specific IGF1R deficiency significantly increases the number of p16-RFP+ senescent cells in the whole brain (Panel C), as well as the percentage of senescent endothelial cells within the total endothelial cell population (Panel D). These findings indicate an elevated burden of p16-induced senescence in the cerebromicrovasculature due to endothelial-specific IGF1R deficiency. The data are presented as mean ± SEM, and statistical significance is indicated on the graphs (n=4–8 animals for each data point). ***p<0.001 (compared to control) with Student’s Two-tailed T-test.
Effects of endothelium-specific disruption of IGF-1 signaling on cerebromicrovascular density
To determine the effects of endothelium-specific depletion of IGFR1 on the structural integrity of the cerebral microcirculation, we compared microvascular densities using two-photon microscopy in the mouse cortex53,54. Briefly, z-stack two-photon images from the WGA-AF594 channel (Figure 3A) went through dimension reduction by maximum intensity projection. After noise removal and contrast enhancement, the images were subjected to supervised machine learning for segmentation then converted into binary mask, Pixel values were measured and after skeletonization, vascular network frame has been measured (Figure 3A). Both vessel area density indices and vessel skeleton density indices (which are independent of the size distribution of vessels in the VOI) were compared. We found that although these vascular density indices tended to decline in the VE-Cadherin-CreERT2/Igf1rfl/fl groups, these differences did not reach statistical significance (Figure 3B–E). Fig. 3F shows the vascular diameter distribution in the experimental groups. Note that VE-Cadherin-CreERT2/Igf1rfl/fl mice exhibited significantly lower density of the microvasculature than age-matched control mice, especially in case of small microvessels. This is consistent with the concept that disruption of IGF-1 input to the endothelium may compromise the structural integrity of the cerebral capillary network.
Disruption of endothelial IGF-1 signaling promotes BBB disruption
Using two-photon microscopy, the relative permeability of the fluorescent tracers was measured. Tracers were injected retro-orbitally, in a decreasing order of molecular weight. The permeability to each tracer was tracked and recorded for 15 minutes. Vascular masks were segmented on the z-stack projections and subtracted from the images to exclusively measure changes in the relative intensity of the extravasated tracer. Figure 4A shows background-corrected fluorescent intensity changes in the brain parenchyma in mice from each experimental group after the administration of different size tracers. The calculation was performed to determine the area under the curve of normalized intensity changes over time, serving as an indicator of relative vascular permeability (Fig. 4B–D). We found that for each tracer the cerebral microcirculation of 18-20-month-old control mice exhibited significantly increased BBB permeability as compared to that in 10-12-month-old mice, extending our recent findings54. For the 40 kDa, 3 kDa, and 0.3 kDa tracers the cerebral microcirculation of 10-12-month-old VE-Cadherin-CreERT2/Igf1rfl/fl mice exhibited significantly increased permeability compared to age-matched control mice (Fig. 4B–D), mimicking the aging phenotype. In 18-20-month-old VE-Cadherin-CreERT2/Igf1rfl/fl mice BBB permeability tended to increase as compared to age-matched control mice, however, this difference reached statistical significance only for the 40 kDa tracer (Fig. 4 B–D). This observation is consistent with the concept that disruption of the BBB due to impaired IGF-1 input to the endothelium and chronological aging is predominantly because of the increased transcellular permeability in aged brain, but in VE-Cadherin-CreERT2/Igf1rfl/fl middle-aged animals there was an increase in all molecular size ranges in compared to age-matched controls.
Figure 4: Endothelial-specific IGF1R deficiency increases barrier permeability, mimicking the aging phenotype.

Panel A: Assessment of cerebromicrovascular permeability to fluorescent, FITC-labeled tracers using multi-photon imaging in brains of middle-aged control, middle-aged endothelial-specific IGF1R deficient, aged control, and aged endothelial-specific IGF1R deficient mice. Changes in tracer fluorescence intensity in the extravascular space and brain parenchyma were analyzed in each group upon retroorbital injection of tracers with decreasing molecular weights. Maximum projected (z-stack) images were obtained after tracer administration, and subtraction of the images of the segmented cerebral microvasculature was performed. Intensity plots derived from average projections of time-stacks show increased extravasation of tracers of different molecular weights in the brains of middle-aged endothelial-specific IGF1R deficient mice compared to control mice. The relative fluorescent intensity scale is shown at the top right. Scale bar: 100 μm. Panel B-D: Summary data for cerebromicrovascular permeability to fluorescent tracers with different molecular weights (B: 40 kDa; C: 3 kda; D: 0.3 kDa) in brains of middle-aged control, middle-aged endothelial-specific IGF1R deficient, aged control, and aged endothelial-specific IGF1R deficient mice. The data reveal significantly increased BBB permeability for the different-sized tracers in middle-aged endothelial-specific IGF1R deficient mice compared to age-matched controls. Data are mean ± SEM. *p<0.05, **p<0.01, ***p<0.001 (n=5 for middle-aged control, n=8 for middle-aged endothelial specific IGF1R deficient, n=16 for aged control and n=12 for aged endothelial specific IGF1R deficient) with Kruskal-Wallis test (non-parametric ANOVA).
Discussion
The key findings of this study are that endothelium-specific disruption of IGF-1 signaling in VE-Cadherin-CreERT2/Igf1rfl/fl mice promotes endothelial senescence, which associates with BBB disruption and compromised structural integrity of the cerebral microcirculation, effectively mimicking the aging phenotype.
The human brain is comprised of a complex network of capillaries stretching over 600 km. Interestingly, the number of endothelial cells in the brain is comparable to that of neurons, and nearly every neuron has its own dedicated capillary. There is growing evidence that age-related impairments in the structure and function of the cerebral microcirculation are causally linked to cognitive decline8,56–66. Our findings highlight the critical impact of anti-geronic IGF-1 signaling on cerebromicrovascular endothelial cells and underline its significance in maintaining the functional and structural integrity of the cerebral microcirculatory system.
Here we demonstrate that disruption of endothelial IGF-1 signaling is associated with increased presence of senescent endothelial cells in the cerebral microcirculation. Cellular senescence is widely recognized as a prominent hallmark of aging50,67,68. With advancing age, there is an accumulation of senescent cells in multiple organs, including the brain and we have evidence that cerebromicrovascular endothelial cells are particularly sensitive to age-related induction of cellular senescence 48,49,68–72. Our findings are in line with emerging evidence suggesting a crucial role of IGF-1 in the modulation of cellular senescence in different age-related vascular diseases, including atherosclerosis73–75. The mechanisms by which IGF-1 signaling prevents induction of senescence are likely multifaceted. Increased oxidative stress and consequential increases in cellular DNA damage are important mechanisms of endothelial senescence. Considering the previously documented protective effects of IGF-1 against oxidative stress and other cellular stressors13,14,16–18,47,76–83,84, we hypothesize that endothelial-specific knockdown of IGF1R would impede these protective effects. Our results accord with emerging evidence that IGF-1 may play a critical role in modulating cellular senescence in various age-related diseases, such as atherosclerosis73–75. Increased endothelial senescence can have detrimental effects on cerebromicrovascular function and may potentially contribute to cognitive impairment50,53.
With advanced aging there is a notable reduction in the density of cerebral capillaries ("microvascular rarefaction"), which plays a significant role in the age-related decrease in regional cerebral blood flow60,85–97. Consequently, this mismatch between the brain's energy requirements and its blood supply has been identified as a causal factor in the pathogenesis of cognitive decline87,89,98–101. IGF-1 is known to confer multifaceted pro-angiogenic and trophic effects, regulating microvascular remodeling102. The findings of the present study, taken together with results of earlier investigations suggest that impaired IGF-1 input to endothelial cells, either due to endocrine IGF-1102 deficiency or disruption of IGF-1 signaling, leads to structural pathologic remodeling of the microcirculation and may promote regression of cerebral microvessels.
One of the limitations of our study is that even if the genetic model is specialized on VECAD expressing endothelial cell specific IGF1R deletion, this is not completely restrict the effect only to brain microvascular endothelial cells within the endothelial cell niche, hence there might be further effects of the IGF1R knockdown in other organs of this mouse model that we did not examine in the present study. Another limitation, similarly to the previous, VECAD is not completely restricted to endothelial cells. Thus, other cells like hematopoietic cells (potentially endothelial progenitors) in the circulation, in the bone marrow, or perivascular interstitial cells around large pial vessels belonging VECAD lineage, can also be effected. IGF1R deletion in these cells could have some level of indirect outcomes on brain vascular endothelial cells. Based on our recently published results circulating factors have striking effect on the vasculature, especially on endothelial cells, thus it is very difficult to completely dissect these mechanisms in a living organism, therefore we were focusing on the brain endothelium-related consequences56,103. It is also important to mention that it is still unclear which brain vascular region is the most effected by endothelial senescence in this accelerated aging model. We assume based on our presented BBB permeability results and our previous studies, that the capillary endothelial cells are mostly affected by increased senescence. In our single cell transcriptomic study, it has been showed that the highest number of senescence endothelial cells were found among capillary endothelial cells, but they were also present in arterioles and venules during chronological aging63. Since during chronological aging circulating IGF1 level is also decreasing (reflecting to our present accelerated aging model) we assume that the distribution of the senescence endothelial cells would be similar. Further single cell sequencing studies can provide more detailed information on the endothelial senescence in this model.
Endothelial cells in the cerebral microvessels play a critical role as key components of the BBB, characterized by the presence of cell-to-cell junctions (adherens and tight junctions) that effectively prevent the leakage of blood-borne factors into the brain parenchyma. Maintaining the integrity and proper function of the BBB is essential for normal brain connectivity, synaptic activity, and information processing104. Our findings demonstrate that disruption of endothelial IGF-1 signaling results in BBB dysfunction18,105, underscoring the critical importance of maintaining a youthful endothelial phenotype in preserving the barrier function of the cerebral microvasculature. The consequences of deficient IGF-1 input to endothelial cells extend beyond BBB disruption, impairing endothelium-mediated neurovascular coupling responses52 and giving rise to associated complications.
In conclusion, our study sheds light on the essential anti-geronic role of IGF-1 signaling in cerebromicrovascular endothelial cells and emphasizes the potentially deleterious consequences of its disruption on the functional and structural integrity of the cerebrovascular system. Further investigations into the specific mechanisms underlying IGF-1's endothelial protective effects and its potential as a therapeutic target may provide new avenues for maintaining cerebromicrovascular health and mitigating age-related cognitive decline.
Funding Statement
This work was financially supported by grants found from the American Heart Association (RG: 916225, ANT: AHA834339), the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295; R01AG070915, K01AG073614), the National Institute of Neurological Disorders and Stroke (R01NS100782), the National Cancer Institute (R01CA255840), the Presbyterian Health Foundation, the Reynolds Foundation, the Oklahoma Nathan Shock Center (P30AG050911), and the Cellular and Molecular GeroScience CoBRE (P20GM125528). Support was also provided by Project no. TKP2021-NKTA-47, implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NKTA funding scheme; by funding through the National Cardiovascular Laboratory Program (RRF-2.3.1-21-2022-00003) provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund; Project no. 135784 implemented with the support provided from the National Research, Development and Innovation Fund of Hungary, financed under the K_20 funding scheme and the European University for Well-Being (EUniWell) program (grant agreement number: 101004093/ EUniWell/EAC-A02-2019 / EAC-A02-2019-1). The funding sources had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Heart Association, or the Presbyterian Health Foundation.
List of abbreviations
- 3MR
trimodality reporter fusion protein
- AUC
Area Under the Curve
- BBB
Blood-Brain Barrier
- BSA
Bovine Serum Albumin
- FACS
Fluorescence-Activated Cell Sorting
- FITC
Fluorescein Isothiocyanate
- IGF-1
Insulin-like Growth Factor-1
- IGF1R
IGF-1 Receptor
- kDa
Kilodalton
- mRFP
Monomeric Red Fluorescent Protein
- PBS
Phosphate-Buffered Saline
- PMT
Photomultiplier Tube
- RT
Room Temperature
- SEM
Standard Error of the Mean
- VCID
Vascular Cognitive Impairment and Dementia
- VECAD
Vascular Endothelial Cadherin
- VOI
Volume of Interest
Footnotes
Disclosure of Competing Interests
The authors declare no competing interests.
Ethical statement
All procedures involved experimental animals were carried out in accordance with the protocol reviewed and approved by the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee (OUHSC IACUC), in a manner verified by them, in designated locations, following all local, state, and federal guidelines for use of animal subjects.
Data availability Statement
All data that support the findings of this study are available in either the main text or from the authors upon reasonable request. Please contact the corresponding authors for access to this data.
References
- 1.van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, Scheltens P. Vascular cognitive impairment. Nat Rev Dis Primers 2018;4:18003. doi: 10.1038/nrdp.2018.3 [DOI] [PubMed] [Google Scholar]
- 2.Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol 2017;312:H1–H20. doi: 10.1152/ajpheart.00581.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verheggen ICM, de Jong JJA, van Boxtel MPJ, Postma AA, Jansen JFA, Verhey FRJ, Backes WH. Imaging the role of blood-brain barrier disruption in normal cognitive ageing. Geroscience 2020. doi: 10.1007/s11357-020-00282-1 [DOI] [PMC free article] [PubMed]
- 4.Verheggen ICM, de Jong JJA, van Boxtel MPJ, Gronenschild E, Palm WM, Postma AA, Jansen JFA, Verhey FRJ, Backes WH. Increase in blood-brain barrier leakage in healthy, older adults. Geroscience 2020;42:1183–1193. doi: 10.1007/s11357-020-00211-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeze WM, Jacobs HIL, de Jong JJ, Verheggen ICM, Gronenschild E, Palm WM, Hoff EI, Wardlaw JM, Jansen JFA, Verhey FR, et al. White matter hyperintensities mediate the association between blood-brain barrier leakage and information processing speed. Neurobiol Aging 2020;85:113–122. doi: 10.1016/j.neurobiolaging.2019.09.017 [DOI] [PubMed] [Google Scholar]
- 6.Noe CR, Noe-Letschnig M, Handschuh P, Noe CA, Lanzenberger R. Dysfunction of the Blood-Brain Barrier-A Key Step in Neurodegeneration and Dementia. Front Aging Neurosci 2020;12:185. doi: 10.3389/fnagi.2020.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Towner RA, Gulej R, Zalles M, Saunders D, Smith N, Lerner M, Morton KA, Richardson A. Rapamycin restores brain vasculature, metabolism, and blood-brain barrier in an inflammaging model. Geroscience 2021;43:563–578. doi: 10.1007/s11357-021-00363-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerkhofs D, Wong SM, Zhang E, Uiterwijk R, Hoff EI, Jansen JFA, Staals J, Backes WH, van Oostenbrugge RJ. Blood-brain barrier leakage at baseline and cognitive decline in cerebral small vessel disease: a 2-year follow-up study. Geroscience 2021;43:1643–1652. doi: 10.1007/s11357-021-00399-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montagne A, Barnes SR, Nation DA, Kisler K, Toga AW, Zlokovic BV. Imaging subtle leaks in the blood-brain barrier in the aging human brain: potential pitfalls, challenges, and possible solutions. Geroscience 2022;44:1339–1351. doi: 10.1007/s11357-022-00571-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li B, Yabluchanskiy A, Tarantini S, Allu SR, Sencan-Egilmez I, Leng J, Alfadhel MAH, Porter JE, Fu B, Ran C, et al. Measurements of cerebral microvascular blood flow, oxygenation, and morphology in a mouse model of whole-brain irradiation-induced cognitive impairment by two-photon microscopy and optical coherence tomography: evidence for microvascular injury in the cerebral white matter. Geroscience 2023. doi: 10.1007/s11357-023-00735-3 [DOI] [PMC free article] [PubMed]
- 11.Toth L, Czigler A, Hegedus E, Komaromy H, Amrein K, Czeiter E, Yabluchanskiy A, Koller A, Orsi G, Perlaki G, et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. Geroscience 2022;44:2771–2783. doi: 10.1007/s11357-022-00623-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higashi Y, Gautam S, Delafontaine P, Sukhanov S. IGF-1 and cardiovascular disease. Growth Horm IGF Res 2019;45:6–16. doi: 10.1016/j.ghir.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulop GA, Ramirez-Perez FI, Kiss T, Tarantini S, Valcarcel Ares MN, Toth P, Yabluchanskiy A, Conley SM, Ballabh P, Martinez-Lemus LA, et al. IGF-1 deficiency Promotes Pathological Remodeling of Cerebral Arteries: A Potential Mechanism Contributing to the Pathogenesis of Intracerebral Hemorrhages in Aging. J Gerontol A Biol Sci Med Sci 2018. doi: 10.1093/gerona/gly144 [DOI] [PMC free article] [PubMed]
- 14.Tarantini S, Valcarcel-Ares NM, Yabluchanskiy A, Springo Z, Fulop GA, Ashpole N, Gautam T, Giles CB, Wren JD, Sonntag WE, et al. Insulin-like growth factor 1 deficiency exacerbates hypertension-induced cerebral microhemorrhages in mice, mimicking the aging phenotype. Aging Cell 2017;16:469–479. doi: 10.1111/acel.12583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tarantini S, Tucsek Z, Valcarcel-Ares MN, Toth P, Gautam T, Giles CB, Ballabh P, Wei JY, Wren JD, Ashpole NM, et al. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age (Dordr) 2016;38:273–289. doi: 10.1007/s11357-016-9931-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, Menyhart A, Farkas E, Sonntag WE, Csiszar A, et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell 2015;14:1034–1044. doi: 10.1111/acel.12372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, Koller A, Sonntag WE, Csiszar A, Ungvari Z. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab 2014;34:1887–1897. doi: 10.1038/jcbfm.2014.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong X, Chang G, Ji XF, Tao DB, Wang YX. The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One 2014;9:e94845. doi: 10.1371/journal.pone.0094845 PONE-D-13-52718 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Sonntag WE, Deak F, Ashpole N, Toth P, Csiszar A, Freeman W, Ungvari Z. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Front Aging Neurosci 2013;5:27. doi: 10.3389/fnagi.2013.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troncoso R, Vicencio JM, Parra V, Nemchenko A, Kawashima Y, Del Campo A, Toro B, Battiprolu PK, Aranguiz P, Chiong M, et al. Energy-preserving effects of IGF-1 antagonize starvation-induced cardiac autophagy. Cardiovasc Res 2012;93:320–329. doi: 10.1093/cvr/cvr321 cvr321 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higashi Y, Sukhanov S, Anwar A, Shai SY, Delafontaine P. Aging, atherosclerosis, and IGF-1. J Gerontol A Biol Sci Med Sci 2012;67:626–639. doi: 10.1093/gerona/gls102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von der Thusen JH, Borensztajn KS, Moimas S, van Heiningen S, Teeling P, van Berkel TJ, Biessen EA. IGF-1 has plaque-stabilizing effects in atherosclerosis by altering vascular smooth muscle cell phenotype. Am J Pathol 2011;178:924–934. doi: 10.1016/j.ajpath.2010.10.007 S0002-9440(10)00099-4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shai SY, Sukhanov S, Higashi Y, Vaughn C, Rosen CJ, Delafontaine P. Low Circulating Insulin-Like Growth Factor I Increases Atherosclerosis in Apoe-Deficient Mice. Am J Physiol Heart Circ Physiol 2011. doi: ajpheart.01081.2010 [pii] 10.1152/ajpheart.01081.2010 [DOI] [PMC free article] [PubMed]
- 25.Prabhu D, Khan SM, Blackburn K, Marshall JP, Ashpole NM. Loss of insulin-like growth factor-1 signaling in astrocytes disrupts glutamate handling. J Neurochem 2019;151:689–702. doi: 10.1111/jnc.14879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Logan S, Pharaoh GA, Marlin MC, Masser DR, Matsuzaki S, Wronowski B, Yeganeh A, Parks EE, Premkumar P, Farley JA, et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-beta uptake in astrocytes. Mol Metab 2018;9:141–155. doi: 10.1016/j.molmet.2018.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Littlejohn EL, Scott D, Saatman KE. Insulin-like growth factor-1 overexpression increases long-term survival of posttrauma-born hippocampal neurons while inhibiting ectopic migration following traumatic brain injury. Acta Neuropathol Commun 2020;8:46. doi: 10.1186/s40478-020-00925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garwood CJ, Ratcliffe LE, Morgan SV, Simpson JE, Owens H, Vazquez-Villasenor I, Heath PR, Romero IA, Ince PG, Wharton SB. Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors. Mol Brain 2015;8:51. doi: 10.1186/s13041-015-0138-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pardo J, Uriarte M, Console GM, Reggiani PC, Outeiro TF, Morel GR, Goya RG. Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci 2016;44:2120–2128. doi: 10.1111/ejn.13278 [DOI] [PubMed] [Google Scholar]
- 30.Labandeira-Garcia JL, Costa-Besada MA, Labandeira CM, Villar-Cheda B, Rodriguez-Perez AI. Insulin-Like Growth Factor-1 and Neuroinflammation. Front Aging Neurosci 2017;9:365. doi: 10.3389/fnagi.2017.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okoreeh AK, Bake S, Sohrabji F. Astrocyte-specific insulin-like growth factor-1 gene transfer in aging female rats improves stroke outcomes. Glia 2017;65:1043–1058. doi: 10.1002/glia.23142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piriz J, Muller A, Trejo JL, Torres-Aleman I. IGF-I and the aging mammalian brain. Exp Gerontol 2011;46:96–99. doi: 10.1016/j.exger.2010.08.022 [DOI] [PubMed] [Google Scholar]
- 33.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci 2012;13:225–239. doi: 10.1038/nrn3209 [DOI] [PubMed] [Google Scholar]
- 34.Muller AP, Fernandez AM, Haas C, Zimmer E, Portela LV, Torres-Aleman I. Reduced brain insulin-like growth factor I function during aging. Mol Cell Neurosci 2012;49:9–12. doi: 10.1016/j.mcn.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 35.Trueba-Saiz A, Cavada C, Fernandez AM, Leon T, Gonzalez DA, Fortea Ormaechea J, Lleo A, Del Ser T, Nunez A, Torres-Aleman I. Loss of serum IGF-I input to the brain as an early biomarker of disease onset in Alzheimer mice. Transl Psychiatry 2013;3:e330. doi: 10.1038/tp.2013.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ascenzi F, Barberi L, Dobrowolny G, Villa Nova Bacurau A, Nicoletti C, Rizzuto E, Rosenthal N, Scicchitano BM, Musaro A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019;18:e12954. doi: 10.1111/acel.12954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williamson TT, Ding B, Zhu X, Frisina RD. Hormone replacement therapy attenuates hearing loss: Mechanisms involving estrogen and the IGF-1 pathway. Aging Cell 2019;18:e12939. doi: 10.1111/acel.12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonntag WE, Ramsey M, Carter CS. Growth hormone and insulin-like growth factor-1 (IGF-1) and their influence on cognitive aging. Ageing Res Rev 2005;4:195–212. doi: S1568-1637(05)00005-X [pii] 10.1016/j.arr.2005.02.001 [DOI] [PubMed] [Google Scholar]
- 39.Sanders JL, Guo W, O'Meara ES, Kaplan RC, Pollak MN, Bartz TM, Newman AB, Fried LP, Cappola AR. Trajectories of IGF-I Predict Mortality in Older Adults: The Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 2018;73:953–959. doi: 10.1093/gerona/glx143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fazekas-Pongor V, Peterfi A, Major D, Szarvas Z, Fekete M, Tabak AG, Csiszar A, Sonntag WE, Austad SN, Ungvari ZI. Decreased lifespan in female "Munchkin" actors from the cast of the 1939 film version of The Wizard of Oz does not support the hypothesis linking hypopituitary dwarfism to longevity. Geroscience 2022;44:2527–2539. doi: 10.1007/s11357-022-00680-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zegarra-Valdivia JA, Fernandes J, Fernandez de Sevilla ME, Trueba-Saiz A, Pignatelli J, Suda K, Martinez-Rachadell L, Fernandez AM, Esparza J, Vega M, et al. Insulin-like growth factor I sensitization rejuvenates sleep patterns in old mice. Geroscience 2022;44:2243–2257. doi: 10.1007/s11357-022-00589-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Connor KG, Tobin JD, Harman SM, Plato CC, Roy TA, Sherman SS, Blackman MR. Serum levels of insulin-like growth factor-I are related to age and not to body composition in healthy women and men. J Gerontol A Biol Sci Med Sci 1998;53:M176–182. [DOI] [PubMed] [Google Scholar]
- 43.Pavlov EP, Harman SM, Merriam GR, Gelato MC, Blackman MR. Responses of growth hormone (GH) and somatomedin-C to GH-releasing hormone in healthy aging men. J Clin Endocrinol Metab 1986;62:595–600. doi: 10.1210/jcem-62-3-595 [DOI] [PubMed] [Google Scholar]
- 44.Ameri P, Canepa M, Fabbi P, Leoncini G, Milaneschi Y, Mussap M, AlGhatrif M, Balbi M, Viazzi F, Murialdo G, et al. Vitamin D modulates the association of circulating insulin-like growth factor-1 with carotid artery intima-media thickness. Atherosclerosis 2014;236:418–425. doi: 10.1016/j.atherosclerosis.2014.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherlala RA, Kammerer CM, Kuiper AL, Wojczynski MK, Ukraintseva SV, Feitosa MF, Mengel-From J, Zmuda JM, Minster RL. Relationship between Serum IGF-1 and BMI differs by Age. J Gerontol A Biol Sci Med Sci 2020. doi: 10.1093/gerona/glaa282 [DOI] [PMC free article] [PubMed]
- 46.Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci 2012;67:599–610. doi: 10.1093/gerona/gls072 gls072 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience 2021;43:2387–2394. doi: 10.1007/s11357-021-00405-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 2020;42:429–444. doi: 10.1007/s11357-020-00177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kiss T, Nyul-Toth A, DelFavero J, Balasubramanian P, Tarantini S, Faakye J, Gulej R, Ahire C, Ungvari A, Yabluchanskiy A, et al. Spatial transcriptomic analysis reveals inflammatory foci defined by senescent cells in the white matter, hippocampi and cortical grey matter in the aged mouse brain. Geroscience 2022;44:661–681. doi: 10.1007/s11357-022-00521-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tarantini S, Balasubramanian P, Delfavero J, Csipo T, Yabluchanskiy A, Kiss T, Nyul-Toth A, Mukli P, Toth P, Ahire C, et al. Treatment with the BCL-2/BCL-xL inhibitor senolytic drug ABT263/Navitoclax improves functional hyperemia in aged mice. Geroscience 2021;43:2427–2440. doi: 10.1007/s11357-021-00440-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yabluchanskiy A, Tarantini S, Balasubramanian P, Kiss T, Csipo T, Fulop GA, Lipecz A, Ahire C, DelFavero J, Nyul-Toth A, et al. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience 2020;42:409–428. doi: 10.1007/s11357-020-00154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tarantini S, Nyul-Toth A, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, Ungvari A, Toth P, Benyo Z, Sonntag WE, et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. Geroscience 2021. doi: 10.1007/s11357-021-00405-2 [DOI] [PMC free article] [PubMed]
- 53.Ahire C, Nyul-Toth A, DelFavero J, Gulej R, Faakye JA, Tarantini S, Kiss T, Kuan-Celarier A, Balasubramanian P, Ungvari A, et al. Accelerated cerebromicrovascular senescence contributes to cognitive decline in a mouse model of paclitaxel (Taxol)-induced chemobrain. Aging Cell 2023:e13832. doi: 10.1111/acel.13832 [DOI] [PMC free article] [PubMed]
- 54.Nyul-Toth A, Tarantini S, DelFavero J, Yan F, Balasubramanian P, Yabluchanskiy A, Ahire C, Kiss T, Csipo T, Lipecz A, et al. Demonstration of age-related blood-brain barrier disruption and cerebromicrovascular rarefaction in mice by longitudinal intravital two-photon microscopy and optical coherence tomography. Am J Physiol Heart Circ Physiol 2021;320:H1370–H1392. doi: 10.1152/ajpheart.00709.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arganda-Carreras I, Kaynig V, Rueden C, Eliceiri KW, Schindelin J, Cardona A, Sebastian Seung H. Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics 2017;33:2424–2426. doi: 10.1093/bioinformatics/btx180 [DOI] [PubMed] [Google Scholar]
- 56.Bickel MA, Csik B, Gulej R, Ungvari A, Nyul-Toth A, Conley SM. Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis. Front Endocrinol (Lausanne) 2023;14:1087053. doi: 10.3389/fendo.2023.1087053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chojdak-Lukasiewicz J, Dziadkowiak E, Zimny A, Paradowski B. Cerebral small vessel disease: A review. Adv Clin Exp Med 2021;30:349–356. doi: 10.17219/acem/131216 [DOI] [PubMed] [Google Scholar]
- 58.Costea L, Meszaros A, Bauer H, Bauer HC, Traweger A, Wilhelm I, Farkas AE, Krizbai IA. The Blood-Brain Barrier and Its Intercellular Junctions in Age-Related Brain Disorders. Int J Mol Sci 2019;20. doi: 10.3390/ijms20215472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Csipo T, Mukli P, Lipecz A, Tarantini S, Bahadli D, Abdulhussein O, Owens C, Kiss T, Balasubramanian P, Nyul-Toth A, et al. Assessment of age-related decline of neurovascular coupling responses by functional near-infrared spectroscopy (fNIRS) in humans. Geroscience 2019;41:495–509. doi: 10.1007/s11357-019-00122-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Prog Neurobiol 2001;64:575–611. doi: S0301-0082(00)00068-X [pii] [DOI] [PubMed] [Google Scholar]
- 61.Fulop GA, Ahire C, Csipo T, Tarantini S, Kiss T, Balasubramanian P, Yabluchanskiy A, Farkas E, Toth A, Nyul-Toth A, et al. Cerebral venous congestion promotes blood-brain barrier disruption and neuroinflammation, impairing cognitive function in mice. Geroscience 2019;41:575–589. doi: 10.1007/s11357-019-00110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fulop GA, Tarantini S, Yabluchanskiy A, Molnar A, Prodan CI, Kiss T, Csipo T, Lipecz A, Balasubramanian P, Farkas E, et al. Role of age-related alterations of the cerebral venous circulation in the pathogenesis of vascular cognitive impairment. Am J Physiol Heart Circ Physiol 2019;316:H1124–H1140. doi: 10.1152/ajpheart.00776.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiss T, Nyul-Toth A, Balasubramanian P, Tarantini S, Ahire C, DelFavero J, Yabluchanskiy A, Csipo T, Farkas E, Wiley G, et al. Single-cell RNA sequencing identifies senescent cerebromicrovascular endothelial cells in the aged mouse brain. Geroscience 2020. doi: 10.1007/s11357-020-00177-1 [DOI] [PMC free article] [PubMed]
- 64.Levit A, Hachinski V, Whitehead SN. Neurovascular unit dysregulation, white matter disease, and executive dysfunction: the shared triad of vascular cognitive impairment and Alzheimer disease. Geroscience 2020;42:445–465. doi: 10.1007/s11357-020-00164-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers Dement 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarantini S, Tran CHT, Gordon GR, Ungvari Z, Csiszar A. Impaired neurovascular coupling in aging and Alzheimer's disease: Contribution of astrocyte dysfunction and endothelial impairment to cognitive decline. Exp Gerontol 2017;94:52–58. doi: 10.1016/j.exger.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001 [DOI] [PubMed] [Google Scholar]
- 68.Dorigatti AO, Riordan R, Yu Z, Ross G, Wang R, Reynolds-Lallement N, Magnusson K, Galvan V, Perez VI. Brain cellular senescence in mouse models of Alzheimer's disease. Geroscience 2022;44:1157–1168. doi: 10.1007/s11357-022-00531-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fulop GA, Kiss T, Tarantini S, Balasubramanian P, Yabluchanskiy A, Farkas E, Bari F, Ungvari Z, Csiszar A. Nrf2 deficiency in aged mice exacerbates cellular senescence promoting cerebrovascular inflammation. Geroscience 2018;40:513–521. doi: 10.1007/s11357-018-0047-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yousefzadeh MJ, Wilkinson JE, Hughes B, Gadela N, Ladiges WC, Vo N, Niedernhofer LJ, Huffman DM, Robbins PD. Heterochronic parabiosis regulates the extent of cellular senescence in multiple tissues. Geroscience 2020;42:951–961. doi: 10.1007/s11357-020-00185-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Csipo T, Lipecz A, Ashpole NM, Balasubramanian P, Tarantini S. Astrocyte senescence contributes to cognitive decline. Geroscience 2020;42:51–55. doi: 10.1007/s11357-019-00140-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ungvari Z, Tarantini S, Nyul-Toth A, Kiss T, Yabluchanskiy A, Csipo T, Balasubramanian P, Lipecz A, Benyo Z, Csiszar A. Nrf2 dysfunction and impaired cellular resilience to oxidative stressors in the aged vasculature: from increased cellular senescence to the pathogenesis of age-related vascular diseases. Geroscience 2019;41:727–738. doi: 10.1007/s11357-019-00107-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Childs BG, Zhang C, Shuja F, Sturmlechner I, Trewartha S, Fierro Velasco R, Baker D, Li H, van Deursen JM. Senescent cells suppress innate smooth muscle cell repair functions in atherosclerosis. Nat Aging 2021;1:698–714. doi: 10.1038/s43587-021-00089-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Childs BG, Baker DJ, Wijshake T, Conover CA, Campisi J, van Deursen JM. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science 2016;354:472–477. doi: 10.1126/science.aaf6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gluchowska A, Cysewski D, Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Podszywalow-Bartnicka P, Sunderland P, Kozlowska E, Sliwinska MA, Dabrowski M, et al. Unbiased proteomic analysis of extracellular vesicles secreted by senescent human vascular smooth muscle cells reveals their ability to modulate immune cell functions. Geroscience 2022. doi: 10.1007/s11357-022-00625-0 [DOI] [PMC free article] [PubMed]
- 76.Bailey-Downs LC, Mitschelen M, Sosnowska D, Toth P, Pinto JT, Ballabh P, Valcarcel-Ares MN, Farley J, Koller A, Henthorn JC, et al. Liver-specific knockdown of IGF-1 decreases vascular oxidative stress resistance by impairing the Nrf2-dependent antioxidant response: a novel model of vascular aging. J Gerontol A Biol Sci Med Sci 2012;67:313–329. doi: 10.1093/gerona/glr164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bailey-Downs LC, Sosnowska D, Toth P, Mitschelen M, Gautam T, Henthorn JC, Ballabh P, Koller A, Farley JA, Sonntag WE, et al. Growth Hormone and IGF-1 Deficiency Exacerbate High-Fat Diet-Induced Endothelial Impairment in Obese Lewis Dwarf Rats: Implications for Vascular Aging. J Gerontol A Biol Sci Med Sci 2012;67:553–564. doi: glr197 [pii] 10.1093/gerona/glr197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Csiszar A, Balasubramanian P, Tarantini S, Yabluchanskiy A, Zhang XA, Springo Z, Benbrook D, Sonntag WE, Ungvari Z. Chemically induced carcinogenesis in rodent models of aging: assessing organismal resilience to genotoxic stressors in geroscience research. Geroscience 2019;41:209–227. doi: 10.1007/s11357-019-00064-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farias Quipildor GE, Mao K, Hu Z, Novaj A, Cui MH, Gulinello M, Branch CA, Gubbi S, Patel K, Moellering DR, et al. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience 2019;41:185–208. doi: 10.1007/s11357-019-00065-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miller LR, Tarantini S, Nyul-Toth A, Johnston MP, Martin T, Bullen EC, Bickel MA, Sonntag WE, Yabluchanskiy A, Csiszar A, et al. Increased Susceptibility to Cerebral Microhemorrhages Is Associated With Imaging Signs of Microvascular Degeneration in the Retina in an Insulin-Like Growth Factor 1 Deficient Mouse Model of Accelerated Aging. Front Aging Neurosci 2022;14:788296. doi: 10.3389/fnagi.2022.788296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tarantini S, Balasubramanian P, Yabluchanskiy A, Ashpole NM, Logan S, Kiss T, Ungvari A, Nyul-Toth A, Schwartzman ML, Benyo Z, et al. IGF1R signaling regulates astrocyte-mediated neurovascular coupling in mice: implications for brain aging. Geroscience 2021;43:901–911. doi: 10.1007/s11357-021-00350-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tarantini S, Giles CB, Wren JD, Ashpole NM, Valcarcel-Ares MN, Wei JY, Sonntag WE, Ungvari Z, Csiszar A. IGF-1 deficiency in a critical period early in life influences the vascular aging phenotype in mice by altering miRNA-mediated post-transcriptional gene regulation: implications for the developmental origins of health and disease hypothesis. Age (Dordr) 2016;38:239–258. doi: 10.1007/s11357-016-9943-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ungvari Z, Sosnowska D, Podlutsky A, Koncz P, Sonntag WE, Csiszar A. Free radical production, antioxidant capacity, and oxidative stress response signatures in fibroblasts from Lewis dwarf rats: effects of life span-extending peripubertal GH treatment. J Gerontol A Biol Sci Med Sci 2011;66:501–510. doi: 10.1093/gerona/glr004 glr004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Higashi Y, Pandey A, Goodwin B, Delafontaine P. Insulin-like growth factor-1 regulates glutathione peroxidase expression and activity in vascular endothelial cells: Implications for atheroprotective actions of insulin-like growth factor-1. Biochim Biophys Acta 2013;1832:391–399. doi: 10.1016/j.bbadis.2012.12.005 S0925-4439(12)00290-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mitschelen M, Garteiser P, Carnes BA, Farley JA, Doblas S, Demoe JH, Warrington JP, Yan H, Nicolle MM, Towner R, et al. Basal and hypercapnia-altered cerebrovascular perfusion predict mild cognitive impairment in aging rodents. Neuroscience 2009;164:918–928. doi: S0306-4522(09)01472-9 [pii] 10.1016/j.neuroscience.2009.08.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riddle DR, Sonntag WE, Lichtenwalner RJ. Microvascular plasticity in aging. Ageing Res Rev 2003;2:149–168. doi: S1568163702000648 [pii] [DOI] [PubMed] [Google Scholar]
- 87.Khan AS, Lynch CD, Sane DC, Willingham MC, Sonntag WE. Growth hormone increases regional coronary blood flow and capillary density in aged rats. J Gerontol A Biol Sci Med Sci 2001;56:B364–371. [DOI] [PubMed] [Google Scholar]
- 88.Lynch CD, Cooney PT, Bennett SA, Thornton PL, Khan AS, Ingram RL, Sonntag WE. Effects of moderate caloric restriction on cortical microvascular density and local cerebral blood flow in aged rats. Neurobiol Aging 1999;20:191–200. doi: S0197458099000329 [pii] [DOI] [PubMed] [Google Scholar]
- 89.Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology 1997;138:3515–3520. [DOI] [PubMed] [Google Scholar]
- 90.Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS. Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 1991;11:684–689. doi: 10.1038/jcbfm.1991.121 [DOI] [PubMed] [Google Scholar]
- 91.Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D. The metabolic topography of normal aging. J Cereb Blood Flow Metab 1996;16:385–398. doi: 10.1097/00004647-199605000-00005 [DOI] [PubMed] [Google Scholar]
- 92.Kawamura J, Terayama Y, Takashima S, Obara K, Pavol MA, Meyer JS, Mortel KF, Weathers S. Leuko-araiosis and cerebral perfusion in normal aging. Exp Aging Res 1993;19:225–240. [DOI] [PubMed] [Google Scholar]
- 93.Krejza J, Mariak Z, Walecki J, Szydlik P, Lewko J, Ustymowicz A. Transcranial color Doppler sonography of basal cerebral arteries in 182 healthy subjects: age and sex variability and normal reference values for blood flow parameters. AJR Am J Roentgenol 1999;172:213–218. [DOI] [PubMed] [Google Scholar]
- 94.Schultz SK, OĽeary DS, Boles Ponto LL, Watkins GL, Hichwa RD, Andreasen NC. Age-related changes in regional cerebral blood flow among young to mid-life adults. Neuroreport 1999;10:2493–2496. [DOI] [PubMed] [Google Scholar]
- 95.Bentourkia M, Bol A, Ivanoiu A, Labar D, Sibomana M, Coppens A, Michel C, Cosnard G, De Volder AG. Comparison of regional cerebral blood flow and glucose metabolism in the normal brain: effect of aging. J Neurol Sci 2000;181:19–28. doi: S0022-510X(00)00396-8 [pii] [DOI] [PubMed] [Google Scholar]
- 96.Hagstadius S, Risberg J. Regional cerebral blood flow characteristics and variations with age in resting normal subjects. Brain Cogn 1989;10:28–43. [DOI] [PubMed] [Google Scholar]
- 97.Pagani M, Salmaso D, Jonsson C, Hatherly R, Jacobsson H, Larsson SA, Wagner A. Regional cerebral blood flow as assessed by principal component analysis and (99m)Tc-HMPAO SPET in healthy subjects at rest: normal distribution and effect of age and gender. Eur J Nucl Med Mol Imaging 2002;29:67–75. doi: 10.1007/s00259-001-0676-2 [DOI] [PubMed] [Google Scholar]
- 98.Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci 2008;63:12–20. doi: 63/1/12 [pii] [DOI] [PubMed] [Google Scholar]
- 99.Sonntag WE, Lynch C, Thornton P, Khan A, Bennett S, Ingram R. The effects of growth hormone and IGF-1 deficiency on cerebrovascular and brain ageing. J Anat 2000;197 Pt 4:575–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Warrington JP, Csiszar A, Johnson DA, Herman TS, Ahmad S, Lee YW, Sonntag WE. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am J Physiol Heart Circ Physiol 2011;300:H736–744. doi: 10.1152/ajpheart.01024.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS One 2012;7:e30444. doi: 10.1371/journal.pone.0030444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lopez-Lopez C, LeRoith D, Torres-Aleman I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc Natl Acad Sci U S A 2004;101:9833–9838. doi: 10.1073/pnas.0400337101 0400337101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kiss T, Tarantini S, Csipo T, Balasubramanian P, Nyul-Toth A, Yabluchanskiy A, Wren JD, Garman L, Huffman DM, Csiszar A, et al. Circulating anti-geronic factors from heterochonic parabionts promote vascular rejuvenation in aged mice: transcriptional footprint of mitochondrial protection, attenuation of oxidative stress, and rescue of endothelial function by young blood. Geroscience 2020. doi: 10.1007/s11357-020-00180-6 [DOI] [PMC free article] [PubMed]
- 104.Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol Rev 2019;99:21–78. doi: 10.1152/physrev.00050.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nowrangi DS, McBride D, Manaenko A, Dixon B, Tang J, Zhang JH. rhIGF-1 reduces the permeability of the blood-brain barrier following intracerebral hemorrhage in mice. Exp Neurol 2019;312:72–81. doi: 10.1016/j.expneurol.2018.11.009 [DOI] [PubMed] [Google Scholar]
- 106.Yabluchanskiy A, Tarantini S, Balasubramanian P, Kiss T, Csipo T, Fulop GA, Lipecz A, Ahire C, DelFavero J, Nyul-Toth A, et al. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. Geroscience 2020. doi: 10.1007/s11357-020-00154-8 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are available in either the main text or from the authors upon reasonable request. Please contact the corresponding authors for access to this data.


