Abstract
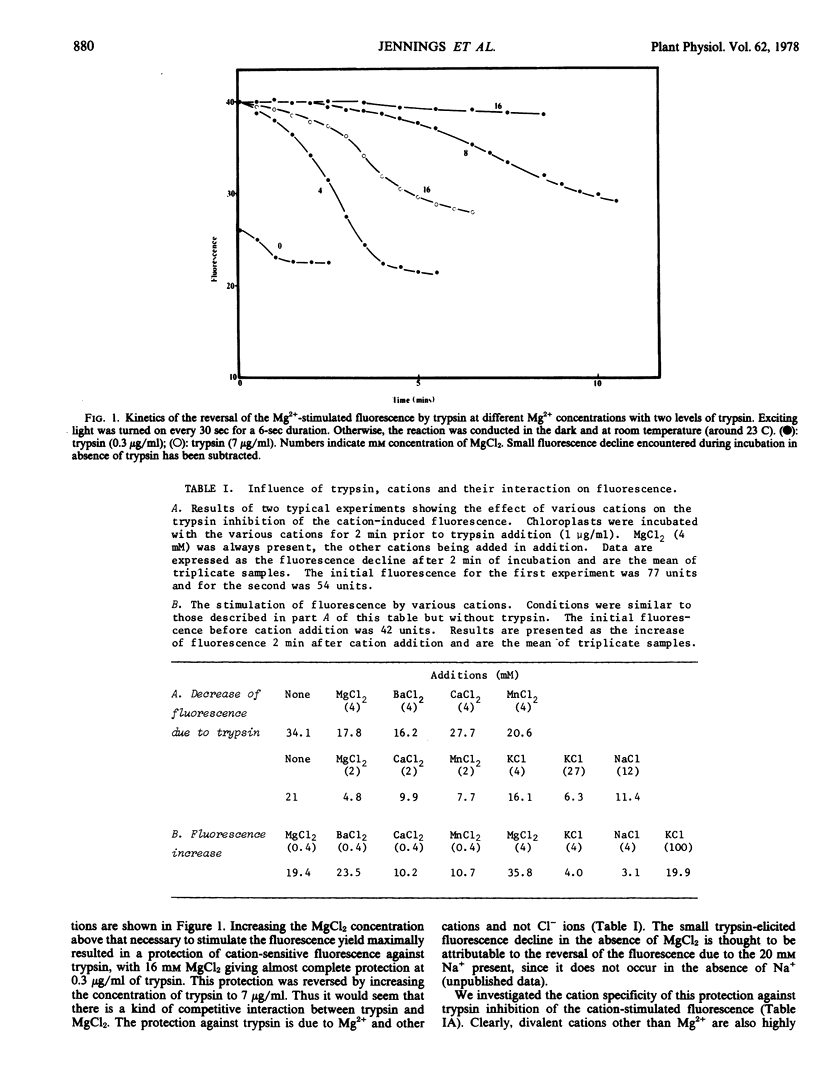
Trypsin digestion of photosynthetic membranes isolated from spinach (Spinacia oleracea L.) leaves eliminates the cation stimulation of chlorophyll fluorescence. High concentrations of cations protect the fluorescence yield against trypsin digestion, and the cation specificity for this protection closely resembles that required for the stimulation of fluorescence by cations. Trypsin digestion reverses cation-induced thylakoid stacking, and the time course of this effect seems to parallel that of the reversal of cation fluorescence. High concentrations of cations protect thylakoid stacking and cation-stimulated fluorescence alike. The cation stimulation of photosytem II photochemistry remains intact after trypsinization has reversed both cation-induced thylakoid stacking and fluorescence yield. It is concluded that cation-stimulated fluorescence yield, and not the cation stimulation of photosystem II photochemistry, is associated with thylakoid membrane stacking.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Argyroudi-Akoyunoglou J. H., Akoyunoglou G. Correlation between cation-induced formation of heavy subchloroplast fractions and cation-induced increase in chlorophyll a fluorescence yield in tricine-washed chloroplasts. Arch Biochem Biophys. 1977 Mar;179(2):370–377. doi: 10.1016/0003-9861(77)90124-2. [DOI] [PubMed] [Google Scholar]
- Bar-Nun S., Schantz R., Ohad I. Appearance and composition of chlorophyll-protein complexes I and II during chloroplast membrane biogenesis in Chlamydomonas reinhardi y-1. Biochim Biophys Acta. 1977 Mar 11;459(3):451–467. doi: 10.1016/0005-2728(77)90045-7. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Okayama S. The photoreduction of C-550 in chloroplasts and its inhibition by lipase. Biochim Biophys Acta. 1971 Aug 6;245(1):237–239. doi: 10.1016/0005-2728(71)90028-4. [DOI] [PubMed] [Google Scholar]
- Davis D. J., Armond P. A., Gross E. L., Arntzen C. J. Differentiation of chloroplast lamellae. Onset of cation regulation of excitation energy distribution. Arch Biochem Biophys. 1976 Jul;175(1):64–70. doi: 10.1016/0003-9861(76)90485-9. [DOI] [PubMed] [Google Scholar]
- Davis D. J., Gross E. L. Protein-protein interactions of the light-harvesting chlorophyll a/b protein. II. Evidence for two stages of cation independent association. Biochim Biophys Acta. 1976 Dec 6;449(3):554–564. doi: 10.1016/0005-2728(76)90164-x. [DOI] [PubMed] [Google Scholar]
- GREEN N. M., NEURATH H. The effects of divalent cations on trypsin. J Biol Chem. 1953 Sep;204(1):379–390. [PubMed] [Google Scholar]
- Gross E. L., Prasher S. H. Correlation between monovalent cation-induced decreases in chlorophyll a fluorescence and chloroplast structural changes. Arch Biochem Biophys. 1974 Oct;164(2):460–468. doi: 10.1016/0003-9861(74)90056-3. [DOI] [PubMed] [Google Scholar]
- Homann P. H. Cation effects on the fluorescence of isolated chloroplasts. Plant Physiol. 1969 Jun;44(6):932–936. doi: 10.1104/pp.44.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Good N. E. Effect of Salts and Electron Transport on the Conformation of Isolated Chloroplasts. II. Electron Microscopy. Plant Physiol. 1966 Mar;41(3):544–552. doi: 10.1104/pp.41.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings R. C., Forti G. The influence of magnesium on the chlorophyll fluorescence yield of isolated chloroplasts. Biochim Biophys Acta. 1974 May 22;347(2):299–310. doi: 10.1016/0005-2728(74)90053-x. [DOI] [PubMed] [Google Scholar]
- Murakami S., Packer L. The role of cations in the organization of chloroplast membranes. Arch Biochem Biophys. 1971 Sep;146(1):337–347. doi: 10.1016/s0003-9861(71)80072-3. [DOI] [PubMed] [Google Scholar]
- Murata N. Control of excitation transfer in photosynthesis. II. Magnesium ion-dependent distribution of excitation energy between two pigment systems in spinach chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):171–181. doi: 10.1016/0005-2728(69)90045-0. [DOI] [PubMed] [Google Scholar]
- Selman B. R., Bannister T. T. Trypsin inhibition of photosystem II. Biochim Biophys Acta. 1971 Dec 7;253(2):428–436. doi: 10.1016/0005-2728(71)90046-6. [DOI] [PubMed] [Google Scholar]