Abstract
Background:
Activation of coagulation and fibrin deposition in the regenerating liver appears to promote adequate liver regeneration in mice. In humans, perioperative hepatic fibrin deposition is reduced in patients that develop liver dysfunction after partial hepatectomy (PHx), but the mechanism underlying reduced fibrin deposition in these patients is unclear.
Methods and Results:
Hepatic deposition of cross-linked (i.e., stabilized) fibrin was evident in livers of mice after 2/3rd partial hepatectomy. Interestingly, hepatic fibrin cross-linking was dramatically reduced in mice after 90% PHx, an experimental setting of failed liver regeneration, despite similar activation of coagulation after 2/3rd or 90% PHx. Likewise, intraoperative activation of coagulation was not reduced in patients who developed liver dysfunction after PHx. Pre-operative fibrinogen plasma concentration was not connected to liver dysfunction after PHx in patients. Rather, pre- and postoperative plasma activity of the transglutaminase coagulation factor XIII (FXIII), which cross-links fibrin, was lower in patients who developed liver dysfunction, compared to those who did not. PHx-induced hepatic fibrin cross-linking and hepatic platelet accumulation were significantly reduced in mice lacking the catalytic subunit of FXIII (FXIII−/− mice) after 2/3rd PHx. This was coupled to a reduction in both hepatocyte proliferation and liver/body weight ratio, as well as an apparent reduction in survival after 2/3rd PHx in FXIII−/− mice.
Conclusions:
The results indicate that FXIII is a critical driver of liver regeneration after PHx and suggest that perioperative plasma FXIII activity may predict post-hepatectomy liver dysfunction. The results may inform strategies to stabilize pro-regenerative fibrin during liver resection.
Keywords: fibrinogen, platelets, coagulation, fibrin, hepatocytes
Introduction
Partial liver resection (e.g., partial hepatectomy [PHx]) is a common surgical procedure used to remove diseased liver tissue (e.g., tumors). The regenerative capability and functional reserve of the remnant liver ensures hepatic function quickly recovers, even after removal of a substantial part of the liver.1 Notably, in approximately 10–15% of patients undergoing partial liver resection, liver regeneration is insufficient and as a consequence, patients can develop post-hepatectomy liver failure (PHLF), a condition where the liver remnant cannot sustain critical hepatic functions.2 Detecting patients at risk of PHLF and developing targeted therapies to improve liver regeneration are continued needs, even though the molecular mechanisms of liver regeneration are increasingly understood3.
Cellular and molecular mechanisms driving liver regeneration after PHx have largely been discovered using the well-characterized experimental setting of 2/3rd PHx in rodents.4 PHx is rapidly followed by a sequence of cell signaling events leading to coordinated cell proliferation and restoration of liver mass and function in 7–10 days in mice.4–6 Standard 2/3rd PHx induces activation of the blood coagulation cascade in mice, indicated by biomarkers of thrombin generation and deposition of the thrombin substrate fibrin(ogen) in the regenerating liver (i.e., remnant) within 30 minutes of surgery7. Reducing thrombin generation using genetic or pharmacologic approaches reduced hepatic fibrin(ogen) deposition and hepatocyte proliferation after PHx in mice7, 8. Likewise, depletion of plasma fibrinogen with ancrod, prior to PHx, significantly reduced hepatic fibrin(ogen) deposition and hepatocyte proliferation after PHx7. Prior studies suggest that fibrin(ogen) plays a pivotal role in promoting rapid accumulation of platelets in the regenerating liver7. Indeed, like fibrin(ogen), platelets rapidly accumulate in the liver remnant after PHx in mice9 and humans.10 Moreover, depleting or inhibiting platelets significantly reduced hepatocyte proliferation and delayed liver regeneration after PHx in mice.9, 11 Overall, experimental and clinical evidence suggests that components of the hemostatic system are amongst the earliest triggers of liver regeneration after PHx.
Rapid hepatic activation of coagulation was also evident in patients undergoing liver resection7, 12. Consistent with observations in mice, a rapid intraoperative increase in hepatic fibrin(ogen) deposition in the regenerating liver of patients undergoing partial liver resection was demonstrated in biopsies taken perioperatively7. Remarkably, hepatic fibrin(ogen) accumulation was reduced in patients who ultimately developed post-hepatectomy liver dysfunction, suggesting a functional connection between rapid hepatic fibrin(ogen) deposition and successful regeneration7. Pre-operative plasma concentrations of clottable fibrinogen were not connected to post-operative liver dysfunction, and the mechanistic basis for this failure of rapid intrahepatic fibrin(ogen) deposition is unknown. We tested the hypothesis that intrahepatic fibrin(ogen) stability and liver regeneration after PHx are driven by coagulation factor FXIII. When activated (i.e., FXIIIa), this multifunctional transglutaminase cross-links multiple proteins including fibrin polymers13, 14. The role of FXIII was determined using experimental settings of PHx-induced liver regeneration (2/3rd PHx) and liver failure (90% PHx) as well as analysis of perioperative samples from patients undergoing partial liver resection.
Materials and methods
Partial hepatectomy (PHx) in mice:
Wild-type C57Bl/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice lacking the factor XIII catalytic A subunit (FXIII-A−/− mice)15, 16 and a matched line of wild-type mice originally generated from heterozygous mice were bred at MSU. Surgeries were performed on male and female mice between 8–14 weeks old (see figure legends). Mice were housed under a 12-h light/dark cycle, fed standard diet (Teklad 8940, Envigo) and provided drinking water ad libitum. All procedures on mice were performed at Michigan State University approved by the Institutional Animal Care and Use Committee of Michigan State University, East Lansing, USA. Age-matched cohorts of mice underwent a standard 2/3rd or extended 90% PHx according to published protocols with some modifications.4, 17 2/3rd PHx was performed in unfasted mice during the light cycle (0800–1300) by resection of the left lateral lobe, the right portion of the median lobe and the left portion of the median lobe using three separate ligatures to preserve the gall bladder. For extended 90% PHx, the lower right lateral lobe was also resected.18 Sham surgery was identical but included gentle manipulation of the liver lobes without removal of liver tissue. Surgical procedures were performed under deep surgical anesthesia induced by isoflurane (Abbott, Chicago, IL, USA) and carprofen (5 mg/kg, s.c.) was administered as analgesic (Pfizer, New York, NY, USA). Blood and liver samples were collected 30 minutes or 48 hours after surgery. Blood was collected under deep surgical anesthesia induced by isoflurane by exsanguination from the inferior vena cava immediately after injection of 3.8% sodium citrate (Merck, Darmstadt, Germany) in the spleen (8ul/g). Blood samples were centrifuged to obtain plasma and were stored at −80°C. Livers were rinsed in PBS and fixed in either 10% neutral-buffered formalin for 96 hours prior to routine processing or snap frozen in liquid nitrogen.
Patient sample collection and cohorts:
In total, 98 patients were recruited at three different hospitals in Vienna, Austria (General Hospital, Clinic Landstraße, Clinic Favoriten) and followed up prospectively over a period of 90 days after surgery. Within this group of 98 patients, blood samples were assessed one day prior to surgery as well as on postoperative day 1 (POD1) and 5 days (POD5) after liver resection in 88 patients. In 25 of these 88 patients, additional intraoperative blood samples were taken from the portal and hepatic vein (draining the regenerating liver lobe), 2 h after induction of liver regeneration. Patient-related data was collected, including baseline characteristics, surgical procedure, perioperative routine laboratory parameters, and baseline liver pathology, and were distributed as illustrated in Table S1 (intraoperative blood sample cohort, N=25) and Table S2 (perioperative blood samples cohort, N=88), and Table S3 (patient demographics). This study was conducted in adherence to the Declaration of Helsinki and was approved by the institutional ethics committee (Medical University of Vienna) and the ethics committee of the city of Vienna (EK#1186/2018 and EK 16-253-0117) and informed consent was obtained from all participants.
Definition of Post-hepatectomy Liver Failure (PHLF):
To evaluate post-hepatectomy liver failure (PHLF) in our patient cohort, the definition published by the International Study Group of Liver Surgery (ISGLS) was used19. Here, PHLF is characterized by an increase in the international normalized ratio (INR) and a concomitant hyperbilirubinemia on or after postoperative day 5 (POD5). Further, this definition classifies the severity of PHLF in grades A-C. Patients suffering from Grade A PHLF only show a deviation in laboratory parameter but do not require a change in clinical management. Grade B on the other hand does require a change in management however, without the need for invasive treatment. Lastly, Grade C is defined as patients needing invasive treatment and at great risk for postoperative mortality19.
Plasma biomarkers of liver injury and coagulation:
Plasma alanine aminotransferase (ALT) activity was determined using commercial reagents (ThermoFisher, Waltham, MA; Pointe Scientific, Canton, MI) according to the manufacturer’s instructions and adapted for measurement in a microplate reader (i.e., final reaction volume 110 μL). Data was collected using an Infinite M200 plate reader (Tecan, Durham, NC). Plasma fibrinogen concentration in mouse plasma was measured by ELISA using two distinct polyclonal antibodies directed against fibrinogen (Capture, A0080 [Agilent]; Detection, ASMFBGN-GF-HRP [Innovative Research, Novi, MI) as described20. Plasma TAT and prothrombin fragment 1+2 concentration were determined using commercial ELISAs (Enzygnost TAT micro; Siemens Healthcare Diagnostics). Plasma D-dimer was determined using a commercial ELISA (Asserachrom D-Di, Diagnostica Stago). FXIII-A concentration in mouse plasma was detected in plasma using capillary western blotting (Wes) using a sheep anti-FXIII-A antibody (1:2000 [SAF13A-IG], Affinity Biologicals, Ancaster, ON) and HRP-conjugated rabbit anti-sheep antibody (1:1000, Jackson Immunoresearch) and other reagents accompanying the Wes Master Kit were used according to the manufacturer’s protocol (ProteinSimple). Prior studies have used this antibody to detect plasma FXIII-A by western blotting21. Plasma FXIIIa activity in human plasma samples was determined as described previously22 and plasma fibrinogen concentration in human plasma was determined using an ELISA22
Measurement of cell proliferation in liver:
Formalin-fixed paraffin embedded liver sections were stained for Ki-67 (SP6 clone, Cell Marque) by the Investigative Histopathology laboratory at Michigan State University, as previously described.7 Slides were scanned using a Virtual Slide System VS110 and ~500 high-power fields randomly sampled images used for quantification of Ki67-positive hepatocyte nuclei. Ki67-positive nuclei were identified using ImageJ (Fiji) (version 1.5w, National Institutes of Health, Bethesda, MD, USA) and expressed as percentage of total hepatocyte nuclei.
Hepatic fibrin(ogen) and platelet accumulation:
The urea-insoluble protein fraction was enriched from snap-frozen mouse liver as described previously and fibrin(ogen) detected using automated capillary western blotting (Wes, Protein Simple, San Jose, CA), as described previously.23 Insoluble protein fractions were resolved using Wes 12–230 kDa (Fibβ) or 66–440 kDa 25-capillary gels (Fibα) (Protein Simple, San Jose, CA). Fibrinogen polypeptides were detected using antibodies selective for each individual fibrinogen chain24 (Proteintech, Chicago, IL) (1:100 dilution for α chain, and 1:1000 dilution for β chain), as described previously.23 A goat anti-rabbit HRP-conjugated secondary antibody (1:400 dilution for detection of Fibα antibody, 1:700 dilution for Fibβ antibody; Jackson Immunoresearch) and other reagents accompanying the Wes Master Kit were used according to the manufacturer’s protocol. Quantification of fibrin(ogen) peak area was performed using Compass for SW software (version 6.0.0, ProteinSimple). Hepatic platelet accumulation was evaluated by western blotting for the platelet-specific integrin αIIB in detergent-soluble liver extracts. Protein samples were diluted in Laemmli sample buffer containing 2-ME and heat denatured for 10 minutes at 95 °C. 10 μg protein was loaded and separated using SDS-PAGE on a 4–12% Bis-Tris gel (Bio-Rad) in Tris/Glycine SDS buffer (Bio-Rad). Proteins were transferred to a PVDF membrane (Millipore Sigma) using the Criterion™ Blotter system (Bio-Rad, Hercules, CA) and membrane was blocked for 1 hour at room temperature in 5% BSA/TBST. Platelets were detected by a recombinant rabbit monoclonal anti-CD41 antibody [EPR17876] (Abcam 181582, 1:1000 dilution in blocking buffer). Peroxidase AffiniPure Goat Anti-Rabbit IgG (H+L) was used as secondary antibody (1:10.000 dilution in 1% BSA/TBST, Jackson ImmunoResearch). For chemiluminescent detection, membranes were incubated with EcoBright Pico HRP substrate (Innovative Solutions, Beverly Hills, MI) and exposed to blue autoradiography film (DOT Scientific). Total protein was evaluated using Revert 700 reagent and imaged using the Odyssey® CLx Infrared Imaging System (Licor, Lincoln, NE, USA). Quantification of bands was performed using the gel analysis tool in Image Studio (Licor).
Transmission electron microscopy
Liver biopsy samples (collected at Medical University of Vienna) were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS). After washing and post fixing in 1% osmium tetroxide (resolved in 3% Potassium hexacyanoferrate), the material was dehydrated and embedded in EPON Resin 812. For ultrastructural assessment, thin sections were cut using an Ultracut UCT ultramicrotome (Leica Microsystems, Austria), mounted on copper grids and further counterstained with uranyl acetate and lead citrate. The sections were then examined at 60 kV in a JEOL JEM-1400 Plus transmission electron microscope, whereas images were obtained by using an Olympus Quemesa bottom-mounted TEM CCD camera and RADIUS - EM Imaging Software (Emsis GmbH, Germany).
Statistics
Statistical analyses were performed using GraphPad Prism v.9 (San Diego, CA, USA) software package. Continuous variables are presented as mean ± SEM. Comparison of two groups was performed using Student’s t test. Comparison of three or more groups was performed using one-way analysis of variance (ANOVA) with Tukey post-hoc test. Results from patient samples were analyzed using a mixed-effects analysis with Ŝídák’s posthoc test, because of missing results for certain samples. A p value of less than 0.05 was considered statistically significant.
Results
Hepatic fibrin(ogen) cross-linking after 2/3rd PHx in mice.
Plasma thrombin-antithrombin (TAT), a biomarker of coagulation cascade activation, increased within 30 minutes after 2/3rd PHx, in agreement with prior studies7, and returned to levels observed in sham mice by 6 hours after PHx in wild-type mice (Supplemental Fig. 1). Prior studies used immunohistochemistry to observe rapid sinusoidal fibrin(ogen) accumulation after 2/3rd PHx in mice and humans7. In agreement with this observation, hepatic levels of fibrin(ogen)-β (Fib-β) increased in the insoluble protein fraction 30 min after 2/3rd PHx (Fig. 1A, C). Thrombin-mediated fibrin polymerization precedes fibrin cross-linking25. Notably, a robust increase in high-molecular weight fibrin(ogen) complexes in the liver remnant, likely cross-linked α-polymer, was evident after 2/3rd PHx (Fig. 1B, D). Importantly, sinusoidal accumulation of fibrin was discernable in intraoperative liver biopsy samples collected from the liver remnant 2 hours after ligation of the portal vein in patients undergoing partial liver resection (red arrows, Supplemental Fig. 2). The results support prior observations to suggest that insoluble cross-linked fibrin polymer (i.e., traditional fibrin clot) formation occurs rapidly after PHx in the liver remnant.
Figure 1. 2/3rd PHx induces rapid accumulation of insoluble cross-linked fibrin in liver.

Male wild-type mice underwent 2/3rd partial hepatectomy (PHx) or sham surgery (see Methods) and livers were collected 30 minutes after the last lobe was resected. Fibrin(ogen) levels were measured in enriched insoluble liver extracts using automated capillary western blotting. Representative digital capillary images show fibrin(ogen) detected by rabbit polyclonal antibodies selective for fibrin(ogen) Bβ chain (A) and Aα chain (B). Quantification of peaks is shown in panels C-D. Results from individual mice are plotted and bars represent mean ± standard error of mean. N=3–6 mice per group. *p<0.05.
Impact of 90% PHx on hepatic fibrin(ogen) deposition.
Notably, hepatic fibrin(ogen) accumulation in the liver remnant is evident in patients undergoing partial liver resection, but this response was attenuated in patients that developed post-operative liver dysfunction7. Seeking the basis for this observation, we compared the rapid fibrin(ogen) deposition in the standard setting 2/3rd PHx in mice with that of 90% PHx (extended hepatectomy), a model of failed liver regeneration, hepatic dysfunction and liver failure18, 26. Both 2/3rd PHx and 90% PHx caused a slight but significant increase in plasma ALT activity (Fig. 2A). Standard 2/3rd PHx significantly increased hepatic fibrin(ogen) accumulation 30 min after PHx, indicated by an increase in insoluble Fib-β (Fig. 2B, D). A similar increase in Fib β chain was observed in mice after 90% PHx (Fig. 2B, D). Interestingly, levels of cross-linked α-polymer (apparent MW ~360 kD) in urea-extracted insoluble protein were markedly reduced in livers of mice after 90% PHx compared to mice that underwent 2/3rd PHx (Fig. 2C, E). The results suggest that cross-linking of hepatic fibrin(ogen) deposits is decreased in liver after 90% PHx compared to 2/3rd PHx.
Figure 2. Hepatic fibrin cross-linking is reduced in mice after extended 90% PHx.

Male wild-type mice underwent 2/3rd partial hepatectomy (PHx), 90% extended PHx, or sham surgery (see Methods) and livers and plasma were collected 30 minutes after the last lobe was resected. (A) Plasma alanine aminotransferase (ALT) activity. Fibrin(ogen) levels were measured in enriched insoluble liver extracts using automated capillary western blotting. Representative digital capillary images show fibrin(ogen) detected by rabbit polyclonal antibodies selective for fibrin(ogen) Bβ chain (B) and Aα chain (C). Quantification of peaks is shown in panels D-E. Cross-linked high molecular weight (HMW) Aα chain was expressed relative to total insoluble fibrin levels (i.e., Bβ chain). Results from individual mice are plotted and bars represent mean ± standard error of mean. N=4–10 mice per group. *p<0.05, ** p<0.01., ****P<0.0001.
Coagulation activation, fibrinogen and FXIII plasma levels in mice and humans after PHx.
Our studies in mice and in humans point to altered fibrin(ogen) deposition/cross-linking as a common mechanism leading to impaired liver regeneration. Prior studies indicate that hepatic fibrin(ogen) deposition requires activation of the coagulation cascade7. Indeed, the extent of coagulation activation and thrombin levels play a key role in penultimate fibrin structure and stability27. Thus, we evaluated biomarkers of coagulation activation in both mice and patients after PHx. Plasma thrombin-antithrombin (TAT) complexes were significantly increased after 2/3rd PHx and similarly increased after 90% PHx compared to sham surgery (Fig. 3A). Likewise, the concentration of markers of coagulation activation (i.e., TAT complexes [normal: 1.6–5.1 ng/ml] and prothrombin fragment 1+2 [normal, 34.4–260.3 pM) measured in plasma collected from the hepatic vein (i.e, blood draining from the regenerating lobe) two hours after induction of regeneration were similar in patients that did or not develop liver dysfunction (Fig. 3B–C). Plasma fibrinogen concentration decreased only slightly after either 2/3rd or 90% PHx (Fig. 4A). Pre-operative plasma fibrinogen antigen concentration was also similar in patients that did or did not develop hepatic dysfunction after PHx (Fig. 4B). Notably, plasma fibrinogen concentration in patients developing liver dysfunction reduced on post-operative day 1 compared to baseline values and remained low on post-operative day 5 (Fig. 4B), whereas fibrinogen levels in patients that did not develop liver dysfunction did not change over time. Collectively, the results suggest that changes in intrahepatic fibrin(ogen) deposition are not attributed to i) insufficient thrombin generation or ii) insufficient fibrinogen. Plasma FXIII circulates in complex with fibrinogen. Upon activation by thrombin, FXIIIa imposes covalent cross-links at specific residues on the fibrin α and γ polypeptides, which increases clot stability and improves resistance to clot lysis28. Plasma FXIII-A antigen concentration was significantly reduced after 2/3rd PHx and 90% PHx in mice, with the greatest reduction evident after 90% PHx (Fig. 4C). Mirroring changes in mice after PHx, plasma FXIIIa activity was reduced on post-operative day 1 in patients after partial liver resection, and FXIIIa activity was lowest in patients who developed liver dysfunction (Fig. 4D). Interestingly, pre-operative plasma FXIIIa activity tended to be lower (p=0.051) in patients that ultimately developed liver dysfunction after surgery (42–101%, [median, 63%]) compared to those who did not (40–294%, [median, 89%]) (Fig. 4D).
Figure 3. Biomarkers of coagulation cascade activation in mice and humans after PHx.
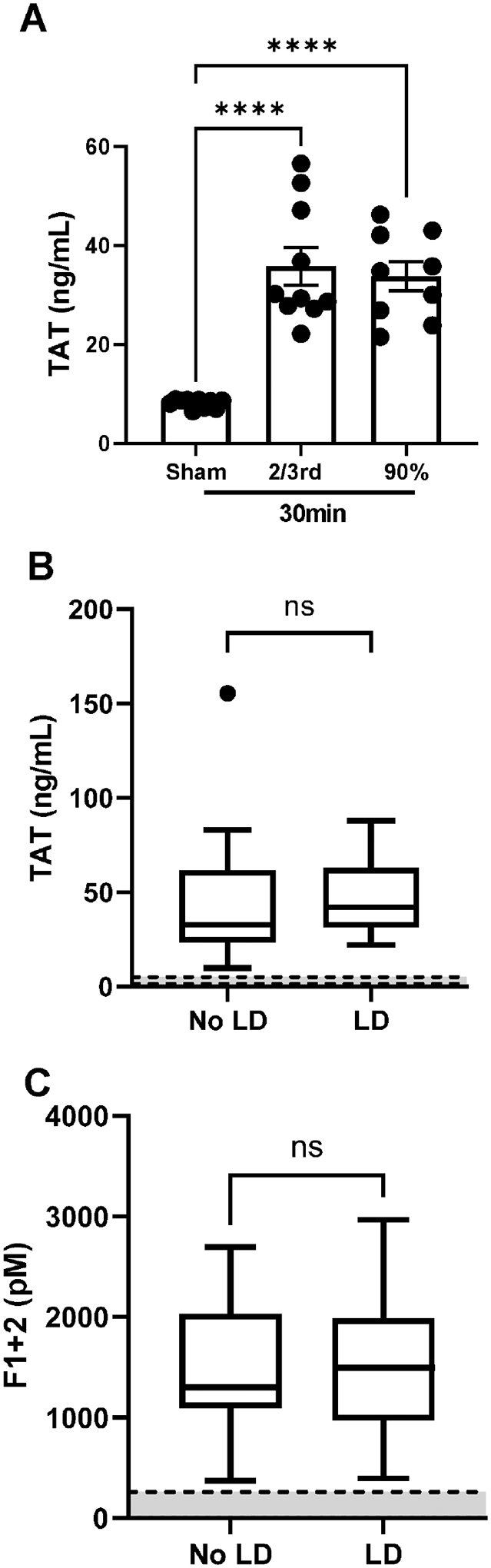
Male wild-type mice underwent 2/3rd partial hepatectomy (PHx), 90% extended PHx, or sham surgery (see Methods) and plasma was collected 30 minutes after the last lobe was resected. (A) Plasma thrombin-antithrombin (TAT) complexes were determined using a commercial ELISA (see Methods). For B-C, the concentration of TAT complexes (B) and prothrombin fragment 1+2 (C) were measured in hepatic vein plasma samples collected intraoperatively from 25 patients that underwent hemihepatectomy using commercial ELISAs (see Methods). For A, results from individual mice are plotted and bars represent mean ± standard error of mean. N=9–10 mice per group. ****P<0.0001. For B-C, results from 25 patients are expressed as Tukey box and whisker plot with the normal range indicated by gray space demarcated by dashed lines.
Figure 4. Plasma fibrinogen and FXIII in mice and humans after PHx.

Male wild-type mice underwent 2/3rd partial hepatectomy (PHx), 90% extended PHx, or sham surgery (see Methods) and plasma was collected 30 minutes after the last lobe was resected. (A) Plasma fibrinogen concentration was determined by ELISA (see Methods). (B) Plasma fibrinogen concentration was determined by ELISA (see Methods) in plasma samples collected from 88 patients one day prior to surgery as well as on postoperative day 1 (POD1) and 5 days (POD5) after liver resection. Liver dysfunction (LD) at 90 days post-op was determined using ISGLS criteria (see Methods). (C) Plasma FXIII-A antigen levels in mice after PHx was determined using capillary-based western blotting. Representative samples are shown in a digital capillary rendering. (D) Plasma FXIII-A activity was determined in 88 patients as above for fibrinogen (see Methods). For A and C, results from individual mice are plotted and bars represent mean ± standard error of mean. N=5–10 mice per group. *P<0.05, ***P<0.001. For B and D, results from 88 patients are expressed as Tukey box and whisker plot. *P<0.05, **P<0.01, ***P<0.001., ****P<0.0001.
FXIII-mediated fibrin(ogen) cross-linking is a key driver of hepatic platelet accumulation after PHx.
We next determined the impact of FXIII deficiency on hepatic fibrin(ogen) cross-linking in mice after 2/3rd PHx, using mice deficient in the catalytic subunit FXIII-A29. Insoluble cross-linked fibrin was evident in livers of wild-type mice 30 minutes after 2/3rd PHx (Fig. 5A–D). Deposition of cross-linked fibrin was dramatically reduced in livers of FXIII-A−/− mice after 2/3rd PHx (Fig 5B, D). Notably, overall levels of insoluble fibrin(ogen) were reduced in livers of FXIII-A−/− mice. Prior studies have shown that plasma fibrinogen depletion reduced hepatic accumulation of platelets after PHx, a key pro-regenerative signal30, 31. Notably, hepatic integrin αIIB levels were significantly reduced in livers of FXIII-A−/− mice compared to wild-type mice after 2/3rd PHx (Fig. E-F). Collectively, the results suggest that stabilization of hepatic fibrin deposits and initial hepatic platelet accumulation after PHx is driven in part by FXIII-dependent fibrin cross-linking.
Figure 5. FXIII drives hepatic fibrin cross-linking and platelet accumulation after PHx.
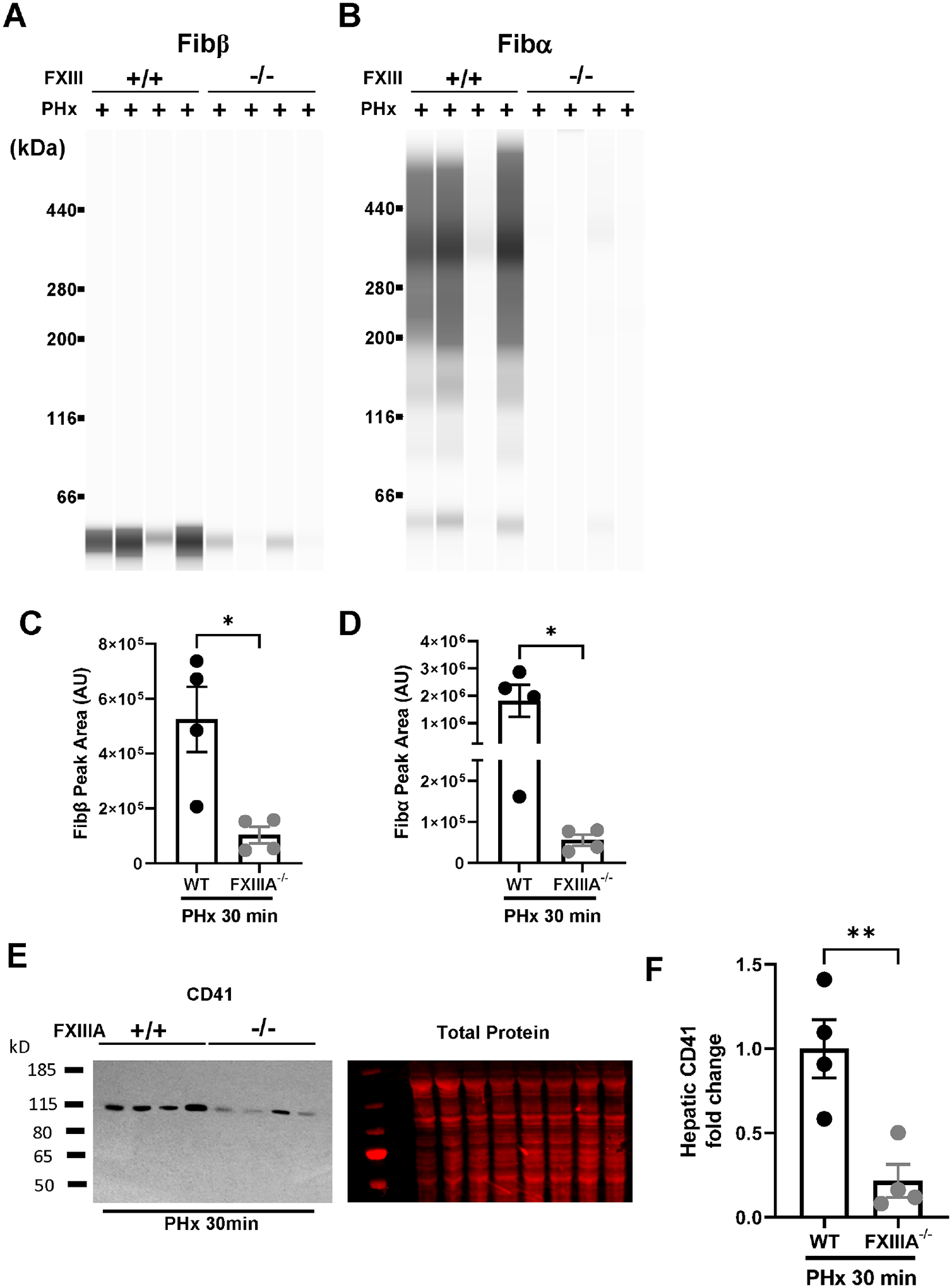
Male wild-type mice and FXIII-A−/− mice underwent 2/3rd partial hepatectomy (PHx (see Methods) and livers and plasma were collected 30 minutes after the last lobe was resected. Fibrin(ogen) levels were measured in enriched insoluble liver extracts using automated capillary western blotting. Representative digital capillary images show fibrin(ogen) detected by rabbit polyclonal antibodies selective for fibrin(ogen) Bβ chain (A) and Aα chain (B). Quantification of peaks is shown in panels C-D. Hepatic levels of the platelet integrin CD41 were determined by western blotting (E-F). Results from individual mice are plotted and bars represent mean ± standard error of mean. N=4 mice per group. *p<0.05, ** p<0.01.
Impact of FXIII deficiency on hepatocyte proliferation after 2/3rd PHx.
Insofar as our studies suggested that i) FXIII-dependent fibrin cross-linking was altered in experimental 90% PHx-induced liver dysfunction and ii) plasma FXIIIa activity was reduced in patients that developed liver dysfunction, we sought to determine the impact of FXIII-A deficiency on liver regeneration after 2/3rd PHx. Proliferating hepatocytes (Ki67+) were abundant in livers of male wild-type mice 48 hours after 2/3rd PHx (Fig. 6A, C) and FXIII-A deficiency significantly reduced the number of Ki67+ hepatocytes (Fig. 6B, C). This corresponded to a reduction in liver-to-body weight ratio 48 hours after 2/3rd PHx in both male and female mice (Fig. 6D). Remarkably, whereas no obvious morbidity was evident in wild-type mice, several FXIII-deficient mice died between 24–48 hours after 2/3rd PHx (Fig. 6E). The results indicate that FXIII deficiency and a lack of hepatic fibrin cross-linking are coupled to reduced hepatocyte proliferation.
Figure 6. FXIII promotes hepatocyte proliferation after PHx.

Male and female wild-type mice and FXIII-A−/− mice underwent 2/3rd partial hepatectomy (PHx (see Methods) and livers were collected 48 hours later. Representative photomicrographs showing immunohistochemical labeling of Ki67-positive (brown, DAB) hepatocytes in (A) wild-type (WT) and (B) FXIII-A−/− mice. (C) Percentage Ki67+ hepatocytes was quantified (see Methods) and (D) Liver/body weight ratio. (E) Observed mortality. Results from individual mice are plotted and bars represent mean ± standard error of mean. Male mice are shown as circles, whereas female mice are shown as squares. *p<0.05, ** p<0.01.
Discussion
Partial hepatectomy induces rapid intrahepatic activation of the coagulation cascade in the regenerating liver remnant7 and hepatic accumulation of fibrin(ogen) has been linked to hepatocyte proliferation and liver regeneration in both humans and mice7, 8. Compared to liver regeneration induced by standard 2/3rd PHx, we found that crosslinking of insoluble hepatic fibrin was altered in mice after 90% PHx, an experimental setting of hepatic dysfunction/failed regeneration. Plasma levels of the transglutaminase FXIIIa, which can crosslink fibrin, were reduced pre- and post-operatively in patients who developed post-hepatectomy liver dysfunction compared to patients that did not develop liver dysfunction. Notably, we found that complete FXIII-A deficiency in mice dramatically reduced hepatic levels of cross-linked fibrin, attenuated hepatic platelet accumulation, and reduced hepatocyte proliferation in mice after standard 2/3rd PHx. These results suggest that reduced FXIIIa activity or altered FXIII-directed fibrin(ogen) crosslinking are associated with reduced liver regeneration and liver dysfunction post-PHx.
Ten to fifteen percent of patients have insufficient regeneration and develop liver dysfunction post-hepatectomy. Intraoperative hepatic fibrin(ogen) deposition, evident in the liver remnant in the first few hours after portal vein ligation, is diminished in patients that develop liver dysfunction7. We sought to uncover which aspect of intrahepatic hemostasis was ‘failing’ in patients that ultimately developed liver dysfunction. One potential explanation for altered fibrin(ogen) accumulation in the liver remnant of patients who ultimately developed liver dysfunction is relatively low pre-operative plasma fibrinogen levels. However, pre-operative fibrinogen concentration was not reduced in patients that developed liver dysfunction compared to those that did not, as measured by ELISA (Fig. 4B) or by Clauss assay, as described previously7. Post-operative changes in plasma fibrinogen may also portend liver dysfunction7, likely reflecting a reduction in de novo expression of fibrinogen by the liver, and perhaps a reduction in continued hepatic fibrinogen deposition. The collective consequences of these and other hemostatic changes could ultimately also connect to the requirement for post-operative transfusion. We also considered the possibility that coagulation activity (e.g., thrombin generation) could be reduced after PHx. However, biomarkers of coagulation activation were roughly equivalent after standard (2/3rd) and 90% PHx in mice. Moreover, there was no association of TAT complexes or prothrombin fragment 1+2 with the propensity to develop liver dysfunction in patients. Thus, a reduction in fibrin(ogen) accumulation in the liver remnant cannot be ascribed to a relative lack of fibrinogen or a failure to generate sufficient amounts of thrombin.
Despite equivalent coagulation activation after standard PHx (2/3rd) and 90% PHx in mice, altered hepatic fibrin cross-linking was evident in the liver remnant 30 min after 90% PHx. The mechanistic basis for this observation is unclear, but we hypothesize this is connected to FXIII, as we found that FXIII was largely responsible for fibrin cross-linking in the liver after PHx in mice. Moreover, we observed reduced FXIIIa activity in patients that developed liver dysfunction compared to patients that did not develop liver dysfunction after PHx. The mechanistic basis for this reduction and how it may potentially relate to patient demographics (Supplemental Table 3), such as surgical differences or tumor type, is not yet known. FXIII is a heterotetramer composed of regulatory (B) and catalytic (A) subunits synthesized by different cell types (i.e., FXIII-A [hematopoietic cells]32, 33; FXIII-B [hepatocytes]32, 34). Deficiency in each FXIII subunit impacts the plasma concentration of the other16, 35–37. Thus, altered expression of either FXIII-A or FXIII-B could explain reduced FXIIIa activity in PHx patients who developed liver dysfunction. Prior transcriptomic analysis did not uncover changes in F13B or F13A1 in livers of patients who did or did not develop liver dysfunction after PHx38. Additional possibilities worth exploring include extrahepatic alterations in FXIII-A production, as may occur in inflammatory disease,13 or the presence of a FXIII inhibitor.
FXIII-deficient mice displayed a substantial reduction in hepatocyte proliferation after PHx. The precise mechanisms linking FXIII to liver regeneration are not known and additional studies are required to pinpoint downstream substrates driving liver regeneration after PHx. Reduced FXIII-dependent fibrin cross-linking may favor premature fibrinolysis. Notably, the HeLiX (Hemorrhage During Liver Resection: traneXamic Acid) trial (NCT02261415) seeks to determine the beneficial effect of tranexamic acid on bleeding during partial liver resection but may very well hint on related proregenerative effects. Notably, FXIIIa cross-links multiple proteins and contributes not only to hemostasis, but also to wound healing14, leaving fibrinogen-independent effects of FXIII fully plausible. Notably, acute depletion of plasma fibrinogen with ancrod also reduced hepatocyte proliferation after PHx7. Moreover, FXIII deficiency reduced fibrin cross-linking and insoluble fibrin accumulation after PHx. The reduction in fibrin cross-linking in FXIII-deficient mice after PHx was associated with a marked reduction in hepatic platelet accumulation. This is important, because multiple studies support the concept that rapid (but transient) platelet accumulation drives liver regeneration in mice and humans30, 31. Although multiple mechanisms alter platelet function in the context of liver regeneration39, FXIII-induced cross-linking stabilizes fibrin40 and this may be critical to drive platelet accumulation in the early moments after PHx. Moreover, multiple studies have shown that FXIII directly contributes to platelet adhesion to fibrin and drives platelet responses secondary to fibrin(ogen)-integrin αIIbβ3 engagement41–43. In addition to platelets, fibrin engagement of leukocyte β2 integrins44 could contribute to liver regeneration by modulating effector functions of either neutrophils or macrophages, both cell types linked to liver regeneration in mice and humans45, 46. Collectively, multiple mechanisms support our results and the concept that FXIII-mediated fibrin cross-linking supports early hepatic platelet accumulation critical for liver regeneration.
In summary, we uncovered a failure of fibrin cross-linking in experimental PHx-induced liver dysfunction and found that complete FXIII deficiency substantially reduced hepatocyte proliferation after standard 2/3rd PHx in mice. Interestingly, plasma FXIII activity was reduced in patients who developed liver dysfunction after PHx compared to those who did not, even preoperatively. The precise basis for this reduction and impact of underlying disease states (e.g., steatotic liver disease) on plasma FXIIIa activity could help identify patients at risk of PHLF. Importantly, correction of plasma FXIII plasma levels could be accomplished using FDA-approved plasma-purified or recombinant FXIII, each of which could be repurposed and administered perioperatively.
Supplementary Material
Acknowledgements and funding.
This research was supported by grants from the National Institutes of Health (NIH) to JPL (R01 DK122813) and support from the USDA National Institute of Food and Agriculture to JPL. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the USDA.
COI Disclosure:
ZW, DG, JA, LGP, HC, AK, BL, LB, AA, PS, TL, and JPL declare no relevant COI for the manuscript beyond research support from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Fausto N, Campbell JS, Riehle KJ: Liver regeneration. Hepatology 2006, 43:S45–53. [DOI] [PubMed] [Google Scholar]
- [2].Mullen JT, Ribero D, Reddy SK, Donadon M, Zorzi D, Gautam S, Abdalla EK, Curley SA, Capussotti L, Clary BM, Vauthey JN: Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg 2007, 204:854–62; discussion 62–4. [DOI] [PubMed] [Google Scholar]
- [3].Michalopoulos GK, Bhushan B: Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol 2021, 18:40–55. [DOI] [PubMed] [Google Scholar]
- [4].Mitchell C, Willenbring H: A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nature protocols 2008, 3:1167–70. [DOI] [PubMed] [Google Scholar]
- [5].Michalopoulos GK: Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. The American journal of pathology 2010, 176:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nevzorova YA, Tolba R, Trautwein C, Liedtke C: Partial hepatectomy in mice. Laboratory animals 2015, 49:81–8. [DOI] [PubMed] [Google Scholar]
- [7].Groeneveld D, Pereyra D, Veldhuis Z, Adelmeijer J, Ottens P, Kopec AK, Starlinger P, Lisman T, Luyendyk JP: Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans. Blood 2019, 133:1245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beier JI, Guo L, Ritzenthaler JD, Joshi-Barve S, Roman J, Arteel GE: Fibrin-mediated integrin signaling plays a critical role in hepatic regeneration after partial hepatectomy in mice. Ann Hepatol 2016, 15:762–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R: Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg 2007, 31:808–16. [DOI] [PubMed] [Google Scholar]
- [10].Starlinger P, Haegele S, Offensperger F, Oehlberger L, Pereyra D, Kral JB, Schrottmaier WC, Badrnya S, Reiberger T, Ferlitsch A, Stift J, Luf F, Brostjan C, Gruenberger T, Assinger A: The profile of platelet alpha-granule released molecules affects postoperative liver regeneration. Hepatology 2016, 63:1675–88. [DOI] [PubMed] [Google Scholar]
- [11].Lesurtel M, Graf R, Aleil B, Walther DJ, Tian Y, Jochum W, Gachet C, Bader M, Clavien PA: Platelet-derived serotonin mediates liver regeneration. Science (New York, NY) 2006, 312:104–7. [DOI] [PubMed] [Google Scholar]
- [12].Starlinger P, Pereyra D, Haegele S, Braeuer P, Oehlberger L, Primavesi F, Kohler A, Offensperger F, Reiberger T, Ferlitsch A, Messner B, Beldi G, Staettner S, Brostjan C, Gruenberger T: Perioperative von Willebrand factor dynamics are associated with liver regeneration and predict outcome after liver resection. Hepatology 2018, 67:1516–30. [DOI] [PubMed] [Google Scholar]
- [13].Dull K, Fazekas F, Torocsik D: Factor XIII-A in Diseases: Role Beyond Blood Coagulation. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Alshehri FSM, Whyte CS, Mutch NJ: Factor XIII-A: An Indispensable “Factor” in Haemostasis and Wound Healing. Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Prasad JM, Gorkun OV, Raghu H, Thornton S, Mullins ES, Palumbo JS, Ko YP, Hook M, David T, Coughlin SR, Degen JL, Flick MJ: Mice expressing a mutant form of fibrinogen that cannot support fibrin formation exhibit compromised antimicrobial host defense. Blood 2015, 126:2047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Souri M, Koseki-Kuno S, Takeda N, Degen JL, Ichinose A: Administration of factor XIII B subunit increased plasma factor XIII A subunit levels in factor XIII B subunit knock-out mice. Int J Hematol 2008, 87:60–8. [DOI] [PubMed] [Google Scholar]
- [17].Mitchell C, Willenbring H: Addendum: A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc 2014, 9. [DOI] [PubMed] [Google Scholar]
- [18].Myronovych A, Murata S, Chiba M, Matsuo R, Ikeda O, Watanabe M, Hisakura K, Nakano Y, Kohno K, Kawasaki T, Hashimoto I, Shibasaki Y, Yasue H, Ohkohchi N: Role of platelets on liver regeneration after 90% hepatectomy in mice. J Hepatol 2008, 49:363–72. [DOI] [PubMed] [Google Scholar]
- [19].Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C, Banting S, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Yokoyama Y, Fan ST, Nimura Y, Figueras J, Capussotti L, Buchler MW, Weitz J: Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011, 149:713–24. [DOI] [PubMed] [Google Scholar]
- [20].Groeneveld DJ, Poole LG, Bouck EG, Schulte A, Wei Z, Williams KJ, Watson VE, Lisman T, Wolberg AS, Luyendyk JP: Robust coagulation activation and coagulopathy in mice with experimental acetaminophen-induced liver failure. J Thromb Haemost 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Byrnes JR, Wilson C, Boutelle AM, Brandner CB, Flick MJ, Philippou H, Wolberg AS: The interaction between fibrinogen and zymogen FXIII-A2B2 is mediated by fibrinogen residues gamma390–396 and the FXIII-B subunits. Blood 2016, 128:1969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ariens RA, Kohler HP, Mansfield MW, Grant PJ: Subunit antigen and activity levels of blood coagulation factor XIII in healthy individuals. Relation to sex, age, smoking, and hypertension. Arterioscler Thromb Vasc Biol 1999, 19:2012–6. [DOI] [PubMed] [Google Scholar]
- [23].Kopec AK, Joshi N, Cline-Fedewa H, Wojcicki AV, Ray JL, Sullivan BP, Froehlich JE, Johnson BF, Flick MJ, Luyendyk JP: Fibrin(ogen) drives repair after acetaminophen-induced liver injury via leukocyte alphaMbeta2 integrin-dependent upregulation of Mmp12. J Hepatol 2017, 66:787–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Poole LG, Pant A, Baker KS, Kopec AK, Cline-Fedewa HM, Iismaa SE, Flick MJ, Luyendyk JP: Chronic liver injury drives non-traditional intrahepatic fibrin(ogen) crosslinking via tissue transglutaminase. J Thromb Haemost 2019, 17:113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pieters M, Wolberg AS: Fibrinogen and fibrin: An illustrated review. Res Pract Thromb Haemost 2019, 3:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Makino H, Togo S, Kubota T, Morioka D, Morita T, Kobayashi T, Tanaka K, Shimizu T, Matsuo K, Nagashima Y, Shimada H: A good model of hepatic failure after excessive hepatectomy in mice. J Surg Res 2005, 127:171–6. [DOI] [PubMed] [Google Scholar]
- [27].Wolberg AS: Thrombin generation and fibrin clot structure. Blood Rev 2007, 21:131–42. [DOI] [PubMed] [Google Scholar]
- [28].Byrnes JR, Wolberg AS: Newly-Recognized Roles of Factor XIII in Thrombosis. Semin Thromb Hemost 2016, 42:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Koseki-Kuno S, Yamakawa M, Dickneite G, Ichinose A: Factor XIII A subunit-deficient mice developed severe uterine bleeding events and subsequent spontaneous miscarriages. Blood 2003, 102:4410–2. [DOI] [PubMed] [Google Scholar]
- [30].Kirschbaum M, Jenne CN, Veldhuis ZJ, Sjollema KA, Lenting PJ, Giepmans BNG, Porte RJ, Kubes P, Denis CV, Lisman T: Transient von Willebrand factor-mediated platelet influx stimulates liver regeneration after partial hepatectomy in mice. Liver Int 2017, 37:1731–7. [DOI] [PubMed] [Google Scholar]
- [31].Lisman T, Porte RJ: Mechanisms of platelet-mediated liver regeneration. Blood 2016, 128:625–9. [DOI] [PubMed] [Google Scholar]
- [32].Wolpl A, Lattke H, Board PG, Arnold R, Schmeiser T, Kubanek B, Robin-Winn M, Pichelmayr R, Goldmann SF: Coagulation factor XIII A and B subunits in bone marrow and liver transplantation. Transplantation 1987, 43:151–3. [DOI] [PubMed] [Google Scholar]
- [33].Beckers CML, Simpson KR, Griffin KJ, Brown JM, Cheah LT, Smith KA, Vacher J, Cordell PA, Kearney MT, Grant PJ, Pease RJ: Cre/lox Studies Identify Resident Macrophages as the Major Source of Circulating Coagulation Factor XIII-A. Arterioscler Thromb Vasc Biol 2017, 37:1494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Consortium GT: The Genotype-Tissue Expression (GTEx) project. Nat Genet 2013, 45:580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Strilchuk AW, Meixner SC, Leung J, Safikhan NS, Kulkarni JA, Russell HM, van der Meel R, Sutherland MR, Owens AP, Palumbo JS, Conway EM, Pryzdial ELG, Cullis PR, Kastrup CJ: Sustained depletion of FXIII-A by inducing acquired FXIII-B deficiency. Blood 2020, 136:2946–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yorifuji H, Anderson K, Lynch GW, Van de Water L, McDonagh J: B protein of factor XIII: differentiation between free B and complexed B. Blood 1988, 72:1645–50. [PubMed] [Google Scholar]
- [37].Byrnes JR L TK, Sharaby S, Campbell RA, Dobson D, Holle LA, Luo M, Kangro K, Homeister J, Aleman M, Luyendyk JP, Kerlin BA, Dumond JB, Wolberg AS: Reciprocal stabilization of coagulation factor XIII-A and -B subunits determines plasma FXIII concentration. Blood 2023, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Starlinger P, Brunnthaler L, McCabe C, Pereyra D, Santol J, Steadman J, Hackl M, Skalicky S, Hackl H, Gronauer R, O’Brien D, Kain R, Hirsova P, Gores GJ, Wang C, Gruenberger T, Smoot RL, Assinger A: Transcriptomic landscapes of effective and failed liver regeneration in humans. JHEP Rep 2023, 5:100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Haegele S, Offensperger F, Pereyra D, Lahner E, Assinger A, Fleischmann E, Gruenberger B, Gruenberger T, Brostjan C, Starlinger P: Deficiency in thrombopoietin induction after liver surgery is associated with postoperative liver dysfunction. PLoS One 2015, 10:e0116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wolberg AS, Sang Y: Fibrinogen and Factor XIII in Venous Thrombosis and Thrombus Stability. Arterioscler Thromb Vasc Biol 2022, 42:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lahav J, Tvito A, Bagoly Z, Dardik R, Inbal A: Factor XIII improves platelet adhesion to fibrinogen by protein disulfide isomerase-mediated activity. Thromb Res 2013, 131:338–41. [DOI] [PubMed] [Google Scholar]
- [42].Kasahara K, Kaneda M, Miki T, Iida K, Sekino-Suzuki N, Kawashima I, Suzuki H, Shimonaka M, Arai M, Ohno-Iwashita Y, Kojima S, Abe M, Kobayashi T, Okazaki T, Souri M, Ichinose A, Yamamoto N: Clot retraction is mediated by factor XIII-dependent fibrinalphaIIbbeta3-myosin axis in platelet sphingomyelin-rich membrane rafts. Blood 2013, 122:3340–8. [DOI] [PubMed] [Google Scholar]
- [43].Mattheij NJ, Swieringa F, Mastenbroek TG, Berny-Lang MA, May F, Baaten CC, van der Meijden PE, Henskens YM, Beckers EA, Suylen DP, Nolte MW, Hackeng TM, McCarty OJ, Heemskerk JW, Cosemans JM: Coated platelets function in platelet-dependent fibrin formation via integrin alphaIIbbeta3 and transglutaminase factor XIII. Haematologica 2016, 101:427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Flick MJ, Du X, Degen JL: Fibrin(ogen)-alpha M beta 2 interactions regulate leukocyte function and innate immunity in vivo. Exp Biol Med (Maywood) 2004, 229:1105–10. [DOI] [PubMed] [Google Scholar]
- [45].Dong Z, Wei H, Sun R, Tian Z: The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol 2007, 4:241–52. [PubMed] [Google Scholar]
- [46].Brandel V, Schimek V, Gober S, Hammond T, Brunnthaler L, Schrottmaier WC, Mussbacher M, Sachet M, Liang YY, Reipert S, Ortmayr G, Pereyra D, Santol J, Rainer M, Walterskirchen N, Ramos C, Gerakopoulos V, Rainer C, Spittler A, Weiss T, Kain R, Messner B, Gruenberger T, Assinger A, Oehler R, Starlinger P: Hepatectomy-induced apoptotic extracellular vesicles stimulate neutrophils to secrete regenerative growth factors. J Hepatol 2022, 77:1619–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


