Osteoarthritis (OA) is the most common form of arthritis and is among the top 10 conditions contributing to Years Lived with Disability (YLDs) 1. To date, no treatments are approved that slow disease progression. Options for treatments that reduce joint pain such as nonsteroidal anti-inflammatory drugs (NSAIDs) are successful in some but of limited use because of toxicity 2. Exercise or weight loss are effective but long term adherence is poor 3. Rates of total knee replacement (TKR) are rising rapidly 4 suggesting that nonsurgical treatments have not successfully alleviated patients’ pain and disability. The burden of OA is comparable in Europe and Asia. New treatments for OA are desperately needed.
Animal models ≠ Human OA
Animal models in which new treatments are screened do not mirror well the disease process in human OA. In most animal models, disease is induced by causing a major untreated joint injury such as damaging the medial meniscus or transecting the anterior cruciate ligament (ACL). Treatments are then tested to see if they prevent OA development.
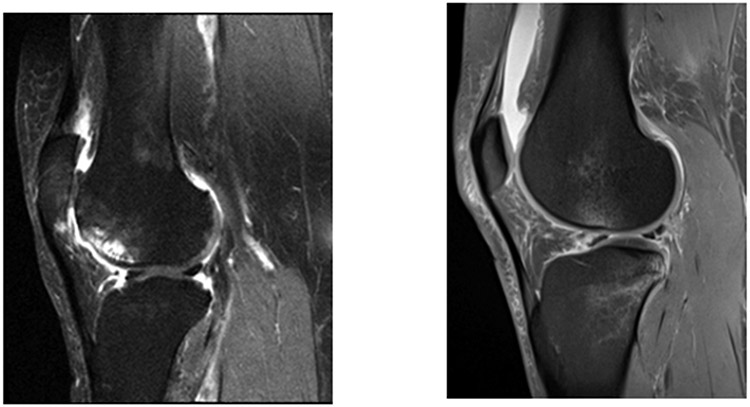
In humans, most cases of OA are not triggered by identifiable injury and evolve over decades in middle and older years. Abnormal joint mechanics develop, and joint shape and other structural changes evolve, resulting in an often irreversibly damaged joint. (see figure 1a for MRI of typical older onset knee with OA). Muscle weakness and malignment are usually present and, if the patient has knee pain, there may be changes in nervous system pathways that magnify pain.
Figure 1a.
Fat Suppressed sagittal MRI of a Typical Knee with KL Grade OA. Knee shows synovitis above and below the patella (in Hoffa’s fat pad), bone marrow lesions (in anterior femur with cysts) and in inferior patella, cartilage loss in patella nad femoral trocheal with thinning in the weight bearing tibial and femoral surfaces and bone attrition in trochela. This patient, if they had sufficient pain, would be eligible for a trial testing treatment slowing progression or cartillage protection. Figure 1b; Fat suppressed sagittal MRI of a badly injured knee postACL tear (tear not shown). Note synovitis and fluid in suprapatellar pouch, tear in posterior horn of lateral menisus, bone marrow lesion with overlying chondral damage. Compared to 1a, no cysts or bone attrition. Cartillage mostly preserved and thick, no osteophytes
One subtype of human OA, accounting for 10-15% of knee OA, develops after major joint injury 6. The most common such injury is an ACL tear which mirrors the changes seen in the animal model of OA but over a much longer timeframe (see figure 1b for MRI of knee with ACL tear before surgery). This disparity between the ACL tear which mimics the animal model for OA and older onset disease may help explain the failure of animal models to identify effective treatments for older onset human OA. Unfortunately, because posttraumatic OA usually takes a decade or longer to develop after joint injury, making it challenging to conduct trials with a sufficiently long follow-up period to test treatments.
However, while most patients recover after sustaining a major joint injury like an ACL tear, a few experience persistent pain and develop OA. We suggest that sufficient numbers of such patients exist and could be identified in advance to form a high-risk group in whom treatments to prevent disease could be tested.
In considering whether changes of OA develop soon after ACL rupture (with or without ACL surgery), we shall adopt the concept that OA consists of pain and structural changes in all joint tissues, especially cartilage loss. Because MRI detects evidence of disease much earlier and more accurately than x-rays 5 6,7, . its use has made evaluating early changes of disease possible.
Evidence of Joint Pain soon after ACL tears
In 2005, Spindler and colleagues created the MOON cohort, a cohort of 2340 persons undergoing ACL reconstructions (ACLR) after traumatic tears with validated patient–reported outcome measures obtained at baseline, 2, 6 and 10 years. A smaller nested cohort of 433 underwent imaging at 2 and 10 years 8,9. The goals of MOON were to characterize long term symptoms (pain) and structural outcomes and factors that increase the risk of poor outcomes. In analyses of 2-year data surveying enrolled subjects using the Knee Injury and Osteoarthritis Outcome Score (KOOS) pain scale, MOON investigators 10,11 reported that of the 80% of ACL reconstruction patients who responded, 26% had at least moderate knee pain on daily activities especially stair climbing and walking and that 16.6% had KOOS pain scores ≤80 (scores on 0-100 scale where 100 is no pain). These numbers suggest that mild to moderate pain is not rare after ACLR. Jones and colleagues have subsequently shown that participants in MOON at highest risk of these pain outcomes at 2 years and later include those whose injuries included chondral defects (OR = 1.52 (95% CI 1.17,1.97), those with elevated BMIs (per unit increase OR = 1.05 (95% CI 1.02,1.07) and especially those with unresolved knee pain at the time of ACLR (OR per unit decrease in pain (0-100 scale) = 0.96 (0.96,0.97). Other studies have also suggested older age and concomitant meniscal injuries increase risk.12. Using the MOON risk factors to select persons at high risk of later pain, we could assemble a cohort at high risk of substantial post ACLR pain. In a cross-cultural comparison of MOON with European databases the baseline KOOS pain was nearly identical 13 and worse pain at baseline was a risk factor for more pain at 10 year in the the European databases 14. However, other nonKOOS pain outcomes in European cohorts may be better at 2 – 5 years than those from MOON.15,16. Overall, given nearly identical baseline KOOS pain and that worse pain at baseline was identified as risk factor for higher pain at 2,6, and 10 years in both MOON and Swedish registry the results should be generalizable.
Evidence of Radiographic OA and Cartilage Loss Developing Soon after ACL Tears
Most long-term studies of persons with ACL tears report that, unlike persistent pain, radiographic changes of OA develop often. A recent international cohort study of 293 adults with ACL tears using tibiofemoral knee x-rays 17 showed that when restricted to the most often used threshold for OA of >=grade 2 (osteophyte and probable narrowing), 21.4% had OA at 5 years. Recent data suggest that this number could be increased by selecting those at highest risk of later OA. In a comprehensive meta-analysis of studies examining radiographic OA after ACL tears, Whittaker et al 18 reported that cartilage injury (OR= 2.31 (95% CI 1.35,3.94)) and partial or complete meniscectomy (OR’s range from 1.87 to 3.14 (95% CI’s 1.45,4.48)) at the time of ACLR increased the risk of later radiographic OA. Older age (OR = 1.36 (95% CI 0.95, 1.96)) and higher BMI (OR = 1.37 (95% CI: 0.83, 2.26)) were also associated with a nonsignficant increased risk of later OA. While the time frame for OA development of studies included in this meta-analysis was mostly 5 years, the MOON cohort released data on risk factors for radiographic OA at 2-3 years after ACLR 19 showing that older age, higher BMI, chondral damage and meniscal surgery were all risk factors for OA development.
While these studies provide evidence that radiographic changes of OA often develop after ACL tears or ACLR, recent data from the KANON study 9 suggest that at 2 years, over 10% of knees developed MRI cartilage loss in the medial and a slightly greater percent experienced loss in the lateral compartment.
Can We Examine Both Pain and Structural Damage Soon After ACL Tear?
Risk factors for pain and for cartilage loss soon after ACL tear are similar (see table 1), suggesting that restricting eligibility of ACL patients to those at high risk of both cartilage loss and pain could be accomplished using the same selection factors. That does not necessarily mean that the patients who experience these outcomes are the same. Older ACLR patients who might be overweight with pain persisting after their ACL tear would be preferred. The presence of chondral damage and/or meniscal damage with their ACL tear would put patients at even higher risk. Only a few patients undergoing ACLR will have all of these risk factors, but a prudent selection of patients at high risk could maximize the numbers of patients eligible while restricting the experiment to those at high risk of outcomes. Enriching trials as suggested here with those at high risk of outcomes has tradeoffs in terms of costs (e.g. fewer recruited at each site might translate into more sites needed) and benefits (e.g. higher number of outcomes.)
Table 1.
Risk Factors for Pain and Cartilage Loss after ACL Tear
| Risk Factors for Pain | Risk Factors for Cartilage Loss |
|---|---|
| Chondral Damage | Chondral Damage |
| Mensical Damage* | Meniscal Damage |
| Older Age | Older Age |
| Higher BMI | Higher BMI |
| Persistent Knee Pain after Tear | ???? |
While not seen in recent analysis, unpublished analyses from MOON show that severe meniscal damage (not as common as meniscal tears) is highly associated with pain. Further, a meta-analysis has shown a strong association between meniscal tears and later OA12
Opportunities Created by Adopting a FastOA Approach to Identify OA Treatments
The US Arthritis Foundation launched the FastOA initiative with FastOA defined as the rapid development of OA in those who have sustained a major joint injury. The initiative involves identifying those at high risk of FastOA and testing treatments to prevent disease.
The approach offers the opportunity to prevent disease and is especially valuable in targeting young adults who, after a knee injury, may have significant joint pain and disability for many years before they become eligible for joint replacement 20.
While there are ongoing small trials testing treatments in those who undergoing ACL reconstruction, the approach suggested is unique in selecting those at high risk of later OA. If those identified by MOON as being at high risk of either cartilage loss or clinically significant pain over two years are selected for study, the sample size needed (alpha ≤ 0.05 two sided, 80-90% power) for a randomized trial will range from 300-800 depending on the anticipated effect size of treatment and the outcomes chosen. The numbers needed are lower if those at higher risk of either pain or cartilage loss outcomes are enrolled and higher with a broader distribution of those with ACL tears many of whom may not get either of these outcomes. If a single primary outcomee (pain or cartilage loss) is selected sample size requirements are roughly 30-40% less than if both pain and cartilage are considered as primary outcomes. This suggests this approach is feasible. For advantages and disadvantages of the approach, see table 2.
Table 2.
Advantages and Disadvantages of Proposed Approach
| Advantages | Disadvantages |
|---|---|
| Mimics animal models of OA where successful treatments have been identified. | Does not necessarily meet regulatory requirements in terms of pain or structure outcomes. |
| May identify treatment for high risk subgroup who suffer for many years with pain | May not identify treatment for older onset OA |
| Data suggests sample size for trial achieveable if high risk patients selected | Actual trial needed to show high risk patients have sufficient number of outcomes to test treatments |
The initiative offers three new ways of approaching treatment development in OA. First, it may provide a model approach for other investigators to test treatments. Second, to the extent that it identifies an effective therapy that prevents posttraumatic OA, it opens a door to testing this therapy in later onset disease. As noted earlier, those with posttraumatic OA constitute a minority of those with OA, and it is unclear whether an effective treatment for this subgroup will be effective for those with more advanced pathology at presentation. Lastly, both imaging and biochemical biomarkers could be developed and validated using FastOA trials, and these could then be used to identify both persons at high risk of rapid development or progression of OA and those likely to respond to treatment including pharmacological and exercise interventions. Given the repeated, expensive and discouraging past failures in the development of effective treatments for OA, a new approach is needed that focuses development efforts on those with early disease.
Acknowledgements:
We are grateful to Dr. Carl Winalski for providing images.
Supported by the Arthritis Foundation and by NIH P30 AR072571
Footnotes
Patient and Public Involvement: Patients and public were not involved in the development or writing of this viewpoint.
Competing Interests: None of the authors declare competing interests.
Contributor Information
David T. Felson, Boston University, Boston, MA USA.
Martin Lotz, Scripps Clinic, LaJolla, CA, USA.
Yuxuan Jin, The Cleveland Clinic, Cleveland, OH.
Morgan Jones, The Cleveland Clinic, Cleveland, OH; The Orthopaedic and Arthritis Center for Outcomes Research and Department of Orthopaedic Surgery, Brigham and Women’s Hospital, Boston, MA USA.
Jason S. Kim, Arthritis Foundation, Atlanta, GA USA.
Kurt Spindler, The Cleveland Clinic, Weston, FL USA.
Data Availability:
The MOON data can be requested from Drs. Spindler or Jones.
Literature Cited
- 1.Long H, Liu Q, Yin H, et al. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis & Rheumatology. 2022;74(7):1172–1183. doi: 10.1002/art.42089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT. Safety of Nonsteroidal Antiinflammatory Drugs. N Engl J Med. 2016;375(26):2595–2596. doi: 10.1056/NEJMe1614257 [DOI] [PubMed] [Google Scholar]
- 3.Chua SD, Messier SP, Legault C, Lenz ME, Thonar EJMA, Loeser RF. Effect of an exercise and dietary intervention on serum biomarkers in overweight and obese adults with osteoarthritis of the knee. Osteoarthritis Cartilage. 2008;16(9):1047–1053. doi: 10.1016/j.joca.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ackerman IN, Bohensky MA, de Steiger R, et al. Substantial rise in the lifetime risk of primary total knee replacement surgery for osteoarthritis from 2003 to 2013: an international, population-level analysis. Osteoarthritis Cartilage. 2017;25(4):455–461. doi: 10.1016/j.joca.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 5.Cibere J, Zhang H, Thorne A, et al. Association of clinical findings with pre-radiographic and radiographic knee osteoarthritis in a population-based study. Arthritis Care Res (Hoboken). 2010;62(12):1691–1698. doi: 10.1002/acr.20314 [DOI] [PubMed] [Google Scholar]
- 6.Guermazi A, Niu J, Hayashi D, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roemer FW, Felson DT, Stefanik JJ, et al. Heterogeneity of cartilage damage in Kellgren and Lawrence grade 2 and 3 knees: the MOST study. Osteoarthritis Cartilage. 2022;30(5):714–723. doi: 10.1016/j.joca.2022.02.614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vega JF, Spindler KP. To MOON and Back: Lessons Learned and Experience Gained Along the Way. Clin Sports Med. 2018;37(3):495–503. doi: 10.1016/j.csm.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roemer FW, Lohmander LS, Englund M, Guermazi A, Akesson A, Frobell R. Development of MRI-defined structural tissue damage after anterior cruciate ligament injury over 5 Years: The KANON Study. Radiology. 2021;299(2):383–393. doi: 10.1148/RADIOL.2021202954 [DOI] [PubMed] [Google Scholar]
- 10.Jones MH, Spindler KP. Risk factors for radiographic joint space narrowing and patient reported outcomes of post-traumatic osteoarthritis after ACL reconstruction: Data from the MOON cohort. Journal of Orthopaedic Research. 2017;35(7):1366–1374. doi: 10.1002/jor.23557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasserstein D, Huston LJ, Nwosu S, Spindler KP. KOOS pain as a marker for significant knee pain two and six years after primary ACL reconstruction: A Multicenter Orthopaedic Outcomes Network (MOON) prospective longitudinal cohort study. Osteoarthritis Cartilage. 2015;23(10):1674–1684. doi: 10.1016/j.joca.2015.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnussen RA, Mansour AA, Carey JL, Spindler KP. Meniscus Status at ACL Reconstruction Is Associated with the Presence of Radiographic Signs of Osteoarthritis at 5-10 Year Follow-up: A Systematic Review. [DOI] [PMC free article] [PubMed]
- 13.Magnussen RA, Granan LP, Dunn WR, et al. Cross-cultural comparison of patients undergoing ACL reconstruction in the United States and Norway. Knee Surgery, Sports Traumatology, Arthroscopy. 2010;18(1):98–105. doi: 10.1007/s00167-009-0919-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamrin Senorski E, Svantesson E, Spindler KP, et al. Ten-Year Risk Factors for Inferior Knee Injury and Osteoarthritis Outcome Score After Anterior Cruciate Ligament Reconstruction: A Study of 874 Patients From the Swedish National Knee Ligament Register. Am J Sports Med. 2018;46(12):2851–2858. doi: 10.1177/0363546518788325 [DOI] [PubMed] [Google Scholar]
- 15.Ahldén M, Samuelsson K, Sernert N, Forssblad M, Karlsson J, Kartus J. The Swedish National Anterior Cruciate Ligament Register. Am J Sports Med. 2012;40(10):2230–2235. doi: 10.1177/0363546512457348 [DOI] [PubMed] [Google Scholar]
- 16.Failla MJ, Logerstedt DS, Grindem H, et al. Does Extended Preoperative Rehabilitation Influence Outcomes 2 Years After ACL Reconstruction? Am J Sports Med. 2016;44(10):2608–2614. doi: 10.1177/0363546516652594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arhos EK, Pohlig RT, di Stasi S, Risberg MA, Snyder-Mackler L, Silbernagel KG. Clinically relevant subgroups exist among athletes who have ruptured their anterior cruciate ligaments: A Delaware-Oslo Cohort Study . Arthritis Care Res (Hoboken). Published online January 16, 2023. doi: 10.1002/acr.25089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittaker JL, Losciale JM, Juhl CB, et al. Risk factors for knee osteoarthritis after traumatic knee injury: A systematic review and meta-analysis of randomised controlled trials and cohort studies for the OPTIKNEE Consensus. Br J Sports Med. 2022;56(24):1406–1421. doi: 10.1136/bjsports-2022-105496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones MH, Oak SR, Andrish JT, et al. Predictors of Radiographic Osteoarthritis 2 to 3 Years After Anterior Cruciate Ligament Reconstruction: Data From the MOON On-site Nested Cohort. Orthop J Sports Med. 2019;7(8):232596711986708. doi: 10.1177/2325967119867085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackerman IN, Bucknill A, Page RS, et al. The substantial personal burden experienced by younger people with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2015;23(8):1276–1284. doi: 10.1016/j.joca.2015.04.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The MOON data can be requested from Drs. Spindler or Jones.