Abstract
Background:
Studies have shown improved survival among individuals with cancer with higher levels of social support. Few studies have investigated social support and overall survival (OS) in individuals with advanced prostate cancer in an international cohort. We investigated the associations of marital status and living arrangements with OS among individuals with advanced prostate cancer in the International Registry for Men with Advanced Prostate Cancer (IRONMAN).
Methods:
IRONMAN is enrolling participants diagnosed with advanced prostate cancer (metastatic hormone-sensitive prostate cancer, mHSPC; castration resistant prostate cancer, CRPC) from 16 countries. Participants in this analysis were recruited between July 2017 and January 2023. Adjusting for demographics and tumor characteristics, the associations were estimated using Cox regression and stratified by disease state (mHSPC, CRPC), age (<70, 70+ years), and continent of enrollment (North America, Europe, Other).
Results:
We included 2,119 participants with advanced prostate cancer, of whom 427 died during up to 5 years of follow-up (median 6 months). Two-thirds had mHSPC. Most were married/in a civil partnership (79%) and 6% were widowed. Very few married participants were living alone (1%), while most unmarried participants were living alone (70%). Married participants had better OS than unmarried participants (adjusted HR: 1.44; 95% CI: 1.02, 2.02). Widowed participants had the worst survival compared to married individuals (adjusted HR: 1.89; 95% CI: 1.22, 2.94).
Conclusions:
Among those with advanced prostate cancer, unmarried and widowed participants had worse OS compared to married participants.
Impact:
This research highlighted the importance of social support in OS within this vulnerable population.
Introduction
Worldwide, prostate cancer is the second most common cancer in men, with almost 1.5 million new cases reported in 2020 (1,2). Prostate cancer caused over 375,000 deaths in 2020, being the leading cause of cancer death in approximately 50 countries and second in the United States (US) (1–3). Survival differs greatly by disease state; the five-year survival rate is nearly 100% for non-advanced prostate cancer yet around 30% for advanced prostate cancer (4). The two main categories of advanced prostate cancer are metastatic hormone-sensitive (mHSPC) and castration resistant prostate cancer (CRPC) (5). While international statistics are scarce, there are over 150,000 individuals with advanced prostate cancer in the US (6). This growing vulnerable population not only are burdened with worse survival but might also be uniquely susceptible or especially responsive to certain factors that impact overall survival (7).
One factor is the degree of social support available to these individuals, such as marital status or living arrangement (8,9). It is well-documented that social support plays an important role in cancer survival, where higher levels of social support are associated with better overall survival across cancer types (10–12). Many studies report protective relationships between being married and prostate cancer survival, specifically (10,13–16). The few studies assessing living arrangements report that individuals with prostate cancer who live alone experienced higher risk of all-cause mortality (17,18). Marital status and living arrangement also represent other social domains. To our knowledge, few studies have investigated this topic in individuals with advanced prostate cancer in an international prospective cohort.
We aimed to investigate the associations of marital status and living arrangement with overall survival among individuals with advanced prostate cancer in the International Registry for Men with Advanced Prostate Cancer (IRONMAN). We also conducted stratified analyses by disease state at enrollment, age at enrollment, and continent of enrollment. We hypothesized that married individuals with advanced prostate cancer would have better overall survival than unmarried individuals, and that unmarried individuals living alone would have the worst outcomes.
Materials and Methods
Study Design and Population
We conducted a prospective cohort study using the IRONMAN registry, an international cohort launched in 2016 that focuses on addressing research gaps in individuals with advanced prostate cancer (19,20). IRONMAN aims to recruit over 5,000 participants from the US, Canada, the United Kingdom (UK), Spain, Switzerland, Sweden, Ireland, Norway, Brazil, Australia, Nigeria, Jamaica, Kenya, and Barbados; South Africa and the Bahamas are pending activation. All recruited participants were newly diagnosed with advanced prostate cancer and could enroll either (a) within 90 days of initiating life-sustaining treatment for mHSPC or (b) within 90 days of initiating treatment for either non-metastatic or metastatic CRPC. Participants completed detailed questionnaires at baseline and were prospectively followed for up to five years for overall survival, clinically significant adverse events, comorbidities, patient-reported outcome measures (PROMs), and other outcome measures.
For this analysis, individuals recruited into IRONMAN between July 2017 and January 2023 were eligible (N = 3,091). We excluded those missing baseline data (N = 546), information on marital status or living arrangement (N = 214), or covariates (N = 187). We also excluded those living in a community home or nursing facility (N = 25), as their level of social support may be functionally different from those living with their relatives or roommates (21). In total, the final analytical sample included 2,119 participants (Figure 1). This research was approved by the Western Institutional Review Board (IRB). All participants provided written informed consent per each site’s local or reliant IRB.
Figure 1.
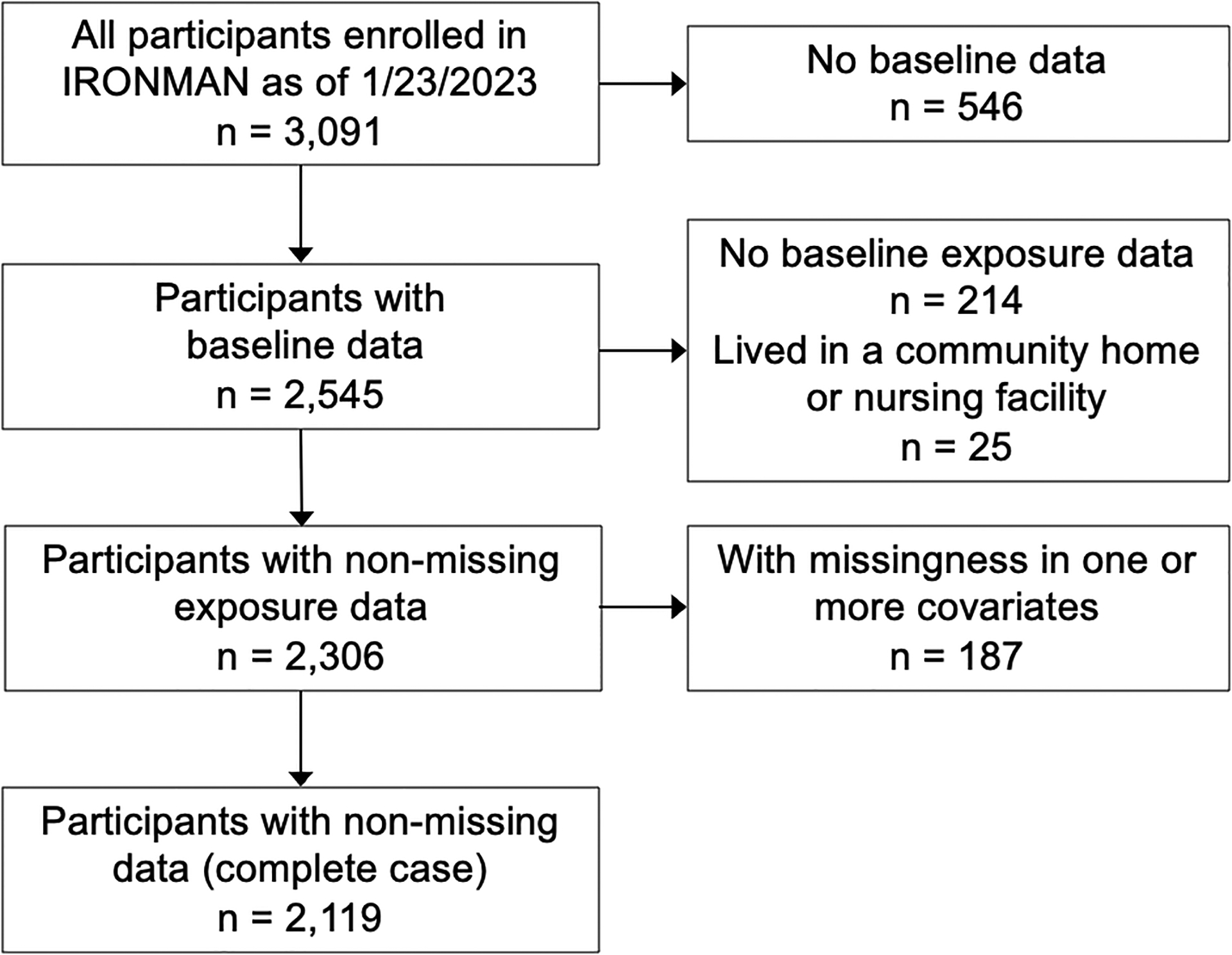
Inclusion and exclusion flowchart of participants in the IRONMAN Registry, 2017–2023. This flowchart illustrates the selection of eligible study participants from the IRONMAN Registry.
Marital Status and Living Arrangement
Marital status and living arrangement were used to measure the level of social support. Both variables were assessed at baseline via electronic patient-reported outcome measures (PROMs) (e-PROMS TrueNTH) or paper questionnaires. Participants reported current marital status as: married, partner/civil partnership, divorced/separated, widowed/surviving partner of civil partnership, or never married. Participants also reported current living arrangement by checking all of the following options that apply: alone, with wife/partner, with other family, assisted living, nursing home, retirement community, or other. Participants who chose “other” wrote in their living situation.
The first exposure was categorized as married (including those who are married or in a civil partnership) or not married (including those who are divorced/separated, widowed, or never married). We additionally examined marital status and living arrangement jointly. As almost all married participants were living with someone, we used three categories: married/not living alone, not married/not living alone (including those who live with at least one other family member or roommate), and not married/living alone.
All-cause Mortality
Follow up started from the date of enrollment and ended on the date of death, censoring due to loss to follow-up, or administrative censoring in January 2023, whichever occurred first. The outcome of interest was all-cause mortality. Both date and cause of death were ascertained from study sites (by linking data from electronic medical records (all sites), regional cancer registries (all sites), notifications of friends and family (all sites), the National Death Index (US sites only)) and physician questionnaires.
Statistical Analysis
We summarized baseline characteristics of the study population by marital status and living arrangement. To examine the relationship between the exposures and all-cause mortality, we conducted time-to-event analyses using Cox regression models for the following exposure definitions: (1) marital status only and (2) marital status and living arrangement jointly. We fit age-adjusted models and fully adjusted models, including living arrangement (living alone, not living alone), age at enrollment (continuous), disease state at enrollment (mHSPC, CRPC), continent of enrollment (North America, Europe, and other), self-reported race (white, non-white), employment status (not working, currently working), smoking status (current non-smoker, current smoker), family history of prostate cancer (yes, no), and prostate specific antigen (PSA) at enrollment (continuous) in the models. We reported hazard ratios (HR) and 95% confidence intervals (CI). We also conducted stratified, fully adjusted analyses by age at enrollment (<70 years old and ≥70 years old), disease state at enrollment (mHSPC and CRPC), and continent of enrollment (North America – US, Canada; Europe – UK, Ireland, Norway, Spain, Sweden, Switzerland; Other – Australia, Brazil, Nigeria, Jamaica, Kenya, Barbados). Lastly, we examined the association between marital status and overall survival using a finer categorization of marital status, which includes married/in a civil partnership, divorced/separated, widowed, and never married. To examine the sensitivity of our findings, we additionally adjusted for education level (less than some college, some college or more), Gleason score at enrollment (6 or less, 7, 8, 9–10), and ECOG (Eastern Cooperative Oncology Group) performance status.
Data Availability
The data generated in this study are not publicly available due to privacy of research participants but are available upon reasonable request from the corresponding author.
Results
This study included 2,119 IRONMAN participants with advanced prostate cancer, of whom 79% were married or in a civil partnership, 6% were widowed, 10% were separated or divorced, and 5% were never married. Few married participants lived alone (1%), while most unmarried participants lived alone (70%). Table 1 summarizes baseline characteristics of all participants, stratified by marital status and living arrangement. The median age at enrollment was 71 years (interquartile range (IQR): 65 – 76 years) and around 20% of the participants were non-white. Two thirds of participants had mHSPC (66%) compared to CRPC (34%).
Table 1.
Baseline characteristics at enrollment of individuals with advanced prostate cancer in IRONMAN, by marital status and living arrangement (N=2,119)
| MARRIED | NOT MARRIED | |||||
|---|---|---|---|---|---|---|
| Living alone (n = 20) | Not living alone (n = 1647) | Total (n = 1667) | Living alone (n = 317) | Not living alone (n = 135) | Total (n = 452) | |
| Age, years (median, IQR) | 73.5 (68.0 – 78.0) | 71.0 (65.0 – 76.0) | 71.0 (65.0 – 76.0) | 70.0 (64.0 – 77.0) | 69.0 (62.5 – 75.5) | 70.0 (63.0 – 76.0) |
| Disease state, n (%) | ||||||
| mHSPC | 14 (70) | 1077 (65) | 1091 (65) | 216 (68) | 94 (70) | 310 (69) |
| CRPC | 6 (30) | 570 (35) | 576 (35) | 101 (32) | 41 (30) | 142 (31) |
| Continent of enrollment, n (%) | ||||||
| North America | 8 (40) | 766 (46) | 774 (46) | 143 (45) | 59 (44) | 202 (45) |
| Europe | 10 (50) | 670 (41) | 680 (41) | 140 (44) | 55 (41) | 195 (43) |
| Other | 2 (10) | 211 (13) | 213 (13) | 34 (11) | 21 (16) | 55 (12) |
| Race, n (%) | ||||||
| White | 14 (70) | 1319 (80) | 1333 (80) | 259 (82) | 94 (70) | 353 (78) |
| Non-white | 6 (30) | 328 (20) | 334 (20) | 58 (18) | 41 (30) | 99 (22) |
| Employment status, n (%) | ||||||
| Not working/Retired | 15 (75) | 1185 (72) | 1200 (72) | 227 (72) | 98 (73) | 325 (72) |
| Currently working | 5 (25) | 462 (28) | 467 (28) | 90 (28) | 37 (27) | 127 (28) |
| Smoking status, n (%) | ||||||
| Non-smokers | 17 (85) | 1501 (91) | 1518 (91) | 1269 (85) | 113 (84) | 382 (85) |
| Current smokers | 3 (15) | 146 (9) | 149 (9) | 48 (15) | 22 (16) | 70 (15) |
| Family history of prostate cancer, n (%) | ||||||
| No | 18 (90) | 1318 (80) | 1336 (80) | 261 (82) | 114 (84) | 375 (83) |
| Yes | 2 (10) | 329 (20) | 331 (20) | 56 (18) | 21 (16) | 77 (17) |
| PSA, ng/ml (median, IQR) | 6.0 (2.9 – 31.1) | 5.4 (1.0 – 23.0) | 5.4 (1.0 – 23.0) | 8.6 (1.6 – 45.0) | 5.9 (0.8 – 26.5) | 7.3 (1.4 – 41.5) |
| Education level, n (%) a | ||||||
| Less than some college | 10 (56) | 732 (50) | 742 (50) | 167 (58) | 79 (63) | 246 (59) |
| Some college or more | 8 (44) | 744 (50) | 752 (50) | 123 (42) | 47 (37) | 170 (41) |
| Missing | 2 | 171 | 173 | 27 | 9 | 36 |
| Gleason Score, n (%) a | ||||||
| 6 or less | 1 (7) | 62 (4) | 63 (4) | 12 (5) | 3 (3) | 15 (4) |
| 7 | 5 (33) | 380 (26) | 385 (26) | 88 (33) | 36 (32) | 124 (33) |
| 8 | 3 (20) | 283 (20) | 286 (20) | 38 (14) | 20 (18) | 58 (15) |
| 9–10 | 6 (40) | 713 (50) | 719 (49) | 125 (48) | 53 (47) | 178 (47) |
| Missing | 5 | 209 | 214 | 54 | 23 | 77 |
| ECOG performance status, n (%) a | ||||||
| 0 | 12 (63%) | 838 (56%) | 850 (56%) | 152 (53%) | 57 (48%) | 209 (51%) |
| 1 | 5 (26%) | 541 (36%) | 546 (36%) | 114 (40%) | 51 (43%) | 165 (41%) |
| 2 | 2 (11%) | 79 (5%) | 81 (5%) | 19 (7%) | 10 (8%) | 29 (7%) |
| 3 | 0 (0%) | 32 (2%) | 32 (2%) | 2 (1%) | 1 (1%) | 3 (1%) |
| 4 | 0 (0%) | 4 (0%) | 4 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Missing | 1 | 153 | 154 | 30 | 16 | 46 |
Abbreviations: IRONMAN, International Registry for Men with Advanced Prostate Cancer; IQR, Interquartile range; mHSPC, Metastatic hormone-sensitive prostate cancer; CRPC, Castration resistant prostate cancer; PSA, Prostate specific antigen; ECOG, Eastern Cooperative Oncology Group.
Among those with non-missing data.
Married participants had lower PSA levels at enrollment, were less likely to be current smokers or have a family history of prostate cancer than unmarried participants but were otherwise comparable regarding age at enrollment, disease state, race, and employment status. Among unmarried participants, those living alone were more likely to be white and had higher PSA levels at enrollment compared to those who were not living alone.
Overall, 427 deaths occurred over a median follow-up of six months (IQR: 12 months; maximum: 60 months). Of these, 333 occurred in the married group, and 94 occurred in the unmarried group. Those who had CRPC and not enrolled from Europe had higher proportion of dying over follow up (Supplementary Table 1). Table 2 presents multivariable-adjusted HRs and 95% CIs for the association between marital status (not married vs. married as a reference group) and all-cause mortality overall and stratified by disease state, age, and continent of enrollment. In the fully adjusted model, individuals who were married/in a civil partnership had better overall survival compared to those unmarried (adjusted HR: 1.44; 95% CI: 1.02, 2.02). The protective association between being married and survival was stronger among those with mHSPC (adjusted HR: 1.63; 95% CI: 1.05, 2.53) than those with CRPC (adjusted HR: 1.14; 95% CI: 0.65, 2.00). The association was also stronger among those aged 70 or older (adjusted HR: 1.67; 95% CI: 1.08, 2.59) than those under 70 and stronger among those from North American sites (adjusted HR: 1.79; 95% CI: 1.09, 2.92).
Table 2.
The association between marital status and all-cause mortality overall and stratified by disease state, age, and continent of enrollment in IRONMAN (N=2,119), 2017–2023a
| Deaths, n | Married | Not Married | |
|---|---|---|---|
| Overall (age adjusted only) | 427 (Married: n=333 Not Married: n=94) |
1.00 (ref) | 1.07 (0.85, 1.35) |
| Overall (fully adjusted) b | 1.00 (ref) | 1.44 (1.02, 2.02) | |
| Disease state | |||
| mHSPC (n = 1,401) | 209 | 1.00 (ref) | 1.63 (1.05, 2.53) |
| CRPC (n = 718) | 218 | 1.00 (ref) | 1.14 (0.65, 2.00) |
| Age group (in years) | |||
| <70 (n = 956) | 175 | 1.00 (ref) | 1.11 (0.64, 1.93) |
| ≥70 (n = 1,163) | 252 | 1.00 (ref) | 1.67 (1.08, 2.59) |
| Continent of enrollment | |||
| North America (n = 976) | 203 | 1.00 (ref) | 1.79 (1.09, 2.92) |
| Europe (n = 875) | 162 | 1.00 (ref) | 1.19 (0.66, 2.16) |
| Other (n = 268) | 62 | 1.00 (ref) | 1.48 (0.63, 3.47) |
Abbreviations: IRONMAN, International Registry for Men with Advanced Prostate Cancer; mHSPC, Metastatic hormone-sensitive prostate cancer; CRPC, Castration resistant prostate cancer.
Hazard ratios (HR) and 95% confidence intervals obtained from Cox proportional hazards model.
Adjusted for living arrangement (living alone vs. not living alone), age at enrollment (continuous), disease state (mHSPC vs. CRPC), continent of enrollment (North America, Europe, and other), self-reported race (white vs. non-white), employment status (not working vs. currently working), smoking status (current non-smoker vs. current smoker), family history of prostate cancer (yes vs. no), and PSA at enrollment (continuous).
Table 3 presents multivariable-adjusted HRs and 95% CIs for the associations between marital status and living arrangements jointly categorized and all-cause mortality. There was no statistically significant difference for overall survival between unmarried participants of either living arrangement and married participants. In the stratified analyses, unmarried participants who were not living alone had worse survival than married participants, specifically among those with mHSPC (adjusted HR: 1.61; 95% CI: 1.01, 2.55), aged 70 or older (adjusted HR: 1.70; 95% CI: 1.08, 2.67), and from North American sites (adjusted HR: 1.80; 95% CI: 1.08, 2.99).
Table 3.
The associations between marital status and living arrangements jointly categorized and all-cause mortality overall and stratified by disease state, age, and continent of enrollment in IRONMAN (N=2,119), 2017–2023a
| Deaths, n | Married | Not married, Living alone | Not married, Not living alone | |
|---|---|---|---|---|
| Overall (age adjusted only) | 427 | 1.00 (ref) | 0.96 (0.73, 1.26) | 1.38 (0.96, 1.97) |
| Overall (fully adjusted) b | 427 | 1.00 (ref) | 0.96 (0.73, 1.26) | 1.41 (0.98, 2.02) |
| Disease state | ||||
| mHSPC (n = 1,401) | 209 | 1.00 (ref) | 0.92 (0.61, 1.36) | 1.61 (1.01, 2.55) |
| CRPC (n = 718) | 218 | 1.00 (ref) | 0.95 (0.64, 1.39) | 1.12 (0.62, 2.03) |
| Age group (in years) | ||||
| <70 (n = 956) | 175 | 1.00 (ref) | 0.73 (0.46, 1.16) | 1.02 (0.56, 1.86) |
| ≥70 (n = 1,163) | 252 | 1.00 (ref) | 1.11 (0.79, 1.57) | 1.70 (1.08, 2.67) |
| Continent of enrollment | ||||
| North America (n = 976) | 203 | 1.00 (ref) | 0.95 (0.63, 1.44) | 1.80 (1.08, 2.99) |
| Europe (n = 875) | 162 | 1.00 (ref) | 1.17 (0.78, 1.75) | 1.14 (0.59, 2.18) |
| Other (n = 268) | 62 | 1.00 (ref) | 0.46 (0.16, 1.28) | 1.46 (0.61, 3.48) |
Abbreviations: IRONMAN, International Registry for Men with Advanced Prostate Cancer; mHSPC, Metastatic hormone-sensitive prostate cancer; CRPC, Castration resistant prostate cancer.
Hazard ratios (HR) and 95% confidence intervals obtained from Cox proportional hazards model.
Adjusted for age at enrollment (continuous), disease state (mHSPC vs. CRPC), continent of enrollment (North America, Europe, and other), self-reported race (white vs. non-white), employment status (not working vs. currently working), smoking status (current non-smoker vs. current smoker), family history of prostate cancer (yes vs. no), and PSA at enrollment (continuous).
Compared to married individuals, widowed participants had the greatest increased risk of death (adjusted HR: 1.89; 95% CI: 1.22, 2.94; Table 4). Those who were divorced/separated (adjusted HR: 1.30; 95% CI: 0.87, 1.94) or never married (adjusted HR: 1.18; 95% CI: 0.67, 2.05) also showed worse survival than married participants (Table 4).
Table 4.
Sensitivity analyses with alternative categorization of marital status, expanded list of covariate adjustment, and modifying follow up time in IRONMAN (N=2,119), 2017–2023a
| Deaths, n | Married | Not Married | |
|---|---|---|---|
| Main analysis (Overall, fully adjustedb) | 427 | 1.00 (ref) | 1.44 (1.02, 2.02) |
| Categorical marital status (n = 2,119) | 427 | — | — |
| Divorced/separated (n = 222) | 43 | 1.00 (ref) | 1.30 (0.87, 1.94) |
| Widowed (n = 123) | 33 | 1.00 (ref) | 1.89 (1.22, 2.94) |
| Never married (n = 107) | 18 | 1.00 (ref) | 1.18 (0.67, 2.05) |
| Additionally adjusting for Education, Gleason score, and ECOG (Binary) (n = 1,489) | 284 | 1.00 (ref) | 1.49 (0.97, 2.22) |
Abbreviations: IRONMAN, International Registry for Men with Advanced Prostate Cancer; ECOG, Eastern Cooperative Oncology Group.
Hazard ratios (HR) and 95% confidence intervals obtained from Cox proportional hazards model.
Adjusted for living arrangement (living alone vs. not living alone), age at enrollment (continuous), disease state (mHSPC vs. CRPC), continent of enrollment (North America, Europe, and other), self-reported race (white vs. non-white), employment status (not working vs. currently working), smoking status (current non-smoker vs. current smoker), family history of prostate cancer (yes vs. no), and PSA at enrollment (continuous).
Discussion
In this international cohort of participants with advanced prostate cancer, being married/in a civil partnership was associated with better overall survival. Widowed participants had the worst survival. The protective association between being married/in a civil partnership and overall survival was stronger among participants with mHSPC disease, aged 70 or older, or from North American sites. Similarly, when examining marital status and living arrangements jointly, unmarried participants who were not living alone had the worst survival among those who had mHSPC, were aged 70 or older, or were from North American sites.
Our findings align with current evidence for other cancers and further support the protective association between marital status/civil partnership and overall survival among individuals with advanced prostate cancer. A US-based meta-analysis reported that unmarried individuals with prostate cancer had increased risk of prostate cancer-specific mortality and shorter overall survival compared to married participants (15). Similarly, being unmarried or being separated/divorced/widowed was associated with higher prostate-cancer-specific mortality and other-cause-specific mortality for all stages of prostate cancer (10,14). Particularly among those who underwent radical prostatectomy, unmarried, compared to married, individuals demonstrated shorter overall survival (13,16). The potential role of social support among individuals with prostate cancer, and with cancer generally, is likely multi-fold, including imparting psychosocial benefits and influencing health behaviors and clinical support (22). Such complexity may offer insight into our finding that unmarried individuals who are living alone fare better than those not living alone. Our study uniquely focused on individuals with advanced prostate cancer, who are at the greatest risk of death among all prostate cancer survivors. While a few prior qualitative studies identified the unique needs and barriers to improving survival in this and other vulnerable prostate cancer populations, such as gay or bisexual individuals, most studies were extremely limited in sample size and exploratory in nature (21,23–26). Our findings built upon existing evidence and highlighted the importance of marital status/civil partnership and overall survival among individuals with advanced prostate cancer.
Most prior studies grouped individuals who were divorced/separated, widowed, or never married into the same category of being unmarried. Few studies have been able to examine the independent associations between those groups and all-cause mortality (15,27). One US-based study found that divorced and never-married people with prostate cancer were at increased risk of all-cause mortality compared to married participants (28), for which our findings were consistent. Additionally, our findings suggested that widowed individuals have the worst overall survival compared to divorced/separated, never married, and married participants. Widowed individuals with advanced prostate cancer might be a subgroup that is particularly susceptible to poor survival.
After jointly examining living arrangement and marital status, we found that overall survival was the worst among unmarried individuals who are not living alone, which was unexpected. Two studies have previously reported that individuals with prostate cancer living alone had significantly increased risk of prostate cancer case-fatality and all-cause mortality (17,18). Our findings might be due to small sample size of a heterogenous population or unmeasured confounding, particularly by socioeconomic status and physical functioning. Without adjusting for these potential confounders, living alone might be an indicator of financial and/or physical ability to live alone, both of which are also related to overall survival (29). Thus, our findings should also be interpreted with potential unmeasured confounding in mind. Though, when we additionally adjusted for education, Gleason score, and ECOG performance status in a sensitivity analysis, results remained similar (Table 4). Still, additional studies should be conducted including these potential confounders.
In addition to what was mentioned above, our study had other potential limitations. First, due to sample size limitaitons, we were unable to examine living arrangements independently or use finer categorization, or examine prostate cancer-specific mortality as a separate outcome of interest. Finer categorization of enrollment site would also be important to elucidate any differences in cultural or social norms surrounding social support. Second, we did not have information on marital or living arrangement satisfaction or quality, which taps into perceived social support, another important but distinct construct from the objective social support examined in this study (30). Also, we did not capture long-term partnerships that did not meet the formal definition of marriage/civil partnership and participants’ other community engagements beyond their household that may also provide social support, or incorporate information on changes to their marital status and living arrangements in the analysis. It would be interesting to further disentangle the types of living arrangements and how levels of social support in each type are associated with overall survival. Third, due to the self-reported and binary nature of the covariates, there was potential for measurement error and residual confounding. However, this was unlikely to change the main results because the measurement error is likely non-differential with respect to the exposure and outcome. Likewise, individuals with metastatic and non-metastatic CRPC were grouped together, and treatment type was not included for analytic purposes. Differences in overall survival between these populations was possible, but minimal after adjusting for other prognostic factors in the analyses. Fourth, the median follow-up time was approximately six months, and site activation varied in time. Thus, we were possibly more likely to observe deaths from sites that activated first. Further work is needed with extended follow-up time.
Our study had notable strengths. To our knowledge, this was the first prospective, quantitative study conducted in a large international cohort that examined social support, using both marital status and living arrangement, and overall survival among individuals with advanced prostate cancer. In addition to marital status, we incorporated information on living arrangement, which captured a more holistic picture of social support. Moreover, given the international nature of IRONMAN, we were able to expand generalizability of our results by conducting stratified analyses by continent of enrollment. We were able to examine whether overall survival differed by continent due to potential differences in attitudes towards marriage and living arrangement.
In summary, we observed a protective association between marital status and overall survival among individuals with advanced prostate cancer and additionally contributed new perspectives regarding living arrangement’s potential role. Future studies could investigate finer categorizations for marital status and living arrangements, including community-based housing or nursing facilities, to capture the heterogeneity among those not living alone. Future research should examine the degree of unmeasured counfounding by socioeconomic status and physical functioning and additionally examine subgroup differences by treatment regimens. Overall, while advanced stage prostate cancer is itself associated with poorer prognoses, this research highlighted that unmarried individuals with advanced prostate cancer may be a vulnerable population. Clinical care teams should pay close attention to the varying levels of social support among individuals with advanced prostate cancer, as some may be at risk for lower overall survival and may benefit from social support interventions.
Supplementary Material
Acknowledgements
IRONMAN is principally supported by the Movember Foundation (collection and management of the data) and sponsored by Prostate Cancer Clinical Trials Consortium (PCCTC) (collection and management of the data, review and approval of the manuscript). Additional funding is provided by Amgen, Astellas, AstraZeneca, Bayer, Janssen, Merck, and Sanofi.
LAM, DJG, DER, KHS, KLP, TH, KAA, DE, RLB, HHC, EH, MP, and JM were supported by the Prostate Cancer Foundation Challenge Awards and the Young Investigator Awards.
EMR was supported by the National Cancer Institute [F30CA264965] and the National Institute of General Medical Sciences [T32GM007753, T32GM144273].
Conflict of Interest Statement:
Karen A. Autio reports grants from Pfizer, grants from Amgen, grants from Astra Zeneca, grants from Parker Institute of Cancer Immunotherapy, and grants from Janssen outside the submitted work. Joaquin Mateo reports grants and personal fees from AstraZeneca, grants and personal fees from Pfizer, grants from Amgen, personal fees from Janssen, personal fees from Roche, and personal fees and other support from Astellas outside the submitted work. Charles W. Githiaka reports grants from the University of Nairobi during the conduct of the study. Kim N. Chi reports grants and personal fees from Janssen, personal fees from Astellas, grants and personal fees from Bayers, grants and personal fees from AstraZenaca, grants from Roche, grants and personal fees from Merck, grants and personal fees from Point Biopharma, grants and personal fees from Amgen, grants and personal fees from BMS, grants and personal fees from Novartis, and grants and personal fees from Pfizer outside the submitted work. Heather H. Cheng reports grants from Astellas, grants from Sanofi, grants from Clovis Oncology, grants from Color Foundation, grants from Janssen, and grants from Promontory Therapeutics outside the submitted work. Ian D. Davis reports unpaid member of advisory boareds for AstraZeneca, Bayer, Roche, Merck/Pfizer, Ipsen, Janssen, Astellas - no payment for this work (all honoraria to ANZUP Cancer Trials Group), Unremunerated director and chair, ANZUP Cancer Trials Group. Anders Bjartell reports personal fees from Janssen, personal fees from Astellas, personal fees from Bayer, personal fees from Sandoz, personal fees from Ipsen, grants and personal fees from Ferring, personal fees from AstraZeneca, and personal fees from Merck Sharp & Dohme outside the submitted work. Elisabeth I. Heath reports a consulting/advisory role, paid travel, and research funding from Astellas Pharma; research funding from Arvinas, AstraZeneca, BioXcel Therapeutics, Bristol‐Myers Squibb, Calibr, Calithera Biosciences Inc, Corcept Therapeutics, Corvis Pharmaceuticals, Daiichi Sankyo Inc, Eisai Inc, Exelixis, Five Prime Therapeutics, Fortis Therapeutics, GlaxoSmithKline, Gilead Sciences Inc, Harpoon Therapeutics, Hoffman‐La Roche, Infinity Pharmaceuticals, iTeos Therapeutics, Merck Sharp & Dohme, Merck, Mirati Therapeutics, Modra Pharmaceuticals, Oncolys BioPharma, Peloton Therapeutics Inc, Pfizer, Pharmacyclics LLC, and POINT Biopharma; honoraria from and Ad Board for Bayer; research funding and paid travel from Caris Life Sciences; research funding from and Steering Committee for Janssen Research & Development LLC; honoraria from, speakers’ bureau, paid travel, and Ad Board for Sanofi; and honoraria, paid travel, research funding from Seattle Genetics. John C. Henegan reports personal fees from Pfizer, Targeted Oncology, Mike Slive Foundation, Exelexis, Sanofi and AVEO outside the submitted work. Terry Hyslop reports grants from PCORI outside the submitted work. Aurelius Omlin reports a consulting/advisory role for AstraZeneca, Astellas, Aventis, Bayer, Janssen, Merck, Molecular Partners, Monrol, MSD, Myriad, Novartis, Pfizer, Roche, Sanofi; reports research support from Teva and Janssen; and travel support from Astellas, Bayers, Janssen, and Sanofi Aventis; speakers’ bureau from Astellas, Bayers, and Janssen. Ray McDermott reports personal fees from Bayer, Sanofi, Janssen, Astellas, BMS, MSD, Pfizer, Novartis, Clovis, Ipsen, and Roche. Andre P. Fay reports honoraria from Pfizer, Novartis, Bristol Myers Squibb, AstraZeneca, Roche, Ipsen, Janssen Oncology, MSD and a consulting/advisory role for Novartis, Roche, Pfizer, Merck Sharp & Dohme, AstraZeneca, Ipsen. Camille Ragin reports grants from Pfizer outside the submitted work. Joel Nowak reports consulting for Janssen Pharma and that Cancer ABCs is funded by Foundation Medicine, AAA - Novartis, Bayer, Sanofi, Pfizer, Dendreon, Janssen Oncology, Blue Earth Diagnostics, Astellas, and Lantheus. Philip W. Kantoff reports that he is cofounder and CEO of Convergent Therapeutics. Daniel J. George reports grants and personal fees from Astellas, AstraZeneca, Exelixis, Janssen, Pfizer, and Sanofi; grants from Novartis; personal fees from AVEO Pharma, Bayer, VMS, Calithera, Eisai, IdeoOncology, Medscape Education, Merck, Myovant, NCI GU Steering Committee, Propella, RevHealth, UroGPO, UroToday, WebMD, and Xcures outside the submitted work. Lorelei A. Mucci reports grants from Janssen, Astra Zeneca, personal fees and other support from Convergent Therapeutics, and personal fees from Bayer outside the submitted work; and she holds equity and serve on the Scientific Advisory Board for Convergent Therapeutics. The other authors has nothing to disclose.
References
- 1.Prostate cancer statistics | World Cancer Research Fund International. [cited 2023 Apr 10]. Available from: https://www.wcrf.org/cancer-trends/prostate-cancer-statistics/ [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7–33. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Facts & Figures 2022| American Cancer Society. [cited 2023 Apr 10]. Available from: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2022.html [Google Scholar]
- 5.Aggarwal RR, Feng FY, Small EJ. Emerging Categories of Disease in Advanced Prostate Cancer and Their Therapeutic Implications. Oncol Williston Park N 2017;31:467–74. [PubMed] [Google Scholar]
- 6.Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate Cancer Incidence and Survival, by Stage and Race/Ethnicity - United States, 2001–2017. MMWR Morb Mortal Wkly Rep 2020;69:1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoshkar Y, Westerberg M, Adolfsson J, Bill-Axelson A, Olsson H, Eklund M, et al. Mortality in men with castration-resistant prostate cancer—A long-term follow-up of a population-based real-world cohort. BJUI Compass 2022;3:173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousefi Nooraie R, Mohile SG, Yilmaz S, Bauer J, Epstein RM. Social networks of older patients with advanced cancer: Potential contributions of an integrated mixed methods network analysis. J Geriatr Oncol 2021;12:855–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Payne N, Palmer Kelly E, Pawlik TM. Assessing structure and characteristics of social networks among cancer survivors: impact on general health. Support Care Cancer Off J Multinatl Assoc Support Care Cancer 2019;27:3045–51. [DOI] [PubMed] [Google Scholar]
- 10.Aizer AA, Chen M-H, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital Status and Survival in Patients With Cancer. J Clin Oncol 2013;31:3869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usta YY. Importance of Social Support in Cancer Patients. Asian Pac J Cancer Prev 2012;13:3569–72. [DOI] [PubMed] [Google Scholar]
- 12.Krajc K, Miroševič Š, Sajovic J, Klemenc Ketiš Z, Spiegel D, Drevenšek G, et al. Marital status and survival in cancer patients: A systematic review and meta-analysis. Cancer Med 2023;12:1685–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan S, Nepple KG, Kibel AS, Sandhu G, Kallogjeri D, Strope S, et al. The association of marital status and mortality among men with early-stage prostate cancer treated with radical prostatectomy: insight into post-prostatectomy survival strategies. Cancer Causes Control CCC 2019;30:871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He H, Han D, Xu F, Lyu J. How socioeconomic and clinical factors impact prostate-cancer-specific and other-cause mortality in prostate cancer stratified by clinical stage: Competing-risk analysis. The Prostate 2022;82:415–24. [DOI] [PubMed] [Google Scholar]
- 15.Guo Z, Gu C, Li S, Gan S, Li Y, Xiang S, et al. Association between Marital Status and Prognosis in Patients with Prostate Cancer: A Meta-Analysis of Observational Studies. Urol J 2020;18:371–9. [DOI] [PubMed] [Google Scholar]
- 16.Huang TB, Zhou GC, Dong CP, Wang LP, Luan Y, Ye JT, et al. Marital status independently predicts prostate cancer survival in men who underwent radical prostatectomy: An analysis of 95,846 individuals. Oncol Lett 2018;15:4737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elovainio M, Lumme S, Arffman M, Manderbacka K, Pukkala E, Hakulinen C. Living alone as a risk factor for cancer incidence, case-fatality and all-cause mortality: A nationwide registry study. SSM - Popul Health 2021;15:100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Z, Nguyen NH, Wang D, Lynch BM, Hodge AM, Bassett JK, et al. Social connectedness and mortality after prostate cancer diagnosis: A prospective cohort study. Int J Cancer 2020;147:766–76. [DOI] [PubMed] [Google Scholar]
- 19.McKay RR, Gold T, Zarif JC, Chowdhury-Paulino IM, Friedant A, Gerke T, et al. Tackling Diversity in Prostate Cancer Clinical Trials: A Report From the Diversity Working Group of the IRONMAN Registry. JCO Glob Oncol 2021;7:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mucci LA, Vinson J, Gold T, Gerke T, Filipenko J, Green RM, et al. IRONMAN: A Novel International Registry of Men With Advanced Prostate Cancer. JCO Glob Oncol 2022;8:e2200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter N, Miller PA, Murphy BR, Payne VJ, Bryant-Lukosius D. Healthcare providers’ perspectives of the supportive care needs of men with advanced prostate cancer. Oncol Nurs Forum 2014;41:421–30. [DOI] [PubMed] [Google Scholar]
- 22.Reamer E, Yang F, Holmes-Rovner M, Liu J, Xu J. Influence of Men’s Personality and Social Support on Treatment Decision-Making for Localized Prostate Cancer. BioMed Res Int 2017;2017:1467056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ralph N, Chambers SK, Laurie K, Oliffe J, Lazenby M, Dunn J. Nurse-Led Supportive Care Intervention for Men With Advanced Prostate Cancer: Healthcare Professionals’ Perspectives. Oncol Nurs Forum 2020;47:33–43. [DOI] [PubMed] [Google Scholar]
- 24.Levy A, Cartwright T. Men’s strategies for preserving emotional well-being in advanced prostate cancer: An interpretative phenomenological analysis. Psychol Health 2015;30:1164–82. [DOI] [PubMed] [Google Scholar]
- 25.Primeau C, Paterson C, Nabi G. A Qualitative Study Exploring Models of Supportive Care in Men and Their Partners/Caregivers Affected by Metastatic Prostate Cancer. Oncol Nurs Forum 2017;44:E241–9. [DOI] [PubMed] [Google Scholar]
- 26.Capistrant BD, Lesher L, Kohli N, Merengwa EN, Konety B, Mitteldorf D, et al. Social Support and Health-Related Quality of Life Among Gay and Bisexual Men With Prostate Cancer. Oncol Nurs Forum 2018;45:439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang SH, Li YN, Li JW, Guo YH, Su XF. Widowed status predicts poor overall survival of Chinese patients with prostate cancer. Transl Cancer Res 2020;9:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abdollah F, Sun M, Thuret R, Abdo A, Morgan M, Jeldres C, et al. The effect of marital status on stage and survival of prostate cancer patients treated with radical prostatectomy: a population-based study. Cancer Causes Control CCC 2011;22:1085–95. [DOI] [PubMed] [Google Scholar]
- 29.Klein J, von dem Knesebeck O. Socioeconomic inequalities in prostate cancer survival: A review of the evidence and explanatory factors. Soc Sci Med 1982 2015;142:9–18. [DOI] [PubMed] [Google Scholar]
- 30.Zhou ES, Kim Y, Rasheed M, Benedict C, Bustillo NE, Soloway M, et al. Marital satisfaction of advanced prostate cancer survivors and their spousal caregivers: the dyadic effects of physical and mental health. Psychooncology 2011;20:1353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are not publicly available due to privacy of research participants but are available upon reasonable request from the corresponding author.


