Abstract
Immune checkpoint inhibitor (ICI) therapy has dramatically changed the clinical landscape for several cancers, and ICI use continues to expand across many cancer types. Low baseline clearance (CL) and/or a large reduction of CL during treatment correlates with better clinical response and longer survival. Similar phenomena have also been reported with other monoclonal antibodies (mAbs) in cancer and other diseases, highlighting a characteristic of mAb clinical pharmacology that is potentially shared among various mAbs and diseases. Though tempting to attribute poor outcomes to low drug exposure and arguably low target engagement due to high CL, such speculation is not supported by the relatively flat exposure-response relationship of most ICIs where a higher dose or exposure is not likely to provide additional benefit. Instead, an elevated and/or increasing CL could be a surrogate marker of the inherent resistant phenotype that cannot be reversed by maximizing drug exposure. The mechanisms connecting ICI clearance, therapeutic efficacy, and resistance are unclear and likely to be multifactorial. Therefore, to explore the potential of ICI CL as an early marker for efficacy, this review highlights the similarities and differences of CL characteristics and CL-response relationships for all FDA-approved ICIs, and we compare and contrast these to selected non-ICI mAbs. We also discuss underlying mechanisms that potentially link mAb CL with efficacy and highlight existing knowledge gaps and future directions where more clinical and preclinical investigations are warranted to clearly understand the value of baseline and/or time-varying CL in predicting response to ICI-based therapeutics.
1. Introduction
Over the last three decades, monoclonal antibodies (mAbs) have revolutionized the treatment modality of various diseases. Compared with small molecule drugs, mAbs are immunoglobulins that can bind to the drug target with high specificity and high affinity to induce favorable treatment outcomes along with fewer off-target toxicities. However, not all patients treated with mAb-based therapies can achieve sufficient response and durable remission, regardless of the corresponding indications(1–3). Furthermore, while toxicities are generally less frequent than with small-molecule drugs, they do occur and can be serious and even life-threatening. Therefore, early biomarkers to help identify patients who are not likely to respond or who are likely to experience serious adverse events would be of significant benefit for clinical decision-making and the selection of alternative therapies.
The clearance (CL) of mAbs has emerged as a potential early marker of treatment outcome. In patients with advanced cancers treated with immune checkpoint inhibitors (ICIs), elevated CL of ICIs at baseline (i.e. ) is associated with decreased survival in several cancer types, but a greater CL decrease within the first few months after treatment initiation (ie. ) is generally associated with better response (Table 1). Additionally, some evidence, though limited, demonstrated that such CL-response phenomena also occur with other non-ICI mAbs for the treatment of various cancers and non-cancer diseases (Table 2). Here, although it is tempting to conclude that the poor response in high CL patients is due to low drug exposure, evidence has suggested that the poor response is not likely to be rescued with increased dose and exposure. For example, the relationship between pembrolizumab exposure (area under the concentration-time curve at steady state over 6 weeks, AUCss-6weeks) and the objective response rate (ORR) was flat across doses of 2–10 mg/kg, indicating that the lowest evaluated dose of 2 mg/kg Q3W to likely be at or near the efficacy plateau (4). Similarly, for nivolumab in advanced melanoma, the relationship of exposure (Cmins) and ORR appeared to plateau at doses ≥ 1 mg/kg (5). The time-averaged concentration after the first dose (Cavg1) was also not a significant predictor of response across the dose range of 0.1–10.0 mg/kg (6). Therefore, at least with ICIs, elevated CL, but not low drug exposure, is linked to poor outcomes, suggesting higher CL is a biomarker for, but not the cause of poor response or short duration of response. This was demonstrated most convincingly by Turner and colleagues in their retrospective analyses of pembrolizumab CL, exposure, and response in patients with melanoma and non-small cell lung cancer where they demonstrated overall survival was strongly associated with baseline clearance (, the clearance calculated from the first one or two doses) and was independent of dose (7,8). Figure 1A demonstrates this in Kaplan-Meier curves showing survival to be significantly improved in patients having the lowest vs. highest quartiles of CL, regardless of whether they received 2 mg/kg or 10 mg/kg.
Table 1.
Summary of ICI clearance and clearance-response relationship.
| Drug name | IgG Type | (L/day) | covariates | Time-varying CL | -outcome relationship | -outcome relationship | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | ALB | TS | Male | eGFR CrCL |
Liver function | PS ≥1 | ADA | Other | Emax | (days) | Covariates | |||||
| Pembrolizumab (7,17,23,33,160) | IgG4 S228P aPD1 | 0.219–0.249 | / | ↓↓ | ↑ | + | ↑ | BIL (↓) | + | / | / | 21.7% | 67.4 | ALB, LDH, TS, ALC (Longitudinal) | -OS (MEL, NSCLC) | -BOR (MEL, NSCLC) |
| Nivolumab (±Ipilimumab) (22,29,32,161–164) | IgG4 S228P aPD1 | 0.225–0.259 | ↑↑ | ↓↓ | / | + | ↑ | / | + | / | Asian (−) AA (+) | 21.3%−27.7% | 52.9–91.7 | ALB on Emax TS on Emax IPI on Emax PS on Emax | -OS -PFS (NSCLC, RCC, MEL) | -BOR (RCC, MEL, NSCLC) |
| Cemiplimab (24) | IgG4 S228P aPD1 | 0.290 | ↑ | ↓↓ | ↑ | / | / | ALT (↓) | / | + | IGG (↑) | 33.6% | 28.9 | Cancer type on Emax Cancer type on Race on | / | -BOR (CSCC) |
| Dostarlimab | IgG4 S228P aPD1 | 0.163 | ↑↑ | ↓↓ | / | + | / | ALT/ALP(↑) | / | / | AGE (↓) | 15.4% | 90.8 | / | / | / |
| Atezolizumab (131,165) | IgG1 Fc mutant* Aglycosylation aPD-L1 | 0.235 | ↑↑ | ↓↓ | ↑ | + | / | ALP(↑) BIL(↓) | / | + | NEU(↑) | 22.0% | 447 | SLD, ALB, WT, ALP, NEU, BIL, ADA (Longitudinal) | / | / |
| Avelumab (25) | IgG1 aPD-L1 | 0.739 | ↑ | ↓ | ↑ | + | ↑ | / | + | + | AGE (↓) CRP(↑) AA(−) | 0–32.1% | 131 | Cancer type on Emax and T50 | / | -BOR (MCC, UC) |
| Durvalumab (62,132) | IgG1 Fc mutant* aPD-L1 | 0.232–0.257 | ↑↑ | ↓↓ | ↑ | + | ↑ | / | + | + | IGG(↑) sPD-L1(↑) LDH(↑) | 16.9% | 173.1 | ALB, TS, IGG, sPD-L1, LDH (Longitudinal) | / | / |
| Relatlimab (+Nivolumab) (FDA PI) | IgG4 S228P Fc mutant* aLAG-3 | 0.167 | ↑↑ | ↓↓ | / | + | ↑ | / | + | / | / | 4.8% | 133 | PS on Emax | / | / |
| Ipilimumab (±Nivolumab) (26,133) | IgG1 aCTLA-4 | 0.338 | ↑↑ | / | / | / | / | / | / | + | LDH (↑↑) | 6.4% (IPI) | 106 | 23.5% (IPI+Nivo) | / | -BOR (MEL, NSCLC, other) |
| Tremelimumab (15,16) | IgG2 aCTLA-4 | 0.26 – 0.31 | / | / | ↑ | + | ↑ | / | + | / | IGG (↑) LDH (↑) CRP (↑) | / | / | / | -OS (MEL) | / |
| Bintrafusp alfa (166) | IgG1# aPD-L1 and aTGF- | 0.379 | / | / | / | / | / | / | / | / | / | / | / | /## | / | / |
(↑/+, positive correlation; ↓/−, negative correlation; /, not reported; WT, weight; ALB, albumin; TS, tumor size; CrCL, serum creatinine clearance; PS, performance status; ADA, anti-drug antibody; Emax, percentage of clearance decrease from baseline to steady state; , time to 50% of maximum reduction of clearance; BIL, bilirubin; AA, African American; Chemo, coadministration with chemotherapy; IPI, ipilimumab; IGG, endogenous IgG; NEU, neutrophil; CRP, c-reactive protein; MEL, melanoma; NSCLC, non-small cell lung cancer; UC, urothelial carcinoma; ALC, actual lymphocyte count; RCC, renal cell carcinoma; MCC, merkel cell carcinoma; CSCC, cutaneous squamous cell carcinoma). References are cited under the drug name.
Fc mutation: Atezolizumab, N297A; Durvalumab, L234F/L235E/P331S; Relatlimab, unknown
IgG1-based bifunctional fusion protein
ALB, CRP, and PLT were found to be influential on time-varying CL, but observed data did not show a notable trend of CL decrease over time.
Table 2.
Summary of clearance of non-ICI mAbs.
| Drug name | IgG Type | (L/day) | covariates | Time-varying CL | -outcome relationship | -outcome relationship | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | ALB | TS | Male | eGFR CrCL | Liver function | PS ≥1 | ADA | Other | ||||||
| Bevacizumab (41,44,167,168) | IgG1 aVGEF | 0.207 | ↑↑ | ↓↓ | / | + | / | ALP(↑) | + | / | combined(−) VGEF (↑) CEA (↑) Total protein (↓) SUVmax change (↑) Extra-hepatic metastases (+) Advanced gastric cancer (+) No prior gastrectomy (+) | No | Trend of lower in CR group | / |
| Trastuzumab (30,40,42,45,169) | IgG1 aHER2 | 0.180–0.230 | ↑↑ | ↓↓ | / | / | / | ALT(↑) | / | / | No prior gastrectomy (+) Metastases sites (↑) | No (Time-dependent increase in Vc was reported in early-stage breast cancer) | / | / |
| Cetuximab (35–39) | IgG1 Chimeric aEGFR | 0.418–0.570 | / | ↓ | / | / | / | / | / | / | BSA (↑) Chemotherapy(−) | Yes (Emax 23.1%; 143.5 days) | CL (global) -OS/PFS (HNSCC and CRC) | -BOR (CRC) |
| Rituximab (46,47,49,170–178) | IgG1 Chimeric aCD20 | 0.14–0.54 | ↑↑ | / | ↑ | + | / | / | / | / | BSA (↑↑) WBC(↑) CD20/CD19 count(↑) Endogenous IgG(↑) Disease type Combined regimens FCGR3A polymorphism | Yes (decrease ~88%; ~ 17 – 34 days) | (Ctrough/AUC on first dose with PFS and BOR) | (A faster increase of CL correlated with no progression) |
| Obinutuzumab (50,179) | IgG1 Glycoengineered aCD20 | 0.238–0.314 | ↑↑ | ↓↓ | ↑ | + | / | / | / | / | Age (↓) Disease type | Yes (decrease ~26.4–32.5%; 9–19 days) | (Cmean correlated with BOR and PFS) | / |
| Ocrelizumab (180) | IgG1 Glycoengineered aCD20 | 0.189–0.218 | ↑↑ | / | / | / | / | / | / | / | / | Yes (decrease ~20%; ~32 weeks) | / | / |
| Infliximab (57–60,181–184) | IgG1 Chimeric | 0.2–0.38 | ↑↑ | ↓↓ | / | + | / | / | / | + | Disease type Time-varying disease activity Time-varying CRP(↑) Azathioprine cotreatment (−) | Yes (linear increase: 0.0348 L/day/year in IBD) | CL at de-escalation-risk of relapse in IBD | / |
| Adalimumab (129,185–189) | IgG1 | 0.24–0.59 | ↑↑ | ↓↓ | / | + | / | / | / | + | Time-varying CRP(↑) Concomitant methotrexate Baseline rheumatoid factor | Yes (Driven by time-varying covariates) | / | / |
| Daratumumab [67–70] | IgG1 aCD38 | 0.171 | ↑↑ | ↓↓ | / | / | / | / | / | / | Disease type (IgG vs. non-IgG multiple myeloma) Drug products (Phase 2 vs. Phase 3 product) | Yes (decrease ~29%) | (Ctrough,max correlated with ORR) | / |
(↑/+, positive correlation; ↓/−, negative correlation; /, not reported; WT, weight; ALB, albumin; TS, tumor size; CrCL, serum creatinine clearance; PS, performance status; ADA, anti-drug antibody; ALP, alkaline phosphatase; ALT, alanine aminotransferase; VEGF, vascular endothelial growth factor; CEA, carcinoembryonic antigen; SUV, standardized uptake value; WBC, white blood cell; HNSCC, head and neck squamous cell carcinoma; CRC, colorectal cancer; BOR, best overall response; IBD, inflammatory bowel diseases). References are cited under the drug name.
Figure 1.

Example CL-response relationship of ICIs. A) Example -OS/PFS relationship for pembrolizumab (Reprinted from Clinical Cancer Research, 2018, 24(23):5841–9, Turner et al., Pembrolizumab Exposure-Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance, with permission from AACR) (7). B) Example of decoupled CL-R vs. E-R across dose levels, within a single dataset. Both E-R and CL-R relationships are apparent within each dose level, but only the CL-R exists across the two dose levels. (Reprinted from Clinical Cancer Research, 2018, 24(23):5841–9, Turner et al., Pembrolizumab Exposure-Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance, with permission from AACR) (7). C) Example /-BOR relationship demonstrating greater decrease in CL over time associates with better clinical response (Used with permission of John Wiley & Sons, from Association of Time-Varying Clearance of Nivolumab with Disease Dynamics and its Implications on Exposure Response Analysis, Liu et al., 101(5), 2017; permission conveyed through Copyright Clearance Center, Inc.) (22). (BOR, best overall response; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; , baseline clearance; , steady-state clearance; / represents magnitude of clearance reduction; OS, overall survival; PFS, progression-free survival.)
It is important to highlight that a positive exposure-response (E-R) relationship is often falsely concluded when data is limited to a single dose level or a small sample size. In fact, if an E-R relationship is observed when only a single dose level is evaluated, then a clearance-response (CL-R) relationship will also be observed(9). Only with case-control analyses or a large dataset consisting of a balanced distribution of baseline factors and pharmacokinetic (PK) data across multiple dose levels can we distinguish whether the true relationship is E-R or CL-R (10,11). This point was also very nicely demonstrated by Turner and colleagues whose analyses clearly showed an apparent and steep E-R within each dose level, 2 mg/kg and 10 mg/kg, though a flat E-R between the two dose levels, despite a 5-fold difference in exposure (AUC) between the two dose levels (see Figure 1B)(7).
The general dogma is that elevated is due to target-mediated CL at baseline, consistent with high disease burden and high target expression, or to catabolic CL, which may be associated with inflammation and/or cachexia, secondary to the advanced stage of disease (7,8). Similarly, decreasing CL over time is attributed to decreasing availability of unbound target (i.e. increasing receptor occupancy, RO, and/or decreased target expression), leading to decreased disease burden (i.e. clinical response), decreased inflammation, and decreased cachexia burden. In cases where CL increases over time, this is typically attributed to increased disease burden (i.e. lack of response or refractory disease), increased target expression, increased target-mediated CL, increased inflammation and cachexia, leading to increased catabolic CL, and/or to the formation of anti-drug antibodies (ADA), leading to ADA-mediated CL of the therapeutic mAb. While this dogma generally allows pharmacologists to successfully model and simulate mAb PK, RO, and efficacy within patient populations, it does not fit across all diseases and with all mAb therapies. For ICIs in particular, at least a significant portion of the high is known to be catabolic in patients(7) and in murine models of cancer cachexia(8). This highlights our poor understanding of the underlying mechanisms driving elevated catabolic CL and changes in catabolic CL with respect to disease status, inflammation, and cachexia.
To provide an overall assessment of the CL-response phenomenon for mAbs, this article summarizes known CL characteristics and CL-R relationships for ICIs and other selected mAbs for the treatment of cancers as well as non-cancer indications such as immune-mediated inflammatory diseases. We also review the potential mechanisms regulating baseline CL elevation and time-varying CL, including antigen-mediated elimination, salvage via the neonatal Fc-receptor (FcRn), a receptor that protects IgG from catabolism and extends their half-life, Fc gamma receptor ()-mediated cellular internalization, and ADA formation. The article also highlights current knowledge gaps and future directions, including the impact of cachexia/inflammation status, the correlation of CL and immune-related adverse events (irAEs), and integration of ICI CL for future biomarker research, with the goal of improving understanding of the CL-response relationship of mAbs in different diseases and the potential value of CL as an early biomarker in mAb-based therapy.
2. Brief overview of mAb drug clearance and the distinction between exposure-response (E-R) and clearance-response (CL-R) relationships
A thorough explanation of CL concepts, the relevant pharmacodynamic models, and most of the math underlying PK and PD of mAb drugs in complex or even simple biological systems are outside the scope of this review. Instead, we summarize only the key concepts we believe are necessary for understanding and appreciating how ICI CL-R relationships (i.e. the correlation between the ICI drug clearance and clinical response or efficacy) may be confused for E-R relationships (correlations between ICI exposure and clinical response) and why even the most experienced pharmacologists can be surprised to learn that a CL-R relationship is present when an E-R relationship is not.
Clearance is defined as the volume (into which a drug is distributed) that is completely cleared of the drug per unit time, which is generally viewed as the rate and extent of drug elimination. For mAb CL, there are two paramount components to be characterized: nonlinear CL and linear CL. The nonlinear CL of mAbs is due primarily to target-mediated drug disposition (TMDD) resulting from high-affinity binding to their target, which can be described by full TMDD models that include important properties such as apparent total target expression, the turnover of the target, and the internalization of the bound drug-target complex (12). Several approximations to the full TMDD models have been proposed (13), and the Michaelis-Menten function is one of the more common approximations used to model the rate of target-mediated elimination (TME),
| (equation 1) |
where is the maximum velocity of the nonlinear elimination process and is constant if target concentration does not change, C is the drug concentration, and is the Michaelis-Menten constant (i.e. drug concentration at which half-maximal TME occurs). Dividing TME by C yields the nonlinear CL,
| (equation 2) |
when , which for mAb therapies may occur when the drug concentration is much higher than the target concentration and the target is effectively saturated, the rate of mAb elimination (TME) approaches a maximum (i.e. ) and the nonlinear CL approaches a minimum.
The linear CL of mAbs includes both the baseline linear CL () and time-varying linear CL can be obtained from the first-dose PK profile and represents the drug elimination capacity at the start of treatment, while refers to the change in CL over time after the start of treatment.
As listed in Table 1 and Table 2, the CL of ICIs and many other mAbs has been extensively evaluated using population pharmacokinetic (PopPK) models with pooled PK data. The base model structure often comprises two-compartments with linear CL, and is in the range of ~0.2 L/day, which is typical CL characteristics of IgGs. is observed in all ICIs and some non-ICI mAbs and is modeled with various methods (14). For ICIs, it is generally modeled using the sigmoidal equation,
| (equation 3) |
where the maximal CL reduction generally reaches 0–30% below baseline, and the time to reach half-maximum after treatment initiation () varies between 50–400 days.
In this way, the total linear CL can be described by the equation,
| (equation 4) |
and then integrated into the differential equations of a two-compartment model to enable the solution and estimation of PK parameters:
| (equation 5) |
| (equation 6) |
where is the drug concentration in the central compartment, is the drug concentration in the peripheral compartment, Q is the intercompartment clearance, is the volume of drug distribution in central compartment, and is the volume of drug distribution in peripheral compartment. For ICIs, the approved clinical dose regimens often achieve high target saturation (i.e. high receptor occupancy, RO) throughout the dosing interval. Therefore, TME often cannot be accurately estimated by data fitting and is not constructed in PopPK models. Only relatlimab exhibits nonlinear CL (or TME) in parallel with linear CL at doses tested in clinical trials, potentially due to undersaturation of the LAG3 target at low concentrations.
Covariates of mAb CL have been investigated and are listed in Table 1 and Table 2. A higher is commonly associated with higher body weight, lower serum albumin, larger tumor size, male sex, and poor performance status. Similarly, is often correlated with baseline covariates such as cancer type, performance status, and baseline tumor size, or longitudinal markers such as on-treatment albumin and tumor size. Although the absolute value of CL itself cannot provide detailed information on drug elimination mechanisms, variations in and may signal differences or changes in the underlying biology that regulates drug elimination capacity. By accurately characterizing the and of a mAb within and across disease populations, we can begin to identify the underlying mechanisms and associated biomarkers linking , , and outcomes from mAb therapies. However, the underlying mechanisms that drive the observed differences in and between each individual, across diseases, and among different mAbs are poorly understood and are discussed in Section 4.
Despite the complexities of mAb PK, one of the most common and useful expressions for CL is
| (equation 7) |
which applies broadly to all drugs and highlights the inverse relationship between CL and AUC, the area under the drug concentration vs. time curve. AUC is a commonly used metric for drug exposure, and if we can determine AUC, then we can easily calculate CL for a given dose.
If CL remains constant across a range of doses (which is generally the case for mAb therapies at clinical dose levels), then the PK is dose-proportional, and the AUC increases/decreases proportionally as the dose increases/decreases. This can be expressed simply by rearranging equation 7 to,
| (equation 8) |
which shows that AUC is equivalent to Dose scaled by the proportionality constant, 1/CL. From equations 7 and 8, for dose-proportional PK, we can know two things for certain. First, that within a population of patients who are all administered a single dose level, that if an apparent E-R relationship exists, then a CL-R relationship must also exist for the same response metric since AUC and 1/CL are perfectly correlated by the constant, Dose, and any observed inter-subject variation in AUC must be due to inter-subject variation in CL. Second, that within a population of patients administered multiple dose levels (e.g. 2 mg/kg and 10 mg/kg), we may still observe both an E-R relationship and a CL-R relationship within each dose level. However, since exposure changes proportionally with dose while CL remains constant across the dose levels, it would not be possible to simultaneously observe both an E-R relationship and a CL-R relationship across the dose levels. Therefore, at least for mAb therapies with dose proportional PK at clinical dose levels, we must resist the urge to conclude E-R relationships when only a single dose level is evaluated.
Probably the most important point to be made in this section is that analyses concluding the presence of E-R relationships have very different implications than analyses concluding CL-R relationships. If we conclude an E-R relationship exists, then we expect that a higher dose will provide higher exposure and therefore an improved response. In contrast, if we conclude a CL-R relationship exists, we recognize that a higher dose and higher exposure will not improve outcomes. Therefore, recognizing a CL-R as a biomarker for outcomes and a flat E-R avoids a likely futile exploration of higher doses, and it opens a path for further exploration of the underlying mechanisms linking mAb CL to poor outcomes.
3. ICI clearance as a marker of therapeutic outcome
3.1. Correlations of ICI clearance and treatment outcome
Evidence has been accumulating that ICI CL is a promising marker for treatment outcomes in patients with advanced cancers. It was first observed in a post hoc analysis of tremelimumab PK and PD in metastatic melanoma that a significant association between baseline CL () and overall survival (OS) was shown with the median OS of 9.6 months for patients with above-median versus 15.8 months for those with below-median (15). It was originally assumed that the poor outcome in the high group was due to low drug exposure, but clinical studies later revealed that an intensified dosing regimen of tremelimumab still failed to provide additional benefits in patients with malignant mesothelioma with high , highlighting that ICI clearance is an independent marker of treatment outcome regardless of drug exposure(16). Similar CL-outcome phenomena were later observed in a post hoc analysis of pembrolizumab PK and PD by Turner et al(7), where an elevated of pembrolizumab was significantly associated with shorter OS regardless of dose levels in NSCLC and advanced melanoma patients. Two recent prospective studies of pembrolizumab and nivolumab also echoed such CL-outcome relationship in advanced cancers despite sparse PK sampling and smaller patient populations(17,18).
Furthermore, the impact of ICI on efficacy endpoints was found to be stronger than other common prognostic covariates, indicating the unique value of as a prognostic marker of outcome. For example, in the study by Turner et al, the and OS relationship of pembrolizumab was tested in univariate and multivariate cox regression models, where the significant -OS association was not fully attenuated after adjustment of baseline prognostic factors such as albumin, performance status, PD-L1 expression positivity and tumor size. Similarly, according to the clinical pharmacology report included in FDA BLA review of Opdualag (nivolumab and relatlimab-rmbw) for treatment of advanced melanoma(19), nivolumab was included as one of the covariates to adjust for the exposure-response relationship of relatlimab. The impact of nivolumab was among one of the greatest compared with other prognostic covariates such as tumor burden, performance status, mutation status, and PD-L1 expression. Compared with patients having median nivolumab (8.7 mL/hr), those with nivolumab at 5th percentile (4.21 mL/hr) were more likely to respond (odds ratio 3.48, 95% CI 1.79–6.77) and more likely to survive (hazard ratio 0.754, 95% CI 0.674–0.843), while those with nivolumab at 95th percentile (17.3 mL/hr) were less likely to respond (odds ratio 0.0916, 95% CI 0.0256–0.327) and more likely to progress (hazard ratio 1.88, 95% CI 1.47–2.42).
Recognizing the prognostic utility of , Wang and colleagues conducted two similar analyses to derive a baseline composite cytokine signature correlated with nivolumab in renal cell carcinoma(20) and advanced melanoma(21), and validated that the predicted based on cytokine signature was also significantly associated with OS. Compared to baseline determination, which requires sample collection and analysis after drug administration, prognostic cytokine signatures determined in blood prior to drug administration would be advantageous. However, both and cytokine signatures as a composite prognostic biomarker may be valuable to balance patient randomization in clinical trials in the future or to aid therapy selection when alternatives to ICIs are available.
In addition to , is also correlated with treatment response, with a larger magnitude of (decreasing clearance over time) corresponding to a deeper response. For example, Liu and colleagues observed in patients who received nivolumab for the treatment of advanced cancers including NSCLC, melanoma, and renal cell carcinoma, the typical CL reduction from baseline was 42.4% for complete response, 35.3% for partial response, 23.8% for stable disease, and 20.1% for progressive disease, respectively(22). We have included data from this study in Figure 1C as a representative example of similar data published throughout the literature. Similar trends of association between and response has been reported in PK/PD analyses of pembrolizumab(23), cemiplimab (24), avelumab (25), and ipilimumab (26), indicating a broad effect across all types of ICIs.
Taken together, the accumulating evidence has demonstrated that the CL-outcome relationship is likely to be a class phenomenon across all ICIs. The value of ICI CL as a promising early marker of treatment outcome and potentially as a marker of pending treatment failure in advanced cancers warrants extensive further investigation.
3.2. Potential underlying impactors of clearance-outcome relationship
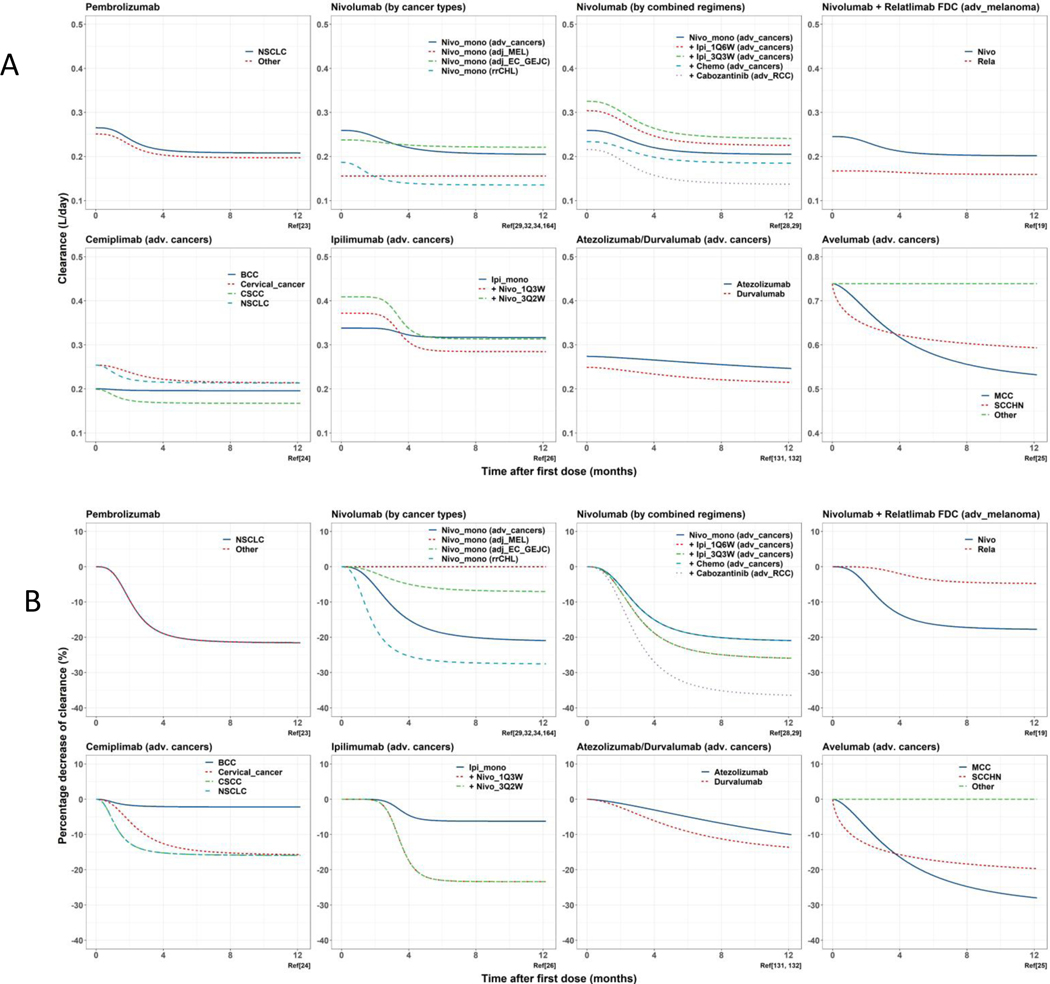
As listed in Table 1, the value of and the magnitude of for ICIs are impacted by various baseline and longitudinal factors. Although those statistically significant covariates are not believed to be clinically meaningful for dose adjustment due to the relatively flat exposure-response relationship for most ICIs(27), it is worthwhile to carefully evaluate and compare the impact of those covariates on ICI CL as they might provide clues to the underlying biology that drives CL differences between and within individual subjects. Therefore, we simulated the typical actual CL and % change of CL from baseline vs time after treatment initiation based on published population PK models of ICIs and plotted by cancer type, disease stage, and treatment regimen.
As depicted in Figure 2A and Figure 2B, the absolute and longitudinal CL change are notably different by mAb type/target (e.g. IgG4/anti-PD1), disease, and combination therapy. As for mAb type/target, the typical CL of anti-PD1s was similar in advanced cancers, where the starts at ~0.25 L/day and was generally decreased by ~20% and reached the plateau at ~ 6 months after treatment initiation. However, as for relatlimab, an IgG4 mAb targeting LAG3, the was 28.3% lower and the CL change was shallower than nivolumab though they were administered together in the same population(19), indicating the potential impact of checkpoint target on ICI CL. As for anti-CTLA4 and anti-PDL1s, although the CL differences were also distinct with anti-PD1s, they both come with the IgG1 backbone which might confound the comparison and needs to be evaluated separately.
Figure 2.
A. Simulation of typical time-dependent change of ICI clearance from PopPK models (actual clearance). (NSCLC, non-small cell lung cancer; EC, esophageal cancer; GEJC, gastroesophageal junction cancer; MEL, melanoma; CHL, Classical Hodgkin Lymphoma; adv., advanced; Chemo, coadministration with chemotherapy; 1Q6W, 1mg every 6 weeks; 3Q3W, 3mg every 3 weeks; 3Q2W, 3 mg every 2 weeks; IPI, ipilimumab; Nivo, nivolumab; Mono, monotherapy; 1L, first line; 2L, second line; Rela, Relatlimab; BCC, basal cell carcinoma; CSCC, cutaneous squamous cell carcinoma; MCC, merkel cell carcinoma; SCCHN, squamous cell carcinoma of head and neck). References (Ref) are provided for each model utilized. B. Simulation of typical time-dependent change of ICI clearance from PopPK models (percentage decrease of clearance). (NSCLC, non-small cell lung cancer; EC, esophageal cancer; GEJC, gastroesophageal junction cancer; MEL, melanoma; CHL, Classical Hodgkin Lymphoma; adv., advanced; Chemo, coadministration with chemotherapy; 1Q6W, 1mg every 6 weeks; 3Q3W, 3mg every 3 weeks; 3Q2W, 3 mg every 2 weeks; IPI, ipilimumab; Nivo, nivolumab; Mono, monotherapy; 1L, first line; 2L, second line; Rela, Relatlimab; BCC, basal cell carcinoma; CSCC, cutaneous squamous cell carcinoma; MCC, merkel cell carcinoma; SCCHN, squamous cell carcinoma of head and neck). References (Ref) are provided for each model utilized.
The structure of mAb backbone could impact the CL in that a stronger interaction of the Fc domain with receptors and/or complements could theoretically induce more internalization, antibody dependent cell-mediated cytotoxicity (ADCC), antibody dependent cell-mediated phagocytosis (ADCP), and/or compliment-dependent cytotoxicity (CDC) effects and therefore potentially elevate mAb CL. In general, IgG1 with wild-type Fc domain is believed to exhibit high affinity leading to more Fc interactions, compared with IgG4. For example, avelumab, an anti-PDL1 with wild-type IgG1 Fc domain, showed the highest typical among all ICIs, while the of atezolizumab and durvalumab, two IgG1 anti-PDL1s with Fc domain mutations (atezolizumab, N298A; durvalumab, L234F/L235E/ P331S) that abrogate receptor interactions, was close to that of anti-PD1 IgG4s. Furthermore, both atezolizumab and durvalumab demonstrated slow and shallow CL change after treatment initiation, which was unique compared with other ICIs, indicating an underlying biology that might be impacted by IgG subtype, mAb target and Fc interactions (Table 1, Figure 2).
The observed differences in ICI CL between ICI monotherapy and ICI combined regimens also need to be highlighted. For example, the nivolumab and cabozantinib combined regimen was approved as first-line treatment for patients with advanced renal cell carcinoma (RCC). Compared with the typical CL of nivolumab monotherapy in advanced cancers, including advanced RCC, the CL of nivolumab when combined with cabozantinib was 16.8% lower, and the maximum CL reduction was 20% less(28). This suggests the underlying mechanisms in cancer patients that link decreased CL and improved response might be altered with cabozantinib coadministration. A similar trend in CL differences was also shown in the nivolumab and chemotherapy combined regimen, where the was ~9% lower compared with nivolumab monotherapy in advanced cancers. Furthermore, the CL of nivolumab and ipilimumab were both affected by combined administration. Depending on the dose levels, the coadministration increases the of nivolumab and ipilimumab by 1–29% and by 0–18%, respectively, and the magnitude of CL reduction increased by 7% and 18% respectively(26,29). The underlying mechanism for this interaction is unknown and likely driven by pharmacodynamics(29). Additionally, the change of CL was not identified in other combined mAb regimens, such as trastuzumab plus pertuzumab and bevacizumab plus trastuzumab(30,31).
The stage of the disease is also associated with variability in CL for ICIs. For example, compared with of nivolumab in advanced cancers, was 40% lower in melanoma patients and longitudinal CL was stable in adjuvant therapy post complete tumor resection, (32). Though it is tempting to conclude that tumor size reduction is the reason for stable CL, incorporating longitudinal tumor size as a CL covariate in PopPK models does not fully account for the variabilities of ICI CL(33). Additionally, of nivolumab in adjuvant gastroesophageal junction cancer (GEJC) is similar to that in advanced cancers, and the magnitude of CL decrease was ~18% greater(34), indicating a generally similar health condition at baseline and a residual disease that could be recovered by adjuvant treatment in GEJC. Therefore, CL is likely driven by underlying changes relative to the disease state that is not fully accounted for by the tumor itself.
3.3. Implications from other mAbs and comparison with ICIs
Interestingly, the underlying biology linking mAb CL with outcomes potentially applies to a broad range of therapeutic mAbs because CL-response relationships similar to those observed with ICIs have been reported in other mAbs for the treatment of cancer and non-cancer diseases. The goal of this section is to review the similarities and differences in CL characteristics of ICIs vs. a selection of other well-studied mAbs to provide a better understanding of the CL-response relationship.
For non-ICI mAbs approved for the treatment of solid tumors, time-dependent CL and the CL-response relationship were rarely reported or explored. Cetuximab is a chimeric IgG1 directed against the epidermal growth factor receptor (EGFR) and is indicated in the treatment of squamous cell carcinoma of the head and neck (HNSCC) and metastatic colorectal cancer (mCRC). Time-dependent linear CL of cetuximab was found in mCRC based on a dataset of 3,821 PK samples from 226 patients(35) where the cetuximab PK was best described by a two-compartment model with parallel Michaelis–Menten and linear CL that changes exponentially over time. It was estimated that the maximum magnitude of linear CL reduction from baseline was around 23.1% and the time to reach half-maximum reduction was around 20.5 weeks, which was similar to the CL characteristics of anti-PD1s. While the role of nonlinear CL in the disposition of cetuximab is mechanistically explained by the presence of target-mediated drug disposition (TMDD) due to the interaction with EGFR, the change of linear CL over time was likely impacted by the change of disease state, as the average magnitude of CL decrease for cetuximab was higher in responders than non-responders. However, a time-dependent change of linear CL was not found for cetuximab in the treatment of HNSCC(36–38) or in mCRC with a small patient dataset(39), indicating a potential impact of tumor type and dataset maturity on the identification of time-dependent CL. In spite of that, a higher global CL was still significantly associated with shorter progression free survival (PFS) in mCRC(39) and poorer OS, PFS, and tumor response in HNSCC(36–38).
Additionally, there was some evidence indicating a CL-response relationship for trastuzumab in advanced gastric or GEJC(11,40), and bevacizumab in mCRC(41). However, trastuzumab PK was not associated with response in HER2+ early breast cancer(42), and bevacizumab serum levels did not predict tumor response in neoadjuvant treatment for early breast cancer(43). These observations could signify a lack of relationship between mAb CL and disease stage at time of treatment.
However, unlike ICIs where combined mAb therapies appear to impact mAb CL, the coadministration of trastuzumab with bevacizumab did not appear to impact the CL of either mAb(30). Similarly, combining chemotherapy with trastuzumab or bevacizumab also did not seem to affect the CL of either mAb(44,45). Therefore, the change of biology with concomitant medications that drives the change of ICI CL might not apply to non-ICI mAbs, which needs to be further investigated.
In non-ICI mAbs for treatment of hematological malignancies, some similarities and differences are noted compared to ICIs in solid tumors. For example, anti-CD20 is a class of mAb therapies where time-dependent CL has been broadly reported. As listed in Table 2, time-dependent linear CL is observed in both type I anti-CD20 (rituximab) and type II anti-CD20 (obinutuzumab), where the maximum CL decrease from baseline varies from 20% to 80%, and time to reach half maximum decrease varies from 9 days to 32 weeks. As for rituximab, time-dependent CL was found in chronic lymphocytic leukemia (CLL)(46), diffuse large B-cell lymphoma (DLBCL)(47), and non-Hodgkin lymphoma (NHL)(48), and the influence of underlying disease on rituximab PK was evident(49). Similar to ICIs, the true exposure-response relationship of rituximab was confounded by underlying biological factors(46), however whether CL could be a better predictor of treatment outcome for rituximab needs to be further evaluated as current evidence is limited (47). Furthermore, whether the underlying confounding factors are similar to that of ICIs (e.g. cachexia) is questionable, as albumin level was not associated with CL or response of rituximab(46). For obinutuzumab (41), the CL (initial and steady-state) and rate of CL decline () were dependent on disease histology (CLL, B-cell lymphoma, DLBCL, or mantle cell lymphoma) and concomitant medications. In general, the initial CL of obinutuzumab was higher in CLL or mantle cell lymphoma than in follicular lymphoma or DLBCL, and the decline of CL was faster in NHL patients than in CLL patients. Although the difference in CL could be due to variations in the level and distribution of target cells between disease types, no association between obinutuzumab exposure and the time course of B-cell counts was observed. Thus it was suggested that the time-dependent CL cannot be fully explained by the rapid depletion of circulating B cells after treatment initiation, and it might be due to other latent factors(50). It is also interesting to highlight that, compared with rituximab, obinutuzumab seems to exhibit a shallower decrease in CL over time. It could be related to the fact that the glycoengineering of obinutuzumab makes it less likely to be internalized via drug-receptor complex (51), but no studies to date have been completed to support or reject this hypothesis.
Daratumumab, an IgG1 mAb targeting CD38, also demonstrated time-dependent linear CL in patients with multiple myeloma (MM) (52). A study by Yan et al. has found that the estimated baseline linear CL of daratumumab was approximately 2-fold higher in IgG MM patients than non-IgG MM patients and the linear CL was correlated with baseline IgG levels(53). Therefore, it has been proposed that the underlying mechanism of CL change might be due to the competition of daratumumab with endogenous IgG for binding to FcRn(54). Since more than 50% of MM patients can produce high levels of IgG M-proteins(55,56), the saturation of FcRn by the hypergammaglobulinemia in MM patients could lead to the increase and time-dependent kinetics in CL of all IgG subclasses, including daratumumab. However, the association between daratumumab CL and treatment outcome is less clear as the clinical outcome is not apparently different between IgG and non-IgG MM patients. It is also unknown whether the proposed mechanism is applicable to ICIs in solid cancers where IgG level is not commonly monitored in clinics.
In non-cancer diseases, the class of mAbs of which time-dependent CL has been broadly reported is anti- mAbs. For example, infliximab is a typical anti- mAb which has proven effective in treatment of various chronic inflammatory diseases. The linear CL of infliximab is found to be time-dependent and is correlated with various covariates such as disease type, disease activity, CRP, cotreatment with immunomodulator, presence of ADAs, etc, suggesting that the CL of infliximab could be also a reflection of overall disease states(57–60). Furthermore, the CL-outcome relationship of infliximab has been studied. In patients with inflammatory bowel diseases, a higher CL at the time of treatment de-escalation was associated with poorer outcome(57). Similarly, an accelerated CL in corticosteroid-refractory acute ulcerative colitis (UC) was associated with poor Week 14 clinical response and Week 54 corticosteroid-free remission rate(60). However, in contrast to ICIs of which the CL tends to decrease over time, the CL of infliximab was observed to decrease in early weeks of treatment for certain patients but increase during later treatment course(59). The acceleration of CL is not mainly due to immunization to infliximab but potentially the fluctuation of disease activity as well.
4. Shared mechanisms between clearance and treatment response with ICI and other mAbs
Therapeutic mAbs are human IgGs that are typically composed of four polypeptide chains, two identical heavy chains, and two identical light chains, linked together by disulfide bonds. The IgG presents two identical antigen-binding (Fab) and one crystallizable (Fc) portion. At the extremity of the variable domain, the Fab portion has a hypervariable region that binds to the specific target antigen with high affinity and specificity. The constant regions of the heavy and light chains determine the isotype and functional properties of the antibody. For example, the Fc region of the antibody can interact with immune cells, such as natural killer cells and macrophages, to trigger a variety of effector functions, including ADCC and CDC. As depicted in Figure 3, the functionality of both the Fab and Fc part of mAbs are, by mechanism, associated with the CL and efficacy of mAbs to some extent, which might help us to better unveil the underlying mechanism of the CL-response relationship.
Figure 3.
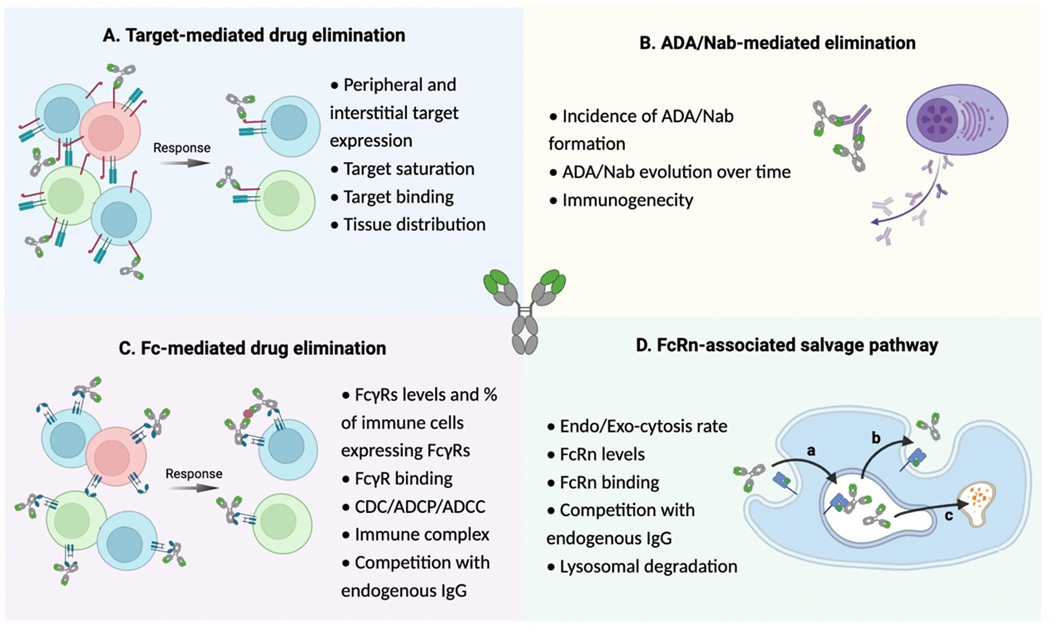
Potential mechanisms of ICI clearance. (mAb, monocloncal antibody; ADA, anti-drug antibody; Nab, neutralizing antibody; CDC, complement-dependent cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; ADCC, antibody-dependent cellular cytotoxicity; FcRn, neonatal Fc receptor. (Image created with BioRender.com.)
4.1. Target engagement
Target-mediated drug disposition is a concept that describes the movement of a drug within the body, influenced by interactions with particular proteins or tissues(61). These interactions allow a drug to either be cleared or actively transported to its target, affecting its overall disposition in the body. The impact of TMDD on mAb PK depends on several factors such as the affinity of the mAb to its target, the target expression level, the mAb dose, the internalization rate of the drug-target complex, and the binding-site barrier in solid tumors.
TMDD can play an important role in mAb PK and the treatment outcome. For example, the interaction between ICIs and checkpoint proteins on the surface of immune cells or cancer cells can lead to target-mediated drug endocytosis as well as induce dynamic changes in the immune microenvironment to maintain a durable anticancer effect. When the drug exposure is not enough to saturate the target, it can result in elevated ICI CL and an unfavorable treatment outcome; When drug exposure is sufficient, TMDD would not be the major contributor to ICI CL, but the dynamic change of target expression after treatment initiation might still impact time-dependent clearance of ICIs, which correlates with treatment efficacy. However, the correlation between ICI CL and its target expression was rarely reported and few studies reported the on-treatment dynamic change of peripheral and tissue target expression. One population pharmacokinetics study of durvalumab found serum PD-L1 expression as one of the significant longitudinal covariates of durvalumab in hematological malignancies, where a decreasing PD-L1 expression in serum was correlated with a decreasing durvalumab CL(62). However, such a relationship was not reported or explored in solid tumors as in other ICIs, potentially due to the logistics and bioanalytical challenges to measure target expression in tumor tissue. Furthermore, although the target engagement is generally believed to be saturated in the systemic circulation at therapeutic doses of ICIs, it is uncertain whether the engagement in tumor tissue is also saturated due to the challenges of measuring drug concentrations and their effects at the site of action in vivo and in real-time. It is also uncertain whether the binging-site barrier negatively affects mAb disposition and efficacy pertaining to the rate of transcapillary transport, the rate of lymphatic outflow, and the antigen density in the context of mAb therapy disposition into solid tumors and therefore the efficacy of treatment (63). However, novel technologies such as positron emission tomography (PET) imaging seems to be promising to elucidate target engagement in tumor. For example, Kumar et al. constructed PET imaging platforms to enable real-time in-vivo measurement of PD-L1 occupancy with different anti-PD-L1s, which provided insights into different PK and PD effects of anti-PD-L1 mAbs within the tumor bed (64,65). Many other radionuclide-conjugated mAbs are currently tested in clinical trials to gain insights into their biodistribution and target expression in humans(66–68). Therefore, it would be of interest to utilize such technology to better understand how and by what extent TMDD contributes to the CL-response phenomenon of mAbs.
4.2. FcRn-mediated mechanisms
The FcRn-mediated salvage pathway is essential for prolonged half-life of therapeutic mAbs. FcRn resides in endothelial cells and hematopoietic cells and functions as a heterodimer comprising an MHC class I-like alpha chain and beta-2-microglobulin. After cellular uptake, FcRn binds the Fc portion of IgG at acidic pH (<~6.0) and shuttles it away from the lysosomal pathway(69,70). Protected from lysosomal degradation, the IgG is recycled back to cell membrane (apical or basolateral for epithelial cells). When the recycling endosome fuses with the cell membrane, pH returns to neutral (7.4), and IgG is released from FcRn.
As shown in Figure 3 the impact of FcRn on mAb CL is dependent on several factors, including endocytosis or exocytosis rate, FcRn level, FcRn binding, competition with endogenous IgG, and lysosomal degradation. The impact of complete or partial loss of FcRn function on mAb CL has been well-characterized in several studies. It is understood that the main cell types responsible for IgG homeostasis are vascular endothelial and bone marrow-derived hemopoietic cells(71,72) and total genetic knockout of FcRn results in decreased circulating IgG and albumin levels(73). However, in cell- or tissue-specific knockouts, FcRn in macrophages is revealed to contribute more significantly to IgG salvage than FcRn in endothelial cells. This is believed to be due to the higher rate of endocytosis and catabolism of IgG in macrophages and other phagocytic hemopoietic cells compared to endothelial cells(74).
In cancer patients, however, the abundance and functionality of FcRn has not been well-characterized, nor has there been a thorough assessment of the relationship between therapeutic mAb CL and FcRn expression or function. In addition to recycling IgG, FcRn also recycles albumin through pH-dependent binding, though at a different, non-IgG competitive binding site(69,75). Since albumin and ICIs are both salvaged by FcRn to yield long half-lives, and since low albumin levels often correlate with higher therapeutic mAb CL(76,77), serum albumin levels may provide some information on the abundance and recycling functionality of FcRn in cancer patients.
Unlike albumin, endogenous IgG competes with ICIs and other therapeutic mAbs for binding to FcRn. Though several studies have reported that a higher endogenous IgG level is correlated with a higher mAb CL(78,79), IgG is not commonly identified as a covariate in PK analyses. This may be due to relatively high endogenous IgG concentrations compared to the therapeutic mAb, and therefore typical variability in IgG levels may not be adequate to produce measurable differences in therapeutic mAb CL across individuals.
Compared to the mechanism of FcRn in regulating mAb CL, the role FcRn plays in anticancer efficacy is less well-understood. Prior studies have evaluated FcRn within the tumor microenvironment, and the cells that express a majority of FcRn are primarily resident and tumor-infiltrating immune cells and not the tumor tissue itself (80). Similarly, FcRn+ dendritic cells within the tumors and adjacent tissue of colorectal cancer patients positively correlated with CD8+ T cell infiltration and were predictive of overall survival(81). Additionally, FcRn has been found to play a role in the differentiation of pro- or anti-inflammatory macrophages(82), which have vastly different roles in the tumor microenvironment. Taken together, these data suggest that elevated FcRn expression within the tumor microenvironment leads to better anti-tumor efficacy. Therefore, a lack of FcRn function could simultaneously correlate with a rapid mAb CL and poor outcome in cancer patients. However, the underlying mechanisms that may link CL and efficacy are still not clear.
A potential mechanism to connect FcRn with anticancer efficacy is that FcRn can affect the albumin consumption by tumor cells, which is important as a source of amino acids for tumor growth (83). As FcRn salvages albumin from lysosomal degradation within cells, the low and undetectable level of FcRn in tumor cells could increase the degradation of albumin and consequently, the level of albumin-derived amino acids in tumor cells. Mice bearing tumors with lower or knockdown expression of FcRn showed significantly higher accumulation of albumin and glutamate in tumor cells and higher tumor growth (84). Another potential mechanism is that FcRn is required for proper antigen- and cross-presentation between professional antigen-presenting cells (APCs), namely dendritic cells and macrophages, to activate naïve CD4+ and CD8+ T cells. For example, mice lacking dendritic cell FcRn exhibit reduced anti-tumor immunity and increased tumor burden in spontaneous models of colorectal cancer(81). This suggests the potential importance of systemic FcRn in anti-tumor immunity and response to mAbs. However, studies to date focused almost exclusively on tumor intrinsic FcRn without evaluating changes in host tissue FcRn in cancer patients. It is also unclear whether the balance of the apparent dual roles for FcRn as a mediator of mAb salvage and as a regulator of antigen presentation may fundamentality shift in cancer patients. Taken together, we hypothesize that a low ICI CL and favorable anticancer efficacy are, to some extent, related to FcRn abundance and proper functioning in recycling and antigen presentation.
4.3. -mediated mechanisms
Another regulating pathway of mAb CL is via the binding of its crystallizable fragment (Fc) to . are a family of receptors that are primarily expressed on leukocytes such as monocytes, macrophages, and dendritic cells. Based on the structure and function, can be classified into inhibitory receptor and activating receptors (, and ) so that the interactions can induce or diminish a milieu of effector functions such as ADCC, ADCP, CDC, induction of cytokines/chemokines, and endocytosis of opsonized targets(85–87). The CL of mAbs can therefore be correlated with interactions in the way that mAbs are internalized into cells after binding to through receptor-mediated endocytosis or phagocytosis and then degraded intracellularly, which might increase the overall CL of mAbs from systemic circulation.
The -mediated mAb elimination can be impacted by a collection of several factors. A stronger binding affinity of mAbs to could lead to more intense interaction and a higher rate of receptor-mediated elimination. It has been well-recognized that different IgG subclasses have distinct binding affinities to each (88,89). For example, hIgG1 Fc fraction can interact with all to induce a broad range of effector functions, but the Fc of hIgG4 only exhibits strong binding to to induce ADCP. Therefore, for mAbs that are designed to directly eliminate circulating antigens or antigen-bearing cells, such as anti-CD20s, anti-EGFRs, and anti-, the backbone of IgG1 is commonly chosen to trigger stronger cellular toxicities and faster endocytosis of target antigen. The Fc structure of those mAbs can be further modified, such as new generation anti-CD20s, to intensify binding and the effector function(90). Moreover, the binding affinity of Fc to is also impacted by polymorphism(91–95), which is primarily involved with and , where a stronger binding variant would induce a much more intense reaction and then leads to better treatment efficacy. Lastly, the competition of endogenous IgG on occupancy could in theory also impact the magnitude of mAb clearance change mediated by .
However, current evidence regarding the role of in the uptake and CL of mAbs is limited and conflicting results have been reported. As reported by Abuqayyas et al, the pharmacokinetics of 8C2 (murine IgG1 targeting topotecan) and MWReg30 (rat IgG1 targeting GPIIb) were not substantially different between wild-type nude mice and its knock-out counterparts(96,97). Similarly, Leabman et al tested the pharmacokinetics of six variants of hIgG1 mAbs with altered binding and found no impact of affinities on mAb CL in cynomolgus monkeys(98). However, another study by Li et al revealed the impact of mouse strains on the clearance of mAbs via interactions by comparing the pharmacokinetics of hIgG1 mAbs in Balb/c, athymic nude, SCID, and NSG mice. The study found a notable CL increase in NSG mice compared with other mice models which can be rescued by eliminating interactions or saturating with hIVIG supplementation(99). More interestingly, the authors found a significantly elevated frequency of granulocytic myeloid cells in the bone marrow and a higher abundance of CD11b+F4/80+ myeloid cells in the spleen and liver of NSG mice, suggesting the potential contribution of those immune cells to mAb CL via interactions. Another study by Oldham et al confirmed the dependency on mouse strains and further elucidated that the rapid clearance in NOD/SCID mice is limited to hIgG1 and mIgG2a isotypes and is primarily attributed to expression(100).
On the other hand, the magnitude of interaction is widely cited to have a profound impact on mAb efficacy (82,101–107). For example, Dahan et al reported the impact of IgG isotype, and subsequent engagement, on the efficacy of anti-PD-1 and PD-L1 therapies in pre-clinical models. Their findings illustrated that for PD-1 therapy, increasing interaction was associated with reduced anti-tumor efficacy, primarily in the form of mediated T cell depletion, whereas optimal PD-L1 efficacy was illustrated in IgG subtypes that had high interaction with due to modulation of myeloid subsets within the tumor microenvironment(101). Similarly, as for anti-CTLA4, a higher interaction with led to a greater reversal of CTLA4-mediated immune exhaustion which ultimately drives better outcomes(108–110). interaction is also important in mAb therapies outside of ICIs, where increasing interaction with helps to drive durable responses(91) For example, patients with FCGR3A 158 V/V genetic polymorphism results in an increased affinity of rituximab for . This polymorphism was associated with increased response rates in patients receiving rituximab treatment for follicular lymphoma(111–113) and diffuse large B cell lymphoma(114), primarily from the increased capacity for ADCC which is believed to be necessary for rituximab efficacy(106).
Taken together, current evidence supports the irrefutable importance of interaction for the efficacy of ICIs, as well as some other mAb treatments. However, importance in the pharmacokinetics of IgG therapies is not yet fully understood. Although there is significant evidence suggesting a potential role for in the clearance and half-life of mAbs, exact mechanisms and implications are still not fully elucidated. More investigations are warranted in the future to comprehensively evaluate the effect of on mAb PK and PD in humans.
4.4. Cellular pinocytosis and nonspecific clearance
Pinocytotic uptake, also referred to as fluid-phase endocytosis, is a nonspecific process and internalizes all extracellular components into endosomes with high turnover rates. Pinocytotic uptake of mAbs occurs throughout the body via endothelial cells and immune cells (monocytes and macrophages) followed by endosomal transit and sorting of antibodies unbound to FcRn, delivery to lysosomes, and enzymatic catabolism, which constitutes the mechanism of target-independent and nonspecific elimination of mAbs (115). Therefore, the CL of mAbs could be impacted by the pinocytic uptake rate, the acidity of endosomes that facilitates mAb recycling by FcRn, as well as intrinsic catabolism rate. The relationship between the above factors and mAb CL is still under in-vitro and/or in-vivo investigations. For example, several studies have suggested that the increased magnitude of pinocytotic uptake is a mechanism for rapid CL and short half-life of mAbs with greater charge-related non-specific binding to cells (116–119). Other studies have investigated whether mAb CL could be modulated by changing the on and/or off-rate of FcRn-binding affinity at pH 6.0 and pH 7.4 in endosomes, indicating that the acidity of endosomal environment could also impact the endosomal sorting of mAbs and mAb CL(120–123). As for treatment outcomes, the association between nonspecific CL pathways and mAb efficacy is not well studied. It is known that macropinocytosis, as a type of pinocytosis accompanied by the formation of large vesicles, is upregulated in some cancer cells to meet high demands for nutrients and amino acids(124), and therefore might increase the nonspecific elimination of mAb in cancer patients. However, evidence is lacking and needs to be studied in future.
4.5. Formation of anti-drug antibodies (ADA)
The generation of ADA generally results from the induction of humoral response by the immune system after the administration of biological agents into humans (125). It is another factor that could potentially impact drug CL, by forming immune complexes to clear drugs out of circulation, and its clinical outcomes, by either neutralizing drug activity or modulating the immune response. The significant impact of ADA on mAb CL and the clear clinical relevance of ADA formation was mostly reported in non-ICI mAbs, such as anti- mAbs for autoimmune inflammatory diseases(126–129). However, as for ICIs, although there was a trend toward lower drug exposure in ADA+ patients, the exposure was believed to be sufficient regardless of ADA status and the impact of ADA status or titer as a longitudinal covariate of ICI CL was generally considered to be minimal and not clinically significant (130–133). On the other hand, although the correlation between ADA status and clinical outcome was reported for ipilimumab in a small-scale study(134), the clinical relevance of ADA status in ICI-treated patients has been extensively reviewed and did not show evidence of clinical relevance with pooled data of other ICIs (5,135–137). Therefore, the ADA profile of ICIs so far cannot provide much insight for us to understand the underlying mechanisms of CL and treatment outcomes. It would be of interest to investigate why the clinical relevance and PK impact of ADA are distinctly different between ICIs and non-ICI mAbs in order to better understand such relationships.
5. Current knowledge gaps and future directions
5.1. Implications of cachexia on the clearance-response relationship
Cachexia is a multifactorial wasting syndrome that occurs in many types of cancer as well as a chronic inflammatory disease(138–140). Patients with cachexia often present with involuntary loss of skeletal muscle mass due to systemic inflammation and aberrant catabolism, though cancer cachexia is highly heterogenous and may be present in any of three consecutive stages, pre-cachexia, cachexia, or refractory cachexia (141). The association between cachexia, treatment response, and mAb CL has been reported in patients with cancers, and we speculate baseline ICI CL () may ultimately serve as an early biomarker for cachexia, even in pre-cachectic patients not yet displaying clinical signs of wasting. On one hand, cachexia is associated with decreased response and poor survival outcomes in various cancer types treated with ICIs(142–146). On the other hand, though the evidence is limited, cachectic patients with advanced melanoma or NSCLC also exhibited rapid CL of pembrolizumab, which was correlated with worse survival outcomes(7). Such elevated catabolic CL of pembrolizumab was replicated in cachectic mouse models, where a decreased liver Fcgrt expression was observed and an altered FcRn-mediated clearance pathway in cachexia was suspected (8). However, more evidence and investigations are still warranted to identify if such phenomena occur across various ICIs and cancer types or even in other mAbs in patients with chronic inflammatory diseases. Due to the lack of consensus on the definition of cachexia and questionable diagnosis criteria, additional effort will be needed in future studies evaluating the apparent link between cachexia, ICI CL, and outcomes to ensure consistent identification of patients with cachexia and additional delineation of cachexia staging. This will require both retrospective and prospective analyses combining prognostic disease biomarkers and utilizing state-of-the art imaging and analytical assessments to evaluate known and suspected biomarkers for cachexia (141,147,148)
5.2. ICI clearance and irAEs
The association between ICI CL and irAEs is largely unknown. However, we speculate that the elevated ICI CL in cachectic patients may correlate with a lower likelihood of developing irAE, considering the immunosuppressive phenotype in cachexia. As for ICI CL, so far only single-center studies reported the association between ICI CL and irAE risk profile and showed no significant difference in ICI CL between patients with grade 1–2 irAE and those with higher grade irAE(17,18). The irAE risk profile in cachectic patients is also currently inconsistent. In a preliminary report of NSCLC patients receiving nivolumab, patients with a low muscle mass developed less any-grade irAE compared to non-low muscle mass patients(149). Conversely, several studies have shown a higher risk of developing grade 3–4 irAE during nivolumab or ipilimumab treatment in patients with low skeletal muscle mass(150,151). It was also found that the combination of low muscle mass (sarcopenia) and high-fat mass (overweight) was associated with more early acute limiting toxicities in melanoma patients treated with nivolumab or pembrolizumab(152). Therefore, prospective studies specifically addressing the relationship between irAE and mAb CL and investigations of the underlying mechanisms associated with CL pathways such as interactions need to be conducted in the future.
5.3. Integrating ICI clearance into future biomarker research
Identification of prognostic and predictive biomarkers and biomarker signatures that can inform clinical decision-making remains a major focus in immuno-oncology. Here we highlight what others have already demonstrated - the potential utility of baseline and time-varying CL of ICIs and other mAb therapies as prognostic biomarkers. We also reviewed the array of potential mechanisms that can cause altered ICI CL in cancer patients, and we propose these mechanisms may provide further insights into mechanisms of resistance to ICI therapies. As mentioned, others have utilized machine learning approaches to identify cytokine signatures that correlate with ICI CL and outcomes(20,21), and the entire immuno-oncology field is investing heavily in similar approaches for discovery of novel biomarkers, combined signatures, or optimized panels of biomarkers that will further aid in prognosis and therapeutic decision-making (153–156). Liquid biopsies are increasingly used to inform machine learning approaches, as they enable analysis of circulating tumor DNA, tumor cells, micro RNA, and exosomes through minimally invasive peripheral blood sampling and have demonstrated utility in early disease detection and prognosis (157–159). While standardization of methods for ICI drug quantification and CL estimation are needed, the cost to incorporate baseline and even time-varying ICI CL is low, especially relative to other sampling, sample analyses and data analyses costs associated with biomarker research involving multi-omics profiling. Thus, integrating sampling and analysis for ICI CL into biomarker research, where this data can be further explored among all other data (clinical, molecular, genetic, imaging, etc.), should be considered and will enable a more comprehensive look at mechanisms linking ICI CL efficacy.
Acknowledgment:
This work is supported by the following National Institutes of Health (NIH) grants: R01CA273924 (T.A. Mace, L.P. Ganesan, D.H. Owen, C.C. Coss, and M. A. Phelps), U24CA247648 (M. A. Phelps), and P30CA016058 (M. A. Phelps).
Author Disclosures:
D.H.O. receives research funding from Merck, Pfizer, Onc.AI, Genentech, Palobiofarma, Turning Point Therapeutic, Bristol Myers Squibb and Travel Funding from Astrazeneca.
Footnotes
No other authors have anything to disclose.
References
- 1.Karasarides M, Cogdill AP, Robbins PB, Bowden M, Burton EM, Butterfield LH, et al. Hallmarks of Resistance to Immune-Checkpoint Inhibitors. Cancer Immunol Res 2022;10(4):372–83 doi 10.1158/2326-6066.CIR-20-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein C, Jamois C, Nielsen T. Anti-CD20 treatment for B-cell malignancies: current status and future directions. Expert Opin Biol Ther 2021;21(2):161–81 doi 10.1080/14712598.2020.1822318. [DOI] [PubMed] [Google Scholar]
- 3.Roda G, Jharap B, Neeraj N, Colombel JF. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin Transl Gastroenterol 2016;7(1):e135 doi 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee M, Turner DC, Felip E, Lena H, Cappuzzo F, Horn L, et al. Systematic evaluation of pembrolizumab dosing in patients with advanced non-small-cell lung cancer. Ann Oncol 2016;27(7):1291–8 doi 10.1093/annonc/mdw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agrawal S, Statkevich P, Bajaj G, Feng Y, Saeger S, Desai DD, et al. Evaluation of Immunogenicity of Nivolumab Monotherapy and Its Clinical Relevance in Patients With Metastatic Solid Tumors. The Journal of Clinical Pharmacology 2017;57(3):394–400 doi 10.1002/jcph.818. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Feng Y, Bajaj G, Gupta M, Agrawal S, Yang A, et al. Quantitative Characterization of the Exposure-Response Relationship for Cancer Immunotherapy: A Case Study of Nivolumab in Patients With Advanced Melanoma. CPT Pharmacometrics Syst Pharmacol 2017;6(1):40–8 doi 10.1002/psp4.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner DC, Kondic AG, Anderson KM, Robinson AG, Garon EB, Riess JW, et al. Pembrolizumab Exposure-Response Assessments Challenged by Association of Cancer Cachexia and Catabolic Clearance. Clin Cancer Res 2018;24(23):5841–9 doi 10.1158/1078-0432.CCR-18-0415. [DOI] [PubMed] [Google Scholar]
- 8.Castillo AMM, Vu TT, Liva SG, Chen M, Xie Z, Thomas J, et al. Murine cancer cachexia models replicate elevated catabolic pembrolizumab clearance in humans. JCSM Rapid Commun 2021;4(2):232–44 doi 10.1002/rco2.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badawi M, Coss CC, Phelps MA. Letter to the Editor: Exposure-response or clearance-response relationship in immune checkpoint therapy?-A comment on ‘correlation between nivolumab exposure and treatment outcomes in non-small-cell lung cancer’ by Basak et al et al. Eur J Cancer 2019;114:25–6 doi 10.1016/j.ejca.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Dai HI, Vugmeyster Y, Mangal N. Characterizing Exposure-Response Relationship for Therapeutic Monoclonal Antibodies in Immuno-Oncology and Beyond: Challenges, Perspectives, and Prospects. Clin Pharmacol Ther 2020;108(6):1156–70 doi 10.1002/cpt.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Zhao H, Garnett C, Rahman A, Gobburu JV, Pierce W, et al. The combination of exposure-response and case-control analyses in regulatory decision making. J Clin Pharmacol 2013;53(2):160–6 doi 10.1177/0091270012445206. [DOI] [PubMed] [Google Scholar]
- 12.Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 2001;28(6):507–32 doi 10.1023/a:1014414520282. [DOI] [PubMed] [Google Scholar]
- 13.Dua P, Hawkins E, van der Graaf PH. A Tutorial on Target-Mediated Drug Disposition (TMDD) Models. CPT Pharmacometrics Syst Pharmacol 2015;4(6):324–37 doi 10.1002/psp4.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petitcollin A, Bensalem A, Verdier MC, Tron C, Lemaitre F, Paintaud G, et al. Modelling of the Time-Varying Pharmacokinetics of Therapeutic Monoclonal Antibodies: A Literature Review. Clin Pharmacokinet 2020;59(1):37–49 doi 10.1007/s40262-019-00816-7. [DOI] [PubMed] [Google Scholar]
- 15.Wang E, Kang D, Bae KS, Marshall MA, Pavlov D, Parivar K. Population pharmacokinetic and pharmacodynamic analysis of tremelimumab in patients with metastatic melanoma. J Clin Pharmacol 2014;54(10):1108–16 doi 10.1002/jcph.309. [DOI] [PubMed] [Google Scholar]
- 16.Baverel P, Roskos L, Tatipalli M, Lee N, Stockman P, Taboada M, et al. Exposure-Response Analysis of Overall Survival for Tremelimumab in Unresectable Malignant Mesothelioma: The Confounding Effect of Disease Status. Clin Transl Sci 2019;12(5):450–8 doi 10.1111/cts.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurkmans DP, Sassen SDT, de Joode K, Putter L, Basak EA, Wijkhuijs AJM, et al. Prospective real-world study on the pharmacokinetics of pembrolizumab in patients with solid tumors. J Immunother Cancer 2021;9(6) doi 10.1136/jitc-2021-002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurkmans DP, Basak EA, van Dijk T, Mercieca D, Schreurs MWJ, Wijkhuijs AJM, et al. A prospective cohort study on the pharmacokinetics of nivolumab in metastatic non-small cell lung cancer, melanoma, and renal cell cancer patients. J Immunother Cancer 2019;7(1):192 doi 10.1186/s40425-019-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Opdualag (nivolumab and relatlimib-rmbw) NDA/BLA Multi-disciplinary Review and Evaluation. In: Administration FaD, editor. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761234Orig1s000MultidisciplineR.pdf2020. [Google Scholar]
- 20.Wang R, Zheng J, Shao X, Ishii Y, Roy A, Bello A, et al. Development of a prognostic composite cytokine signature based on the correlation with nivolumab clearance: translational PK/PD analysis in patients with renal cell carcinoma. J Immunother Cancer 2019;7(1):348 doi 10.1186/s40425-019-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R, Shao X, Zheng J, Saci A, Qian X, Pak I, et al. A Machine-Learning Approach to Identify a Prognostic Cytokine Signature That Is Associated With Nivolumab Clearance in Patients With Advanced Melanoma. Clin Pharmacol Ther 2020;107(4):978–87 doi 10.1002/cpt.1724. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Yu J, Li H, Liu J, Xu Y, Song P, et al. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin Pharmacol Ther 2017;101(5):657–66 doi 10.1002/cpt.656. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Yu J, Liu C, Liu J, Subramaniam S, Zhao H, et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J Pharmacokinet Pharmacodyn 2017;44(5):403–14 doi 10.1007/s10928-017-9528-y. [DOI] [PubMed] [Google Scholar]
- 24.Yang F, Paccaly AJ, Rippley RK, Davis JD, DiCioccio AT. Population pharmacokinetic characteristics of cemiplimab in patients with advanced malignancies. J Pharmacokinet Pharmacodyn 2021;48(4):479–94 doi 10.1007/s10928-021-09739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilkins JJ, Brockhaus B, Dai H, Vugmeyster Y, White JT, Brar S, et al. Time-Varying Clearance and Impact of Disease State on the Pharmacokinetics of Avelumab in Merkel Cell Carcinoma and Urothelial Carcinoma. CPT Pharmacometrics Syst Pharmacol 2019;8(6):415–27 doi 10.1002/psp4.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanghavi K, Zhang J, Zhao X, Feng Y, Statkevich P, Sheng J, et al. Population Pharmacokinetics of Ipilimumab in Combination With Nivolumab in Patients With Advanced Solid Tumors. CPT Pharmacometrics Syst Pharmacol 2020;9(1):29–39 doi 10.1002/psp4.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centanni M, Moes D, Troconiz IF, Ciccolini J, van Hasselt JGC. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin Pharmacokinet 2019;58(7):835–57 doi 10.1007/s40262-019-00748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamuro L, Hu Z, Passarell J, Barcomb H, Zhang J, Goldstein S, et al. Exposure-Response Analysis to Support Nivolumab Once Every 4 Weeks Dosing in Combination with Cabozantinib in Renal Cell Carcinoma. Clin Cancer Res 2022;28(8):1603–13 doi 10.1158/1078-0432.CCR-21-3149. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Sanghavi K, Shen J, Zhao X, Feng Y, Statkevich P, et al. Population Pharmacokinetics of Nivolumab in Combination With Ipilimumab in Patients With Advanced Malignancies. CPT Pharmacometrics Syst Pharmacol 2019;8(12):962–70 doi 10.1002/psp4.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petitcollin A, Azzopardi N, Pierga JY, Ternant D, Navarro-Teulon I, Desvignes C, et al. Population pharmacokinetics and exposure-response relationship of trastuzumab and bevacizumab in early-stage breast cancer. Eur J Clin Pharmacol 2021;77(12):1861–73 doi 10.1007/s00228-021-03179-w. [DOI] [PubMed] [Google Scholar]
- 31.Cortes J, Swain SM, Kudaba I, Hauschild M, Patel T, Grincuka E, et al. Absence of pharmacokinetic drug-drug interaction of pertuzumab with trastuzumab and docetaxel. Anticancer Drugs 2013;24(10):1084–92 doi 10.1097/CAD.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 32.Hamuro L, Statkevich P, Bello A, Roy A, Bajaj G. Nivolumab Clearance Is Stationary in Patients With Resected Melanoma on Adjuvant Therapy: Implications of Disease Status on Time-Varying Clearance. Clin Pharmacol Ther 2019;106(5):1018–27 doi 10.1002/cpt.1502. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Sun Y, Yu J, Liu C, Liu J, Wang Y. Semimechanistically Based Modeling of Pembrolizumab Time-Varying Clearance Using 4 Longitudinal Covariates in Patients With Non-Small Cell Lung Cancer. J Pharm Sci 2019;108(1):692–700 doi 10.1016/j.xphs.2018.10.064. [DOI] [PubMed] [Google Scholar]
- 34.Tsujimoto TI A, Zhao Y, Feng Y, Gao L. MODEL-BASED POPULATION PHARMACOKINETIC ANALYSIS OF NIVOLUMAB AS ADJUVANT TREATMENT IN PATIENTS WITH ESOPHAGEAL OR GASTROESOPHAGEAL JUNCTION CANCER (CHECKMATE-577). American Society of Clinical Pharmacology and Therapeutics: Clinical Pharmacology and Therapeutics; 2022. [DOI] [PubMed]
- 35.Grisic AM, Khandelwal A, Bertolino M, Huisinga W, Girard P, Kloft C. Semimechanistic Clearance Models of Oncology Biotherapeutics and Impact of Study Design: Cetuximab as a Case Study. CPT Pharmacometrics Syst Pharmacol 2020;9(11):628–38 doi 10.1002/psp4.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dirks NL, Nolting A, Kovar A, Meibohm B. Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol 2008;48(3):267–78 doi 10.1177/0091270007313393. [DOI] [PubMed] [Google Scholar]
- 37.Pointreau Y, Azzopardi N, Ternant D, Calais G, Paintaud G. Cetuximab Pharmacokinetics Influences Overall Survival in Patients With Head and Neck Cancer. Ther Drug Monit 2016;38(5):567–72 doi 10.1097/FTD.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 38.Le Louedec F, Alix-Panabieres C, Lafont T, Allal BC, Garrel R, Digue L, et al. Cetuximab pharmacokinetic/pharmacodynamics relationships in advanced head and neck carcinoma patients. Br J Clin Pharmacol 2019;85(6):1357–66 doi 10.1111/bcp.13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azzopardi N, Lecomte T, Ternant D, Boisdron-Celle M, Piller F, Morel A, et al. Cetuximab pharmacokinetics influences progression-free survival of metastatic colorectal cancer patients. Clin Cancer Res 2011;17(19):6329–37 doi 10.1158/1078-0432.CCR-11-1081. [DOI] [PubMed] [Google Scholar]
- 40.Cosson VF, Ng VW, Lehle M, Lum BL. Population pharmacokinetics and exposure-response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol 2014;73(4):737–47 doi 10.1007/s00280-014-2400-5. [DOI] [PubMed] [Google Scholar]
- 41.Caulet M, Lecomte T, Bouche O, Rollin J, Gouilleux-Gruart V, Azzopardi N, et al. Bevacizumab Pharmacokinetics Influence Overall and Progression-Free Survival in Metastatic Colorectal Cancer Patients. Clin Pharmacokinet 2016;55(11):1381–94 doi 10.1007/s40262-016-0406-3. [DOI] [PubMed] [Google Scholar]
- 42.Quartino AL, Hillenbach C, Li J, Li H, Wada RD, Visich J, et al. Population pharmacokinetic and exposure-response analysis for trastuzumab administered using a subcutaneous “manual syringe” injection or intravenously in women with HER2-positive early breast cancer. Cancer Chemother Pharmacol 2016;77(1):77–88 doi 10.1007/s00280-015-2922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabatier R, Pierga JY, Cure H, Abulnaja R, Lambaudie E, Bidard FC, et al. Circulating Tumor Cells and Bevacizumab Pharmacokinetics during Neoadjuvant Treatment Combining Chemotherapy and Bevacizumab for Early Breast Cancer: Ancillary Analysis of the AVASTEM Trial. Cancers (Basel) 2021;13(1) doi 10.3390/cancers13010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han K, Peyret T, Marchand M, Quartino A, Gosselin NH, Girish S, et al. Population pharmacokinetics of bevacizumab in cancer patients with external validation. Cancer Chemother Pharmacol 2016;78(2):341–51 doi 10.1007/s00280-016-3079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruno R, Washington CB, Lu JF, Lieberman G, Banken L, Klein P. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 2005;56(4):361–9 doi 10.1007/s00280-005-1026-z. [DOI] [PubMed] [Google Scholar]
- 46.Gibiansky E, Gibiansky L, Chavanne C, Frey N, Jamois C. Population pharmacokinetic and exposure-response analyses of intravenous and subcutaneous rituximab in patients with chronic lymphocytic leukemia. CPT Pharmacometrics Syst Pharmacol 2021;10(8):914–27 doi 10.1002/psp4.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rozman S, Grabnar I, Novakovic S, Mrhar A, Jezersek Novakovic B. Population pharmacokinetics of rituximab in patients with diffuse large B-cell lymphoma and association with clinical outcome. Br J Clin Pharmacol 2017;83(8):1782–90 doi 10.1111/bcp.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jumbe NS, Valente N, Joshi A, Woo M, Ren S, Kheoh T, et al. Rituximab Exhibits a Long Half-Life Based on a Population Pharmacokinetic Analysis in Non-Hodgkin’s Lymphoma (NHL) Patients. Blood 2007;110(11):2371- doi 10.1182/blood.V110.11.2371.2371. [DOI] [Google Scholar]
- 49.Bensalem A, Cartron G, Specks U, Mulleman D, Gyan E, Cornec D, et al. The Influence of Underlying Disease on Rituximab Pharmacokinetics May be Explained by Target-Mediated Drug Disposition. Clin Pharmacokinet 2022;61(3):423–37 doi 10.1007/s40262-021-01081-3. [DOI] [PubMed] [Google Scholar]
- 50.Gibiansky E, Gibiansky L, Carlile DJ, Jamois C, Buchheit V, Frey N. Population Pharmacokinetics of Obinutuzumab (GA101) in Chronic Lymphocytic Leukemia (CLL) and Non-Hodgkin’s Lymphoma and Exposure-Response in CLL. CPT Pharmacometrics Syst Pharmacol 2014;3(10):e144 doi 10.1038/psp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tobinai K, Klein C, Oya N, Fingerle-Rowson G. A Review of Obinutuzumab (GA101), a Novel Type II Anti-CD20 Monoclonal Antibody, for the Treatment of Patients with B-Cell Malignancies. Adv Ther 2017;34(2):324–56 doi 10.1007/s12325-016-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu XS, Yan X, Puchalski T, Lonial S, Lokhorst HM, Voorhees PM, et al. Clinical Implications of Complex Pharmacokinetics for Daratumumab Dose Regimen in Patients With Relapsed/Refractory Multiple Myeloma. Clin Pharmacol Ther 2017;101(6):721–4 doi 10.1002/cpt.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan X, Clemens PL, Puchalski T, Lonial S, Lokhorst H, Voorhees PM, et al. Influence of Disease and Patient Characteristics on Daratumumab Exposure and Clinical Outcomes in Relapsed or Refractory Multiple Myeloma. Clin Pharmacokinet 2018;57(4):529–38 doi 10.1007/s40262-017-0598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacobs JFM, Mould DR. The Role of FcRn in the Pharmacokinetics of Biologics in Patients With Multiple Myeloma. Clin Pharmacol Ther 2017;102(6):903–4 doi 10.1002/cpt.665. [DOI] [PubMed] [Google Scholar]
- 55.Landgren O, Hofmann JN, McShane CM, Santo L, Hultcrantz M, Korde N, et al. Association of Immune Marker Changes With Progression of Monoclonal Gammopathy of Undetermined Significance to Multiple Myeloma. JAMA Oncol 2019;5(9):1293–301 doi 10.1001/jamaoncol.2019.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc 2003;78(1):21–33 doi 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 57.Petitcollin A, Brochard C, Siproudhis L, Tron C, Verdier MC, Lemaitre F, et al. Pharmacokinetic Parameters of Infliximab Influence the Rate of Relapse After De-Escalation in Adults With Inflammatory Bowel Diseases. Clin Pharmacol Ther 2019;106(3):605–15 doi 10.1002/cpt.1429. [DOI] [PubMed] [Google Scholar]
- 58.Passot C, Mulleman D, Bejan-Angoulvant T, Aubourg A, Willot S, Lecomte T, et al. The underlying inflammatory chronic disease influences infliximab pharmacokinetics. MAbs 2016;8(7):1407–16 doi 10.1080/19420862.2016.1216741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petitcollin A, Leuret O, Tron C, Lemaitre F, Verdier MC, Paintaud G, et al. Modeling Immunization To Infliximab in Children With Crohn’s Disease Using Population Pharmacokinetics: A Pilot Study. Inflamm Bowel Dis 2018;24(8):1745–54 doi 10.1093/ibd/izy129. [DOI] [PubMed] [Google Scholar]
- 60.Kevans D, Murthy S, Mould DR, Silverberg MS. Accelerated Clearance of Infliximab is Associated With Treatment Failure in Patients With Corticosteroid-Refractory Acute Ulcerative Colitis. J Crohns Colitis 2018;12(6):662–9 doi 10.1093/ecco-jcc/jjy028. [DOI] [PubMed] [Google Scholar]
- 61.An G. Concept of Pharmacologic Target-Mediated Drug Disposition in Large-Molecule and Small-Molecule Compounds. J Clin Pharmacol 2020;60(2):149–63 doi 10.1002/jcph.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogasawara K, Newhall K, Maxwell SE, Dell’Aringa J, Komashko V, Kilavuz N, et al. Population Pharmacokinetics of an Anti-PD-L1 Antibody, Durvalumab in Patients with Hematologic Malignancies. Clin Pharmacokinet 2020;59(2):217–27 doi 10.1007/s40262-019-00804-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Osdol W, Fujimori K, Weinstein JN. An analysis of monoclonal antibody distribution in microscopic tumor nodules: consequences of a “binding site barrier”. Cancer Res 1991;51(18):4776–84. [PubMed] [Google Scholar]
- 64.Kumar D, Mishra A, Lisok A, Kureshi R, Shelake S, Plyku D, et al. Pharmacodynamic measures within tumors expose differential activity of PD(L)-1 antibody therapeutics. Proc Natl Acad Sci U S A 2021;118(37) doi 10.1073/pnas.2107982118. [DOI] [PMC free article] [PubMed]
- 65.Kumar D, Lisok A, Dahmane E, McCoy M, Shelake S, Chatterjee S, et al. Peptide-based PET quantifies target engagement of PD-L1 therapeutics. J Clin Invest 2019;129(2):616–30 doi 10.1172/JCI122216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Vries EGE, Kist de Ruijter L, Lub-de Hooge MN, Dierckx RA, Elias SG, Oosting SF. Integrating molecular nuclear imaging in clinical research to improve anticancer therapy. Nat Rev Clin Oncol 2019;16(4):241–55 doi 10.1038/s41571-018-0123-y. [DOI] [PubMed] [Google Scholar]
- 67.Bensch F, van der Veen EL, Lub-de Hooge MN, Jorritsma-Smit A, Boellaard R, Kok IC, et al. (89)Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med 2018;24(12):1852–8 doi 10.1038/s41591-018-0255-8. [DOI] [PubMed] [Google Scholar]
- 68.Vento J, Mulgaonkar A, Woolford L, Nham K, Christie A, Bagrodia A, et al. PD-L1 detection using (89)Zr-atezolizumab immuno-PET in renal cell carcinoma tumorgrafts from a patient with favorable nivolumab response. J Immunother Cancer 2019;7(1):144 doi 10.1186/s40425-019-0607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oganesyan V, Damschroder MM, Cook KE, Li Q, Gao C, Wu H, et al. Structural insights into neonatal Fc receptor-based recycling mechanisms. J Biol Chem 2014;289(11):7812–24 doi 10.1074/jbc.M113.537563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the Site and Dynamics of IgG Salvage by the MHC Class I-Related Receptor, FcRn. The Journal of Immunology 2004;172(4):2021–9 doi 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 71.Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci U S A 2009;106(8):2788–93 doi 10.1073/pnas.0810796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Akilesh S, Christianson GJ, Roopenian DC, Shaw AS. Neonatal FcR expression in bone marrow-derived cells functions to protect serum IgG from catabolism. J Immunol 2007;179(7):4580–8 doi 10.4049/jimmunol.179.7.4580. [DOI] [PubMed] [Google Scholar]
- 73.Challa DK, Wang X, Montoyo HP, Velmurugan R, Ober RJ, Ward ES. Neonatal Fc receptor expression in macrophages is indispensable for IgG homeostasis. MAbs 2019;11(5):848–60 doi 10.1080/19420862.2019.1602459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richter WF, Christianson GJ, Frances N, Grimm HP, Proetzel G, Roopenian DC. Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice. MAbs 2018;10(5):803–13 doi 10.1080/19420862.2018.1458808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt MM, Townson SA, Andreucci AJ, King BM, Schirmer EB, Murillo AJ, et al. Crystal structure of an HSA/FcRn complex reveals recycling by competitive mimicry of HSA ligands at a pH-dependent hydrophobic interface. Structure 2013;21(11):1966–78 doi 10.1016/j.str.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 76.Mould DR. The Pharmacokinetics of Biologics: A Primer. Dig Dis 2015;33 Suppl 1:61–9 doi 10.1159/000437077. [DOI] [PubMed] [Google Scholar]
- 77.Fasanmade AA, Adedokun OJ, Olson A, Strauss R, Davis HM. Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther 2010;48(5):297–308 doi 10.5414/cpp48297. [DOI] [PubMed] [Google Scholar]
- 78.Fahey JL, Robinson AG. Factors Controlling Serum Gamma-Globulin Concentration. J Exp Med 1963;118(5):845–68 doi 10.1084/jem.118.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pyzik M, Sand KMK, Hubbard JJ, Andersen JT, Sandlie I, Blumberg RS. The Neonatal Fc Receptor (FcRn): A Misnomer? Front Immunol 2019;10:1540 doi 10.3389/fimmu.2019.01540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dalloneau E, Baroukh N, Mavridis K, Maillet A, Gueugnon F, Courty Y, et al. Downregulation of the neonatal Fc receptor expression in non-small cell lung cancer tissue is associated with a poor prognosis. Oncotarget 2016;7(34):54415–29 doi 10.18632/oncotarget.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baker K, Rath T, Flak MB, Arthur JC, Chen Z, Glickman JN, et al. Neonatal Fc receptor expression in dendritic cells mediates protective immunity against colorectal cancer. Immunity 2013;39(6):1095–107 doi 10.1016/j.immuni.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamamy J, Larue A, Mariot J, Dhommee C, Demattei MV, Delneste Y, et al. The neonatal Fc receptor expression during macrophage differentiation is related to autophagy. Front Immunol 2022;13:1054425 doi 10.3389/fimmu.2022.1054425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stehle G, Sinn H, Wunder A, Schrenk HH, Stewart JC, Hartung G, et al. Plasma protein (albumin) catabolism by the tumor itself--implications for tumor metabolism and the genesis of cachexia. Crit Rev Oncol Hematol 1997;26(2):77–100 doi 10.1016/s1040-8428(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 84.Swiercz R, Mo M, Khare P, Schneider Z, Ober RJ, Ward ES. Loss of expression of the recycling receptor, FcRn, promotes tumor cell growth by increasing albumin consumption. Oncotarget 2017;8(2):3528–41 doi 10.18632/oncotarget.13869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu R, Oldham RJ, Teal E, Beers SA, Cragg MS. Fc-Engineering for Modulated Effector Functions-Improving Antibodies for Cancer Treatment . Antibodies (Basel) 2020;9(4) doi 10.3390/antib9040064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chenoweth AM, Wines BD, Anania JC, Mark Hogarth P. Harnessing the immune system via FcgammaR function in immune therapy: a pathway to next-gen mAbs. Immunol Cell Biol 2020;98(4):287–304 doi 10.1111/imcb.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen X, Song X, Li K, Zhang T. FcgammaR-Binding Is an Important Functional Attribute for Immune Checkpoint Antibodies in Cancer Immunotherapy. Front Immunol 2019;10:292 doi 10.3389/fimmu.2019.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014;5:520 doi 10.3389/fimmu.2014.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009;113(16):3716–25 doi 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 90.Oflazoglu E, Audoly LP. Evolution of anti-CD20 monoclonal antibody therapeutics in oncology. MAbs 2010;2(1):14–9 doi 10.4161/mabs.2.1.10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 2013;6:1 doi 10.1186/1756-8722-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tout M, Gagez AL, Lepretre S, Gouilleux-Gruart V, Azzopardi N, Delmer A, et al. Influence of FCGR3A-158V/F Genotype and Baseline CD20 Antigen Count on Target-Mediated Elimination of Rituximab in Patients with Chronic Lymphocytic Leukemia: A Study of FILO Group. Clin Pharmacokinet 2017;56(6):635–47 doi 10.1007/s40262-016-0470-8. [DOI] [PubMed] [Google Scholar]
- 93.Nishio M, Endo T, Fujimoto K, Yamamoto S, Obara M, Yamaguchi K, et al. FCGR3A-158V/F polymorphism may correlate with the levels of immunoglobulin in patients with non-Hodgkin’s lymphoma after rituximab treatment as an adjuvant to autologous stem cell transplantation. Eur J Haematol 2009;82(2):143–7 doi 10.1111/j.1600-0609.2008.01174.x. [DOI] [PubMed] [Google Scholar]
- 94.Geva R, Vecchione L, Kalogeras KT, Jensen BV, Lenz HJ, Yoshino T, et al. FCGR polymorphisms and cetuximab efficacy in chemorefractory metastatic colorectal cancer: an international consortium study. Gut 2015;64(6):921–8 doi 10.1136/gutjnl-2014-307234. [DOI] [PubMed] [Google Scholar]
- 95.Ternant D, Berkane Z, Picon L, Gouilleux-Gruart V, Colombel JF, Allez M, et al. Assessment of the Influence of Inflammation and FCGR3A Genotype on Infliximab Pharmacokinetics and Time to Relapse in Patients with Crohn’s Disease. Clin Pharmacokinet 2015;54(5):551–62 doi 10.1007/s40262-014-0225-3. [DOI] [PubMed] [Google Scholar]
- 96.Abuqayyas L, Balthasar JP. Application of knockout mouse models to investigate the influence of FcgammaR on the tissue distribution and elimination of 8C2, a murine IgG1 monoclonal antibody. Int J Pharm 2012;439(1–2):8–16 doi 10.1016/j.ijpharm.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 97.Abuqayyas L, Zhang X, Balthasar JP. Application of knockout mouse models to investigate the influence of on the pharmacokinetics and anti-platelet effects of MWReg30, a monoclonal anti-GPIIb antibody. International Journal of Pharmaceutics 2013;444(1–2):185–92 doi 10.1016/j.ijpharm.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leabman MK, Meng YG, Kelley RF, DeForge LE, Cowan KJ, Iyer S. Effects of altered FcgammaR binding on antibody pharmacokinetics in cynomolgus monkeys. MAbs 2013;5(6):896–903 doi 10.4161/mabs.26436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li F, Ulrich ML, Shih VF-S, Cochran JH, Hunter JH, Westendorf L, et al. Mouse Strains Influence Clearance and Efficacy of Antibody and Antibody–Drug Conjugate Via Interaction. Molecular Cancer Therapeutics 2019;18(4):780–7 doi 10.1158/1535-7163.Mct-18-0977. [DOI] [PubMed] [Google Scholar]
- 100.Oldham RJ, Mockridge CI, James S, Duriez PJ, Chan HTC, Cox KL, et al. FcgammaRII (CD32) modulates antibody clearance in NOD SCID mice leading to impaired antibody-mediated tumor cell deletion. J Immunother Cancer 2020;8(1) doi 10.1136/jitc-2020-000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015;28(3):285–95 doi 10.1016/j.ccell.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 102.Moreno-Vicente J, Willoughby JE, Taylor MC, Booth SG, English VL, Williams EL, et al. Fc-null anti-PD-1 monoclonal antibodies deliver optimal checkpoint blockade in diverse immune environments. J Immunother Cancer 2022;10(1) doi 10.1136/jitc-2021-003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med 2017;9(389) doi 10.1126/scitranslmed.aal3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang T, Song X, Xu L, Ma J, Zhang Y, Gong W, et al. The binding of an anti-PD-1 antibody to FcgammaRIota has a profound impact on its biological functions. Cancer Immunol Immunother 2018;67(7):1079–90 doi 10.1007/s00262-018-2160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019;36(2):168–78 e4 doi 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arnould L, Gelly M, Penault-Llorca F, Benoit L, Bonnetain F, Migeon C, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 2006;94(2):259–67 doi 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu Y ZY, Du X, et al. 231 Molecular insights on safety and anti-tumor activity of a non-irAE-inducing anti-CTLA-4 monoclonal antibody ONC-392. Journal for ImmunoTherapy of Cancer 2021. doi doi: 10.1136/jitc-2021-SITC2021.231. [DOI]
- 108.Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 2018;33(4):649–63 e4 doi 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Du X, Tang F, Liu M, Su J, Zhang Y, Wu W, et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy. Cell Res 2018;28(4):416–32 doi 10.1038/s41422-018-0011-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alan J. Korman JE, John Loffredo, Jose, Rahima Akter, Raja Vuyyuru, Natalie Bezman, Paula So, Robert Graziano, Kimberly Tipton, James West, Bryan Irving, Mark Selby. Next-generation anti-CTLA-4 antibodies [abstract]. n: Proceedings of the American Association for Cancer Research Annual Meeting 2017. 2017 doi doi: 10.1158/1538-7445.AM2017-SY09-01. [DOI]
- 111.Persky DO, Dornan D, Goldman BH, Braziel RM, Fisher RI, Leblanc M, et al. Fc gamma receptor 3a genotype predicts overall survival in follicular lymphoma patients treated on SWOG trials with combined monoclonal antibody plus chemotherapy but not chemotherapy alone. Haematologica 2012;97(6):937–42 doi 10.3324/haematol.2011.050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol 2003;21(21):3940–7 doi 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 113.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002;99(3):754–8 doi 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 114.Kim DH, Jung HD, Kim JG, Lee JJ, Yang DH, Park YH, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood 2006;108(8):2720–5 doi 10.1182/blood-2006-01-009480. [DOI] [PubMed] [Google Scholar]
- 115.Ovacik M, Lin K. Tutorial on Monoclonal Antibody Pharmacokinetics and Its Considerations in Early Development. Clin Transl Sci 2018;11(6):540–52 doi 10.1111/cts.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Igawa T, Tsunoda H, Tachibana T, Maeda A, Mimoto F, Moriyama C, et al. Reduced elimination of IgG antibodies by engineering the variable region. Protein Eng Des Sel 2010;23(5):385–92 doi 10.1093/protein/gzq009. [DOI] [PubMed] [Google Scholar]
- 117.Bumbaca D, Boswell CA, Fielder PJ, Khawli LA. Physiochemical and biochemical factors influencing the pharmacokinetics of antibody therapeutics. AAPS J 2012;14(3):554–8 doi 10.1208/s12248-012-9369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Datta-Mannan A, Lu J, Witcher DR, Leung D, Tang Y, Wroblewski VJ. The interplay of non-specific binding, target-mediated clearance and FcRn interactions on the pharmacokinetics of humanized antibodies. MAbs 2015;7(6):1084–93 doi 10.1080/19420862.2015.1075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Datta-Mannan A, Thangaraju A, Leung D, Tang Y, Witcher DR, Lu J, et al. Balancing charge in the complementarity-determining regions of humanized mAbs without affecting pI reduces non-specific binding and improves the pharmacokinetics. MAbs 2015;7(3):483–93 doi 10.1080/19420862.2015.1016696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Datta-Mannan A, Chow CK, Dickinson C, Driver D, Lu J, Witcher DR, et al. FcRn affinity-pharmacokinetic relationship of five human IgG4 antibodies engineered for improved in vitro FcRn binding properties in cynomolgus monkeys. Drug Metab Dispos 2012;40(8):1545–55 doi 10.1124/dmd.112.045864. [DOI] [PubMed] [Google Scholar]
- 121.Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Wroblewski VJ. Monoclonal antibody clearance. Impact of modulating the interaction of IgG with the neonatal Fc receptor. J Biol Chem 2007;282(3):1709–17 doi 10.1074/jbc.M607161200. [DOI] [PubMed] [Google Scholar]
- 122.Datta-Mannan A, Witcher DR, Tang Y, Watkins J, Jiang W, Wroblewski VJ. Humanized IgG1 variants with differential binding properties to the neonatal Fc receptor: relationship to pharmacokinetics in mice and primates. Drug Metab Dispos 2007;35(1):86–94 doi 10.1124/dmd.106.011734. [DOI] [PubMed] [Google Scholar]
- 123.Dall’Acqua WF, Kiener PA, Wu H. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J Biol Chem 2006;281(33):23514–24 doi 10.1074/jbc.M604292200. [DOI] [PubMed] [Google Scholar]
- 124.Xiao F, Li J, Huang K, Li X, Xiong Y, Wu M, et al. Macropinocytosis: mechanism and targeted therapy in cancers. Am J Cancer Res 2021;11(1):14–30. [PMC free article] [PubMed] [Google Scholar]
- 125.Vaisman-Mentesh A, Gutierrez-Gonzalez M, DeKosky BJ, Wine Y. The Molecular Mechanisms That Underlie the Immune Biology of Anti-drug Antibody Formation Following Treatment With Monoclonal Antibodies. Front Immunol 2020;11:1951 doi 10.3389/fimmu.2020.01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Benucci M, Li Gobbi F, Meacci F, Manfredi M, Infantino M, Severino M, et al. Antidrug antibodies against TNF-blocking agents: correlations between disease activity, hypersensitivity reactions, and different classes of immunoglobulins. Biologics: Targets and Therapy 2015. doi 10.2147/btt.S69606. [DOI] [PMC free article] [PubMed]
- 127.Pecoraro V, De Santis E, Melegari A, Trenti T. The impact of immunogenicity of TNFalpha inhibitors in autoimmune inflammatory disease. A systematic review and meta-analysis. Autoimmun Rev 2017;16(6):564–75 doi 10.1016/j.autrev.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 128.Strand V, Balsa A, Al-Saleh J, Barile-Fabris L, Horiuchi T, Takeuchi T, et al. Immunogenicity of Biologics in Chronic Inflammatory Diseases: A Systematic Review. BioDrugs 2017;31(4):299–316 doi 10.1007/s40259-017-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berends SE, Strik AS, Van Selm JC, Lowenberg M, Ponsioen CY, D’Haens GR, et al. Explaining Interpatient Variability in Adalimumab Pharmacokinetics in Patients With Crohn’s Disease. Ther Drug Monit 2018;40(2):202–11 doi 10.1097/FTD.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 130.Wu B, Sternheim N, Agarwal P, Suchomel J, Vadhavkar S, Bruno R, et al. Evaluation of atezolizumab immunogenicity: Clinical pharmacology (part 1). Clin Transl Sci 2022;15(1):130–40 doi 10.1111/cts.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Marchand M, Zhang R, Chan P, Quarmby V, Ballinger M, Sternheim N, et al. Time-dependent population PK models of single-agent atezolizumab in patients with cancer. Cancer Chemother Pharmacol 2021;88(2):211–21 doi 10.1007/s00280-021-04276-4. [DOI] [PubMed] [Google Scholar]
- 132.Baverel PG, Dubois VFS, Jin CY, Zheng Y, Song X, Jin X, et al. Population Pharmacokinetics of Durvalumab in Cancer Patients and Association With Longitudinal Biomarkers of Disease Status. Clin Pharmacol Ther 2018;103(4):631–42 doi 10.1002/cpt.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng Y, Masson E, Dai D, Parker SM, Berman D, Roy A. Model-based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma. Br J Clin Pharmacol 2014;78(1):106–17 doi 10.1111/bcp.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kverneland AH, Enevold C, Donia M, Bastholt L, Svane IM, Nielsen CH. Development of anti-drug antibodies is associated with shortened survival in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2018;7(5):e1424674 doi 10.1080/2162402X.2018.1424674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peters S, Galle PR, Bernaards CA, Ballinger M, Bruno R, Quarmby V, et al. Evaluation of atezolizumab immunogenicity: Efficacy and safety (Part 2). Clin Transl Sci 2022;15(1):141–57 doi 10.1111/cts.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van Vugt MJH, Stone JA, De Greef R, Snyder ES, Lipka L, Turner DC, et al. Immunogenicity of pembrolizumab in patients with advanced tumors. J Immunother Cancer 2019;7(1):212 doi 10.1186/s40425-019-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Enrico D, Paci A, Chaput N, Karamouza E, Besse B. Antidrug Antibodies Against Immune Checkpoint Blockers: Impairment of Drug Efficacy or Indication of Immune Activation? Clin Cancer Res 2020;26(4):787–92 doi 10.1158/1078-0432.CCR-19-2337. [DOI] [PubMed] [Google Scholar]
- 138.Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Primers 2018;4:17105 doi 10.1038/nrdp.2017.105. [DOI] [PubMed] [Google Scholar]
- 139.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12(5):489–95 doi 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 140.Schmidt SF, Rohm M, Herzig S, Berriel Diaz M. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer 2018;4(12):849–60 doi 10.1016/j.trecan.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 141.Ni J, Zhang L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag Res 2020;12:5597–605 doi 10.2147/CMAR.S261585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fujii H, Makiyama A, Iihara H, Okumura N, Yamamoto S, Imai T, et al. Cancer Cachexia Reduces the Efficacy of Nivolumab Treatment in Patients With Advanced Gastric Cancer. Anticancer Res 2020;40(12):7067–75 doi 10.21873/anticanres.14734. [DOI] [PubMed] [Google Scholar]
- 143.Jo H, Yoshida T, Horinouchi H, Yagishita S, Matsumoto Y, Shinno Y, et al. Prognostic significance of cachexia in advanced non-small cell lung cancer patients treated with pembrolizumab. Cancer Immunol Immunother 2022;71(2):387–98 doi 10.1007/s00262-021-02997-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roch B, Coffy A, Jean-Baptiste S, Palaysi E, Daures JP, Pujol JL, et al. Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 2020;143:19–26 doi 10.1016/j.lungcan.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 145.Rounis K, Makrakis D, Tsigkas AP, Georgiou A, Galanakis N, Papadaki C, et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: results from a prospective, observational study. Transl Lung Cancer Res 2021;10(8):3538–49 doi 10.21037/tlcr-21-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takenaka Y, Oya R, Takemoto N, Inohara H. Predictive impact of sarcopenia in solid cancers treated with immune checkpoint inhibitors: a meta-analysis. J Cachexia Sarcopenia Muscle 2021;12(5):1122–35 doi 10.1002/jcsm.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cao Z, Zhao K, Jose I, Hoogenraad NJ, Osellame LD. Biomarkers for Cancer Cachexia: A Mini Review. Int J Mol Sci 2021;22(9) doi 10.3390/ijms22094501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Han J, Harrison L, Patzelt L, Wu M, Junker D, Herzig S, et al. Imaging modalities for diagnosis and monitoring of cancer cachexia. EJNMMI Res 2021;11(1):94 doi 10.1186/s13550-021-00834-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cortellini A, Verna L, Porzio G, Bozzetti F, Palumbo P, Masciocchi C, et al. Predictive value of skeletal muscle mass for immunotherapy with nivolumab in non-small cell lung cancer patients: A “hypothesis-generator” preliminary report. Thorac Cancer 2019;10(2):347–51 doi 10.1111/1759-7714.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Daly LE, Power DG, O’Reilly A, Donnellan P, Cushen SJ, O’Sullivan K, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer 2017;116(3):310–7 doi 10.1038/bjc.2016.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hirsch L, Bellesoeur A, Boudou-Rouquette P, Arrondeau J, Thomas-Schoemann A, Kirchgesner J, et al. The impact of body composition parameters on severe toxicity of nivolumab. Eur J Cancer 2020;124:170–7 doi 10.1016/j.ejca.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 152.Heidelberger V, Goldwasser F, Kramkimel N, Jouinot A, Huillard O, Boudou-Rouquette P, et al. Sarcopenic overweight is associated with early acute limiting toxicity of anti-PD1 checkpoint inhibitors in melanoma patients. Invest New Drugs 2017;35(4):436–41 doi 10.1007/s10637-017-0464-x. [DOI] [PubMed] [Google Scholar]
- 153.Kong J, Ha D, Lee J, Kim I, Park M, Im SH, et al. Network-based machine learning approach to predict immunotherapy response in cancer patients. Nat Commun 2022;13(1):3703 doi 10.1038/s41467-022-31535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Benzekry S, Grangeon M, Karlsen M, Alexa M, Bicalho-Frazeto I, Chaleat S, et al. Machine Learning for Prediction of Immunotherapy Efficacy in Non-Small Cell Lung Cancer from Simple Clinical and Biological Data. Cancers (Basel) 2021;13(24) doi 10.3390/cancers13246210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xie J, Luo X, Deng X, Tang Y, Tian W, Cheng H, et al. Advances in artificial intelligence to predict cancer immunotherapy efficacy. Front Immunol 2022;13:1076883 doi 10.3389/fimmu.2022.1076883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu Z, Lin G, Yan Z, Li L, Wu X, Shi J, et al. Predictive mutation signature of immunotherapy benefits in NSCLC based on machine learning algorithms. Front Immunol 2022;13:989275 doi 10.3389/fimmu.2022.989275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Halner A, Hankey L, Liang Z, Pozzetti F, Szulc D, Mi E, et al. DEcancer: Machine learning framework tailored to liquid biopsy based cancer detection and biomarker signature selection. iScience 2023;26(5):106610 doi 10.1016/j.isci.2023.106610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rolfo C, Castiglia M, Hong D, Alessandro R, Mertens I, Baggerman G, et al. Liquid biopsies in lung cancer: the new ambrosia of researchers. Biochim Biophys Acta 2014;1846(2):539–46 doi 10.1016/j.bbcan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 159.Diaz LA, Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 2014;32(6):579–86 doi 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ahamadi M, Freshwater T, Prohn M, Li CH, de Alwis DP, de Greef R, et al. Model-Based Characterization of the Pharmacokinetics of Pembrolizumab: A Humanized Anti-PD-1 Monoclonal Antibody in Advanced Solid Tumors. CPT Pharmacometrics Syst Pharmacol 2017;6(1):49–57 doi 10.1002/psp4.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bajaj G, Gupta M, Feng Y, Statkevich P, Roy A. Exposure-Response Analysis of Nivolumab in Patients With Previously Treated or Untreated Advanced Melanoma. J Clin Pharmacol 2017;57(12):1527–33 doi 10.1002/jcph.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors. CPT Pharmacometrics Syst Pharmacol 2017;6(1):58–66 doi 10.1002/psp4.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang J, Cai J, Bello A, Roy A, Sheng J. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Chinese Patients With Previously Treated Advanced Solid Tumors, Including Non-Small Cell Lung Cancer. J Clin Pharmacol 2019;59(10):1415–24 doi 10.1002/jcph.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang X, Ludwig EA, Passarell J, Bello A, Roy A, Hruska MW. Population Pharmacokinetics and Exposure - Safety Analyses of Nivolumab in Patients With Relapsed or Refractory Classical Hodgkin Lymphoma. J Clin Pharmacol 2019;59(3):364–73 doi 10.1002/jcph.1324. [DOI] [PubMed] [Google Scholar]
- 165.Shemesh CS, Chanu P, Jamsen K, Wada R, Rossato G, Donaldson F, et al. Population pharmacokinetics, exposure-safety, and immunogenicity of atezolizumab in pediatric and young adult patients with cancer. J Immunother Cancer 2019;7(1):314 doi 10.1186/s40425-019-0791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wilkins JJ, Vugmeyster Y, Dussault I, Girard P, Khandelwal A. Population Pharmacokinetic Analysis of Bintrafusp Alfa in Different Cancer Types. Adv Ther 2019;36(9):2414–33 doi 10.1007/s12325-019-01018-0. [DOI] [PubMed] [Google Scholar]
- 167.Han K, Jin J, Maia M, Lowe J, Sersch MA, Allison DE. Lower exposure and faster clearance of bevacizumab in gastric cancer and the impact of patient variables: analysis of individual data from AVAGAST phase III trial. AAPS J 2014;16(5):1056–63 doi 10.1208/s12248-014-9631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 2008;62(5):779–86 doi 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- 169.Bernadou G, Campone M, Merlin JL, Gouilleux-Gruart V, Bachelot T, Lokiec F, et al. Influence of tumour burden on trastuzumab pharmacokinetics in HER2 positive non-metastatic breast cancer. Br J Clin Pharmacol 2016;81(5):941–8 doi 10.1111/bcp.12875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bensalem A, Mulleman D, Paintaud G, Azzopardi N, Gouilleux-Gruart V, Cornec D, et al. Non-Linear Rituximab Pharmacokinetics and Complex Relationship between Rituximab Concentrations and Anti-Neutrophil Cytoplasmic Antibodies (ANCA) in ANCA-Associated Vasculitis: The RAVE Trial Revisited. Clin Pharmacokinet 2020;59(4):519–30 doi 10.1007/s40262-019-00826-5. [DOI] [PubMed] [Google Scholar]
- 171.Blasco H, Chatelut E, de Bretagne IB, Congy-Jolivet N, Le Guellec C. Pharmacokinetics of rituximab associated with CHOP chemotherapy in B-cell non-Hodgkin lymphoma. Fundam Clin Pharmacol 2009;23(5):601–8 doi 10.1111/j.1472-8206.2009.00714.x. [DOI] [PubMed] [Google Scholar]
- 172.Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol 2005;45(7):792–801 doi 10.1177/0091270005277075. [DOI] [PubMed] [Google Scholar]
- 173.Tout M, Casasnovas O, Meignan M, Lamy T, Morschhauser F, Salles G, et al. Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: a Lymphoma Study Association report. Blood 2017;129(19):2616–23 doi 10.1182/blood-2016-10-744292. [DOI] [PubMed] [Google Scholar]
- 174.Ternant D, Monjanel H, Venel Y, Prunier-Aesch C, Arbion F, Colombat P, et al. Nonlinear pharmacokinetics of rituximab in non-Hodgkin lymphomas: A pilot study. Br J Clin Pharmacol 2019;85(9):2002–10 doi 10.1111/bcp.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Muller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, et al. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood 2012;119(14):3276–84 doi 10.1182/blood-2011-09-380949. [DOI] [PubMed] [Google Scholar]
- 176.Cornec D, Kabat BF, Mills JR, Cheu M, Hummel AM, Schroeder DR, et al. Pharmacokinetics of rituximab and clinical outcomes in patients with anti-neutrophil cytoplasmic antibody associated vasculitis. Rheumatology (Oxford) 2018;57(4):639–50 doi 10.1093/rheumatology/kex484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lavezzi SM, de Jong J, Neyens M, Cramer P, Demirkan F, Fraser G, et al. Systemic Exposure of Rituximab Increased by Ibrutinib: Pharmacokinetic Results and Modeling Based on the HELIOS Trial. Pharm Res 2019;36(7):93 doi 10.1007/s11095-019-2605-8. [DOI] [PubMed] [Google Scholar]
- 178.Liu S, Wang Z, Chen R, Huang H, Wang X, Peng C, et al. Rituximab exposure-response in triweekly R-CHOP treatment in DLBCL: A loading dose is recommended to improve clinical outcomes. Clin Transl Sci 2022;15(3):680–90 doi 10.1111/cts.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gibiansky E, Gibiansky L, Buchheit V, Frey N, Brewster M, Fingerle-Rowson G, et al. Pharmacokinetics, exposure, efficacy and safety of obinutuzumab in rituximab-refractory follicular lymphoma patients in the GADOLIN phase III study. Br J Clin Pharmacol 2019;85(9):1935–45 doi 10.1111/bcp.13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Gibiansky E, Petry C, Mercier F, Gunther A, Herman A, Kappos L, et al. Ocrelizumab in relapsing and primary progressive multiple sclerosis: Pharmacokinetic and pharmacodynamic analyses of OPERA I, OPERA II and ORATORIO. Br J Clin Pharmacol 2021;87(6):2511–20 doi 10.1111/bcp.14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schrapel C, Kovar L, Selzer D, Hofmann U, Tran F, Reinisch W, et al. External Model Performance Evaluation of Twelve Infliximab Population Pharmacokinetic Models in Patients with Inflammatory Bowel Disease. Pharmaceutics 2021;13(9) doi 10.3390/pharmaceutics13091368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Eser A, Reinisch W, Schreiber S, Ahmad T, Boulos S, Mould DR. Increased Induction Infliximab Clearance Predicts Early Antidrug Antibody Detection. J Clin Pharmacol 2021;61(2):224–33 doi 10.1002/jcph.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fasanmade AA, Adedokun OJ, Blank M, Zhou H, Davis HM. Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin Ther 2011;33(7):946–64 doi 10.1016/j.clinthera.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 184.Konecki C, Feliu C, Cazaubon Y, Giusti D, Tonye-Libyh M, Brixi H, et al. External Evaluation of Population Pharmacokinetic Models and Bayes-Based Dosing of Infliximab. Pharmaceutics 2021;13(8) doi 10.3390/pharmaceutics13081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kang J, Eudy-Byrne RJ, Mondick J, Knebel W, Jayadeva G, Liesenfeld KH. Population pharmacokinetics of adalimumab biosimilar adalimumab-adbm and reference product in healthy subjects and patients with rheumatoid arthritis to assess pharmacokinetic similarity. Br J Clin Pharmacol 2020;86(11):2274–85 doi 10.1111/bcp.14330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ternant D, Ducourau E, Fuzibet P, Vignault C, Watier H, Lequerre T, et al. Pharmacokinetics and concentration-effect relationship of adalimumab in rheumatoid arthritis. Br J Clin Pharmacol 2015;79(2):286–97 doi 10.1111/bcp.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sanchez-Hernandez JG, Perez-Blanco JS, Rebollo N, Munoz F, Prieto V, Calvo MV. Biomarkers of disease activity and other factors as predictors of adalimumab pharmacokinetics in inflammatory bowel disease. Eur J Pharm Sci 2020;150:105369 doi 10.1016/j.ejps.2020.105369. [DOI] [PubMed] [Google Scholar]
- 188.Mostafa NM, Nader AM, Noertersheuser P, Okun M, Awni WM. Impact of immunogenicity on pharmacokinetics, efficacy and safety of adalimumab in adult patients with moderate to severe chronic plaque psoriasis. J Eur Acad Dermatol Venereol 2017;31(3):490–7 doi 10.1111/jdv.13884. [DOI] [PubMed] [Google Scholar]
- 189.Ternant D, Karmiris K, Vermeire S, Desvignes C, Azzopardi N, Bejan-Angoulvant T, et al. Pharmacokinetics of adalimumab in Crohn’s disease. Eur J Clin Pharmacol 2015;71(9):1155–7 doi 10.1007/s00228-015-1892-1. [DOI] [PubMed] [Google Scholar]