Abstract
Objective:
To investigate the association between standard pure tone and speech audiometry with neuroimaging characteristics reflective of aging and dementia in older adults.
Study Design:
Prospective population-based study.
Setting:
Single tertiary care referral center.
Methods:
Participants from the Mayo Clinic Study of Aging 60 years old or older with normal cognition or mild cognitive impairment, baseline neuroimaging, and a behavioral audiogram associated with neuroimaging were eligible for study. Imaging modalities included structural MRI (sMRI) and fluid attenuated inversion recovery MRI (FLAIR-MRI; N=605), diffusion tensor imaging MRI (DTI-MRI; N=444), and fluorodeoxyglucose-positron emission tomography (FDG-PET; N=413). Multivariable logistic and linear regression models were used to evaluate associations with neuroimaging outcomes.
Results:
Mean (SD) pure tone average (PTA) was 33 (15) dB HL and mean (SD) word recognition score (WRS) was 91% (14). There were no significant associations between audiometric performance and cortical thinning assessed by sMRI. Each 10-dB increase in PTA was associated with increased likelihood of abnormal white-matter hyperintensity (WMH) from FLAIR-MRI (odds ratio 1.26, p=0.02). From DTI-MRI, participants with <100% WRSs had significantly lower fractional anisotropy in the genu of the corpus callosum (parameter estimate [PE] −0.012, p=0.008) compared to those with perfect WRSs. From FDG-PET, each 10% decrease in WRSs was associated with decreased uptake in the anterior cingulate cortex (PE −0.013, p=0.001).
Conclusion:
Poorer audiometric performance was not significantly associated with cortical thinning but was associated with white matter damage relevant to cerebrovascular disease (increased abnormal WMH, decreased corpus callosum diffusion). These neuroimaging results suggest a pathophysiologic link between hearing loss and cerebrovascular disease.
Keywords: Hearing loss, neuroimaging, dementia, mild cognitive impairment
INTRODUCTION
With a globally aging population, mild cognitive impairment (MCI) and dementia are steadily increasing, with estimates of dementia prevalence reaching 1 in 85 persons by 2050.1 Given these progressive trends, studies have sought to identify modifiable risk factors as targets for early intervention and disease mitigation. While hearing loss among older patients has historically been considered an inevitable sequala of aging, recent research identifies hearing loss as potentially the largest modifiable risk factor for development of dementia.2,3 These recommendations derive from data that demonstrated an increased likelihood of developing dementia among patients with increasingly poor audiometric performance.4–6
Several underlying mechanisms have been hypothesized to account for the association between hearing loss and cognitive impairment. It has been hypothesized that hearing loss may directly lead to cortical atrophy through the increased cognitive demands for processing auditory information.7 However, as social isolation, depression, and loneliness have all been associated with cognitive decline, several groups have suggested an indirect link between hearing loss and declining cognitive performance over time.8,9
High-resolution neuroimaging is increasingly used to examine the functional and anatomic connectivity changes accompanying hearing loss in cognitively impaired individuals.10–12 Several imaging modalities have been employed for neuroanatomical characterization, including structural MRI (sMRI), fluid attenuated inversion recovery MRI (FLAIR-MRI), diffusion tensor imaging MRI (DTI-MRI), and fluorodeoxyglucose-positron emission tomography (FDG-PET). In addition to structural characterization, WMH has been found to be a marker for late cerebrovascular disease (CVD).13,14 DTI-MRI is employed to measure fractional anisotropy (FA), a marker of axonal diameter, myelin structure, or fiber density in a given area, that has been associated with early CVD.15 FDG-PET is employed to measure glucose utilization, and while neurometabolism decreases globally with age, specific hypometabolic patterns associate with dementia.16
The Mayo Clinic Study of Aging (MCSA) is a prospective, population-based study initiated in 2004 to comprehensively evaluate the epidemiology and risk factors of MCI and dementia in Olmsted County, Minnesota. Using the MCSA, the primary aim of the current work was to investigate the association between standard pure tone and speech audiometry with neuroimaging characteristics reflective of aging and dementia in older adults.
MATERIALS AND METHODS
Participant Selection
Following approval from Institutional Review Board (IRB: 20–004354) a review was undertaken. Participants who enrolled in the MCSA from November 2004 to December 2019 with a status of cognitively unimpaired or MCI were eligible for study. Characteristics from 4 imaging modalities were of interest: 1 and 2) sMRI and FLAIR-MRI using a 3T GE scanner, in use in the MCSA from August 2005 to December 2017; 3) the DTI-MRI sequence, in use from November 2009 to December 2017; and 4) FDG-PET, in use from January 2009 to the end of the current study. Imaging studies of interest were performed as a part of the MCSA for research purposes to examine neuroanatomic changes associated with MCI and dementia on prospectively enrolled MCSA participants. The first available imaging date of each modality was selected to represent the baseline imaging for each participant. Because the most significant brain changes with aging occur after 60 years of age, we restricted to participants 60 years old or older at the baseline imaging date. An audiogram within 5 years of the baseline imaging date was identified to represent the degree of hearing loss associated with baseline imaging. For participants with multiple audiograms, the audiogram closest to the baseline imaging date was selected.
Covariates
Covariates assessed at the MCSA visit associated with the baseline imaging date included age, sex, years of education, smoking status, diabetes, hypertension, apolipoprotein E (APOE) ε4 carriership, and hearing rehabilitation defined as hearing aid or cochlear implant use.17 Except for hearing rehabilitation, the demographic and clinical covariates were ascertained during the MCSA visit associated with the imaging date or by medical record review. Hearing rehabilitation was ascertained by electronic retrieval of patient-provided information from surveys completed during Mayo Clinic visits and searches of diagnosis and procedure codes for hearing aids and cochlear implants, as previously described.17
Hearing Loss Exposures
The exposures studied included air-conduction pure tone averages (PTAs; 0.5, 1, 2, and 3 kilohertz) and word recognition scores (WRSs) from the audiogram, as previously described.17 Air-conduction PTAs were categorized as normal hearing or mild, moderate, severe, and profound hearing loss by ≤25, 26–39, 40–69, 70–89, and ≥90 decibels hearing loss (dB HL), respectively. Subjective hearing difficulties that interfere with daily activities were assessed during the interview with the participants’ study partners (“informants,” selected by the participant) during the visit associated with the imaging date. The informants were asked “Does [the participant] have significant hearing difficulties that interfere with daily activities?”
Neuroimaging Outcomes
The neuroimaging outcomes of interest were collected prospectively from neuroimaging modalities employed per MCSA protocol and included frontal lobe, parietal lobe, occipital lobe, temporal lobe, and superior temporal thicknesses from sMRI, WMH as a percentage of total intracranial volume as continuous and dichotomized as abnormal versus normal from FLAIR-MRI, corpus callosum FA and the subcomponents of body of the corpus callosum, genu corpus callosum, and splenium corpus callosum from DTI-MRI, and anterior cingulate cortex from FDG-PET.13 Except for WMH as a percentage of total intracranial volume, higher values of the continuous neuroimaging outcomes studied are considered more favorable. We defined abnormal WMH as ≥1.62% after mapping the cutoff from a previously published method to our current values.18
Statistical Methods
Cross-sectional associations of PTA, WRS, and hearing difficulties interfering with daily activities with baseline neuroimaging outcomes were evaluated using linear regression models for the continuous outcomes and logistic regression models for the dichotomous outcome. Associations with longitudinal changes in neuroimaging outcomes over time were evaluated using linear mixed-effects models with random participant-specific intercepts and slopes using an unstructured covariance structure. All regression models were adjusted for the baseline covariates of age, sex, years of education, smoking status, diabetes, hypertension, APOE ε4 carriership, and hearing rehabilitation.17 Statistical analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC). All tests were two-sided and p-values <0.05 were considered statistically significant.
RESULTS
In total, 706 participants with a status of cognitively unimpaired or MCI were included in the current study: 605 participants with sMRI and FLAIR-MRI, 444 with DTI-MRI, and 413 with FDG-PET (Table 1). Participants could be included in multiple cohorts; for example, 303 participants were included in the sMRI, FLAIR-MRI, DTI-MRI, and FDG-PET cohorts, although the baseline imaging dates were not necessarily the same across all 4 modalities. Median duration (in absolute value) from the audiogram to the baseline neuroimaging date was 1.5 years (IQR 0.7–2.6) for sMRI and FLAIR-MRI, 1.5 years (IQR 0.7–2.7) for DTI-MRI, and 1.7 years (IQR 0.7–3.0) for FDG-PET.
Table 1:
Summary of covariates, hearing loss exposures, and neuroimaging outcomes by imaging modality
| sMRI/FLAIR-MRI | DTI-MRI | FDG-PET | |
|---|---|---|---|
| Features* | N=605 | N=444 | N=413 |
|
| |||
| Covariates | |||
| Age in years | 77 (7.4) | 77 (8.0) | 76 (8.2) |
| Male sex | 323 (53) | 240 (54) | 230 (56) |
| Years of education | |||
| ≤12 | 193 (32) | 118 (27) | 99 (24) |
| 13–16 | 269 (44) | 217 (49) | 203 (49) |
| >16 | 143 (24) | 109 (25) | 111 (27) |
| Smoking status | |||
| Never | 305 (50) | 229 (52) | 213 (52) |
| Current or former | 300 (50) | 215 (48) | 200 (48) |
| Diabetes | 114 (19) | 82 (18) | 75 (18) |
| Hypertension | 437 (72) | 314 (71) | 285 (69) |
| APOE ε4 carriership (N=604:443:409) | 152 (25) | 116 (26) | 108 (26) |
| Hearing rehabilitation | 261 (43) | 205 (46) | 179 (43) |
| Hearing Loss Exposures | |||
| PTA in dB HL (N=605:444:412) | 33 (15) | 32 (15) | 31 (15) |
| PTA ≥40 dB HL (N=605:444:412) | 208 (34) | 137 (31) | 115 (28) |
| PTA >25 dB HL (N=605:444:412) | 414 (68) | 289 (65) | 266 (65) |
| WRS in % (N=592:432:398) | 91 (14) | 91 (13) | 92 (12) |
| WRS <90% (N=592:432:398) | 143 (24) | 109 (25) | 96 (24) |
| WRS <100% (N=592:432:398) | 284 (48) | 210 (49) | 188 (47) |
| Informant-based hearing difficulties (N=600:441:408) | 203 (34) | 150 (34) | 141 (35) |
| Cognitive Status | |||
| Unimpaired | 525 (87) | 386 (87) | 352 (85) |
| MCI | 80 (13) | 58 (13) | 61 (15) |
| Neuroimaging Outcomes | |||
| Frontal lobe thickness in mm (N=601) | 2.36 (0.12) | - | - |
| Parietal lobe thickness in mm (N=601) | 2.11 (0.12) | - | - |
| Occipital lobe thickness in mm (N=601) | 1.77 (0.10) | - | - |
| Temporal lobe thickness in mm (N=601) | 2.67 (0.16) | - | - |
| Superior temporal thickness in mm (N=601) | 2.46 (0.17) | - | - |
| White-matter hyperintensity (N=595) | |||
| Normal | 430 (72) | - | - |
| Abnormal | 165 (28) | - | - |
| Corpus callosum in FA (N=443) | - | 0.622 (0.040) | - |
| Body of the corpus callosum in FA | - | 0.577 (0.045) | - |
| Genu corpus callosum in FA | - | 0.583 (0.047) | - |
| Splenium corpus callosum in FA (N=443) | - | 0.678 (0.043) | - |
| Anterior cingulate cortex in SUVR | - | - | 1.285 (0.093) |
Summarized with mean (SD) or n (%). Sample sizes for features with missing data are indicated in parentheses.
APOE=apolipoprotein E; dB HL=decibels hearing level; DTI-MRI=diffusion tensor imaging MRI; FA=fractional anisotropy, a scalar value between 0 and 1; FDG-PET=fluorodeoxyglucose-positron emission tomography; FLAIR-MRI=fluid attenuated inversion recovery MRI; MCI=mild cognitive impairment; PTA=pure tone average; sMRI=structural MRI; SUVR=standard uptake value ratio; WRS=word recognition score
Cross-sectional Analysis
Cross-sectional associations of hearing loss with baseline neuroimaging outcomes indicated that none of the hearing loss exposures studied were significantly associated with cortical thickness measures from sMRI. However, each 10-dB HL increase in PTA was associated with a significantly increased likelihood of abnormal WMH (odds ratio 1.26; p=0.02) from FLAIR-MRI. PTA >25 dB HL, representing any degree of hearing loss, was also significantly associated with abnormal WMH (odds ratio 1.88; p=0.03) (Table 2; Figure 1). Although WMH as a percentage of total intracranial volume was primarily analyzed as abnormal versus normal, associations of hearing loss with this outcome analyzed as continuous (after transforming to the natural log scale to satisfy the underlying assumption of normality) were also explored; the results were comparable to those summarized in Table 2. From DTI-MRI, participants with <100% WRSs had significantly lower FA in the corpus callosum as a whole (parameter estimate −0.008; p=0.04) and specifically within the body (parameter estimate −0.010; p=0.03) and genu (parameter estimate −0.012; p=0.008) of the corpus callosum when compared with participants with perfect WRSs (Table 3; Figure 2). From FDG-PET, each 10% decrease in WRSs was associated with a significantly decreased uptake in the anterior cingulate cortex (parameter estimate −0.013; p=0.001) (Table 4; Figure 3).
Table 2:
Associations of hearing loss exposures with baseline sMRI and FLAIR-MRI neuroimaging outcomes
| PE (95% CI)* | P-value* | |
|---|---|---|
| Hearing Loss Exposures | Frontal Lobe Thickness in mm | |
|
| ||
| PTA as continuous (10-dB HL increase) | 0.00 (−0.01 to 0.01) | 0.8 |
| PTA ≥40 vs <40 dB HL | 0.01 (−0.01 to 0.04) | 0.3 |
| PTA >25 vs ≤25 dB HL | 0.00 (−0.03 to 0.02) | 0.8 |
| WRS as continuous (10% decrease) | 0.00 (−0.01 to 0.01) | 0.4 |
| WRS <90 vs ≥90% | 0.01 (−0.02 to 0.03) | 0.7 |
| WRS <100 vs 100% | 0.00 (−0.02 to 0.02) | 0.8 |
| Informant-based hearing difficulties (yes vs no) | −0.01 (−0.03 to 0.01) | 0.4 |
|
| ||
| Parietal Lobe Thickness in mm | ||
|
| ||
| PTA as continuous (10-dB HL increase) | 0.00 (−0.01 to 0.01) | 0.8 |
| PTA ≥40 vs <40 dB HL | 0.00 (−0.02 to 0.02) | 0.9 |
| PTA >25 vs ≤25 dB HL | 0.00 (−0.02 to 0.02) | 0.9 |
| WRS as continuous (10% decrease) | 0.00 (−0.01 to 0.01) | 0.8 |
| WRS <90 vs ≥90% | 0.00 (−0.03 to 0.02) | 0.8 |
| WRS <100 vs 100% | 0.00 (−0.02 to 0.02) | 0.9 |
| Informant-based hearing difficulties (yes vs no) | −0.02 (−0.04 to 0.00) | 0.06 |
|
| ||
| Occipital Lobe Thickness in mm | ||
|
| ||
| PTA as continuous (10-dB HL increase) | 0.00 (−0.01 to 0.01) | 0.5 |
| PTA ≥40 vs <40 dB HL | 0.00 (−0.02 to 0.01) | 0.6 |
| PTA >25 vs ≤25 dB HL | 0.00 (−0.02 to 0.02) | 0.8 |
| WRS as continuous (10% decrease) | 0.00 (−0.01 to 0.01) | 0.9 |
| WRS <90 vs ≥90% | −0.01 (−0.03 to 0.01) | 0.2 |
| WRS <100 vs 100% | 0.00 (−0.02 to 0.02) | 0.8 |
| Informant-based hearing difficulties (yes vs no) | −0.01 (−0.03 to 0.01) | 0.3 |
|
| ||
| Temporal Lobe Thickness in mm | ||
|
| ||
| PTA as continuous (10-dB HL increase) | 0.00 (−0.01 to 0.01) | 0.7 |
| PTA ≥40 vs <40 dB HL | 0.00 (−0.03 to 0.03) | 0.9 |
| PTA >25 vs ≤25 dB HL | 0.00 (−0.03 to 0.03) | 0.9 |
| WRS as continuous (10% decrease) | 0.00 (−0.01 to 0.01) | 0.9 |
| WRS <90 vs ≥90% | 0.01 (−0.02 to 0.04) | 0.6 |
| WRS <100 vs 100% | 0.00 (−0.02 to 0.03) | 0.8 |
| Informant-based hearing difficulties (yes vs no) | −0.02 (−0.04 to 0.01) | 0.19 |
|
| ||
| Superior Temporal Thickness in mm | ||
|
| ||
| PTA as continuous (10-dB HL increase) | 0.00 (−0.01 to 0.01) | 0.5 |
| PTA ≥40 vs <40 dB HL | −0.02 (−0.05 to 0.01) | 0.2 |
| PTA >25 vs ≤25 dB HL | 0.01 (−0.02 to 0.04) | 0.6 |
| WRS as continuous (10% decrease) | 0.00 (−0.01 to 0.01) | 0.6 |
| WRS <90 vs ≥90% | 0.00 (−0.03 to 0.03) | 0.8 |
| WRS <100 vs 100% | 0.00 (−0.02 to 0.03) | 0.8 |
| Informant-based hearing difficulties (yes vs no) | −0.02 (−0.05 to 0.01) | 0.18 |
|
| ||
| Odds Ratio (95% CI)* | P-value* | |
| Abnormal White-matter Hyperintensity | ||
|
| ||
| PTA as continuous (10-dB HL increase) | 1.26 (1.04 to 1.54) | 0.02 |
| PTA ≥40 vs <40 dB HL | 1.35 (0.80 to 2.27) | 0.3 |
| PTA >25 vs ≤25 dB HL | 1.88 (1.06 to 3.31) | 0.03 |
| WRS as continuous (10% decrease) | 1.10 (0.94 to 1.29) | 0.2 |
| WRS <90 vs ≥90% | 1.15 (0.69 to 1.90) | 0.6 |
| WRS <100 vs 100% | 1.09 (0.69 to 1.72) | 0.7 |
| Informant-based hearing difficulties (yes vs no) | 1.48 (0.93 to 2.34) | 0.1 |
PEs, 95% CIs, and p-values from multivariable linear regression models and odds ratios, 95% CIs, and p-values from multivariable logistic regression models adjusted for age, sex, years of education, smoking status, diabetes, hypertension, APOE ε4 carriership, and hearing rehabilitation. Higher values of the continuous sMRI outcomes are considered more favorable.
APOE=apolipoprotein E; dB HL=decibels hearing level; FLAIR-MRI=fluid attenuated inversion recovery MRI; PE=parameter estimate; PTA=pure tone average; sMRI=structural MRI; WRS=word recognition score
Figure 1:

Select fluid attenuated inversion recovery MRI (FLAIR-MRI) demonstrating abnormal white matter hyperintensity (WMH) in participants with worsening pure tone average (PTA). (A) A patient with good hearing (PTA <15 dB HL) and normal WMH. (B) A patient with poor hearing (PTA >50 dB HL) and abnormal WMH.
Table 3:
Associations of hearing loss exposures with baseline DTI-MRI neuroimaging outcomes
| PE (95% CI)* | P-value* | |
|---|---|---|
| Hearing Loss Exposures | Corpus Callosum in FA | |
|
| ||
| PTA as continuous (10-dB HL increase) | −0.002 (−0.005 to 0.001) | 0.18 |
| PTA ≥40 vs <40 dB HL | −0.007 (−0.016 to 0.002) | 0.13 |
| PTA >25 vs ≤25 dB HL | −0.008 (−0.016 to 0.001) | 0.07 |
| WRS as continuous (10% decrease) | −0.001 (−0.005 to 0.002) | 0.4 |
| WRS <90 vs ≥90% | −0.005 (−0.014 to 0.004) | 0.3 |
| WRS <100 vs 100% | −0.008 (−0.016 to 0.000) | 0.04 |
| Informant-based hearing difficulties (yes vs no) | −0.004 (−0.012 to 0.003) | 0.3 |
|
| ||
| Body of the Corpus Callosum in FA | ||
|
| ||
| PTA as continuous (10-dB HL increase) | −0.002 (−0.005 to 0.002) | 0.5 |
| PTA ≥40 vs <40 dB HL | −0.009 (−0.019 to 0.002) | 0.11 |
| PTA >25 vs ≤25 dB HL | −0.006 (−0.016 to 0.004) | 0.2 |
| WRS as continuous (10% decrease) | −0.001 (−0.005 to 0.003) | 0.5 |
| WRS <90 vs ≥90% | −0.002 (−0.012 to 0.009) | 0.8 |
| WRS <100 vs 100% | −0.010 (−0.019 to −0.001) | 0.03 |
| Informant-based hearing difficulties (yes vs no) | 0.000 (−0.009 to 0.008) | 0.9 |
|
| ||
| Genu Corpus Callosum in FA | ||
|
| ||
| PTA as continuous (10-dB HL increase) | −0.001 (−0.005 to 0.002) | 0.5 |
| PTA ≥40 vs <40 dB HL | −0.006 (−0.017 to 0.004) | 0.2 |
| PTA >25 vs ≤25 dB HL | −0.010 (−0.020 to 0.000) | 0.05 |
| WRS as continuous (10% decrease) | −0.002 (−0.005 to 0.002) | 0.4 |
| WRS <90 vs ≥90% | −0.005 (−0.015 to 0.005) | 0.4 |
| WRS <100 vs 100% | −0.012 (−0.021 to −0.003) | 0.008 |
| Informant-based hearing difficulties (yes vs no) | −0.005 (−0.014 to 0.004) | 0.3 |
|
| ||
| Splenium Corpus Callosum in FA | ||
|
| ||
| PTA as continuous (10-dB HL increase) | −0.003 (−0.007 to 0.001) | 0.10 |
| PTA ≥40 vs <40 dB HL | −0.007 (−0.017 to 0.003) | 0.2 |
| PTA >25 vs ≤25 dB HL | −0.008 (−0.018 to 0.001) | 0.09 |
| WRS as continuous (10% decrease) | −0.002 (−0.005 to 0.002) | 0.4 |
| WRS <90 vs ≥90% | −0.008 (−0.018 to 0.002) | 0.11 |
| WRS <100 vs 100% | −0.006 (−0.014 to 0.003) | 0.2 |
| Informant-based hearing difficulties (yes vs no) | −0.007 (−0.015 to 0.002) | 0.11 |
PEs, 95% CIs, and p-values from multivariable linear regression models adjusted for age, sex, years of education, smoking status, diabetes, hypertension, APOE ε4 carriership, and hearing rehabilitation. Higher values of the DTI-MRI outcomes are considered more favorable.
APOE=apolipoprotein E; dB HL=decibels hearing level; DTI-MRI=diffusion tensor imaging MRI; FA=fractional anisotropy, a scalar value between 0 and 1; PE=parameter estimate; PTA=pure tone average; WRS=word recognition score
Figure 2:

Select diffusion tensor imaging MRI (DTI-MRI) demonstrating decreased fractional anisotropy (FA) of the corpus callosum in participants with worsening word recognition scores (WRS). (A) Good hearing (WRS 100%) with high FA in the genu of the corpus callosum. (B) Poorer hearing (WRS <80%) with low FA in the genu of the corpus callosum.
Table 4:
Associations of hearing loss exposures with baseline FDG-PET neuroimaging outcomes
| PE (95% CI)* | P-value* | |
|---|---|---|
| Hearing Loss Exposures | Anterior Cingulate Cortex in SUVR | |
|
| ||
| PTA as continuous (10-dB HL increase) | −0.002 (−0.011 to 0.006) | 0.5 |
| PTA ≥40 vs <40 dB HL | −0.013 (−0.035 to 0.010) | 0.3 |
| PTA >25 vs ≤25 dB HL | 0.004 (−0.017 to 0.025) | 0.7 |
| WRS as continuous (10% decrease) | −0.013 (−0.021 to −0.005) | 0.001 |
| WRS <90 vs ≥90% | −0.018 (−0.040 to 0.003) | 0.09 |
| WRS <100 vs 100% | −0.008 (−0.027 to 0.010) | 0.4 |
| Informant-based hearing difficulties (yes vs no) | −0.012 (−0.030 to 0.006) | 0.2 |
PEs, 95% CIs, and p-values from multivariable linear regression models adjusted for age, sex, years of education, smoking status, diabetes, hypertension, APOE ε4 carriership, and hearing rehabilitation. Higher values of anterior cingulate cortex are considered more favorable.
APOE=apolipoprotein E; dB HL=decibels hearing level; FDG-PET=fluorodeoxyglucose-positron emission tomography; PE=parameter estimate; PTA=pure tone average; SUVR=standard uptake value ratio; WRS=word recognition score
Figure 3:
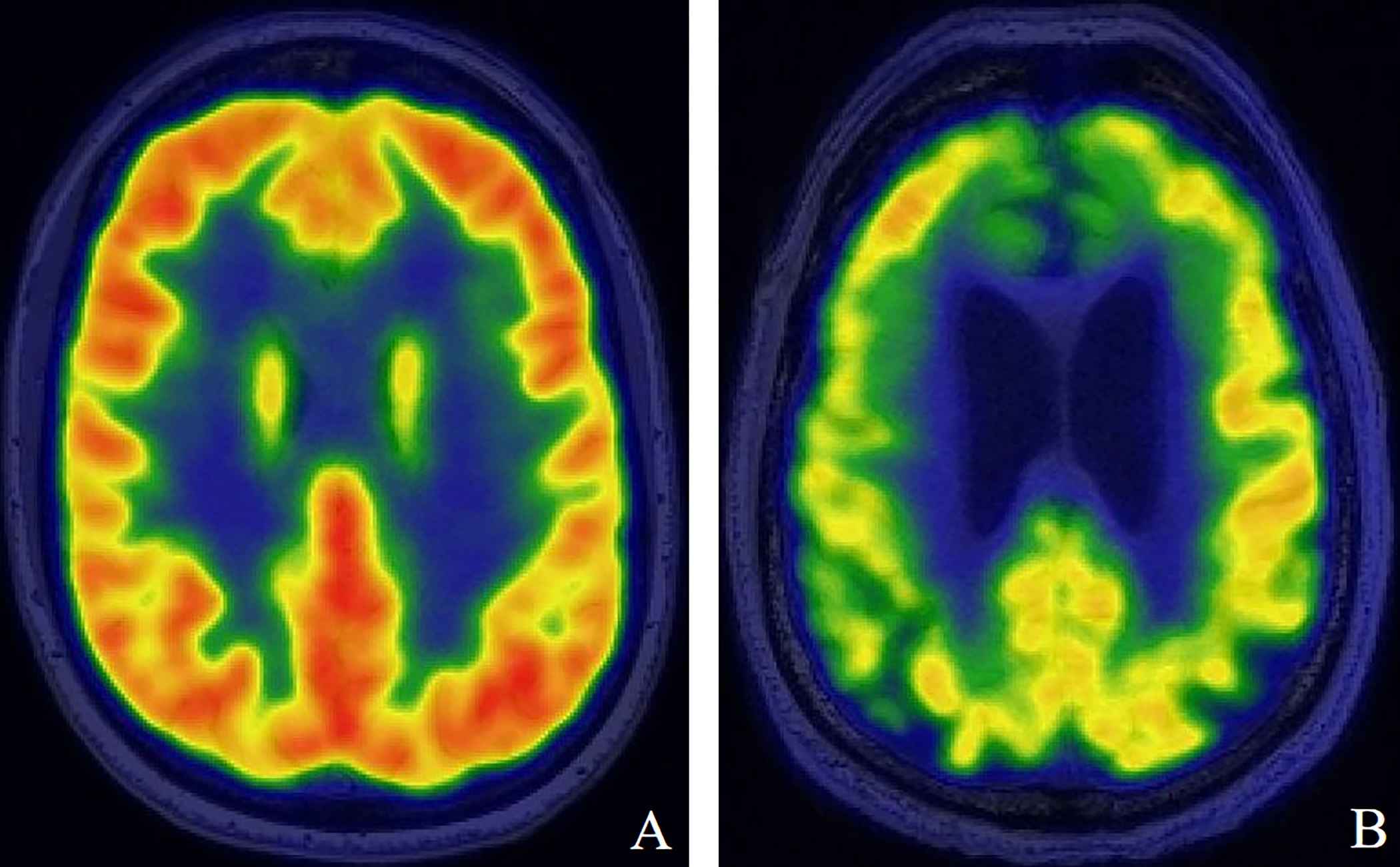
Select fluorodeoxyglucose-positron emission tomography (FDG-PET) imaging demonstrating decreased metabolism within the anterior cingulate cortex in participants with worsening word recognition scores (WRS). (A) Good hearing (WRS 100%) with high FDG uptake in the anterior cingulate cortex. (B) Poorer hearing (WRS <80%) with low FDG uptake in the anterior cingulate cortex.
Longitudinal Analysis
A summary of the participant cohorts available for associations of hearing loss with longitudinal changes in neuroimaging outcomes over time is shown in Supplemental Table 1. Associations of PTA, WRS, and hearing difficulties interfering with daily activities with longitudinal changes in sMRI and FLAIR-MRI, DTI-MRI, and FDG-PET neuroimaging outcomes over time are summarized in Supplemental Tables 2, 3, and 4, respectively. The parameter estimates in these tables represent the rates of change in neuroimaging outcomes per year for each level of exposure studied. For example, the parameter estimates for changes in frontal lobe thickness from sMRI were −0.004 and −0.005 for participants with normal hearing (PTA ≤25 dB HL) and participants with hearing loss (PTA>25 dB HL), respectively (Supplemental Table 2). Put another way, frontal lobe thickness decreased by 0.004 mm per year, on average, for those with normal hearing compared with 0.005 mm per year for those with hearing loss. These estimates are expected to be negative, representing declines in frontal lobe thickness over time, and indeed the 95% CIs around both estimates indicate statistically significant declines. However, the p-value of 0.4 for the interaction term between hearing loss and time indicates that the rate of decline was not significantly steeper for participants with hearing loss compared with those with normal hearing. There were no statistically significant differences between participants with better or poorer audiometric performance when looking at rates of deterioration over time in the sMRI, FLAIR-MRI, DTI-MRI, and FDG-PET neuroimaging outcomes under study.
DISCUSSION
In this prospective, population-based cohort study, worsening audiometric performance was associated with specific neuroimaging findings on cross-sectional assessment. Worsening PTA was not significantly associated with cortical thinning but was significantly associated with abnormal WMH, and worsening WRS was significantly associated with decreased FA in the body and genu of the corpus callosum as well as decreased FDG-PET uptake in the anterior cingulate cortex. When assessing associations of hearing loss with declines in neuroimaging outcomes over time, we did not identify any statistically significant differences in the rate of deterioration of brain area thickness, WMH, FA of the corpus callosum, or FDG-PET uptake in the anterior cingulate cortex across a range of audiometric measures.
In the present cohort, we used sMRI to measure thickness of each lobe of the brain. Due to the anatomic localization of the auditory cortex within the temporal lobe, previous authors have specifically examined this area for imaging associations with hearing loss. Two recent studies based on the Baltimore Longitudinal Study of Aging reported significant temporal lobe volume differences when comparing participants with hearing loss to normal hearing participants, and steeper rates of volume loss in participants with greater degrees of hearing loss in midlife.12,19 In our data, we did not find any significant differences in temporal lobe thickness when comparing a range of audiometric performance characteristics, although we did not specifically look at the volume of the temporal lobe.
Using FLAIR-MRI, we identified a significant association between audiometric performance and WMH. Interestingly, while WMH has been implicated in a wide range of cognitive deficits ranging from MCI to dementia, there are limited data on the associations of hearing loss with this outcome. In patients with sudden sensorineural hearing loss, worse hearing outcomes were found in patients who had periventricular WMH, although the underlying mechanisms are not well understood.20,21 Eckert et al. reported a significant association between low-frequency hearing loss and periventricular WMH among aging women with a history of hypertension.22 Our finding of an association of worsening PTA with abnormal WMH suggests an underlying pathophysiologic connection between PTA and CVD, although causality cannot be inferred from these findings.
With DTI-MRI sequences, we identified a significant association between worsening audiometric performance and decreased FA in the genu and body of the corpus callosum. Broadly, the corpus callosum is the primary connection of white matter projections between the right and left cerebral hemispheres, with a topographical organization where auditory, visual, and somatosensory information are conducted posteriorly and higher cognitive functions communicate anteriorly.23 Armstrong et al. compared mean diffusivity and FA of the corpus callosum between hearing impaired and normal hearing patients, and while they found decreased mean diffusivity in the body of the corpus callosum, they did not find statistically significant changes in FA between these patient groups.24 FA of the corpus callosum has been implicated in CVD, with hypothesized differences in white matter microstructural injury associating with severity of neurologic dysfunction in the post-mortem state.15 Taken in combination with the WMH findings, the changes in primarily FA of the genu of the corpus callosum observed in participants with WRS <100% further suggest a connection between hearing loss and CVD.
We found decreasing metabolic function within the anterior cingulate cortex with decreasing WRS. While the understanding of anterior cingulate cortex function continues to evolve, this area is primarily thought to be involved with emotional and cognitive processing25 and is a region that is relevant to cognitive resilience.26 Hypometabolism within the anterior cingulate cortex can be seen with advancing age, although larger decreases within this area are also seen with CVD and vascular dementia and are more specific to this etiology in comparison to Alzheimer’s disease.27,28 Again, here we see the overlap between anatomic areas of hearing loss and markers of vascular dementia.
We did not observe significant differences between participants with better and poorer audiometric performance at baseline in rates of change over time within the neuroanatomic areas of interest in this study. Specifically, there were no statistically significant associations of hearing loss with increases in WMH, decreases in FA of the corpus callosum, or decreases in FDG-PET uptake in the anterior cingulate cortex over time, in contrast to the significant associations observed on cross-sectional analysis. The lack of statistically significant differences in rates of change by the degree of audiometric performance is consistent across the selected neuroimaging outcomes of interest within this study. The median duration of follow-up between the initial scan and the last follow-up scan ranged from 3 to 4 years across imaging modalities, which may not be sufficient time to distinguish slowly progressive neurologic changes between groups. This is especially true with CVD where the rate of brain changes is slower compared to neurodegenerative disorders. Therefore, further longitudinal neuroimaging follow-up, as well as longitudinal hearing changes over time, could better delineate the neuroimaging associations that we observed to be significant on cross-sectional analysis.
There are several limitations to our study. Firstly, MCSA participants are drawn from the population of Olmsted County, which has an improved access to healthcare, elevated life span, and higher socioeconomic status than the general U.S. population, limiting the generalizability of our results. Secondly, there is a selection bias in the current study cohort who underwent standard audiometry as a result of concerns for hearing dysfunction as compared with the MCSA cohort as a whole.17 Finally, as these data were collected over a large time period in a standardized fashion within the MCSA, we were limited in our ability to adjust for potential confounding variables that were not collected as a part of the participant intake process.
CONCLUSION
In conclusion, this prospective, population-based study demonstrates associations between poorer audiometric performance and neuroimaging findings that overlap substantially with neuroimaging markers of CVD. These results therefore suggest a pathophysiologic link between hearing loss and CVD, potentially involving injury to white matter microstructure indicated in abnormal WMH and decreased FA of the corpus callosum, as well as decreased executive processing with depressed metabolic function in the anterior cingulate cortex. These changes exist within the complex network of neuroanatomic connectivity, and further study is required to delineate the underlying mechanism of hearing loss and its relationship with cognitive deterioration.
Supplementary Material
FUNDING:
The present study received internal departmental funding, without commercial support or sponsorship. The Mayo Clinic Study of Aging was supported by the National Institutes of Health (U01 AG006786, P30 AG062677, R37 AG011378, R01 AG041851, R01 NS097495), the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic, the Mayo Foundation for Medical Education and Research, the Liston Award, the GHR Foundation, and the Schuler Foundation, and used the resources of the Rochester Epidemiology Project (REP) medical records linkage system, which is supported by the National Institute on Aging (NIA AG058738), the Mayo Clinic Research Committee, and fees paid annually by REP users.
Footnotes
CONFLICT OF INTEREST: The authors report no relevant conflict of interest in submitting this article for publication. Maria Vassilaki has received research funding from F. Hoffmann-La Roche Ltd and Biogen in the past and consulted for F. Hoffmann-La Roche Ltd; she currently receives research funding from the National Institutes of Health and has equity ownership in Johnson and Johnson, Medtronic, Merck, and Amgen.
ETHICS STATEMENT: This study was approved by the Mayo Clinic Institutional Review Board (IRB 20–004354)
This article was presented at the AAO-HNSF 20223Annual Meeting & OTO Experience, Nashville, TN, September 30- October 4, 2023.
REFERENCES
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. Dec 17 2005;366(9503):2112–7. doi: 10.1016/S0140-6736(05)67889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. Aug 8 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. Dec 16 2017;390(10113):2673–2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 4.Deal JA, Betz J, Yaffe K, et al. Hearing Impairment and Incident Dementia and Cognitive Decline in Older Adults: The Health ABC Study. J Gerontol A Biol Sci Med Sci. May 1 2017;72(5):703–709. doi: 10.1093/gerona/glw069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. Feb 2011;68(2):214–20. doi: 10.1001/archneurol.2010.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology. Oct 9 2012;79(15):1583–90. doi: 10.1212/WNL.0b013e31826e263d [DOI] [PubMed] [Google Scholar]
- 7.Park SY, Ho SH, Hong YJ, et al. The Effect of Hearing Loss on Cognitive Function in Subjective Cognitive Decline. Dement Geriatr Cogn Disord. 2022;51(4):348–356. doi: 10.1159/000526230 [DOI] [PubMed] [Google Scholar]
- 8.Lawrence BJ, Jayakody DMP, Bennett RJ, Eikelboom RH, Gasson N, Friedland PL. Hearing Loss and Depression in Older Adults: A Systematic Review and Meta-analysis. Gerontologist. Apr 2 2020;60(3):e137–e154. doi: 10.1093/geront/gnz009 [DOI] [PubMed] [Google Scholar]
- 9.Shukla A, Harper M, Pedersen E, et al. Hearing Loss, Loneliness, and Social Isolation: A Systematic Review. Otolaryngol Head Neck Surg. May 2020;162(5):622–633. doi: 10.1177/0194599820910377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peelle JE, Troiani V, Grossman M, Wingfield A. Hearing loss in older adults affects neural systems supporting speech comprehension. J Neurosci. Aug 31 2011;31(35):12638–43. doi: 10.1523/JNEUROSCI.2559-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckert MA, Cute SL, Vaden KI, Jr., Kuchinsky SE, Dubno JR. Auditory cortex signs of age-related hearing loss. J Assoc Res Otolaryngol. Oct 2012;13(5):703–13. doi: 10.1007/s10162-012-0332-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin FR, Ferrucci L, An Y, et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage. Apr 15 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vemuri P, Lesnick TG, Przybelski SA, et al. Development of a cerebrovascular magnetic resonance imaging biomarker for cognitive aging. Ann Neurol. Nov 2018;84(5):705–716. doi: 10.1002/ana.25346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. Aug 2 2011;77(5):461–8. doi: 10.1212/WNL.0b013e318227b227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen AT, Kouri N, Labuzan SA, et al. Neuropathologic scales of cerebrovascular disease associated with diffusion changes on MRI. Acta Neuropathol. Dec 2022;144(6):1117–1125. doi: 10.1007/s00401-022-02465-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shivamurthy VK, Tahari AK, Marcus C, Subramaniam RM. Brain FDG PET and the diagnosis of dementia. AJR Am J Roentgenol. Jan 2015;204(1):W76–85. doi: 10.2214/AJR.13.12363 [DOI] [PubMed] [Google Scholar]
- 17.Marinelli JP, Lohse CM, Fussell WL, et al. Association between hearing loss and development of dementia using formal behavioural audiometric testing within the Mayo Clinic Study of Aging (MCSA): a prospective population-based study. Lancet Healthy Longev. Dec 2022;3(12):e817–e824. doi: 10.1016/S2666-7568(22)00241-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graff-Radford J, Aakre JA, Knopman DS, et al. Prevalence and Heterogeneity of Cerebrovascular Disease Imaging Lesions. Mayo Clin Proc. Jun 2020;95(6):1195–1205. doi: 10.1016/j.mayocp.2020.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armstrong NM, An Y, Doshi J, et al. Association of Midlife Hearing Impairment With Late-Life Temporal Lobe Volume Loss. JAMA Otolaryngol Head Neck Surg. Sep 1 2019;145(9):794–802. doi: 10.1001/jamaoto.2019.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JW, Kim D, Lee S, et al. The Clinical Value of Periventricular White Matter Hyperintensity on MRI in Sudden Sensorineural Hearing Loss. Ann Otol Rhinol Laryngol. Mar 2022;131(3):244–251. doi: 10.1177/00034894211018925 [DOI] [PubMed] [Google Scholar]
- 21.Luo D, Li G, Qi W, Chen D. [Association of sudden sensorineural hearing loss and its prognosis with the brain white matter hyperintensity]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. Jul 2022;36(7):523–527. doi: 10.13201/j.issn.2096-7993.2022.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckert MA, Kuchinsky SE, Vaden KI, Cute SL, Spampinato MV, Dubno JR. White matter hyperintensities predict low frequency hearing in older adults. J Assoc Res Otolaryngol. Jun 2013;14(3):425–33. doi: 10.1007/s10162-013-0381-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein A, Covington BP, Mahabadi N, Mesfin FB. Neuroanatomy, Corpus Callosum. StatPearls. 2023. [PubMed] [Google Scholar]
- 24.Armstrong NM, Williams OA, Landman BA, Deal JA, Lin FR, Resnick SM. Association of Poorer Hearing With Longitudinal Change in Cerebral White Matter Microstructure. JAMA Otolaryngol Head Neck Surg. Nov 1 2020;146(11):1035–1042. doi: 10.1001/jamaoto.2020.2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jumah FR, Dossani RH. Neuroanatomy, Cingulate Cortex. StatPearls. 2023. [PubMed] [Google Scholar]
- 26.Arenaza-Urquijo EM, Przybelski SA, Lesnick TL, et al. The metabolic brain signature of cognitive resilience in the 80+: beyond Alzheimer pathologies. Brain. Apr 1 2019;142(4):1134–1147. doi: 10.1093/brain/awz037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berti V, Mosconi L, Pupi A. Brain: normal variations and benign findings in fluorodeoxyglucose-PET/computed tomography imaging. PET Clin. Apr 2014;9(2):129–40. doi: 10.1016/j.cpet.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerrouche N, Herholz K, Mielke R, Holthoff V, Baron JC. 18FDG PET in vascular dementia: differentiation from Alzheimer’s disease using voxel-based multivariate analysis. J Cereb Blood Flow Metab. Sep 2006;26(9):1213–21. doi: 10.1038/sj.jcbfm.9600296 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


