Abstract
Introduction:
Neurofibromatosis 1 and schwannomatosis are characterized by potential lifelong morbidity and life-threatening complications. To date, however, diagnostic and predictive biomarkers are an unmet need in this patient population. The inclusion of biomarker discovery correlatives in neurofibromatosis 1/schwannomatosis clinical trials enables study of low-incidence disease. The implementation of a common data model would further enhance biomarker discovery by enabling effective concatenation of data from multiple studies.
Methods:
The Response Evaluation in Neurofibromatosis and Schwannomatosis biomarker working group reviewed published data on emerging trends in neurofibromatosis 1 and schwannomatosis biomarker research and developed recommendations in a series of consensus meetings.
Results:
Liquid biopsy has emerged as a promising assay for neurofibromatosis 1/schwannomatosis biomarker discovery and validation. In addition, we review recommendations for a range of biomarkers in clinical trials, neurofibromatosis 1/schwannomatosis–specific data annotations, and common data models for data integration.
Conclusion:
These Response Evaluation in Neurofibromatosis and Schwannomatosis consensus guidelines are intended to provide best practices for the inclusion of biomarker studies in neurofibromatosis 1/schwannomatosis clinical trials, data, and sample annotation and to lay a framework for data harmonization and concatenation between trials.
Keywords: Biomarker, neurofibromatosis, schwannomatosis, cell-free DNA, consensus guidelines, liquid biopsy, cytokines, open science
Introduction
Neurofibromatosis 1 (NF1) and schwannomatosis (SWN), which now includes NF2-related SWN,1 are rare neurocutaneous syndromes characterized by the potential for lifelong morbidly and life-threatening complications.2–4 The identification of biomarkers for diagnosis of disease-associated manifestations, prediction of treatment response, and markers of response to treatment is an unmet need. The inclusion of biomarker discovery correlatives in NF1/SWN clinical trials presents an opportunity to power studies of a low-incidence population in a biologically relevant manner.
The development of effective therapies for NF1/SWN may itself be hindered by the lack of validated biomarkers. The cost of bringing novel therapeutics to market, estimated to be $1.3 billion per novel therapeutic from discovery through clinical trials,5,6 is especially challenging for relatively rare diseases like NF1/SWN where powering clinical trials7,8 and demonstrating market demand9 remains difficult. Identifying novel biomarkers in NF1 and SWN may therefore not only aid in diagnostics but also in patient compliance,10 selection, and stratification on intervention trials, thereby optimizing trial design and improving odds of detecting response in biologically targeted subpopulations. With advances in molecular stratification, however, a new obstacle arises: already small sample numbers are further subdivided into still smaller trial cohorts, thereby constraining power for novel biomarker discovery.11,12 These challenges in biomarker discovery and validation in NF1/SWN may be attenuated through effective integration of multiple data sets from different clinical trials and studies.13 Data harmonization, however, requires deliberate and uniform data collection, data structure and annotation, as well as transparency in data processing.
The goal of this article is to provide guidance for inclusion of biomarker endpoints in NF1/SWN clinical trials and to offer a framework to promote data sharing and collaboration through a standardized approach for data collection and reporting. Effective collaboration fosters community between stakeholders (patients, patient support groups, clinicians, researchers and industry) by promoting altruistic practices from investigators and effectively increasing the potential impact of each individual participant’s contributions.14
Methods
The Response Evaluation in Neurofibromatosis and Schwannomatosis (REiNS) International Collaboration was established 2011 to define and develop informative, reliable, and meaningful endpoints for clinical trials for NF1/SWN. The REiNS biomarker working group, which is comprised of community stakeholders, neurologists, neurosurgeons, industry representatives, oncologists, and pediatricians, met during a series of meetings in 2021–2023 to establish recommendations for the incorporation of biomarker discovery and validation correlatives in NF1/SWN clinical trials as well as to update our previously published3 standards of practice for data annotation in NF1/SWN biomarker research. In addition, the group performed a systematic literature search for emerging trends in NF1/SWN biomarker research since our 2016 update,3 reviewing and summarizing representative studies.
Results
Emerging biomarker technologies for NF1/SWN
Per the Food and Drug Administration’s (FDA)-National Institutes of Health (NIH) Biomarkers, Endpoints, and other Tools working group, biomarkers can be classified as diagnostic, monitoring, pharmacodynamic/response, predictive, safety, or susceptibility/risk biomarkers.15 Liquid biopsies, a term coined to describe the non-invasive study of analytes in human biofluids, has rapidly emerged as a non-invasive biomarker assay applicable to all Biomarkers, Endpoints, and other Tools biomarker categories.16–21 Circulating nucleic acids (e.g. cell-free DNA (cfDNA)), circulating proteins including cytokines and cell populations including circulating tumor cells, may be collected from multiple biofluids including blood, cerebrospinal fluid, urine, and tears.22 Although the application of this technology to cancer predispositions and rare disease has been recent, its potential has been quickly realized. For example, since the first publication in 2021 applying cfDNA to NF1,17 at least four additional publications have described the application of circulating DNA in NF117,24,25 (Table 1).
Table 1.
Summary of representative liquid biopsy studies in NF1.
| Study | NF1/SWN | Circulating analyte | Patient populationa | Primary findings |
|---|---|---|---|---|
| Blakeley et al.26 | Circulating protein | NF2 with vestibular schwannomas (n = 14) | • Bevacizumab treatment was associated with decreased free vascular endothelial growth factor (not bound to bevacizumab) and increased placental growth factor in plasma. • Hearing responses were inversely associated with baseline plasma hepatocyte growth factor (p = 0.019). |
|
| Gross et al.27 | NF1 | Circulating proangiogenic hematopoietic stem/progenitor cell populations (pCHSPC) |
PN (n = 42) | • The mean ratio of proangiogenic to non-angiogenic circulating hematopoietic stem/progenitor cells (pCHSPC/nCHSPC) measured at baseline and cycle 3 was reduced in subjects with PR to selumetinib versus subjects with SD or PD; discriminating between PR from SD/PD with 96.5% accuracy. |
| Szymanski et al.17 | NF1 | cfDNA | Healthy (n = 16), PN (n = 23), MPNST (n = 46) |
• Copy number analysis of in silico size-selected cfDNA fragments distinguished patients with PN versus MPNSTon pretreatment (91% specificity, 75% sensitivity, AUC 0.83), and serial (91% specificity, 83% sensitivity, AUC 0.89) cfDNA samples using ULP-WGS • cfDNA fragment length differs between MPNST, PN, and unaffected patients • MPNST cfDNA had focal copy number changes in MPNST-associated loci SUZ12, SMARCA2, CDKN2A/B, and chromosome arms 6p, 9p, 1q, 7p, 8q, and 17q. PN and MPNST cfDNA had focal loss of NF1, unaffected cfDNA did not. • cfDNA derived tumor fraction changes dynamically and correlates with clinical outcome in small case series. Increased tumor fraction predated radiographic evidence of progression, metastases, and recurrence. |
| Kallionpää et al.24 | NF1 | cfDNA | Healthy (n = 12), NF1 no PN (n = 13), PN (n = 6) | • No significant changes in cfDNA median plasma concentrations between healthy controls, patients with NF1, and patients with NF1 and a PN. |
| Fisher et al.28 | NF1 | cytokine | PN (n = 19) | • MIB-MS of GEM PN tissue treated with cabozantinib demonstrated shifts in MET, DDR1/2 and AXL kinases • Increased soluble AXL (sAXL) in plasma by ELISA associated with tumor response |
| Mattox et al.25 | NF1 | cfDNA | Healthy (n = 883), benign neurofibroma (n = 28), plexiform neurofibroma (n = 9), MPNST (n = 12) | • GAS RealSeqS amplicon–based sequencing distinguished PN from MPNSTwith 33% sensitivity and 97% specificity. Inclusion of subchromosomal CNA improved sensitivity to 50%, 97% specificity. • Subchromosomal focal copy number changes in MPNST-associated TERT, TP53, and SUZ12 loci. • GAS did not correlate with tumor volume, previous therapy, or PET positivity. • Low GAS score correlated with survival. PN with the highest GAS progressed to MPNSTwithin 25 months of blood draw. |
| Cortes-Ciriano et al.23 | NF1 | cfDNA | MPNST (n = 14)b | • Tissue loss of H3K27me3 associated with lower levels of immune cell infiltration. Tissue retention of H3K27me3 leads to genomic instability with an immune cell-rich phenotype. • MPNST tissue LOH of chromosome arm 5q, 11q, 7p, and 22q or amplification of 2q and 9p associated with poor prognosis. • Novel chromosomal aberrations associated with poor prognosis in tissue are detectable upon analysis of an external cfDNA data set. • Tissue chromosome 8 amplification described as a surrogate for H3K27me3. Chromosome 8 amplifications are detectable in an external cfDNA data set. |
NF1: neurofibromatosis 1; SWN: schwannomatosis; PN: plexiform neurofibroma; PR: partial response; SD: stable disease; PD: progressive disease; cfDNA, cell free DNA; MPNST: malignant peripheral nerve sheath tumor; AUC: area under the curve; ULP-WGS: ultra low-pass whole genome sequencing; MIB-MS: multiplexed inhibitor bead–mass spectrometry; GEM: genetically engineered mouse; DDR1/2: discoidin domain receptor tyrosine kinase (RTK) 1/2; ELISA: enzyme-linked immunoassay; GAS: genome wide aneuploidy score; CNA: copy number analysis; LOH: loss of heterozygosity; MET: mesenchymal epithlial transition RTK; PET: positron emission tomography imaging.
Study size reflects only subjects with circulating biomarker analytes.
Reanalysis of cfDNA data from Szymanski et al.
cfDNA has garnered specific interest as a biomarker in NF1/SWN due in part to its ability to characterize tumors with spatial heterogeneity29 and its rapid clearance from body fluids enabling “real-time” analysis.22 Many additional liquid biomarkers have been studied including additional DNA, RNA, and protein–based approaches.30 As an example, circulating cytokines may provide orthogonal insights into tumor growth, inflammation, and response to treatment. A recent clinical trial of the receptor tyrosine kinase inhibitor, cabozantinib, in plexiform neurofibroma (PN), for instance, showed an association with increased soluble AXL (sAXL) and tumor shrinkage.28 Defining molecular signatures in the circulation of patients with NF1 and SWN through establishment of collaborative biobanks and databases has the potential to enable diagnosis, therapy selection, and treatment monitoring of disease-related morbidities by phlebotomy.
Finally, liquid biopsy may be of particular benefit to patients with rare conditions like NF1/SWN, where geographic dispersion is a barrier to effective care. Due to relatively few patients with the disease at any given community or regional center,31,32 individual institutions may have a limited number of patients with NF1/SWN. The lack of highly specialized medical expertise in the local community and logistical challenges related to travel may, in turn, lead to delayed diagnosis of disease-related complications and malignancies, resulting in increased morbidity and mortality. For example, one recent meta-analysis of seven non-NF1/SWN-related, common cancer types found that a 1-month delay in curative treatment increases risk of mortality by 6%–13%.33 cfDNA collected with preservative, however, is stable for up to 14 days,34 overcoming geographic and logistical barriers in rare-tumor care by allowing samples to be collected by phlebotomists in remote or underserved regions and then analyzed by centers with expertise. However, consideration must be given to the timing and method of collection including the collection tube used as it can impact downstream analyses.35 Some studies suggest that dry blood spots, which could be collected at home, may also be a potential source of cfDNA.36 If NF1/SWN tumors can be molecularly characterized from blood drawn at regional labs, this would expand patient access to expertise at clinical centers of excellence for underserved and isolated populations.37,38
Recommendations for incorporating biomarker discovery and validation in clinical trials
Molecular characterization of tissue is invaluable for the identification of candidate biomarkers and molecular stratification of study participants. Post hoc analysis may reveal predictive markers for treatment response or drug toxicities. In addition, a priori knowledge from tissue can aid the identification of candidate circulating biomarkers.22 When tissue biopsy or resection is clinically indicated, we therefore recommend the collection of additional research unstained slides and, if sufficient material, flash frozen and formalin-fixed paraffin-embedded tissue for downstream analysis. Sample processing and storage should be implemented per the NCI Best Practices39 and Johns Hopkins NF1 Biorepository standard operating protocols.40
In addition, many institutions are now employing standard of care molecular profiling of tissues, and many patients are opting to send surgical specimens to outside vendors for additional genomic characterization. When possible, these additional molecular data should be extracted from reports and cataloged in a database to help inform biomarker selection, development, and validation.
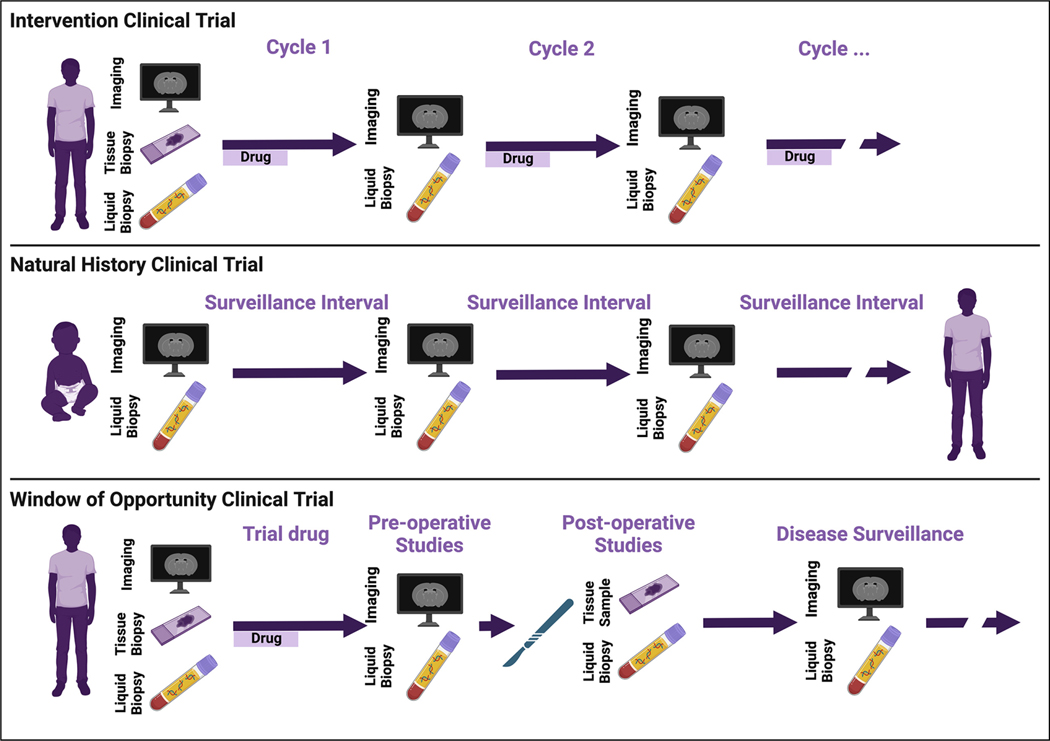
For evaluation of circulating biomarkers, minimum recommended times of sample collection are (1) upon enrollment in the study, (2) at the time of tissue biopsies or resections, (3) at the time of suspected or confirmed disease progression, and (4) at the time of planned imaging studies (Figure 1). Anchoring circulating biomarker samples to standard of care tissue and imaging evaluations is needed for evaluation of the metrics’ sensitivity and specificity as well as to improve the interpretability of interceding, unpaired samples. Specifically, pairing circulating analytes with tissue studies enables evaluation of whether detected circulating biomarkers accurately recapitulate the genomic, metabolomic, or proteomic landscape of the target tissue. Pairing with imaging allows correlation with established radiographic markers of response, for example, RECIST criteria.41
Figure 1.
Incorporation of molecular biomarkers in NF1/SWN clinical trials.
Exploratory correlatives with tissue and circulating biomarkers in interventional trials, windows of opportunity trials, and natural history studies hold promise for enhancing diagnosis, prognostication, and treatment stratification of patients with NF1/SWN.
Trials with local control, including resections in window of opportunity trials or standard of care interventions on natural history studies, provide opportunity for discovering biomarkers of minimum residual disease or transformation. Multiple fields of oncology have described cfDNA, for instance, as an accurate marker of residual disease.42–49 Indeed, it has been deemed reliable enough in many settings as to be a criterion for the addition of adjuvant therapy.46,50 Circulating tumor DNA (ctDNA) concentrations continue to decrease for up to 3 days postoperatively, even with total resection.44 Detection of ctDNA from residual disease, however, may remain blunted for up to 4 weeks postoperatively by relative increases in cfDNA released from healthy tissue during operative trauma.51 We therefore recommend that cfDNA be collected preoperatively, approximately 72 h postoperatively and, if ctDNA is not detected at 72 h, repeated 4 weeks postoperatively. To enable direct comparison of candidate biomarker performances and potential multi-omic integration,18 additional circulating biomarkers including cytokines and circulating immune markers should be collected on this same schedule (Table 2).
Table 2.
Summary of recommendations for incorporation of biomarker correlatives in NF1/SWN clinical trials.
| • When feasible, unstained research tissue samples should be collected at study enrollment and all clinical tissue evaluations. |
| • Circulating biomarkers should be collected, at a minimum, with: |
| ∘ Study enrollment |
| ∘ Tissue biopsy or resection |
| ■ Circulating biomarkers (cfDNA, cytokines, proteins): preoperatively, 72 h postoperatively, 4 weeks postoperatively |
| ∘ Suspected or confirmed disease progression |
| ∘ Imaging studies |
Recommendations for facilitating data harmonization
In addition to implementing biomarker correlatives for the evaluation of endpoints on individual clinical trials, opportunity exists to broaden the understanding of NF1/SWN biology through concatenation of data from multiple studies. These collaborative efforts would have the potential to power drug development for disease states otherwise deprioritized by industry.5 A hurdle to meaningful multi-site data integration, however, is harmonization of institutional data structures into a shared, common data model with common representation of terminologies, vocabularies, and coding schemes in sample and clinical annotation. The potential of multi-modal, multi-study data harmonization through the adoption of common data model (Figure 2) to accelerate research in rare diseases has already been endorsed by multiple consortia including the European Joint Initiative Toward Semantic Interoperability in Rare Disease Research52 and Project Data Sphere Initiative.14 For NF1/SWN, efforts to develop a common data model include the NF-Open Science Initiative’s NF Data Portal53 with an open-source metadata dictionary54 based on the NCI Thesaurus,55 Experimental Factor Ontology,56 and Global Alliance for Genomics and Health57–59 biomedical ontologies but tailored to NF1 and SWN experimental data terms.53 The metadata dictionary defines minimum data elements and shared data language. Importantly, as a living document welcoming contributions and input from NF1/SWN community members, it enables dynamic ontology management as new technologies and disease insights emerge. It is our recommendation that NF1/SWN experimental data, whether deposited on the NF Data Portal associated Synapse repository or alternative public data repositories, be annotated according to the NF-Open Science Initiative’s metadata dictionary. For cfDNA data, it is particularly important to include labels for the type of collection tube, library kit, and adapters used as well as the number of cycles of polymerase chain reaction (PCR) libraries underwent.
Figure 2.
Common data models and annotation ontology enables powered biomarker discovery and validation through the integration of multi-modal data from multiple studies and institutions.
To power biomarker discovery in rare diseases like NF1 and SWN, standardized data collection, annotation, and processing must be widely adopted. This would enable data concatenation and harmonization. Importantly, resultant data, including raw molecular data, should be publicly available and disseminated through data repositories.
In addition to a common data model in experimental data, common ontologies must be adopted for clinical correlates. Minimal clinical and demographic elements are outlined in our previous guidelines.3 It is essential that minimal sample annotation also includes current diagnostic criteria for paired datapoints. For instance, when matched tissue is available, liquid biopsy studies describing peripheral nerve sheath tumors should include comment on all consensus histological features of atypical neurofibroma and malignant peripheral nerve sheath tumors to allow for uniform labeling of correlated samples.60 Annotation of matched imaging files should include apparent diffusion coefficient (ADC) and standardized uptake value (SUV),61 if available, as well as tumor measurements or volumetrics. We recommend adoption of the Integration of Observational Medical Outcomes Partnership Oncology Module62 standardized vocabulary with NF1/SWN-specific terms from the 2016 REiNS Biomarker Guidelines3 for clinical annotations. The field, however, would benefit in the future from development of a central NF1/SWN specific clinical common data model or Observational Medical Outcomes Partnership module.
While prospective multi-trial data sets will benefit from the implementation of a common data model, an additional need for hypothesis generation and better powering future biomarker studies is the harmonization of existing NF1/SWN data sets. Extraction, transformation, and loading processes lift the data from its original source, cleans and de-duplicates the data, and then integrates the data into a common data model.63 Extraction, transformation, and loading of existing study data while maintaining previous data structures, as opposed to construction of a novel unified data-entry system, would reduce cost and risk of transcription or coding errors during data re-entry. Implementation of templated extraction, transformation, and loading processes, however, require development of data-specific algorithms and would likely require centralized, disease-specific platforms or repositories resourced for extraction, transformation, and loading process development and maintenance These recommendations are summarized in Table 3.
Table 3.
Summary of recommended best practices for data annotation.
| • Clinical minimal annotations per 2016 REiNS Biomarker guidelines |
| ∘ Standardized vocabulary using the Observational Medical Outcomes Partnership Oncology Module supplemented by 2016 REiNS Biomarker guideline terms |
| • Sample annotation should describe samples of interest as well as matched datapoints/studies |
| ∘ Time from sample collection to sample processing/storage |
| ∘ Minimal annotation of paired tissue should: |
| ■ Detail timing relative to the candidate biomarker collection |
| ■ Document timing relative to administration of last dose of therapeutic agent (if applicable) |
| ■ Document timing from tissue collection to processing/storage |
| ■ Address all criteria of consensus histologic or genomic guidelines |
| ∘ Minimal annotation of paired imaging should: |
| ■ Detail timing relative to the candidate biomarker collection |
| ■ Detail imaging modality and anatomic locations |
| ■ Include ADC if DWI performed |
| ■ Include SUV if PET performed |
| ■ Include sum of the longest diameter and, if available, volumetrics |
| ■ Include RECIST category, if relevant |
| • NF1/SWN experimental data should be annotated per the NF OSI metadata dictionary ontology and data structure. |
| • We recommend harmonization of existing NF1/SWN data through funding and maintenance of extraction, transformation and loading processes with disease-specific terms and dictionaries on a central NF1/SWN data repository. |
REiNS: Response Evaluation in Neurofibromatosis and Schwannomatosis; NF1: neurofibromatosis 1; SWN: schwannomatosis; ADC: apparent diffusion coefficient; SUV: standardized uptake value; DWI: diffusion weighted imaging; OSI: open science inititiave.
Standards for data reproducibility and usability
The FAIR Guiding Principles for scientific data management and stewardship provide consensus standards for data reusability, emphasizing the core themes of making scholarly data findable, accessible, interoperable, and reusable.64 The REiNS biomarker working group has integrated these themes into guidelines pertinent for NF1/SWN research and data.
Batch effect from technical, non-biologic variations during sample collection, processing, and preparation can result in erroneous conclusions and hinder the reproducibility and generalizability of findings.65–68 This challenge is amplified when combining datasets,69,70 as we propose, to better power analyses of rare disease states and treatment effectiveness. To minimize batch effect and enhance the validity and reliability of NF1/SWN biomarker research, it is imperative that preprocessing conditions, detailed methodology, and complete analytic pipelines are described and publicly available. In design of combined-data set experiments, propensity score methods should be considered to balance patient characteristics from separate trials.14 Furthermore, when publishing analyses from combined data sets, best practices include statistical analysis to determine whether institution or data set of origin, technical variations in methodology, and timing of sample collection are significant covariates.17 These comparisons require public and comprehensive metadata.
Research papers’ materials and methods rarely provide sufficient details for step-by-step replication;71 however, granularity (e.g. cycles of PCR) can have significant impact on the interpretability of specific analyses.68 Reproducibility can be enhanced by depositing detailed protocols in repositories such as Nature Protocol Exchange72 or protocol.io,73 both of which provide permanent citable identifiers. Alternatively, protocols may be published in journals including Structured Transparent Accessible Reproducible Protocols, bio-protocol, or Current Protocols, which aim specifically to disseminate step-by-step, reproducible methods.
In addition, the use of versioned, standardized computational pipelines74 improves reproducibility, interoperability, and accelerates scientific discovery.74,75 Established workflows, including peer-reviewed Nextflow nf-core pipelines,74 are already being used in efforts by NF-Open Science Initiative to standardize reprocessed data from deposited NF/SWN genomic and transcriptomic data.76 When standardized workflows are not used, custom scripts and code for data analysis should be published to version-controlled repositories with permanent, citable identifiers.75 To address compatibility issues in published pipelines resulting from changed dependencies and software versions, local computing environments can be immortalized in portable and easily distributed saved image files called containers.77–79 This enables the preservation of the software versions and dependencies that were used for initial analyses, thereby promoting reproducibility as well as facilitating the deconstruction of processed published data for data concatenation or repurposing in novel applications. We therefore recommend that investigators provide publicly available containers using Docker,78 Singularity,77 or similar platforms accompanying all custom scripts and code used for analysis.
Finally, preanalytic processing and variations in reference data sets can augment non-biologic differences in data sets.68 To help mitigate these batch effects when concatenating data, we recommend that raw data formats (e.g. FASTQ) as opposed to preprocessed formats (e.g. BAM) should be deposited in public data repositories. To facilitate reproducibility, all relevant citable protocol identifiers, scripts and associated containers should be included in the data provenance in the repository. These recommendations are summarized in Table 4.
Table 4.
Summary of recommended best practices for data reproducibility and usability.
| • Publication of step-by-step methodology |
| ∘ Publication in protocol journal or protocol repository, for example, Protocols.io or Nature Protocol Exchange |
| ∘ Permanent citable identifier |
| • Use of consensus pipeline or publication of all custom scripts and code used in data analysis, for example, on GitHub |
| • Preservation and publication of local computing environment using containers |
| • Data sharing of raw files in public access repositories with needed metadata |
| ∘ Notation of protocol identifier in data’s provenance |
| • Linked data annotation per Table 3 recommendations |
Conclusion
The discovery and validation of diagnostic, predictive, and response biomarkers are an unmet need in NF1 and SWN. The identification and validation of effective biomarkers have the potential to improve patient outcomes through improved diagnostics, risk adaptive disease surveillance, molecularly guided therapies and, potentially, improved success of clinical trials through guided subgrouping and earlier determination of treatment benefit or toxicity. Biomarker discovery has, however, been hindered by low-incidence disease, non-standardized procedures, and still developing technologies that have resulted in underpowered studies. The inclusion of exploratory biomarker correlatives in NF1 and SWN clinical trials holds promise for improving statistical power of biomarker studies. The implementation of a common data model, building off disease-specific experimental metadata dictionaries from the NF-Open Science Initiative,53 paired with best practices in methods, data and analysis sharing, would further enhance biomarker discovery by enabling multi-modal, multi-study data sets. The field would benefit from harmonization of clinical labels and terms through the development of an independent NF1/SWN common data model or extension of existing common data model with NF1/SWN-specific modules. Finally, support for NF1/SWN-specific central repositories with resources for the development and maintenance of extraction, transformation, and loading processes would decrease data management burdens on institutions with well-established data infrastructures and would improve the incorporation of existing studies’ data.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: Figures were created using BioRender. R.T.S. is supported by the National Cancer Institute (NCI) Center for Cancer Research Intramural Research Program (1ZIABC011722-04), Neurofibromatosis Therapeutic Acceleration Program (NTAP) (230116), and Children’s Tumor Foundation (CTF-2022-10-002). D.G.E. is supported by the Manchester National Institute for Health Research (NIHR) Biomedical Research Centre (IS-BRC-1215-20007). C.B. is supported by NIH grants R37CA230400 and U01CA230691. C.O.H. is funded by the charity Brain Tumour Research. S.D.R. is supported by NTAP (2004757180) and NINDS (5K08NS128266-02). H.S. and Infixion Bioscience are supported by the National Institutes of Health (NIH) (R43NS117234, R43NS124424, and R43NS127718).
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.T.S. has patent filings related to cancer biomarkers. C.B. is a consultant to Depuy-Synthes, Bionaut Labs, Haystack Oncology, Privo Technologies, and Galectin Therapeutics. C.B. is a co-founder of OrisDx and Belay Diagnostics. C.O.H. has received research support from BergenBio. J.O.B. serves on the advisory board of SpringWorks Therapeutics. HS is CEO of Infixion Bioscience. V.G. is Executive Vice President and CEO of the NYS Academy of Family Physicians and a member of the Board of Directors of the Neural Stem Cell Institute.
References
- 1.Plotkin SR, Messiaen L, Legius E, et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: an international consensus recommendation. Genet Med 2022; 24(9): 1967–1977. [DOI] [PubMed] [Google Scholar]
- 2.Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 2002; 39: 311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanemann CO, Blakeley JO, Nunes FP, et al. Current status and recommendations for biomarkers and biobanking in neurofibromatosis. Neurology 2016; 87: S40–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotkin SR, Stemmer-Rachamimov AO, Barker FG II, et al. Hearing improvement after bevacizumab in patients with neurofibromatosis type 2. N Engl J Med 2009; 361: 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karpen SR, White JK, Mullin AP, et al. Effective data sharing as a conduit for advancing medical product development. Ther Innov Regul Sci 2021; 55(3): 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wouters OJ, McKee M and Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009–2018. JAMA 2020; 323: 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Augustine EF, Adams HR and Mink JW. Clinical trials in rare disease: challenges and opportunities. J Child Neurol 2013; 28(9): 1142–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pizzamiglio C, Vernon HJ, Hanna MG, et al. Designing clinical trials for rare diseases: unique challenges and opportunities. Nat Rev Methods Primers 2022; 2(1): s43586–022-00100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yates N and Hinkel J. The economics of moonshots: value in rare disease drug development. Clin Transl Sci 2022; 15(4): 809–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pauler DK, Gower KB, Goodman PJ, et al. Biomarker-based methods for determining noncompliance in a prevention trial. Control Clin Trials 2002; 23(6): 675–685. [DOI] [PubMed] [Google Scholar]
- 11.Berry DA. The Brave New World of clinical cancer research: adaptive biomarker-driven trials integrating clinical practice with clinical research. Mol Oncol 2015; 9(5): 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman R, Ventz S, McDunn J, et al. Leveraging external data in the design and analysis of clinical trials in neuro-oncology. Lancet Oncol 2021; 22(10): e456–e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo T. Current status and perspectives of patient-derived rare cancer models. Hum Cell 2020; 33(4): 919–929. [DOI] [PubMed] [Google Scholar]
- 14.Green AK, Reeder-Hayes KE, Corty RW, et al. The project data sphere initiative: accelerating cancer research by sharing data. Oncologist 2015; 20(5): 464–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cagney DN, Sul J, Huang RY, et al. The FDA NIH e(BEST) resource in neuro-oncology. Neuro Oncol 2018; 20: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chabon JJ, Hamilton EG, Kurtz DM, et al. Integrating genomic features for non-invasive early lung cancer detection. Nature 2020; 580(7802): 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szymanski JJ, Sundby RT, Jones PA, et al. Cell-free DNA ultra-low-pass whole genome sequencing to distinguish malignant peripheral nerve sheath tumor (MPNST) from its benign precursor lesion: a cross-sectional study. PLoS Med 2021; 18(8): e1003734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JD, Li L, Wang Y, et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018; 359: 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cristiano S, Leal A, Phallen J, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 2019; 570(7761): 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol 2020; 31: 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen SY, Singhania R, Fehringer G, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature 2018; 563(7732): 579–583. [DOI] [PubMed] [Google Scholar]
- 22.Sundby RT, Pan A and Shern JF. Liquid biopsies in pediatric oncology: opportunities and obstacles. Curr Opin Pediatr 2022; 34(1): 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cortes-Ciriano I, Steele CD, Piculell K, et al. Genomic Patterns of malignant peripheral nerve sheath tumor (MPNST) evolution correlate with clinical outcome and are detectable in cell-free DNA. Cancer Discov 2023; 13: 654–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallionpää RA, Ahramo K, Aaltonen M, et al. Circulating free DNA in the plasma of individuals with neurofibromatosis type 1. Am J Med Genet A 2021; 185(4): 1098–1104 [DOI] [PubMed] [Google Scholar]
- 25.Mattox AK, Douville C, Silliman N, et al. Detection of malignant peripheral nerve sheath tumors in patients with neurofibromatosis using aneuploidy and mutation identification in plasma. Elife 2022; 11: e74238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blakeley JO, Ye X, Duda DG, et al. Efficacy and biomarker study of bevacizumab for hearing loss resulting from neurofibromatosis type 2-associated vestibular schwannomas. J Clin Oncol 2016; 34: 1669–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross AM, Wolters PL, Dombi E, et al. Selumetinib in children with inoperable plexiform neurofibromas. N Engl J Med 2020; 382: 1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher MJ, Shih CS, Rhodes SD, et al. Cabozantinib for neurofibromatosis type 1-related plexiform neurofibromas: a phase 2 trial. Nat Med 2021; 27(1): 165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira B, Chen CT, Goyal L, et al. Cell-free DNA captures tumor heterogeneity and driver alterations in rapid autopsies with pre-treated metastatic cancer. Nature Commun 2021; 12: 3199–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ignatiadis M, Sledge GW and Jeffrey SS. Liquid biopsyenters the clinic—implementation issues and future challenges. Nat Rev Clin Oncol 2021; 18(5): 297–312. [DOI] [PubMed] [Google Scholar]
- 31.Greenlee RT, Goodman MT, Lynch CF, et al. The occurrence of rare cancers in U.S. adults, 1995–2004. Public Health Rep 2010; 125(1): 28–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharifnia T, Hong AL, Painter CA, et al. Emerging opportunities for target discovery in rare cancers. Cell Chem Biol 2017; 24: 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanna TP, King WD, Thibodeau S, et al. Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ 2020; 371: m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parackal S, Zou D, Day R, et al. Comparison of roche cell-free DNA collection tubes ((R)) to streck cell-free DNA BCT ((R)) s for sample stability using healthy volunteers. Pract Lab Med 2019; 16: e00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maass KK, Schad PS, Finster AME, et al. From sampling to sequencing: a liquid biopsy pre-analytic workflow to maximize multi-layer genomic information from a single tube. Cancers 2021; 13(12): 3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heider K, Wan JCM, Hall J, et al. Detection of ctDNA from dried blood spots after DNA size selection. Clin Chem 2020; 66: 697–705. [DOI] [PubMed] [Google Scholar]
- 37.Cai C, Gaffney A, McGregor A, et al. Racial and ethnic disparities in outpatient visit rates across 29 specialties. JAMA Intern Med 2021; 181: 1525–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reisner SL, Mateo C, Elliott MN, et al. Analysis of reported health care use by sexual orientation among youth. JAMA Netw Open 2021; 4: e2124647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institutes of Health. NCI best practices for biospecimen resources, 2016, https://biospecimens.cancer.-gov/bestpractices/
- 40.Pollard K, Banerjee J, Doan X, et al. A clinically andgenomically annotated nerve sheath tumor biospecimen repository. Sci Data 2020; 7: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 2017; 7(12): 1394–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chauhan PS, Shiang A, Alahi I, et al. Urine cell-free DNA multi-omics to detect MRD and predict survival in bladder cancer patients. NPJ Precis Oncol 2023; 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen K, Zhao H, Shi Y, et al. Perioperative dynamic changes in circulating tumor DNA in patients with lung cancer (DYNAMIC). Clin Cancer Res 2019; 25: 7058–7067. [DOI] [PubMed] [Google Scholar]
- 45.Chin RI, Chen K, Usmani A, et al. Detection of solid tumor molecular residual disease (MRD) using circulating tumor DNA (ctDNA). Mol Diagn Ther 2019; 23(3): 311–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Henriksen TVV, Tarazona N, Frydendahl A, et al. Serial circulating tumor DNA analysis to assess recurrence risk, benefit of adjuvant therapy, growth rate and early relapse detection in stage III colorectal cancer patients. J Clin Oncol 2021; 39: 3540–3540. [Google Scholar]
- 47.Killock D. DYNAMIC insights into MRD responses early after resection of NSCLC. Nat Rev Clin Oncol 2019; 16(11): 661. [DOI] [PubMed] [Google Scholar]
- 48.Pellini B, Pejovic N, Feng W, et al. CtDNA MRD detection and personalized oncogenomic analysis in oligometastatic colorectal cancer from plasma and urine. JCO Precis Oncol 2021; 5: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vrabel D, Sedlarikova L, Besse L, et al. Dynamics of tumor-specific cfDNA in response to therapy in multiple myeloma patients. Eur J Haematol 2020; 104(3): 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Powles T, Assaf ZJ, Davarpanah N, et al. CtDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021; 595: 432–437. [DOI] [PubMed] [Google Scholar]
- 51.Henriksen TV, Reinert T, Christensen E, et al. The effect of surgical trauma on circulating free DNA levels in cancer patients-implications for studies of circulating tumor DNA. Mol Oncol 2020; 14(8): 1670–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abaza H, Kadioglu D, Martin S, et al. Domain-specific common data elements for rare disease registration: conceptual approach of a European joint initiative toward semantic interoperability in rare disease research. JMIR Med Inform 2022; 10: e32158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allaway RJ, La Rosa S, Verma S, et al. Engaging a community to enable disease-centric data sharing with the NF data portal. Sci Data 2019; 6: 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vu AN, Banerjee J and Allaway R. nf-osi/nf-metadata-dictionary: v7.1.8, 2023, https://zenodo.org/record/8121888
- 55.Ceusters W, Smith B and Goldberg L. A terminological and ontological analysis of the NCI thesaurus. Methods Inf Med 2005; 44(4): 498–507. [PubMed] [Google Scholar]
- 56.Malone J, Holloway E, Adamusiak T, et al. Modeling sample variables with an experimental factor ontology. Bioinformatics 2010; 26: 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Courtot M, Cherubin L, Faulconbridge A, et al. BioSamples database: an updated sample metadata hub. Nucleic Acids Res 2019; 47: D1172–D1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawson J, Cabili MN, Kerry G, et al. The data use ontology to streamline responsible access to human biomedical datasets. Cell Genom 2021; 1: 100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorogood A, Rehm HL, Goodhand P, et al. International federation of genomic medicine databases using GA4GH standards. Cell Genom 2021; 1(2): 100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miettinen MM, Antonescu CR, Fletcher CDM, et al. Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview. Hum Pathol 2017; 67: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahlawat S, Blakeley JO, Rodriguez FJ, et al. Imaging biomarkers for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Neurology 2019; 93: e1076–e1084. [DOI] [PubMed] [Google Scholar]
- 62.Belenkaya R, Gurley MJ, Golozar A, et al. Extending the OMOP common data model and standardized vocabularies to support observational cancer research. JCO Clin Cancer Inform 2021; 5: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Quiroz JC, Chard T, Sa Z, et al. Extract, transform, load framework for the conversion of health databases to OMOP. PLoS ONE 2022; 17: e0266911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilkinson MD, Dumontier M, Aalbersberg IJ, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data 2016; 3: 160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009; 55(4): 611–622. [DOI] [PubMed] [Google Scholar]
- 66.Johnson WE, Li C and Rabinovic A. Adjusting batcheffects in microarray expression data using empirical Bayes methods. Biostatistics 2007; 8(1): 118–127. [DOI] [PubMed] [Google Scholar]
- 67.Sharma M, Verma RK, Kumar S, et al. Computational challenges in detection of cancer using cell-free DNA methylation. Comput Struct Biotechnol J 2022; 20: 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tom JA, Reeder J, Forrest WF, et al. Identifying and mitigating batch effects in whole genome sequencing data. BMC Bioinform 2017; 18: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sims AH, Smethurst GJ, Hey Y, et al. The removal of multiplicative, systematic bias allows integration of breast cancer gene expression datasets—improving meta-analysis and prediction of prognosis. BMC Med Genomics 2008; 1: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soneson C, Gerster S and Delorenzi M. Batch effect confounding leads to strong bias in performance estimates obtained by cross-validation. PLoS ONE 2014; 9(6): e100335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siegel V. Share the details of your experimental methods. Mol Biol Cell 2020; 31: 2879–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Introducing the new protocol exchange site. Nat Protoc 2019; 14(7): 1945. [DOI] [PubMed] [Google Scholar]
- 73.Teytelman L, Stoliartchouk A, Kindler L, et al. Protocols. Io: virtual communities for protocol development and discussion. PLoS Biol 2016; 14: e1002538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ewels PA, Peltzer A, Fillinger S, et al. The nf-core framework for community-curated bioinformatics pipelines. Nat Biotechnol 2020; 38(3): 276–278. [DOI] [PubMed] [Google Scholar]
- 75.Wilson G, Bryan J, Cranston K, et al. Good enough practices in scientific computing. PLoS Comput Biol 2017; 13(6): e1005510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Banerjee J, Grande BM, Scott S, et al. Accelerating exploration of the genomic landscape of neurofibromatosis using harmonized genomic data. Philadelphia, PA: Children’s Tumor Foundation, 2022. [Google Scholar]
- 77.Kurtzer GM, Sochat V and Bauer MW. Singularity: scientific containers for mobility of compute. PLoS ONE 2017; 12(5): e0177459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Merkel D. Docker: lightweight Linux containers for consistent development and deployment. Linux J 2014; 2014: 2. [Google Scholar]
- 79.Mitra-Behura S, Fiolka RP and Daetwyler S. Singularity containers improve reproducibility and ease of use in computational image analysis workflows. Front Bioinform 2021; 1: 757291. [DOI] [PMC free article] [PubMed] [Google Scholar]